Linezolid (LZD) has been used for the treatment of nosocomial and community-acquired pneumonia, as well as complicated skin and soft-tissue infection caused by methicillin-resistant Staphylococcus aureus (MRSA), including vancomycin (VCM)-intermediate S. aureus (VISA) (5, 10, 12). Since the first report of a VISA strain in 1997, VISA infection has been reported in many countries (2, 7). Because VISA tends to exhibit cross-resistance to some anti-MRSA agents, such as teicoplanin and daptomycin (4, 9, 11), we evaluated the in vitro activity of LZD toward VISA strains isolated from various countries of the world. During the susceptibility tests, quite unexpectedly, we noticed that the LZD MIC was relatively low for VISA clinical strains compared to that for VCM-susceptible S. aureus (VSSA). To confirm this phenomenon, we compared VCM and LZD MICs for clinical VSSA and VISA strains, with a total number of 47 VSSA strains and 43 VISA strains (3), including 28 VISA strains from the network on antimicrobial resistance in S. aureus (http://www.narsa.net/content/default.jsp). To evaluate VCM and LZD susceptibilities more precisely, the MICs were also determined using Etest strips (AB Biodisk, Sweden). The test confirmed a significant inverse relationship of VCM and LZD susceptibilities between VSSA and VISA. The MICs of VCM for VSSA and VISA were 1.65 ± 0.38 and 5.16 ± 1.42 mg/liter, and those of LZD for VSSA and VISA were 2.14 ± 0.81 and 1.46 ± 0.51 mg/liter, respectively (Student's t test; P < 0.01 for VISA results versus VSSA results for both VCM and LZD).
To further confirm the phenomenon, we evaluated the VCM and LZD MICs for 15 isogenic sets of clinical VISA strains, their passage-derived VCM-susceptible strains, and VISA phenotypic revertant strains using Etest strips (3, 4). An inverse relationship between VCM and LZD susceptibilities was again observed in most of these isogenic triple sets of VISA and their derivatives (Table 1). Regression analysis with the above 15 triple sets confirmed the existence of a negative correlation between VCM and LZD MICs, with a correlation coefficient of −0.628 (P < 0.01).
TABLE 1.
VCM and LZD susceptibility profiles for VISA strains and their derivativesa
| Strain | MIC (mg/liter) of drug for indicated strain
|
|||||
|---|---|---|---|---|---|---|
| VCM
|
LZD
|
|||||
| O | P | PR | O | P | PR | |
| Mu50 | 6 | 2.5 | 8 | 0.38 | 2 | 1.5 |
| MI | 8 | 1.25 | 4 | 1.5 | 3 | 1.5 |
| NJ | 8 | 3 | 5 | 1.25 | 3 | 2 |
| PC | 6 | 2 | 4 | 2 | 4 | 2 |
| IL | 6 | 2 | 4 | 2 | 4 | 2 |
| AMC11094 | 6 | 2 | 4 | 2 | 3 | 2 |
| 99/3759-V | 5 | 2 | 8 | 1 | 3 | 1.5 |
| 99/3700-W | 4 | 1.5 | 3 | 1 | 3 | 1.25 |
| LIM2 | 4 | 2 | 4 | 1 | 2 | 1.5 |
| 28160 | 4 | 1 | 3 | 1.5 | 3 | 2 |
| BR1 | 6 | 1.5 | 3 | 1.25 | 1.5 | 1.5 |
| BR2 | 6 | 2 | ND | 1 | 2 | ND |
| BR3 | 8 | 2 | 4 | 1 | 2 | 2 |
| BR4 | 6 | 1.5 | 4 | 1.5 | 3 | 2 |
| BR5-1 | 5 | 1.25 | 4 | 1.5 | 3 | 3 |
| Mean ± SDb | 5.86 ± 1.30 | 1.83 ± 0.49 | 4.42 ± 1.54 | 1.32 ± 0.44 | 2.76 ± 0.7 | 1.83 ± 0.41 |
MICs were determined using Etest strips, and each result was read after a 24-h incubation at 37°C. O, parent VISA strain; P, vancomycin-susceptible derivative of VISA strain obtained by passage of VISA strain on drug-free medium; PR, phenotypic revertant: vancomycin-resistant derivative of P strain obtained by vancomycin selection (3). ND, not determined.
