Abstract
We studied the effects of a neuraminidase inhibitor (oseltamivir) and an inhibitor of influenza virus polymerases (ribavirin) against two highly pathogenic H5N1 influenza viruses. In vitro, A/Vietnam/1203/04 virus (clade 1) was highly susceptible to oseltamivir carboxylate (50% inhibitory concentration [IC50] = 0.3 nM), whereas A/Turkey/15/06 virus (clade 2.2) had reduced susceptibility (IC50 = 5.5 nM). In vivo, BALB/c mice were treated with oseltamivir (1, 10, 50, or 100 mg/kg of body weight/day), ribavirin (37.5, 55, or 75 mg/kg/day), or the combination of both drugs for 8 days, starting 4 h before virus inoculation. Monotherapy produced a dose-dependent antiviral effect against the two H5N1 viruses in vivo. Three-dimensional analysis of the drug-drug interactions revealed that oseltamivir and ribavirin interacted principally in an additive manner, with several exceptions of marginal synergy or marginal antagonism at some concentrations. The combination of ribavirin at 37.5 mg/kg/day and oseltamivir at 1 mg/kg/day and the combination of ribavirin at 37.5 mg/kg/day and oseltamivir at 10 mg/kg/day were synergistic against A/Vietnam/1203/04 and A/Turkey/15/06 viruses, respectively. These optimal oseltamivir-ribavirin combinations significantly inhibited virus replication in mouse organs, prevented the spread of H5N1 viruses beyond the respiratory tract, and abrogated the cytokine response (P < 0.01). Importantly, we observed clear differences between the efficacies of the drug combinations against two H5N1 viruses: higher doses were required for the protection of mice against A/Turkey/15/06 virus than for the protection of mice against A/Vietnam/1203/04 virus. Our preliminary results suggest that oseltamivir-ribavirin combinations can have a greater or lesser antiviral effect than monotherapy, depending on the H5N1 virus and the concentrations used.
The spread of highly pathogenic avian influenza A (H5N1) viruses from Asia to the Middle East, Europe, and Africa poses the threat of an influenza pandemic (44, 46). Of the influenza A viruses circulating in birds, viruses of the H5N1 subtype are currently of the greatest public health concern because of an increasing number of infected humans, high mortality rates (>60%), and the emergence of multiple distinguishable clades (44, 46, 47). On the basis of the phylogenetic analysis of hemagglutinin (HA), H5N1 viruses can be divided into 10 distinct clades; the most diverse clade, clade 2, can be further subdivided into five subclades, and all clades and subclades differ in their antigenic characteristics (46, 47). Besides supportive care, treatment options for humans infected with avian H5N1 influenza virus are limited and uncertain. In the absence of clinical trials evaluating the efficacies of drugs against H5N1 influenza viruses, preclinical animal studies offer a suitable experimental approach.
Specific anti-influenza virus agents such as neuraminidase (NA) inhibitors and, to a lesser degree, M2 ion-channel blockers (adamantanes) are recommended for use for the management of H5N1 human infection and could possibly play a role in the initial response to pandemic influenza, especially if an effective strain-specific vaccine is unavailable (4, 10, 24, 25, 27, 50). However, the emergence of drug-resistant variants is one of the disadvantages of using antiviral therapy. Recently, up to 95% of clade 1 avian H5N1 influenza viruses have been found to be resistant to adamantanes, although most representatives from other clades remain adamantane sensitive (2, 10, 15). Unlike M2 ion-channel blockers, NA inhibitors appear to be associated with a lower frequency of resistance. Resistant H5N1 virus strains either can emerge naturally or can be developed under selection pressure from antiviral drugs (10, 11, 19, 25, 27). Oseltamivir-resistant H5N1 viruses with the H274Y or the N294S NA mutation have recently been identified in infected patients during or after treatment (5, 21). The N294S NA amino acid change was also detected in two patients before the administration of antiviral therapy, and the source of this NA mutation is still under investigation (34).
H5N1 influenza viruses differ from seasonal human H1N1 or H3N2 viruses mainly in that they have high replication efficiencies; are disseminated beyond the respiratory tract, causing multiorgan failure; and induce hypercytokinemia (1, 28). Because the disease caused by highly pathogenic H5N1 influenza viruses can be very severe in some cases, the current strategies approved for use for the treatment of seasonal influenza may be not optimal; and other options, such as the use of a combination of drugs, must be explored. In our previous in vitro studies, treatment with a combination of an NA inhibitor and an M2 ion-channel blockers resulted in an additive and synergistic reduction of the extracellular virus yield and prevented or reduced the emergence of H5N1 drug-resistant variants (8, 17). Importantly, oseltamivir combined with amantadine or rimantadine was more effective than oseltamivir used singly in preventing the mortality of BALB/c mice infected with H5N1 or H9N2 viruses (16, 22).
The broad-spectrum antiviral agent ribavirin, a nucleoside analogue, is an inhibitor of influenza A and B virus infections in vitro and in animal models (4, 7, 12, 18, 20, 37, 38, 39). To date, at least two mechanisms of action have been proposed for ribavirin: ribavirin monophosphate decreases the intracellular concentration of GTP because of competitive inhibition of IMP dehydrogenase, and ribavirin triphosphate inhibits the function of virus-coded RNA polymerases, which are necessary for the initiation and elongation of viral mRNAs (4, 6, 7, 38, 48). The latter antiviral activity of ribavirin may provide additional benefits against highly pathogenic H5N1 influenza viruses by inhibiting replication efficiency, a key factor in their pathogenesis (28, 36, 42).
A previous study has shown that in vitro ribavirin was effective against two low pathogenic H5N1 strains in a concentration range that was similar to that required for the inhibition of replication of seasonal human influenza virus strains (38). The activity of ribavirin alone or in combination with other antiviral agents against highly pathogenic H5N1 viruses has not been studied, but the activity of ribavirin against influenza viruses of the H1N1 and H3N2 subtypes has been investigated in vitro and in vivo (14, 18, 23, 39, 40, 45). Ribavirin combined with rimantadine or zanamivir exerted an additive antiviral effect and, at specific concentrations, a synergistic antiviral effect to reduce the yield of influenza viruses in Madin-Darby canine kidney (MDCK) cells (14, 23). In a mouse model, the use of ribavirin with the M2 ion-channel blocker amantadine enhanced the survival of the mice, and the combination was significantly more effective than either drug alone (45). Ribavirin interacted additively and synergistically with the NA inhibitor peramivir to reduce the levels of H1N1 influenza virus infection in vitro and in vivo (23, 40).
In the present study, our goal was to determine whether ribavirin could be combined additively, synergistically, or antagonistically with oseltamivir for use as prophylaxis against infections caused by highly pathogenic H5N1 influenza viruses in mice. We used two H5N1 viruses isolated from fatally infected humans from different geographical areas. The viruses represent two different clades on the H5 HA phylogenetic tree: clade 1 (A/Vietnam/1203/04) and clade 2.2 (A/Turkey/15/06).
MATERIALS AND METHODS
Compounds.
