Abstract
Sitamaquine (WR6026), an 8-aminoquinoline derivative, is a new antileishmanial oral drug. As a lipophilic weak base, it rapidly accumulates in acidic compartments, represented mainly by acidocalcisomes. In this work, we show that the antileishmanial action of sitamaquine is unrelated to its level of accumulation in these acidic vesicles. We have observed significant differences in sitamaquine sensitivity and accumulation between Leishmania species and strains, and interestingly, there is no correlation between them. However, there is a relationship between the levels of accumulation of sitamaquine and acidotropic probes, acidocalcisomes size, and polyphosphate levels. The Leishmania major AP3δ-null mutant line, in which acidocalcisomes are devoid of their usual polyphosphate and proton content, is unable to accumulate sitamaquine; however, both the parental strain and the AP3δ-null mutants showed similar sensitivities to sitamaquine. Our findings provide clear evidence that the antileishmanial action of sitamaquine is unrelated to its accumulation in acidocalcisomes.
In the absence of effective vaccines against leishmaniasis, the main weapon to control the disease relies exclusively on chemotherapy. Although pentavalent antimonials have been the first-line treatment for many years, the emergence of antimony resistance compromised their use. Alternative treatments such as amphotericin B, paromomycin, and more recently, miltefosine have replaced antimonials for use in disease control (1). Sitamaquine (WR6026) is a new antileishmanial oral drug currently in phase 2b clinical trials by GlaxoSmithKline (16, 28). Sitamaquine is a lipophilic weak base that rapidly accumulates in acidic compartments of Leishmania spp., mainly in acidocalcisomes. It has been suggested, using permeabilized Leishmania parasites, that the alkalinization produced by sitamaquine in the acidocalcisomes could be involved in its antileishmanial action (27); however, its mechanism of action is still unknown. In addition to this, it has been reported that Leishmania species show different sensitivities to sitamaquine in vitro (12). This characteristic could affect sitamaquine effectiveness and should be considered in future treatments.
Acidocalcisomes are dense acidic organelles with a high concentration of phosphorus present as pyrophosphate and polyphosphate (polyP) complexed with calcium and other elements, including sodium, magnesium, and zinc, and are the main calcium storage compartments of trypanosomatids (6). Acidocalcisomes, conserved from bacteria to humans, could play an important role in Leishmania physiology. Their functions include (i) storage for polyP and calcium, which could be involved in energy sources and signaling processes, respectively; (ii) pH homeostasis, in which polyP could be involved in intracellular pH regulation through the H+ produced from its hydrolysis; and (iii) osmoregulation, in which acidocalcisomes respond to osmotic stress by changing their sodium and chloride content. Some of these functions are related to the presence of several pumps and exchangers in the acidocalcisome membrane. Pumps that have been described are calcium pumps (Ca2+-ATPase), two proton pumps, a vacuolar-type H+-ATPase (V-H+-ATPase) and a vacuolar-type H+-pyrophosphatase (V-H+-PPase), and Na+/H+ and Ca2+/H+ exchangers (6).
The aim of this study was to determine if there was a correlation between sitamaquine sensitivity and accumulation in different Leishmania species. Furthermore, we have found an explanation for the differences observed in sitamaquine accumulation between the Leishmania species L. donovani and L. tropica. We have identified the fact that acidocalcisomes play a key role in the accumulation of sitamaquine in nonpermeabilized parasites and that they can be considered the main factor which determines the differences shown by Leishmania strains in terms of sitamaquine accumulation but not the antileishmanial potency of the drug.
MATERIALS AND METHODS
Leishmania strains and culture.
L. donovani MHOM/ET/67/L82, L. donovani MHOM/IN/80/DD8, L. tropica MHOM/SU/60/LCR-L39, L. tropica MHOM/SU/74/SAF-K27, Leishmania infantum MHOM/ES/1993/BCN-99, Leishmania mexicana M9012, and Leishmania braziliensis MHOM/PE/03/LH2419 promastigotes were grown at 28°C in RPMI 1640-modified medium (Invitrogen, Carlsbad, CA) and supplemented with 20% heat-inactivated fetal bovine serum (Invitrogen). So that they worked under the same growth stage conditions, all the parasite strains were always collected after 48 h of growth, by centrifugation, and washed in phosphate-buffered saline (PBS; 1.2 mM KH2PO4, 8.1 mM Na2HPO4, 130 mM NaCl, and 2.6 mM KCl, adjusted to pH 7). The final concentration of parasites was determined using a Coulter Counter Z1 system.
The Leishmania major (MHOM/JL/80/Friedlin) promastigote lines, the wild type, the AP3δ-null mutant, and the AP3δ-null mutant transfected with the AP3δ gene (designated AP3 complemented) were from J. Mottram (4).
Chemical compounds.
Sitamaquine [N,N-diethyl-N′-(6-methoxy-4-methylquinolin-8-yl)hexane-1,6-diamine] dihydrochloride and benzene ring U-14C-labeled sitamaquine ([14C]sitamaquine; 2.07 GBq/mmol) were provided by GlaxoSmithKline (Greenford, United Kingdom). Ammonium chloride, monensin sodium salt, nigericin sodium salt, sodium azide, 2-deoxy-d-glucose, resazurin sodium salt (Alamar Blue), and DAPI (4′,6-diamidino-2-phenylindole dilactate) were purchased from Sigma. Lysotracker Green DND-26, Lysotracker Red DND-99, and Lysosensor Yellow-Blue DND-160 were from Invitrogen.
Determination of sitamaquine accumulation.
