Abstract
Naegleria fowleri is a ubiquitous, pathogenic free-living amoeba; it is the most virulent Naegleria species and causes primary amoebic meningoencephalitis (PAME) in laboratory animals and humans. Although amphotericin B is currently the only agent available for the treatment of PAME, it is a very toxic antibiotic and may cause many adverse effects on other organs. In order to find other potentially therapeutic agents for N. fowleri infection, the present study was undertaken to evaluate the in vitro and in vivo efficacies of miltefosine and chlorpromazine against pathogenic N. fowleri. The result showed that the growth of the amoeba was effectively inhibited by treatment with amphotericin B, miltefosine, and chlorpromazine. When N. fowleri trophozoites were treated with amphotericin B, miltefosine, and chlorpromazine, the MICs of the drug were 0.78, 25, and 12.5 μg/ml, respectively, on day 2. In experimental meningoencephalitis of mice that is caused by N. fowleri, the survival rates of mice treated with amphotericin B, miltefosine, and chlorpromazine were 40, 55, and 75%, respectively, during 1 month. The average mean time to death for the amphotericin B, miltefosine, and chlorpromazine treatments was 17.9 days. In this study, the effect of drugs was found to be optimal when 20 mg/kg was administered three times on days 3, 7, and 11. Finally, chlorpromazine had the best therapeutic activity against N. fowleri in vitro and in vivo. Therefore, it may be a more useful therapeutic agent for the treatment of PAME than amphotericin B.
Naegleria fowleri, a small free-living amoeba, causes primary amoebic meningoencephalitis (PAME), a fulminant and rapidly fatal disease that affects mainly children and young adults (7, 22). It has been associated with swimming or bathing in amoeba-contaminated warm waters. The period between contact with the organism and onset of clinical symptoms such as fever, headache, and rhinitis may vary from 2 to 3 days to as long as 7 to 15 days. The infection results from the introduction of amoeba-containing water into the nasal cavity and the subsequent passage of these organisms to the central nervous system via the olfactory apparatus (6, 7, 8).
Many drugs have been screened for in vitro and in vivo therapeutic activity against N. fowleri. Artemisinin and its derivatives have been evaluated for activity against experimental PAME, and the efficacy of these compounds has been compared to that of amphotericin B (11). Other antimicrobial drugs, such as clotrimazole, itraconazole, fluconazole, ketoconazole, and chlorpromazine, have also been tested, with various degrees of efficacy in vitro (15, 26). Azithromycin has been described for effective treatment of experimental PAME in mice (10). Recently, miltefosine, which was developed and used as an anticancer drug, and voriconazole, which was used in systemic fungal infections, were found to be effective in in vitro studies (20).
Until now, amphotericin B is the only agent with established clinical efficacy in PAME, and successes have been reported in some patients treated with amphotericin B alone or in combination with other drugs (1, 2, 14, 17, 23, 29). However, amphotericin B is a very toxic agent and has some side effects such as organ damage (9, 16, 18). Moreover, not all patients treated with amphotericin B have survived PAME (24).
In the present study, we investigated effects of amphotericin B, miltefosine, and chlorpromazine in vitro to identify potentially effective drugs for N. fowleri infection. Furthermore, we showed that miltefosine and chlorpromazine had better therapeutic effects in in vivo experiments than did amphotericin B.
MATERIALS AND METHODS
Culture of N. fowleri.
N. fowleri trophozoites (Carter NF69 strain, ATCC 30215) were cultured under axenic conditions in Nelson's medium at 37°C (31). Before N. fowleri trophozoites were used, their pathogenicity or cytotoxicity was tested in mice or target cells, respectively (12).
Pharmaceutical agents.
Amphotericin B and chlorpromazine were purchased from Sigma-Aldrich, Inc. (St. Louis, MO). Miltefosine was purchased from Cayman Chemical (Ann Arbor, MI). For in vitro study, amphotericin B (250 μg/ml), miltefosine (10 mg/ml), and chlorpromazine (10 mg/ml) were dissolved in Nelson's medium. For in vivo study, amphotericin B (2 mg/ml), miltefosine (10 mg/ml), and chlorpromazine (10 mg/ml) were dissolved in saline. All drug solutions were sterilized by filtration through a 0.22-μm-pore-size filter (Millipore, Molsheim, France).
Determination of MIC of drugs against N. fowleri.