The NA inhibitor oseltamivir carboxylate [(3R,4R,5S)-4-acetamido-5-amino-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxylic acid] and the prodrug oseltamivir phosphate [oseltamivir; ethyl(3R,4R,5S)-4-acetamido-5-amino-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxylate] were provided by F. Hoffmann-La Roche, Ltd. (Basel, Switzerland). Zanamivir [5-acetamido-4-guanidino-6-(1,2,3-trihydroxypropyl)-5,6-dihydro-4H-pyran-2-carboxylic acid] was provided by the R. W. Johnson Pharmaceutical Research Institute (Raritan, NJ). Ribavirin (1-β-d-ribofuranosyl-1,2,4-triazole-3-carboxamine) was obtained from Sigma-Aldrich, Inc. (St. Louis, MO). The compounds were dissolved in sterile distilled water, and aliquots were frozen at −70°C until they were used.
Viruses and cells.
The H5N1 influenza viruses A/Vietnam/1203/04 and A/Turkey/15/06 were obtained from the World Health Organization collaborating laboratories. Stock viruses were grown in the allantoic cavities of 10-day-old embryonated chicken eggs for 32 h at 36°C, and aliquots were stored at −70°C until they were used. Experiments with highly pathogenic H5N1 influenza viruses were conducted in an animal biosafety level 3+ containment facility approved by the U.S. Department of Agriculture.
MDCK cells were obtained from the American Type Culture Collection (Manassas, VA) and were maintained as described previously (8).
Infectivity of H5N1 viruses.
The yield of H5N1 viruses was determined by the plaque assay in MDCK cells, as described previously (13). Briefly, MDCK cells were inoculated with serial 10-fold dilutions of influenza viruses. After a 1-h incubation, the cells were overlaid with minimal essential medium containing 0.9% agar and 4% bovine serum albumin. After 3 days of incubation at 37°C, the cells were stained with 0.1% crystal violet in 10% formaldehyde solution, and the numbers of PFU per milliliter were determined. In our study, both H5N1 viruses grew to comparable titers: ∼108.5 PFU/ml in MDCK cells.
NA enzyme inhibition assay.
NA activity was determined by the method described by Potier et al. (29). Briefly, H5N1 viruses and various concentrations of oseltamivir carboxylate or zanamivir were preincubated for 30 min at 37°C before the substrate 2′-(4-methylumbelliferyl)-α-D-N-acetylneuraminic acid (Sigma, St. Louis, MO) was added. After 1 h, the reaction was terminated by adding 14 mM NaOH, and the fluorescence was quantitated with a Perkin-Elmer fluorimeter (model LS50B) with an excitation wavelength of 360 nm and an emission wavelength of 448 nm. The 50% inhibitory concentration (IC50) was defined as the concentration of NA inhibitor necessary to reduce the activity NA by 50% relative to that in a reaction mixture containing virus but no inhibitor.
Assessment of drug efficacy in vivo.
Female 6-week-old BALB/c mice (Jackson Laboratories, Bar Harbor, ME) were anesthetized with isoflurane and were intranasally inoculated with 50 μl of 10-fold serial dilutions of A/Vietnam/1203/04 (H5N1) or A/Turkey/15/06 (H5N1) virus in phosphate-buffered saline (PBS). The 50% mouse lethal dose (MLD50) was calculated after a 21-day observation period. For the A/Vietnam/1203/04 (H5N1) and the A/Turkey/15/06 (H5N1) viruses, the MLD50s/ml were ∼1 PFU and 4 PFU, respectively. Groups of 15 mice each were then given oseltamivir (1, 10, 50, or 100 mg/kg of body weight/day) or ribavirin (37.5, 55, or 75 mg/kg/day) by oral gavage twice daily for 8 days. In the combination treatment experiments, oseltamivir was coadministered with ribavirin on the same schedule. Virus-inoculated control mice received sterile PBS (placebo). The first drug dose was given 4 h before intranasal inoculation with 5 MLD50/mouse of A/Vietnam/1203/04 (H5N1) or 5 MLD50/mouse of A/Turkey/15/06 (H5N1) virus; these doses were equivalent to ∼4 and 20 PFU/mouse, respectively. Survival and weight change were observed; animals that showed signs of severe disease and weight loss of >25% were humanely killed. Three mice each in the experimental and the placebo groups were killed on day 3 after inoculation; and the lungs, brains, and spleens were removed, homogenized, and suspended in 1 ml of PBS. Virus from each organ was titrated by inoculation of embryonated chicken eggs with serial dilutions of the suspensions. The titers were calculated by the method of Reed and Muench (32) and are expressed as the mean log10 50% egg-infective dose (EID50)/ml ± standard deviation (SD). The limit of virus detection was 0.75 log10 EID50/ml. For calculation of the mean, samples with a virus titer of <0.75 log10 EID50/ml were assigned a value of 0.
As controls for drug toxicity, five mice were given the highest dose of each drug used alone and in combination and were observed for survival, weight change, and overt toxic effects. All studies were approved by the St. Jude Children's Research Hospital Animal Care and Use Committee and were conducted according to applicable laws and guidelines.
Cytokine/chemokine analysis.
The concentrations of interleukin 1 alpha (IL-1α), IL-6, monocyte chemotactic protein 1 (MCP-1), gamma interferon inducible protein (IP-10), tumor necrosis factor alpha (TNF-α), and alpha interferon (IFN-α) in homogenates of lungs collected on day 3 after inoculation with H5N1 virus from five mice from the treatment and the placebo groups were measured by a specific enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN). The cytokine induction profiles in mock-infected mice treated with oseltamivir and ribavirin (alone or in combination) or PBS (placebo) as well as in completely clean mice were also assayed.
Statistical analysis and synergy determinations.
The susceptibility of influenza A (H5N1) viruses to NA inhibitors was compared by an unpaired two-tailed t test. The virus titers in the internal organs of the mice (lungs, brain, and spleen) and the levels of cytokines/chemokines were compared by analysis of variance (ANOVA). The Kaplan-Meier method was used to estimate the probability of survival. The log-rank test was used to compare the survival distributions of the placebo and treatment groups (43). The proportional hazards model was used to determine the death hazard ratios for the treatment and placebo groups (3). A probability value of 0.05 was prospectively chosen to indicate that the findings of these analyses were not the result of chance alone.
The data from the animal studies with drug combinations were analyzed by using the MacSynergy II software program, provided by Mark N. Prichard (30, 31). Theoretical additive interactions were calculated from the dose-response curves for each drug used individually. This calculated additive surface was then subtracted from the experimentally determined dose-response surface to give regions of nonadditive interactions. The confidence intervals around the experimental dose-response surface were used to evaluate the data statistically, and the volumes of the peaks were calculated and used to quantify the volume of synergy (or antagonism) produced. The guidelines for the volumes of the synergy determinations, expressed as μm2 (μm × μm × %) at a 95% confidence level, were as follows: 0 to 25 μm2 unit %, insignificant synergy or antagonism; 25 to 50 μm2 unit %, minor but significant synergy or antagonism; 50 to 100 μm2 unit %, moderate synergy or antagonism; >100 μm2 unit %, strong synergy or antagonism. Synergy plots were made at the 95% confidence limit.
RESULTS
Susceptibilities of H5N1 viruses to NA inhibitors in vitro.