Leishmania parasites washed twice with PBS were resuspended in HEPES-buffered saline (HBS; 21 mM HEPES, 0.7 mM Na2HPO4, 137 mM NaCl, 5 mM KCl, and 6 mM dextrose, adjusted to pH 7). A final concentration of 2 × 107 parasites per ml was incubated at 28°C or 4°C with 5 μM [14C]sitamaquine for 15 min in the presence or absence of different concentrations of nonradioactive sitamaquine. Afterwards, samples were removed and placed on ice. The parasites were spun down in a microcentrifuge and washed in PBS or in PBS containing 100 μΜ sitamaquine for 10 min on ice, followed by two washes with PBS to remove the radiolabeled drug adhered to the cell surface, as previously described for the chloroquine uptake assays in yeast (10). Finally, the cell pellet was resuspended in 0.1 ml of 1% Triton X-100. Eight microliters of the sample were used for protein determination with a Bradford kit (Bio-Rad), and the remaining volume was used to determine cell-associated radioactivity by liquid scintillation counting.
Energy, protein, pH, and H+ gradient dependence in the sitamaquine uptake process.
Parasite suspensions were prepared as described above. For the energy depletion study, parasites were preincubated for 30 min at 28°C in HBS buffer without glucose, with 5 mM 2-deoxy-d-glucose and 20 mM sodium azide as previously described (2). For protein modification, parasites were treated with 1 mM N-ethylmaleimide (NEM) for 15 min on ice, centrifuged, and resuspended in fresh HBS as previously described (22). H+ gradient dependence was determined with parasites pretreated at 28°C in HBS with 20 mM NH4Cl for 1 min and 10 μM of the ionophores nigericin and monensin for 10 min. Finally, 2 × 107 parasites per ml were incubated at 28°C with 5 μM [14C]sitamaquine for 15 min in HBS for parasites pretreated with NEM or in HBS without glucose for energy depletion studies. Parasites preincubated with ionophores and NH4Cl were incubated with 5 μM [14C]sitamaquine for 5 min in HBS. The influence of extracellular pH in drug uptake was established with parasites incubated at 28°C with radiolabeled sitamaquine in HBS adjusted to different pHs. Samples were then removed and placed on ice. The parasites were spun down and washed in PBS containing 100 μΜ sitamaquine for 10 min on ice, followed by two washes with PBS. The amount of drug incorporated into the cells was determined as described above.
Sitamaquine sensitivity assay.
The sensitivity of Leishmania parasites to sitamaquine was determined after a 72-h incubation at 28°C in the presence of increasing concentrations of sitamaquine (24). The concentration of sitamaquine necessary to inhibit the parasites growth by 50% (EC50) was calculated by the Alamar Blue method (20) using a spectrofluorometer (Molecular Devices Ltd., Wokingham, United Kingdom) at an excitation and emission wavelength of 530 nm and 585 nm, respectively.
Amastigote sensitivity in vitro.
Late-stage promastigotes of wild-type and AP3δ-null mutant L. major lines were used to infect peritoneal macrophages from BALB/c mice (Charles River Ltd.) at a ratio of 1:5 macrophages/parasites, as previously described (25). After 4 h of infection, excess parasites were removed by washing with serum-free medium. The infected macrophage cultures were maintained at 37°C with 5% CO2 with different sitamaquine concentrations in RPMI 1640 medium plus 10% heat-inactivated fetal bovine serum. After 72 h, samples were fixed for 20 min at 4°C with 2% (wt/vol) paraformaldehyde in PBS, followed by permeabilization with 0.1% Triton X-100 in PBS for 10 min. Intracellular parasites were detected by nuclear staining (Prolong-Gold antifade reagent with DAPI; Invitrogen). The percentage of infection and the mean number of amastigotes by infected macrophages were calculated in 200 macrophages/well. Three independent experiments were performed with duplicates.
pH determination of acidic organelles.
The measurements of acidic vesicle pHs in different Leishmania strains and species were carried out using a modification of a previously described assay for the determination of lysosomal pH in fibroblasts (15). The pH-sensitive fluorescent probe Lysosensor Yellow-Blue DND-160 (Molecular Probes) was used at 50 μM for 5 min at 28°C with 1.5 × 108 parasites per ml, previously washed with PBS and resuspended in HBS glucose buffer. Excess dye was removed with cold PBS washing. Finally, 1 × 107 parasites per ml were resuspended in 2 ml of morpholineethanesulfonic buffer at pH 7 (5 mM NaCl, 115 mM KCl, 1.2 mM MgSO4, 25 mM morpholineethanesulfonic acid), and transferred into magnetically stirred four-window cuvettes at 28°C. The fluorescence emission intensity ratios at 490 and 530 nm were measured in an Aminco-Bowman series 2 spectrometer using excitation at 360 nm (emission and excitation bandwidths were set to 4 nm).
Flow cytometry analysis.
Parasites (4 × 106 cells/ml) were labeled with 100 nM of the acidotropic dye Lysotracker Green DND-26 in HBS buffer at 28°C. After a 10-min incubation, parasites were treated with 20 mM NH4Cl for 1, 5, and 8 min or with 1, 10, and 30 μM sitamaquine for 15 min at 28°C. Washed parasites were resuspended in PBS, and the cellular fluorescence intensity of the probe was measured by flow cytometry in a FACScan flow cytometer (Becton-Dickinson, San Jose, CA) equipped with an argon laser operating at 488 nm. The cells were gated to eliminate dead cells and debris, and the cell fluorescence was quantified by scanning the emissions between 515 and 545 nm (FL-1) by using Cell Quest software.
Confocal microscopy analysis.