Serial twofold dilutions of the drugs were prepared in Nelson's medium. Mitefosine and chlorpromazine at concentrations of 1.56, 3.125, 6.25, 12.5, 25, 50, and 100 μg/ml were applied directly in sterile 96-well culture plates (Nunc A/S, Roskilde, Denmark). Amphotericin B at concentrations of 0.39, 0.78, 1.56, 3.125, 6.25, 12.5, and 25 μg/ml was used. N. fowleri trophozoites at a density of 104/ml was added at volume of 100 μl to each well, and the plates were then sealed and incubated at 37°C. The amoebae were treated with each drug at a volume of 100 μl for 6 days. Control wells received 100 μl of Nelson's medium. Cell growth was determined on days 2, 4, and 6. Morphological features of N. fowleri trophozoites were observed by a light microscope, and amoebae were counted using a hemacytometer by trypan blue staining. All tests were performed in triplicate and repeated at least three times.
LDH release assay after drug treatment.
To evaluate the amoebicidal activities (cytotoxicity against N. fowleri) of three drugs, a lactate dehydrogenase (LDH) release assay was performed according to a previous study (12). N. fowleri trophozoites were cultured as a monolayer in Nelson's medium using 96-well culture plates at 37°C. The details of experiments were as follows: 104 of N. fowleri trophozoites only or 104 of N. fowleri trophozoites treated with each drug whose concentration ranging from 1.56 to 100 μg/ml (miltefosine and chlorpromazine) or 0.39 to 25 μg/ml (amphotericin B). The optical density of the supernatant containing LDH released from N. fowleri was measured on days 2, 4, and 6. For the LDH release assay, 50 μl of reacted supernatant in each well was transferred onto 96-well assay plates (Nunc A/S). After 50 μl of the reconstituted assay buffer in CytoTox96 nonradioactive cytotoxicity assay kit (Promega, Madison, WI) was added, the plate was incubated for 30 min at room temperature, and then 50 μl of stop solution was added. The reactions were read at 490 nm with a enzyme-linked immunosorbent assay reader. The in vitro cytotoxicity was calculated as follows: cytotoxicity (%) = [(sample release − spontaneous release)/(maximum release − spontaneous release)] × 100.
Experimental PAME in mice.
The N. fowleri trophozoites cultured for 3 days in Nelson's medium at 37°C were harvested and inoculated into mice. N. fowleri trophozoites (104) were inoculated intranasally into 7-week-old BALB/c female mice (KIST, Daejeon, Republic of Korea) under secobarbital anesthetic. Intranasal inoculation of N. fowleri trophozoites showed 100% mortality of mice. All of the following experiments were carried out with 20 mice per group. When infected mice died or death was apparent, an autopsy or a biopsy was performed. In the brains of mice, PAME was observed grossly, and trophozoites were recovered from tissue cultivation.
Analysis of BUN level in mice sera.
The blood urea nitrogen (BUN) level from mouse sera after treatment with each drug was measured in order to check renal toxicity. The analyses were performed at the Department of Clinical Pathology, Ajou University Hospital, with the chemical analyzer, Hitachi 747. Blood samples were collected once a day for 3 days from mice treated with each drug at 5, 10, 20, and 50 mg/kg or with saline as a negative control. Histopathological examination of the kidneys and livers was performed for the toxicity evaluation after treatment with the scheduled dosage of drugs.
Dosage of drugs for treatment of N. fowleri-infected mice.
Drug treatments began intraperitoneally at day 3 after N. fowleri inoculation and were repeated on days 7 and 11. The control group received 100 μl of sterile saline solution. The treatment groups received 100 μl each of drug in concentrations of 20 mg/kg (miltefosine and chlorpromazine) or 10 mg/kg (amphotericin B).
Survival rate and MTD of mice for evaluation of drug effect.
Mice were held for 1 month after inoculation of N. fowleri, and the cumulative percentage of death was recorded on a daily basis. The mean time to death (MTD) was also determined for each treatment group. In order to verify the cause of death, brain tissue from dead mice was cultured at 37°C in Nelson's medium, containing penicillin (500 U/ml) and streptomycin (500 μg/ml), with 25-cm2 culture flasks, and trophozoites were observed microscopically.
Histopathological examination of mice tissues.
The organs of dead or live mice were histopathologically evaluated to confirm whether amoebae existed or not. The mouse organs (liver, kidney, and brain) were fixed in 10% buffered neutral formalin and then carefully dissected. Each fragment of tissues was included in paraffin. Paraffin-embedded tissues on glass slides were stained with hematoxylin and eosin and examined with a light microscope. If possible, a complete cross-section of each organ was evaluated (liver, kidney, and brain).
Statistical analysis.
Statistical differences between groups or samples were determined by using a Student two-sample t test. The difference was considered significant when P was <0.05.
RESULTS
Effects of drugs against N. fowleri in vitro.