The susceptibilities of influenza A/Vietnam/1203/04 (H5N1) and A/Turkey/15/06 (H5N1) viruses to oseltamivir carboxylate and zanamivir were evaluated in a fluorescence-based NA enzyme inhibition assay (data not shown). The A/Vietnam/1203/04 (H5N1) virus was highly susceptible to both NA inhibitors: the mean IC50s ± SDs were 0.3 ± 0.1 nM and 0.9 ± 0.2 nM for oseltamivir carboxylate and zanamivir, respectively. In contrast, the A/Turkey/15/06 (H5N1) virus had a significantly reduced susceptibility to oseltamivir carboxylate (18-fold increase; IC50 = 5.5 ± 1.9 nM) compared with the susceptibility of the A/Vietnam/1203/04 strain (P < 0.05) and remained highly susceptible to zanamivir (IC50 = 0.8 ± 0.1 nM).
Efficacy and mode of interaction of oseltamivir and ribavirin in mice inoculated with A/Vietnam/1203/04 (H5N1) virus.
Table 1 presents the efficacies of oseltamivir and ribavirin (survival, time to death, and risk of death) against lethal A/Vietnam/1203/04 (H5N1) virus infection in mice. All control animals died 6 to 13 days after inoculation with 5 MLD50 of H5N1 influenza virus. Oseltamivir administration for 8 days had a dose-dependent effect: treatment with 10 mg/kg/day rather than 1 mg/kg/day resulted in higher survival rates (80 and 30%, respectively) and a lower risk of death (0.07 and 0.44, respectively). Ribavirin given alone orally at 37.5 and 75 mg/kg/day was significantly protective (50% and 70% survivors, respectively) and caused significant delays in the time to death (P < 0.01) (Table 1).
TABLE 1.
Effect of treatment with oseltamivir, ribavirin, and their combinations on the survival of mice inoculated with A/Vietnam/1203/04 (H5N1) influenza virus
| Oseltamivir (mg/kg/day) | Results obtained with the following ribavirin dose (mg/kg/day)a:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0
|
37.5
|
75
|
|||||||
| No. of survivors/total no. of mice (%) | Day of death (mean ± SD) | Hazard ratiob | No. of survivors/total of mice (%) | Day of death (mean ± SD) | Hazard ratio | No. of survivors/total of mice (%) | Day of death (mean ± SD) | Hazard ratio | |
| 0 | 0/10 (0) | 10.9 ± 0.7 | 1 | 5/10 (50)* | 16.7 ± 1.4* | 0.21* | 7/10 (70)** | 14.9 ± 0.9* | 0.11* |
| 1 | 3/10 (30) | 11.8 ± 0.7 | 0.44 | 9/10 (90)**,°°,† | 15 | 0.03*,° | 9/10 (90)**, °° | 10 | 0.03*,° |
| 10 | 8/10 (80)** | 12.8 ± 0.3 | 0.07* | 7/10 (70)** | 14.2 ± 0.6*,† | 0.11* | 9/10 (90)** | 19 | 0.03* |
Boldface type indicates a higher percentage of survivors, a longer time to death, or a lower risk of death compared with the values obtained when either compound was used alone. *, P < 0.01, compared with the results for the placebo-treated control group; **, P < 0.001 compared with the results for the placebo-treated control group; °, P < 0.05, compared with the results for the group treated with oseltamivir alone; °°, P < 0.01, compared with the results for the group treated with oseltamivir alone; †, P < 0.05 compared with the results for the group treated with ribavirin alone.
An estimate of the relative risk of death.
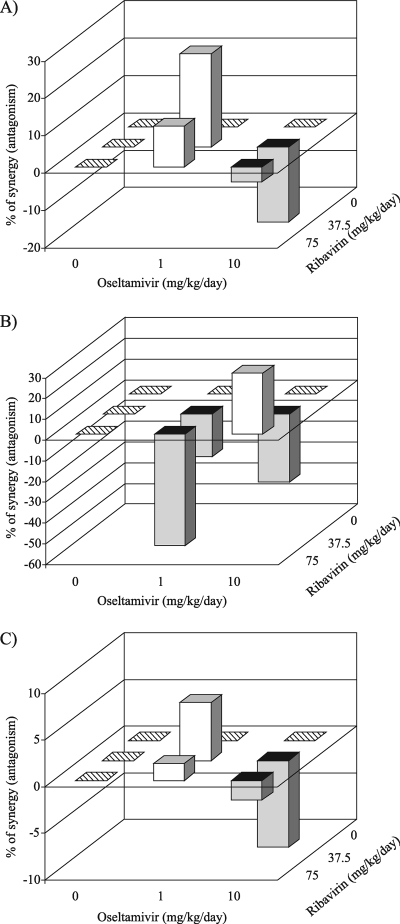
We tested if the antiviral activities of the agents tested alone could be combined and whether the resulting activity would be synergistic or antagonistic against A/Vietnam/1203/04 (H5N1) virus. Statistically significant increases in survival and risk of death (P < 0.01) were seen on comparison of the groups treated with the combination (1 mg/kg/day oseltamivir and 37.5 and 75 mg/kg/day ribavirin) to the groups treated with 1 mg/kg/day oseltamivir only (Table 1). Evaluation of the survival data presented in Table 1 by using the independent-effects three-dimensional model (MacSynergy II) (30, 31) demonstrated that the significant volume of synergy (25 μm2 unit %) was generated only for the combination of 37.5 mg/kg/day ribavirin and 1 mg/kg/day oseltamivir (Fig. 1A). However, values near the threshold of 25 μm2 unit % were at the very lower limits of significant synergy. The results for longevity were significantly lower than the expected values when 10 mg/kg/day oseltamivir was combined with 37.5 mg/kg/day ribavirin (−34 μm2 unit %), indicative of antagonism (Fig. 1B). Therefore, although there may be pockets of marginal synergy or marginal antagonism within the combination profiles for all pairs of compounds used against A/Vietnam/1203/04 (H5N1) virus, there seemed to be a general trend toward an additive oseltamivir-ribavirin interaction (Fig. 1).
FIG. 1.
Three-dimensional plots showing the interaction of oseltamivir and ribavirin on the effect of treatment on the number of survivors (A), the mean day of death (B), and the risk of death (C) for mice infected with influenza A/Vietnam/1203/04 (H5N1) virus. Treatments were given twice daily for 8 days starting 4 h before virus exposure. Light bars, synergy; dark bars, antagonism. The synergy or antagonism for the various drug combinations, although minor, was significant at the 95% confidence level.
Efficacy and mode of interaction of oseltamivir and ribavirin in mice inoculated with A/Turkey/15/06 (H5N1) virus.
Table 2 presents the efficacies of oseltamivir and ribavirin used alone or in combination against lethal infection with A/Turkey/15/06 (H5N1) virus in mice. Oseltamivir at 0.1 and 1 mg/kg/day had no significant effect on the survival of animals infected with A/Turkey/15/06 (H5N1) virus (data not shown). Of the three oseltamivir regimens (10, 50, and 100 mg/kg/day), significantly enhanced survival (P < 0.05) was observed only in mice that received 100 mg/kg/day (Table 2). Mice that received 50 and 100 mg/kg/day oseltamivir had a higher survival rate and a lower risk of death than those that received 10 mg/kg/day or placebo. Treatment with 55 and 75 mg/kg/day ribavirin but not with 37.5 mg/kg/day ribavirin protected 50% of the infected animals and decreased the risk of death (Table 2).
TABLE 2.
