Abstract
The Rel/NF-κB transcription factors are constitutively activated in many human cancers. The Rel proteins in this family are implicated in leukemia/lymphomagenesis, but the mechanism is not completely understood. Previous studies showed that the transcription activation domains (TADs) of the viral oncoprotein v-Rel and its cellular Rel/NF-κB homologues c-Rel and RelA are key determinants of their different transforming activities in primary lymphocytes. Substitution of a Rel TAD for that of RelA conferred a strong transforming phenotype upon RelA, which otherwise failed to transform cells. To gain insights into protein interactions that influence cell transformation by the Rel TADs, we identified factors that interact with the TAD of v-Rel, the most oncogenic member of the Rel/NF-κB family. We report that the coactivator for transcription factors AP-1 and estrogen receptors, CAPERα, interacts with the v-Rel TAD and potently synergizes v-Rel-mediated transactivation. Importantly, coexpression of CAPERα markedly reduced and delayed v-Rel's transforming activity in primary lymphocytes, whereas a dominant-negative mutant enhanced the kinetics of v-Rel-mediated transformation. Furthermore, small interfering RNA-mediated knockdown of CAPERα in v-Rel-transformed lymphocytes significantly enhanced colony formation in soft agar. Since the potency of Rel-mediated transactivation is an important determinant of lymphocyte transformation, as is Rel's ability to induce transcriptional repression, these data suggest that CAPERα's interaction with the Rel TAD could modulate Rel/NF-κB's transforming activity by facilitating expression or dampening repression of specific gene subsets important for oncogenesis. Overall, this study identifies CAPERα as a new transcriptional coregulator for v-Rel and reveals an important role in modulating Rel's oncogenic activity.
The Rel/NF-κB family of transcription factors is key for immune and inflammatory responses and also controls cell proliferation and apoptosis. The v-Rel oncoprotein of reticuloendotheliosis virus strain T (Rev-T) is the most potent oncogenic member of this family. Both v-Rel and its cellular homologue c-Rel transform primary splenic lymphocytes in vitro and cause fatal leukemia/lymphomas in chickens and transgenic mice (5, 9, 17, 22, 23, 33, 38, 39). This agrees with the implication of Rel/NF-κB in the pathogenesis and chemoresistance of many hematopoietic and solid tumors, including primary mediastinal B-cell lymphomas (PMBCL) and classical Hodgkin's lymphoma, in which constitutively high levels of nuclear c-Rel protein are necessary for tumor cell survival and proliferation (3, 4, 14, 18, 20, 27, 49). Additionally, some PMBCL and follicular lymphomas harbor c-rel gene mutations that decrease c-Rel's transactivation potency and enhance its transforming activity in primary chicken lymphocytes (45). This is consistent with the increased transforming phenotype conferred by certain mutations in v-Rel and c-Rel and indicates that modulation of Rel's transcriptional activity can significantly affect its oncogenicity (12, 43, 44).
The Rel proteins bind to κB DNA sites as homo- or heterodimers with other NF-κB family members via their N-terminal Rel homology domain (RHD), which also mediates nuclear localization and association with inhibitory IκB subunits. Their C-terminal regions carry transactivation domains (TADs) that control cellular gene expression. Analyses focusing on the effects of v-Rel and c-Rel proteins on cellular gene expression have provided important insights into the mechanisms by which Rel/NF-κB is involved in cancer (reviewed in reference 18). Studies revealed that their C-terminal TADs greatly influence their transforming potential in primary lymphocytes. While RelA failed to transform chicken lymphoid cells on its own, replacement of RelA's TAD with that of either v-Rel or c-Rel conferred a strong transforming phenotype both in vitro and in vivo (13). Recent work showed that in addition to activating expression of antiapoptotic and proproliferative genes, the Rel TADs can also lead to gene-specific transcriptional repression of genes such as those for SH3BGRL and the B-cell receptor signaling molecules BCAP and BLNK, and this activity is as important for lymphocyte transformation by v-Rel as is its transactivation function (19, 35). Additionally v-Rel can promote expression and alternative splicing of telomerase reverse transcriptase (TERT), which is also involved in lymphocyte transformation (24). However, little is known about the cellular factors that associate with the Rel TADs and that modulate its transcriptional and oncogenic activities.
Here we report that coactivator of activating protein-1 (AP-1) and estrogen receptors (CAPERα), also known as RNA-binding region (RNP1 or RRM) containing protein 2 (RNPC2), RNA-binding motif protein 39 (RBM39), or hepatocellular carcinoma 1.4 (HCC1.4), interacts with the v-Rel TAD (vTAD) and strongly modulates its transcriptional and transforming activities. Originally cloned as an autoantigen in a patient with liver cirrhosis that progressed to hepatocellular carcinoma (25), CAPERα was previously described as a specific coactivator for JUN/AP-1 and estrogen receptors alpha and beta (ERα and ERβ) that also interacts with transcriptional coactivator ASC-2 (NCoA6) (26). CAPERα shares homology with the SR family splicing factors U2AF65 and PUF60 (11) and was also implicated in steroid hormone receptor-mediated alternative splicing, although its abilities to modulate transcription and alternative splicing are distinct and separable (11). Our studies show that CAPERα is a novel transcriptional coregulator for v-Rel that strongly suppresses its transforming activity, uncovering a tumor suppressor role for CAPERα in regulating Rel's oncogenic activity.
MATERIALS AND METHODS
Yeast two-hybrid screen.
We used a cytosolic yeast two-hybrid screen based on the Ras recruitment system (a gift of Ami Aronheim, BioRap Technologies Ltd., Haifa, Israel) to isolate factors that interact with the vTAD (amino acids 314 to 503). We used a Myc-Ras(61)-vTAD bait to screen a myristylated (Myr) human pre-B cell leukemia cDNA library in the pMyrXR vector (Stratagene no. 975210-41) in the temperature-sensitive strain of Saccharomyces cerevisiae Cdc25-2, as described previously (1). Colonies containing the bait and interacting cDNAs were selected for growth on galactose medium lacking uracil, leucine, and methionine at 37°C; confirmed by retransformation with isolated target cDNAs; and subjected to DNA sequence analysis (Molecular Resource Facility, UMDNJ-NJMS, Newark, NJ).
Cloning of CAPERα and mutagenesis.
CAPERα cDNA (1,592 nucleotides; GenBank accession number NM_184234) was isolated by one-step reverse transcriptase PCR (RT-PCR) (Roche) of RNA from the human acute lymphocytic lymphoma cell line REH with primers CTGGGATCCATGGCAGACGATATTGATATTG and ATAAGAATGCGGCCGCTATCATCGTCTACTTGGAACCAG. Wild-type and mutant CAPERα cDNAs were cloned into pcDNA3.1HisC (Invitrogen) with N-terminal His6 and Xpress tags. CAPERα was fused to a C-terminal Flag tag by PCR amplification with primers CTGGGATCCATGGCAGACGATATTGATATTG and ATAAGAATGCGGCCGCTATCACTTATCGTCGTCATCCTTGTAATCTCGTCTACTTGGAACCAGTAG and cloned into pcDNA3.1(+) (Invitrogen). A CAPERα mutant lacking the putative v-Rel interaction domain (vRID) (amino acids 310 to 359) was created using the QuikChange mutagenesis kit (Stratagene) with primers GCCAAAAAGGCTTTGGAACAAGCAAGACTTGCAGAGGGTACAGG and CCTG TACCCTCTGCAAGTCTTGCTTGTTCCAAAGCCTTTTTGGC (ΔvRID mutant). Mutant vRID was amplified with primers CGTGGATCCCTTAATGGATTTGAACTAGCAGGA and ATAAGAATGCGGCCGCTATCACATTAACTGAAGACGACC. The N-terminal region of CAPERα (amino acids 1 to 291) was fused to a C-terminal Flag tag using primers CTGGGATCCATGGCAGACGATATTGATATTG and ATAAGAATGCGGCTATCACTTATCGTCGTCATCCTTGTAATCCTTGGATCGACCAGTTTCACTG (N-CAPERα-Flag). The C-terminal region of CAPERα (amino acids 406 to 530) was similarly generated using primers CGTGGATCCGCCACCATGGAAGCTTCAGCTTTAGCTGCAGC and ATAAGAATGCGGCCGCTATCACTTATCGTCGTCATCCTTGTAATCTCGTCTACTTGGAACCAGTAG (CAPERα-C-Flag). Glutathione S-transferase (GST)-CAPERα was generated by subcloning the CAPERα cDNA into pGEX-4T-1 (GE Healthcare). All constructs were verified by DNA sequencing.
