Abstract
Two TolC homologs, PM0527 and PM1980, were identified for Pasteurella multocida. A pm0527 mutant displayed increased susceptibility to a range of chemicals, including rifampin (512-fold) and acridine orange (128-fold). A pm1980 mutant showed increased susceptibility to rifampin, ceftazidime, and vancomycin.
Pasteurella multocida is a gram-negative bacterial pathogen that can cause disease in a wide range of animals (10, 40). Like other pathogens, P. multocida must survive within diverse host niches during the infection process. To this end, pathogens have evolved different systems for the import and export of certain molecules to help them survive and disseminate within the host. Efflux pumps actively export substances from the bacterial cell. These systems can be specific for one substrate or can transport a range of structurally dissimilar compounds. TolC family export systems typically export several unrelated substances, including molecules produced by the host, such as bile (26, 35), indicating that these systems might have a role in facilitating bacterial survival in particular niches. Efflux systems that transport several compounds can also be associated with multidrug resistance (32). While these systems have been found in many species, there are no data for P. multocida.
Efflux systems consist of multiple protein components. Some multidrug resistance efflux systems comprise three proteins, viz., a transporter, accessory protein, and outer membrane protein channel (32). These tripartite systems are often encoded as a single operon. However, some efflux systems, such as AcrAB from Escherichia coli (27), have the outer membrane component encoded elsewhere on the chromosome (25).
Envelope proteins of the TolC family are key components of both the type I secretion system and efflux pumps. The crystal structure of E. coli TolC revealed a channel-tunnel that spans the bacterial outer membrane and periplasm, providing a large exit duct for protein export and multidrug efflux when recruited by the substrate-engaged inner membrane complexes (5, 6).
There is accumulating evidence that efflux pumps that confer clinically relevant antibiotic resistance are important for bacterial pathogenesis. The reported properties associated with pump expression include adherence to, and invasion of, host cells by Salmonella enterica and colonization and persistence in chickens both by S. enterica (9) and by Campylobacter jejuni (26).
In this study we have characterized two outer membrane proteins, encoded by the genes pm0527 and pm1980, predicted to be TolC homologues in P. multocida.
PM0527 was recently identified experimentally as an outer membrane protein (8) with a predicted molecular mass of 50 kDa. PM0527 showed similarity to a number of bacterial TolC proteins, including those from Haemophilus influenzae (HI1462; 65% identity), C. jejuni (CmeC; 22% identity), and E. coli (TolC; 22% identity). PM1980, a predicted 52-kDa protein, showed similarity to Mannheimia succiniciproducens TolC (41% identity), E. coli CusC (26% identity), and H. influenzae HI1462 (21% identity). Each of the candidate genes encoding these proteins was inactivated in a tetracycline-resistant derivative of a P. multocida VP161 strain (AL435) (for strains, see Table S1 in the supplemental material) as described previously (16, 17, 18, 29) using single-crossover insertional mutagenesis (primers are listed in Table S2 in the supplemental material). Each mutation was confirmed by PCR (95°C, 5 min; 30 cycles of 95°C for 30 s, 54°C for 30 s, 72°C for 2 min; and finally 72°C for 5 min). Each mutant strain was complemented in trans with the intact gene generated using flanking oligonucleotides (for primers, see Table S2 in the supplemental material). The amplified DNA fragments were ligated into the SalI- and BamHI-digested expression vector pAL99 (for plasmids, see Table S1 in the supplemental material) such that transcription of the gene was driven by the constitutive P. multocida tpiA promoter. As a control, the vector pAL99 was transformed separately into each mutant (for strains, see Table S1 in the supplemental material).
As a secondary confirmation of the mutants, we used Western blotting with chicken antiserum raised against recombinant PM0527 and recombinant PM1980 (4). For immunoblotting, approximately 108 whole cells were loaded in each lane, separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis, and transferred to a polyvinylidene difluoride membrane. For PM0527, the antiserum recognized a protein of 50 kDa in the wild-type strain (Fig. 1, lane 1) which was absent in the mutant (Fig. 1, lane 2). Importantly, this protein was restored in the complemented strain (Fig. 1, lane 3) but not in the mutant strain transformed with vector only (Fig. 1, lane 4), confirming the identity of the 50-kDa protein as PM0527. The high level of PM0527 in the complemented strain is due to the multicopy gene dosage effect. These data are consistent with the PCR data showing that the pm0527 mutant expresses no PM0527. The antiserum produced against PM1980 failed to detect a protein of the predicted size in the P. multocida wild-type strain grown in vitro.
