Abstract
Several genotypic interpretation scores have been proposed for the evaluation of susceptibility to lopinavir/ritonavir (LPV/r) but have not been compared using an independent data set. This study was a retrospective multicenter cohort of patients initiating LPV/r-based therapy. The virologic response (VR) was defined as a viral load of <500 copies/ml at week 24. The genotypic interpretation scores surveyed were the LPV mutation score, the ViroLogic score, the ATU score, the Stanford database score, and the International AIDS Society-USA mutation list. Of the 103 patients included in the analysis, 76% achieved VR at 24 weeks. For scores with clinical breakpoints defined (LPV mutation, ATU, ViroLogic, and Stanford), over 80% of the patients below the breakpoints achieved VR, while 50% or less above the breakpoints responded. Protease mutations at positions 10, 54, and 82 and at positions 54, 84, and 90 were associated with a lack of VR in the univariate and multivariate analyses, respectively. The area under the receiver-operator characteristic curves for the five genotypic interpretation scores studied ranged from 0.73 to 0.76. The study confirms that the currently available genotypic interpretation scores which are widely used by clinicians performed similarly well and can be effectively used to predict the virologic activity of LPV/r in treatment-experienced patients.
The treatment of human immunodeficiency virus (HIV)-infected individuals with combination antiretroviral therapy has significantly reduced the morbidity and mortality associated with HIV (13, 14). However, the efficacy of antiretroviral therapy (ART) can be impaired by several factors, including the development of antiretroviral-resistant HIV quasispecies (18).
Lopinavir/ritonavir (LPV/r), approved by the FDA in 2000, has been widely used in the management of treatment-naïve and treatment-experienced patients (7, 17). Genotypic interpretation of the impact of protease mutations on ritonavir-boosted protease inhibitors (PI/r) is complicated, as clinically relevant resistance generally requires multiple mutations and can develop through the interplay of major and minor mutations in a variety of patterns. Phenotypic resistance testing is generally considered easier to interpret for boosted PIs; however, it is more expensive than genotypic resistance testing and, in clinical studies, has not been shown to improve effectiveness outcomes above that achieved using genotypic resistance testing (3). Several genotypic interpretation scores have been developed, but there is no consensus on their relative value.
The LPV mutation score was developed by comparing the genotypes and phenotypes of 112 viral isolates from early clinical trials (10). Mutations at 11 amino acid positions in protease were found to be correlated with increased phenotypic resistance to LPV. Later studies linked a reduction in LPV/r activity to the presence of six or more LPV mutations (9).
The ViroLogic score was developed using clinical samples available from the Monogram Biosciences (then ViroLogic) database, for which both a genotype and phenotype for LPV/r had been performed (n = 1,482) (16). Parkin et al. evaluated “discordant” samples for which the genotype showed susceptibility according to the LPV mutation score (i.e., less than six LPV/r-associated mutations), but the phenotype showed meaningful clinical reduced susceptibility (i.e., change of >10-fold) to identify additional mutations that contributed to decreased LPV susceptibility. The weighted ViroLogic score consists of 29 mutations at 18 protease positions. Viruses with a score of 7 or more are considered to have reduced susceptibility to LPV. Although this score was based on many more isolates than the LPV mutation score, the investigators were limited to making genotype-phenotype correlations, as they did not have access to the corresponding virologic response (VR) data for the isolates.
Recently, a large database from France (the ATU database) was used to evaluate VR to LPV/r in 792 treatment-experienced individuals and generate ATU scores (11). Within this patient set, mutations at 10 amino acid positions in protease were found to better predict VR than the original LPV mutation scores. The breakpoints of 0 to 2, 3 to 5, and ≥6 mutations best distinguished responders and nonresponders.
In addition to these studies, many Web-based interpretation systems (Agence nationale de recherches sur le SIDA, http://www.hivfrenchresistance.org; geno2pheno, http://www.geno2pheno.org/cgi-bin/geno2pheno.pl; REGA genotypic resistance interpretation system, http://www.kuleuven.ac.be/rega/cev/links/rega_algorithm/) and lists of mutations from various organizations can be used to predict susceptibility to LPV/r. We chose to evaluate the scores generated by the Stanford University HIV drug resistance database (http://hivdb.stanford.edu) and International AIDS Society-USA (IAS-USA) given their widespread availability and usage. The Stanford database generates a weighted score using 52 mutations at 18 different positions in protease and produces five different outputs (susceptible, potential low-level resistance, low-level resistance, intermediate-level resistance, and high-level resistance), depending on the score. The IAS-USA score for LPV/r includes 32 mutations at 17 positions with mutations at 3 positions considered major (8). In contrast to other prediction systems, the IAS-USA score does not define clinical breakpoints.
