Abstract
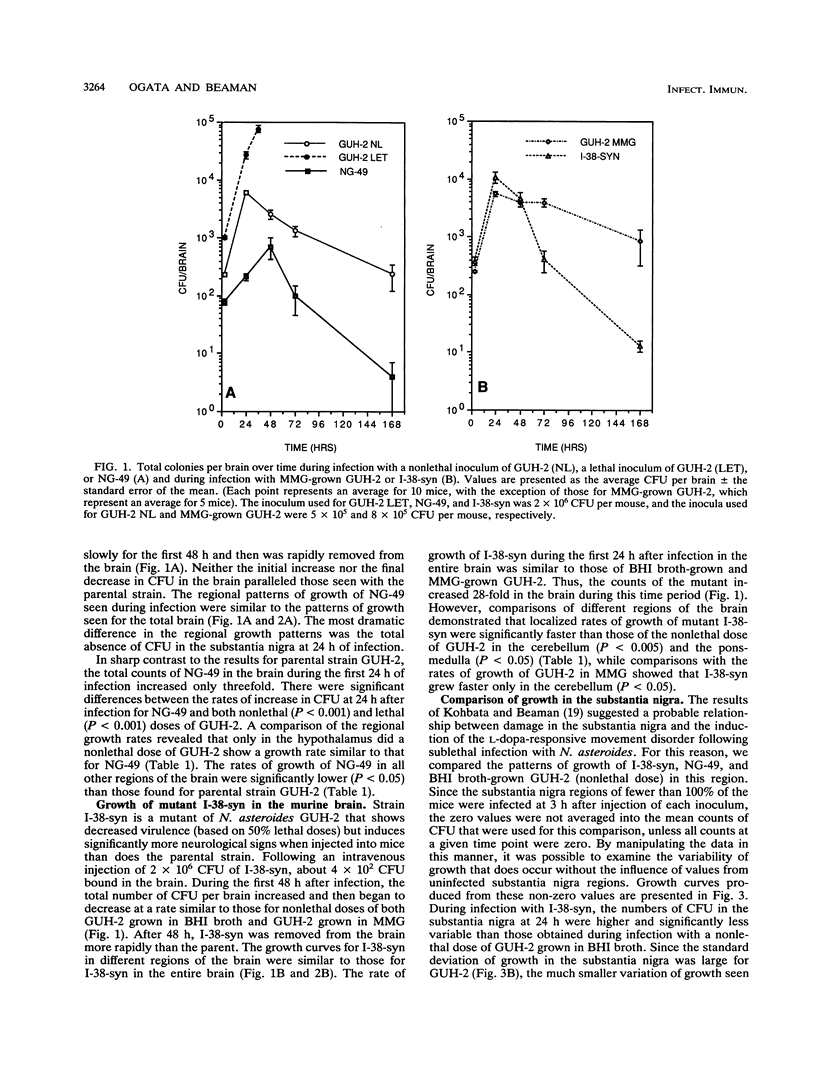
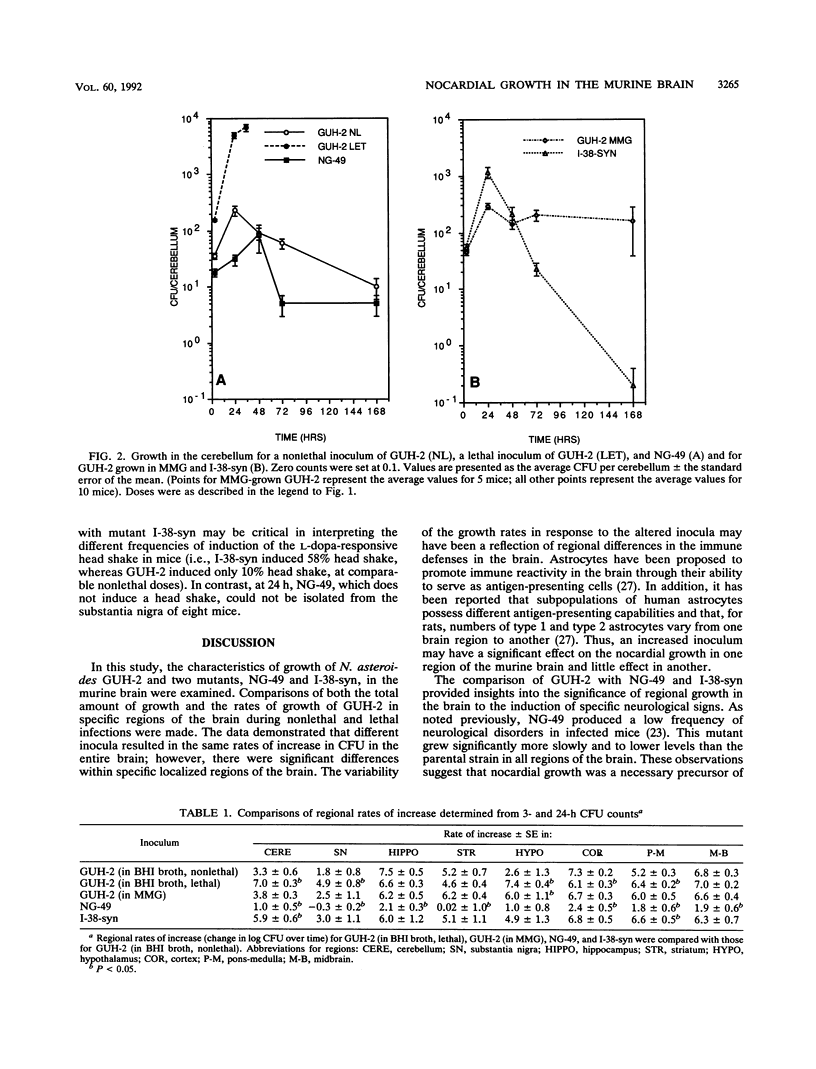
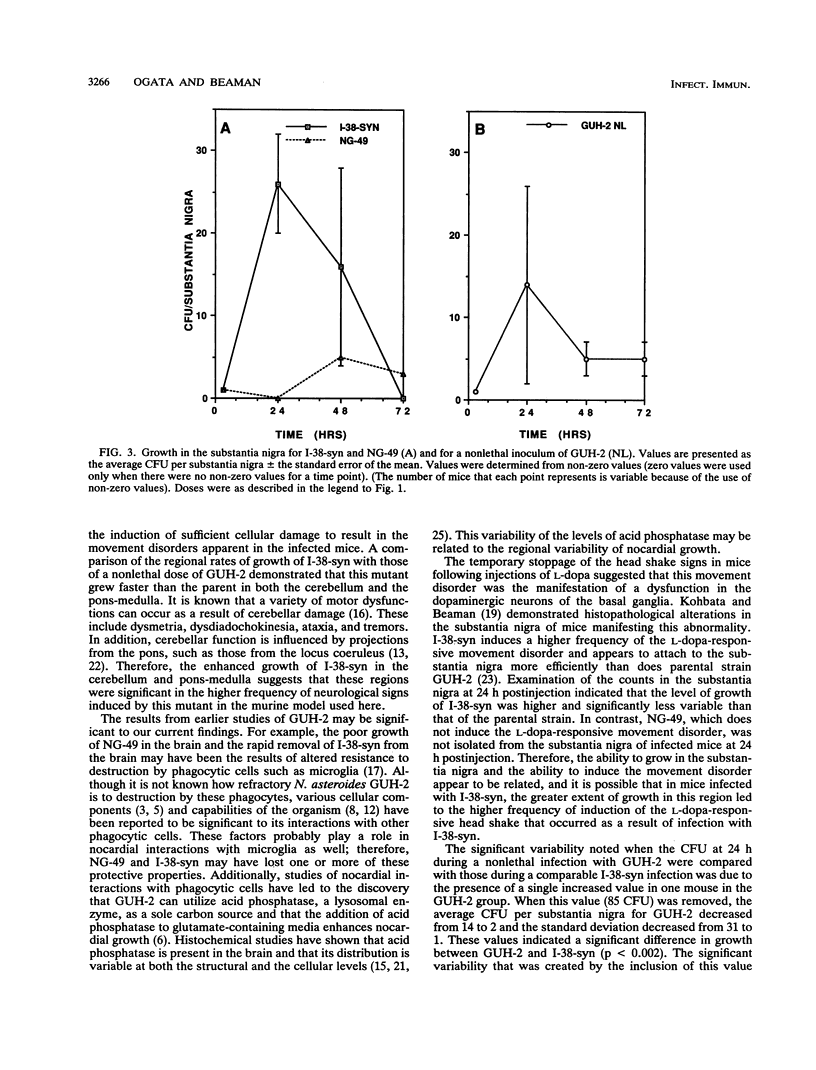
The growth of Nocardia asteroides GUH-2 and two mutants (NG-49 and I-38-syn) in regions of the brains of BALB/c mice was determined by microdissection and viable counting. GUH-2 grew throughout the murine brain but at different growth rates that depended on the specific location. The rate of increase in total CFU per brain during GUH-2 infection was unaffected by the inoculum size; however, in five of eight brain regions, an alteration in the inoculum size resulted in altered nocardial growth rates. Mutant NG-49 showed a significantly slower rate of increase in total CFU per brain than did the parental strain, GUH-2, and significantly decreased growth rates in seven brain regions. Mutant I-38-syn showed a rate of increase in total CFU per brain similar to that of the parental strain; however, this mutant grew significantly faster in the cerebellum and pons-medulla. Growth appeared to be a necessary precursor to the cellular damage that resulted in the variety of neurological disorders observed in mice infected with N. asteroides GUH-2, because mutant NG-49 exhibited a decreased ability to grow in specific regions of the brain and did not induce signs of neurological damage. In contrast, mutant I-38-syn induced neurological signs in a larger percentage of the infected animals than did parental strain GUH-2 and grew better in certain regions of the brain than did the parental strain. Furthermore, there appeared to be a relationship between the growth of N. asteroides in the substantia nigra and the induction of an L-dopa-responsive head shake that was observed in some of the mice following a sublethal intravenous injection of N. asteroides GUH-2.