The acidocalcisome accumulation of the acidotropic dye Lysotracker Red DND-99 was determined by confocal microscopy analysis, essentially as previously described (21). Lysotracker Red DND-99 (75 nM) was added to 4 × 106 parasites/ml maintained in HBS buffer, and, after a 10-min incubation at 28°C, the parasites were washed with PBS and analyzed with an Axiovert confocal microscope (TCS SP5 model; Leica), operating with a He/Ne laser (633 nm) and coupled to MRC1024 model confocal scanning laser equipment.
Determination of polyP levels in Leishmania lines.
Fluorescence staining using DAPI is commonly used for nucleic acid detection (using an excitation wavelength at 360 nm, with a peak of emission wavelength at 475 nm), but it is known that DAPI also binds and stains other polyanions such as polyP (3), using an excitation wavelength at 415 nm with a peak of emission wavelength at 525 nm. We used DAPI staining to quantify the acidocalcisomal polyP content in different Leishmania species and strains. Leishmania parasites (2 × 107) were resuspended in 1 ml of PBS and incubated for 10 min at room temperature with 10 μg/ml DAPI. After two washes with PBS, parasites in 2 ml of PBS were transferred into magnetically stirred four-window cuvettes at 28°C. Cell density determined at 600 nm was equilibrated in all the samples before fluorescence measurement. Sample fluorescence was calculated by an emission spectrum (from 450 to 650 nm) using excitation at 415 nm in an Aminco-Bowman series 2 spectrometer.
Statistical analysis.
Experiments were performed three times in duplicate. Statistical significance was calculated by using Student's t test. Significance was considered P values of <0.05.
RESULTS AND DISCUSSION
Uptake of sitamaquine.
We studied the uptake of [14C]sitamaquine at 4°C and 28°C in L. donovani strain L82 to find out if the mechanism used by sitamaquine to cross the plasma membrane is temperature dependent. No significant differences in [14C]sitamaquine accumulation at 4°C and 28°C were observed (Fig. 1A); also, [14C]sitamaquine accumulation decreases in a similar way at both temperatures after washing with a nonradioactive drug. Furthermore, we did not observe a decrease in [14C]sitamaquine uptake when cells were pretreated with NEM, which is considered a protein inhibitor, as it forms covalent bonds with protein sulfhydryl groups (18), or when parasites were ATP depleted (data not shown). In addition, sitamaquine entry in Leishmania is a nonsaturable process. It does not saturate either when the nonradioactive sitamaquine concentrations are increased (Fig. 1B) or when the concentration of [14C]sitamaquine is increased (data not shown). These results confirm the fact that a transporter protein does not mediate the sitamaquine entry process. We propose that in a first step, sitamaquine would be retained in the outer side of the plasma membrane, and subsequently, it would cross the membrane, reaching the cytosol by a process independent of energy and endocytosis. Recent, studies of the interaction of sitamaquine with membrane lipids of L. donovani suggest that the hydrophobic interaction between the aromatic ring of sitamaquine, a positively charged drug, and alkyl chains of membrane phospholipids leads to insertion of sitamaquine into a monolayer, mediating the drug entry in the parasite (9). Sitamaquine is a weak base, and its uptake was dependent on the extracellular pH. At basic pHs, such as pH 8, there was a higher sitamaquine uptake than at less basic pHs such as pH 7 to 7.3 (Fig. 1C). Consequently, all data suggest that sitamaquine seems to cross the plasma membrane by a diffusion process driven by its chemical potential gradient and possible pH gradient of intracellular organelles as previously described for the aminoquinolines amodiaquine and chloroquine in mammalian cells (14).
FIG. 1.
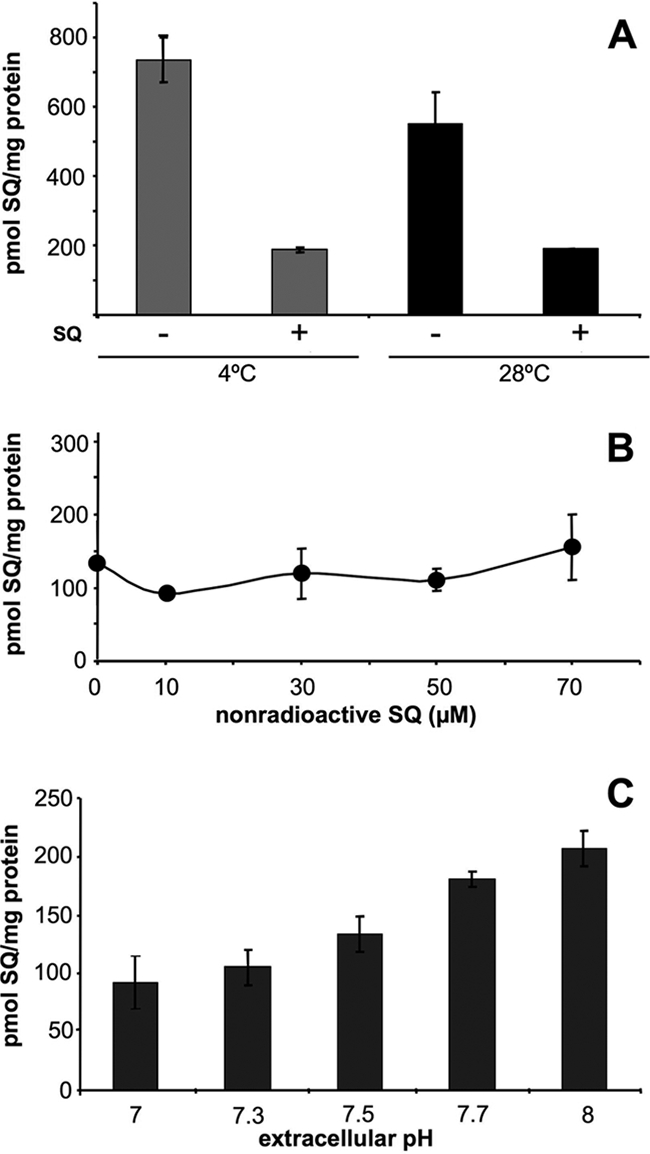
Uptake of sitamaquine in L. donovani parasites. (A) Effect of temperature on [14C]sitamaquine uptake. Cells were incubated with 5 μM [14C]sitamaquine for 15 min at 4°C and 28°C, with (+) and without (−) subsequent washing with 100 μM nonradioactive sitamaquine. No significant differences were observed in sitamaquine accumulation at 4°C and 28°C (P > 0.05). (B) Effect of nonradioactive sitamaquine on [14C]sitamaquine uptake. Cells were incubated with 5 μM [14C]sitamaquine and at the same time with increasing concentrations of nonradioactive sitamaquine at 28°C. After 15 min, cells were washed with 100 μM sitamaquine. (C) Effect of extracellular pH on [14C]sitamaquine uptake. Cells were incubated with [14C]sitamaquine in HBS at several pHs and processed as described above. Results are means ± standard deviations of three independent experiments. SQ, sitamaquine.
