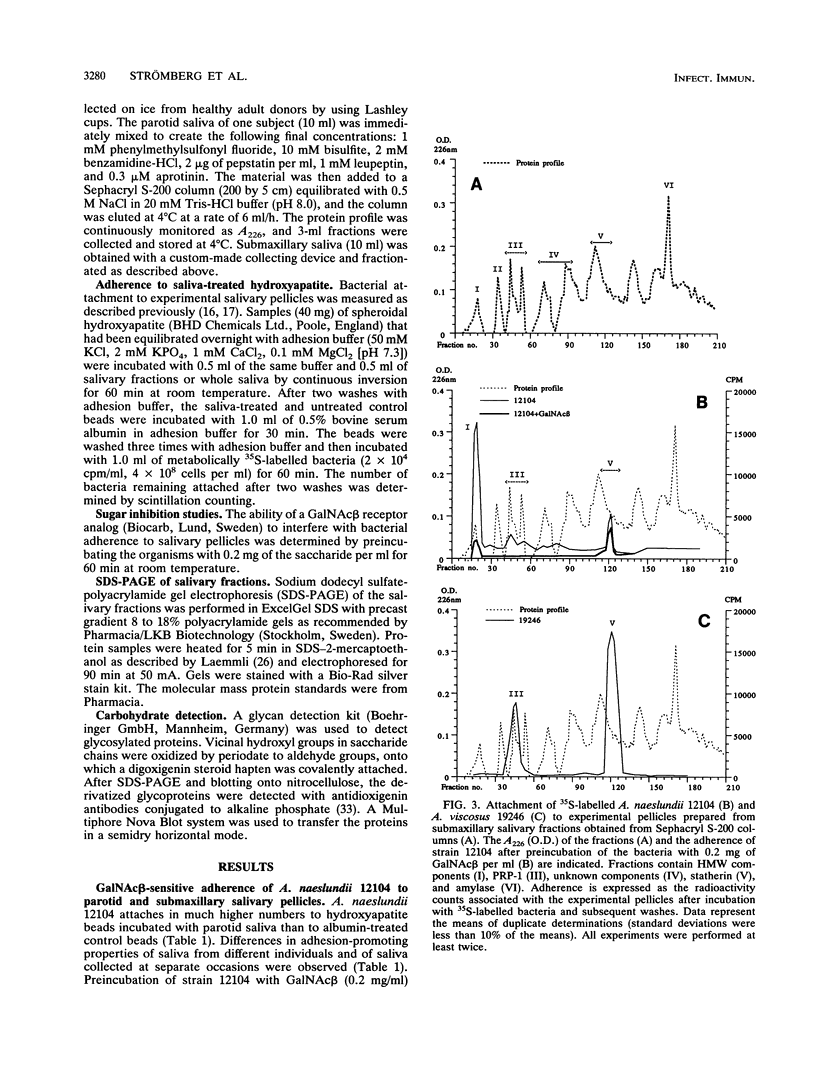
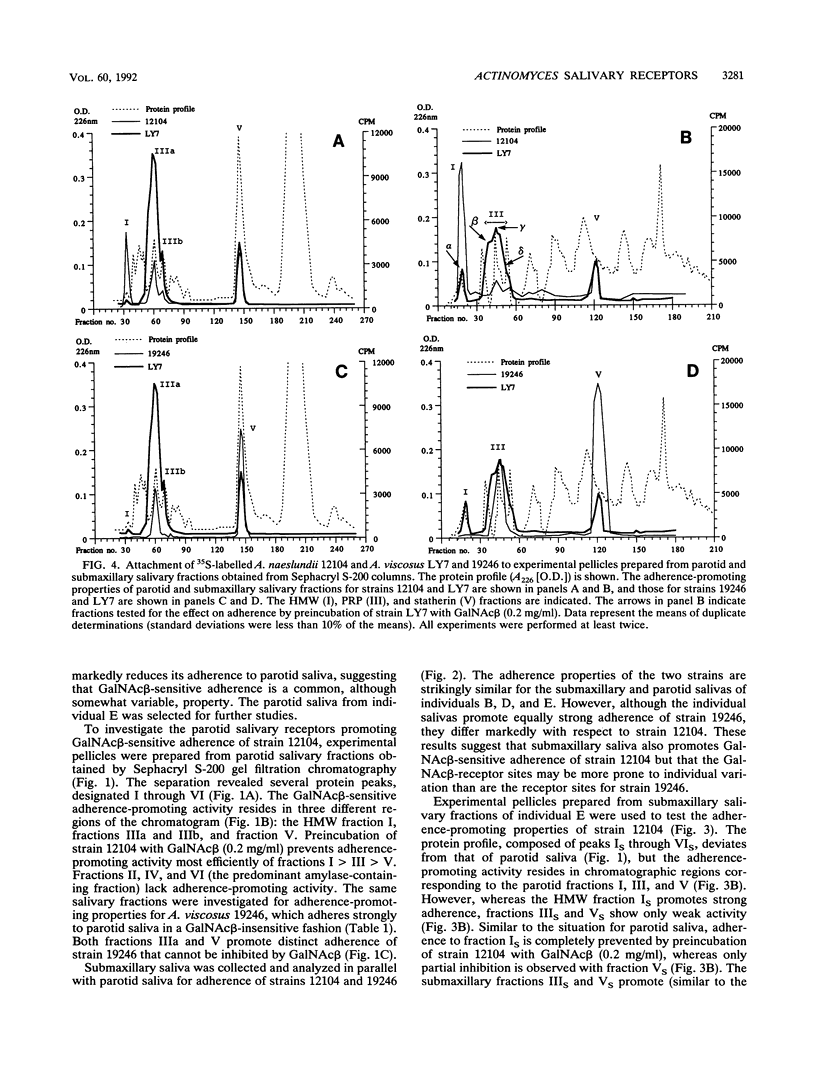
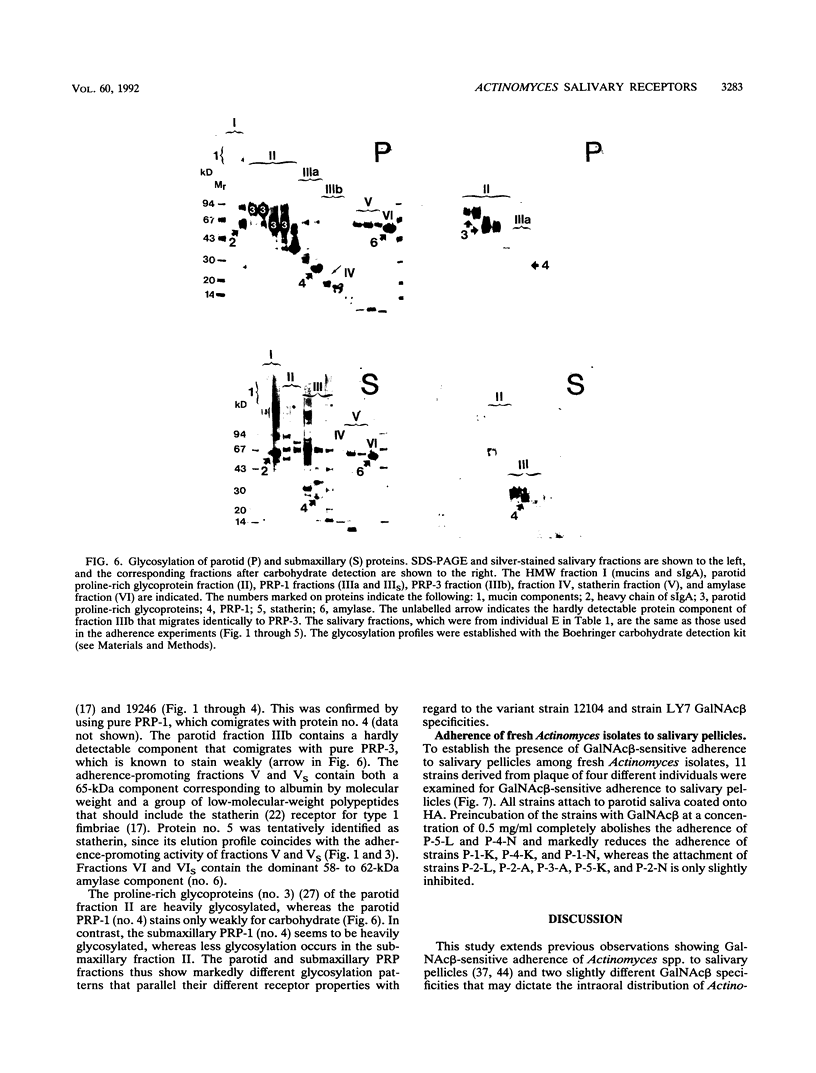
Abstract
The receptors for GalNAc beta 1-3Gal alpha Oethyl (GalNAc beta)-sensitive adherence of Actinomyces strains to salivary pellicles were investigated. Parotid and submaxillary saliva from one individual was size fractionated and utilized in hydroxyapatite adherence assays with Actinomyces naeslundii 12104 and A. viscosus 19246 and LY7 with and without GalNAc beta. Three parotid salivary fractions, the high-molecular-weight, acidic proline-rich protein (PRP), and statherin fractions, promote GalNAc beta-sensitive adherence of strain 12104, whereas only the high-molecular-weight fraction of submaxillary saliva promotes such adherence. In contrast, strain LY7, possessing a variant GalNAc beta specificity, shows GalNAc beta-sensitive adherence to the leading and trailing regions of the submaxillary PRP fractions but less distinct adherence to the parotid and submaxillary high-molecular-weight fractions. In addition, the PRP and statherin fractions promote adherence of strains LY7 and 19246 that is not inhibited by GalNAc beta. However, whereas strain LY7 binds more strongly to the PRP fraction than to the statherin fraction, strain 19246 binds preferentially to the statherin fractions of parotid and submaxillary saliva. These salivary protein fractions were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunostained to detect glycosylated proteins. The different salivary receptor properties are paralleled by different glycosylation patterns. The variable GalNAc beta specificities may have evolved to match different salivary glycosylation patterns, and PRP and statherin binding properties seem to be heterogeneous among the Actinomyces strains.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brennan M. J., Cisar J. O., Sandberg A. L. A 160-kilodalton epithelial cell surface glycoprotein recognized by plant lectins that inhibit the adherence of Actinomyces naeslundii. Infect Immun. 1986 Jun;52(3):840–845. doi: 10.1128/iai.52.3.840-845.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan M. J., Cisar J. O., Vatter A. E., Sandberg A. L. Lectin-dependent attachment of Actinomyces naeslundii to receptors on epithelial cells. Infect Immun. 1984 Nov;46(2):459–464. doi: 10.1128/iai.46.2.459-464.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisar J. O., David V. A., Curl S. H., Vatter A. E. Exclusive presence of lactose-sensitive fimbriae on a typical strain (WVU45) of Actinomyces naeslundii. Infect Immun. 