Abstract
Moxifloxacin- and rifapentine-based regimens are under investigation for the treatment of tuberculosis. However, rifapentine may induce enzymes that metabolize moxifloxacin, resulting in decreased moxifloxacin concentrations. In this phase I, two-period, sequential-design study, 13 subjects received 400 mg moxifloxacin daily for 4 days followed by daily moxifloxacin coadministered with 900 mg rifapentine thrice weekly. Pharmacokinetic analyses were performed after the 4th and 19th doses of moxifloxacin and after the 1st and 7th doses of rifapentine. For moxifloxacin, the mean area under the concentration-time curve from 0 to 24 h (AUC0-24) decreased by 17.2% (P = 0.0006) when the drug was coadministered with rifapentine, and the mean half-life (t1/2) decreased from 11.1 to 8.9 h (P = 0.0033). For rifapentine, the mean AUC0-48 after seven thrice-weekly doses decreased by 20.3% (P = 0.0035) compared to the AUC0-48 after the first dose, and the mean t1/2 decreased from 18.5 to 14.8 h (P = 0.0004). The AUC0-48 for the 25-desacetyl-rifapentine metabolite diminished 21%. Two days after completing the study drugs, one subject developed a fever and hepatitis, and another developed a flu-like illness with a rash. In conclusion, rifapentine modestly reduced moxifloxacin concentrations. Changes consistent with rifapentine autoinduction of metabolism were seen. Adverse reactions in two subjects may have represented rifamycin hypersensitivity syndrome, although some features were atypical.
Tuberculosis (TB) is a leading cause of infectious disease death worldwide (7). Novel treatment strategies are needed to shorten the duration of treatment required for cure (currently 6 months), improve cure rates, and prevent the emergence of drug resistance.
Moxifloxacin (MXF) and the long-lived rifamycin derivative rifapentine (RPT) are being studied for TB treatment. RPT is approved for use in combination TB treatment, but a dose of 600 mg (∼10 mg/kg of body weight) once weekly during the continuation phase of treatment has been associated with unacceptably high relapse rates in some populations (3, 31; Hoechst Marion Roussel, FDA new-drug application 21-024). In a mouse model of TB treatment, RPT administered thrice weekly at 15 mg/kg/dose in combination with MXF decreases to 3 months the duration of treatment required for cure (20, 21, 23, 25). The optimal dose of RPT for the treatment of TB in humans has not been determined.
Several pharmacologic issues regarding the concomitant use of RPT and MXF merit investigation. First, MXF is metabolized by sulfation and glucuronidation (Avelox package insert), and RPT may induce the enzymes responsible for these biotransformations (5). Coadministration of MXF with rifampin, a more potent inducer than RPT, results in reduced MXF concentrations (19, 32). In addition, RPT may induce its own metabolism, decreasing its plasma concentrations and those of its active metabolite, 25-desacetyl-RPT (14, 23). Finally, the tolerability of 900 mg of RPT administered thrice weekly in combination with MXF has not been evaluated.
We conducted a pharmacokinetic (PK) study of healthy volunteers to evaluate the PK interaction between RPT and MXF, to assess whether RPT induces its own metabolism, and to evaluate the tolerability of high-dose RPT in combination with MXF.
MATERIALS AND METHODS
Study population.
Subjects were healthy adults aged 18 to 65 recruited at the Johns Hopkins Hospital in Maryland. Subjects were eligible if they had a negative human immunodeficiency virus antibody test and normal serum aspartate aminotransferase, total bilirubin, creatinine, uric acid, potassium, and albumin measurements. Those with hemoglobin levels of <12.0 g/dl (men) or <11.0 g/dl (women), neutrophil counts of <1,250/mm3, platelet counts of <125,000/mm3, corrected QT interval of >0.44 s upon electrocardiography, or a positive pregnancy test were excluded. Other exclusion criteria were breastfeeding, known intolerance to study drugs, use of rifamycin or fluoroquinolone antibiotics in the preceding 30 days, prior gastrointestinal surgery, chronic illness, illicit drug use, and current use of medications. The study was approved by the Institutional Review Board of the Johns Hopkins University School of Medicine. All subjects provided written informed consent.
Experimental protocol. (i) Study design.