FIG. 1.
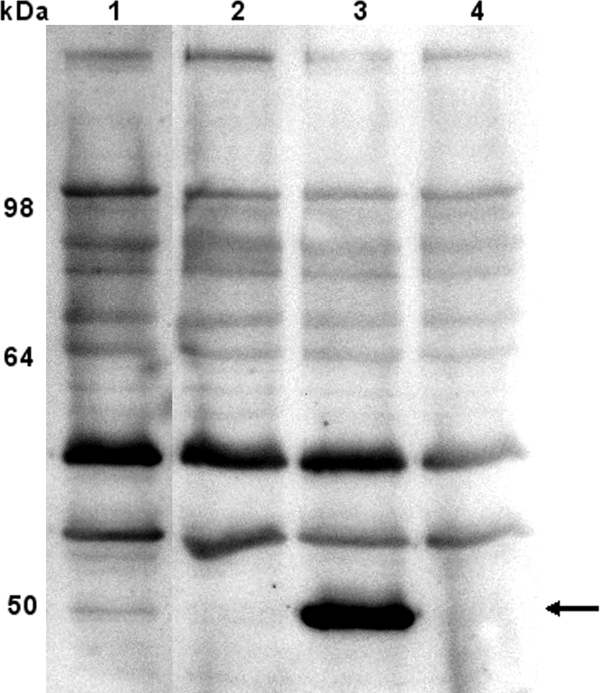
Immunoblot analysis of PM0527 TolC expression in P. multocida whole-cell lysate probed with chicken antiserum against recombinant PM0527. Lanes: 1, AL435 parent strain; 2, pm0527 mutant; 3, complemented mutant; 4, mutant complemented with empty vector. The positions of standard molecular mass markers are shown on the left. The 50-kDa PM0527 is indicated with an arrow. Prebleed serum showed no reactivity.
To determine whether the proteins PM0527 and PM1980 were involved in drug efflux, we tested the susceptibilities of parent (AL435), mutant (pm0527 and pm1980), and complemented strains to various chemicals (Table 1). The MICs were defined in twofold dilution steps in triplicate using the standard microtiter broth dilution method (20) in brain heart infusion broth. Microtiter plates were incubated for 24 h under constant aeration at 37°C. The antibiotics and other compounds used in this study were purchased from Sigma Chemical Co., Merck, or BDH Chemicals. Compared to the parent, AL435, the pm0527 mutant showed significantly increased susceptibilities to many compounds (Table 1). For the pm0527 mutant, the MICs of aminoglycoside decreased fourfold for gentamicin; the MICs of two bacteristatic antibiotics, rifampin and trimethoprim, decreased 512-fold and 32-fold, respectively, while the MICs of ceftazidime, novobiocin, vancomycin, and lincomycin decreased 16-fold, 16-fold, 8-fold, and 4-fold, respectively. Furthermore, the MICs of two macrolides decreased eightfold (erythromycin) and twofold (spiramycin) and the MICs of sulfathiazole, cycloheximide, and polymyxin B decreased fourfold, twofold, and fourfold, respectively. MICs of the bile salts taurocholic acid, sodium deoxycholate, chloramphenicol, dithiothreitol, HgCl2, fusaric acid, and mucin were decreased slightly (two-fold) but reproducibly for the pm0527 mutant. Furthermore, the MICs of the dyes acridine orange, crystal violet, and ethidium bromide and the detergent SDS decreased 128-fold, 2-fold, 4-fold, and 4-fold, respectively. The pm0527 mutant demonstrated levels of resistance similar to those of wild-type P. multocida for neomycin sulfate, nalidixic acid, deoxycholic acid, cyclophosphamide, the heavy metals CoCl2, CuCl2, and ZnCl2, and the surfactants Triton X-100 and Tween 20, suggesting that these compounds are not substrates of the PM0527 TolC efflux system. Complementation with the full-length pm0527 gene restored resistance to the majority of the compounds to approximately wild-type levels, indicating that the observed phenotype was not due to polar effects (Table 1).