There is no correlation between sitamaquine uptake and sensitivity in different Leishmania species.
Sensitivity to sitamaquine was assessed with different Leishmania species, obtaining EC50 values ranging from 9.5 to 19.8 μM (Fig. 2A). Under our experimental conditions, L. infantum and L. donovani were the most and the least sensitive species, respectively. Similarly, variability of sitamaquine susceptibility was previously reported for promastigotes of different Leishmania species (12), showing EC50 values ranging from 5.7 to 75.7 μM. In addition, we studied the uptake of [14C]sitamaquine in these Leishmania species to find out if the sensitivity observed could be caused by dissimilarities in the uptake of sitamaquine. Results showed significant differences between Leishmania species in terms of [14C]sitamaquine accumulation (Fig. 2B). However, the values corresponding to the uptake of sitamaquine did not correlate with the sensitivity to the drug.
FIG. 2.
Sitamaquine sensitivity and uptake in different Leishmania species. (A) Differences in sensitivity to sitamaquine between Leishmania species, compared with L. donovani 82, as assessed by Alamar Blue. (B) Differences in sitamaquine uptake between Leishmania species, compared with L. donovani L82, using 5 μM [14C]sitamaquine for 15 min at 28°C and then washed with 100 μM nonradioactive sitamaquine as described in Materials and Methods. Statistical significance using Student's t test was considered for P values of <0.05. Values for L. donovani L82 versus those of L. mexicana M9012, L. braziliensis LH2419, and L. tropica LRC were significantly different (P < 0.02, P < 0.0005, and P < 0.0003, respectively). Values for L. infantum BCN99 versus L. mexicana M9012, L. braziliensis LH2419, and L. tropica LRC were significantly different (P < 0.04, P < 0.0006, and P < 0.0001, respectively). Values for L. mexicana M9012 versus L. braziliensis LH2419 and L. tropica LRC were significantly different (P < 0.0005 and P < 0.00007, respectively). Data are the means ± standard deviations of five independent experiments. SQ, sitamaquine.
To determine if the level of sitamaquine uptake is an intrinsic feature of each species of Leishmania, we assessed their behavior in terms of [14C]sitamaquine uptake by different strains of L. donovani and L. tropica, which showed the lowest and the highest rates of sitamaquine uptake, respectively. We obtained different levels of sitamaquine uptake in each strain, which suggests that sitamaquine uptake is an intrinsic feature of each Leishmania strain (Fig. 3A). The sensitivity assays to sitamaquine in L. donovani L82, L. donovani dd8, L. tropica LRC, and L. tropica k27 reaffirmed the fact that there is no correlation between sitamaquine uptake and sensitivity (Fig. 3B). On the other hand, when cells were treated with 20 mM NH4Cl, which induces rapid alkalinization in acidic organelles (18), the differences observed for the uptake of sitamaquine between Leishmania strains completely disappeared (Fig. 3A). The alkalinization produced by NH4Cl was checked by flow cytometry in all the strains assayed. A decrease in the fluorescence of cells loaded with 100 nM Lysotracker Green was observed after the addition of 20 mM NH4Cl (Fig. 3C). Furthermore, we observed the same behavior when the cells were incubated with the ionophores monensin and nigericin (Fig. 3A), which enable exchange of sodium and potassium ions with protons, respectively, affecting the pH gradient in the cell (23). These data suggest that differences in sitamaquine uptake between Leishmania strains could be related to differences at the level of acidic organelles, such as lysosomes or acidocalcisomes. Consequently, we continued the study to find out what determines the differences in sitamaquine accumulation in Leishmania strains.
FIG. 3.

Correlation between sitamaquine uptake and the pH of acidic organelles in different Leishmania strains. (A) [14C]sitamaquine uptake in different Leishmania strains. Accumulation assays were determined as described in the legend to Fig. 2. Parasites were pretreated with 20 mM NH4Cl for 1 min and with the ionophores nigericin and monensin at 10 μM for 10 min at 28°C. [14C]sitamaquine uptake was determined for 5 min as described in Materials and Methods. (B) Determination of the sensitivity to sitamaquine in L. tropica and L. donovani strains by Alamar Blue assay. Statistical significance using Student's t test was considered for P values of <0.05. Values for L. donovani L82 versus those of L. donovani dd8, L. tropica LRC, and L. tropica k27 were significantly different (P < 0.05, P < 0.0005, and P < 0.0004, respectively). Values for L. donovani dd8 versus those of L. tropica LRC and L. tropica k27 were significantly different (P < 0.001 and P < 0.02, respectively). (C) Effect of NH4Cl on Lysotracker Green fluorescence by flow cytometry. A representative graph is shown. Fluorescence of L. donovani L82 after incubation with 100 nM Lysotracker Green for 10 min is shown in black. Decrease in fluorescence produced by 20 mM NH4Cl at 1, 5, and 8 min is represented by the gray lines (a, b and c, respectively). The dotted line corresponds to parasites’ autofluorescence. Data are the means ± standard deviations of three independent experiments. SQ, sitamaquine.