Effect of treatment with oseltamivir, ribavirin, and their combinations on the survival of mice inoculated with A/Turkey/15/06 (H5N1) influenza virus
| Oseltamivir dose (mg/kg/day) | Results obtained with the following ribavirin dose (mg/kg/day)a:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0
|
37.5
|
55
|
75
|
|||||||||
| No. of survivors/total no. of mice (%) | Day of death (mean ± SD) | Hazard ratiob | No. of survivors/ total no. of mice (%) | Day of death (mean ± SD) | Hazard ratio | No. of survivors/total no. of mice (%) | Day of death (mean ± SD) | Hazard ratio | No. of survivors/total no. of mice (%) | Day of death (mean ± SD) | Hazard ratio | |
| 0 | 0/10 (0) | 9.7 ± 0.3 | 1 | 1/10 (10) | 10.4 ± 0.2 | 0.57 | 5/10 (50)* | 10.6 ± 0.2 | 0.18** | 5/10 (50)** | 12.2 ± 0.4* | 0.15** |
| 10 | 2/10 (20) | 10.7 ± 0.6 | 0.47 | 5/10 (50)*,† | 11.4 ± 0.3* | 0.16**,† | 5/10 (50)** | 11.0 ± 0.0*,° | 0.15**,° | 3/10 (30)* | 10.8 ± 0.1† | 0.28* |
| 50 | 3/10 (30) | 11.7 ± 0.8* | 0.27* | 5/10 (50)** | 12.5 ± 0.7*,†† | 0.16**,† | 7/10 (70)**,° | 13.8 ± 0.2*,°°,†† | 0.07** | 7/10 (70)**,° | 15.3 ± 0.6*,°°,†† | 0.07** |
| 100 | 9/10 (90)** | 15 | 0.02** | 9/10 (90)**,†† | 10 | 0.02**,†† | 7/10 (70)** | 11.7 ± 0.3*,°° | 0.08** | 8/10 (80)** | 14.0 ± 0.0*,†† | 0.05** |
Boldface type indicates a higher percentage of survivors, a longer time to death, or a lower risk of death compared with the values obtained when either compound was used alone. *, P < 0.01 compared with the results for the placebo-treated control group; **, P < 0.001 compared with the results for the placebo-treated control group; °, P < 0.05, compared with the results for the group treated with oseltamivir alone; °°, P < 0.01 compared with the results for the group treated with oseltamivir alone; †, P < 0.05 compared with the results for the group treated with ribavirin alone; ††, P < 0.01 compared with the results for the group treated with ribavirin alone.
An estimate of the relative risk of death.
We administered oseltamivir (10, 50, and 100 mg/kg/day) together with ribavirin (37.5, 55, and 75 mg/kg/day) to mice to assay whether their combination would be more beneficial than either drug administered alone. Significant improvements in the rate of survival, the mean day of death, and the relative risk of death (P < 0.05) were seen in mice treated with 37.5, 55, or 75 mg/kg/day ribavirin and 50 mg/kg/day oseltamivir (Table 2). Other combinations were marginally effective, except for 10 mg/kg/day oseltamivir combined with 37.5 mg/kg/day ribavirin (Table 2).
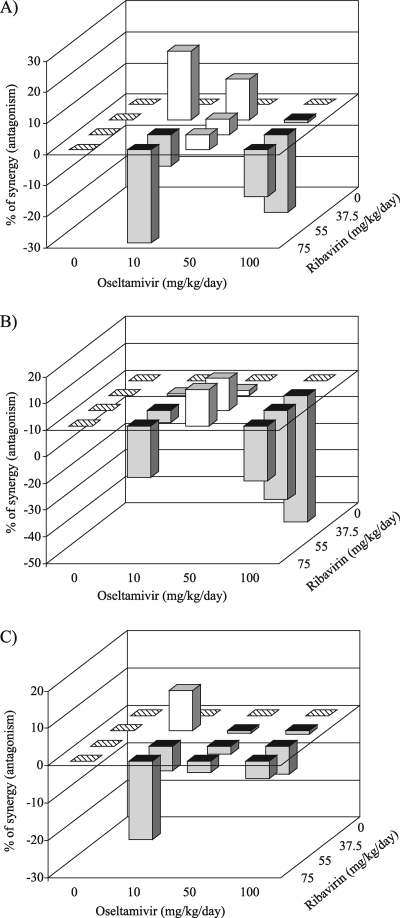
Figure 2 gives a three-dimensional analysis of the survival data. Most drug combinations provided additive antiviral effects on the number of survivors, the mean day of death, and the relative risk of death in mice challenged with influenza A/Turkey/15/06 (H5N1) virus. However, there were some instances of antagonism. The combination of 37.5 or 55 mg/kg/day ribavirin with 100 mg/kg/day oseltamivir showed minor but significant antagonism with respect to the greater longevity of infected mice (Fig. 2B).
FIG. 2.
Three-dimensional plots showing the interaction of oseltamivir and ribavirin on the effect of treatment on the number of survivors (A), the mean day of death (B), and the risk of death (C) for mice infected with influenza A/Turkey/15/06 (H5N1) virus. Treatments were given twice daily for 8 days starting 4 h before virus exposure. Light bars, synergy; dark bars, antagonism. The synergy or antagonism for several drug combinations, although minor, was significant at the 95% confidence level.
Over an observation period of 21 days, an 8-day administration of 100 mg/kg/day oseltamivir and 75 mg/kg/day ribavirin alone or in combination to uninfected control mice did not lead to toxicity, as assessed by weight loss (or the suppression of weight gain; data not shown).
Effect of optimal oseltamivir-ribavirin combinations on replication of H5N1 viruses in mouse organs.
Survival curves revealed that oseltamivir and ribavirin reached maximum synergy at 37.5 mg/kg/day for ribavirin and 1 mg/kg/day for oseltamivir and at 37.5 mg/kg/day for ribavirin and 10 mg/kg/day for oseltamivir against A/Vietnam/1203/04 (H5N1) and A/Turkey/15/06 (H5N1) viruses, respectively (Fig. 1A and 2A).
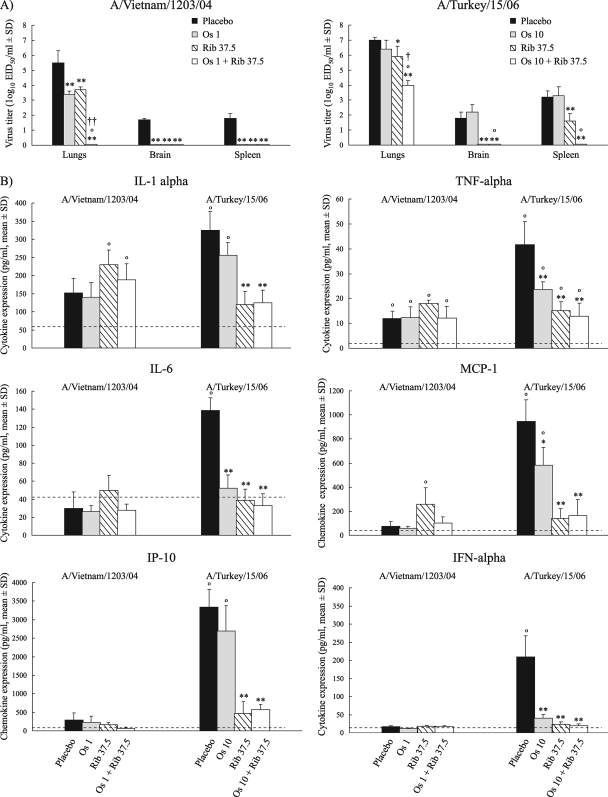
We investigated the efficacies of the optimal oseltamivir-ribavirin combinations in inhibiting virus replication in the lungs and spleens and in preventing the spread of H5N1 viruses to the brains of mice on day 3 postinoculation. In control untreated mice, virus was detected in the lungs (5 to 7 log10 EID50/ml), brains (1.5 to 2 log10 EID50/ml), and spleens (2 to 3.5 log10 EID50/ml) after challenge with either A/Vietnam/1203/04 (H5N1) or A/Turkey/15/06 (H5N1) virus (Fig. 3A). Oseltamivir (1 mg/kg/day) or ribavirin (37.5 mg/kg/day) used alone significantly inhibited A/Vietnam/1203/04 (H5N1) virus replication in the lungs compared to the levels of replication in the lungs of the control untreated animals (P < 0.01). Importantly, the H5N1 virus was not detected in the lungs, brains, or spleens of mice when combination treatment was administered (Fig. 3A).