TABLE 1.
Susceptibilities of P. multocida AL435, its tolC mutants (pm0527 and pm1980 strains), and their complemented strains to different compounds
| Group | Compounds | MIC (μg/ml) of compound for:
|
Fold differencea | MIC (μg/ml) of compound for pm1980 strain
|
Fold differencea | |||
|---|---|---|---|---|---|---|---|---|
| AL435 (parent) | pm0527 strain
|
|||||||
| Mutant | Complemented | Mutant | Complemented | |||||
| Antibiotics | ||||||||
| Aminoglycoside | Gentamicin | 20 | 5 | 20 | 4 | 40 | 40 | 0.5 |
| Neomycin sulfate | 125 | 125 | 125 | 1 | 1,000 | 500 | 0.125 | |
| Bacteriostatic | Chloramphenicol | 1 | 0.5 | 1 | 2 | 1 | 1 | 1 |
| Trimethoprim | 8 | 0.25 | 0.5 | 32 | 2 | 1 | 4 | |
| Rifampin | 50 | 0.1 | 0.2 | 512 | 0.78 | 0.78 | 64 | |
| Beta-lactam | Ceftazidime | 250 | 15.6 | 7.8 | 16 | 31.2 | 31.2 | 8 |
| Coumarin | Novobiocin | 40 | 2.5 | 40 | 16 | 20 | 40 | 2 |
| Fluoroquinolone | Nalidixic acid | 25 | 25 | 25 | 1 | 25 | 12.5 | 1 |
| Glycopeptide | Vancomycin | 1,000 | 125 | 250 | 8 | 250 | 250 | 4 |
| Lincosamide | Lincomycin | 100 | 25 | 25 | 4 | 100 | 50 | 1 |
| Macrolides | Erythromycin | 3.8 | 0.47 | 1.88 | 8 | 1.88 | 1.88 | 2 |
| Spiramycin | 25 | 12.5 | 25 | 2 | 25 | 25 | 1 | |
| Sulfinamide | Sulfathiazole | 2,500 | 625 | 1,250 | 4 | 2,500 | 2,500 | 1 |
| Other antibiotics | Cycloheximide | 500 | 250 | 500 | 2 | 500 | 500 | 1 |
| Polymyxin B | 12.5 | 3.125 | 6.25 | 4 | 12.5 | 6.25 | 1 | |
| Bile salts | Deoxycholic acid | 625 | 625 | 312.5 | 1 | 625 | 625 | 1 |
| Sodium deoxycholate | 625 | 312.5 | 312.5 | 2 | 625 | 625 | 1 | |
| Taurocholic acid | 3,125 | 1,562.5 | 781.25 | 2 | 1,562.5 | 1,562.5 | 2 | |
| Chemicals | ||||||||
| Alkylating agent | Cyclophosphamide | 4,000 | 4,000 | 2,000 | 1 | 2,000 | 2,000 | 2 |
| Redox agent | Dithiothreitol | 4,000 | 2,000 | 2,000 | 2 | 2,000 | 4,000 | 2 |
| Dyes | Acridine orange | 30 | 0.234 | 15 | 128 | 30 | 30 | 1 |
| Crystal violet | 12.5 | 3.125 | 6.25 | 4 | 12.5 | 12.5 | 1 | |
| Ethidium bromide | 20 | 5 | 10 | 4 | 10 | 10 | 2 | |
| Metals | CoCl2 | 1,250 | 1,250 | 1,250 | 1 | 1,250 | 1,250 | 1 |
| CuCl2 | 312.5 | 312.5 | 312.5 | 1 | 312.5 | 312.5 | 1 | |
| HgCl2 | 2.5 | 1.25 | 2.5 | 2 | 2.5 | 5 | 1 | |
| ZnCl2 | 62.5 | 62.5 | 62.5 | 1 | 62.5 | 125 | 1 | |
| Surfactants | SDS | 50 | 12.5 | 25 | 4 | 25 | 25 | 2 |
| Triton X-100 | 40,000 | 40,000 | 40,000 | 1 | 20,000 | 40,000 | 2 | |
| Tween 20 | 40,000 | 40,000 | 40,000 | 1 | 40,000 | 40,000 | 1 | |
| Toxin | Fusaric acid | 100 | 50 | 50 | 2 | 50 | 100 | 2 |
| Mucosal protein | Mucin | 8,000 | 4,000 | 8,000 | 2 | 8,000 | 8,000 | 1 |
n-fold difference between MICs for mutant strain and strain AL435.