Other plasmids used in this study included 4× AP-1-luciferase (10), interleukin-6 (IL-6)-κB-luciferase (47), and p73-luciferase (36) (gifts from N. Colburn, NCI Frederick Cancer Research and Development Center, Frederick, MD; A Rabson, Cancer Institute of New Jersey, NJ; and W Liu, Mayo Clinic, Rochester, MN, respectively); GST-TBP (50); cytomegalovirus (CMV) vectors expressing the RHD of v-Rel (mutant v-HincII) (29), mouse c-Rel, human c-Rel, human RelA, or a RelA/v-Rel hybrid protein (13); and expression vectors for c-Jun and c-Fos (pCB6+:c-jun and pCB6+:c-fos) (2) (gifts from T. Curran, St. Jude Children's Research Hospital, Memphis, TN), E2F1 (pRC-CMV HA E2F1) (36) (a gift from W. Liu, Mayo Clinic, Rochester, MN), or IκBαM (47). For lymphocyte transformation assays, CAPERα, vRID, or green fluorescent protein (GFP) was coexpressed with v-Rel using the bicistronic avian spleen necrosis virus-derived retroviral vector pUC-pJD214-IRES-v-Rel (12).
Cell culture, transfection, and luciferase assays.
The 293T human embryonic kidney cell line, primary chicken embryo fibroblasts (CEFs), v-Rel-transformed chicken spleen cells (CSC), and the DT40 chicken pre-B-cell line were cultured as described previously (12, 19). Luciferase assays were performed with extracts from 293T cells (1 × 106) transfected with calcium phosphate using a total of 6.01 μg DNA, including 0.01 μg of hRL-null Renilla luciferase DNA as a control, as described previously (12). Extracts were prepared at 48 h posttransfection and analyzed for protein concentration and dual-luciferase activity (Promega Corp., Madison, WI).
GST pull-down, coimmunoprecipitation, and Western blot analyses.
Extracts from 293T cells transfected with Rel expression vectors using Lipofectamine 2000 (Invitrogen) were prepared at 48 h posttransfection, quantitated for equal protein amounts, and used in GST pull-down assays with GST-CAPERα as described previously (40) in buffers supplemented with 10 mM sodium fluoride, 1 mM sodium orthovanadate, 1 mM dithiothreitol, 2 mM phenylmethylsulfonyl fluoride, 10 mM p-nitrophenyl phosphate, 10 mM sodium molybdate, 10 mM β-glycerophosphate, 10 mM benzamidine, and fresh 1× Complete protease inhibitor cocktail (Boehringer-Mannheim/Roche), followed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting.
Coimmunoprecipitation assays were carried out with extracts from 293T cells (3.5 × 106 to 4 × 106 cells) cotransfected with 6 μg each of pCMV-v-Rel or pcDNA3.1-HisC-Xpress-CAPERα and either pcDNA3.1-CAPERα-Flag, ΔvRID-Flag, N-CAPERα-Flag or Flag-Mcl-1 control using Lipofectamine 2000 (Invitrogen) or with extracts from v-Rel-transformed CSCs (107 cells). Cell lysates (1 mg) prepared at 48 h posttransfection were immunoprecipitated as described previously (30) with anti-v-Rel N-terminal antibody no. 1967 (50), preimmune serum, anti-CAPERα (BL462; Bethyl Laboratories), or anti-FlagM2 (Sigma) and protein A/G Sepharose (Amersham Biosciences), followed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting with antibodies to Flag, Rel, or Xpress and enhanced chemiluminescence (Amersham-Pharmacia). Other extracts used for immunoblotting were prepared in EBC lysis buffer (50 mM Tris [pH 8.0], 120 mM NaCl, 0.5% NP-40, 10% glycerol) containing 1× Complete protease inhibitor cocktail. Antibodies for immunoblotting were against human CAPERα (BL462; Bethyl Laboratories), the RHD (SC-6955; Santa-Cruz Biotechnology), v-Rel (no. 1967) (50), Xpress and Myc (Invitrogen), Flag (F-3165; Sigma), GFP (TP401; Torrey Pines Biolabs), c-Jun (SC-45; Santa-Cruz Biotechnology), or actin (Sigma).
Transformation of primary splenic lymphocytes.
CSCs were transformed with virus harvested from CEFs cotransfected with retroviral vectors coexpressing enhanced GFP (EGFP), Xpress-vRID, or CAPERα-Flag along with v-Rel in pUCpJD214-IRES2-v-Rel, as described previously (19). Cells (5% input) were seeded in soft agar, and transformed colonies were scored after 2 weeks. The remaining cells were used for Western blotting to verify protein expression. The results of three experiments were calculated as mean ± standard deviation. Animals were used according to the National Cancer Institute Animal Care and Use Committee guidelines under an approved animal study protocol.
siRNA-mediated knockdown and RT-PCR.
Small interfering RNA (siRNA) oligonucleotides for chicken CAPERα (GenBank accession number XM_425690) were designed with the pSicoOligomaker 1.5 program (48), synthesized, and annealed (siRNA no.233 sense [ATGATTTCTGATAGAAATT] and antisense [GGATGATTTCTGATAGAAATT] [Ambion]). CAPERα siRNA no. 233 (2.25 μg) or an siRNA negative control (Ambion, NC 1) was electroporated into v-Rel-transformed CSCs (105) using Ambion's siPORT electroporation kit at 400 V and 1 μF with a GenePulser (Bio-Rad). Electroporation of a Cy3-labeled control siRNA estimated 97.5% transfection efficiency as determined by flow cytometry. At 72 h postelectroporation, cells were analyzed by RT-PCR using primers CGTGGATCCGCCACCATGGGATATGGATTTATTACATTTTCTG and ATAAGAATGCGGCCGCTATCACATTAACTGAAGACGACC (24 cycles). v-Rel-transformed CSCs and control avian leukosis virus (ALV)-transformed DT40 chicken pre-B cells (105) were electroporated with chicken CAPERα siRNA no. 233 as described above, and 104 cells were seeded into soft agar. Transformed colonies were scored after 2 weeks.
RESULTS
CAPERα is a novel vTAD-interacting factor.
To better understand how protein interactions with the Rel TADs influence the potent transforming activity of v-Rel, we screened a human pre-B-cell leukemia cDNA library fused to an N-terminal Myr tag for factors that could interact with a vTAD bait, using a modified cytosolic yeast two-hybrid screen (6). In this system, interaction of a Myr-tagged cellular protein with the vTAD bait fused to amino acids 1 to 61 of constitutively active Ras confers membrane localization to Myc-Ras(61)-vTAD, allowing survival of the Cdc25-2 temperature-sensitive strain of S. cerevisiae at the restrictive temperature (37°C). Screening of 3 × 105 colonies yielded 372 colonies, which represented 24 potential target cDNAs based on growth on Gal-ULM conditional medium at the restrictive temperature. Eleven cDNAs showed specific interaction with Myc-Ras(61)-vTAD. These corresponded to five different interacting candidates as determined by DNA sequence analysis. This study focuses on one of them, CAPERα, which was previously described as a specific transcriptional coactivator for AP-1 and steroid hormone receptors (11, 26).