1984 Nov;46(2):453–458. doi: 10.1128/iai.46.2.453-458.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisar J. O., Kolenbrander P. E., McIntire F. C. Specificity of coaggregation reactions between human oral streptococci and strains of Actinomyces viscosus or Actinomyces naeslundii. Infect Immun. 1979 Jun;24(3):742–752. doi: 10.1128/iai.24.3.742-752.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisar J. O., Sandberg A. L., Mergenhagen S. E. The function and distribution of different fimbriae on strains of Actinomyces viscosus and Actinomyces naeslundii. J Dent Res. 1984 Mar;63(3):393–396. doi: 10.1177/00220345840630030701. [DOI] [PubMed] [Google Scholar]
- Cisar J. O., Vatter A. E., Clark W. B., Curl S. H., Hurst-Calderone S., Sandberg A. L. Mutants of Actinomyces viscosus T14V lacking type 1, type 2, or both types of fimbriae. Infect Immun. 1988 Nov;56(11):2984–2989. doi: 10.1128/iai.56.11.2984-2989.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W. B., Wheeler T. T., Cisar J. O. Specific inhibition of adsorption of Actinomyces viscosus T14V to saliva-treated hydroxyapatite by antibody against type 1 fimbriae. Infect Immun. 1984 Feb;43(2):497–501. doi: 10.1128/iai.43.2.497-501.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W. B., Wheeler T. T., Lane M. D., Cisar J. O. Actinomyces adsorption mediated by type-1 fimbriae. J Dent Res. 1986 Sep;65(9):1166–1168. doi: 10.1177/00220345860650091001. [DOI] [PubMed] [Google Scholar]
- Costello A. H., Cisar J. O., Kolenbrander P. E., Gabriel O. Neuraminidase-dependent hamagglutination of human erythrocytes by human strains of Actinomyces viscosus and Actinomyces naeslundii. Infect Immun. 1979 Nov;26(2):563–572. doi: 10.1128/iai.26.2.563-572.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen R. P., Banting D. W., Fillery E. D. Longitudinal microbiological investigation of a hospitalized population of older adults with a high root surface caries risk. J Dent Res. 1985 Dec;64(12):1377–1381. doi: 10.1177/00220345850640121001. [DOI] [PubMed] [Google Scholar]
- Ellen R. P. Establishment and distribution of Actinomyces viscosus and Actinomyces naeslundii in the human oral cavity. Infect Immun. 1976 Nov;14(5):1119–1124. doi: 10.1128/iai.14.5.1119-1124.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen R. P., Fillery E. D., Chan K. H., Grove D. A. Sialidase-enhanced lectin-like mechanism for Actinomyces viscosus and Actinomyces naeslundii hemagglutination. Infect Immun. 1980 Feb;27(2):335–343. doi: 10.1128/iai.27.2.335-343.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen R. P., Segal D. N., Grove D. A. Relative proportions of Actinomyces viscosus and Actinomyces naeslundii in dental plaques collected from single sites. J Dent Res. 1978 Apr;57(4):550–550. doi: 10.1177/00220345780570040201. [DOI] [PubMed] [Google Scholar]