We tested the second TolC homologue, PM1980, for susceptibility to the same set of compounds. Compared with the parent, AL435, the pm1980 mutant showed increased susceptibility to a small number of antimicrobials (Table 1). The MICs of rifampin, ceftazidime, trimethoprim, and vancomycin decreased 64-fold, 8-fold, 4-fold, and 4-fold, respectively. MICs of novobiocin, erythromycin, taurocholic acid, cyclophosphamide, dithiothreitol, ethidium bromide, SDS, Triton X-100, and fusaric acid were decreased slightly (twofold).
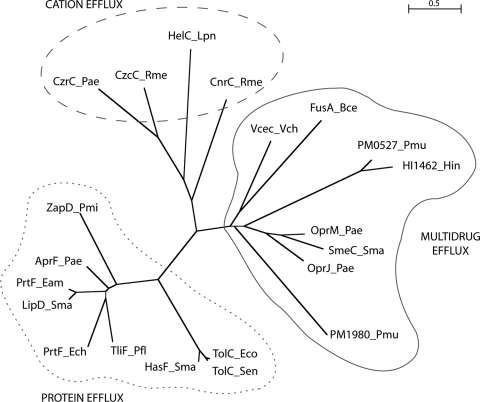
A phylogenetic analysis was performed to investigate the evolutionary relationships of PM0527 and PM1980 to 21 functionally characterized TolC family members (Fig. 2; Table 2). Multiple sequence alignment of the TolC sequences was produced using the ClustalX software program (eBiotools software) (36). Manual curation of the multiple sequence alignment was performed using the Seaview software program (14). All sequences in the alignment were cropped to the common topology core equivalent to residues 24 to 430 of the E. coli TolC protein (sequence identifier, P02930). Domain boundaries and multiple sequence alignment validation used a structural alignment of structures of E. coli TolC (PDB identifier, 1EK9), Vibrio cholerae Vcec (PDB identifier, 1YC9), and Pseudomonas aeruginosa OprM (PDB identifier, 1WP1) (aligned using the MUSTANG software program [21]). The multiple sequence alignment was used to produce a boot-strapped neighbor-joining tree, using 1,000 bootstrap trials (36). The neighbor-joining tree was drawn using the NJplot software program (31). The tree shows clustering of TolC members into clades with conserved efflux function: multidrug, cation, or protein efflux. PM0527 and PM1980 are phylogenetically most related to the TolC proteins with multidrug efflux function. Sequence alignment of the 21 TolC family members is shown in Table S3 in the supplemental material.
FIG. 2.
Location of PM0527 and PM1980 proteins in the TolC family phylogenetic tree. The TolC homologues fall into three major clades, which reflect the efflux function of the TolC proteins: multidrug, cation, or protein efflux. The distance bar indicates 50 substitutions per 100 residues. Proteins are labeled as “protein name_species name” according to details in Table 2.
TABLE 2.