FIG. 3.
Effect of treatment with oseltamivir (Os), ribavirin (Rib), and their combinations on virus titers (A) and cytokine/chemokine expression (B) of mice infected with influenza A/Vietnam/1203/04 (H5N1) or A/Turkey/15/06 (H5N1) viruses on day 3 postinoculation. (A) Oseltamivir and ribavirin (alone or in combination) or PBS was administered by oral gavage twice daily, starting 4 h before inoculation of 6-week-old BALB/c mice with 5 MLD50/mouse of H5N1 virus. Each data bar represents the mean virus titer ± SD (log10 EID50/ml) in the organs of three mice. *, P < 0.05, compared with the results for the placebo-treated control group, one-way ANOVA; **, P < 0.01 compared with the results for the placebo-treated control group, one-way ANOVA; °, P < 0.001 compared with the results for the group treated with oseltamivir alone, one-way ANOVA; †, P < 0.05, compared with the results for the group treated with ribavirin alone, one-way ANOVA; ††, P < 0.001 compared with the results for the group treated with ribavirin alone, one-way ANOVA. (B) The expression of cytokines (IL-1α, IL-6, TNF-α, IFN-α), and chemokines (MCP-1, IP-10) in lung homogenates was determined by enzyme-linked immunosorbent assay on day 3 postinoculation. The means and SDs of the results are based on experiments with five mice. The dashed line indicates the level of cytokine/chemokine expression in mock-infected mice treated with oseltamivir and ribavirin (alone or in combination) or PBS (placebo). The cytokine induction profile in completely clean mice was also measured and is comparable to that of mock-infected mice. *, P < 0.05 compared with the results for the placebo-treated infected control group, one-way ANOVA; **, P < 0.01 compared with the results for the placebo-treated infected control group, one-way ANOVA; °, P < 0.01 compared with the results for the treated mock-infected control groups, one-way ANOVA.
Oseltamivir at 10 mg/kg/day failed to significantly inhibit virus replication in the internal organs of animals infected with A/Turkey/15/06 (H5N1) virus on day 3 postinoculation (P < 0.05) (Fig. 3A). Ribavirin at 37.5 mg/kg/day reduced the virus titers in the lungs by ∼1 log10 EID50/ml. Combination therapy completely eliminated virus replication in the brains and spleens of the mice and significantly reduced the virus titers in the lungs compared with those in the lungs of the control and monotherapy groups (P < 0.05) (Fig. 3A). Taken together, our results indicated that optimal oseltamivir-ribavirin combination regimens are more beneficial than monotherapy in decreasing the virus load in mouse lungs and in preventing the spread of both H5N1 viruses beyond the respiratory tract.
Effect of optimal oseltamivir-ribavirin combinations on cytokine/chemokine expression in lungs of mice inoculated with H5N1 viruses.
The excessive production of proinflammatory cytokines/chemokines upon infection with highly pathogenic H5N1 influenza viruses is believed to contribute to increased pathogenicity and mortality in humans and mice (28, 42). To assess the effect of antiviral treatment on the elimination of an excessive cytokine response that could inflict immune damage to mouse tissues, we determined the levels of production of several proinflammatory cytokines/chemokines in the lungs of mice infected with H5N1 viruses and treated prophylactically with optimal oseltamivir-ribavirin combinations (Fig. 3B).
We observed that the levels of induction of all cytokines tested were significantly higher in the control group than in mock-infected mice on day 3 after inoculation with 5 MLD50 of A/Turkey/15/06 (H5N1) influenza virus (Fig. 3B, dashed line [P < 0.01]). Administration of 37.5 mg/kg/day ribavirin alone or in combination abrogated the IP-10 and IL-1α responses relative to those in the placebo group, which correlated with the viral titers in the mouse organs (Fig. 3). Both antiviral compounds used alone significantly decreased the levels of MCP-1, TNF-α, IL-6, and IFN-α (P < 0.01); for IL-6 and IFN-α, treatment caused the responses to return to those observed in mock-infected mice (Fig. 3B).
In contrast to the abundant production of proinflammatory cytokines/chemokines induced by A/Turkey/15/06 (H5N1) virus, the induction of all cytokines except TNF-α was negligible in the lungs of mice infected with A/Vietnam/1203/04 (H5N1) virus on day 3 postinoculation (Fig. 3B). The mean magnitude of the responses of IL-1α, TNF-α, IL-6, MCP-1, IP-10, and IFN-α showed that oseltamivir and ribavirin used singly or in combination did not affect the level of inflammation compared with that in the placebo group. However, the amounts of IL-1α, TNF-α, and MCP-1 secreted in the lung homogenates of infected mice treated with 37.5 mg/kg/day ribavirin were significantly higher (P < 0.01) than those in mock-infected mice (Fig. 3B).
DISCUSSION
This study is the first, to our knowledge, to have assessed the effectiveness of oseltamivir-ribavirin combination chemotherapy against highly pathogenic H5N1 influenza viruses in vivo and focused on oral treatments, because both drugs are active by the oral route. We have previously shown that amantadine-oseltamivir combination chemotherapy can provide a survival advantage over that from single-agent treatment for mice inoculated with influenza A/Vietnam/1203/04 (H5N1) virus (16). However, because most H5N1 isolates from clade 1 are adamantane resistant, other combination regimens need to be investigated.
In this study we observed that the susceptibilities to NA inhibitors of two H5N1 viruses belonging to two distinct clades of the H5 HA phylogenetic tree may differ significantly. The NA enzyme inhibition assay, which is a more accurate predictor of in vivo susceptibility (50), showed that the A/Turkey/15/06 (H5N1) virus, which belongs to clade 2.2, was 18-fold less susceptible to oseltamivir carboxylate than the A/Vietnam/1203/04 (H5N1) virus, which belongs to clade 1, with no decrease in susceptibility to zanamivir detected. This finding correlated with the recent observation that H5N1 viruses from clade 2 isolated in 2005 demonstrate 25- to 30-fold decreases in susceptibility to oseltamivir carboxylate compared with the susceptibilities of clade 1 viruses (26). Alignment of the NA protein sequences of A/Vietnam/1203/04 (H5N1) and A/Turkey/15/06 (H5N1) viruses revealed changes in 14 amino acids (data not shown). None of these residues has previously been shown to confer resistance to NA inhibitors in vitro or in clinical studies (24, 33). Interestingly, no difference in sensitivity to zanamivir was observed between the two H5N1 isolates, suggesting that the reduced binding to oseltamivir carboxylate of strain A/Turkey/15/06 (H5N1) is because of its altered interaction with the hydrophobic pentyl ether group of the drug. The structural role of at least the N247S and the H252Y residues (N2 numbering) in the NA of strain A/Turkey/15/06 (H5N1) in altering the susceptibility to oseltamivir carboxylate cannot be discounted, because they are adjacent to position 274 and an H274Y change confers oseltamivir-specific resistance (24, 33).