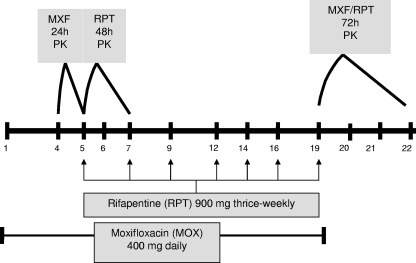
This was a multiple-dose, two-period, sequential-design PK study. Subjects received 400 mg MXF once daily on study days 1 to 4. On day 4, a 24-h MXF PK analysis was performed with 10 ml of blood collected into a green-top (sodium and heparin) Vacutainer tube before drug ingestion and at 0.5, 1.0, 1.5, 2, 3, 4, 6, 8, 12, and 24 h after ingestion (Fig. 1). On day 5, subjects continued on 400 mg MXF daily and RPT was added at a dose of 900 mg thrice weekly. On day 5, after the first dose of RPT, a 48-h RPT PK analysis was performed with 10 ml of blood collected in a green-top tube before drug ingestion and at 2, 4, 6, 8, 10, 12, 24, 34, and 48 h after ingestion. From days 6 to 19, participants continued on 400 mg MXF daily and 900 mg RPT thrice weekly. On day 19, after 19 doses of MXF and 7 doses of RPT, a 72-h MXF and RPT PK analysis was performed. Study drugs were administered following a continental breakfast (750 kcal, 20% fat). Other medications, alcohol, and smoking were not permitted.
FIG. 1.
Schematic of the dosing regimen and pharmacokinetic sample collection.
(ii) Safety monitoring.
On days 3, 9, 12, 15, 18, and 33, subjects underwent a directed review of systems to identify adverse events. A comprehensive metabolic panel was performed and a complete blood count taken on the subjects on days 3, 12, and 18. Signs and symptoms were graded according to the National Cancer Institute Common Toxicity Criteria, version 2.0 (http://ctep.cancer.gov/forms/CTCv20_4-30-992.pdf).
Drug concentration analysis. (i) Determination of plasma concentrations of MXF.
Plasma concentrations of MXF were determined using a validated high-performance liquid chromatography (HPLC) assay. Samples were measured using a system consisting of a ThermoFinnegan P4000 HPLC pump (San Jose, CA) with an AS3000 fixed-volume autosampler, McPherson model FL-750 fluorescence detector, Gateway E series computer (Poway, CA), and ChromQuest HPLC data management system. The plasma standard curve ranged from 0.20 to 15 μg/ml. The lower limit of quantification was 0.02 μg/ml. Absolute recovery of MXF from plasma was 90%. Within-sample precision (percent coefficient of variation) was 6.33%, and overall validation precision across all standards was 1.59 (10 standard) to 5.13% (5 standard). All assays used an internal standard, difloxacin. No interferences were observed with 90 commonly used medications.
(ii) Determination of plasma concentrations of RPT and its metabolite 25-desacetyl-RPT.
Plasma concentrations of RPT and 25-desacetyl-RPT were determined using a validated HPLC assay. Samples were measured using a system consisting of a ThermoFinnegan P4000 HPLC pump with an AS3000 fixed-volume autosampler, model UV2000 UV detector, Gateway E series computer, and ChromQuest data management system. The plasma standard curve ranged from 0.50 to 50 μg/ml. The lower limit of quantification was 0.10 μg/ml. The absolute recovery of RPT and 25-desacetyl-RPT from plasma was 95%. Within-sample precision was 3.61%, and overall validation precision across all standards was 3.49% (50 standard) to 10.65% (0.50 standard) for RPT; those of the metabolite were similar. All assays used an internal standard, trimipramine. No interferences were observed with 90 medications.
PK and statistical evaluation. (i) Sample size.
It was estimated that a sample size of 10 subjects would achieve 80% power to test whether or not coadministration of RPT resulted in an MXF area under the concentration-time curve (AUC) that differed from that observed with MXF alone by ≥10%, assuming an MXF AUC standard deviation of 5.4 μg · h/ml (twice that observed in previous studies of healthy volunteers) and a significance level of 0.05 using a two-sided one-sample t test (Avelox package insert). It was estimated that 11 subjects would be needed to determine bioequivalence with 80% power at a 3% one-sided significance level using two one-sided tests if the treatment mean was 90% of the reference mean, with equivalence defined as 80% to 125% of the reference mean (30). Fifteen subjects were enrolled to ensure that there would be 11 evaluable subjects.
(ii) PK parameters and statistical evaluation.
PK parameters, including the mean AUC, maximum plasma concentration (Cmax), time to maximum plasma concentration (Tmax), half-life (t1/2), clearance as a function of bioavailability (CL/F), and volume of distribution (V/F) were calculated using noncompartmental methods with WinNonlin software, version 4.1 (Pharsight, Cary, NC). Comparisons involving AUC were restricted to a common exposure time for all patients of 24 (MXF) or 48 (RPT and desacetyl-RPT) hours. Descriptive and statistical analyses were performed using Intercooled Stata 9.0 software (StataCorp LP, College Station, TX). The calculated geometric mean ratio (GMR) and a 90% confidence interval (CI) were used to compare the Cmax, Tmax, AUC from 0 to 24 h (AUC0-24), and t1/2 values of MXF alone and in the presence of RPT. P values using two-sided, one-sample t tests with an α of 0.05 are reported. To estimate the effect of RPT on its own metabolism, similar procedures were performed using the PK parameters for RPT on study days 5 and 19.
RESULTS
Subjects.
