Abstract
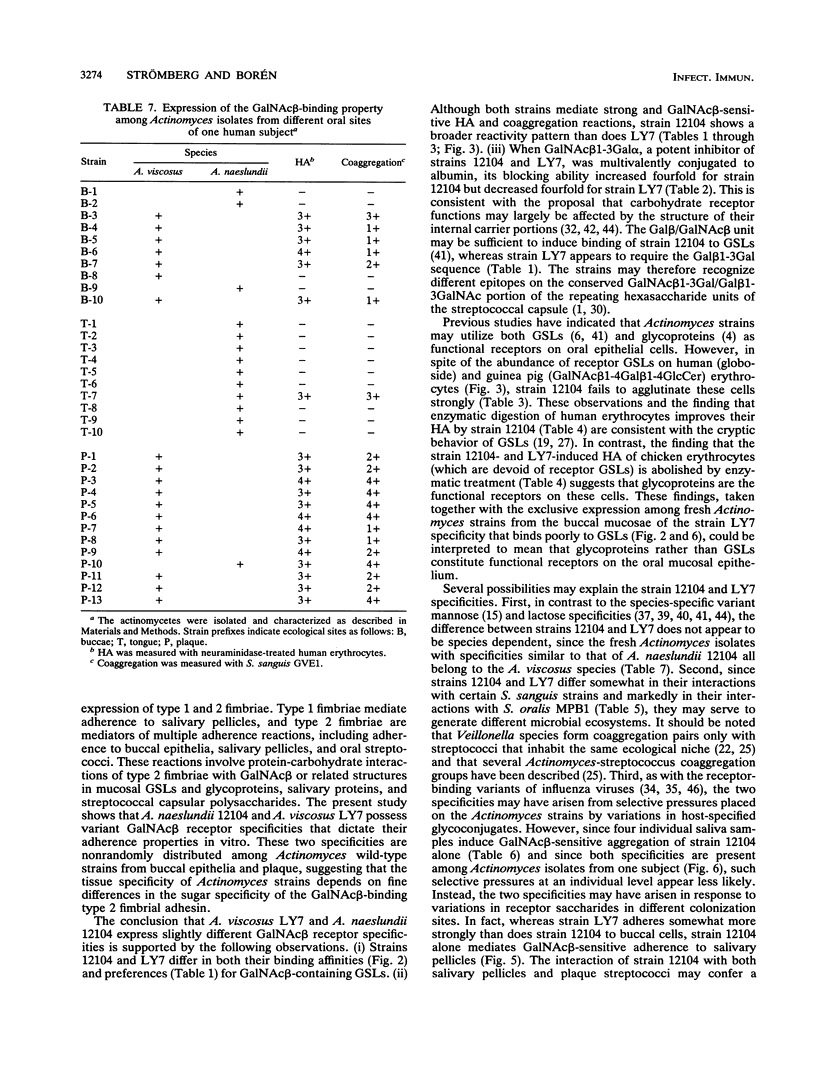
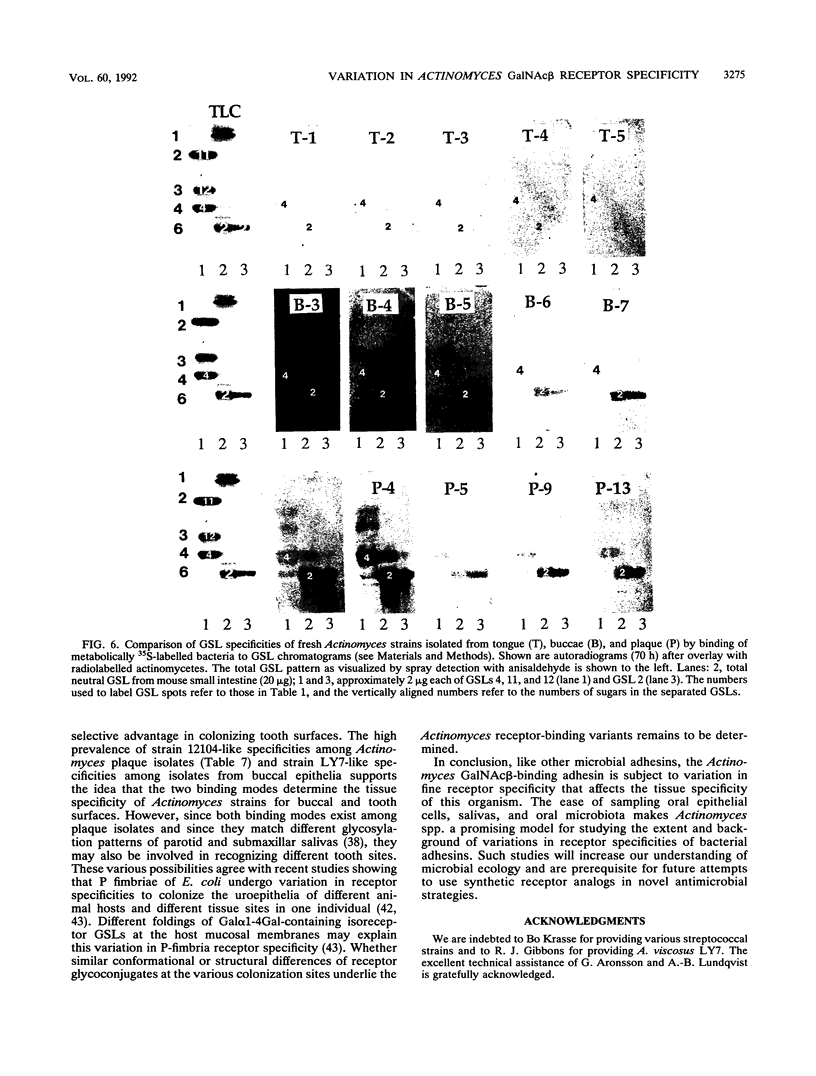
Actinomyces naeslundii 12104 and A. viscosus LY7 were compared for receptor specificities and adherence properties because these relate to their oral colonization sites. Both strains bind GalNAc beta-containing glycosphingolipids (GSLs) in a GalNAc beta 1-3Gal alpha Oethyl-sensitive fashion but differ with respect to the number of cells bound to GSLs and the effect of neighboring sugar groups on the binding. Their hemagglutination and saccharide inhibition profiles confirms the existence of two receptor specificities (for example, when GalNAc beta 1-3Gal alpha Oethyl is multivalently conjugated to albumin, its inhibitory activity increases fourfold toward strain 12104 but decreases fourfold toward strain LY7). Trypsin or chymotrypsin treatment of human erythrocytes, which possess receptor GSLs, improves their hemagglutination with strain 12104. In contrast, the same treatment of chicken erythrocytes, which lack receptor GSLs, abolishes their hemagglutination. These findings suggest that both GSLs and glycoproteins act as functional receptors on eukaryotic cells. The strains also differ with respect to the following GalNAc beta 1-3Gal alpha Oethyl-sensitive adherence properties: (i) strain LY7 adheres somewhat better than does strain 12104 to buccal epithelial cells; (ii) in spite of their similar overall coaggregation patterns with streptococci, strain 12104 coaggregates with Streptococcus oralis MPB1 but strain LY7 does not; (iii) strain 12104 alone shows GalNAc beta-sensitive saliva aggregation and adherence to saliva-coated hydroxyapatite. The GSL binding patterns of fresh Actinomyces isolates reveal a high prevalence of LY7-like specificities among buccal isolates, whereas 12104-like specificities are most prevalent among plaque isolates. These findings strongly suggest that fresh Actinomyces isolates use fine specificity for GalNAc beta-containing glycoconjugates in recognition and subsequent colonization of specific oral surfaces.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeygunawardana C., Bush C. A., Cisar J. O. Complete structure of the cell surface polysaccharide of Streptococcus oralis ATCC 10557: a receptor for lectin-mediated interbacterial adherence. Biochemistry. 1991 Jul 2;30(26):6528–6540. doi: 10.1021/bi00240a025. [DOI] [PubMed] [Google Scholar]
- Abeygunawardana C., Bush C. A., Cisar J. O. Complete structure of the polysaccharide from Streptococcus sanguis J22. Biochemistry. 1990 Jan 9;29(1):234–248. doi: 10.1021/bi00453a032. [DOI] [PubMed] [Google Scholar]