LPV/r has been the standard boosted PI for treatment-experienced patients with PI-related resistance mutations. However, with the recent approval of tipranavir and darunavir for PI-experienced patients, it has become increasingly important for clinicians to be able to choose between these newer PI/r's and LPV/r for these patients. Newer PIs have activity against strains resistant to LPV/r (2, 6), but in LPV/r-susceptible patients, darunavir has not shown a statistically significant improvement in response (12). Given the many different interpretation tools available, it can be difficult for the clinician to decide when LPV/r will be the best choice or when one of the newer PI/r's should be used.
In this study, we used an independent multicenter cohort to evaluate VR to LPV/r and to better define the factors that influence VR. We performed univariate and multivariate analyses to determine which mutations were associated with virologic failure. In addition, we used our cohort to determine how well each genotypic interpretation score predicted VR to LPV/r and which genotypic interpretation score performed best (17a).
MATERIALS AND METHODS
Recruitment and data collection.
The Clinic-Based Investigator Group is comprised of 20 academic, private, and public clinics. Participating clinics (n = 8) retrospectively identified previously LPV/r-naive patients who had received an LPV/r-based regimen, had a baseline viral load and genotype, and had follow-up viral loads. Participating clinics were sent a standardized seven-page data acquisition form.
Inclusion criteria.
Patients were included for analysis if they had at least one viral load determination within 12 weeks prior to the initiation of LPV/r treatment, a baseline genotype within 24 weeks prior to and up to 3 weeks after LPV/r initiation, at least one viral load measurement during the first 18 weeks on LPV/r, and at least one viral load measurement between 18 and 30 weeks after LPV/r initiation (used as the 24-week viral load).
Definitions.
Given the use of different viral load assays having different lower limits of quantification, the VR was defined by a viral load of <500 copies/ml at 24 weeks of therapy (i.e., the lower limit of quantification of the least sensitive assay).
A genotypic susceptibility score for the ART was calculated using the Stanford HIV drug resistance database. For each drug used in the new LPV/r-based regimen, a score was given based on the interpretation of the genotype (1, susceptible; 0.75, potential low-level resistance; 0.5, low-level resistance; 0.25, intermediate resistance; and 0, high-level resistance). The genotypic susceptibility score was calculated by adding the activity of all the antiretroviral medications used in the regimen. In addition, a partial genotypic susceptibility score was defined as the genotypic susceptibility score minus the PI's contribution to the regimen.
Scores.
Not all mutations used to calculate the scores were captured in the data acquisition form. Not all the labs performing genotypic resistance testing reported all the mutations of interest in their genotype reports, while some of the mutations of interest had not been described at the time of the study.
The LPV mutation score (10) is the unweighted sum of the numbers of mutations appearing at amino acid positions 10, 20, 24, 46, 53, 54, 63, 71, 82, 84, and 90.
The ViroLogic (16) score is the weighted sum of the following mutations: 10I/F, 16E, 20I/M, 32I, 33F, 34Q, 43T, 46L/I, 47V, 48 M/V, 50V, 54A/M/S/T/V, 58E, 63T, 73T, 74S, 82A/F/S, and 89I/M. All mutations were given a weight of one except for those at positions 50, 54, and 82, which were given a weight of three. The following mutations were not included in the data acquisition form and were excluded from the calculations: 16E, 20I, 34Q, 43T, 48M, 54S, 54T, 63T, 82S, 89I, and 89M.
The ATU (11) score is the unweighted sum of mutations appearing at positions 10, 20, 24, 33, 36, 47, 48, 54, 82, and 84.
The Stanford score is the weighted sum of the following mutations (the points for each mutation are in parentheses): 10F (4), 10I (2), 10R (2), 10V (2), 10Y (2), 11I (2), 24I (2), 24F (1), 32I (10), 32A (5), 33F (5), 46I (12), 46L (12), 46V (10), 47V (10), 47A (40), 48V (10), 48M (10), 48A (5), 48S (5), 48T (5), 50V (20), 53L (3), 53Y (2), 54L (12), 54M (12), 54S (10), 54T (10), 54V (12), 54A (10), 71T (1), 71V (3), 71I (2), 73C (2), 73S (2), 73T (2), 73A (2), 76V (8), 82A (20), 82F (20), 82S (20), 82T (20), 82M (10), 82L (10), 82C (10), 84A (10), 84V (12), 84C (10), 89V (1), 89I (1), 89T (1), and 90M (10). The following mutations were not included in the data acquisition form and were excluded from the calculations: 10R, 10Y, 11I, 24F, 32A, 46V, 47A, 48A, 48M, 48S, 48T, 53Y, 54S, 54T, 71I, 76V, 82S, 82C, 82L, 82M, 84A, 84C, 89I, 89T, and 89V.
