Abstract
Daptomycin is approved for the treatment of complicated skin and soft tissue infections, including diabetic wounds of the lower extremities, at a dose of 4 mg/kg of body weight once daily. For such localized tissue infections, drug concentrations in the interstitial space are an important determinant of successful therapy. In the diabetic population, peripheral arterial disease may limit antibiotic penetration into the target tissue. The objective of this study was to describe and compare the pharmacokinetic profiles of daptomycin in the interstitial fluid of soft tissues in diabetic and healthy volunteers by using in vivo microdialysis. Twelve subjects (six diabetic and six healthy) received a single 4-mg/kg dose of daptomycin intravenously. Samples of plasma and tissue were simultaneously collected over 24 h. Diabetic and healthy groups were matched in mean age (±10 years), gender ratio, mean weight (±10 kg), and creatinine clearance rate (±20 ml/min/1.73 m2). Pharmacokinetic parameters for plasma were similar between groups (P > 0.05). The mean peak drug concentrations ± standard deviations in tissue were 4.3 ± 3.3 μg/ml and 3.8 ± 1.4 μg/ml for diabetic and healthy subjects, respectively. The degree of tissue penetration, defined as the ratio of the area under the free drug concentration-time curve for tissue to that for plasma, was 0.93 ± 0.61 for diabetic subjects and 0.74 ± 0.09 for healthy subjects (P = 0.46). Daptomycin at 4 mg/kg penetrated well into the soft tissue, reaching concentrations approximately 70 to 90% of those of the free drug in plasma. Moreover, these free, bioactive concentrations in tissue exceeded the MICs for staphylococci and streptococci over the 24-h dosing interval.
Wound infections of the lower extremities frequently affect the diabetic population and are a significant cause of morbidity, hospitalization, and amputation (12). Gram-positive organisms, particularly Staphylococcus aureus, are the primary causative pathogens in these complicated skin infections. As the extent of antibiotic resistance has grown, the prevalence of methicillin-resistant S. aureus (MRSA) in skin and soft tissue infections (SSTI) has risen alarmingly, resulting in reports of worse outcomes for infections caused by MRSA than for infections caused by other bacteria (20).
Anti-MRSA agents like daptomycin are increasingly in demand, particularly in light of recent queries regarding vancomycin's efficacy and continued utility (7). Daptomycin is approved for the treatment of complicated SSTI, including diabetic wound infections, at an intravenous dose of 4 mg/kg of body weight once daily (1, 13). While the pharmacokinetic profile of daptomycin in plasma has been reported previously, for localized tissue infections, drug concentrations in the interstitial space rather than those in plasma determine clinical outcomes. Moreover, diabetic patients also typically suffer from peripheral arterial disease (PAD), which may further limit antibiotic concentrations in infected tissues, as was shown previously for vancomycin by Skhirtladze and colleagues (18). To date, there are no available studies evaluating the pharmacokinetics of daptomycin at the infection site (i.e., tissue). The objective of this study was to describe the pharmacokinetic profile of daptomycin in the interstitial fluid of soft tissues by using in vivo microdialysis and to compare the degrees of penetration between healthy and diabetic volunteers.
MATERIALS AND METHODS
Study protocol.
This study was a comparative single-dose pharmacokinetic study of diabetic and healthy adult volunteers. The study protocol was reviewed and approved by the Institutional Review Board at Hartford Hospital. Written informed consent was obtained from all participants prior to the study. Subjects were admitted to the Clinical Research Center at Hartford Hospital the evening prior to the beginning of the study (i.e., the day of administration of the study medication) and kept under fasting conditions for 9 h before the start of drug administration until the completion of the dose.
Volunteers.
Twelve volunteers were enrolled, with six subjects in each group. Diabetic subjects were required to have a documented medical history of type 1 or type 2 diabetes for which they were actively receiving insulin or oral antihyperglycemic agents. All other medications were allowed during the study period except for HMG (3-hydroxy-3-methylglutaryl)-coenzyme A reductase inhibitors. Healthy volunteers, in comparison, had to be free of medical conditions and medications, not including birth control and vitamins. Those eligible for the healthy group were enrolled based on matching the mean values for the diabetic group characteristics of age (±10 years), gender ratio, weight (±10 kg), and creatinine clearance rate (CLCR; ±20 ml/min/1.73 m2).