- Bock K., Breimer M. E., Brignole A., Hansson G. C., Karlsson K. A., Larson G., Leffler H., Samuelsson B. E., Strömberg N., Edén C. S. Specificity of binding of a strain of uropathogenic Escherichia coli to Gal alpha 1----4Gal-containing glycosphingolipids. J Biol Chem. 1985 Jul 15;260(14):8545–8551. [PubMed] [Google Scholar]
- Brennan M. J., Cisar J. O., Sandberg A. L. A 160-kilodalton epithelial cell surface glycoprotein recognized by plant lectins that inhibit the adherence of Actinomyces naeslundii. Infect Immun. 1986 Jun;52(3):840–845. doi: 10.1128/iai.52.3.840-845.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan M. J., Cisar J. O., Vatter A. E., Sandberg A. L. Lectin-dependent attachment of Actinomyces naeslundii to receptors on epithelial cells. Infect Immun. 1984 Nov;46(2):459–464. doi: 10.1128/iai.46.2.459-464.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan M. J., Joralmon R. A., Cisar J. O., Sandberg A. L. Binding of Actinomyces naeslundii to glycosphingolipids. Infect Immun. 1987 Feb;55(2):487–489. doi: 10.1128/iai.55.2.487-489.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisar J. O., Sandberg A. L., Mergenhagen S. E. The function and distribution of different fimbriae on strains of Actinomyces viscosus and Actinomyces naeslundii. J Dent Res. 1984 Mar;63(3):393–396. doi: 10.1177/00220345840630030701. [DOI] [PubMed] [Google Scholar]
- Cisar J. O., Vatter A. E., Clark W. B., Curl S. H., Hurst-Calderone S., Sandberg A. L. Mutants of Actinomyces viscosus T14V lacking type 1, type 2, or both types of fimbriae. Infect Immun. 1988 Nov;56(11):2984–2989. doi: 10.1128/iai.56.11.2984-2989.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W. B., Wheeler T. T., Cisar J. O. Specific inhibition of adsorption of Actinomyces viscosus T14V to saliva-treated hydroxyapatite by antibody against type 1 fimbriae. Infect Immun. 1984 Feb;43(2):497–501. doi: 10.1128/iai.43.2.497-501.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W. B., Wheeler T. T., Lane M. D., Cisar J. O. Actinomyces adsorption mediated by type-1 fimbriae. J Dent Res. 1986 Sep;65(9):1166–1168. doi: 10.1177/00220345860650091001. [DOI] [PubMed] [Google Scholar]
- Dzandu J. K., Deh M. E., Wise G. E. A re-examination of the effects of chymotrypsin and trypsin on the erythrocyte membrane surface topology. Biochem Biophys Res Commun. 1985 Jan 16;126(1):50–58. doi: 10.1016/0006-291x(85)90569-8. [DOI] [PubMed] [Google Scholar]
- Ellen R. P. Establishment and distribution of Actinomyces viscosus and Actinomyces naeslundii in the human oral cavity. Infect Immun. 1976 Nov;14(5):1119–1124. doi: 10.1128/iai.14.5.1119-1124.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen R. P., Segal D. N., Grove D. A. Relative proportions of Actinomyces viscosus and Actinomyces naeslundii in dental plaques collected from single sites. J Dent Res. 1978 Apr;57(4):550–550. doi: 10.1177/00220345780570040201. [DOI] [PubMed] [Google Scholar]