We evaluated the in vivo efficacies of oseltamivir and ribavirin used alone and in combination against influenza A (H5N1) viruses. Both variants were isolated from fatal human cases and were highly pathogenic and neurotropic in mice without prior adaptation (9, 49). Low doses of both compounds (≤10 mg/kg/day for oseltamivir and 37.5 mg/kg/day for ribavirin) were more effective against A/Vietnam/1203/04 (H5N1) virus than A/Turkey/15/06 (H5N1) virus. For lethal A/Vietnam/1203/04 (H5N1) virus infection, our results are consistent with those of Yen et al. (49), who inoculated mice with 5 MLD50 of the H5N1 virus and achieved a survival rate of 80% after 8 days of administration of 10 mg/kg/day oseltamivir. However, mice infected with strain A/Turkey/15/06 (H5N1) and given the same oseltamivir regimen had a survival rate of 20% and high viral titers in their organs. Orally administered ribavirin reduced the rates of morbidity and mortality among mice infected with H5N1 viruses; however, 37.5 mg/kg/day ribavirin resulted in 50% survival among animals infected with A/Vietnam/1203/04 (H5N1) virus but failed to protect mice infected with the A/Turkey/15/06 (H5N1) strain. Our results therefore demonstrate that the doses of oseltamivir and ribavirin, when they are used alone, required to produce significant protection and to lower the risk of death in mice infected with different H5N1 viruses may differ.
Some of these differences might be explained by the unique biological characteristics of the viruses. Our data suggest that the reduced susceptibility of strain A/Turkey/15/06 (H5N1) to oseltamivir carboxylate in the NA enzyme inhibition assay could be an important factor in the circulating viral phenotype, which may implicate viral susceptibility to NA inhibitors in a mouse animal model. In contrast, the reasons for the disparity in the antiviral action of ribavirin in vivo remain uncertain. Our results did not show a consistent pattern of efficacy of the drugs against the two H5N1 influenza viruses, and moreover, the concentrations of ribavirin that inhibited virus replication in mice were higher than those that inhibited seasonal influenza viruses of the H1N1 and H3N2 subtypes (38, 39, 40). These findings suggest that additional studies are required to test the effectiveness of ribavirin against rapidly evolving H5N1 influenza viruses in an effort to identify all the mechanisms responsible for its antiviral activity in vivo.
In this study, we demonstrated that combination treatment can have a greater or a lesser antiviral effect than monotherapy in vivo (P < 0.05). Our three-dimensional approach allowed the complete analysis of all drug concentrations tested and the resulting biological effects and is one of the most suitable models for the assessment of drug interactions (23, 30, 31). Our results indicate that anti-influenza virus agents with different mechanisms of antiviral action interact principally in an additive manner, with several exceptions of marginal synergy or marginal antagonism. This simple additivity indicates that the antiviral mechanisms of oseltamivir and ribavirin are unrelated, are independent of each other in a mouse animal model, and likely involve inhibition of influenza virus NA activity and RNA polymerase, respectively.
However, we did observe minor synergism between oseltamivir and ribavirin. The lowest concentrations of both drugs, 37.5 mg/kg/day for ribavirin and 1 mg/kg/day for oseltamivir for A/Vietnam/1203/04 (H5N1) and 37.5 mg/kg/day for ribavirin and 10 mg/kg/day for oseltamivir for A/Turkey/15/06 (H5N1) virus, synergistically improved the survival rates among mice infected with the two viruses. In the two combination therapy groups, the virus titers in the lungs declined significantly on day 3 postinoculation compared with the titers in the control and monotherapy groups (P < 0.05). Notably, the spread of both neurotropic H5N1 viruses to the brain was completely eliminated. This fact may be attributable to the complete prevention of virus spread beyond the respiratory tract. Our data could suggest that the level of replication of residual virus in the lungs during the optimal combination regimens was not quite sufficient to initiate viral spread to the brain. Therefore, whereas oseltamivir and ribavirin used singly have a limited ability to cross the blood-brain barrier (7, 41), their combination might more effectively decrease the virus load in the lungs and, therefore, prevent the dissemination of neurovirulent H5N1 influenza viruses to the central nervous system. Encephalitis and encephalopathy have been reported in patients infected with H5N1 virus. The detection of A/Vietnam/1203/04 (H5N1) and A/Turkey/15/06 (H5N1) viruses in the brains of infected mice and ferrets (9, 49) suggests that different approaches such as combined therapy are needed to facilitate the antiviral activity of each drug against highly pathogenic and neurotropic H5N1 influenza viruses.
In the present study, we investigated the relationship between the magnitude of viral replication and the levels of cytokine/chemokine expression in the lungs of mice infected with H5N1 influenza viruses and treated with oseltamivir and ribavirin singly or in combination. The cytokine responses in the lungs of mice infected with A/Vietnam/1203/04 (H5N1) virus were not as high as those in the lungs of mice infected with strain A/Turkey/15/06 (H5N1) on day 3 postinfection. This finding may be partially attributable to the incomplete activation of macrophages and monocytes, which are the major sources of MCP-1, IL-6, IL-1α, TNF-α, and INF-α during inflammation, because their activation is dependent on the virus load. We used a fivefold smaller amount of A/Vietnam/1203/04 (H5N1) virus than A/Turkey/15/06 (H5N1) virus (quantified by PFU), which might have led to the insufficient induction of cytokines/chemokines on day 3 postinfection, which is relatively early in the course of infection. In contrast, A/Turkey/15/06 (H5N1) virus was a more potent inducer of all cytokines/chemokines studied. Treatment with ribavirin alone or in combination significantly abrogated the integrated response of all cytokines and chemokines, which correlated with the significant reduction in the viral titers in the mouse lungs. These results not only demonstrate the correlation between the inhibition of A/Turkey/15/06 (H5N1) infection and the elimination of the cytokine/chemokine responses due to the antiviral activity of ribavirin but also suggest that cytokines and chemokines play a role in the pathogenesis of H5N1 influenza (42). Our data also suggest that inhibition of the excessive cytokine/chemokine production by ribavirin antiviral treatment might help in offering protection against some lethal H5N1 influenza viruses (35).
The use of antiviral monotherapy to control infections caused by highly pathogenic influenza viruses has several limitations, and therefore, there is a need to assess other approaches in direct comparative studies, such as the use of drug combinations and their efficacies. We demonstrated that oseltamivir and ribavirin interacted principally in an additive manner, although some instances of marginal synergy or marginal antagonism were observed with certain drug concentrations. The finding of a slight but reproducible antagonistic activity of both drugs raises concern about the specificity of the antiviral effects found with this combination. We also demonstrated clear differences between the efficacies of the drug combinations required to protect mice infected with the two different H5N1 strains. Therefore, our results suggest that the combination of oseltamivir and ribavirin against other avian H5N1 influenza viruses should be tested further in an effort to identify all possible factors that affect the antiviral effect of the combination in vivo.