The IAS-USA (8) score is the unweighted sum of the following mutations: 10F/I/R/V, 20M/R, 24I, 32I, 33F, 46I/L, 47A/V, 50V, 53L, 54V/L/A/M/T/S, 63P, 71V/T, 73S, 76V, 82A/F/T/S, 84V, and 90M. The following mutations were not included in the data acquisition form and were excluded from the calculations: 10R, 47A, 54S, 54T, 76V, and 82S.
Laboratory methods.
A variety of genotype methods were used across the participating sites. Thirty-six percent of the genotypes were run at hospital-based labs, and 64% were run at commercial reference labs.
Statistical analysis.
Virologic responses were evaluated in predefined demographic and clinical subgroups. Chi-square tests were used to assess statistical significance within each subgroup at an α level of 0.05 (two-tailed).
Fisher's exact test was used to evaluate the association between VR and the presence or absence of mutations in protease at each position of interest. The Benjamini-Hochberg procedure was used to correct for multiple comparisons. Statistical significance was assessed at an α level of 0.05 (two-tailed) (1).
Multivariate stepwise logistic regression models were used to assess the association between VR and demographic characteristics, clinical predictors, and mutations in protease. Demographic characteristics consisted of age, clinic, and gender. Clinical predictors included baseline log10 viral load, baseline CD4+ T-cell count, and partial genotypic susceptibility score. Mutations in protease found to be significant from the univariate analysis and the 20 mutations thought a priori to be important to LPV/r resistance (based on the LPV mutation, ATU, ViroLogic, Stanford, and IAS-USA scores) were included in the starting model. The criterion for entry into the stepwise models was at an α level of 0.10. Predictors were retained in the stepwise models if they remained significant at an α level of 0.05.
To compare the performance of the different scores, receiver-operator characteristic (ROC) curves were constructed and their corresponding areas under the curve (AUC) were calculated. The AUC of the ROC curve is used to evaluate the predictive value of a given test. An AUC of 1 denotes a perfect test, while an AUC of 0.5 reflects a test without discriminatory power. The precision of the AUC estimates was evaluated by constructing 95% bootstrap confidence intervals (5,000 replicates) (4).
All statistical analyses were done using SAS 9.1.3 (Cary, NC). Institutional review board approval was obtained from participating centers.
RESULTS
Patient characteristics.
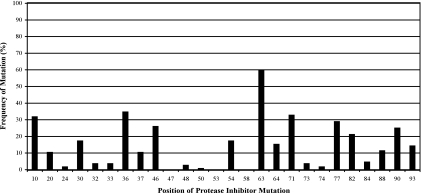
A total of 103 patients were included in the study. The median age was 42.2 years (Table 1). The patients were predominantly male (75%) and Caucasian (64%). Patients were enrolled from the United States (77%) and from Italy (23%), with the majority being treated in academic centers (69%). The majority of the patients were antiretroviral (97%) and PI experienced (79%). The most commonly prescribed PI prior to enrollment was nelfinavir (55%) followed by indinavir (43%). The median baseline viral load and CD4+ T-cell count were 17,000 copies/ml and 247 cells/μl, respectively. The median numbers of PI, nonnucleoside reverse transcriptase inhibitor, and nucleoside reverse transcriptor inhibitor mutations as defined by the IAS-USA were 3, 1, and 4, respectively. The frequencies of PI mutations are summarized in Fig. 1.
TABLE 1.