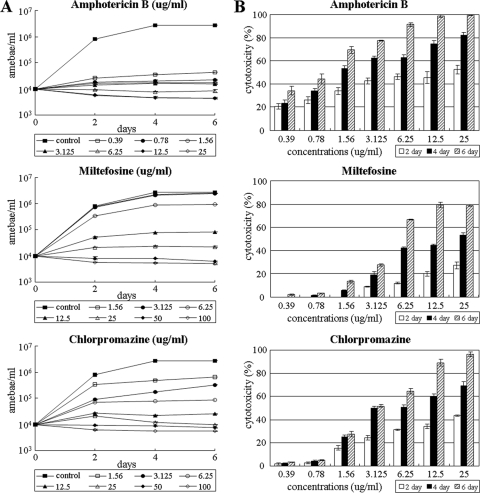
The amoeba growth curves of N. fowleri during incubation at 37°C were calculated by counting the trophozoites in each well. After 6 days of incubation, the control group treated with Nelson's medium produced 2.7 × 106 amoebae/well, which was a 274-fold increase compared to the start value (P < 0.01) (Fig. 1A). When drug was added, the growth of N. fowleri was inhibited (Fig. 1A), and the lowest concentrations of drugs that completely inhibited amoebic growth (MIC100) on days 2, 4, and 6 are shown in Table 1. Amphotericin B at 0.39 μg/ml completely suppressed the amoeba proliferation (100%) on day 2. The MIC100 of amphotericin B was 0.78 μg/ml on days 2, 4, and 6, respectively. Miltefosine also effectively inhibited the growth during the experimental period, with an MIC100 of 25 μg/ml. Under the same conditions, chlorpromazine showed 100% growth inhibition at a concentration of 12.5 μg/ml on days 2, 4, and 6 (Table 1).
FIG. 1.
In vitro effects of three drugs against N. fowleri. (A) Growth curves of N. fowleri after treatment with each drug. N. fowleri trophozoites were cultured at a density of 104/ml in the absence (control) or presence of each drug. (B) The amoebicidal activity of three drugs against N. fowleri was evaluated by LDH release assay. Amoebae were treated with miltefosine and chlorpromazine at concentrations from 1.56 to 100 μg/ml and with amphotericin B at concentrations from 0.39 to 25 μg/ml. The optical density was measured on days 2, 4, and 6, respectively. Values are means ± the standard errors of three experiments performed in triplicate.
TABLE 1.
MICs of the drugs tested for N. fowleri
| Drug | MIC100 (μg/ml)a
|
||
|---|---|---|---|
| Day 2 | Day 4 | Day 6 | |
| Amphotericin B | 0.78 | 0.78 | 0.78 |
| Miltefosine | 25.00 | 25.00 | 25.00 |
| Chlorpromazine | 12.50 | 12.50 | 12.50 |
MIC100, the lowest drug concentration that caused a 100% inhibition of growth. Complete inhibition of trophozoite growth in Nelson's medium was scored on days 2, 4, and 6.
To further investigate the amoebicidal activities of each drug, the cytotoxicity against N. fowleri was examined by LDH assay (Fig. 1B). Amphotericin B showed 99.5% cytotoxicity at a concentration of 25 μg/ml on day 6 and >50% cytotoxicity at concentrations of >1.56 μg/ml on day 4. Miltefosine showed 79.3% cytotoxicity at a concentration of 50 μg/ml on day 6, whereas the result on day 2 showed <30% cytotoxicity in all concentrations (P < 0.01). Chlorpromazine showed 64.4% cytotoxicity at a concentration of 25 μg/ml on day 6 and 69.1% cytotoxicity at a concentration of 100 μg/ml on day 4 (Fig. 1B).
Determination of drug dosage and administration method for in vivo experiments.
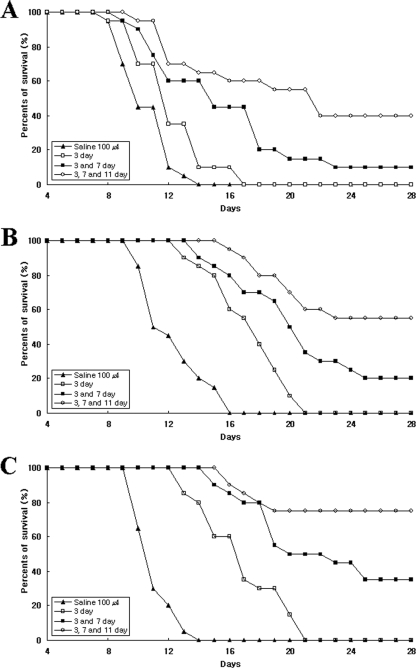
To decide the dose, frequency, and intervals of drug administration, an in vivo preliminary study was performed. At first, the inoculum of 104 amoeba trophozoites produced 100% mortality in normal mice. At 3 days postinoculation of the N. fowleri, two groups (20 mice each) were intraperitoneally injected with chlorpromazine and miltefosine at doses of 10, 20, and 50 mg/kg, respectively. One group (20 mice) was intraperitoneally injected with amphotericin B at doses of 5, 10, and 20 mg/kg, respectively. The most effective dose of the drugs was 20 mg/kg, except for amphotericin B (10 mg/kg). Also, the best effect was observed when the drugs were administered into mice three times at 4-day intervals (see Fig. 3).