Prior to enrollment, inclusion-exclusion criteria were verified and a general physical examination including a review of medical history and an assessment of vital signs (temperature [oral or tympanic], blood pressure, heart rate, and respiratory rate) was performed by the study physician. Clinical laboratory data, including complete blood cell counts and the results of serum electrolyte panels, serum creatinine measurements, liver function panels, creatine phosphokinase (CPK) measurements, and urine analyses, were also collected. Serum pregnancy tests were conducted for females with childbearing potential. Subjects were excluded if they were <18 years of age or were pregnant or breast-feeding or if they had a history of hypersensitivity to the study medication (daptomycin) or anesthetics (lidocaine or lidocaine derivatives); a body mass index (weight in kilograms per square meter) of >30; a CLCR of <30 ml/min/1.73 m2 as calculated by the Cockcroft-Gault equation (5); aspartate aminotransferase, an alanine aminotransferase, or alkaline phosphatase level of >2 times the upper limit of the normal range; a CPK level of >5 times the upper limit of the normal range; or anemia as defined by a hematocrit value of <30%. For those originally meeting the criteria, clinical lab results were retested the evening prior to the receipt of the study drug to confirm continued adherence to inclusion-exclusion conditions. Physical exams, assessments of vital signs, and clinical lab tests were performed again at the end of the study to evaluate any study-related adverse events. The consumption of alcohol or caffeinated products was prohibited throughout the study period, and participants were discouraged from excessive movement during the study.
The ankle brachial index (ABI) was measured for all those enrolled to assess for lower-extremity PAD by using a handheld Doppler device (MedaSonics CardioBeat blood flow 5.12-MHz Doppler device with stethoscope; CooperSurgical Inc., Trumbull, CT) in accordance with guidelines from the American College of Cardiology and the American Heart Association (11). An ABI value of <0.90 was interpreted to indicate mild to moderate PAD, and a value of <0.40 was interpreted to indicate severe PAD.
Microdialyis procedure.
A microdialysis probe (CMA 60 microdialysis catheter; CMA Microdialysis AB, Solna, Sweden) with a membrane length of 30 mm and a molecular mass cutoff of 20 kDa was inserted under sterile conditions into the subcutaneous layer of the thigh via a guidance cannula, following the local injection of 0.5% lidocaine solution. Once implanted in tissue, the microdialysis system was connected, flushed (15 μl/min for 5 min), and then continuously perfused with lactated Ringer's solution at a flow rate of 2 μl/min with a microinfusion pump (CMA 107 microdialysis pump; CMA Microdialysis AB, Solna, Sweden). Preliminary testing determined that lidocaine would not interfere with the detection of daptomycin in dialysate fluid.
The principles of microdialysis have been described in detail previously (3, 14, 15, 17). In brief, microdialysis is based on the continuous sampling of the unbound (i.e., microbiologically active) fraction of analytes from the interstitial space that diffuse through the semipermeable membrane at the tip of the microdialysis probe. The probe is constantly perfused with physiological solution, allowing analytes to filter out of the interstitial space into the probe and ultimately collect in the perfusion fluid. Equilibrium between the interstitium and the perfusion fluid across the probe membrane is generally incomplete; therefore, the concentration obtained with the probe (Cdialysate) reflects only a fraction of the absolute concentration in the interstitium. Thus, microdialysis probes must be calibrated and the concentrations must be corrected for probe recovery, which is assessed by retrodialysis.
Microdialysis probe recovery. (i) In vivo retrodialysis.
The calibration of the microdialysis probes was conducted in vivo for each subject; retrodialysis was performed over a 1-h interval at 2 μl/min, after a baseline sampling period of at least 40 min. Daptomycin solutions for calibration were freshly prepared within 24 h of retrodialysis by diluting daptomycin to the desired concentration with lactated Ringer's solution and were stored refrigerated until use. Daptomycin was added to the perfusate at a concentration (Cperfusate) of 60 μg/ml, and its rate of disappearance through the membrane was determined as the level of recovery. The level of in vivo recovery was calculated as follows: % recovery in vivo = 100 − (100 × Cdialysate/Cperfusate). Following retrodialysis, the microdialysis system was washed out with lactated Ringer's solution for at least 40 min before the study drug was systemically administered.
(ii) In vitro retrodialysis.
Retrodialysis experiments were additionally conducted in vitro to evaluate probe recovery by gain and loss over a 24-h period. For recovery by gain, microdialysis probes (n = 2) were placed into a glass beaker of lactated Ringer's solution containing daptomycin at a concentration (Cmedium) of 60 μg/ml and perfused with blank Ringer's solution. For recovery by loss, probes (n = 2) were placed into a glass beaker of blank Ringer's solution and perfused with 60-μg/ml daptomycin solution. In vitro studies utilized the same daptomycin concentration employed in vivo, and the assay mixtures were kept under the same experimental conditions; probes were perfused at 2 μl/min for 24 h at room temperature. Dialysate samples of approximately 240 μl were collected every 2 h for the first 6 h and then at 8 and 10 h and 22 and 24 h after the start of drug administration. The level of recovery by gain was calculated as follows: % recovery by gain = (Cdialysate/Cmedium) × 100. The level of recovery by loss was determined similarly to the level of in vivo recovery: % recovery by loss = 100 − (100 × Cdialysate/Cperfusate).
Study medication.