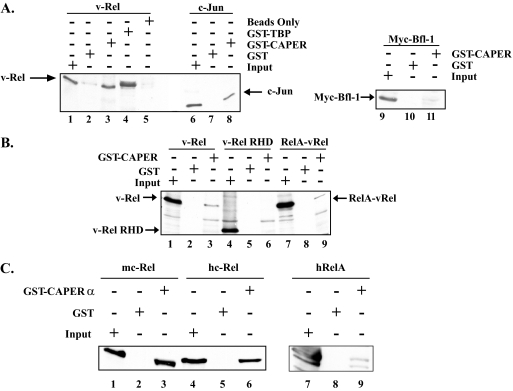
CAPERα was isolated five times in the screen with the Myc-Ras(61)-vTAD bait and in overlapping fragments. Four of these spanned amino acids 310 to 530 of CAPERα, and the other spanned amino acids 116 to 359. GST pull-down assays confirmed interaction of v-Rel with GST-CAPERα (Fig. 1A, lanes 1 to 5), similar to its previously reported interaction with GST-TBP (lane 4) (50). GST-CAPERα also interacted with c-Jun, consistent with prior studies (lanes 6 to 8) (26). In contrast, it failed to associate with a Myc-Bfl-1 negative control (lanes 9 to 11). The fact that GST-CAPERα could associate with a hybrid protein comprised of the N-terminal RHD of RelA fused to the vTAD (RelA-vRel) but failed to pull down the N-terminal RHD of v-Rel confirmed that CAPERα interacts with the C-terminal TAD of v-Rel (Fig. 1B, compare lanes 1 to 3 and 7 to 9 with lanes 4 to 6). Similar to its interaction with v-Rel, GST-CAPERα could also associate with the cellular NF-κB family proteins mouse c-Rel, human c-Rel, and human RelA (Fig. 1C).
FIG. 1.
GST pull-down assays of Rel proteins with CAPERα. (A) GST pull-down assay of v-Rel, c-Jun, or Myc-Bfl-1 expressed in 293T cells with GST, GST-CAPERα, or GST-TBP as a positive control, followed by immunoblotting with antibodies to v-Rel, c-Jun, or Myc tag. (B) GST pull-down assays were performed as for panel A with extracts expressing v-Rel, the v-Rel RHD, or a hybrid RelA/v-Rel protein, using GST-CAPERα or GST as a control. The blot was probed with antibodies specific for the RHD. (C) GST pull-down assays were performed as for panel A with extracts expressing mouse c-Rel (mc-Rel), human c-Rel (hc-Rel), or the human RelA (hRelA) protein. The blot was probed with antibodies specific for the RHD (lanes 1 to 6) or the C terminus of hRelA (lanes 7 to 9).
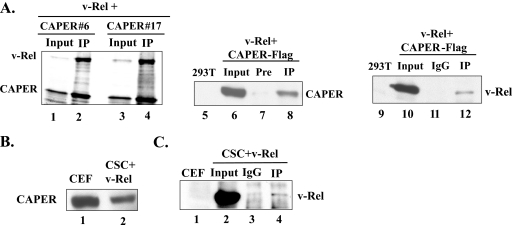
Coimmunoprecipitation assays verified the interaction of v-Rel with CAPERα, as seen by coimmunoprecipitation of v-Rel produced by in vitro translation with Xpress-tagged CAPERα fragments 6 and 17 isolated in the yeast two-hybrid screen (amino acids 116 to 359 and 310 to 530) (Fig. 2A, lanes 1 to 4; Fig. 4A). v-Rel similarly associated with CAPERα-Flag in vivo as seen by immunoprecipitation of transiently transfected 293T cells with anti-v-Rel followed by immunoblotting with anti-Flag (lanes 5 to 8) and, conversely, by immunoprecipitation with anti-CAPERα followed by immunoblotting with an anti-RHD antibody (lanes 9 to 12).
FIG. 2.
Coimmunoprecipitation assays of v-Rel with CAPERα. (A) Coimmunoprecipitation of radiolabeled v-Rel with Xpress-tagged CAPERα fragment 6 or 17 produced by in vitro translation, using anti-Xpress antibodies (lanes 1 to 4) and in vivo coimmunoprecipitation of v-Rel and CAPERα-Flag expressed in 293T cells with preimmune (Pre) or anti-v-Rel (lanes 7 and 8), IgG or anti-CAPERα (lanes 11 and 12), followed by immunoblotting with anti-FlagM2 (lanes 5 to 8) or anti-RHD (lanes 9 to 12). Nontransfected cells served as a control. One hundred micrograms of total protein was loaded as input. (B) Western blot of endogenous chicken CAPERα in CEFs and v-Rel-transformed CSCs (CSC+v-Rel) using an anti-CAPERα antibody. (C) In vivo coimmunoprecipitation of v-Rel with endogenous chicken CAPERα in v-Rel-transformed CSCs, with anti-CAPERα or IgG as a control, followed by immunoblotting with anti-RHD. Extracts from CEFs were used as a negative control. Input protein (100 μg) was loaded as a control.
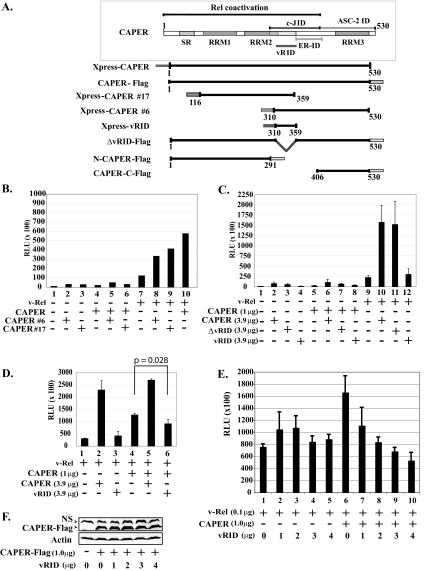
FIG. 4.
CAPERα sequences important for coactivation with v-Rel. (A) Schematic representation of wild-type CAPERα and mutants. SR, serine-arginine-rich region; RRM, RNA recognition motif; c-JID, c-Jun interaction domain; ER-ID, estrogen receptor interaction domain; ASC-2 ID, ASC-2 interaction domain; vRID, putative v-Rel interaction domain. (B) CAPERα fragments 6 and 17 synergize v-Rel-mediated activation. Luciferase assays were carried out as for Fig. 3A with extracts from 293T cells cotransfected with v-Rel (0.1 μg) alone or together with CAPERα (1.0 μg) and/or CAPERα fragments (no. 6 or 17) (3.9 μg) and an IL-6κB-luciferase reporter (1.0 μg) and hRL-null Renilla control (0.01 μg). The averages from three experiments are shown. (C) Like CAPERα, CAPERα mutant ΔvRID synergizes v-Rel-mediated activation, in contrast to vRID. Luciferase assays were carried out as for panel B with CAPERα mutants (ΔvRID or vRID). (D) vRID acts in a dominant-negative fashion. Luciferase assays were carried out as for panel B with extracts from cells cotransfected with v-Rel (0.1 μg) alone or together with CAPERα (1.0 μg) alone or together with excess CAPERα or vRID (3.9 μg). (E) Transfection of increasing amounts of vRID leads to dose-dependent inhibition of CAPER-mediated coactivation of v-Rel. Cells were cotransfected with v-Rel (0.1 μg) alone or together with CAPERα (1 μg) along with increasing amounts of vRID (0 to 4 μg). (F) Western blot showing that transfection of increasing amounts of vRID does not alter the levels of CAPERα-Flag. NS, nonspecific band.
Since antibodies to human CAPERα could detect endogenous chicken CAPERα by immunoblotting of CEFs and of v-Rel-transformed CSCs (Fig. 2B), we investigated their interaction in v-Rel-transformed CSCs. Interestingly, coimmunoprecipitation assays with anti-CAPERα failed to show a significant interaction between endogenous chicken CAPERα and endogenous v-Rel in v-Rel-transformed CSCs compared to the immunoglobulin G (IgG) control (Fig. 2C, compare lanes 4 and 3). This suggests that if these proteins interact endogenously in v-Rel-transformed CSCs, their interaction is very weak, and it raises the possibility that CAPERα might negatively influence v-Rel's biological activity (see below).