FIG. 3.
Survival curve of N. fowleri-infected mice treated with amphotericin B (A), miltefosine (B), or chlorpromazine (C). Mice were inoculated intranasally with 104 N. fowleri trophozoites. At 3 days postinoculation, the groups were treated with 10 mg of amphotericin B/kg or 20 mg of miltefosine and chlorpromazine/kg, respectively. Each group (n = 20 mice) was treated with drugs once (day 3), twice (days 3 and 7), or three times (days 3, 7, and 11). The control group received 100 μl of saline.
BUN levels in mice.
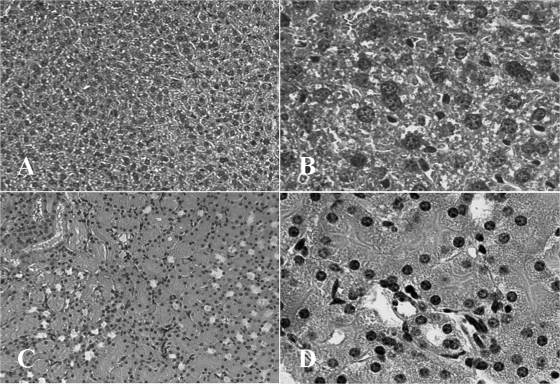
BUN levels in mouse sera were measured to investigate the toxicity of liver and kidney due to drug treatment (Table 2). When mice were treated with miltefosine or chlorpromazine at a dose of 50 mg/kg, the BUN levels were 82.4 and 57.5 mg/dl, respectively, which were out of the physiological range in normal mouse (15 to 40 mg/dl). However, BUN levels at doses of 10 and 20 mg/kg were within the physiological range in normal mouse. In the case of amphotericin B treatment, the BUN levels in mice were 27.4, 38.6, and 44.1 mg/dl at doses of 5, 10, and 20 mg/kg, respectively. The control mice treated with 100 μl of saline had BUN levels of 34.1 mg/dl (Table 2). Pathologically, the hepatocytes of mice treated with chlorpromazine at a dose of 20 mg/kg were of normal size and shape and showed no morphological changes (Fig. 2A and B); there was no degeneration of the renal tubules or necrosis in the cortex of the kidney, and the renal cortical tubules were of normal size and shape (Fig. 2C and D). In the case of amphotericin B or miltefosine treatment, we obtained microscopic results similar to those obtained with chlorpromazine (data not shown).
TABLE 2.
BUN analysis in mouse serum after drug treatment
| Drug | Dose (mg/kg) | Mean BUNa level (mg/dl) ± SE |
|---|---|---|
| Amphotericin B | 5 | 27.4 ± 7.18 |
| 10 | 38.6 ± 6.73 | |
| 20 | 44.1 ± 6.44 | |
| Miltefosine | 10 | 29.6 ± 8.28 |
| 20 | 34.6 ± 5.37 | |
| 50 | 82.4 ± 5.29 | |
| Chlorpromazine | 10 | 36.3 ± 2.53 |
| 20 | 38.4 ± 6.86 | |
| 50 | 57.5 ± 3.32 | |
| Control (saline)b | 34.1 ± 2.90 |
The reference value of BUN in mice ranges from 15 to 40 mg/dl. Values represent the means of three experiments.
The control group was treated with 100 μl of saline.
FIG. 2.
Liver and kidney tissue of mice treated with chlorpromazine. (A and B) Liver tissue from a mouse administered 20 mg of chlorpromazine/kg had hepatocytes of normal size and shape (A, ×100; B, ×400). (C and D) Kidney from a mouse administered 20 mg of chlorpromazine/kg had renal cortical tubules of normal size and shape (C, ×100; D, ×400). The tissues were stained with hematoxylin and eosin.
Survival rate and MTD of N. fowleri-infected mice after drug treatment.