- Firon N., Ofek I., Sharon N. Carbohydrate-binding sites of the mannose-specific fimbrial lectins of enterobacteria. Infect Immun. 1984 Mar;43(3):1088–1090. doi: 10.1128/iai.43.3.1088-1090.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahnberg L., Olsson J., Krasse B., Carlén A. Interference of Salivary immunoglobulin A antibodies and other salivary fractions with adherence of Streptococcus mutans to hydroxyapatite. Infect Immun. 1982 Aug;37(2):401–406. doi: 10.1128/iai.37.2.401-406.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Hay D. I., Cisar J. O., Clark W. B. Adsorbed salivary proline-rich protein 1 and statherin: receptors for type 1 fimbriae of Actinomyces viscosus T14V-J1 on apatitic surfaces. Infect Immun. 1988 Nov;56(11):2990–2993. doi: 10.1128/iai.56.11.2990-2993.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Hay D. I. Human salivary acidic proline-rich proteins and statherin promote the attachment of Actinomyces viscosus LY7 to apatitic surfaces. Infect Immun. 1988 Feb;56(2):439–445. doi: 10.1128/iai.56.2.439-445.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakomori S. Aberrant glycosylation in tumors and tumor-associated carbohydrate antigens. Adv Cancer Res. 1989;52:257–331. doi: 10.1016/s0065-230x(08)60215-8. [DOI] [PubMed] [Google Scholar]
- Hansson G. C., Karlsson K. A., Larson G., Strömberg N., Thurin J. Carbohydrate-specific adhesion of bacteria to thin-layer chromatograms: a rationalized approach to the study of host cell glycolipid receptors. Anal Biochem. 1985 Apr;146(1):158–163. doi: 10.1016/0003-2697(85)90410-5. [DOI] [PubMed] [Google Scholar]
- Heeb M. J., Marini A. M., Gabriel O. Factors affecting binding of galacto ligands to Actinomyces viscosus lectin. Infect Immun. 1985 Jan;47(1):61–67. doi: 10.1128/iai.47.1.61-67.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes C. V., Kolenbrander P. E., Andersen R. N., Moore L. V. Coaggregation properties of human oral Veillonella spp.: relationship to colonization site and oral ecology. Appl Environ Microbiol. 1988 Aug;54(8):1957–1963. doi: 10.1128/aem.54.8.1957-1963.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson K. A., Strömberg N. Overlay and solid-phase analysis of glycolipid receptors for bacteria and viruses. Methods Enzymol. 1987;138:220–232. doi: 10.1016/0076-6879(87)38019-x. [DOI] [PubMed] [Google Scholar]
- Kolenbrander P. E. Intergeneric coaggregation among human oral bacteria and ecology of dental plaque. Annu Rev Microbiol. 1988;42:627–656. doi: 10.1146/annurev.mi.42.100188.003211. [DOI] [PubMed] [Google Scholar]