CAPERα coactivates v-Rel-mediated transcription.
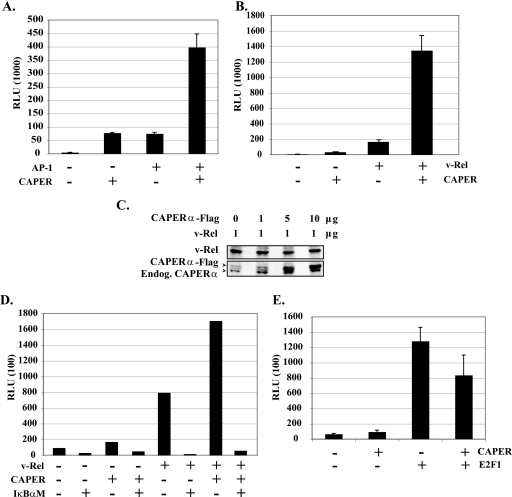
Since CAPERα was described as a selective coactivator for transcription factors AP-1 and estrogen receptors (26), its association with the v-Rel TAD led us to investigate its effect on v-Rel-mediated transcription. Consistent with its interaction with c-Jun, CAPERα efficiently synergized transactivation of a 4× AP-1-luciferase reporter by c-Jun and c-Fos (Fig. 3A). Enhancement of basal AP-1-luciferase activity most likely resulted from CAPERα effects on endogenous AP-1 complexes. Importantly, CAPERα strongly enhanced v-Rel-mediated activation of an IL-6-κB-luciferase reporter by approximately eightfold, in contrast to CAPERα alone (Fig. 3B). Since overexpression of CAPERα-Flag did not affect the expression levels of v-Rel (Fig. 3C), these data suggest genuine transcriptional synergy between CAPERα and v-Rel. This synergy was greatly reduced by coexpression of a dominant inhibitor of NF-κB (IκBαM), indicating that the ability of CAPERα to enhance IL-6-κB-luciferase expression is Rel/NF-κB dependent (Fig. 3D). In contrast, CAPERα failed to enhance E2F1-mediated transcriptional activation of a p73-luciferase reporter (Fig. 3E). This is consistent with previous work showing that CAPERα is not a general transcriptional coactivator but rather is selective for certain transcription factors such as AP-1 and ERα/β but not for thyroid hormone receptor, retinoid acid receptor, p53, or serum response factor (26). Together, these results validate CAPERα as a new transcriptional coactivator for v-Rel.
FIG. 3.
Specific coactivation of v-Rel- and AP-1-mediated transcription by CAPERα. (A) CAPERα coactivates AP-1-mediated transactivation. Luciferase assays were performed in 293T cells cotransfected with vectors encoding AP-1 complex components c-Jun and c-Fos (0.2 μg each) along with CAPERα-Flag (4.0 μg), a 4× AP-1-luciferase reporter (1.6 μg), and the hRL-null Renilla internal control (0.01 μg). The total amount of transfected DNA was kept constant (6.01 μg) by addition of pCMV (for transcription factors) or pcDNA3.1(+) DNA (for coactivators). Luciferase activity was normalized to total protein concentration and internal Renilla luciferase activity and is represented as relative light units (RLU). The averages from three independent assays are shown with standard deviations. (B) CAPERα strongly synergizes v-Rel-mediated transactivation. Assays were performed as for panel A in cells cotransfected with v-Rel (0.4 μg) and/or CAPERα-Flag DNA (4.0 μg), along with an IL-6κB-luciferase reporter (1.6 μg) and hRL-null Renilla internal control (0.01 μg). The data represent the averages from three independent assays. (C) Western blot showing that transfection of increasing amounts of CAPERα-Flag does not alter v-Rel expression levels, as seen by probing with an antibody to the RHD. (D) Coactivation of IL-6-κB-luciferase expression by CAPERα is Rel/NF-κB dependent. Luciferase assays were carried out as for panel A in cells cotransfected with v-Rel (0.4 μg) and/or CAPERα (1.2 μg) expression vectors in the presence or absence of IκBαM (3.6 μg), together with IL-6κB-luciferase (0.8 μg) and the hRL-null Renilla control (0.01 μg). (E) CAPERα fails to synergize E2F1-mediated transactivation. Luciferase assays were performed in cells transfected with an expression vector for transcription factor E2F1 (0.4 μg), CAPERα (4.0 μg), or both, along with a p73-luciferase reporter (1.6 μg) and hRL-null Renilla internal control (0.01 μg). The averages from three independent experiments are shown.
CAPERα's N-terminal and central regions are involved in coactivation of v-Rel-mediated transcription.
We used mutagenesis to identify the domains of CAPERα necessary for activation of NF-κB-dependent transcription (Fig. 4A). Similar to full-length CAPERα, the two CAPERα fragments that were isolated in the two-hybrid screen (CAPERα 6 and 17) synergistically enhanced v-Rel-mediated transactivation of a luciferase reporter (Fig. 4B, bars 8 and 9). This suggested that the overlapping region between these fragments might be important for interaction of CAPERα with the v-Rel TAD (CAPERα amino acids 310 to 359). Surprisingly, however, a CAPERα mutant deleted of this putative vRID (ΔvRID) retained the ability to enhance v-Rel-mediated transactivation (Fig. 4C, bar 11). This suggests that additional sequences in CAPERα can promote its interaction with v-Rel. Conversely, a CAPERα mutant consisting of only the putative vRID failed to coactivate with v-Rel (bar 12). In fact, the vRID mutant reduced synergistic activation of IL-6-κB-luciferase by CAPERα plus v-Rel, suggesting that it acts in a dominant-negative fashion (Fig. 4D, compare bar 6 with bars 4 and 5). Despite the modest inhibition seen in bar 6 versus 4, analysis by Student's t test showed that repression of CAPER-induced v-Rel coactivation by vRID is significant (P value of 0.028). Moreover, titration experiments showed that transfection of increasing amounts of vRID leads to inhibition of CAPER-induced v-Rel coactivation in a dose-dependent manner (Fig. 4E). Since expression of vRID did not reduce the levels of CAPERα-Flag (Fig. 4F), these data support the conclusion that vRID acts in a dominant-negative fashion.
We then analyzed the activities of mutants comprised of the N-terminal or C-terminal region of CAPERα (N-CAPERα and CAPERα-C) (Fig. 4A). The N terminus of CAPERα extending up to the previously described c-Jun interaction domain (26) strongly enhanced v-Rel's transcriptional activity (N-CAPERα, amino acids 1 to 291 (Fig. 5A, bar 3 versus bar 1). This mutant showed no dominant-negative effect when coexpressed with CAPERα (bar 7 versus bars 2 and 6). In contrast, a C-terminal mutant spanning from the end of the c-Jun interaction domain to the C-terminal end of CAPERα failed to enhance v-Rel-mediated transactivation (mutant CAPERα-C, amino acids 406 to 430) (Fig. 4A and 5A, bar 4).
FIG. 5.
The N-terminal region of CAPERα is required for coactivation with v-Rel. (A) Luciferase assays in cells cotransfected with v-Rel (0.1 μg), CAPERα (1.0 μg) alone or together with excess CAPERα (3.9 μg) or mutant N-CAPERα or CAPERα-C (3.9 μg), along with IL-6κB-luciferase reporter (1.0 μg). The averages and standard deviations from three independent experiments are shown. (B) Coimmunoprecipitation of CAPERα mutants with v-Rel or with wild-type CAPERα. Extracts from 293T cells cotransfected with equal amounts of v-Rel and either CAPERα-Flag, ΔvRID-Flag, or N-CAPERα-Flag DNA (6 μg each) were immunoprecipitated with anti-v-Rel (lanes 3, 6, and 9) or preimmune serum (lanes 2, 5, and 8), followed by immunoblotting with anti-Flag. (C) CAPERα forms homodimers. Coimmunoprecipitation assays were performed in cells cotransfected with equal amounts of Xpress-CAPERα and either CAPERα-Flag, ΔvRID-Flag, N-CAPERα-Flag, or Flag-Mcl-1 control (6 μg each), followed by immunoprecipitation with anti-Flag or IgG and immunoblotting with anti-Xpress. (D) Coimmunoprecipitation assays were performed as for panel C. The blot was reprobed with anti-Flag to determine the amount of immunoprecipitated Flag-tagged proteins. Arrowheads point to immunoprecipitated CAPER-Flag, ΔvRID-Flag, N-CAPERα-Flag, or Flag-Mcl-1 and to low levels of Xpress-CAPER coimmunoprecipitated with N-CAPERα-Flag. Total protein (100 μg) was loaded as input (In).