The mice were treated with each drug on days 3, 7, and 11 after N. fowleri inoculation. The survival rate of mice given amphotericin B three times was 40%, and the MTD was 17.3 days. However, the survival rate of mice given amphotericin B two times was 10% (Fig. 3A). The survival rates of mice administered miltefosine two times and three times were 20 and 55%, respectively (Fig. 3B). The survival rate of mice administered chlorpromazine three times was stably maintained to 75% throughout experimental periods (1 month) (Fig. 3C). Otherwise, the survival rate of mice administered the drug two times was 35%. Thus, the average MTDs with amphotericin B, chlorpromazine, and miltefosine were 17.3, 17.2, and 19.3 days, respectively, compared to 11.2 days for control mice (P < 0.01) (Table 3).
TABLE 3.
Survival and MTD of N. fowleri-infected mice treated with various drugs
| Groupa | No. of mice | No. of surviving miceb (%) | MTDc (days) |
|---|---|---|---|
| Amphotericin B | 20 | 8 (40) | 17.3 |
| Miltefosine | 20 | 11 (55) | 19.3 |
| Chlorpromazine | 20 | 15 (75) | 17.2 |
| Controld | 20 | 0 (0) | 11.2 |
All groups were treated with drugs three times at days 3, 7, and 11, respectively. The control group received 100 μl of saline under the same conditions.
Mice were held for 28 days after inoculation, and the cumulative percentage was recorded on a daily basis.
The MTD was calculated based only on dead mice.
Histopathological findings of N. fowleri-infected mice after drug treatment.
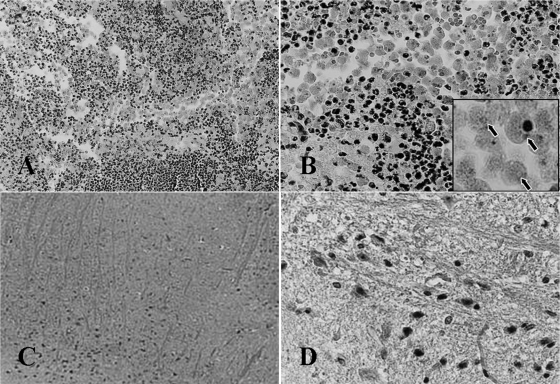
To confirm the death of mice, mouse brain tissue was stained by hematoxylin and eosin and observed under a light microscope and also cultured with Nelson's medium at 37°C in 25-cm2 culture flasks. Both staining and culture revealed no N. fowleri trophozoites in the brain tissues of mice who lived through the experimental period (1 month) (Fig. 4C and D). On the other hand, however, N. fowleri trophozoites surrounded by many inflammatory cells were found in the brain tissues of mice that died during the experimental periods. In addition, many N. fowleri trophozoites were observed upon cultivation of the brain tissue (Fig. 4A and B).
FIG. 4.
Brain tissue of mice infected with N. fowleri trophozoites. (A and B) The brain tissue of dead mouse after drug treatment shows N. fowleri trophozoites and inflammatory cells. In the boxed area in panel B, arrows indicate N. fowleri trophozoites surrounded by inflammatory cells (A, ×50; B, ×400). (C and D) The brain tissue of mice that survived during the experimental periods (1 month) after drug treatment had no N. fowleri trophozoites and showed no change (C, ×50; D, ×400). The tissues were stained by hematoxylin and eosin.
DISCUSSION
Because of rapid progression of disease, difficulty in diagnosis, and lack of effective therapeutic agents, N. fowleri infections are difficult to control, and the mortality of patients is greater than 95% (22). Some investigators have reported cases successfully treated with amphotericin B, fluconazole, rifampin, and chloramphenicol (27, 29). Although amphotericin B is the only agent for the treatment of PAME, amphotericin B is a very toxic antibiotic, and it may cause renal toxicity, electrolyte disturbances, hematopoietic effects, and other organ damage, as well as chills, fever, nausea, vomiting, and headache (9, 16, 18). Most drugs used for the treatment of PAME are not curative or show only slight protection. Therefore, to find other potentially effective drugs for treatment, the in vitro and in vivo efficacies of various drugs against pathogenic N. fowleri should be evaluated.
In the present study, the growth curve of N. fowleri treated with three drugs, amphotericin B, miltefosine, and chlorpromazine, and the amoebicidal activity against each drug were tested. Amphotericin B and chlorpromazine showed 100% growth inhibition at concentrations of 0.78 and 12.5 μg/ml, respectively, on day 6. Miltefosine effectively sustained the growth inhibition throughout experimental period (6 days) and increased a little in amoebicidal activity, a finding that was similar to those of a previous study (20). Our results confirmed that all of the drugs used here effectively inhibited amoeba proliferation and had amoebicidal activity against N. fowleri.