Acknowledgments
We are grateful to Mark N. Prichard for providing the MacSynergy II software program. We thank Julie G. Groff for illustrations, Vani Shanker for editorial assistance, and Jennifer L. McClaren and Cedric Proctor for technical assistance. We also thank Thi Dung Nguyen for providing influenza A/Vietnam/1203/04 (H5N1) virus and Alan Douglas, Ahmet Faik Oner, Sukru Arslar, and Ali Bay for providing influenza A/Turkey/15/06 (H5N1) virus.
This study was supported by grants A195357 and A157570 and under contract HHSN266200700005C from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, U.S. Department of Health and Human Services, and by the American Lebanese Syrian Associated Charities. While the study reported herein did not utilize corporate funding, Natalia A. Ilyushina, Elena A. Govorkova, and Robert G. Webster are currently performing a different research study funded by F. Hoffmann-La Roche, Ltd.
Footnotes
Published ahead of print on 25 August 2008.
REFERENCES
- 1.Beigel, J. H., J. Farrar, A. M. Han, F. G. Hayden, R. Hyer, M. D. de Jong, S. Lochindarat, T. K. Nguyen, T. H. Nguyen, T. H. Tran, A. Nicoll, S. Touch, and K. Y. Yuen. 2005. Avian influenza A (H5N1) infection in humans. N. Engl. J. Med. 353:1374-1385. [DOI] [PubMed] [Google Scholar]
- 2.Cheung, C. L., J. M. Rayner, G. J. Smith, P. Wang, T. S. Naipospos, J. Zhang, K. Y. Yuen, R. G. Webster, J. S. Peiris, Y. Guan, and H. Chen. 2006. Distribution of amantadine-resistant H5N1 avian influenza variants in Asia. J. Infect. Dis. 193:1626-1629. [DOI] [PubMed] [Google Scholar]
- 3.Cox, D. R. 1972. Regression models and life-tables. J. R. Stat. Soc. B 34:187-220. [Google Scholar]
- 4.de Clercq, E. 2006. Antiviral agents active against influenza A viruses. Nat. Rev. 5:1015-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Jong, M. D., T. T. Tran, H. K. Truong, M. H. Vo, G. J. Smith, V. C. Nguyen, V. C. Bach, T. Q. Phan, Q. H. Do, Y. Guan, J. S. Peiris, T. H. Tran, and J. Farrar. 2005. Oseltamivir resistance during treatment of influenza A (H5N1) infection. N. Engl. J. Med. 353:2667-2672. [DOI] [PubMed] [Google Scholar]
- 6.Eriksson, B., B. Helgstrand, N. G. Johansson, A. Larsson, A. Misiorny, J. O. Noren, L. Phillipson, K. Stenberg, G. Stening, S. Stridh, and B. Oberg. 1977. Inhibition of influenza virus ribonucleic acid polymerase by ribavirin triphosphate. Antimicrob. Agents Chemother. 11:946-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilbert, B. E., and V. Knight. 1986. Biochemistry and clinical applications of ribavirin. Antimicrob. Agents Chemother. 30:201-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Govorkova, E. A., H. B. Fang, M. Tan, and R. G. Webster. 2004. Neuraminidase inhibitor-rimantadine combinations exert additive and synergistic anti-influenza virus effects in MDCK cells. Antimicrob. Agents Chemother. 48:4855-4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Govorkova, E. A., N. A. Ilyushina, D. A. Boltz, A. Douglas, N. Yilmaz, and R. G. Webster. 2007. Efficacy of oseltamivir therapy in ferrets inoculated with different clades of H5N1 influenza virus. Antimicrob. Agents Chemother. 51:1414-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayden, F. G. 2006. Antiviral resistance in influenza viruses—implications for management and pandemic response. N. Engl. J. Med. 354:785-788. [DOI] [PubMed] [Google Scholar]
- 11.Hayden, F. G., and A. J. Hay. 1992. Emergence and transmission of influenza A viruses resistant to amantadine and rimantadine. Curr. Top. Microbiol. Immunol. 176:119-130. [DOI] [PubMed] [Google Scholar]
- 12.Hayden, F. G., C. A. Sable, J. D. Connor, and J. Lane. 1996. Intravenous ribavirin by constant infusion for serious influenza and parainfluenza virus infection. Antivir. Ther. 1:51-56. [PubMed] [Google Scholar]
- 13.Hayden, F. G., K. M. Cote, and R. G. Douglas. 1980. Plaque inhibition assay for drug susceptibility testing of influenza viruses. Antimicrob. Agents Chemother. 17:865-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayden, F. G., R. G. Douglas, Jr., and R. Simons. 1980. Enhancement of activity against influenza viruses by combinations of antiviral agents. Antimicrob. Agents Chemother. 18:536-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ilyushina, N. A., E. A. Govorkova, and R. G. Webster. 2005. Detection of amantadine-resistant variants among avian influenza viruses isolated in North America and Asia. Virology 341:102-106. [DOI] [PubMed] [Google Scholar]
- 16.Ilyushina, N. A., E. Hoffmann, R. Salomon, R. G. Webster, and E. A. Govorkova. 2007. Amantadine-oseltamivir combination therapy for H5N1 influenza virus infection in mice. Antivir. Ther. 12:363-370. [PubMed] [Google Scholar]
- 17.Ilyushina, N. A., N. V. Bovin, R. G. Webster, and E. A. Govorkova. 2006. Combination chemotherapy, a potential strategy for reducing the emergence of drug-resistant influenza A variants. Antivir. Res. 70:121-131. [DOI] [PubMed] [Google Scholar]
- 18.Khare, G. P., R. W. Sidwell, J. T. Witkowski, L. N. Simon, and R. K. Robins. 1973. Suppression by 1-β-d-ribofuranosyl-1,2,4-triazole-3-carboxamine (virazole, ICN 1229) of influenza virus-induced infections in mice. Antimicrob. Agents Chemother. 3:517-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiso, M., K. Mitamura, Y. Sakai-Tagawa, K. Shiraishi, C. Kawakami, K. Kimura, F. G. Hayden, N. Sugaya, and Y. Kawaoka. 2004. Resistant influenza A viruses in children treated with oseltamivir: descriptive study. Lancet 364:759-765. [DOI] [PubMed] [Google Scholar]
- 20.Knight, V., and B. E. Gilbert. 1987. Ribavirin aerosol treatment of influenza. Infect. Dis. Clin. N. Am. 1:441-457. [PubMed] [Google Scholar]
- 21.Le, Q. M., M. Kiso, K. Someya, Y. T. Sakai, T. H. Nguyen, K. H. Nguyen, N. D. Pham, H. H. Ngyen, S. Yamada, Y. Muramoto, T. Horimoto, A. Takada, H. Goto, T. Suzuki, Y. Suzuki, and Y. Kawaoka. 2005. Avian flu: isolation of drug-resistant H5N1 virus. Nature 437:1108. [DOI] [PubMed] [Google Scholar]
- 22.Leneva, I. A., N. Roberts, E. A. Govorkova, O. G. Goloubeva, and R. G. Webster. 2001. The neuraminidase inhibitor GS4104 (oseltamivir phosphate) is efficacious against A/Hong Kong/156/97 (H5N1) and A/Hong Kong/1074/99 (H9N2) influenza viruses. Antivir. Res. 48:101-115. [DOI] [PubMed] [Google Scholar]
- 23.Madren, L. K., C. Shipman, and F. G. Hayden. 1995. In vitro inhibitory effects of combinations of anti-influenza agents. Antivir. Chem. Chemother. 6:109-113. [Google Scholar]
- 24.McKimm-Breschkin, J. L. 2000. Resistance of influenza viruses to neuraminidase inhibitors—a review. Antivir. Res. 47:1-17. [DOI] [PubMed] [Google Scholar]
- 25.McKimm-Breschkin, J. L. 2005. Management of influenza virus infections with neuraminidase inhibitors: detection, incidence, and implications of drug resistance. Treat. Respir. Med. 4:107-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McKimm-Breschkin, J. L., P. Selleck, T. Usman, and M. Johnson. 2007. Reduced sensitivity of influenza A (H5N1) to oseltamivir. Emerg. Infect. Dis. 13:1354-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monto, A. S. 2003. The role of antivirals in the control of influenza. Vaccine 21:1796-1800. [DOI] [PubMed] [Google Scholar]
- 28.Peiris, J. S. M., M. D. de Jong, and Y. Guan. 2007. Avian influenza virus (H5N1): a threat to human health. Clin. Mircobiol. Rev. 20:243-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Potier, M., L. Mameli, M. Belislem, L. Dallaire, and S. B. Melanxon. 1979. Fluorometric assay of neuraminidase with a sodium (4-methylumbelliferyl-α-d-N-acetylneuraminate) substrate. Anal. Biochem. 94:287-296. [DOI] [PubMed] [Google Scholar]
- 30.Prichard, M. N., and C. Shipman, Jr. 1990. A three-dimensional model to analyze drug-drug combinations. Antivir. Res. 14:181-206. [DOI] [PubMed] [Google Scholar]
- 31.Prichard, M. N., K. R. Aseltine, and C. Shipman, Jr. 1992. MacSynergy™ II. University of Michigan, Ann Arbor.