Since the N-terminal region of CAPERα was necessary for synergistic activation with v-Rel, we examined whether it was also required for their interaction by coimmunoprecipitation in 293T cells cotransfected with v-Rel and Flag-tagged CAPERα, mutant ΔvRID, or N-CAPERα. Mutant ΔvRID coimmunoprecipitated with v-Rel, indicating that although amino acids 310 to 359, which overlap between CAPERα fragments 6 and 17, may be involved in CAPERα's interaction with v-Rel, they are not exclusively responsible for association with v-Rel (Fig. 5B, lanes 5 and 6). Surprisingly despite its ability to coactivate v-Rel-mediated transcription, mutant N-CAPERα did not show significant interaction with v-Rel compared to its background immunoprecipitation with the preimmune control (Fig. 5B, lanes 8 and 9). Of potential relevance in this regard, our studies revealed that CAPERα can form homodimers as seen by coimmunoprecipitation of Xpress-tagged CAPERα with either CAPERα-Flag, ΔvRID or, N-CAPERα (Fig. 5C, lanes 2, 4, and 9, and D, lanes 2, 5, and 8). Although the amount of Xpress-CAPER coprecipitating with N-CAPERα-Flag appeared to be significantly less than that with CAPERα-Flag or ΔvRID, reprobing with anti-Flag also revealed less efficient immunoprecipitation of N-CAPERα-Flag (Fig. 5D). In contrast to its association with these proteins, Xpress-CAPERα failed to associate with a Flag-Mcl-1 control (Fig. 5D, lane 11). This suggests that the ability of N-CAPERα to coactivate v-Rel-mediated transcription may result from its ability to form dimers with endogenous CAPERα. Together these results pinpoint an important role for the N-terminal and central regions of CAPERα in coactivating v-Rel-mediated transcription.
CAPERα antagonizes v-Rel's potent transforming activity.
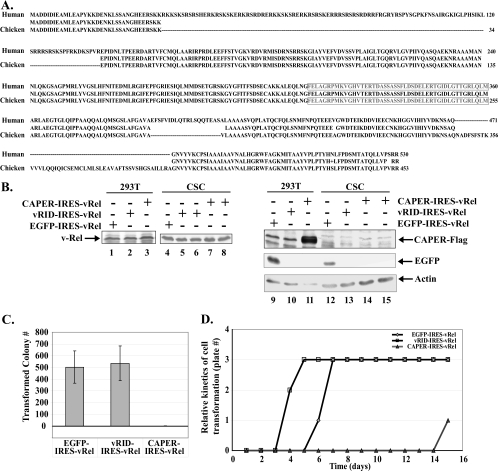
The human and chicken CAPERα proteins are highly related (69% identity) and are identical in the putative vRID region (Fig. 6A). We thus investigated whether CAPERα influences v-Rel's potent transforming activity in primary chicken lymphocytes by coexpressing CAPERα, mutant vRID, or an EGFP control along with v-Rel in primary CSCs, using a bicistronic avian retroviral vector driving v-Rel from an internal ribosome entry site (pUC19-pJD214-IRES-vRel). Immunoblots verified that v-Rel was expressed efficiently from all of these constructs, as were CAPERα-Flag and EGFP (Fig. 6B, lanes 1 to 3, 9, and 11). Although the very small size of Xpress-vRID (∼9.5 kDa) precluded its detection by immunoblotting (data not shown), it displayed a dominant-negative effect in luciferase assays (Fig. 4D and E). Importantly, CAPERα dramatically inhibited v-Rel's transforming activity, as primary CSCs infected with CAPERα-IRES-vRel rarely gave rise to transformed colonies (Fig. 6C). This was in stark contrast to the EGFP-IRES-v-Rel control, which led to abundant colony formation. Moreover, the few colonies that arose from cells infected with CAPERα-IRES-vRel did so with markedly delayed kinetics compared to the EGFP-IRES-vRel control (Fig. 6D). Indeed, the soft agar medium of a single dish out of three containing cells expressing CAPERα-IRES-vRel only began to acidify at day 14 postseeding, compared to day 6 to 7 in those expressing the EGFP-IRES-vRel control. Only two of the seven colonies that ever arose from CSCs infected with CAPERα-IRES-vRel could be propagated in liquid culture, and both failed to express CAPERα-Flag although they expressed v-Rel at levels equivalent to those in CSCs transformed by the EGFP-IRES-vRel control (Fig. 6B, lanes 4, 7, 8, 14, and 15). This indicates that CAPERα is detrimental to cell transformation by v-Rel.
FIG. 6.
CAPERα inhibits v-Rel's transforming activity in lymphocytes. (A) Sequence alignment of the human and chicken CAPERα proteins. The putative vRID (amino acids 310 to 359) is boxed. (B) Immunoblot showing expression of v-Rel, CAPERα-Flag, and EGFP from JD214 retroviral vectors encoding CAPERα-Flag-IRES-v-Rel, Xpress-vRID-IRES-vRel, and EGFP-IRES-vRel in transfected 293T cells and in primary CSCs transformed by EGFP-IRES-vRel, Xpress-vRID-IRES-vRel, and CAPERα-Flag-IRES-v-Rel. The blot was reprobed with antiactin as a control. (C) Effects of CAPERα, vRID, or EGFP control on v-Rel's transforming efficiency in primary chicken lymphocytes. The average numbers of colonies forming in soft agar in four independent assays performed in duplicate are shown with standard deviations. (D) Effect of CAPERα, vRID, or EGFP control on the kinetics of lymphoid cell transformation by v-Rel, as determined by acidification of the agar medium over time.
In contrast to CAPERα, the dominant-negative vRID mutant coexpressed with v-Rel transformed cells as efficiently as the EGFP-IRES-vRel control as measured by colony formation in soft agar (Fig. 6C). In addition, vRID reproducibly accelerated the kinetics of cell transformation, as seen by faster acidification of the agar medium compared to the EGFP-IRES-vRel positive control (Fig. 6D). Since v-Rel was expressed at equivalent levels in lymphoid cells transformed by all of these constructs (Fig. 6B, lanes 4 to 8), these data indicate that CAPERα adversely affects cell transformation by v-Rel by interfering with initiation and/or maintenance of cell transformation, whereas the dominant-negative effect of mutant vRID is associated with accelerated kinetics of transformation.
CAPERα knockdown promotes colony formation by v-Rel-transformed CSCs.
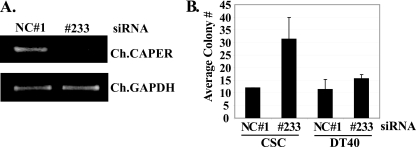
In complementary studies, we investigated how endogenous CAPERα affects the transformed phenotype of established v-Rel-transformed CSCs by silencing endogenous chicken CAPERα with siRNA. Electroporation of siRNA 233 significantly knocked down endogenous chicken CAPERα compared to a nonspecific control siRNA (NC 1), as seen by RT-PCR (Fig. 7A). Importantly, silencing of CAPERα reproducibly enhanced (∼3-fold) the ability of v-Rel-transformed CSCs to form colonies in soft agar compared to the NC 1 siRNA control (Fig. 7B). This effect appeared to be specific, since siRNA 233 had no significant effect on the growth of control ALV-transformed chicken DT40 pre-B cells in soft agar (Fig. 7B). Hence, reducing endogenous CAPERα levels selectively enhances the growth of v-Rel-transformed lymphocytes in soft agar, a phenotype that is a strong indicator of cell transformation. These results are consistent with our finding that dominant-negative vRID enhances the kinetics of v-Rel-mediated transformation (Fig. 6D). This also validates our data showing that CAPERα interferes with v-Rel's transforming activity (Fig. 6C) and rules out the possibility that the antagonistic effects of CAPERα in these assays resulted from artifacts due to overexpression. Overall, these results uncovered a novel interplay between the transcriptional coactivator CAPERα and v-Rel and suggest that lymphocyte transformation by v-Rel may require selection against endogenous interaction between v-Rel and CAPERα.