Daptomycin was supplied by Cubist Pharmaceuticals (Lexington, MA). Aliquots of 500 mg of daptomycin powder for injection were reconstituted with 10 ml of 0.9% sodium chloride and further diluted with 0.9% sodium chloride to a total volume of 50 ml according to the instructions of the manufacturer (package insert for daptomycin for injection; Cubist Pharmaceuticals Inc., Lexington, MA). All doses were prepared by the Department of Pharmacy at Hartford Hospital within 3 h of the administration time and were refrigerated until use.
Each subject received a single intravenous dose of daptomycin at 4 mg/kg over 0.5 h through a peripheral catheter placed in the cephalic or antecubital vein. Daptomycin doses were administered once the washout period following microdialysis probe calibration was completed.
Sample collection.
Venous blood was obtained through a peripheral intravenous catheter from the arm other than the dosing arm at the following time points: 0 (the start of infusion), 0.5 (the end of infusion), 1, 2, 3, 4, 6, 8, 12, and 24 h. Blood samples were collected in a 10-ml BD Vacutainer (Becton, Dickinson and Company, Franklin Lakes, NJ) containing sodium heparin. Blood samples were then immediately centrifuged (2,000 × g for 10 min) to obtain the separated plasma fractions, which were stored in polypropylene tubes at −80°C until analysis.
Simultaneously, dialysate samples of approximately 120 μl were obtained every hour for the first 8 h and then at 11 and 12 h and 23 and 24 h after the start of drug administration. Dialysate samples were collected in 200-μl microvials (CMA Microdialysis AB, Solna, Sweden), which were additionally stored within polypropylene tubes at −80°C to protect against evaporation until analysis.
Protein binding studies.
For each subject, protein binding was assessed by three independent tests using Centrifree ultrafiltration devices with 30-kDa molecular mass cutoff filters per the package insert from the manufacturer (Millipore Corporation, Billerica, MA). An extra blood sample at the time of the peak drug concentration (0.5 h) was collected into a 10-ml BD Vacutainer containing sodium heparin and then immediately centrifuged (2,000 × g for 10 min) to obtain the separated plasma. Exactly 0.9 ml of plasma was transferred into each of three ultrafiltration devices and centrifuged for 40 min at 25°C and 2,000 × g to generate an ultrafiltrate volume of approximately 250 μl. In addition, an aliquot of plasma was retained for the determination of the total drug concentration in plasma (Cplasma).
The adsorption of daptomycin to the ultrafiltration membrane during the filtration process was investigated using aqueous standards. Analytical-grade daptomycin (Cubist Pharmaceuticals, Lexington, MA) was used to prepare aqueous solutions of daptomycin at 15, 50, 60, and 70 μg/ml with 0.9% sodium chloride. Assessed with three to six independent tests for each concentration, samples were ultrafiltrated and analyzed in the same manner described above for plasma samples. The percentage of the drug adhering to the ultrafiltration membrane was found to decrease with increasing daptomycin concentrations. Therefore, a linear regression line was formed to correlate the unbound fraction of the drug (y) to the daptomycin concentration (x) (y = 0.131x + 85.8; R2 = 0.818). Once the percentage of membrane binding was determined for each subject, ultrafiltrate drug concentrations (Cultrafiltrate values) were corrected. The level of protein binding was then calculated as follows: % protein binding = 100 − (100 × Cultrafiltrate/Cplasma).
Analytical procedures.
Concentrations of daptomycin in plasma and 0.9% sodium chloride were determined by a validated high-performance liquid chromatography (HPLC) assay at the Center for Anti-Infective Research and Development by methods described previously (8). The assay results were linear over a range from 1 to 100 μg/ml (R2 = 0.999). Intra- and interday coefficients of variation (CV) for the low (4-μg/ml)- and high (80-μg/ml)-concentration quality control samples were <7%.
Concentrations in dialysate and calibration standards for retrodialysis were assayed by liquid chromatography-tandem mass spectrometry (LC-MS-MS) by Cubist Pharmaceuticals. Lactated Ringer's solution was utilized as the matrix for calibration curves and quality controls. The calibration curve was linear over a range from 0.01 to 5 μg/ml (R2 = 0.993) by the linear regression model. Calibration curves, quality controls, and dialysate samples were prepared with 5 to 10% CHAPS {3-[(3-cholamidoprophly)-dimethylammonio]-1-propanesulfonate} solution (50 mM in 0.1% formic acid water). In brief, a 25-μl dialysate sample was mixed with 100 μl of an internal standard (10 μg/ml) in a solution of 0.1% trifluoric acetic acid in acetonitrile, dried, reconstituted with 100 μl of 0.1% formic acid water, and then analyzed. Intra- and interday CV for the low (0.03-μg/ml)- and high (4.0-μg/ml)-concentration quality control samples were <7%. The limit of quantification was 0.01 μg/ml.