Mean result for each strain category (P < 0.01 [result for O group versus result for P group and result for P group versus result for PR group for both VCM and LZD]).
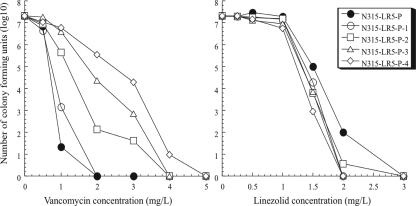
Finally, to further confirm this negative correlation, we generated another set of isogenic strains from the VCM-susceptible laboratory strain N315LR5-P1 (VCM MIC = 0.75 mg/liter). The strain is a heterogeneously methicillin-resistant derivative of N315 with its mecI gene inactivated and its penicillinase plasmid eliminated (1, 8). The strain was cultivated in gradual increments of VCM concentrations, and isogenic resistant mutants were selected from ascending VCM concentrations of 1, 2, 3, and 4 mg/liter. This set of isogenic laboratory-derived strains with gradual increments of VCM resistance was subjected to LZD MIC determination. Consistent with results of the first two investigations, we found that the derivative strains with higher VCM resistance had greater susceptibility to LZD and vice versa (Table 2). Analysis of resistant subpopulations (population analysis [6]) of the derivative strains also showed an inverse relationship between VCM and LZD susceptibilities (Fig. 1), indicating that N315LR5-P1 becomes more susceptible to LZD as it acquires VCM resistance. We carried out the same experiment using chloramphenicol, clindamycin, azithromycin, and quinupristin-dalfopristin. Unlike the case with LZD, there was no clear correlation between results for VCM and those for the above antibiotics. Taken together, the results presented here show a curious negative correlation between VCM and LZD susceptibilities in MRSA. The phenomenon seems to reveal a weakness of VISA posed by its vancomycin resistance mechanism, which might provide a hint for developing a new strategy in the treatment of VISA infection.
TABLE 2.
VCM and LZD MICs for N315-LR5-P1 and its derivatives
| Strain | MIC (mg/liter) of drug with indicated mediuma
|
|||
|---|---|---|---|---|
| VCM
|
LZD
|
|||
| MH | BHI | MH | BHI | |
| N315-LR5-P1 | 0.75 | 1.25 | 3 | 3 |
| N315-LR5-P1-1 | 1.25 | 2 | 2.5 | 2 |
| N315-LR5-P1-2 | 2 | 3 | 2 | 2 |
| N315-LR5-P1-3 | 2.5 | 4 | 1.75 | 2 |
| N315-LR5-P1-4 | 3 | 5 | 1.5 | 1.5 |
MH, Mueller-Hinton broth; BHI, brain heart infusion broth.
FIG. 1.
Antibiotic-resistant subpopulation profiles of N315LR5-P1 and its vancomycin-resistant derivatives. Note that the order of their antibiotic resistance patterns for vancomycin (left) and linezolid (right) is inverted.
Acknowledgments
This work was supported by a Grant-in-Aid for 21st Century COE Research and a Grant-in-Aid for Scientific Research on Priority Areas (13226114 and 18590438) from the Ministry of Education, Science, Sports, Culture and Technology of Japan.
Footnotes
Published ahead of print on 18 August 2008.
REFERENCES
- 1.Asada, K., Y. Inaba, E. Tateda-Suzuki, K. Kuwahara-Arai, T. Ito, and K. Hiramatsu. 1995. Evolution and resistance expression of MRSA. Evaluation of beta-lactam antibiotics against a set of isogenic strains with different types of phenotypic expression. Acta Biochim. Pol. 42:517-524. [PubMed] [Google Scholar]
- 2.Cui, L., and K. Hiramatsu. 2003. Vancomycin-resistant Staphylococcus aureus, p. 187-212. In A. C. Fluit and F. J. Schmitz (ed.), MRSA: current perspectives. Caister Academic Press, Norfolk, England.