FIG. 7.
Knockdown of endogenous CAPERα promotes colony formation by v-Rel-transformed CSCs. (A) RT-PCR showing efficacy of siRNA 233-mediated knockdown of endogenous chicken CAPERα at 72 h after electroporation of v-Rel-transformed CSCs, compared a nonspecific control siRNA (NC 1). Chicken GAPDH (glyceraldehyde-3-phosphate dehydrogenase) mRNA levels were analyzed as a control. (B) Effect of siRNA-mediated knockdown of endogenous chicken CAPERα or the NC 1 siRNA control on the ability of v-Rel-transformed CSCs or the control ALV-transformed DT40 chicken pre-B cell line to form colonies in soft agar. The averages from three independent experiments are shown with standard deviations.
DISCUSSION
NF-κB's interaction with coactivators and corepressors is crucial for its ability to modulate transcription in a gene-specific manner and has an impact on its biological activity (reviewed in reference 21). For instance, association of RelA with coactivators CBP/p300, P/CAF, or members of the SRC family increases κB site-dependent gene activation, whereas its association with repressive histone deacetylases leads to gene-specific NF-κB-dependent transcriptional repression and plays an important role in regulating RelA's antiapoptotic activity (see, for example, references 7, 8, 16, and 41). Since the Rel TADs are critical determinants of Rel's transforming potential, transcriptional regulators that engage in interactions with Rel TADs are likely to strongly influence Rel's oncogenic activity. Here we report a novel and functional interaction between the TAD of the potent NF-κB oncoprotein v-Rel and the transcriptional coactivator CAPERα and show that CAPERα strongly affects v-Rel's transforming activity in primary lymphocytes. This illustrates an important role for CAPERα in modulating the oncogenic activity of Rel/NF-κB.
Distinct role for the N terminus of CAPERα.
The region of CAPERα involved in coactivating v-Rel-mediated transcription differs from that previously implicated in coactivation of transcription factors c-Jun (AP-1) and ERα and ERβ. While c-Jun and ERα/β associate with the C-terminal half of mouse CAPERα (amino acids 291 to 406 and 355 to 406, respectively) (26), our studies implicate the N-terminal region of CAPERα (amino acids 1 to 291) in coactivating v-Rel-mediated transcription. Surprisingly, mutant N-CAPERα did not show significant association with v-Rel. Although this might be due to technical difficulties arising from higher background association of N-CAPERα with IgG compared to wild-type CAPERα (Fig. 5B, lane 8 versus lane 2), we do not rule out the possibility that N-CAPERα might coactivate v-Rel transcription indirectly, via heterodimer formation with endogenous CAPERα. Indeed, we showed that CAPERα can form homodimers, a characteristic shared by a limited number of transcriptional coregulators, including the coactivator of thyroid hormone receptor and retinoid X receptor NRIF3 (34).
The N-terminal half of CAPERα has a serine-arginine-rich region similar to that found in a large group of factors involved in pre-mRNA splicing (amino acids 41 to 90). It also contains two of the three RNA recognition motifs found in CAPERα (amino acids 153 to 230, 250 to 328, and 445 to 508) (Fig. 4A), which are typically implicated in protein-RNA or protein-protein interactions. These may allow CAPERα to interact with other factors to modulate transcription, mediate its association with v-Rel, and/or participate in CAPERα dimer formation. Indeed, we found that although mutant vRID had a dominant-negative effect on v-Rel coactivation by CAPERα, deletion of amino acids 310 to 359 in mutant ΔvRID did not eliminate its ability to associate with v-Rel and synergize its transcription. This suggests that although the putative vRID may participate in CAPERα's interaction with v-Rel, other sequences such as those found in the N terminus are likely to also be involved.
CAPERα coactivation of v-Rel-mediated transcription.
CAPERα is a rather selective coactivator of certain transcription factors, and it synergized v-Rel- and AP-1-mediated transcription but failed to enhance transactivation by E2F1 in our assays. This agrees with prior work showing that CAPERα selectively coactivates transcription mediated by AP-1 and steroid hormone receptors but not that induced by thyroid hormone receptor, retinoid acid receptor, p53, or serum response factor (11, 26). Its ability to synergistically enhance transcription by v-Rel and AP-1 is interesting, since both factors regulate the expression of genes involved in the oncogenic process and AP-1 can directly interact with Rel/NF-κB to synergistically activate gene expression (42, 46). Additionally, c-Jun is a transcriptional target of v-Rel and is essential as part of the AP-1 complex for v-Rel's ability to transform lymphocytes (15, 28). Both c-Jun and JunB are aberrantly expressed in malignant Hodgkin/Reed-Sternberg cells of Hodgkin's lymphoma, which depend on Rel/NF-κB for survival, and synergize with NF-κB (37). It is thus tempting to speculate that CAPERα might help to integrate the transcriptional activities of Rel/NF-κB and AP-1 on certain promoters.
CAPERα was previously isolated by virtue of its interaction with the general coactivator ASC-2 (26), which interacts with multiple transcriptional regulators, including SRC-1, CBP/p300, nuclear receptors, AP-1, and NF-κB (31, 32). While ASC-2 was previously shown to potentiate NF-κB-driven transcription and relieve its trans-repression and that of AP-1 by nuclear hormone receptors (32), CAPERα might coactivate transcription driven by v-Rel independently of ASC-2, since ASC-2 associates with the C terminus of CAPERα, which is absent in N-CAPERα (amino acids 406 to 530) (Fig. 4A) (26). In this scenario CAPERα would be likely to recruit different cofactors to enhance transcription mediated by AP-1, nuclear hormone receptors, and v-Rel. On the other hand, since N-CAPERα may coactivate v-Rel by forming heterodimers with endogenous CAPERα, we do not rule out the possibility that ASC-2 might contribute to coactivation by CAPERα and v-Rel. Future studies will help to elucidate the mechanism by which CAPERα coactivates v-Rel-mediated transcription.
CAPERα inhibition of Rel's oncogenic activity.
In addition to its effect on v-Rel-induced transcription, CAPERα strongly suppressed v-Rel's transforming activity. This is unlikely to result from overexpression artifacts, since coexpression of the dominant-negative mutant vRID consistently accelerated the kinetics of v-Rel-induced transformation. Additionally, endogenous CAPERα knockdown with siRNA markedly enhanced the transformed phenotype of v-Rel-transformed CSCs, as seen by colony formation in agar. These results agree with our coimmunoprecipitation data showing that only very low levels of v-Rel are found in complex with endogenous CAPERα in transformed CSCs (Fig. 2C). This also agrees with the apparent selection that we observed against high-level expression of CAPERα in CSCs transformed by CAPERα-IRES-vRel (Fig. 6B, lanes 14 and 15). This antagonistic effect of CAPERα is unlikely to result from a global inhibitory effect on lymphocyte transformation, since silencing CAPERα with siRNA had no significant effect on the ability of ALV-transformed DT40 cells to form colonies in agar. These data rather support the notion that CAPERα's inhibitory effects are specific to Rel-mediated transformation.