- Firon N., Ofek I., Sharon N. Carbohydrate-binding sites of the mannose-specific fimbrial lectins of enterobacteria. Infect Immun. 1984 Mar;43(3):1088–1090. doi: 10.1128/iai.43.3.1088-1090.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahnberg L., Olsson J., Krasse B., Carlén A. Interference of Salivary immunoglobulin A antibodies and other salivary fractions with adherence of Streptococcus mutans to hydroxyapatite. Infect Immun. 1982 Aug;37(2):401–406. doi: 10.1128/iai.37.2.401-406.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Hay D. I., Cisar J. O., Clark W. B. Adsorbed salivary proline-rich protein 1 and statherin: receptors for type 1 fimbriae of Actinomyces viscosus T14V-J1 on apatitic surfaces. Infect Immun. 1988 Nov;56(11):2990–2993. doi: 10.1128/iai.56.11.2990-2993.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Hay D. I. Human salivary acidic proline-rich proteins and statherin promote the attachment of Actinomyces viscosus LY7 to apatitic surfaces. Infect Immun. 1988 Feb;56(2):439–445. doi: 10.1128/iai.56.2.439-445.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Nygaard M. Interbacterial aggregation of plaque bacteria. Arch Oral Biol. 1970 Dec;15(12):1397–1400. doi: 10.1016/0003-9969(70)90031-2. [DOI] [PubMed] [Google Scholar]
- Gillece-Castro B. L., Prakobphol A., Burlingame A. L., Leffler H., Fisher S. J. Structure and bacterial receptor activity of a human salivary proline-rich glycoprotein. J Biol Chem. 1991 Sep 15;266(26):17358–17368. [PubMed] [Google Scholar]
- Hay D. I., Bennick A., Schlesinger D. H., Minaguchi K., Madapallimattam G., Schluckebier S. K. The primary structures of six human salivary acidic proline-rich proteins (PRP-1, PRP-2, PRP-3, PRP-4, PIF-s and PIF-f). Biochem J. 1988 Oct 1;255(1):15–21. doi: 10.1042/bj2550015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeb M. J., Marini A. M., Gabriel O. Factors affecting binding of galacto ligands to Actinomyces viscosus lectin. Infect Immun. 1985 Jan;47(1):61–67. doi: 10.1128/iai.47.1.61-67.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto E., Hay D. I., Gibbons R. J. A human salivary protein which promotes adhesion of Streptococcus mutans serotype c strains to hydroxyapatite. Infect Immun. 