Ultrafiltrate samples for the evaluation of plasma protein binding were also run by LC-MS-MS by the methods described above, except that 200 mM phosphate buffer was used as the matrix. The consistency of plasma data obtained by LC-MS-MS with those obtained by HPLC was examined by additionally assaying samples of plasma with peak drug concentrations (previously analyzed by HPLC) by LC-MS-MS. The CV among peak plasma drug concentrations determined by HPLC and LC-MS-MS were ≤21%.
Pharmacokinetic analysis. (i) Plasma.
The total daptomycin concentrations in plasma from each subject were analyzed by compartmental methods using the WinNonlin software program (version 5.0; Pharsight Corporation, Mountain View, CA). A two-compartment model was selected for all subjects based on a visual inspection of the fit and the goodness of the fit measured by the Akaike information criterion. The maximum concentration of daptomycin (Cmax) was estimated by a visual inspection of the concentration-time profile. The area under the concentration-time curve from 0 h to infinity (AUC0-∞) was calculated as dose/(V1 × k10), where V1 is the volume of distribution in the central compartment and k10 is the rate constant of elimination from the central compartment. The half-life (t1/2) was calculated as 0.693/β, where β is the rate constant of the terminal elimination phase. Clearance was calculated as dose/AUC0-∞, and the volume of distribution at steady state was calculated as follows: V1 + (k12/k21 × V1), where k12 and k21 are transfer rate constants. Individual protein binding percentages were applied to each subject's AUC to determine free, bioactive drug concentrations.
(ii) Tissue.
Concentrations for all dialysate samples were corrected for the level of in vivo recovery calculated for each patient prior to the pharmacokinetic analysis with the following equation: Ctissue = 100 × (Cdialysate/% recovery in vivo), where Ctissue is the absolute drug concentration in the interstitium. Tissue drug concentration data for each subject were analyzed using a noncompartmental approach with WinNonlin. The Cmax and the minimum concentration (Cmin) were estimated by the visual inspection of the concentration-time profile. The time to reach the peak concentration reflected the time at the end of the period sample collection, as did all the time points for dialysate samples. The AUC0-∞ was calculated as follows: AUC0-last + Clast/λz, where AUC0-last is the area under the concentration-time curve from 0 h to the time of the last quantifiable concentration (Clast) determined using the log-linear trapezoidal rule. The terminal elimination rate constant (λz) was estimated by linear regression analysis of the terminal portion of the concentration-time curve by using no fewer than three datum points, and t1/2 was calculated as 0.693/λz. The percentage of penetration into tissue was calculated with AUC0-∞ results determined for tissue (AUCtissue) and plasma (using the free drug concentration [fAUCplasma]), as follows: AUCtissue/fAUCplasma × 100.
Statistical analysis.
All pharmacokinetic parameters and indices for tissue penetration were compared between diabetic and healthy groups by the t test or Mann-Whitney rank sum test for nonnormally distributed data. A P value of <0.05 was considered statistically significant.
RESULTS
Volunteers.
Twelve subjects meeting the respective criteria for the diabetic and healthy groups were enrolled in and completed the study. The groups of diabetic and healthy volunteers were relatively similar in mean age, gender ratio, and mean weight, as intended (Table 1). The average length of diabetes during which pharmacological treatment was necessary was 9.8 ± 5.5 years. Two subjects, one of whom had type 1 diabetes, were actively receiving insulin. Lower-extremity PAD, as assessed by ABI methods, was not detected in any subject. Serum creatinine values did not differ notably between groups, although healthy subjects expectedly had a greater CLCR.
TABLE 1.
Characteristics of diabetic and healthy subjectsa
| Characteristic | Value for:
|
|
|---|---|---|
| Diabetic subjects (n = 6) | Healthy subjects (n = 6) | |
| Age (yr) | 53 ± 10 | 40 ± 11 |
| Males (% [no.]) | 33.3 (2) | 33.3 (2) |
| Race (% [no.]) | ||
| African-American | 50.0 (3) | 16.7 (1) |
| Caucasian | 33.3 (2) | 66.7 (4) |
| Hispanic | 16.7 (1) | 16.7 (1) |
| Height (in.) | 65.4 ± 2.8 | 64.8 ± 5.1 |
| Weight (kg) | 76.9 ± 10.0 | 71.4 ± 10.0 |
| Body mass index (kg/m2) | 28.0 ± 4.2 | 26.4 ± 2.0 |
| ABI | ||
| Right | 1.09 ± 0.16 | 1.24 ± 0.17 |
| Left | 1.12 ± 0.15 | 1.22 ± 0.16 |
| Serum creatinine concnb (mg/dl) | 0.9 ± 0.2 | 0.8 ± 0.2 |
| CLCRb (ml/min/1.73 m2) | 75.8 ± 27.7 | 96.2 ± 25.2 |
Data are reported as means ± standard deviations, unless otherwise specified.