Although the mechanism by which CAPERα antagonizes v-Rel's transforming activity is not fully understood, it is possible that transcriptional coactivation by CAPERα increases v-Rel's transactivation potency beyond an optimal level for cell transformation. Indeed work from Gilmore's group and ours unveiled an inverse correlation between the strength of the v-Rel- and c-Rel TADs and their transforming efficiency (12, 43, 44). The fact that naturally occurring c-rel gene mutations in PMBCL and follicular lymphoma specimens decrease c-Rel's transactivation potency and enhance its transforming activity in chicken lymphocytes is also consistent with the idea that too much Rel transcriptional activity can be detrimental to cell transformation (45). This raises the possibility that CAPERα might enhance Rel-induced activation of specific genes beyond an acceptable level and that this is detrimental to its oncogenic activity.
Alternatively, CAPERα might preclude efficient gene-specific repression by v-Rel, as recent reports from Bose's group and ours showed that v-Rel-induced downregulation of genes such as those for SH3BGRL and the B-cell receptor signaling molecules BLNK and BCAP is important for its transforming activity (19, 35). While it remains to be determined whether CAPERα modulates expression of these or other Rel-regulated genes, it is conceivable that it compromises Rel's transforming activity by enhancing activation and/or dampening repression of specific Rel-regulated genes. Since CAPERα is a bifunctional protein that can also modulate alternative splicing, as seen in response to steroid hormone receptor activation (11), it could also affect alternative splicing of genes important for cell transformation by v-Rel. Support for this idea stems from recent evidence that v-Rel can promote alternative splicing of TERT to produce full-length TERT, which is necessary in v-Rel-transformed lymphocytes (24). In this scenario, CAPERα might compromise production of full-length TERT, given its antagonistic effect on cell transformation. Future studies will help to elucidate the mechanism by which CAPERα suppresses v-Rel's transforming activity.
Finally, our finding that inhibition of CAPERα enhances v-Rel's transforming activity agrees with preliminary data indicating that CAPERα knockdown in the Hodgkin Reed-Sternberg-derived cell line KM-H2 causes a significant reduction in the number of cells in G0/G1 phase and a concomitant increase in cells accumulating in S and G2/M (data not shown). Since these cells depend on constitutive Rel/NF-κB activity for proliferation and survival (20), these results suggest a potential tumor suppressor role for CAPERα toward Rel's oncogenic activity. In summary, we uncovered a novel and functional interaction between the coactivator CAPERα and the Rel TAD that influences v-Rel's transcriptional activity and strongly affects its oncogenicity. Future studies aimed at understanding how CAPERα functions in this context will help to further elucidate how Rel/NF-κB participates in the control of lymphoid cell survival, proliferation, and malignant transformation.
Acknowledgments
This work was supported by a grant from the New Jersey Commission on Cancer Research and partially by Public Health Service grant CA054999 from the National Cancer Institute to C.G.
We thank A. Aronheim, T. Curran, N. H. Colburn, R. Hrdlickova, W. Liu, and A. B. Rabson for gifts of reagents and protocols and M. J. Simmons, Y. Fan, and N. Gupta for fruitful discussions.
Footnotes
Published ahead of print on 27 August 2008.
REFERENCES
- 1.Aronheim, A. 2001. Membrane recruitment systems for analysis of protein-protein interactions. Methods Mol. Biol. 177319-328. [DOI] [PubMed] [Google Scholar]
- 2.Baker, S. J., T. K. Kerppola, D. Luk, M. T. Vandenberg, D. R. Marshak, T. Curran, and C. Abate. 1992. Jun is phosphorylated by several protein kinases at the same sites that are modified in serum-stimulated fibroblasts. Mol. Cell. Biol. 124694-4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bargou, R., F. Emmerich, D. Krappmann, K. Bommert, M. Mapara, W. Arnold, H. Royer, E. Grinstein, A. Greiner, C. Scheidereit, and B. Dorken. 1997. Constitutive nuclear factor-kappaB-RelA activation is required for proliferation and survival of Hodgkin's disease tumor cells. J. Clin. Investig. 1002961-2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barth, T. F., J. I. Martin-Subero, S. Joos, C. K. Menz, C. Hasel, G. Mechtersheimer, R. M. Parwaresch, P. Lichter, R. Siebert, and P. Mooller. 2003. Gains of 2p involving the REL locus correlate with nuclear c-Rel protein accumulation in neoplastic cells of classical Hodgkin lymphoma. Blood 1013681-3686. [DOI] [PubMed] [Google Scholar]
- 5.Beug, H., H. Muller, S. Grieser, G. Doederlein, and T. Graf. 1981. Hematopoietic cells transformed in vitro by REVT avian reticuloendotheliosis virus express characteristics of very immature lymphoid cells. Virology 115295-309. [DOI] [PubMed] [Google Scholar]
- 6.Broder, Y. C., S. Katz, and A. Aronheim. 1998. The ras recruitment system, a novel approach to the study of protein-protein interactions. Curr. Biol. 81121-1124. [DOI] [PubMed] [Google Scholar]
- 7.Campbell, K. J., and N. D. Perkins. 2006. Regulation of NF-kappaB function. Biochem. Soc. Symp. 73165-180. [DOI] [PubMed] [Google Scholar]
- 8.Campbell, K. J., S. Rocha, and N. D. Perkins. 2004. Active repression of antiapoptotic gene expression by RelA(p65) NF-kappa B. Mol. Cell 13853-865. [DOI] [PubMed] [Google Scholar]
- 9.Carrasco, D., C. A. Rizzo, K. Dorfman, and R. Bravo. 1996. The v-rel oncogene promotes malignant T-cell leukemia/lymphoma in transgenic mice. EMBO J. 153640-3650. [PMC free article] [PubMed] [Google Scholar]
- 10.Dong, Z., R.-H. Xu, J. Kim, S.-N. Zhan, W.-Y. Ma, N. H. Colburn, and H.-F. Kung. 1996. AP-1/Jun is required for early Xenopus development and mediates mesoderm induction by fibroblast growth factor but not by activin. J. Biol. Chem. 2719942-9946. [DOI] [PubMed] [Google Scholar]
- 11.Dowhan, D. H., E. P. Hong, D. Auboeuf, A. P. Dennis, M. M. Wilson, S. M. Berget, and B. W. O'Malley. 2005. Steroid hormone receptor coactivation and alternative RNA splicing by U2AF65-related proteins CAPERalpha and CAPERbeta. Mol. Cell 17429-439. [DOI] [PubMed] [Google Scholar]
- 12.Fan, Y., and C. Gelinas. 2007. An optimal range of transcription potency is necessary for efficient cell transformation by c-Rel to ensure optimal nuclear localization and gene-specific activation. Oncogene 264038-4043. [DOI] [PubMed] [Google Scholar]
- 13.Fan, Y., B. Rayet, and C. Gélinas. 2004. Divergent C-terminal transactivation domains of Rel/NF-kB proteins are critical determinants of their oncogenic potential in lymphocytes. Oncogene 231030-1042. [DOI] [PubMed] [Google Scholar]
- 14.Feuerhake, F., J. L. Kutok, S. Monti, W. Chen, A. S. LaCasce, G. Cattoretti, P. Kurtin, G. S. Pinkus, L. de Leval, N. L. Harris, K. J. Savage, D. Neuberg, T. M. Habermann, R. Dalla-Favera, T. R. Golub, J. C. Aster, and M. A. Shipp. 2005. NFkappaB activity, function, and target-gene signatures in primary mediastinal large B-cell lymphoma and diffuse large B-cell lymphoma subtypes. Blood 1061392-1399. [DOI] [PubMed] [Google Scholar]