Known TolC homologues for which functional data are publisheda
| Name | Organism | NCBI accession no. | Function | Protein Data Bank structure code | Reference |
|---|---|---|---|---|---|
| ZapD_Pmi | Proteus mirabilis | AAC33452 | Protein efflux | 39 | |
| AprF_Pae | Pseudomonas aeruginosa | CAA45857 | Protein efflux | 12 | |
| PrtF_Eam | Erwinia amylovora | CAB42876 | Protein efflux | 41 | |
| LipD_Sma | Serratia marcescens | BAA25796 | Protein efflux | 3 | |
| PrtF_Ech | Erwinia chrysanthemi | CAA37344 | Protein efflux | 23 | |
| TliF_Pfl | Pseudomonas fluorescens | AAD09855 | Protein efflux | 1 | |
| HasF_Sma | Serratia marcescens | CAA67136 | Protein efflux | 7 | |
| TolC_Eco | Escherichia coli | P02930 | Protein efflux | 1EK9/1TQ | 22 |
| TolC_Sen | Salmonella enterica | AAL22060 | Protein efflux | 28 | |
| PM1980_Pmu | Pasteurella multocida | NP_246919 | Multidrug efflux | This study | |
| OprJ_Pae | Pseudomonas aeruginosa | AAB41958 | Multidrug efflux | 34 | |
| SmeC_Sma | Stenotrophomonas maltophilia | AAD51346 | Multidrug efflux | 24 | |
| OprM_Pae | Pseudomonas aeruginosa | Q51487 | Multidrug efflux | 1WP1 | 2 |
| HI1462_Hin | Haemophilus influenzae | P45217 | Multidrug efflux | ||
| PM0527_Pmu | Pasteurella multocida | NP_245464 | Multidrug efflux | This study | |
| FusA_Bce | Burkholderia cepacia | P24126 | Multidrug efflux | 38 | |
| Vcec_Vch | Vibrio cholerae | ZP_01680658 | Multidrug efflux | 1YC9 | 13 |
| CzrC_Pae | Pseudomonas aeruginosa | CAB56469 | Cation efflux | 19 | |
| CzcC_Rme | Ralstonia metallidurans | CAA67082 | Cation efflux | 30 | |
| CnrC_Cme | Ralstonia metallidurans | CAB82451 | Cation efflux | 15 | |
| HelC_Lpn | Legionella pneumophila | CAH15280 | Cation efflux | 11 |
Database annotation, NCBI database (http://www.ncbi.nlm.nih.gov) accession number, function, and reference are also given.
These results clearly demonstrated that PM0527 and PM1980 are TolC homologues which contribute to the intrinsic resistance of P. multocida to diverse antimicrobial agents. This conclusion is based on several lines of evidence. First, PM0527 shares significant sequence and predicted structural similarity with many known tripartite efflux systems in gram-negative bacterial pathogens. Second, inactivation of the pm0527 and pm1980 proteins by insertional mutagenesis substantially increased the susceptibility of P. multocida to structurally diverse antimicrobial agents (Table 1). Furthermore, complementation of the mutants with the intact genes restored the resistance to numerous compounds. Phylogenetic analysis showed that both the PM0527 and PM1980 proteins align with other TolC homologues with specific drug efflux function. To determine if either efflux pump might also be involved in the export of proteins, supernatants from 30 ml of overnight brain heart infusion cultures were concentrated 300-fold by ultrafiltration (10-kDa cutoff) and examined by SDS-polyacrylamide gel electrophoresis. We observed no difference in the profiles of secreted proteins between the wild type and mutants. Together, these findings define the active role of PM0527 and PM1980 in the export of chemical compounds and antimicrobial agents.
The genomic location of pm0527 in P. multocida resembles that of acrAB in E. coli and tolC in H. influenzae, where the gene encoding the channel-tunnel is unlinked to those encoding the proteins of the inner-membrane complex (27, 37). Of particular interest is the high functional and sequence identity of PM0527 with the H. influenzae TolC homologue, HI1462. PM0527 and HI1462 share 65% sequence identity (from PM0527 residues 1 to 452), and the HI1462 mutant showed a susceptibility profile similar to that of the pm0527 mutant against 9 out of 12 compounds tested (37). The model of HI1462 (37) predicts that a pair of oppositely charged residues (R396 and E397) forms a circular network of salt bridges at the periplasmic tunnel entrance. In addition, R397 in HI1462 was reported to be responsible for the anion selectivity (33). This pair of oppositely charged residues is also found in the PM0527 sequence (residues R414 and D415) (Table 3), suggesting a common efflux mechanism. Interestingly, although PM1980 shares only 20% sequence identity with HI1462, it also contains a conserved pair of oppositely charged residues (D363 and R364), albeit in reverse orientation (Table 3).
TABLE 3.
Sequence region that is predicted to form a circular network of salt bridges at the periplasmic tunnel entrancea
| Name | Organism | NCBI accession no. | Salt bridges | Position | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HI1462_Hin | Haemophilus influenzae | P45217 | G | V | S | E | L | R | E | W | L | V | A | A | 391-402 |
| PM0527_Pmu | Pasteurella multocida | NP_245464 | G | V | S | P | L | R | D | W | L | S | A | A | 409-420 |
| PM1980_Pmu | Pasteurella multocida | NP_246919 | G | D | Y | T | F | D | R | V | L | Q | A | R | 358-369 |
Database annotation, NCBI database (http://www.ncbi.nlm.nih.gov) accession numbers are given. Underlining indicates acidic residues, and boldface indicates basic residues.