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adair J. C., Amber I. J., Johnston J. M. Peritonsillar abscess caused by Nocardia asteroides. J Clin Microbiol. 1987 Nov;25(11):2214–2215. doi: 10.1128/jcm.25.11.2214-2215.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman B. L., Black C. M., Doughty F., Beaman L. Role of superoxide dismutase and catalase as determinants of pathogenicity of Nocardia asteroides: importance in resistance to microbicidal activities of human polymorphonuclear neutrophils. Infect Immun. 1985 Jan;47(1):135–141. doi: 10.1128/iai.47.1.135-141.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman B. L., Moring S. E. Relationship among cell wall composition, stage of growth, and virulence of Nocardia asteroides GUH-2. Infect Immun. 1988 Mar;56(3):557–563. doi: 10.1128/iai.56.3.557-563.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman L., Beaman B. L. Monoclonal antibodies demonstrate that superoxide dismutase contributes to protection of Nocardia asteroides within the intact host. Infect Immun. 1990 Sep;58(9):3122–3128. doi: 10.1128/iai.58.9.3122-3128.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman L., Paliescheskey M., Beaman B. L. Acid phosphatase stimulation of the growth of Nocardia asteroides and its possible relationship to the modification of lysosomal enzymes in macrophages. Infect Immun. 1988 Jun;56(6):1652–1654. doi: 10.1128/iai.56.6.1652-1654.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman H., Wichmann T., DeLong M. R. Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science. 1990 Sep 21;249(4975):1436–1438. doi: 10.1126/science.2402638. [DOI] [PubMed] [Google Scholar]