Values reflect laboratory results obtained the evening prior to the patients’ receipt of the study drug.
Two errors occurred during the administration of the study medication. One volunteer received an extra 1.4% of the original 4-mg/kg dose. In another volunteer, the dose was infused over 20 min rather than 30 min. Both discrepancies were accounted for during the pharmacokinetic analysis.
Four adverse events were reported during the study. Three subjects complained of mild to moderate headaches, and one complained of light-headedness. These conditions subsided during the 3-day study and were not considered to be drug related. CPK elevations to >2 times the upper limit of the normal range were observed in three subjects; however, these elevations occurred prior to drug administration. Two of these subjects had increased CPK levels of 403 and 479 U/liter, while the third had a remarkably high value of 2,602 U/liter. All abnormal CPK values in these volunteers decreased to levels within the acceptable range before the admittance of these subjects into the study. None of the 12 participants experienced a discernible rise in CPK levels following the receipt of the study medication. All other laboratory results were either within normal limits or clinically insignificant (as determined by the study physician) over the course of the study.
Microdialysis probe recovery. (i) In vivo retrodialysis.
Mean in vivo recovery levels for daptomycin in subcutaneous tissue were 34 and 31% for diabetic and healthy volunteers, respectively.
(ii) In vitro recovery.
Mean in vitro recovery levels for gain were 34, 37, 36, 35, and 30% at 2, 4, 6, 10, and 24 h. Mean recovery levels for loss were 33, 33, 31, 30, and 30% at 2, 4, 6, 10, and 24 h. Probe recovery levels for daptomycin were consistent over the 24-h sampling period and equivalent between gain and loss.
Pharmacokinetic analyses.
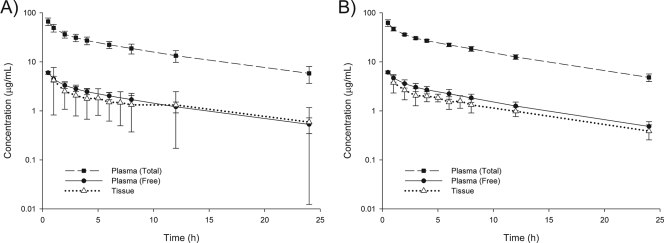
The average daptomycin doses administered were 308 ± 42 and 286 ± 40 mg for diabetic and healthy groups, respectively (P = 0.36). Errors in dosing administration for the two aforementioned volunteers were not found to influence plasma or tissue results at any time point compared to the results for the other participants. The concentration-time profiles of the drug in plasma and the interstitium for diabetic and healthy groups are displayed in Fig. 1.
FIG. 1.
Concentration-time profiles for plasma and tissue samples from diabetic (A) and healthy (B) subjects following a single 4-mg/kg dose of daptomycin. Time points for concentrations in tissue reflect the end time of the sample collection period. Data are reported as means ± standard deviations of results for six subjects per group.
(i) Plasma.
The levels of protein binding were 91% ± 2% and 90% ± 2% for diabetic and healthy subjects, respectively (P = 0.57). No statistical difference in plasma pharmacokinetic parameters between groups was noted, although the mean AUC0-∞ and t1/2 values for diabetic subjects were higher than those for healthy subjects due to a lower clearance rate in the diabetic group (Table 2).
TABLE 2.
Pharmacokinetic parameters for drug concentrations in plasma and tissue samples following a single 4-mg/kg dose of daptomycin
| Sample type and parametera | Valueb for:
|
P value | |
|---|---|---|---|
| Diabetic subjects (n = 6) | Healthy subjects (n = 6) | ||
| Plasmac | |||
| Cmax (μg/ml) | 67.8 ± 11.1 | 62.4 ± 9.8 | 0.40 |
| AUC0-∞ (μg·h/ml) | 496.7 ± 123.1 | 450.5 ± 43.0 | 0.41 |
| t1/2 (h) | 9.3 ± 1.6 | 8.1 ± 0.6 | 0.13 |
| CL (ml/h/kg) | 8.4 ± 1.7 | 9.0 ± 0.9 | 0.50 |
| Vss (ml/kg) | 101.7 ± 18.2 | 97.6 ± 5.4 | 0.94 |
| Tissue | |||
| Cmax (μg/ml) | 4.3 ± 3.3 | 3.8 ± 1.4 | 0.73 |
| Tmax (h) | 1.5 ± 0.8 | 1.3 ± 0.8 | 0.70 |
| AUC0-∞ (μg·h/ml) | 45.1 ± 40.6 | 33.5 ± 8.1 | 0.82 |
| t1/2 (h) | 12.4 ± 4.7 | 8.8 ± 1.8 | 0.24 |
| Cmin (μg/ml) | 0.59 ± 0.58 | 0.39 ± 0.13 | 0.94 |
| Penetration ratio (AUCtissue/fAUCplasma) | 0.93 ± 0.61 | 0.74 ± 0.09 | 0.46 |
CL, clearance; Vss, steady-state volume of distribution; Tmax, time to reach peak concentration.