Fifteen subjects, including nine African Americans, four Caucasians, one Hispanic, and one Asian, were enrolled; 3 were female and 12 were male. One subject withdrew for personal reasons after receiving two doses of MXF. Another withdrew after the first PK analysis, citing grade 1 side effects and job obligations. Thirteen subjects completed the study, three of whom were women. The mean age was 43.2 years (range, 24 to 64 years). The mean dose was 5.27 mg/kg for MXF and 11.9 mg/kg for RPT.
PK interaction between MXF and RPT.
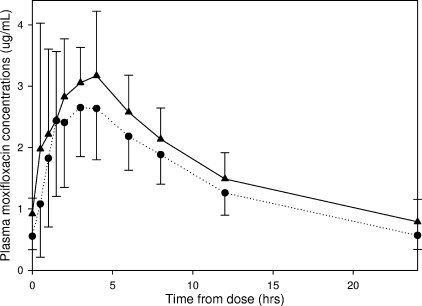
Figure 2 shows mean MXF plasma concentration-time curves after four doses of MXF dosed at 400 mg once daily and after 15 days of 400 mg MXF once daily plus 900 mg RPT thrice weekly. The mean MXF AUC0-24 was 41.9 μg · h/ml for MXF alone and 34.4 μg · h/ml for MXF coadministered with RPT (P = 0.0006) (Table 1). The AUCday 19/AUCday 4 GMR was 0.83 (90% CI, 0.77 to 0.89). The MXF t1/2 was 11.1 h on day 4 versus 8.9 h on day 19 (P = 0.0033), and CL/F increased from 7.89 liters/h to 10.1 liters/h (P = 0.0003). The Cmaxs and Tmaxs on days 4 and 19 were not significantly different.
FIG. 2.
Steady-state mean MXF plasma concentrations versus time curves after four doses of MXF dosed at 400 mg once daily (▴) and after 15 days of 400 mg MXF once daily plus 900 mg RPT thrice weekly (•). Values shown represent arithmetic means with standard error bars.
TABLE 1.
PK parameters for MXF administered alone at a dosage of 400 mg by mouth daily or coadministered with RPT at a dosage of 900 mg by mouth thrice weekly
| PK parameter (unit) | MXF alonea | MXF with RPTa | GMRb | 90% CI | P valuec |
|---|---|---|---|---|---|
| AUC0-24 (μg · h/ml) | 41.9 (10.2) | 34.4 (7.3) | 0.83 | 0.77-0.89 | 0.0006 |
| Cmax (μg/ml) | 4.03 (1.5) | 3.33 (0.67) | 0.85 | 0.75-0.97 | 0.054 |
| Tmax (h) | 2.37 (1.3) | 2.60 (1.5) | 1.12 | 0.70-1.78 | 0.68 |
| t1/2 (h) | 11.1 (3.1) | 8.94 (1.5) | 0.81 | 0.74-0.90 | 0.0033 |
| CL/F (liter/h) | 7.89 (1.9) | 10.1 (1.9) | 1.30 | 1.19-1.43 | 0.0003 |
| V (liter) | 120.8 (24.2) | 127.4 (16.6) | 1.06 | 0.99-1.14 | 0.16 |
Values are means (± standard deviations).
GMRs of PKs of MXF coadministered with RPT to those of MXF alone.
P values of paired t tests comparing PKs of MXF coadministered with RPT to those of MXF alone. α = 0.05.
Autoinduction of metabolism by RPT.
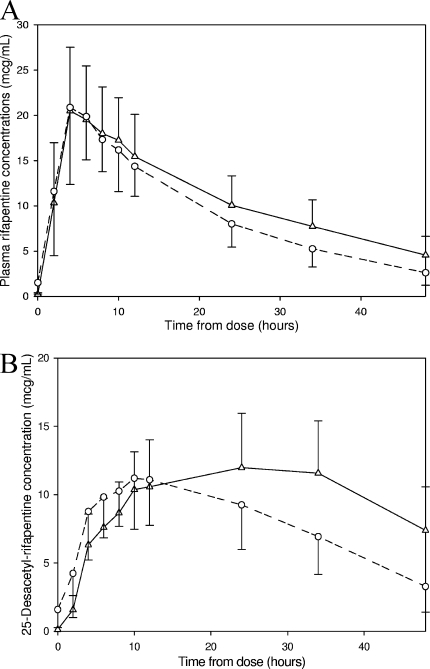
Figure 3 shows mean RPT and 25-desacetyl-RPT plasma concentration-time curves after one dose of RPT and after seven doses of 900 mg RPT thrice weekly. After one dose (day 5), the mean RPT AUC0-48 and t1/2 were 512.9 μg · h/ml and 18.5 h, respectively, and after seven doses (day 19), these values were 410.2 μg · h/ml and 14.8 h, respectively (P values of 0.0035 and 0.0004, respectively) (Table 2). The AUCday 19/AUCday 5 GMR for RPT was 0.80 (90% CI, 0.71 to 0.89). The mean CL/F increased from 1.59 liters/h to 2.17 liters/h (P = 0.0010). The Cmaxs, Tmaxs, and V/Fs were not significantly different on day 5 versus day 19. The AUC0-48 of 25-desacetyl-RPT decreased from 461.7 μg · h/ml after a single dose to 369.9 μg · h/ml after multiple doses of RPT (P = 0.0198). Interestingly, the Tmax for 25-desacetyl-RPT decreased from a mean of 24.9 h to a mean of 10.8 h. Upon visual inspection of the mean concentration-time plots, it appeared that elimination of the metabolite was increased after multiple doses of RPT. However, the t1/2 for the metabolite could not be calculated because the number of data points on the downward slope of the day 5 concentration-time plot was too small to accurately estimate this parameter for 5 of the 13 subjects. In those for whom accurate estimates could be made, the t1/2 changed from 24.1 h on day 5 to 11.4 h on day 19.