Acidic organelles such as acidocalcisomes are involved in the differences observed for sitamaquine accumulation of Leishmania strains.
Vercesi et al. (27) suggested that sitamaquine induces extensive alkalinization in the acidocalcisomes of permeabilized L. donovani parasites. Our studies of [14C]sitamaquine accumulation in the presence of NH4Cl and the ionophores monensin and nigericin also suggested a relationship between [14C]sitamaquine uptake and acidic organelles. Furthermore, we studied the possible implication of acidic organelles in the differences observed for the accumulation of [14C]sitamaquine in Leishmania strains. First, we studied the ability of sitamaquine to displace the accumulation of Lysotracker Green mediated by the alkalinization of acidic organelles. Lysotracker Green is a fluorescent acidotropic probe used to label acidic organelles in live cells. Lysotracker was previously found to label mainly acidocalcisomes rather than multivesicular tubules, the lysosomal compartment of Leishmania (21). In all the strains assessed, sitamaquine reduced the fluorescence of Lysotracker Green measured by flow cytometry analysis (Fig. 4A). Spectrofluorometric studies did not show a quenching phenomenon between Lysotracker Green and sitamaquine (data not shown). These results confirm the role of acidic organelles, acidocalcisomes, in the accumulation of sitamaquine in Leishmania. They also suggest an important role for these organelles in the differences observed for [14C]sitamaquine accumulation between Leishmania strains. However, significant differences between Leishmania strains were observed in terms of accumulation of Lysotracker Green, in spite of the variable behavior of L. tropica strain k27 (Fig. 4B). These differences could be due to different pHs in acidic organelles of the strains, or they also could be explained by differences in the volume (size and/or number) of acidic organelles.
FIG. 4.
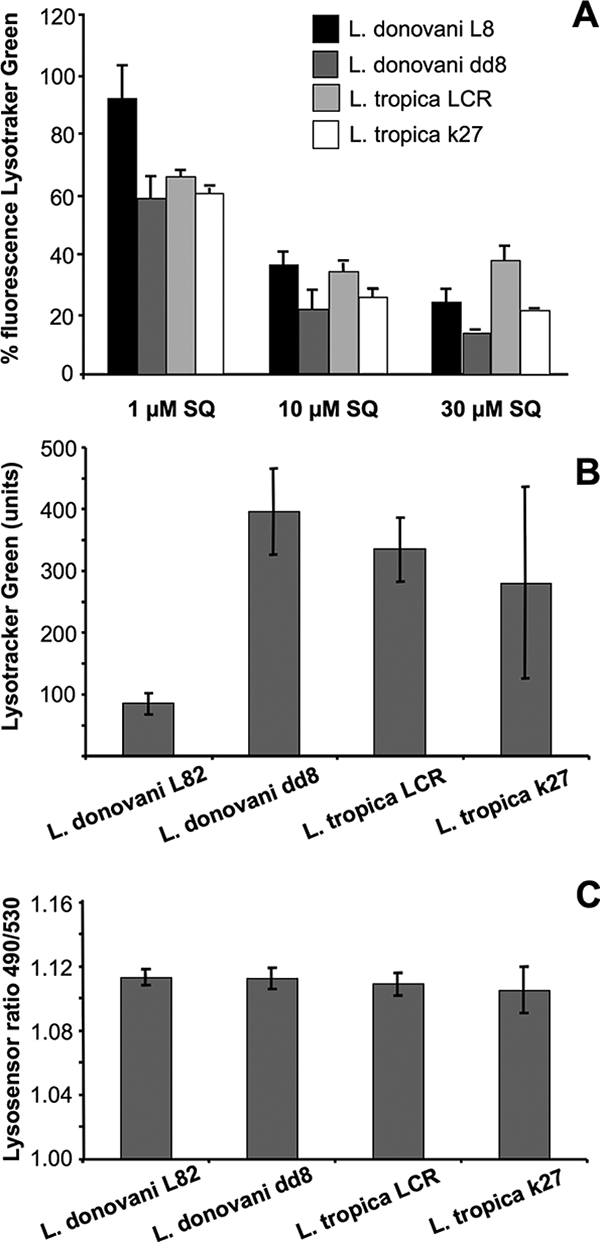
Lysotracker Green and Lysosensor Yellow-Blue accumulation in Leishmania strains. (A) Effect of sitamaquine on Lysotracker Green fluorescence by flow cytometry. Parasites were labeled for 10 min with 100 nM Lysotracker Green. Afterwards, several concentrations of sitamaquine were added as described in Materials and Methods. Sample fluorescence is represented as the percentage of treated compared to nontreated parasites. (B) Lysotracker Green accumulation in Leishmania strains. Units are mean fluorescent units. Values for L. donovani L82 versus those for L. donovani dd8 and L. tropica LRC were significantly different (P < 0.0007 and P < 0.0005, respectively) using Student's t test. (C) Lysosensor Yellow-Blue 490 nm/530 nm ratio values in Leishmania strains. Data are the means ± standard deviations of three independent experiments. SQ, sitamaquine.