- Kolenbrander P. E., Williams B. L. Lactose-reversible coaggregation between oral actinomycetes and Streptococcus sanguis. Infect Immun. 1981 Jul;33(1):95–102. doi: 10.1128/iai.33.1.95-102.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampio A., Rauvala H., Gahmberg C. G. Exposure of major neutral glycolipids in red cells to galactose oxidase. Effect of neuraminidase. Eur J Biochem. 1986 Jun 16;157(3):611–616. doi: 10.1111/j.1432-1033.1986.tb09709.x. [DOI] [PubMed] [Google Scholar]
- McIntire F. C., Crosby L. K., Barlow J. J., Matta K. L. Structural preferences of beta-galactoside-reactive lectins on Actinomyces viscosus T14V and Actinomyces naeslundii WVU45. Infect Immun. 1983 Aug;41(2):848–850. doi: 10.1128/iai.41.2.848-850.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire F. C., Crosby L. K., Vatter A. E., Cisar J. O., McNeil M. R., Bush C. A., Tjoa S. S., Fennessey P. V. A polysaccharide from Streptococcus sanguis 34 that inhibits coaggregation of S. sanguis 34 with Actinomyces viscosus T14V. J Bacteriol. 1988 May;170(5):2229–2235. doi: 10.1128/jb.170.5.2229-2235.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkkinen J., Rogers G. N., Korhonen T., Dahr W., Finne J. Identification of the O-linked sialyloligosaccharides of glycophorin A as the erythrocyte receptors for S-fimbriated Escherichia coli. Infect Immun. 1986 Oct;54(1):37–42. doi: 10.1128/iai.54.1.37-42.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchett T. J., Brossmer R., Rose U., Paulson J. C. Recognition of monovalent sialosides by influenza virus H3 hemagglutinin. Virology. 1987 Oct;160(2):502–506. doi: 10.1016/0042-6822(87)90026-2. [DOI] [PubMed] [Google Scholar]
- Revis G. J., Vatter A. E., Crowle A. J., Cisar J. O. Antibodies against the Ag2 fimbriae of Actinomyces viscosus T14V inhibit lactose-sensitive bacterial adherence. Infect Immun. 1982 Jun;36(3):1217–1222. doi: 10.1128/iai.36.3.1217-1222.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers G. N., Paulson J. C., Daniels R. S., Skehel J. J., Wilson I. A., Wiley D. C. Single amino acid substitutions in influenza haemagglutinin change receptor binding specificity. Nature. 1983 Jul 7;304(5921):76–78. doi: 10.1038/304076a0. [DOI] [PubMed] [Google Scholar]
- Rogers G. N., Paulson J. C. Receptor determinants of human and animal influenza virus isolates: differences in receptor specificity of the H3 hemagglutinin based on species of origin. Virology. 1983 Jun;127(2):361–373. doi: 10.1016/0042-6822(83)90150-2. [DOI] [PubMed] [Google Scholar]
- Sharon N., Lis H. Lectins as cell recognition molecules. Science. 1989 Oct 13;246(4927):227–234. doi: 10.1126/science.2552581. [DOI] [PubMed] [Google Scholar]