FIG. 3.
(A) Mean plasma RPT concentrations versus time of RPT after a single 900-mg dose (▵) versus after seven doses of 900 mg thrice weekly (○). (B) Mean plasma 25-desacetyl-RPT concentrations versus time after a single 900-mg dose of RPT (▵) versus after seven doses of 900 mg given thrice weekly (○). Values shown represent arithmetic means with standard error bars.
TABLE 2.
PK parameters of RPT after a single dose of 900 mg administered orally versus after seven doses of 900 mg administered thrice weekly
| PK parameter (unit) | Single dose of RPTa | Multiple doses of RPTa | GMRb | 90% CI | P valuec |
|---|---|---|---|---|---|
| AUC0-48 (μg · h/ml) | 512.9 (148) | 410.2 (127) | 0.80 | 0.71-0.89 | 0.0035 |
| Cmax (μg/ml) | 21.9 (6.0) | 21.2 (7.2) | 0.96 | 0.86-1.06 | 0.45 |
| Tmax (h) | 5.08 (1.9) | 4.77 (1.73) | 0.93 | 0.75-1.14 | 0.55 |
| t1/2 (h) | 18.5 (3.4) | 14.8 (4.4) | 0.78 | 0.72-0.86 | 0.0004 |
| CL/F (liter/h) | 1.59 (0.63) | 2.17 (0.93) | 1.36 | 1.20-1.55 | 0.0010 |
| V (liter) | 40.7 (13) | 43.7 (13) | 1.07 | 0.97-1.18 | 0.26 |
Values are means (± standard deviations).
GMRs of PKs of MXF coadministered with RPT to those of MXF alone.
P values of paired t tests comparing PKs of MXF coadministered with RPT to those of MXF alone. α = 0.05.
Safety and tolerability of high-dose RPT with MXF.
There were no serious adverse events or grade 3 or 4 toxicities. Two subjects, both Caucasian women, experienced grade 2 adverse events after the completion of study medications. One developed a fever of 39.3°C accompanied by nausea, anorexia, and hepatitis (alanine aminotransferase, 135 IU/liter). Her symptoms began on study day 20, 36 h after her final dose of study drugs; she had been asymptomatic with normal liver chemistries on day 18. Her symptoms resolved without specific intervention by day 22, and her alanine aminotransferase level was normal on day 33. Evaluation for infectious etiologies was unrevealing. Her MXF AUC0-24 was 68.3 μg · h/ml on day 4 and 55.3 μg · h/ml on day 19, the highest values among participants. Her RPT AUC0-48 on day 19 was 456.15 μg · h/ml (75th percentile), and her 25-desacetyl-RPT AUC0-48 was 364.5 μg · h/ml (50th percentile). The second subject developed a fever of 38.6°C with fatigue, malaise, and nausea on study day 21, 2 days after her last dose of study drugs. Her liver chemistries were normal, and laboratory testing failed to reveal an infectious source. She subsequently developed an urticarial rash and headache. Symptoms resolved over the next few days without specific intervention, and she felt well at the time of the final study visit on day 35. Her RPT AUC0-48 after multiple doses was 223.3 μg · h/ml, and her 25-desacetyl-RPT AUC0-48 was 172.1 μg · h/ml, both significantly lower than the values for any other participant. Her day 19 MXF AUC0-48 was 29.2 μg · h/ml (10th percentile). Both subjects received a single dose of acetaminophen for symptomatic relief.
DISCUSSION
Rifamycins induce the activities of phase II enzymes such as glucuronosyltransferase and sulfotransferase and may reduce concentrations of drugs metabolized by these pathways, including MXF (5). In our study, the MXF AUC was decreased by 17% and CL/F was increased by 30%. These data suggest that RPT induces the metabolism of MXF but to a lesser extent than rifampin, which decreased the MXF AUC by 27% in a study of healthy volunteers and by 31% in a study of patients with active TB (19, 32). Given that RPT is a less potent inducer of metabolism than rifampin, this is not unexpected (5).