PM0527 appears to be the predominant TolC protein in P. multocida. Compared with PM0527, the level of resistance conferred by PM1980 was relatively moderate, and it may be masked by the function of PM0527 in wild-type P. multocida.
In conclusion, data from the present study demonstrated that PM0527 and PM1980 are TolC proteins of P. multocida, since their corresponding mutants show susceptibility to a range of substances. Based on our functional analyses and amino acid sequence similarity, both PM0527 and PM1980 can be classified as components of efflux pump systems of the resistance nodulation family. The characterized proteins are likely components of a tripartite efflux system, but the precise nature of protein interactions within the different TolC complexes must await future studies.
Supplementary Material
Acknowledgments
This work was supported by the Australian Research Council, Canberra, Australia. Michelle Dunstone is an NHMRC Peter Doherty Training Fellow.
We thank Marietta John and Ian McPherson for excellent technical assistance.
Footnotes
Published ahead of print on 25 August 2008.
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Ahn, J. H., J. G. Pan, and J. S. Rhee. 1999. Identification of the tliDEF ABC transporter specific for lipase in Pseudomonas fluorescens SIK W1. J. Bacteriol. 181:1847-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akama, H., M. Kanemaki, M. Yoshimura, T. Tsukihara, T. Kashiwagi, H. Yoneyama, S. Narita, A. Nakagawa, and T. Nakae. 2004. Crystal structure of the drug discharge outer membrane protein, OprM, of Pseudomonas aeruginosa: dual modes of membrane anchoring and occluded cavity end. J. Biol. Chem. 279:52816-52819. [DOI] [PubMed] [Google Scholar]
- 3.Akatsuka, H., E. Kawai, K. Omori, and T. Shibatani. 1995. The three genes lipB, lipC, and lipD involved in the extracellular secretion of the Serratia marcescens lipase which lacks an N-terminal signal peptide. J. Bacteriol. 177:6381-6389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Hasani, K., J. Boyce, V. P. McCarl, S. Bottomley, I. Wilkie, and B. Adler. 2007. Identification of novel immunogens in Pasteurella multocida. Microb. Cell Fact. 6:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersen, C., C. Hughes, and V. Koronakis. 2000. Chunnel vision. Export and efflux through bacterial channel-tunnels. EMBO Rep. 1:313-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andersen, C., C. Hughes, and V. Koronakis. 2001. Protein export and drug efflux through bacterial channel-tunnels. Curr. Opin. Cell Biol. 13:412-416. [DOI] [PubMed] [Google Scholar]
- 7.Binet, R., and C. Wandersman. 1996. Cloning of the Serratia marcescens hasF gene encoding the Has ABC exporter outer membrane component: a TolC analogue. Mol. Microbiol. 22:265-273. [DOI] [PubMed] [Google Scholar]
- 8.Boyce, J. D., P. A. Cullen, V. Nguyen, I. Wilkie, and B. Adler. 2006. Analysis of the Pasteurella multocida outer membrane sub-proteome and its response to the in vivo environment of the natural host. Proteomics 6:870-880. [DOI] [PubMed] [Google Scholar]
- 9.Buckley, A. M., M. A. Webber, S. Cooles, L. P. Randall, R. M. La Ragione, M. J. Woodward, and L. J. Piddock. 2006. The AcrAB-TolC efflux system of Salmonella enterica serovar Typhimurium plays a role in pathogenesis. Cell Microbiol. 8:847-856. [DOI] [PubMed] [Google Scholar]