Baseline patient demographics
| Demographica | Value |
|---|---|
| Gender (n = 101) (%) | |
| Male | 76 |
| Female | 24 |
| Age (yr) (n = 103) (median, range) | 42 (18-71) |
| HIV risk factor (n = 99) (%) | |
| Men who have sex with men | 53 |
| Heterosexual transmission | 31 |
| Injection drug use | 17 |
| Blood products | 2 |
| Unknown | 9 |
| CDC stage (n = 103) (%) | |
| A (asymptomatic) | 33 |
| B (symptomatic) | 17 |
| C (AIDS-defining illness) | 50 |
| Race (n = 99) (%) | |
| Caucasian | 64 |
| Black | 20 |
| Latino | 12 |
| Asian | 1 |
| Other | 3 |
| Clinic setting (n = 103) (%) | |
| United States | 77 |
| Italy | 23 |
| Academic | 70 |
| Public | 22 |
| Private | 9 |
| Baseline log10 viral load (n = 103) (median, SD) | 4.23 (0.81) |
| Baseline CD4 count (n = 102) (median, SD) | 247 (214) |
| ART experienced (n = 103) (%) | 97 |
| PI experienced | 79 |
| NNRTI experienced | 55 |
| NRTI experienced | 94 |
| No. of protease inhibitors (median, range) | 1 (0-6) |
| Amprenavir (%) | 7 |
| Indinavir (%) | 43 |
| Nelfinavir (%) | 55 |
| Ritonavir (%) | 31 |
| Saquinavir (%) | 26 |
| No. of NNRTIs (median, range) | 1 (0-3) |
| No. of NRTIs (median, range) | 4 (0-6) |
| No. of PI mutations (median, range) | 3 (0-7) |
| No. of NNRTI mutations (median, range) | 1 (0-3) |
| No. of NRTI mutations (median, range) | 4 (0-12) |
CDC, Centers for Disease Control; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptor inhibitor; SD, standard deviation.
FIG. 1.
Frequency of PI mutations by position.
Virologic/immunologic response.
The median CD4+ T-cell count increase from the baseline was 63 cells/μl. Overall, 76% of patients achieved VR at 24 weeks (Table 2). As expected, more ART- and PI-naive patients experienced VR at week 24 compared to their ART- and PI-experienced counterparts (ART naive versus ART experienced, 100% versus 75%; PI naive versus PI experienced, 82% versus 74%); however, these differences were not statistically significant (P = 0.32 and P = 0.45, respectively). There were no significant differences between the responses in patients across the different clinic settings or by gender or race. There was a trend toward an improved response in those with baseline viral loads less than 100,000 copies/ml (80% versus 60%; P = 0.068), but there appeared to be no difference in VR in patients with CD4 T-cell counts of <200 cells/μl compared to those with ≥200 cells/μl (71% versus 79%, respectively; P = 0.34).
TABLE 2.
VR by subgroup
| Subgroup | No. of patients/total no. of patients (%) | P valuea |
|---|---|---|
| Overall | 78/103 (76) | |
| ART exposure | ||
| ART naive | 3/3 (100) | 0.32 |
| ART experienced | 75/100 (75) | |
| PI naive | 18/22 (82) | 0.45 |
| PI experienced | 60/81 (74) | |
| Clinic | ||
| United States | 62/79 (78) | 0.24 |
| Italy | 16/24 (67) | |
| Academic | 54/72 (75) | 0.97 |
| Public | 17/22 (77) | |
| Private | 7/9 (78) | |
| Gender | ||
| Male | 59/77 (77) | 0.57 |
| Female | 17/24 (71) | |
| Race | ||
| Caucasian | 46/63 (73) | 0.38 |
| Black | 17/20 (85) | |
| Latino | 9/12 (75) | |
| Asian | 0/1 (0) | |
| Other | 3/3 (100) | |
| Genotypic susceptibility score | ||
| <1 | 5/13 (38) | 0.0046 |
| 1-<1.5 | 10/14 (71) | |
| 1.5-<2 | 19/21 (90) | |
| ≥2 | 44/55 (80) | |
| Baseline viral load | ||
| <100,000 | 66/83 (80) | 0.068 |
| ≥100,000 | 12/20 (60) | |
| CD4 count | ||
| <200 | 32/45 (71) | 0.34 |
| >200 | 46/58 (79) | |
| LPV mutation score | ||
| <6 | 75/93 (81) | 0.0004 |
| >6 | 3/10 (30) | |
| ViroLogic score | ||
| <7 | 73/87 (84) | <0.0001 |
| ≥7 | 5/16 (31) | |
| ATU score | ||
| <3 | 68/83 (82) | 0.0028 |
| ≥3 | 10/20 (50) | |
| Stanford score | ||
| Susceptible and potential low-level resistance | 59/68 (87) | 0.0003 |
| Low-level resistance | 8/11 (73) | |
| Intermediate- and high-level resistance | 11/24 (46) |
P value from chi-square test.