The clinical implications of diminished MXF drug concentrations for patients receiving combination TB chemotherapy that includes MXF are unclear. In studies of fluoroquinolones for the treatment of infections caused by gram-negative bacilli, the 24-h AUC/MIC ratio should exceed 90 to 125 to achieve a bacteriological and clinical cure, whereas for Streptococcus pneumoniae, the goal AUC/MIC is 25 to 35 (2, 6, 26). For Mycobacterium tuberculosis, the ratio of the AUC to the MIC (AUC/MIC) was the pharmacodynamic (PD) parameter that best correlated with efficacy in a mouse model (27). However, it is not known how the PDs of MXF provided as monotherapy relates to its PDs as a part of combination TB treatment.
Using data from our study and assuming that MXF's MIC90 for M. tuberculosis isolates is 0.5 μg/ml (13), the mean Cmax/MIC90 is 6.66, and the 24-h AUC/MIC90 is 68.8 (with an unbound AUC0-24/MIC90 of 34.4 to 48.1, assuming 30 to 50% concentration-independent protein binding) (Avelox package insert) for MXF at a dose of 400 mg daily when coadministered with 900 mg RPT thrice weekly. However, for M. tuberculosis, the AUC/MIC and Cmax/MIC ratios of MXF needed to achieve a cure and prevent the emergence of resistance in humans are unknown. In an in vitro hollow-fiber PD infection model of TB, the ratio of the unbound MXF AUC to the MIC needed for the suppression of drug resistance was 53, a concentration that the investigators estimated would be reached in 59%, 86%, and 93% of patients taking 400, 600, and 800 mg, respectively, of MXF alone daily (10). These data, together with data from dose fractionation studies of mice (27), suggest that an MXF dose of 400 mg daily is on the steep part of the dose-response curve for M. tuberculosis killing and suppression of resistance. Decreases in concentration could result in diminished efficacy or a more rapid emergence of resistance.
Our finding that the thrice-weekly administration of RPT at 900 mg/dose results in a reduction in RPT concentrations after 14 days is consistent with autoinduction of metabolism. Previous studies found that repeated administration of rifampin or rifabutin increased the CL/Fs of these agents, either by inducing intestinal and hepatic metabolism or by enhancing biliary secretion (29). The rifampin AUC decreases by 33% to 45% with repeated dosing (1, 17). In one study of RPT in healthy volunteers, subjects received RPT doses of 150, 300, or 600 mg on day 1 and then the same daily dose on days 4 to 10 or 600 mg RPT every 3 days for a total of four doses. Single-dose and steady-state AUC, Cmax, and t1/2 values for RPT and its 25-desacetyl derivative were similar after a single dose and at steady state, suggesting that autoinduction of metabolism by RPT did not occur (14). A subsequent murine study showed a 25% reduction in AUC and a 44% reduction in t1/2 after treatment with twice-weekly RPT at a dose of 15 mg/kg for 5 weeks versus after a single dose (23). In our study, there was a 20% decrease in the RPT 48-h AUC and a 36% increase in CL/F but no change in Cmax after seven doses of 900 mg of RPT dosed thrice weekly; the CL/F of 25-desacetyl-RPT was similarly enhanced, whether due to induction of its metabolizing enzymes or more-rapid biliary secretion. Taken together, these data indicate that high doses of RPT administered intermittently for more than 2 weeks can result in autoinduction of metabolism, leading to diminished concentrations of RPT and its active metabolite. The clinical implications of a 20% decrease in exposure to RPT coupled with a 20% diminished exposure to 25-desacetyl-RPT are unclear.
PK/PD parameters predictive of TB treatment success with RPT have not been established. RPT is highly protein bound, and total drug plasma concentrations may be poorly predictive of activity against M. tuberculosis. For example, although RPT reaches a plasma Cmax/MIC ratio of 375 when given at 600 mg once weekly during the continuation phase of TB treatment, clinical trials using that dose have shown unacceptably high relapse rates in patients with cavitary disease or low CD4 counts (3, 31). In mice and hollow-fiber models, the PD measure most predictive of rifampin activity is the AUC/MIC ratio (9, 12). In humans, current rifampin dosing regimens result in exposures that are on the steep part of the dose-response curve, as evidenced by the reduction in activity resulting from a reduction in dose from the currently recommended 600 mg (∼10 mg/kg) to 450 mg (∼7.5 mg/kg) (16).
The maximization of rifamycin exposures is a major goal of efforts to develop improved rifamycin-based TB treatments. For 600 mg rifampin administered daily 5 days per week, total rifampin exposure over 1 week, accounting for autoinduction, is approximately 383.3 μg · h/ml (or, corrected for protein binding of 80% [4; rifampin capsules, USP package insert; Versapharm Incorporated], the unbound AUC is 76.7 μg · h/ml) (1, 17). Using data from our study, total RPT exposure over 1 week at a dose of 900 mg thrice weekly, accounting for autoinduction, is 1,230 μg · h/ml (or, corrected for protein binding of 97% [22], the unbound RPT AUC is 36.9 μg · h/ml). However, RPT is more potent, with an MIC90 for M. tuberculosis of 0.06, compared to an MIC90 for rifampin of 0.25 (11). Further study is needed to determine the PK/PD parameter that correlates best with treatment response in humans and threshold values for efficacy. However, it is notable that RPT exposures similar to those herein described have been achieved in a murine TB treatment model and are associated with greater efficacy of a RPT-based regimen than that of a rifampin-based regimen (25).