To study the role of acidic organelle pHs in the differences observed for Lysotracker Green accumulation, we used Lysosensor Yellow/Blue, a marker used to measure acidic organelle pHs (5, 15). The 490/530-nm ratio values obtained showed no statistically significant differences between acidic organelle pHs in Leishmania strains (Fig. 4C). Consequently, variations observed for Lysotracker Green accumulation are not explained by differences in acidic organelle pHs; thus, they may be explained by another mechanism, such as differences in the volumes of these organelles in Leishmania strains.
Leishmania strains show differences in the sizes of acidic organelles.
We studied the size of acidic organelles in Leishmania strains after labeling the parasites with Lysotracker Red. We determine the accumulation of Lysotracker Red in the different strains, comparing their fluorescence intensities by confocal microscopy. We observed differences in the sizes of acidic organelles between the strains. Thus, the L. tropica LRC and L. donovani dd8 strains showed bigger acidic organelles, while the L. donovani L82 and L. tropica k27 strains showed smaller ones (Fig. 5). We found a correlation between Lysotracker Red and Lysotracker Green fluorescence intensities and [14C]sitamaquine accumulation (compare the results shown in Fig. 3A, 4B, and 5). These results suggest a possible relationship between acidic organelle volume and [14C]sitamaquine accumulation. With other aminoquinolines such as chloroquine, changes in the volume of the digestive vacuole of P. falciparum were associated with drug accumulation and sensitivity in different parasite strains (13).
FIG. 5.
Size of acidic organelles in Leishmania strains. Differences in the sizes of acidic organelles of Leishmania strains labeled with 75 nM Lysotracker Red were determined by confocal microscopy as described in Materials and Methods. Top row shows details of acidic organelles in Leishmania strains at the optimal voltage used to visualize each strain. Inserted values are volts. Bottom row shows corresponding differential interference contrast images.
Leishmania strains show differences in polyP levels.
Based on the fact that acidocalcisomes contain particularly high levels of polyP (7), we studied polyP levels in Leishmania strains (3). We used DAPI fluorescence at 525 nm after an excitation at 415 nm to measure polyP levels (Fig. 6A). We obtained higher levels of polyP with L. tropica LRC and L. donovani dd8 and lower levels with L. tropica k27 and L. donovani L82 (Fig. 6B). These results showed a good correlation between polyP levels, Lysotracker Green/Lysotracker Red accumulation, and [14C]sitamaquine accumulation.
FIG. 6.
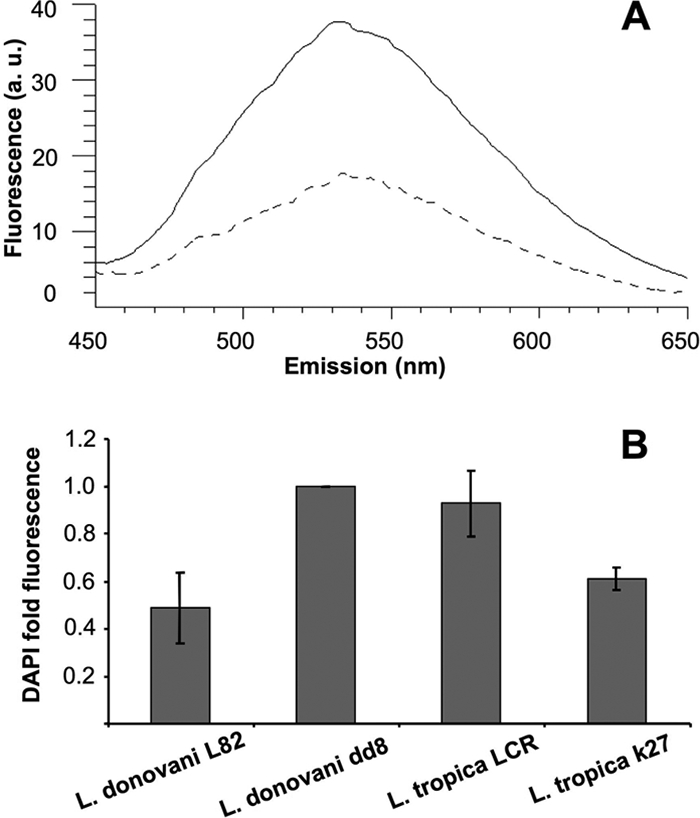
Determination of polyP levels in Leishmania strains. (A) Representative emission spectra of polyP-bound DAPI in L. donovani dd8 (continuous line) and L. donovani L82 (dashed line) after sample excitation at 415 nm. Parasites were incubated for 10 min with 10 μg/ml DAPI as described in Materials and Methods. (B) PolyP levels in different Leishmania strains were determined by spectral integration from 450 to 650 nm as described above and compared with those in L. donovani dd8. Values for L. donovani L82 versus those for L. donovani dd8 and L. tropica k27 were significantly different (P < 0.004 and P < 0.0001, respectively), using Student's t test. Results represent means ± standard deviations of four independent experiments. a. u., arbitrary units.
Accumulation and sensitivity to sitamaquine in Leishmania major AP3δ-null mutant.