Virologic response was improved in patients with higher baseline genotypic susceptibility scores. In particular, patients with genotypic susceptibility scores of <1, 1 to <1.5, 1.5 to <2, and ≥2 had virologic responses of 38%, 71%, 90%, and 80%, respectively (P = 0.0046).
Importantly, all of the LPV/r scores with defined breakpoints (the LPV mutation score, ViroLogic score, ATU score, and Stanford score) were able to distinguish VR with similar accuracy. Using the LPV mutation score, 81% of patients with a score less than 6 experienced VR at week 24 compared to only 30% of patients with a score of 6 or greater (P = 0.0004). For the ViroLogic score, 84% of patients with a score less than 7 experienced VR at week 24 compared to only 31% of patients with a score of 7 or greater (P < 0.0001). Similarly, 82% of patients with an ATU score less than 3 experienced VR at week 24 compared to only 50% of patients with an ATU score of 3 or greater (P = 0.0028). Using the Stanford score, 87% versus 73% versus 46% of patients responded with increasing levels of resistance to LPV/r (susceptible and potential low-level resistance versus low-level resistance versus intermediate- and high-level resistance, respectively) (P = 0.0003).
Univariate analyses.
In the univariate analyses, mutations at positions 10, 54, and 82 were significantly associated with a lack of VR (P = 0.020, P = 0.00145, and P = 0.014, respectively). Additionally, there was a trend toward a significant association with a lack of VR and mutations at positions 30 and 90 (P = 0.074 and P = 0.074, respectively) (Table 3).
TABLE 3.
Univariate predictors of VR
| Mutationa | Adjusted P valueb |
|---|---|
| PR10 | 0.02022c |
| PR20 | 1.00000 |
| PR24 | 0.98158 |
| PR30 | 0.07356 |
| PR33 | 1.00000 |
| PR36 | 0.84976 |
| PR46 | 0.84976 |
| PR47 | 1.00000 |
| PR48 | 0.84976 |
| PR50 | 1.00000 |
| PR53 | 1.00000 |
| PR54 | 0.00145c |
| PR63 | 1.00000 |
| PR71 | 0.71325 |
| PR82 | 0.01376c |
| PR84 | 0.71325 |
| PR90 | 0.07356 |
PR, protease position.
P value from Fisher's exact test, adjusted by the Benjamini-Hochberg procedure.
Indicates a P of <0.05.
Multivariate analysis.
In the multivariate analysis, an improved VR was associated with a lower baseline viral load. In addition, a lack of VR was associated with mutations at positions 54, 84, and 90 (Table 4).
TABLE 4.
Multivariate predictors of VR
| Variablea | Odds ratio (95% Wald confidence limit) |
|---|---|
| Log10 viral load | 0.270 (0.075-0.965) |
| PR54 | 0.046 (0.006-0.324) |
| PR84 | 0.005 (<0.001-0.309) |
| PR90 | 0.099 (0.014-0.689) |
PR, protease position.
Evaluation of scoring systems.
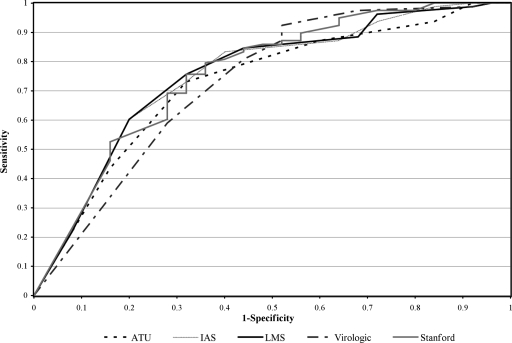
ROC curves for each of the scores are presented in Fig. 2. Each of the genotypic interpretation scores appears to have performed reasonably well in our cohort. AUCs for the ROC curves were similar, with values ranging from 0.73 to 0.76 and their 95% confidence intervals having substantive overlap (Table 5). Prediction error and misclassification rates were calculated and also showed no clear advantage for any genotypic interpretation score (data not shown).
FIG. 2.
ROC curves for genotypic interpretation systems. The ROC curves depict the tradeoff between the true positive rate (sensitivity) and false positive rate (1-specificity) of each genotypic interpretation system, as the clinical cutoff is varied.
TABLE 5.