Concerns about adverse immunoallergic effects have limited the use of high-dose, intermittent rifampin therapy. Rifampin hypersensitivity syndrome (RHS) is characterized by flu-like symptoms but is occasionally associated with renal failure, hemolytic anemia, thrombocytopenia, or hypotension. RHS increases with increasing rifampin dose, occurs more commonly in women than men, and is nearly exclusively seen with intermittent dosing (8, 18). These hypersensitivity responses have not been described with RPT use. In our study, two female participants developed adverse events, one characterized by fever and clinical hepatitis and the other by fever, malaise, headache, and rash. Curiously, both syndromes occurred approximately 2 days after administration of the last dose of study drugs, a time course that is atypical for RHS. The subject with fever and hepatitis had high plasma MXF concentrations, raising the possibility of MXF-induced liver injury (28).
One limitation of our study is that concentrations of MXF's major metabolites, M1 and M2, were not determined, as the development and validation of the assay method for these compounds were cost prohibitive. The determination of metabolite concentrations would help to definitively establish that diminished MXF concentrations during the second period of the study resulted from induction of metabolism by RPT. Although it is possible that the reduction in MXF concentrations was a consequence of diminished medication adherence, we do not believe that nonadherence played a role, as pill counts and medication diaries were closely monitored and did not show changes in adherence. Another possible mechanism for decreased MXF concentrations is protein binding displacement, but because MXF is poorly protein bound, this is unlikely. Finally, altered drug transport could have played a role in this interaction but was not assessed. A second potential limitation of our study is that RPT autoinduction was evaluated while subjects received concomitant MXF. However, both RPT PK analyses were performed while MXF was at steady state, and MXF does not affect the PKs of RPT in mice (24). Third, our study was performed with healthy volunteers, and given that cytokines and inflammatory patterns in patients with infection can sometimes alter the V/F and CL/F, these PK parameters may be different in TB patients. However, RPT PK parameter estimates and their variability have been shown to be similar in TB patients and healthy volunteers (15). Finally, our sample size was too small to meaningfully analyze subgroups, such as females.
In conclusion, RPT coadministration was associated with a 17% decrease in the plasma MXF AUC. RPT at high doses appeared to induce its own metabolism, leading to 20%-lower concentrations of RPT and its active metabolite. Both MXF and RPT were generally well tolerated, but after completion of study drugs, a flu-like syndrome with a rash occurred in one participant, and fever with hepatitis occurred in another. These episodes may represent an atypical form of RHS. Further evaluation of the tolerability of high-dose RPT is warranted.
Acknowledgments
We thank Teresa Parsons and James Johnson for their help with processing analytical samples. We also thank Craig Hendrix and Ying Cao for their thoughtful assistance with the pharmacological and statistical analyses of the data. In addition, we express our appreciation to the General Clinical Research Center at the Johns Hopkins Hospital for their support. We also thank the study subjects for their participation.
This study was supported by grants from the National Institutes of Health, including U19AI045432 (R. E. Chaisson), K23AI51528 (S. E. Dorman), K24AI01637 (R. E. Chaisson), and 5 T32 GM066691 (K. Dooley; principal investigator, Theresa Shapiro). None of the authors has a conflict of interest.
Footnotes
Published ahead of print on 2 September 2008.