Data are reported as means ± standard deviations.
Plasma parameters are based on total drug concentrations.
(ii) Tissue.
The time-course of drug concentrations in tissue closely paralleled that of free drug concentrations in plasma (Fig. 1). The Cmax for tissue (≈4 μg/ml) was considerably lower than that for plasma, occurring within 2 h of the dose (Table 2). Concentrations in the interstitium were generally ≥1 μg/ml for both groups for up to 12 h following dose administration. Similar to the values for plasma, the mean AUC0-∞ and t1/2 values for tissue in diabetic subjects were greater than those for tissue in healthy subjects, although no meaningful difference between groups was detected (Table 2). In particular, the mean t1/2 in tissue for diabetics (12.4 h) was longer than the t1/2 in plasma for either group. The degrees of tissue penetration, defined as the AUCtissue/fAUCplasma ratios, were 93% ± 61% and 74% ± 9% for diabetic and healthy subjects, respectively (P = 0.46).
DISCUSSION
Herein, we determined the pharmacokinetic profile of daptomycin at the target site (i.e., tissue) by means of in vivo microdialysis. We further explored the penetration of daptomycin by comparing diabetics against healthy persons.
Firstly, plasma pharmacokinetic data and protein binding levels for our diabetic and healthy subjects were consistent with what has been reported previously for single-dose studies of daptomycin at 4 mg/kg in healthy volunteers (9, 22). There is no available pharmacokinetic study with diabetics exclusively for comparison, but no difference between our study groups was found.
Secondly, penetration into the subcutaneous tissue was rapid, as peak concentrations were observed within 2 h of the dose administration. Daptomycin accumulated in the interstitial fluid, where levels were sustained through 12 h before considerably declining. Concentrations in the tissue were 93 and 74% of free drug concentrations in plasma for diabetic and healthy subjects, respectively. These levels are greater than the 68% penetration reported by Wise et al., who used the inflammatory blister fluid method and the same single 4-mg/kg dose (21). While the value reported by these investigators is somewhat lower than those noted in the present study, the difference may be due to the utilization of total drug concentrations for the calculation of the AUCs for tissue and plasma. Thus, the percentage reported by Wise et al. may underestimate the level of penetration as calculated by free drug comparisons. Moreover, daptomycin protein binding to serum albumin has been shown previously to be weak (Kd [dissociation constant], 90.3 μM) and reversible, which may contribute to the greater penetration estimated from our data for free drug concentrations in plasma, since we assessed protein binding at a single time point (10). To date, no other tissue penetration data are available for daptomycin in humans.
The penetration of other anti-MRSA agents commonly utilized for skin infections into the soft tissues has been investigated previously by the microdialysis technique. Median tissue-to-plasma drug concentration ratios were 0.1 and 0.3, respectively, for vancomycin in diabetic and nondiabetic patients, while the AUCtissue/AUCplasma ratios were 0.44 and 1.45, respectively (18). For linezolid, mean AUCtissue/fAUCplasma ratios were 1.4 and 0.9 for single- and multiple-dose regimens, respectively, in healthy volunteers (6). It should be emphasized that a definition for the level of tissue penetration consistent among studies is lacking; methods of determining the degree of penetration differ in the use of single- versus multiple-dose data, free versus total drug concentrations in plasma, and the concentration per time point versus the overall AUC in the calculation of ratios of AUCs for tissue to those for plasma, proving comparisons to be difficult.
Lastly, no difference in the degree of tissue penetration between our diabetic and healthy subjects was observed, although data for the diabetics were noticeably more variable. One comparative pharmacokinetic study of vancomycin revealed significantly less tissue penetration in diabetic patients than in nondiabetic subjects when the penetration results were calculated as the ratios of the drug concentrations in tissue to those in plasma (a median tissue-to-plasma drug concentration ratio of 0.1 [range, 0.01 to 0.45] for diabetics versus a median of 0.3 [range, 0.46 to 0.94] for healthy subjects; P = 0.002) (18). These data were collected at steady state following continuous infusions of vancomycin at 80 to 120 mg/h, with no differences found in plasma drug concentrations between groups. Subjects were patients undergoing cardiac surgery, and all diabetic subjects had received insulin for at least 5 years. Unfortunately, the degree of microvascular damage in the diabetic study population is unknown, as no objective tests were performed to show evidence of PAD. Interestingly, the authors focused on the median concentration points rather than the overall drug exposure (AUC) to define the penetration levels, which were reported to be 0.44 (range, 0.08 to 2.02) and 1.45 (range, 0.35 to 3.72) for diabetic and nondiabetic groups, respectively, when calculated as ratios of AUCs for tissue to those for plasma, and no corresponding statistical results are disclosed. The penetration of linezolid into tissue in patients with diabetic foot infections has also been studied previously; however, that investigation was performed with tissue specimens and not in vivo microdialysis (19).