- Black C. M., Paliescheskey M., Beaman B. L., Donovan R. M., Goldstein E. Acidification of phagosomes in murine macrophages: blockage by Nocardia asteroides. J Infect Dis. 1986 Dec;154(6):952–958. doi: 10.1093/infdis/154.6.952. [DOI] [PubMed] [Google Scholar]
- Buggy B. P. Nocardia asteroides meningitis without brain abscess. Rev Infect Dis. 1987 Jan-Feb;9(1):228–231. doi: 10.1093/clinids/9.1.228. [DOI] [PubMed] [Google Scholar]
- CARLILE W. K., HOLLEY K. E., LOGAN G. B. Fatal acute disseminated nocardiosis in a child. JAMA. 1963 May 11;184:477–480. doi: 10.1001/jama.1963.03700190095013. [DOI] [PubMed] [Google Scholar]
- Curry W. A. Human nocardiosis. A clinical review with selected case reports. Arch Intern Med. 1980 Jun;140(6):818–826. doi: 10.1001/archinte.140.6.818. [DOI] [PubMed] [Google Scholar]
- Davis-Scibienski C., Beaman B. L. Interaction of Nocardia asteroides with rabbit alveolar macrophages: association of virulence, viability, ultrastructural damage, and phagosome-lysosome fusion. Infect Immun. 1980 May;28(2):610–619. doi: 10.1128/iai.28.2.610-619.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrichs E. Cerebellar cortical and nuclear afferents from the feline locus coeruleus complex. Neuroscience. 1988 Oct;27(1):77–91. doi: 10.1016/0306-4522(88)90220-5. [DOI] [PubMed] [Google Scholar]
- FRIEDE R. L., KNOLLER M. A QUANTITATIVE MAPPING OF ACID PHOSPHATASE IN THE BRAIN OF THE RHESUS MONKEY. J Neurochem. 1965 May;12:441–450. doi: 10.1111/j.1471-4159.1965.tb04244.x. [DOI] [PubMed] [Google Scholar]
- Giulian D. Ameboid microglia as effectors of inflammation in the central nervous system. J Neurosci Res. 1987;18(1):155-71, 132-3. doi: 10.1002/jnr.490180123. [DOI] [PubMed] [Google Scholar]