- 32.Reed, L. J., and H. Muench. 1938. A simple method for estimating fifty percent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 33.Russell, R. J., L. F. Haire, D. J. Stevens, P. J. Collins, Y. P. Lin, G. M. Blackburn, A. J. Hay, S. J. Gamblin, and J. J. Skehel. 2006. The structure of H5N1 avian influenza neuraminidase suggests new opportunities for drug design. Nature 443:45-49. [DOI] [PubMed] [Google Scholar]
- 34.Saad, M. D., B. R. Boynton, K. C. Earhart, M. M. Mansour, H. L. Niman, N. M. Elsayed, A. L. Nayel, A. S. Abdelghani, H. M. Essmat, E. M. Labib, E. A. Ayoub, and M. R. Monteville. 2007. Detection of oseltamivir resistance mutation N294S in humans with influenza A H5N1, p. 228. Abstr. Options Control Influenza VI, Toronto, Ontario, Canada.
- 35.Salomon, R., E. Hoffmann, and R. G. Webster. 2007. Inhibition of the cytokine response does not protect against lethal H5N1 influenza infection. Proc. Natl. Acad. Sci. USA 30:12479-12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salomon, R., J. Franks, E. A. Govorkova, N. A. Ilyushina, H.-L. Yen, D. Post, J. Humberd, M. Trichet, J. E. Rehg, R. Webby, R. G. Webster, and E. Hoffmann. 2006. The polymerase complex genes contribute to the high virulence of the human H5N1 influenza virus isolate A/Vietnam/1203/04. J. Exp. Med. 203:689-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sidwell, R. W., J. H. Huffman, G. P. Khare, L. B. Allen, J. T. Witkowski, and R. K. Robins. 1972. Broad-spectrum antiviral activity of virazole: 1-β-d-ribofuranosyl-1,2,4-triazole-3-carboxamine. Science 177:705-706. [DOI] [PubMed] [Google Scholar]
- 38.Sidwell, R. W., K. W. Bailey, M. H. Wong, D. L. Barnard, and D. F. Smee. 2005. In vitro and in vivo influenza virus—inhibitory effects of viramidine. Antivir. Res. 68:10-17. [DOI] [PubMed] [Google Scholar]
- 39.Smee, D. F., M. H. Wong, K. W. Bailey, and R. W. Sidwell. 2006. Activities of oseltamivir and ribavirin used alone and in combination against infections in mice with recent isolates of influenza A (H1N1) and B viruses. Antivir. Chem. Chemother. 17:185-192. [DOI] [PubMed] [Google Scholar]
- 40.Smee, D. F., K. W. Bailey, A. C. Morrison, and R. W. Sidwell. 2002. Combination treatment of influenza A virus infections in cell culture and in mice with the cyclopentane neuraminidase inhibitor RWJ-270201 and ribavirin. Chemotherapy 48:88-93. [DOI] [PubMed] [Google Scholar]
- 41.Sweeny, D. J., G. Lynch, A. M. Bidgood, W. Lew, K. Y. Wang, and K. C. Cundy. 2000. Metabolism of the influenza neuraminidase inhibitor prodrug oseltamivir in the rat. Drug Metab. Dispos. 28:737-741. [PubMed] [Google Scholar]
- 42.Szretter, K. J., S. Gandappa, X. Lu, C. Smith, W. J. Shieh, S. R. Saki, S. Sambhara, T. M. Tumpey, and J. M. Katz. 2007. Role of host cytokine responses in the pathogenesis of avian H5N1 influenza viruses in mice. J. Virol. 81:2736-2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Venables, W. N., and B. D. Ripley. 1997. Modern applied statistics, p. 223-242, 297-321, 345-350. Springer, New York, NY.
- 44.Webster, R. G., and E. A. Govorkova. 2006. H5N1 influenza-continuing evolution and spread. N. Engl. J. Med. 355:2174-2177. [DOI] [PubMed] [Google Scholar]
- 45.Wilson, S. Z., V. Knight, P. R. Wyde, S. Drake, and R. B. Couch. 1980. Amantadine and ribavirin aerosol treatment of influenza A and B infection in mice. Antimicrob. Agents Chemother. 17:642-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.World Health Organization. 2007. Towards a unified nomenclature system for the highly pathogenic H5N1 avian influenza viruses (EPR). World Health Organization, Geneva, Switzerland. http://www.who.int/csr/disease/avian_influenza/guidelines/nomenclature/en/index.html.
- 47.World Health Organization. 2008. H5N1 avian influenza: timeline of major events. World Health Organization, Geneva, Switzerland. http://www.who.int/csr/disease/ avian_influenza/ai_timeline/en/index.html.
- 48.Wray, S. K., B. E. Gilbert, and V. Knight. 1985. Effect of ribavirin triphosphate on primer generation and elongation during influenza virus transcription in vitro. Antivir. Res. 5:39-48. [DOI] [PubMed] [Google Scholar]
- 49.Yen, H.-L., A. S. Monto, R. G. Webster, and E. A. Govorkova. 2005. Virulence may determine the necessary duration and dosage of oseltamivir treatment for highly pathogenic A/Vietnam/1203/04 influenza virus in mice. J. Infect. Dis. 192:665-672. [DOI] [PubMed] [Google Scholar]
- 50.Zambon, M., and F. G. Hayden. 2001. Position statement: global neuraminidase inhibitor susceptibility network. Antivir. Res. 49:147-156. [DOI] [PubMed] [Google Scholar]