Although the pharmacodynamic profile of daptomycin has been elucidated previously according to plasma exposures in murine infection models (16), the pharmacodynamics of the compound in the human interstitium remain to be determined. Based on our observations in the present study, free daptomycin concentrations in the subcutaneous tissue surpass the breakpoint (1 μg/ml) for commonly encountered skin pathogens like S. aureus and Streptococcus spp. (4) for about 50% of the dosing interval. Moreover, considering that daptomycin MICs for most S. aureus and Staphylococcus epidermidis strains and beta-hemolytic streptococci are 0.25 μg/ml or lower, as reported in a 2006 U.S. surveillance study of 5,297 isolates, the free drug concentration-time profile in tissue indicates that daptomycin concentrations can be expected to exceed the MICs for these organisms for the entire dosing interval, given the Cmin results (2).
In conclusion, daptomycin at 4 mg/kg penetrates well into the soft tissue, with concentrations of approximately 70 to 90% of free drug concentrations in plasma. Our data on the drug distribution in tissue, gathered with the use of the in vivo microdialysis technique, are supportive of daptomycin's apparent clinical effectiveness in complicated SSTI.
Acknowledgments
We thank the staff at the Center for Anti-Infective Research and Development and Hartford Hospital for their assistance in the preparation and conduct of the study.
This work was supported by an investigator-initiated research grant from Cubist Pharmaceuticals Inc., Lexington, MA. D. P. Nicolau and J. L. Kuti have received other research grants from Cubist Pharmaceuticals, and D. P. Nicolau has also acted as a consultant for Cubist Pharmaceuticals.
Footnotes
Published ahead of print on 8 September 2008.
REFERENCES
- 1.Arbeit, R. D., D. Maki, F. P. Tally, E. Campanaro, B. I. Eisenstein, and the Daptomycin 98-01 and 99-01 Investigators. 2004. The safety and efficacy of daptomycin for the treatment of complicated skin and skin-structure infections. Clin. Infect. Dis. 38:1673-1681. [DOI] [PubMed] [Google Scholar]
- 2.Castanheira, M., R. N. Jones, and H. S. Sader. 2008. Update of the in vitro activity of daptomycin tested against 6710 Gram-positive cocci isolated in North America (2006). Diagn. Microbiol. Infect. Dis. 61:235-239. [DOI] [PubMed] [Google Scholar]
- 3.Chaurasia, C. S., M. Müller, E. D. Bashaw, E. Benfeldt, J. Bolinder, R. Bullock, P. M. Bungay, E. C. M. DeLange, H. Derendorf, W. F. Elmquist, M. Hammarlund-Udenaes, C. Joukhadar, D. L. Kellogg, Jr., C. E. Lunte, C. H. Nordstrom, H. Rollema, R. J. Sawchuk, B. W. Y. Cheung, V. P. Shah, L. Stahle, U. Ungerstedt, D. F. Welty, and H. Yeo. 2007. AAPS-FDA workshop white paper: microdialysis principles, application, and regulatory perspectives report from the joint AAPS-FDA workshop, November 4-5, 2005, Nashville, TN. AAPS J. 9:e48-e59. [Google Scholar]
- 4.Clinical and Laboratory Standards Institute. 2008. Performance standards for antimicrobial susceptibility testing, 18th informational supplement. M100-S18. Clinical and Laboratory Standards Institute, Wayne, PA.