1989 Dec;57(12):3702–3707. doi: 10.1128/iai.57.12.3702-3707.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander P. E., Williams B. L. Lactose-reversible coaggregation between oral actinomycetes and Streptococcus sanguis. Infect Immun. 1981 Jul;33(1):95–102. doi: 10.1128/iai.33.1.95-102.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levine M. J., Reddy M. S., Tabak L. A., Loomis R. E., Bergey E. J., Jones P. C., Cohen R. E., Stinson M. W., Al-Hashimi I. Structural aspects of salivary glycoproteins. J Dent Res. 1987 Feb;66(2):436–441. doi: 10.1177/00220345870660020901. [DOI] [PubMed] [Google Scholar]
- Li J., Ellen R. P. Relative adherence of Bacteroides species and strains to Actinomyces viscosus on saliva-coated hydroxyapatite. J Dent Res. 1989 Sep;68(9):1308–1312. doi: 10.1177/00220345890680090301. [DOI] [PubMed] [Google Scholar]
- McIntire F. C., Crosby L. K., Barlow J. J., Matta K. L. Structural preferences of beta-galactoside-reactive lectins on Actinomyces viscosus T14V and Actinomyces naeslundii WVU45. Infect Immun. 1983 Aug;41(2):848–850. doi: 10.1128/iai.41.2.848-850.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyvad B., Kilian M. Microbiology of the early colonization of human enamel and root surfaces in vivo. Scand J Dent Res. 1987 Oct;95(5):369–380. doi: 10.1111/j.1600-0722.1987.tb01627.x. [DOI] [PubMed] [Google Scholar]
- Nyvad B., Kilian M. Microflora associated with experimental root surface caries in humans. Infect Immun. 1990 Jun;58(6):1628–1633. doi: 10.1128/iai.58.6.1628-1633.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shannessy D. J., Voorstad P. J., Quarles R. H. Quantitation of glycoproteins on electroblots using the biotin-streptavidin complex. Anal Biochem. 1987 May 15;163(1):204–209. doi: 10.1016/0003-2697(87)90114-x. [DOI] [PubMed] [Google Scholar]
- Qureshi J. V., Gibbons R. J. Differences in the adsorptive behavior of human strains of Actinomyces viscosus and Actinomyces naeslundii to saliva-treated hydroxyapatite surfaces. Infect Immun. 1981 Jan;31(1):261–266. doi: 10.1128/iai.31.1.261-266.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J., Gibbons R. J. Attachment of Bacteroides melaninogenicus subsp. asaccharolyticus to oral surfaces and its possible role in colonization of the mouth and of periodontal pockets. Infect Immun. 1978 Jan;19(1):254–264. doi: 10.1128/iai.19.1.254-264.