Abstract
Retapamulin, the first pleuromutilin antimicrobial agent approved for the topical treatment of skin infections in humans, was tested against 987 clinical isolates representing 30 species and/or resistance groups. MICs were determined along with disk diffusion zone diameters using a 2-μg disk. Population distribution and MIC versus disk zone diameter scattergrams were analyzed to determine microbiological MIC cutoff values and inhibition zone correlates. Minimum bactericidal concentrations were performed on a smaller subset of key species. The retapamulin MIC90 against 234 Staphylococcus aureus isolates and 110 coagulase-negative staphylococci was 0.12 μg/ml. Retapamulin MIC90s ranged from 0.03 to 0.06 μg/ml against beta-hemolytic streptococci including 102 Streptococcus pyogenes, 103 Streptococcus agalactiae, 59 group C Streptococcus, and 71 group G Streptococcus isolates. The MIC90 against 55 viridans group streptococci was 0.25 μg/ml. Retapamulin had very little activity against 151 gram-negative bacilli and most of the Enterococcus species tested. Based on the data from this study, for staphylococci, MICs of ≤0.5, 1, and ≥2 μg/ml with corresponding disk diffusion values of ≥20 mm, 17 to 19 mm, and ≤16 mm can be proposed for susceptible, intermediate, and resistant microbiological cutoffs, respectively. For beta-hemolytic streptococci, a susceptible-only MIC of ≤0.25 μg/ml with a corresponding disk diffusion value of ≥15 mm can be proposed for susceptible-only microbiological cutoffs.
Pleuromutilins are a class of antimicrobial agents derived naturally from the compound pleuromutilin, which is produced by Pleurotus mutilus, an edible mushroom (15). Retapamulin is a semisynthetic derivative of the pleuromutilin compound isolated through fermentation from Clitopilus passeckerians (formerly Pleurotus passeckerianus) (13). Retapamulin and other agents in the class have a novel mechanism of activity in that they selectively inhibit protein synthesis by binding to a site on the 50S subunit of the bacterial ribosome through an interaction that is different from those of other antibiotics that target the ribosome, such as macrolides, mupirocin, or fusidic acid (6, 10, 13, 24). Two types of target modifications have been reported to affect susceptibility to pleuromutilins. In experimental serial passage studies, in vitro mechanisms of resistance have been identified as mutations in rplC encoding ribosomal protein L3 (first-, second-, and third-step mutations) in Staphylococcus aureus. In comparison to wild-type strains, first- and second-step mutations cause a slight (4- to 32-fold) increase in retapamulin MICs (9, 15). Third-step mutations acquire a third mutation in rplC; these mutants require a longer exposure time and exhibit severe growth defects and fast-growing revertants (7, 11, 16). The faster-growing revertants typically have only slightly (two- to fourfold) increased MICs, suggesting that mutations occurring in rplC during therapy are unlikely (7, 11). Susceptibility to pleuromutilins can also be affected by the Cfr rRNA methyltransferase, which confers cross-resistance to phenicols, lincosamides, and streptogramin A in staphylococci (17). While first identified in coagulase-negative staphylococci of animal origin, two S. aureus clinical isolates carrying cfr has recently been reported (18, 22). A non-target-specific efflux mechanism has also been implicated to cause reduced susceptibility to retapamulin (8).
Previous studies documented the in vitro activities of retapamulin against a variety of clinical species including the predominant pathogens associated with impetigo and other skin and soft tissue infections (12, 14, 19, 20). Data presented here are in agreement with data reported in previously published studies.
Retapamulin ointment (1%) was recently approved by the Food and Drug Administration (FDA) as a topical formulation for the treatment of impetigo in adults and pediatric patients aged 9 months and older due to Staphylococcus aureus (methicillin-susceptible isolates only) or Streptococcus pyogenes infection (10). Retapamulin ointment (1%) was also recently approved in Europe and other countries for the treatment of impetigo and secondarily infected open wounds (8). Clinical studies are being planned at this time to determine if the drug will be useful for other skin and soft tissue infections, including infections caused by methicillin-resistant Staphylococcus aureus. Neither the FDA nor the Clinical and Laboratory Standards Institute (CLSI) publish or set interpretive criteria for susceptibility tests of topical agents. This study was undertaken to determine the microbiological cutoff values for interpreting MIC and disk diffusion tests using a selected set of recently isolated clinical isolates and stock isolates representing a wide range of species and antibiotic resistance groups (23).