In the in vivo study, chlorpromazine was the most effective drug among the three drugs against experimental N. fowleri infection. The in vivo activity and low toxicity of chlorpromazine, therefore, make it a potentially attractive drug for the treatment of PAME. In a mouse model, experiments were performed by treating of mice with drugs three times, on days 3, 7, and 11 postinfection, a treatment regimen that may more closely resemble the clinical setting, because patients do not present with symptoms until several days after infection (10). Although amphotericin B treatment resulted in 40% survival among N. fowleri-infected mice, chlorpromazine led to 75% survival in N. fowleri-infected mice and, furthermore, the effect in the in vivo setting was maintained for a long time, indicating that the protective effect of chlorpromazine is better than that of amphotericin B. On the other hand, miltefosine was associated with a 55% survival rate. Although amphotericin B showed the best in vitro amoebicidal activity (cytotoxicity) against N. fowleri, the survival rate of mice treated with amphotericin B was 40%. However, the survival rates of mice treated with miltefosine or chlorpromazine were 55 and 75%.
It is very important to reduce the side effects of drugs, such as liver or renal toxicity, when a patient is treated with drugs. Our experimental setting (doses of 10 or 20 mg/kg, 4-day-interval, and three administrations) induced no liver and renal toxicity of mice and also resulted in a high survival rate among N. fowleri-infected mice. In our setting, the BUN levels of mice treated with each drug were within the physiological range in normal mouse (15 to 40 mg/dl) (3). Hepatocytes and renal cortical tubules of mouse also were of normal size and shape and showed no morphological changes. Thus, no toxicity of the liver and kidney in mice treated with drugs was observed. However, it is not known at present whether the dose, frequency, and intervals of administration are critical factors for successful treatment in PAME patients.
Miltefosine, originally developed as an antineoplastic, is used for the treatment of visceral and cutaneous leishmaniasis (25), Chagas' disease (19), cryptococcosis (30), and Trichomonas vaginalis infection (4). Schuster et al. (20) reported that miltefosine showed effective in vitro activity against free-living amoebae, such as N. fowleri, Acanthamoeba spp., and Balamuthia mandrillaris. Recently, miltefosine has been used in the case of an Acanthamoeba granulomatous amoebic encephalitis infection in a patient (28). Walochnik et al. (28) reported that the patient that was finally diagnosed with miliary tuberculosis, tuberculous meningitis, granulomatous amoebic encephalitis, and Acanthamoeba skin lesions that did not improve after tuberculostatic treatment or treatment with streptomycin, fluconazole, trimethoprim-sulfamethoxazole, amphotericin B, flucytosine, and sulfadiazine, which are drugs that are potentially effective against Acanthamoeba spp. However, the patient was finally treated successfully with oral and topical miltefosine and intrathecal and intravenous amikacin. In our study, miltefosine was shown to have amoebicidal activity in N. fowleri and increased the survival rate against PAME in experimental mice model.
Chlorpromazine developed as an antiemetic agent (5) is used for the clinical management of patients in shock (32) and for reducing the replication of adenovirus (13). Since 1984, it has been shown that chlorpromazine has in vitro activity against pathogenic free-living amoebae such as N. fowleri, Acanthamoeba culbertsoni, and Acanthamoeba polyphaga (21). Particularly, Schuster and Mandel (21) reported that chlorpromazine showed effective in vitro activity against Acanthamoeba spp. Mattana et al. (15) also demonstrated the in vitro effectiveness of chlorpromazine against Acanthamoeba castellanii. In the present study, chlorpromazine showed in vivo efficiency as well as amoebicidal activity against N. fowleri. Also, when the N. fowleri-infected mice were treated with 20 mg of chlorpromazine/kg three times at 4-day intervals, the survival rate for mice with PAME was 75%. At that time, no liver and kidney toxicities in mice were observed. Unfortunately, the mechanism of chlorpromazine action is still unclear. However, we suggest that the activity of chlorpromazine may change amoeba calcium regulatory protein or may be due to the lipophilic action of the drugs on the amoeba plasma membrane. The accumulation of chlorpromazine in the central nervous system makes it a potentially useful chemotherapeutic agent for the treatment of PAME in humans that is caused by N. fowleri (15, 21).
Further studies are needed to investigate the mechanisms of action of chlorpromazine or miltefosine and whether chlorpromazine exhibits additive, synergistic, or antagonistic effects in mouse experiments when combined with miltefosine or other drugs. Finally, on the basis of both in vitro and in vivo activities against N. fowleri, chlorpromazine and miltefosine may be candidate drugs for the treatment of N. fowleri infection.
Acknowledgments
This study was supported by grants from the Zoonosis Research Center, Wonkwang University, Iksan Chonbuk, Republic of Korea.
Footnotes
Published ahead of print on 2 September 2008.