Area under ROC curve (95% confidence interval) for genotypic interpretation scores
| Genotypic interpretation score | Area under ROC curve (95% confidence interval)a |
|---|---|
| Lopinavir mutation | 0.75 (0.62-0.86) |
| ViroLogic | 0.73 (0.59-0.83) |
| ATU | 0.73 (0.60-0.83) |
| Stanford | 0.76 (0.62-0.86) |
| IAS-USA | 0.75 (0.62-0.85) |
An area under the ROC curve of 1 denotes a perfect test, while an AUC of 0.5 reflects a test without discriminatory power. The precision of the AUC estimates was evaluated by constructing 95% bootstrap confidence intervals (5,000 replicates) (5).
DISCUSSION
With the availability of newer PIs, it becomes increasingly important to know when LPV/r will have full activity. Prior to the approval of tipranavir and darunavir, oftentimes, patients with viral isolates having high-level resistance to LPV/r received LPV/r due to limited alternative options. The TITAN study suggests that patients with limited LPV resistance do better on darunavir (12). However, the superiority of darunavir (77%) versus LPV (68%) in patients achieving viral loads of <400 copies/ml at 48 weeks was primarily driven by the 72% failure rate in the LPV/r arm in patients with viral isolates with a LPV change of >10-fold (versus the 28% failure rate for the darunavir group in patients with viral isolates with a LPV change of >10-fold). There was no significant difference in response for patients with viral isolates with a LPV change of <10-fold. This phenotypic breakpoint is roughly approximated by the genotypic breakpoints used in the various scoring systems (9).
Various resistance scores have been derived that posit to be superior to other available scoring systems in predicting susceptibility to LPV/r. However, no independent data set has been used to evaluate the relative merits of the different scores. The score created based on a given data set will always outperform a score created using a different data set as there is always some degree of model overfitting. Even if a portion of the data set is used as a training data set and the remainder is used as a validation data set, the similarities of the treatment histories between the training and validation data sets will likely bias the evaluation in favor of one's own scoring system. This occurs because the treatment history of the patient population has a major impact on which mutations will be found to be associated with LPV/r resistance. For instance, with the ViroLogic score, the protease mutation 50V was found to be associated with decreased LPV susceptibility (16). Our study did not find this correlation, as only 7% of the patients from our cohort had received amprenavir, which selects for this mutation. By using an independent data set to evaluate the relative merits of multiple different resistance scores, we have removed the inherent bias that is created when a data set is used to both create and test the performance of a scoring system.
In our study, 76% of the patients achieved a viral load of less than 500 copies/ml at 24 weeks. Currently, the gold standard for combination antiretroviral therapy is to achieve a viral load of less than 50 copies/ml, because suppression to this level has been found to be most durable (15). However, at the time the data from our cohort were ascertained (2001 to 2002), viral load assays sensitive to 50 copies/ml were not widely used.
All the scores with defined breakpoints (the LPV mutation, ViroLogic, ATU, and Stanford scores) effectively predicted patients who achieved VR. Using any of these scores, over 80% of the patients who had viral isolates which fell below the breakpoints achieved VR, while 50% or less of the patients with viral isolates at or above the breakpoints achieved VR.
With our relatively small data set, we did not identify uncommon mutations that may be associated with reduced VR to LPV/r. The results from our multivariate analysis showed an association between mutations at positions 54, 84, and 90 in protease and a lack of VR. Additionally, in the univariate analysis, mutations at positions 10, 54, and 82 were associated with a lack of VR. Mutations at position 82 are often considered more important than mutations at positions 54, 84, and 90 (9, 16; Stanford University HIV drug resistance database [http://hivdb.stanford.edu]). In our data set, mutations at position 82 frequently occurred in conjunction with 54V, thus likely obscuring the effect of a mutation at position 82 in the multivariate analysis. The five mutations found to be significant in our univariate or multivariate analyses constitute a subset of the mutations included in the LPV mutation score and are found relatively commonly in PI-experienced individuals (Stanford University HIV sequence database [http://hivdb.stanford.edu/cgii-bin/MutPrevBySubtypeRx.cgi]).
Comparing the shape of the ROC curves and the corresponding AUCs of the different models, all the models performed reasonably well without any one being clearly superior. This is similar to the finding in a recent paper by Fox et al. which compared four different genotypic interpretation scores for PIs and found that all of the scores effectively predicted the viral load response to a diverse set of PIs and that none of the scores performed significantly better than the others (5).