(Portions of this work were presented at the 45th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, DC, December 2005.)
MATERIALS AND METHODS
Retapamulin powder was provided by GlaxoSmithKline. The compound was dissolved in dimethyl sulfoxide and diluted in 10% β-cyclodextrin. The final 1:10 dilution of the working stock solution was made in cation-adjusted Mueller-Hinton broth. Mupirocin was also provided by GlaxoSmithKline and was dissolved and diluted in water. Broth microdilution panels were prepared and frozen at −70°C until the day of testing. Commercially prepared disks of 2 μg of retapamulin were used for disk diffusion tests on Mueller-Hinton agar (nonfastidious isolates) or Mueller-Hinton agar with 5% sheep blood (streptococci).
A total of 987 bacterial isolates representing 30 species or phenotypic resistance groups were selected from a stock collection derived from recent clinical isolates. An additional 13 staphylococcal isolates with known retapamulin MICs of ≥1 μg/ml were also provided from GlaxoSmithKline's isolate collection for testing. The individual species for staphylococcal and streptococcal isolates and the number of strains of each grouping for all organisms tested are listed in Table 1. The following quality control organisms were tested on each test day: S. aureus ATCC 25923 (disk test only), S. aureus ATCC 29213 (dilution test only), and Streptococcus pneumoniae ATCC 49619. Previously published quality control ranges for susceptibility testing of retapamulin were used (21).
TABLE 1.
Susceptibilities of aerobic bacteria to retapamulin and mupirocin
| Species (no. of isolates) | MIC (μg/ml) of:
|
|||||
|---|---|---|---|---|---|---|
| Retapamulin
|
Mupirocin
|
|||||
| 50% | 90% | Range | 50% | 90% | Range | |
| Gram-positive strains | ||||||
| All staphylococcal strains combined (344) | 0.12 | 0.12 | 0.03-128 | 0.25 | 1 | 0.03->32 |
| All coagulase-negative staphylococcal strains combined (110) | 0.12 | 0.12 | 0.03-16 | 0.25 | >32 | 0.03->32 |
| Coagulase-negative staphylococci, methicillin resistant (56) | 0.06 | 0.12 | 0.03-1 | 0.25 | >32 | 0.12->32 |
| Coagulase-negative staphylococci, methicillin sensitive (54) | 0.12 | 4 | 0.03-16 | 0.25 | 32 | 0.03->32 |
| All Staphylococcus aureus strains combined (234) | 0.12 | 0.12 | 0.03-128 | 0.25 | 0.5 | 0.12->32 |
| Staphylococcus aureus, methicillin resistant (111) | 0.12 | 0.12 | 0.06-4 | 0.25 | 2 | 0.12->32 |
| Staphylococcus aureus, methicillin sensitive (103) | 0.12 | 0.12 | 0.06-128 | 0.25 | 0.5 | 0.12-1 |
| Staphylococcus aureus, vancomycin intermediate (20) | 0.06 | 0.12 | 0.03-1 | 0.25 | 1 | 0.12-32 |
| All streptococcal strains combined (390) | 0.03 | 0.06 | 0.16-8 | 0.5 | 2 | 0.06->32 |
| Group C beta-hemolytic streptococci (59) | 0.016 | 0.03 | 0.016-0.06 | 0.5 | 1 | 0.12-1 |
| Group G beta-hemolytic streptococci (71) | 0.03 | 0.06 | 0.016-0.06 | 0.25 | 0.5 | 0.12-2 |
| Streptococcus agalactiae (103) | 0.03 | 0.03 | 0.016-0.25 | 1 | 2 | 0.25-2 |
| Streptococcus pyogenes (102) | 0.016 | 0.03 | 0.016-0.06 | 0.12 | 0.25 | 0.06-0.25 |
| Viridans group streptococci (55) | 0.06 | 0.25 | 0.016-8 | 1 | 2 | 0.25->32 |
| All enterococcal strains combined (102) | 64 | 128 | 0.03-256 | 32 | >32 | 0.5->32 |
| All gram-negative bacilli combined (151) | 256 | >512 | 0.016->512 | >32 | >32 | 16->32 |
MICs were determined for all strains by the CLSI reference method (3, 4). Concentrations tested were serial twofold dilutions ranging from 512 to 0.016 μg/ml for retapamulin and 32 to 0.03 μg/ml for mupirocin. Disk diffusion tests were performed along with MIC determinations using one inoculum preparation. The CLSI disk diffusion reference method was used (5). For all S. aureus isolates demonstrating retapamulin MICs of ≥1, the presence of mutations in rplC were determined according to previously reported procedures (11).