REFERENCES
- 1.Anderson, K., and A. Jamieson. 1972. Primary amoebic meningoencephalitis. Lancet 19:379. [DOI] [PubMed] [Google Scholar]
- 2.Apley, J., S. K. Clarke, A. P. Roome, S. A. Sandry, G. Saygi, B. Silk, and D. C. Warhurst. 1970. Primary amoebic meningoencephalitis in Britain. BMJ 7:596-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aubrecht, J., M. E. Goad, E. M. Simpson, and R. H. Schiestl. 1997. Expression of Hygr in transgenic mice causes resistance to toxic effects of hygromycin B in vivo. J. Pharmacol. Exp. Ther. 281:992-997. [PubMed] [Google Scholar]
- 4.Blaha, C., M. Duchene, H. Aspock, and J. Walochnik. 2006. In vitro activity of hexadecylphosphocholine (miltefosine) against metronidazole-resistant and -susceptible strains of Trichomonas vaginalis. J. Antimicrob. Chemother. 57:273-278. [DOI] [PubMed] [Google Scholar]
- 5.Brand, E. D., T. D. Harris, H. L. Borison, and L. S. Goodman. 1954. The anti-emetic activity of 10-(γ-dimethylaminopropyl)-2-chlorophenothiazine (chlorpromazine) in dog and cat. J. Pharmacol. Exp. Ther. 110:86-92. [PubMed] [Google Scholar]
- 6.Carter, R. F. 1968. Primary amoebic meningoencephalitis: clinical, pathological, and epidemiological features of six fatal cases. J. Pathol. Bacteriol. 96:1-25. [DOI] [PubMed] [Google Scholar]
- 7.Carter, R. F. 1970. Description of a Naegleria species isolated from two cases of primary amoebic meningoencephalitis and of the experimental pathological changes induced by it. J. Pathol. 100:217-244. [DOI] [PubMed] [Google Scholar]
- 8.Carter, R. F. 1972. Primary amoebic meningo-encephalitis. An appraisal of present knowledge. Trans. R. Soc. Trop. Med. Hyg. 66:193-213. [DOI] [PubMed] [Google Scholar]
- 9.Goodman, J. S., and M. G. Koenig. 1970. Amphotericin B: specifics of administration. Mod. Treat. 7:581-595. [PubMed] [Google Scholar]
- 10.Goswick, S. M., and G. M. Brenner. 2003. Activities of azithromycin and amphotericin B against Naegleria fowleri in vitro and in a mouse model of primary amebic meningoencephalitis. Antimicrob. Agents Chemother. 17:524-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta, S., P. K. Ghosh, G. P. Dutta, and R. A. Vishwakarma. 1995. In vivo study of artemisinin and its derivatives against primary amebic meningoencephalitis caused by Naegleria fowleri. J. Parasitol. 81:1012-1013. [PubMed] [Google Scholar]
- 12.Jeong, S. R., S. Y. Kang, S. C. Lee, K. J. Song, K. I. Im, and H. J. Shin. 2004. Decreasing effect of an anti-Nfa1 polyclonal antibody on the in vitro cytotoxicity of pathogenic Naegleria fowleri. Korean J. Parasitol. 42:35-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanerva, A., M. Raki, T. Ranki, M. Särkioja, J. Koponen, R. A. Desmond, A. Helin, U. H. Stenman, H. Isoniemi, K. Höckerstedt, A. Ristimäki, and A. Hemminki. 2007. Chlorpromazine and apigenin reduce adenovirus replication and decrease replication associated toxicity. J. Gene Med. 9:3-9. [DOI] [PubMed] [Google Scholar]
- 14.Loschiavo, F., T. Ventura-Spagnolo, E. Sessa, and P. Bramanti. 1993. Acute primary meningoencephalitis from entamoeba Naegleria fowleri: report of a clinical case with a favorable outcome. Acta Neurol. 15:333-340. [PubMed] [Google Scholar]
- 15.Mattana, A., G. Biancu, L. Alberti, A. Accardo, G. Delogu, P. L. Fiori, and P. Cappuccinelli. 2004. In vitro evaluation of the effectiveness of the macrolide rokitamycin and chlorpromazine against Acanthamoeba castellanii. Antimicrob. Agents Chemother. 48:4520-4527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCurdy, D. K., M. Frederic, and J. R. Elkinton. 1968. Renal tubular acidosis due to amphotericin B. N. Engl. J. Med. 278:124-130. [DOI] [PubMed] [Google Scholar]
- 17.Poungvarin, N., and P. Jariya. 1991. The fifth nonlethal case of primary amoebic meningoencephalitis. J. Med. Assoc. Thai. 74:112-115. [PubMed] [Google Scholar]