- 10.Cardenas, M., A. R. Fernandez de Henestrosa, S. Campoy, A. M. Perez de Rozas, J. Barbe, I. Badiola, and M. Llagostera. 2001. Virulence of Pasteurella multocida recA mutants. Vet. Microbiol. 80:53-61. [DOI] [PubMed] [Google Scholar]
- 11.Cazalet, C., C. Rusniok, H. Bruggemann, N. Zidane, A. Magnier, L. Ma, M. Tichit, S. Jarraud, C. Bouchier, F. Vandenesch, F. Kunst, J. Etienne, P. Glaser, and C. Buchrieser. 2004. Evidence in the Legionella pneumophila genome for exploitation of host cell functions and high genome plasticity. Nat. Genet. 36:1165-1173. [DOI] [PubMed] [Google Scholar]
- 12.Duong, F., A. Lazdunski, B. Cami, and M. Murgier. 1992. Sequence of a cluster of genes controlling synthesis and secretion of alkaline protease in Pseudomonas aeruginosa: relationships to other secretory pathways. Gene 121:47-54. [DOI] [PubMed] [Google Scholar]
- 13.Federici, L., D. Du, F. Walas, H. Matsumura, J. Fernandez-Recio, K. S. McKeegan, M. I. Borges-Walmsley, B. F. Luisi, and A. R. Walmsley. 2005. The crystal structure of the outer membrane protein VceC from the bacterial pathogen Vibrio cholerae at 1.8 Å resolution. J. Biol. Chem. 280:15307-15314. [DOI] [PubMed] [Google Scholar]
- 14.Galtier, N., M. Gouy, and C. Gautier. 1996. SEAVIEW and PHYLO_WIN: two graphic tools for sequence alignment and molecular phylogeny. Comput. Appl. Biosci. 12:543-548. [DOI] [PubMed] [Google Scholar]
- 15.Grass, G., C. Grosse, and D. H. Nies. 2000. Regulation of the cnr cobalt and nickel resistance determinant from Ralstonia sp. strain CH34. J. Bacteriol. 182:1390-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harper, M., J. D. Boyce, A. D. Cox, F. St Michael, I. W. Wilkie, P. J. Blackall, and B. Adler. 2007. Pasteurella multocida expresses two lipopolysaccharide glycoforms simultaneously, but only a single form is required for virulence: identification of two acceptor-specific heptosyl I transferases. Infect. Immun. 75:3885-3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harper, M., J. D. Boyce, I. W. Wilkie, and B. Adler. 2003. Signature-tagged mutagenesis of Pasteurella multocida identifies mutants displaying differential virulence characteristics in mice and chickens. Infect. Immun. 71:5440-5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harper, M., A. D. Cox, F. St Michael, I. W. Wilkie, J. D. Boyce, and B. Adler. 2004. A heptosyltransferase mutant of Pasteurella multocida produces a truncated lipopolysaccharide structure and is attenuated in virulence. Infect. Immun. 72:3436-3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hassan, M. T., D. van der Lelie, D. Springael, U. Romling, N. Ahmed, and M. Mergeay. 1999. Identification of a gene cluster, czr, involved in cadmium and zinc resistance in Pseudomonas aeruginosa. Gene 238:417-425. [DOI] [PubMed] [Google Scholar]
- 20.Jorgensen, J. H., D. F. Sahm, and J. A. Washington. 1999. Antimicrobial susceptibility test: dilution and disk diffusion methods, p. 1526-1543. In R. P. Murray, J. E. Baron, A. M. Pfaller, C. F. Tenover, and H. R. Yolken (ed.), Manual of clinical microbiology, 7th ed. American Society for Microbiology, Washington, DC.