REFERENCES
- 1.Acocella, G., A. Nonis, G. Perna, E. Patane, G. Gialdroni-Grassi, and C. Grassi. 1988. Comparative bioavailability of isoniazid, rifampin, and pyrazinamide administered in free combination and in a fixed triple formulation designed for daily use in antituberculosis chemotherapy. II. Two-month, daily administration study. Am. Rev. Respir. Dis. 138:886-890. [DOI] [PubMed] [Google Scholar]
- 2.Ambrose, P. G., D. M. Grasela, T. H. Grasela, J. Passarell, H. B. Mayer, and P. F. Pierce. 2001. Pharmacodynamics of fluoroquinolones against Streptococcus pneumoniae in patients with community-acquired respiratory tract infections. Antimicrob. Agents Chemother. 45:2793-2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benator, D., M. Bhattacharya, L. Bozeman, W. Burman, A. Cantazaro, R. Chaisson, F. Gordin, C. R. Horsburgh, J. Horton, A. Khan, C. Lahart, B. Metchock, C. Pachucki, L. Stanton, A. Vernon, M. E. Villarino, Y. C. Wang, M. Weiner, and S. Weis for the Tuberculosis Trials Consortium. 2002. Rifapentine and isoniazid once a week versus rifampicin and isoniazid twice a week for treatment of drug-susceptible pulmonary tuberculosis in HIV-negative patients: a randomised clinical trial. Lancet 360:528-534. [DOI] [PubMed] [Google Scholar]
- 4.Boman, G., and V. A. Ringberger. 1974. Binding of rifampicin by human plasma proteins. Eur. J. Clin. Pharmacol. 7:369-373. [DOI] [PubMed] [Google Scholar]
- 5.Burman, W. J., K. Gallicano, and C. Peloquin. 2001. Comparative pharmacokinetics and pharmacodynamics of the rifamycin antibacterials. Clin. Pharmacokinet. 40:327-341. [DOI] [PubMed] [Google Scholar]
- 6.Drusano, G. L., S. L. Preston, C. Fowler, M. Corrado, B. Weisinger, and J. Kahn. 2004. Relationship between fluoroquinolone area under the curve: minimum inhibitory concentration ratio and the probability of eradication of the infecting pathogen, in patients with nosocomial pneumonia. J. Infect. Dis. 189:1590-1597. [DOI] [PubMed] [Google Scholar]
- 7.Frieden, T. R., T. R. Sterling, S. S. Munsiff, C. J. Watt, and C. Dye. 2003. Tuberculosis. Lancet 362:887-899. [DOI] [PubMed] [Google Scholar]
- 8.Grosset, J., and S. Leventis. 1983. Adverse effects of rifampin. Rev. Infect. Dis. 5(Suppl. 3):S440-S450. [DOI] [PubMed] [Google Scholar]
- 9.Gumbo, T., A. Louie, M. R. Deziel, W. Liu, L. M. Parsons, M. Salfinger, and G. L. Drusano. 2007. Concentration-dependent Mycobacterium tuberculosis killing and prevention of resistance by rifampin. Antimicrob. Agents Chemother. 51:3781-3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gumbo, T., A. Louie, M. R. Deziel, L. M. Parsons, M. Salfinger, and G. L. Drusano. 2004. Selection of a moxifloxacin dose that suppresses drug resistance in Mycobacterium tuberculosis, by use of an in vitro pharmacodynamic infection model and mathematical modeling. J. Infect. Dis. 190:1642-1651. [DOI] [PubMed] [Google Scholar]
- 11.Heifets, L. B., P. J. Lindholm-Levy, and M. A. Flory. 1990. Bactericidal activity in vitro of various rifamycins against Mycobacterium avium and Mycobacterium tuberculosis. Am. Rev. Respir. Dis. 141:626-630. [DOI] [PubMed] [Google Scholar]
- 12.Jayaram, R., S. Gaonkar, P. Kaur, B. L. Suresh, B. N. Mahesh, R. Jayashree, V. Nandi, S. Bharat, R. K. Shandil, E. Kantharaj, and V. Balasubramanian. 2003. Pharmacokinetics-pharmacodynamics of rifampin in an aerosol infection model of tuberculosis. Antimicrob. Agents Chemother. 47:2118-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ji, B., N. Lounis, C. Maslo, C. Truffot-Pernot, P. Bonnafous, and J. Grosset. 1998. In vitro and in vivo activities of moxifloxacin and clinafloxacin against Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 42:2066-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keung, A., K. Reith, M. G. Eller, K. A. McKenzie, L. Cheng, and S. J. Weir. 1999. Enzyme induction observed in healthy volunteers after repeated administration of rifapentine and its lack of effect on steady-state rifapentine pharmacokinetics: part I. Int. J. Tuberc. Lung Dis. 3:426-436. [PubMed] [Google Scholar]
- 15.Langdon, G., J. J. Wilkins, P. J. Smith, and H. McIlleron. 2004. Consecutive-dose pharmacokinetics of rifapentine in patients diagnosed with pulmonary tuberculosis. Int. J. Tuberc. Lung Dis. 8:862-867. [PubMed] [Google Scholar]
- 16.Long, M. W., D. E. Snider, Jr., and L. S. Farer. 1979. U.S. Public Health Service Cooperative trial of three rifampin-isoniazid regimens in treatment of pulmonary tuberculosis. Am. Rev. Respir. Dis. 119:879-894. [DOI] [PubMed] [Google Scholar]