- 18.Proffitt, R. T., A. Satorius, S. M. Chiang, L. Sullivan, and J. P. Adler-Moore. 1991. Pharmacology and toxicology of a liposomal formulation of amphotericin B (AmBisome) in rodents. J. Antimicrob. Chemother. 28(Suppl. B):49-61. [DOI] [PubMed] [Google Scholar]
- 19.Saraiva, V. B., D. Gibaldi, J. O. Previato, L. Mendonca-Previato, M. T. Bozza, C. G. Freire-De-Lima, and N. Heise. 2002. Proinflammatory and cytotoxic effects of hexadecylphosphocholine (miltefosine) against drug-resistant strains of Trypanosoma cruzi. Antimicrob. Agents Chemother. 46:3472-3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schuster, F. L., B. J. Guglielmo, and G. S. Visvesvara. 2006. In vitro activity of miltefosine and voriconazole on clinical isolates of free-living amebas: Balamuthia mandrillaris, Acanthamoeba spp., and Naegleria fowleri. J. Eukaryot. Microbiol. 53:121-126. [DOI] [PubMed] [Google Scholar]
- 21.Schuster, F. L., and N. Mandel. 1984. Phenothiazine compounds inhibit in vitro growth of pathogenic free-living amoebae. Antimicrob. Agents Chemother. 25:109-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schuster, F. L., and G. S. Visvesvara. 2004. Free-living amoebae as opportunistic and non-opportunistic pathogens of humans and animals. Int. J. Parasitol. 34:1001-1027. [DOI] [PubMed] [Google Scholar]
- 23.Seidel, J. S., P. Harmatz, G. S. Visvesvara, A. Cohen, J. Edwards, and J. Turner. 1982. Successful treatment of primary amebic meningoencephalitis. N. Engl. J. Med. 11:346-348. [DOI] [PubMed] [Google Scholar]
- 24.Stevens, A. R., S. T. Shulman, T. A. Lansen, M. J. Cichon, and E. Willaert. 1981. Primary amoebic meningoencephalitis: a report of two cases and antibiotic and immunologic studies. J. Infect. Dis. 143:193-199. [DOI] [PubMed] [Google Scholar]
- 25.Sundar, S., T. K. Jha, C. P. Thakur, J. Engel, H. Sindermann, C. Fischer, K. Junge, A. Bryceson, and J. Berman. 2002. Oral miltefosine for Indian visceral leishmaniasis. N. Engl. J. Med. 347:1739-1746. [DOI] [PubMed] [Google Scholar]
- 26.Tiewcharoen, S., V. Junnu, and S. Suvoutho. 2003. Effect of antifungal drugs on pathogenic Naegleria spp. isolated from natural water sources. J. Med. Assoc. Thai. 86:876-882. [PubMed] [Google Scholar]
- 27.Vargas-Zepeda, J., A. V. Gomez-Alcala, J. A. Vasquez-Morales, L. Licea-Amaya, J. F. De Jonckheere, and F. Lares-Villa. 2005. Successful treatment of Naegleria fowleri meningoencephalitis by using intravenous amphotericin B, fluconazole and rifampicin. Arch. Med. Res. 36:83-86. [DOI] [PubMed] [Google Scholar]
- 28.Walochnik, J., A. Aichelburg, O. Assadian, A. Steuer, G. S. Visvesvara, N. Vetter, and H. Aspöck. 2008. Granulomatous amoebic encephalitis caused by Acanthamoeba amoebae of genotype T2 in a human immunodeficiency virus-negative patient. J. Clin. Microbiol. 46:338-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang, A., R. Kay, W. S. Poon, and H. K. Ng. 1993. Successful treatment of amoebic meningoencephalitis in a Chinese living in Hong Kong. Clin. Neurol. Neurosurg. 95:249-252. [DOI] [PubMed] [Google Scholar]
- 30.Widmer, F., L. C. Wright, D. Obando, R. Handke, R. Ganendren, D. H. Ellis, and T. C. Sorrell. 2006. Hexadecylphosphocholine (miltefosine) has broad-spectrum fungicidal activity and is efficacious in a mouse model of cryptococcosis. Antimicrob. Agents Chemother. 50:414-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Willaert, E. 1971. Isolement et culture in vitro des amibes de genre Naegleria. Ann. Soc. Belg. Med. Trop. 51:701-708. [PubMed] [Google Scholar]
- 32.Winnie, A. P., and V. J. Collins. 1965. Clinical management of the patient in shock. Pharmacologic adjuncts to the management of shock. Clin. Anesth. 2:59-77. [PubMed] [Google Scholar]