- 21.Konagurthu, A. S., J. C. Whisstock, P. J. Stuckey, and A. M. Lesk. 2006. MUSTANG: a multiple structural alignment algorithm. Proteins 64:559-574. [DOI] [PubMed] [Google Scholar]
- 22.Koronakis, V., A. Sharff, E. Koronakis, B. Luisi, and C. Hughes. 2000. Crystal structure of the bacterial membrane protein TolC central to multidrug efflux and protein export. Nature 405:914-919. [DOI] [PubMed] [Google Scholar]
- 23.Letoffe, S., P. Delepelaire, and C. Wandersman. 1990. Protease secretion by Erwinia chrysanthemi: the specific secretion functions are analogous to those of Escherichia coli alpha-haemolysin. EMBO J. 9:1375-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li, X. Z., L. Zhang, and K. Poole. 2002. SmeC, an outer membrane multidrug efflux protein of Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 46:333-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin, J., L. O. Michel, and Q. Zhang. 2002. CmeABC functions as a multidrug efflux system in Campylobacter jejuni. Antimicrob. Agents Chemother. 46:2124-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin, J., O. Sahin, L. O. Michel, and Q. Zhang. 2003. Critical role of multidrug efflux pump CmeABC in bile resistance and in vivo colonization of Campylobacter jejuni. Infect. Immun. 71:4250-4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma, D., D. N. Cook, M. Alberti, N. G. Pon, H. Nikaido, and J. E. Hearst. 1993. Molecular cloning and characterization of acrA and acrE genes of Escherichia coli. J. Bacteriol. 175:6299-6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McClelland, M., K. E. Sanderson, J. Spieth, S. W. Clifton, P. Latreille, L. Courtney, S. Porwollik, J. Ali, M. Dante, F. Du, S. Hou, D. Layman, S. Leonard, C. Nguyen, K. Scott, A. Holmes, N. Grewal, E. Mulvaney, E. Ryan, H. Sun, L. Florea, W. Miller, T. Stoneking, M. Nhan, R. Waterston, and R. K. Wilson. 2001. Complete genome sequence of Salmonella enterica serovar Typhimurium LT2. Nature 413:852-856. [DOI] [PubMed] [Google Scholar]
- 29.Miller, V. L., and J. J. Mekalanos. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 170:2575-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nies, D. H. 1992. CzcR and CzcD, gene products affecting regulation of resistance to cobalt, zinc, and cadmium (czc system) in Alcaligenes eutrophus. J. Bacteriol. 174:8102-8110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perriere, G., and M. Gouy. 1996. WWW-query: an on-line retrieval system for biological sequence banks. Biochimie 78:364-369. [DOI] [PubMed] [Google Scholar]
- 32.Piddock, L. J. 2006. Multidrug-resistance efflux pumps—not just for resistance. Nat. Rev. Microbiol. 4:629-636. [DOI] [PubMed] [Google Scholar]
- 33.Polleichtner, G., and C. Andersen. 2006. The channel-tunnel HI1462 of Haemophilus influenzae reveals differences to Escherichia coli TolC. Microbiology 152:1639-1647. [DOI] [PubMed] [Google Scholar]
- 34.Poole, K., N. Gotoh, H. Tsujimoto, Q. Zhao, A. Wada, T. Yamasaki, S. Neshat, J. Yamagishi, X. Z. Li, and T. Nishino. 1996. Overexpression of the mexC-mexD-oprJ efflux operon in nfxB-type multidrug-resistant strains of Pseudomonas aeruginosa. Mol. Microbiol. 21:713-724. [DOI] [PubMed] [Google Scholar]
- 35.Prouty, A. M., I. E. Brodsky, S. Falkow, and J. S. Gunn. 2004. Bile-salt-mediated induction of antimicrobial and bile resistance in Salmonella typhimurium. Microbiology 150:775-783. [DOI] [PubMed] [Google Scholar]
- 36.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trepod, C. M., and J. E. Mott. 2004. Identification of the Haemophilus influenzae tolC gene by susceptibility profiles of insertionally inactivated efflux pump mutants. Antimicrob. Agents Chemother. 48:1416-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Utsumi, R., T. Yagi, S. Katayama, K. Katsuragi, K. Tachibana, H. Toyoda, S. Ouchi, K. Obata, Y. Shibano, and M. Noda. 1991. Molecular cloning and characterization of the fusaric acid-resistance gene from Pseudomonas cepacia. Agric. Biol. Chem. 55:1913-1918. [PubMed] [Google Scholar]
- 39.Wassif, C., D. Cheek, and R. Belas. 1995. Molecular analysis of a metalloprotease from Proteus mirabilis. J. Bacteriol. 177:5790-5798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilkie, I. W., S. E. Grimes, D. O'Boyle, and A. J. Frost. 2000. The virulence and protective efficacy for chickens of Pasteurella multocida administered by different routes. Vet. Microbiol. 72:57-68. [DOI] [PubMed] [Google Scholar]
- 41.Zhang, Y., D. D. Bak, H. Heid, and K. Geider. 1999. Molecular characterization of a protease secreted by Erwinia amylovora. J. Mol. Biol. 289:1239-1251. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.