Minimum bactericidal concentrations (MBCs) were determined for a subset of strains consisting of 55 isolates of coagulase-negative staphylococci, 52 isolates of Staphylococcus aureus, and 51 isolates of Streptococcus pyogenes. The MBCs were determined according to the methods outlined by the CLSI (1). The MBC was defined as the lowest concentration of the compound that produced a ≥99.9% killing (≥3-log10) drop in CFU/ml compared to that of the starting inoculum.
RESULTS AND DISCUSSION
Table 1 summarizes the MICs of retapamulin and mupirocin for all 987 bacterial isolates. Retapamulin had excellent in vitro activity against Staphylococcus and Streptococcus species, minimal in vitro activity against the 102 enterococci, and relatively little in vitro activity against the 151 enteric gram-negative bacilli (Table 1). The retapamulin MIC90 for all staphylococcal strains combined was 0.12 μg/ml. This same MIC90 was found for all S. aureus strains combined and for each of the S. aureus subcategories (methicillin susceptible, methicillin-resistant, and vancomycin intermediate). The activity of the compound was the same against coagulase-negative staphylococci, including methicillin-resistant isolates, with the exception of methicillin-susceptible coagulase-negative staphylococci, against which the MIC90 was 4 μg/ml.
Retapamulin was fourfold more active in vitro than mupirocin against methicillin-susceptible S. aureus (retapamulin MIC90 of 0.12 μg/ml versus mupirocin MIC90 of 0.5 μg/ml) and 16-fold more active in vitro than mupirocin against methicillin-resistant S. aureus (retapamulin MIC90 of 0.12 μg/ml versus mupirocin MIC90 of 2 μg/ml). Mupirocin was less active against coagulase-negative staphylococci (mupirocin MIC90 of ≥32 μg/ml versus retapamulin MIC90 of 0.12 μg/ml).
Retapamulin also exhibited excellent in vitro activity against all species or groups of streptococci under study (Table 1). The greatest in vitro activity was noted for the beta-hemolytic strains, with MIC90s in the 0.03- to 0.06-μg/ml range. The compound was slightly less active in vitro against viridans group streptococci, with an MIC90 of 0.25 μg/ml. On a gram-for-gram basis, retapamulin was 32-fold more active in vitro than mupirocin against all streptococcal strains combined (MIC90s of 0.06 μg/ml versus 2 μg/ml, respectively).
Retapamulin showed minimal activity in vitro against the enterococci, with an MIC90 of 128 μg/ml for all strains combined. Retapamulin also exhibited very little activity in vitro against all of the fermentative and nonfermentative gram-negative isolates, as demonstrated by MIC90s of ≥512 μg/ml.