- 5.Cockcroft, D. W., and M. H. Gault. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31-41. [DOI] [PubMed] [Google Scholar]
- 6.Dehghanyar, P., C. Büger, M. Zeitlinger, F. Islinger, F. Kovar, M. Müller, C. Kloft, and C. Joukhadar. 2005. Penetration of linezolid into soft tissues of healthy volunteers after single and multiple doses. Antimicrob. Agents Chemother. 49:2367-2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deresinski, S. 2007. Counterpoint: vancomycin and Staphylococcus aureus—an antibiotic enters obsolescence. Clin. Infect. Dis. 44:1543-1548. [DOI] [PubMed] [Google Scholar]
- 8.DeRyke, C. A., C. Sutherland, B. Zhang, D. P. Nicolau, and J. L. Kuti. 2006. Serum bactericidal activities of high-dose daptomycin with and without coadministration of gentamicin against isolates of Staphylococcus aureus and Enterococcus species. Antimicrob. Agents Chemother. 50:3529-3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dvorchik, B. H., D. Brazier, M. F. DeBruin, and R. D. Arbeit. 2003. Daptomycin pharmacokinetics and safety following administration of escalating doses once daily to healthy subjects. Antimicrob. Agents Chemother. 47:1318-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eisenstein, B. I. 2004. Lipopeptides, focusing on daptomycin, for the treatment of gram-positive infections. Expert. Opin. Investig. Drugs 13:1159-1169. [DOI] [PubMed] [Google Scholar]
- 11.Hirsch, A. T., Z. J. Haskal, N. R. Hertzer, C. W. Bakal, M. A. Creager, J. L. Halperin, L. F. Hiratzka, W. R. Murphy, J. W. Olin, J. B. Puschett, K. A. Rosenfield, D. Sacks, J. C. Stanley, L. M. Taylor, Jr., C. J. White, J. White, R. A. White, E. M. Antman, S. C. Smith, Jr., C. D. Adams, J. L. Anderson, D. P. Faxon, V. Fuster, R. J. Gibbons, S. A. Hunt, A. K. Jacobs, R. Nishimura, J. P. Ornato, R. L. Page, B. Riegel, American Association for Vascular Surgery, Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, ACC/AHA Task Force on Practice Guidelines Writing Committee To Develop Guidelines for the Management of Patients with Peripheral Arterial Disease, American Association of Cardiovascular and Pulmonary Rehabilitation, National Heart, Lung, and Blood Institute, Society for Vascular Nursing, TransAtlantic Inter-Society Consensus, and Vascular Disease Foundation. 2006. ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee To Develop Guidelines for the Management of Patients with Peripheral Arterial Disease)—endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 113:e463-e654. [DOI] [PubMed] [Google Scholar]
- 12.Lipsky, B. A., A. R. Berendt, H. G. Deery, J. M. Embil, W. S. Joseph, A. W. Karchmer, J. L. LeFrock, D. P. Lew, J. T. Mader, C. Noden, and J. S. Tan. 2004. Diagnosis and treatment of diabetic foot infections. Clin. Infect. Dis. 39:885-910. [DOI] [PubMed] [Google Scholar]
- 13.Lipsky, B. A., and U. Stoutenburgh. 2005. Daptomycin for treating infected diabetic foot ulcers: evidence from a randomized, controlled trial comparing daptomycin with vancomycin or semi-synthetic penicillins for complicated skin and skin-structure infections. J. Antimicrob. Chemother. 55:240-245. [DOI] [PubMed] [Google Scholar]
- 14.Müller, M. 2002. Science, medicine, and the future: microdialysis. Br. Med. J. 324:588-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Müller, M., O. Haag, T. Burgdorff, A. Georgopoulos, W. Weninger, B. Jansen, G. Stanek, H. Pehamberger, E. Agneter, and H. G. Eichler. 1996. Characterization of peripheral-compartment kinetics of antibiotics by in vivo microdialysis. Antimicrob. Agents Chemother. 40:2703-2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Safdar, N., D. Andes, and W. A. Craig. 2004. In vivo pharmacodynamic activity of daptomycin. Antimicrob. Agents Chemother. 48:63-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt, S., R. Banks, V. Kumar, K. H. Rand, and H. Derendorf. 2008. Clinical microdialysis in skin and soft tissues: an update. J. Clin. Pharmacol. 48:351-364. [DOI] [PubMed] [Google Scholar]
- 18.Skhirtladze, K., D. Hutschala, T. Fleck, F. Thalhammer, M. Ehrlich, T. Vukovich, M. Müller, and E. M. Tschernko. 2006. Impaired target site penetration of vancomycin in diabetic patients following cardiac surgery. Antimicrob. Agents Chemother. 50:1372-1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stein, G. E., S. Schooley, C. A. Peloquin, A. Missavage, and D. H. Havlichek. 2007. Linezolid tissue penetration and serum activity against strains of methicillin-resistant Staphylococcus aureus with reduced vancomycin susceptibility in diabetic patients with foot infections. J. Antimicrob. Chemother. 60:819-823. [DOI] [PubMed] [Google Scholar]
- 20.Vardakas, K. Z., M. Horianopoulou, and M. E. Falagas. 2008. Factors associated with treatment failure in patients with diabetic foot infections: an analysis of data from randomized controlled trials. Diabetes Res. Clin. Pract. 80:344-351. [DOI] [PubMed] [Google Scholar]
- 21.Wise, R., T. Gee, J. M. Andrews, B. Dvorchik, and G. Marshall. 2002. Pharmacokinetics and inflammatory fluid penetration of intravenous daptomycin in volunteers. Antimicrob. Agents Chemother. 46:31-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woodworth, J. R., E. H. Nyhart, Jr., G. L. Brier, J. D. Wolny, and H. R. Black. 1992. Single-dose pharmacokinetics and antibacterial activity of daptomycin, a new lipopeptide antibiotic, in healthy volunteers. Antimicrob. Agents Chemother. 36:318-325. [DOI] [PMC free article] [PubMed] [Google Scholar]