- 3.Cui, L., X. Ma, K. Sato, K. Okuma, F. C. Tenover, E. M. Mamizuka, C. G. Gemmell, M. N. Kim, M. C. Ploy, N. El-Solh, V. Ferraz, and K. Hiramatsu. 2003. Cell wall thickening is a common feature of vancomycin resistance in Staphylococcus aureus. J. Clin. Microbiol. 41:5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cui, L., E. Tominaga, H. M. Neoh, and K. Hiramatsu. 2006. Correlation between reduced daptomycin susceptibility and vancomycin resistance in vancomycin-intermediate Staphylococcus aureus. Antimicrob. Agents Chemother. 50:1079-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diekema, D. J., and R. N. Jones. 2001. Oxazolidinone antibiotics. Lancet 358:1975-1982. [DOI] [PubMed] [Google Scholar]
- 6.Hiramatsu, K., N. Aritaka, H. Hanaki, S. Kawasaki, Y. Hosoda, S. Hori, Y. Fukuchi, and I. Kobayashi. 1997. Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogeneously resistant to vancomycin. Lancet 350:1670-1673. [DOI] [PubMed] [Google Scholar]
- 7.Hiramatsu, K., M. Kapi, Y. Tajima, L. Cui, S. Trakulsomboon, and T. Iso. 2004. Advances in vancomycin resistance: research in Staphylococcus aureus, p. 289-298. In M. Alekshun, P. McDermott, and D. White (ed.), Frontiers in antibiotic resistance: a tribute to Stuart B. Levy. ASM Press, Washington, DC.
- 8.Kuroda, M., T. Ohta, I. Uchiyama, T. Baba, H. Yuzawa, I. Kobayashi, L. Cui, A. Oguchi, K. Aoki, Y. Nagai, J. Lian, T. Ito, M. Kanamori, H. Matsumaru, A. Maruyama, H. Murakami, A. Hosoyama, Y. Mizutani-Ui, N. K. Takahashi, T. Sawano, R. Inoue, C. Kaito, K. Sekimizu, H. Hirakawa, S. Kuhara, S. Goto, J. Yabuzaki, M. Kanehisa, A. Yamashita, K. Oshima, K. Furuya, C. Yoshino, T. Shiba, M. Hattori, N. Ogasawara, H. Hayashi, and K. Hiramatsu. 2001. Whole genome sequencing of methicillin-resistant Staphylococcus aureus. Lancet 357:1225-1240. [DOI] [PubMed] [Google Scholar]
- 9.Patel, J. B., L. A. Jevitt, J. Hageman, L. C. McDonald, and F. Tenover. 2006. An association between reduced susceptibility to daptomycin and reduced susceptibility to vancomycin in Staphylococcus aureus. Clin. Infect. Dis. 42:1652-1653. [DOI] [PubMed] [Google Scholar]
- 10.Ruef, C. 2004. Epidemiology and clinical impact of glycopeptide resistance in Staphylococcus aureus. Infection 32:315-327. [DOI] [PubMed] [Google Scholar]
- 11.Sakoulas, G., J. Alder, C. Thauvin-Eliopoulos, R. C. Moellering, Jr., and G. M. Eliopoulos. 2006. Induction of daptomycin heterogeneous susceptibility in Staphylococcus aureus by exposure to vancomycin. Antimicrob. Agents Chemother. 50:1581-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wunderink, R., J. Rello, S. Cammarata, R. Croos-Dabrera, and M. Kollef. 2003. Linezolid vs vancomycin: analysis of two double-blind studies of patients with methicillin-resistant Staphylococcus aureus nosocomial pneumonia. Chest 124:1789-1797. [PubMed] [Google Scholar]