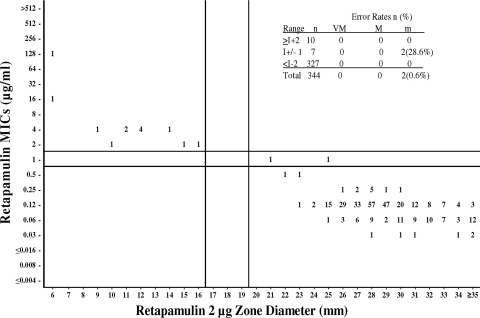
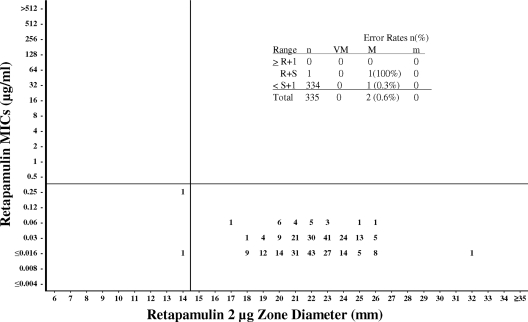
Since the FDA and the CLSI have not set breakpoints or interpretive criteria for topical antimicrobial agents, we looked at the population distributions of MICs to determine microbiological cutoff values for MICs and then compared the MICs to the disk diffusion zone diameters to determine where the corresponding susceptible, intermediate, and resistant zone diameters would fall within the population (23). These microbiological cutoffs are proposed based on microbiological data and are not derived from pharmacokinetic, pharmacodynamic, or clinical data. Based on the data from this study, MICs of ≤0.5, 1, and ≥2 μg/ml can be proposed for susceptible, intermediate, and resistant microbiological cutoffs, respectively, for staphylococci, and a susceptible-only MIC cutoff of ≤0.25 μg/ml can be proposed for beta-hemolytic streptococci. As can be seen in Table 1 and Fig. 1 and 2, 95.6% of the staphylococci would be considered susceptible to retapamulin at a concentration of ≤0.5 μg/ml, and 100% of beta-hemolytic streptococci would be considered susceptible at a concentration of ≤0.25 μg/ml. These data agree with data from previous studies of this drug against these species (14, 19, 20).
FIG. 1.
Retapamulin broth microdilution MICs (μg/ml) versus disk diffusion zone diameter (mm) using 2-μg disks. Data for all Staphylococcus species (n = 344) are shown. MIC microbiological cutoffs are as follows: susceptible (S), ≤0.5μg/ml; intermediate (I), 1 μg/ml; resistant (R), ≥2 μg/ml. Disk microbiological cutoffs are as follows: susceptible, ≥20 mm; intermediate, 17 to 19 mm; resistant, ≤16 mm. Error rates: VM, very major (MIC R, disk zone S); M, major (MIC S, disk zone R); M, minor (MIC S or R, disk zone I, or MIC I, disk zone S or R).
FIG. 2.
Retapamulin broth microdilution MICs (μg/ml) versus disk diffusion zone diameter (mm) using 2-μg disks. Data for all beta-hemolytic Streptococcus species (n = 335) are shown. The susceptible-only MIC microbiological cutoff was ≤0.25 μg/ml. The susceptible-only disk microbiological cutoff was ≥15 mm. Error rates: VM, very major (MIC R, disk zone S); M, major (MIC S, disk zone R); M, minor (MIC S or R, disk zone I, or MIC I, disk zone S or R).
Six S. aureus isolates demonstrated retapamulin MICs of ≥1 μg/ml and were inhibited at 1 μg/ml (n = 1), 2 μg/ml (n = 1), 4 μg/ml (n = 3), and 128 μg/ml (n = 1). Therefore, these isolates were further characterized to determine the presence of any mutations in rplC. With the exception of one isolate (retapamulin MIC of 1 μg/ml), none of the other five S. aureus isolates had mutations in rplC, and the reason for the reduced susceptibility to retapamulin is not known at this point.
Figures 1 and 2 are scattergrams of retapamulin MICs versus disk diffusion zone diameters obtained using a 2-μg retapamulin disk for staphylococci and beta-hemolytic streptococci, respectively. Disk diffusion microbiological cutoff values are proposed by attempting to obtain a maximum separation of susceptible and resistant strains while at the same time minimizing very major, major, and minor error rates (2). Using these criteria, microbiological disk diffusion cutoff values of ≤0.5, 1, and ≥2 μg/ml are proposed for staphylococci. Using these cutoffs, there were no very major errors or major errors and only 0.6% minor errors for all staphylococcal isolates (Fig. 1). These error rates are within the acceptable ranges specified by the CLSI (2). The ≥20-mm susceptible breakpoint also falls within the 15- to 25-mm range, which is considered to be ideal by the same CLSI guideline and agrees with data from previous disk diffusion studies (19).
For streptococci, a microbiological disk diffusion cutoff value of ≥15 mm is proposed. Traditionally, when only a susceptible population is observed, the breakpoints are established by taking the point at which 95% of the data are included and subtracting 2 to 3 mm. The ≥15-mm disk breakpoint proposed here for streptococci also agrees with these criteria. The two primary target species for this compound (S. aureus and S. pyogenes) produced MIC results that were 97.4% and 100% within the proposed susceptible MIC range, respectively.