To confirm that sitamaquine is accumulated in acidic vesicles such as acidocalcisomes and that there is no correlation between uptake and sensitivity to sitamaquine, we used the L. major AP3δ-null mutant line in which acidocalcisomes lacked several membrane proteins (transporters or ion channels), were devoid of their usual polyP and proton content, and had a higher acidocalcisomal pH (4). AP3 is a heterodimeric protein complex mediator of protein transport (such as integral membrane proteins) to the lysosomes and lysosome-related organelles, such as acidocalcisomes (4). Using the L. major AP3δ-null mutant line and L. major cell line in which the AP3δ gene was complemented from the ribosomal locus in the L. major AP3δ-null mutant (designated AP3-complemented), we studied sitamaquine accumulation and sensitivity. [14C]sitamaquine uptake was significantly reduced in the AP3δ-null mutant parasites (more than 95% reduction versus control parasites), while in the AP3-complemented lines, the [14C]sitamaquine accumulation was significantly increased (Fig. 7A). To further confirm if there was a correlation between sitamaquine uptake and sensitivity, we studied the sensitivity to sitamaquine by using the AP3δ-null mutant and the AP3-complemented L. major lines. The results clearly show that there are no significant differences in sitamaquine sensitivity in the promastigote forms of L. major lines (Fig. 7B). Similarly, to assess sitamaquine sensitivity of intracellular amastigotes, mouse peritoneal macrophages were infected with late-stage promastigotes of wild-type and AP3δ-null mutant parasites. Sensitivities to sitamaquine of the intracellular amastigotes of L. major lines were similar, with EC50 values of 4.3 ± 0.6 μM and 3.9 ± 0.4 μM for the wild-type and AP3δ-null mutant lines, respectively, and similar to those previously described (12). Overall, these data confirm that sitamaquine accumulates in acidic vesicles such as acidocalcisomes and that there is no correlation between uptake and sensitivity to sitamaquine in Leishmania parasites. This conclusion is similar to those obtained from studies of the mechanism of action of drugs that accumulate in acidocalcisomes: diamidines against Trypanosoma brucei (17, 19) and N-alkyl and N-aryl-biphosphonates against parasites of the order Kinetoplastida and the phylum Apicomplexa (8, 11). Thus, the accumulation of drugs in acidocalcisomes may not predict in vitro activity and seems to be a widespread phenomenon.
FIG. 7.

Accumulation and sensitivity to sitamaquine in L. major AP3δ-null mutant lines. (A) Sitamaquine uptake in the L. major AP3δ-null mutant line and AP3-complemented parasites, using 5 μM [14C]sitamaquine for 15 min as described in Materials and Methods. (B) Sitamaquine sensitivity in the L. major lines by Alamar Blue assay after 72 h of incubation at 28°C in the presence of increasing concentrations of sitamaquine is shown. Results represent means ± standard deviations of three independent experiments. SQ, sitamaquine.
Acknowledgments
This work was supported by the Spanish grants SAF2006-02093 (to F.G.) and ISCIII-Red de Investigación Cooperativa en Enfermedades Tropicales (RICET) RD06/0021/0002 (FG) and by the Plan Andaluz de Investigación (Cod. BIO130).
We thank GlaxoSmithKline (Greenford, United Kingdom) for the sitamaquine and [14C]sitamaquine used throughout this research work.
We also thank Jeremy Mottram (University of Glasgow, Glasgow, Scotland, United Kingdom) for the AP3δ-null mutant and AP3-complemented L. major lines used in the manuscript, Roberto Docampo (University of Georgia, Athens, GA) for advice on all aspects concerning acidocalcisomes, and Ana Muñoz Gomez for assistance with the manuscript.
C.L.M. has a fellowship from the Ministerio de Educación y Ciencia (Spain).
Footnotes
Published ahead of print on 15 September 2008.
REFERENCES
- 1.Alvar, J., S. Croft, and P. Olliaro. 2006. Chemotherapy in the treatment and control of leishmaniasis. Adv. Parasitol. 61:223-274. [DOI] [PubMed] [Google Scholar]
- 2.Araujo-Santos, J. M., F. Gamarro, S. Castanys, A. Herrmann, and T. Pomorski. 2003. Rapid transport of phospholipids across the plasma membrane of Leishmania infantum. Biochem. Biophys. Res. Commun. 306:250-255. [DOI] [PubMed] [Google Scholar]
- 3.Aschar-Sobbi, R., A. Y. Abramov, C. Diao, M. E. Kargacin, G. J. Kargacin, R. J. French, and E. Pavlov. 2008. High sensitivity, quantitative measurements of polyphosphate using a new DAPI-based approach. J. Fluoresc. 18:859-866. [DOI] [PubMed] [Google Scholar]
- 4.Besteiro, S., D. Tonn, L. Tetley, G. H. Coombs, and J. C. Mottram. 2008. The AP3 adaptor is involved in the transport of membrane proteins to acidocalcisomes of Leishmania. J. Cell Sci. 121:561-570. [DOI] [PubMed] [Google Scholar]
- 5.Diwu, Z., C. S. Chen, C. Zhang, D. H. Klaubert, and R. P. Haugland. 1999. A novel acidotropic pH indicator and its potential application in labeling acidic organelles of live cells. Chem. Biol. 6:411-418. [DOI] [PubMed] [Google Scholar]
- 6.Docampo, R., W. de Souza, K. Miranda, P. Rohloff, and S. N. Moreno. 2005. Acidocalcisomes: conserved from bacteria to man. Nat. Rev. Microbiol. 3:251-261. [DOI] [PubMed] [Google Scholar]