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromberg N., Deal C., Nyberg G., Normark S., So M., Karlsson K. A. Identification of carbohydrate structures that are possible receptors for Neisseria gonorrhoeae. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4902–4906. doi: 10.1073/pnas.85.13.4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strömberg N., Borén T. Actinomyces tissue specificity may depend on differences in receptor specificity for GalNAc beta-containing glycoconjugates. Infect Immun. 1992 Aug;60(8):3268–3277. doi: 10.1128/iai.60.8.3268-3277.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strömberg N., Karlsson K. A. Characterization of the binding of Actinomyces naeslundii (ATCC 12104) and Actinomyces viscosus (ATCC 19246) to glycosphingolipids, using a solid-phase overlay approach. J Biol Chem. 1990 Jul 5;265(19):11251–11258. [PubMed] [Google Scholar]
- Strömberg N., Karlsson K. A. Characterization of the binding of propionibacterium granulosum to glycosphingolipids adsorbed on surfaces. An apparent recognition of lactose which is dependent on the ceramide structure. J Biol Chem. 1990 Jul 5;265(19):11244–11250. [PubMed] [Google Scholar]
- Strömberg N., Marklund B. I., Lund B., Ilver D., Hamers A., Gaastra W., Karlsson K. A., Normark S. Host-specificity of uropathogenic Escherichia coli depends on differences in binding specificity to Gal alpha 1-4Gal-containing isoreceptors. EMBO J. 1990 Jun;9(6):2001–2010. doi: 10.1002/j.1460-2075.1990.tb08328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strömberg N., Nyholm P. G., Pascher I., Normark S. Saccharide orientation at the cell surface affects glycolipid receptor function. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9340–9344. doi: 10.1073/pnas.88.20.9340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams B. L., Pantalone R. M., Sherris J. C. Subgingival microflora and periodontitis. J Periodontal Res. 1976 Feb;11(1):1–18. doi: 10.1111/j.1600-0765.1976.tb00045.x. [DOI] [PubMed] [Google Scholar]
- Wold A. E., Mestecky J., Tomana M., Kobata A., Ohbayashi H., Endo T., Edén C. S. Secretory immunoglobulin A carries oligosaccharide receptors for Escherichia coli type 1 fimbrial lectin. Infect Immun. 1990 Sep;58(9):3073–3077. doi: 10.1128/iai.58.9.3073-3077.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylber L. J., Jordan H. V. Development of a selective medium for detection and enumeration of Actinomyces viscosus and Actinomyces naeslundii in dental plaque. J Clin Microbiol. 1982 Feb;15(2):253–259. doi: 10.1128/jcm.15.2.253-259.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]