- Higashide E., Asai M., Ootsu K., Tanida S., Kozai Y., Hasegawa T., Kishi T., Sugino Y., Yoneda M. Ansamitocin, a group of novel maytansinoid antibiotics with antitumour properties from Nocardia. Nature. 1977 Dec 22;270(5639):721–722. doi: 10.1038/270721a0. [DOI] [PubMed] [Google Scholar]
- Kohbata S., Beaman B. L. L-dopa-responsive movement disorder caused by Nocardia asteroides localized in the brains of mice. Infect Immun. 1991 Jan;59(1):181–191. doi: 10.1128/iai.59.1.181-191.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moises H. C., Woodward D. J. Potentiation of GABA inhibitory action in cerebrllum by locus coeruleus stimulation. Brain Res. 1980 Jan 27;182(2):327–344. doi: 10.1016/0006-8993(80)91192-0. [DOI] [PubMed] [Google Scholar]
- Ogata S. A., Beaman B. L. Adherence of Nocardia asteroides within the murine brain. Infect Immun. 1992 May;60(5):1800–1805. doi: 10.1128/iai.60.5.1800-1805.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer D. L., Harvey R. L., Wheeler J. K. Diagnostic and therapeutic considerations in Nocardia asteroides infection. Medicine (Baltimore) 1974 Sep;53(5):391–401. doi: 10.1097/00005792-197409000-00005. [DOI] [PubMed] [Google Scholar]
- Sethi J. S., Tanwar R. K. Comparative distribution of acid phosphatase and simple esterase in the mouse neocortex and hippocampal formation. Acta Anat (Basel) 1989;135(4):323–329. doi: 10.1159/000146776. [DOI] [PubMed] [Google Scholar]
- Tsuboi R., Yamaguchi T., Matsuda K., Ogawa H. Extracellular proteinase production and the pathogenicity of Nocardiae. Arch Dermatol Res. 1989;281(1):78–80. doi: 10.1007/BF00424279. [DOI] [PubMed] [Google Scholar]
- Yong V. W., Yong F. P., Olivier A., Robitaille Y., Antel J. P. Morphologic heterogeneity of human adult astrocytes in culture: correlation with HLA-DR expression. J Neurosci Res. 1990 Dec;27(4):678–688. doi: 10.1002/jnr.490270428. [DOI] [PubMed] [Google Scholar]