- 7.Docampo, R., and S. N. Moreno. 1999. Acidocalcisome: a novel Ca2+ storage compartment in trypanosomatids and apicomplexan parasites. Parasitol. Today 15:443-448. [DOI] [PubMed] [Google Scholar]
- 8.Docampo, R., and S. N. Moreno. 2001. Bisphosphonates as chemotherapeutic agents against trypanosomatid and apicomplexan parasites. Curr. Drug Targets Infect. Disord. 1:51-61. [DOI] [PubMed] [Google Scholar]
- 9.Duenas-Romero, A. M., P. M. Loiseau, and M. Saint-Pierre-Chazalet. 2007. Interaction of sitamaquine with membrane lipids of Leishmania donovani promastigotes. Biochim. Biophys. Acta 1768:246-252. [DOI] [PubMed] [Google Scholar]
- 10.Emerson, L. R., M. E. Nau, R. K. Martin, D. E. Kyle, M. Vahey, and D. F. Wirth. 2002. Relationship between chloroquine toxicity and iron acquisition in Saccharomyces cerevisiae. Antimicrob. Agents Chemother. 46:787-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferella, M., Z. H. Li, B. Andersson, and R. Docampo. 2008. Farnesyl diphosphate synthase localizes to the cytoplasm of Trypanosoma cruzi and T. brucei. Exp. Parasitol. 119:308-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garnier, T., M. B. Brown, M. J. Lawrence, and S. L. Croft. 2006. In-vitro and in-vivo studies on a topical formulation of sitamaquine dihydrochloride for cutaneous leishmaniasis. J. Pharm. Pharmacol. 58:1043-1054. [DOI] [PubMed] [Google Scholar]
- 13.Gligorijevic, B., T. Bennett, R. McAllister, J. S. Urbach, and P. D. Roepe. 2006. Spinning disk confocal microscopy of live, intraerythrocytic malarial parasites. 2. Altered vacuolar volume regulation in drug resistant malaria. Biochemistry 45:12411-12423. [DOI] [PubMed] [Google Scholar]
- 14.Hawley, S. R., P. G. Bray, B. K. Park, and S. A. Ward. 1996. Amodiaquine accumulation in Plasmodium falciparum as a possible explanation for its superior antimalarial activity over chloroquine. Mol. Biochem. Parasitol. 80:15-25. [DOI] [PubMed] [Google Scholar]
- 15.Holopainen, J. M., J. Saarikoski, P. K. Kinnunen, and I. Jarvela. 2001. Elevated lysosomal pH in neuronal ceroid lipofuscinoses (NCLs). Eur. J. Biochem. 268:5851-5856. [DOI] [PubMed] [Google Scholar]
- 16.Kinnamon, K. E., E. A. Steck, P. S. Loizeaux, W. L. Hanson, W. L. Chapman, Jr., and V. B. Waits. 1978. The antileishmanial activity of lepidines. Am. J. Trop. Med. Hyg. 27:751-757. [DOI] [PubMed] [Google Scholar]
- 17.Lanteri, C. A., R. R. Tidwell, and S. R. Meshnick. 2008. The mitochondrion is a site of trypanocidal action of the aromatic diamidine DB75 in bloodstream forms of Trypanosoma brucei. Antimicrob. Agents Chemother. 52:875-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marchesini, N., and R. Docampo. 2002. A plasma membrane P-type H(+)-ATPase regulates intracellular pH in Leishmania mexicana amazonensis. Mol. Biochem. Parasitol. 119:225-236. [DOI] [PubMed] [Google Scholar]
- 19.Mathis, A. M., A. S. Bridges, M. A. Ismail, A. Kumar, I. Francesconi, M. Anbazhagan, Q. Hu, F. A. Tanious, T. Wenzler, J. Saulter, W. D. Wilson, R. Brun, D. W. Boykin, R. R. Tidwell, and J. E. Hall. 2007. Diphenyl furans and aza analogs: effects of structural modification on in vitro activity, DNA binding, and accumulation and distribution in trypanosomes. Antimicrob. Agents Chemother. 51:2801-2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mikus, J., and D. Steverding. 2000. A simple colorimetric method to screen drug cytotoxicity against Leishmania using the dye Alamar Blue. Parasitol. Int. 48:265-269. [DOI] [PubMed] [Google Scholar]
- 21.Mullin, K. A., B. J. Foth, S. C. Ilgoutz, J. M. Callaghan, J. L. Zawadzki, G. I. McFadden, and M. J. McConville. 2001. Regulated degradation of an endoplasmic reticulum membrane protein in a tubular lysosome in Leishmania mexicana. Mol. Biol. Cell 12:2364-2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perez-Victoria, F. J., S. Castanys, and F. Gamarro. 2003. Leishmania donovani resistance to miltefosine involves a defective inward translocation of the drug. Antimicrob. Agents Chemother. 47:2397-2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanchez, C. P., W. D. Stein, and M. Lanzer. 2007. Is PfCRT a channel or a carrier? Two competing models explaining chloroquine resistance in Plasmodium falciparum. Trends Parasitol. 23:332-339. [DOI] [PubMed] [Google Scholar]
- 24.Seifert, K., S. Matu, F. J. Perez-Victoria, S. Castanys, F. Gamarro, and S. L. Croft. 2003. Characterisation of Leishmania donovani promastigotes resistant to hexadecylphosphocholine (miltefosine). Int. J. Antimicrob. Agents 22:380-387. [DOI] [PubMed] [Google Scholar]
- 25.Seifert, K., F. J. Pérez-Victoria, M. Stettler, M. P. Sánchez-Cañete, S. Castanys, F. Gamarro, and S. L. Croft. 2007. Inactivation of the miltefosine transporter, LdMT, causes miltefosine resistance that is conferred to the amastigote stage of Leishmania donovani and persists in vivo. Int. J. Antimicrob. Agents 30:229-235. [DOI] [PubMed] [Google Scholar]
- 26.Reference deleted.
- 27.Vercesi, A. E., C. O. Rodrigues, R. Catisti, and R. Docampo. 2000. Presence of a Na(+)/H(+) exchanger in acidocalcisomes of Leishmania donovani and their alkalization by antileishmanial drugs. FEBS Lett. 473:203-206. [DOI] [PubMed] [Google Scholar]
- 28.Yeates, C. 2002. Sitamaquine (GlaxoSmithKline/Walter Reed Army Institute). Curr. Opin. Investig. Drugs 3:1446-1452. [PubMed] [Google Scholar]