- 17.Loos, U., E. Musch, J. C. Jensen, G. Mikus, H. K. Schwabe, and M. Eichelbaum. 1985. Pharmacokinetics of oral and intravenous rifampicin during chronic administration. Klin. Wochenschr. 63:1205-1211. [DOI] [PubMed] [Google Scholar]
- 18.Martinez, E., J. Collazos, and J. Mayo. 1999. Hypersensitivity reactions to rifampin. Pathogenetic mechanisms, clinical manifestations, management strategies, and review of the anaphylactic-like reactions. Medicine (Baltimore) 78:361-369. [DOI] [PubMed] [Google Scholar]
- 19.Nijland, H. M., R. Ruslami, A. J. Suroto, D. M. Burger, B. Alisjahbana, R. van Crevel, and R. E. Aarnoutse. 2007. Rifampicin reduces plasma concentrations of moxifloxacin in patients with tuberculosis. Clin. Infect. Dis. 45:1001-1007. [DOI] [PubMed] [Google Scholar]
- 20.Nuermberger, E. L., T. Yoshimatsu, S. Tyagi, R. J. O'Brien, A. N. Vernon, R. E. Chaisson, W. R. Bishai, and J. H. Grosset. 2004. Moxifloxacin-containing regimen greatly reduces time to culture conversion in murine tuberculosis. Am. J. Respir. Crit. Care Med. 169:421-426. [DOI] [PubMed] [Google Scholar]
- 21.Nuermberger, E. L., T. Yoshimatsu, S. Tyagi, K. Williams, I. Rosenthal, R. J. O'Brien, A. A. Vernon, R. E. Chaisson, W. R. Bishai, and J. H. Grosset. 2004. Moxifloxacin-containing regimens of reduced duration produce a stable cure in murine tuberculosis. Am. J. Respir. Crit. Care Med. 170:1131-1134. [DOI] [PubMed] [Google Scholar]
- 22.Reith, K., A. Keung, P. C. Toren, L. Cheng, M. G. Eller, and S. J. Weir. 1998. Disposition and metabolism of 14C-rifapentine in healthy volunteers. Drug Metab. Dispos. 26:732-738. [PubMed] [Google Scholar]
- 23.Rosenthal, I. M., K. Williams, S. Tyagi, C. A. Peloquin, A. A. Vernon, W. R. Bishai, J. H. Grosset, and E. L. Nuermberger. 2006. Potent twice-weekly rifapentine-containing regimens in murine tuberculosis. Am. J. Respir. Crit. Care Med. 174:94-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenthal, I. M., K. Williams, S. Tyagi, A. A. Vernon, C. A. Peloquin, W. R. Bishai, J. H. Grosset, and E. L. Nuermberger. 2005. Weekly moxifloxacin and rifapentine is more active than the Denver regimen in murine tuberculosis. Am. J. Respir. Crit. Care Med. 172:1457-1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenthal, I. M., M. Zhang, K. N. Williams, C. A. Peloquin, S. Tyagi, A. A. Vernon, W. R. Bishai, R. E. Chaisson, J. H. Grosset, and E. L. Nuermberger. 2007. Daily dosing of rifapentine cures tuberculosis in three months or less in the murine model. PLoS Med. 4:E344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schentag, J. J., A. K. Meagher, and A. Forrest. 2003. Fluoroquinolone AUIC break points and the link to bacterial killing rates. Part 2: human trials. Ann. Pharmacother. 37:1478-1488. [DOI] [PubMed] [Google Scholar]
- 27.Shandil, R. K., R. Jayaram, P. Kaur, S. Gaonkar, B. L. Suresh, B. N. Mahesh, R. Jayashree, V. Nandi, S. Bharath, and V. Balasubramanian. 2007. Moxifloxacin, ofloxacin, sparfloxacin, and ciprofloxacin against Mycobacterium tuberculosis: evaluation of in vitro and pharmacodynamic indices that best predict in vivo efficacy. Antimicrob. Agents Chemother. 51:576-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soto, S., L. Lopez-Roses, S. Avila, A. Lancho, A. Gonzalez, E. Santos, and B. Urraca. 2002. Moxifloxacin-induced acute liver injury. Am. J. Gastroenterol. 97:1853-1854. [DOI] [PubMed] [Google Scholar]
- 29.Strolin Benedetti, M., and P. Dostert. 1994. Induction and autoinduction properties of rifamycin derivatives: a review of animal and human studies. Environ. Health Perspect. 102(Suppl. 9):101-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.U.S. Department of Health and Human Services, Food and Drug Administration. 2003. Guidance for industry: bioavailability and bioequivalence studies for orally administered drug products—general considerations. U.S. Department of Health and Human Services, Food and Drug Administration, Washington, DC.
- 31.Vernon, A., W. Burman, D. Benator, A. Khan, and L. Bozeman for the Tuberculosis Trials Consortium. 1999. Acquired rifamycin monoresistance in patients with HIV-related tuberculosis treated with once-weekly rifapentine and isoniazid. Lancet 353:1843-1847. [DOI] [PubMed] [Google Scholar]
- 32.Weiner, M., W. Burman, C.-C. Luo, C. A. Peloquin, M. Engle, S. Goldberg, V. Agarwal, and A. Vernon. 2007. Effects of rifampin and multidrug resistance gene polymorphism on concentrations of moxifloxacin. Antimicrob. Agents Chemother. 51:2861-2866. [DOI] [PMC free article] [PubMed] [Google Scholar]