Retapamulin demonstrated bacteriostatic activity with MBC90s ranging from 16 μg/ml for S. pyogenes up to 64 μg/ml for coagulase-negative staphylococci and 128 μg/ml for S. aureus (data not shown). The MBC90s were considerably higher than the MIC90s for each group, and this is reflected by the MBC90-to-MIC90 ratios of 256, 512, and 1,024 for S. pyogenes, coagulase-negative staphylococci, and S. aureus, respectively. The mupirocin MBC90 was ≥32 μg/ml for each of the three groups (data not shown). These findings are in agreement with data from previously published studies (20).
Retapamulin had excellent in vitro activity against streptococcal and staphylococcal skin pathogens. The in vitro activity of retapamulin against these strains was superior to that of mupirocin. Retapamulin MICs against staphylococci did not increase with resistance to oxacillin or vancomycin. Retapamulin showed only minimal in vitro activity against the enterococci and all gram-negative species tested. Retapamulin was bacteriostatic in that the MBC90s were ≥256-fold greater than the MIC90s of the strains tested. Microbiological cutoff interpretive values for MIC and disk diffusion of ≤0.5, 1, and 2 μg/ml and ≥20, 17 to 19, and ≤16 mm, respectively, are proposed for staphylococci, and susceptible-only MIC and disk diffusion values of ≤0.25 μg/ml and ≥15 mm are proposed for beta-hemolytic streptococci (Table 2).
TABLE 2.
Proposed microbiological cutoffs for interpreting MIC and disk diffusion susceptibility tests
| Susceptibility | Staphylococci
|
Beta-hemolytic streptococci
|
||
|---|---|---|---|---|
| MIC (μg/ml) | Disk diffusion 2-μg disk (mm) | MIC (μg/ml) | Disk diffusion 2-μg disk (mm) | |
| Susceptible | ≤0.5 | ≥20 | ≤0.25 | ≥15 |
| Intermediate | 1 | 17-19 | ||
| Resistant | ≥2 | ≤16 | ||
Acknowledgments
This study was sponsored by GlaxoSmithKline.
Testing to determine mutations in rplC was conducted by Dan Gentry at GlaxoSmithKline in Collegeville, PA.
Footnotes
Published ahead of print on 25 August 2008.
REFERENCES
- 1.Clinical and Laboratory Standards Institute. 1999. Methods for determining bactericidal activity of antimicrobial agents; approved guideline. Document M26-A. CLSI, Wayne, PA.
- 2.Clinical and Laboratory Standards Institute. 2001. Development of in vitro susceptibility testing criteria and quality control parameters; approved guideline, 2nd ed. Document M23-A2. CLSI, Wayne, PA.
- 3.Clinical and Laboratory Standards Institute. 2007. Performance standard for antimicrobial susceptibility testing. Document M100-S17. CLSI, Wayne, PA.
- 4.Clinical and Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 7th ed. Document M7-A7. CLSI, Wayne, PA.
- 5.Clinical and Laboratory Standards Institute. 2006. Performance standards for antimicrobial disk susceptibility tests; approved standard, 9th ed. Document M2-A9. CLSI, Wayne, PA.
- 6.Daum, R. S., S. Kar, and P. Kirkpatrick. 2007. Retapamulin. Nat. Rev. Drug Discov. 6:865-866. [Google Scholar]
- 7.Davidovich, C., A. Bashan, T. Auerbach-Nevo, R. D. Yaggie, R. R. Gontarek, and A. Yonath. 2007. Induced-fit tightens pleuromutilins binding to ribosomes and remote interactions enable their selectivity. Proc. Natl. Acad. Sci. USA 104:4291-4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.European Medicines Agency. 2007. Altargo: summary of product characteristics. European Medicines Agency, London, United Kingdom. http://www.emea.europa.eu/humandocs/PDFs/EPAR/altargo/H-757-PI-en.pdf.
- 9.Reference deleted.
- 10.Food and Drug Administration. 2007. FDA labeling information Altabax (retapamulin). Food and Drug Administration, Washington, DC. http://www.fda.gov/cder/foi/label/2007/022055lbl.pdf.