One limitation of this study is that not all the mutations included in the scores were captured by our data acquisition form. While all of the mutations used to calculate the LPV mutation score and ATU score were collected, we did not acquire information on some of the mutations used to calculate the ViroLogic, Stanford, and IAS-USA scores. This could lead these latter three scores to underperform compared to the LPV mutation score and ATU score. However, the mutations that were not collected are relatively uncommon. For the ViroLogic score, we collected data on 18 mutations which are on average present in 14% of PI-experienced patients. The 11 mutations for which we do not have data are present in only 3% of PI-experienced patients. Similarly, for the Stanford and IAS-USA mutations, the mutations for which we do not have data are present in 1% and 2% of PI-experienced patients, respectively (Stanford University HIV sequence database [http://hivdb.stanford.edu/cgii-bin/MutPrevBySubtypeRx.cgi]).
In conclusion, deciding which genotypic interpretation score to use for predicting the response for LPV/r can be intimidating to the average clinician. However, we found that the LPV mutation, ATU, ViroLogic, Stanford, and IAS-USA scores are all able to distinguish between patients who are more or less likely to respond to LPV/r. There was no substantial difference in the performance of the scores compared using data from our independent clinical cohort.
From this study we can, therefore, recommend to clinicians any of the scores evaluated as effective tools in deciding when LPV/r will be active. The fact that all scoring systems tested were roughly equivalent in their performance, should provide some comfort to prescribing clinicians who may be daunted by the task of choosing between the various genotypic scoring systems that are available. From this analysis, using an independent clinical cohort, we conclude that all of the scores evaluated are equally predictive of virologic response.
Acknowledgments
Financial support for this project was provided by Abbott Laboratories, Abbott Park, IL.
Footnotes
Published ahead of print on 18 August 2008.
REFERENCES
- 1.Benjamini, Y., and Y. Hochberg. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B. 57:289-300. [Google Scholar]
- 2.Clotet, B., N. Bellos, J. M. Molina, D. Cooper, J. C. Goffard, A. Lazzarin, A. Wöhrmann, C. Katlama, T. Wilkin, R. Haubrich, C. Cohen, C. Farthing, D. Jayaweera, M. Markowitz, P. Ruane, S. Spinosa-Guzman, E. Lefebvre, and POWER 1 and 2 study groups. 2007. Efficacy and safety of darunavir-ritonavir at week 48 in treatment-experienced patients with HIV-1 infection in POWER 1 and 2: a pooled subgroup analysis of data from two randomised trials. Lancet 369:1169-1178. [DOI] [PubMed] [Google Scholar]
- 3.Dunn, D. T., H. Green, C. Loveday, A. Rinehart, A. Pillay, M. Fisher, S. McCormack, A. G. Babiker, J. H. Darbyshire, and Evaluation of Resistance Assays (ERA) Trial Investigators. 2005. A randomized controlled trial of the value of phenotypic testing in addition to genotypic testing for HIV drug resistance: evaluation of resistance assays (ERA) trial investigators. J. Acquir. Immune Defic. Syndr. 38:553-559. [DOI] [PubMed] [Google Scholar]
- 4.Efron, B., and R. Tibshirani. 1993. Introduction to the bootstrap. Chapman & Hall/CRC Press, Boca Raton, FL.
- 5.Fox, Z. V., A. M. Geretti, J. Kjaer, U. B. Dragsted, A. N. Phillips, J. Gerstoft, S. Staszewski, B. Clotet, V. von Wyl, and J. D. Lundgren. 2007. The ability of four genotypic interpretation systems to predict virological response to ritonavir-based protease inhibitors. AIDS 21:2033-2042. [DOI] [PubMed] [Google Scholar]
- 6.Hicks, C. B., P. Cahn, D. A. Cooper, S. L. Walmsley, C. Katlama, B. Clotet, A. Lazzarin, M. A. Johnson, D. Neubacher, D. Mayers, H. Valdez, and RESIST investigator group. 2006. Durable efficacy of tipranavir-ritonavir in combination with an optimised background regimen of antiretroviral drugs for treatment-experienced HIV-1-infected patients at 48 weeks in the randomized evaluation of strategic intervention in multi-drug resistant patients with tipranavir (RESIST) studies: an analysis of combined data from two randomised open-label trials. Lancet 368:466-475. [DOI] [PubMed] [Google Scholar]