- Stromberg N., Deal C., Nyberg G., Normark S., So M., Karlsson K. A. Identification of carbohydrate structures that are possible receptors for Neisseria gonorrhoeae. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4902–4906. doi: 10.1073/pnas.85.13.4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strömberg N., Borén T., Carlén A., Olsson J. Salivary receptors for GalNAc beta-sensitive adherence of Actinomyces spp.: evidence for heterogeneous GalNAc beta and proline-rich protein receptor properties. Infect Immun. 1992 Aug;60(8):3278–3286. doi: 10.1128/iai.60.8.3278-3286.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strömberg N., Karlsson K. A. Characterization of the binding of Actinomyces naeslundii (ATCC 12104) and Actinomyces viscosus (ATCC 19246) to glycosphingolipids, using a solid-phase overlay approach. J Biol Chem. 1990 Jul 5;265(19):11251–11258. [PubMed] [Google Scholar]
- Strömberg N., Karlsson K. A. Characterization of the binding of propionibacterium granulosum to glycosphingolipids adsorbed on surfaces. An apparent recognition of lactose which is dependent on the ceramide structure. J Biol Chem. 1990 Jul 5;265(19):11244–11250. [PubMed] [Google Scholar]
- Strömberg N., Marklund B. I., Lund B., Ilver D., Hamers A., Gaastra W., Karlsson K. A., Normark S. Host-specificity of uropathogenic Escherichia coli depends on differences in binding specificity to Gal alpha 1-4Gal-containing isoreceptors. EMBO J. 1990 Jun;9(6):2001–2010. doi: 10.1002/j.1460-2075.1990.tb08328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strömberg N., Nyholm P. G., Pascher I., Normark S. Saccharide orientation at the cell surface affects glycolipid receptor function. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9340–9344. doi: 10.1073/pnas.88.20.9340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strömberg N., Ryd M., Lindberg A. A., Karlsson K. A. Studies on the binding of bacteria to glycolipids. Two species of Propionibacterium apparently recognize separate epitopes on lactose of lactosylceramide. FEBS Lett. 1988 May 9;232(1):193–198. doi: 10.1016/0014-5793(88)80415-0. [DOI] [PubMed] [Google Scholar]
- Weis W., Brown J. H., Cusack S., Paulson J. C., Skehel J. J., Wiley D. C. Structure of the influenza virus haemagglutinin complexed with its receptor, sialic acid. Nature. 1988 Jun 2;333(6172):426–431. doi: 10.1038/333426a0. [DOI] [PubMed] [Google Scholar]
- Zylber L. J., Jordan H. V. Development of a selective medium for detection and enumeration of Actinomyces viscosus and Actinomyces naeslundii in dental plaque. J Clin Microbiol. 1982 Feb;15(2):253–259. doi: 10.1128/jcm.15.2.253-259.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]