- 11.Gentry, D. R., S. F. Rittenhouse, L. McCloskey, and D. J. Holmes. 2007. Stepwise exposure of Staphylococcus aureus to pleuromutilins is associated with stepwise acquisition of mutations in rplC and minimally affects susceptibility to retapamulin. Antimicrob. Agents Chemother. 51:2048-2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldstein, E. J. C., D. M. Citron, C. V. Merriam, Y. A. Warren, K. L. Tyrrell, and H. T. Fernandez. 2006. Comparative in vitro activities of retapamulin (SB-275833) against 141 clinical isolates of Propionibacterium spp., including 117 P. acnes isolates. Antimicrob. Agents Chemother. 50:379-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hunt, E. 2000. Pleuromutilin antibiotics. Drugs Future 25:1163-1168. [Google Scholar]
- 14.Jones, R. N., T. R. Fritsche, H. S. Sader, and J. E. Ross. 2006. Activity of retapamulin (SB-275833), a novel pleuromutilin, against selected resistant gram-positive cocci. Antimicrob. Agents Chemother. 50:2583-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kavanagh, F., A. Hervey, and W. J. Robbins. 1951. Antibiotic substances from Basidiomycetes: VIII Pleurotus Mutilus (Fr.) Sacc. and Pleurotus Passeckerianus Pilat. Proc. Natl. Acad. Sci. USA 37:570-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kosowska-Shick, K., C. Clark, K. Credito, P. McGhee, B. Dewasse, T. Bogdanovich, and P. C. Appelbaum. 2006. Single and multistep resistance selection studies on the activity of retapamulin compared to other agents against Staphylococcus aureus and Streptococcus pyogenes. Antimicrob. Agents Chemother. 50:765-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Long, K. S., J. Poehlsgaard, C. Kehrenberg, S. Schwarz, and B. Vester. 2006. The cfr rRNA methyltransferase confers resistance to phenicols, lincosamides, oxazolidinones, pleuromutilins, and streptogramin A antibiotics. Antimicrob. Agents Chemother. 50:2500-2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mendes, R. E., L. Deshpande, M. Castanheira, J. DiPersio, M. Saubolle, and R. N. Jones. 7 April 2008. First report of cfr-mediated resistance to linezolid in human staphylococcal clinical isolates recovered in the United States. Antimicrob. Agents Chemother. doi: 10.1128/AAC.00231-08. [DOI] [PMC free article] [PubMed]
- 19.Pankuch, G. A., G. Lin, D. B. Hoellman, C. E. Good, M. R. Jacobs, and P. C. Appelbaum. 2006. Activity of retapamulin against Streptococcus pyogenes and Staphylococcus aureus evaluated by agar dilution, microdilution, E-test, and disk diffusion methodologies. Antimicrob. Agents Chemother. 50:1727-1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rittenhouse, S., S. Biswas, J. Broskey, L. McCloskey, T. Moore, S. Vasey, J. West, M. Zalacain, R. Zonis, and D. Payne. 2006. Selection of retapamulin, a novel pleuromutilin for topical use. Antimicrob. Agents Chemother. 50:3882-3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ross, J. E., and R. N. Jones. 2005. Quality control guidelines for susceptibility testing of retapamulin (SB-275833) by reference and standardized methods. J. Clin. Microbiol. 43:6212-6213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toh, S. M., L. Xiong, C. A. Arias, M. V. Villegas, K. Lolans, J. Quinn, and A. S. Mankin. 2007. Acquisition of a natural resistance gene renders a clinical strain of methicillin-resistant Staphylococcus aureus resistant to the synthetic antibiotic linezolid. Mol. Microbiol. 64:1506-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turnidge, J., and D. L. Paterson. 2007. Setting and revising antibacterial susceptibility breakpoints. Clin. Microbiol. Rev. 20:391-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yan, K., L. Madden, A. E. Choudhry, C. S. Voigt, R. A. Copeland, and R. R. Gontarek. 2006. Biochemical characterization of the interactions of the novel pleuromutilin derivative retapamulin with bacterial ribosomes. Antimicrob. Agents Chemother. 50:3875-3881. [DOI] [PMC free article] [PubMed] [Google Scholar]