- 7.Johnson, M., G. Beatriz, C. Rodriguez, J. Coco, E. DeJesus, A. Lazzarin, K. Lichtenstein, A. Rightmire, S. Sankoh, and R. Wilber. 2005. Atazanvir plus ritonavir or saquinavir, and lopinavir/ritonavir in patients experiencing multiple virologic failures. AIDS 19:685-694. [DOI] [PubMed] [Google Scholar]
- 8.Johnson, V. A., F. Brun-Vezinet, B. Clotet, H. F. Günthard, D. R. Kuritzkes, D. Pillay, J. M. Schapiro, and D. D. Richman. 2007. Update of the drug resistance mutations in HIV-1: 2007. Top. HIV Med. 15:119-125. [PubMed] [Google Scholar]
- 9.Kempf, D. J., J. D. Isaacson, M. S. King, S. C. Brun, J. Sylte, B. Richards, B. Bernstein, R. Rode, and E. Sun. 2002. Analysis of the virological response with respect to baseline viral phenotype and genotype in protease inhibitor-experienced HIV-1-infected patients receiving lopinavir/ritonavir therapy. Antivir. Ther. 7:165-174. [PubMed] [Google Scholar]
- 10.Kempf, D. J., J. D. Isaacson, M. S. King, S. C. Brun, Y. Xu, K. Real, B. M. Bernstein, A. J. Japour, E. Sun, and R. Rode. 2001. Identification of genotypic changes in human immunodeficiency virus protease that correlate with reduced susceptibility to the protease inhibitor lopinavir among viral isolates from protease inhibitor-experienced patients. J. Virol. 75:7462-7469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.King, M. S., R. Rode, I. Cohen-Codar, V. Calvez, A.-G. Marcelin, G. J. Hanna, and D. J. Kempf. 2007. Predictive genotypic algorithm for virologic response to lopinavir-ritonavir in protease inhibitor-experienced patients. Antimicrob. Agents Chemother. 51:3067-3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madruga, J. V., D. Berger, M. McMurchie, F. Suter, D. Banhegyi, K. Ruxrungtham, D. Norris, E. Lefebvre, M. P. de Béthune, F. Tomaka, M. De Pauw, T. Vangeneugden, S. Spinosa-Guzman, and TITAN study group. 2007. Efficacy and safety of darunavir-ritonavir compared with that of lopinavir-ritonavir at 48 weeks in treatment-experienced, HIV-infected patients in TITAN: a randomised controlled phase III trial. Lancet 370:49-58. [DOI] [PubMed] [Google Scholar]
- 13.Mocroft, A., S. Vella, T. L. Benfield, A. Chiesi, V. Miller, P. Gargalianos, A. d'Arminio Monforte, I. Yust, J. N. Bruun, A. N. Phillips, J. D. Lundgren, et al. 1998. Changing patterns of mortality across Europe in patients infected with HIV-1. Lancet 352:1725-1730. [DOI] [PubMed] [Google Scholar]
- 14.Palella, F. J. J., K. M. Delaney, A. C. Moorman, M. O. Loveless, J. Fuhrer, G. A. Satten, D. J. Aschman, S. D. Holmberg, et al. 1998. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N. Engl. J. Med. 338:853-860. [DOI] [PubMed] [Google Scholar]
- 15.Panel on antiretroviral guidelines for adults and adolescents. 29 January 2008, posting date. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services, Washington, DC. http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf.
- 16.Parkin, N., C. Chappey, and C. Petropoulos. 2003. Improving lopinavir genotype algorithm through phenotype correlations: novel mutation patterns and amprenavir cross-resistance. AIDS 17:955-961. [DOI] [PubMed] [Google Scholar]
- 17.Walmsley, S., B. Bernstein, M. King, J. Arribas, G. Beall, P. Ruane, M. Johnson, D. Johnson, R. Lalonde, A. Japour, S. Brun, E. Sun, and M98-863 Study Team. 2002. Lopinavir-ritonavir versus nelfinavir for the initial treatment of HIV infection. N. Engl. J. Med. 346:2039-2046. [DOI] [PubMed] [Google Scholar]
- 17a.Zolopa, A., H. Rice, J. Nadler, J. Schaenman, T. Hawkins, C. Cohen, R. Rode, and D. Kempf. 2003. Genotypic predictors of response to lopinavir/ritonavir in clinical practice, abstr. no. 823. Second Int. AIDS Soc. Conf. HIV Pathog. Treat., Paris, France, 13 to 16 July 2003.
- 18.Zolopa, A. R., R. W. Shafer, A. Warford, J. G. Montoya, P. Hsu, D. Katzenstein, T. C. Merigan, and B. Efron. 1999. HIV-1 genotypic resistance patterns predict response to saquinavir-ritonavir therapy in patients in whom previous protease inhibitor therapy had failed. Ann. Intern. Med. 131:813-821. [DOI] [PMC free article] [PubMed] [Google Scholar]