- 15.Fujii, M., T. Minamino, M. Nomura, K. Miyamoto, J. Tanaka, and M. Seiki. 1997. v-Rel activates the proto-oncogene c-Jun promoter: a correlation with its transforming activity. Leukemia 11(Suppl. 3)402-404. [PubMed] [Google Scholar]
- 16.Gao, Z., P. Chiao, X. Zhang, X. Zhang, M. A. Lazar, E. Seto, H. A. Young, and J. Ye. 2005. Coactivators and corepressors of NF-kappaB in IkappaB alpha gene promoter. J. Biol. Chem. 28021091-21098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilmore, T. D., C. Cormier, J. Jean-Jacques, and M. E. Gapuzan. 2001. Malignant transformation of primary chicken spleen cells by human transcription factor c-Rel. Oncogene 207098-7103. [DOI] [PubMed] [Google Scholar]
- 18.Gilmore, T. D., D. Kalaitzidis, M. C. Liang, and D. T. Starczynowski. 2004. The c-Rel transcription factor and B-cell proliferation: a deal with the devil. Oncogene 232275-2286. [DOI] [PubMed] [Google Scholar]
- 19.Gupta, N., J. Delrow, A. Drawid, A. M. Sengupta, G. Fan, and C. Gelinas. 2008. Repression of B-cell linker (BLNK) and B-cell adaptor for phosphoinositide 3-kinase (BCAP) is important for lymphocyte transformation by rel proteins. Cancer Res. 68808-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hinz, M., P. Loser, S. Mathas, D. Krappmann, B. Dorken, and C. Scheidereit. 2001. Constitutive NF-kappaB maintains high expression of a characteristic gene network, including CD40, CD86, and a set of antiapoptotic genes in Hodgkin/Reed-Sternberg cells. Blood 972798-2807. [DOI] [PubMed] [Google Scholar]
- 21.Hoffmann, A., G. Natoli, and G. Ghosh. 2006. Transcriptional regulation via the NF-kappaB signaling module. Oncogene 256706-6716. [DOI] [PubMed] [Google Scholar]
- 22.Hrdlickova, R., J. Nehyba, and E. H. Humphries. 1994. In vivo evolution of c-rel oncogenic potential. J. Virol. 682371-2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hrdlickova, R., J. Nehyba, and E. H. Humphries. 1994. v-rel induces expression of three avian immunoregulatory surface receptors more efficiently than c-rel. J. Virol. 68308-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hrdlickova, R., J. Nehyba, A. S. Liss, and H. R. Bose, Jr. 2006. Mechanism of telomerase activation by v-Rel and its contribution to transformation. J. Virol. 80281-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imai, H., E. K. Chan, K. Kiyosawa, X. D. Fu, and E. M. Tan. 1993. Novel nuclear autoantigen with splicing factor motifs identified with antibody from hepatocellular carcinoma. J. Clin. Investig. 922419-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jung, D. J., S. Y. Na, D. S. Na, and J. W. Lee. 2002. Molecular cloning and characterization of CAPER, a novel coactivator of activating protein-1 and estrogen receptors. J. Biol. Chem. 2771229-1234. [DOI] [PubMed] [Google Scholar]
- 27.Kim, H. J., N. Hawke, and A. S. Baldwin. 2006. NF-kappaB and IKK as therapeutic targets in cancer. Cell Death Differ. 13738-747. [DOI] [PubMed] [Google Scholar]
- 28.Kralova, J., A. S. Liss, W. Bargmann, and H. R. Bose, Jr. 1998. AP-1 factors play an important role in transformation induced by the v-rel oncogene. Mol. Cell. Biol. 182997-3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar, S., A. B. Rabson, and C. Gelinas. 1992. The RxxRxRxxC motif conserved in all Rel/κB proteins is essential for the DNA-binding activity and redox regulation of the v-Rel oncoprotein. Mol. Cell. Biol. 123094-3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kwon, H. J., E. H. Breese, E. Vig-Varga, Y. Luo, Y. Lee, M. G. Goebl, and M. A. Harrington. 2004. Tumor necrosis factor alpha induction of NF-κB requires the novel coactivator SIMPL. Mol. Cell. Biol. 249317-9326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee, S. K., S. L. Anzick, J. E. Choi, L. Bubendorf, X. Y. Guan, Y. K. Jung, O. P. Kallioniemi, J. Kononen, J. M. Trent, D. Azorsa, B. H. Jhun, J. H. Cheong, Y. C. Lee, P. S. Meltzer, and J. W. Lee. 1999. A nuclear factor, ASC-2, as a cancer-amplified transcriptional coactivator essential for ligand-dependent transactivation by nuclear receptors in vivo. J. Biol. Chem. 27434283-34293. [DOI] [PubMed] [Google Scholar]
- 32.Lee, S. K., S. Y. Na, S. Y. Jung, J. E. Choi, B. H. Jhun, J. Cheong, P. S. Meltzer, Y. C. Lee, and J. W. Lee. 2000. Activating protein-1, nuclear factor-kappaB, and serum response factor as novel target molecules of the cancer-amplified transcription coactivator ASC-2. Mol. Endocrinol. 14915-925. [DOI] [PubMed] [Google Scholar]
- 33.Lewis, R. B., J. McClure, B. Rup, D. W. Niesel, R. F. Garry, J. D. Hoelzer, K. Nazerian, and H. R. Bose, Jr. 1981. Avian reticuloendotheliosis virus: identification of the hematopoietic target cell for transformation. Cell 25421-431. [DOI] [PubMed] [Google Scholar]
- 34.Li, D., F. Wang, and H. H. Samuels. 2001. Domain structure of the NRIF3 family of coregulators suggests potential dual roles in transcriptional regulation. Mol. Cell. Biol. 218371-8384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Majid, S. M., A. S. Liss, M. You, and H. R. Bose. 2006. The suppression of SH3BGRL is important for v-Rel-mediated transformation. Oncogene 25756-768. [DOI] [PubMed] [Google Scholar]
- 36.Mason, R. P., D. M. Moisey, and L. Shajenko. 1992. Cholesterol alters the binding of Ca2+ channel blockers to the membrane lipid bilayer. Mol. Pharmacol. 41315-321. [PubMed] [Google Scholar]
- 37.Mathas, S., M. Hinz, I. Anagnostopoulos, D. Krappmann, A. Lietz, F. Jundt, K. Bommert, F. Mechta-Grigoriou, H. Stein, B. Dorken, and C. Scheidereit. 2002. Aberrantly expressed c-Jun and JunB are a hallmark of Hodgkin lymphoma cells, stimulate proliferation and synergize with NF-kappa B. EMBO J. 214104-4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nehyba, J., R. Hrdlickova, and E. H. Humphries. 1994. Evolution of the oncogenic potential of v-rel: rel-induced expression of immunoregulatory receptors correlates with tumor development and in vitro transformation. J. Virol. 682039-2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Romieu-Mourez, R., D. W. Kim, S. M. Shin, E. G. Demicco, E. Landesman-Bollag, D. C. Seldin, R. D. Cardiff, and G. E. Sonenshein. 2003. Mouse mammary tumor virus c-rel transgenic mice develop mammary tumors. Mol. Cell. Biol. 235738-5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ryo, A., F. Suizu, Y. Yoshida, K. Perrem, Y. C. Liou, G. Wulf, R. Rottapel, S. Yamaoka, and K. P. Lu. 2003. Regulation of NF-kappaB signaling by Pin1-dependent prolyl isomerization and ubiquitin-mediated proteolysis of p65/RelA. Mol. Cell 121413-1426. [DOI] [PubMed] [Google Scholar]
- 41.Sheppard, K. A., D. W. Rose, Z. K. Haque, R. Kurokawa, E. McInerney, S. Westin, D. Thanos, M. G. Rosenfeld, C. K. Glass, and T. Collins. 1999. Transcriptional activation by NF-κB requires multiple coactivators. Mol. Cell. Biol. 196367-6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shyu, Y. J., C. D. Suarez, and C. D. Hu. 2008. Visualization of AP-1 NF-kappaB ternary complexes in living cells by using a BiFC-based FRET. Proc. Natl. Acad. Sci. USA 105151-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Starczynowski, D. T., J. G. Reynolds, and T. D. Gilmore. 2003. Deletion of either C-terminal transactivation subdomain enhances the in vitro transforming activity of human transcription factor REL in chicken spleen cells. Oncogene 226928-6936. [DOI] [PubMed] [Google Scholar]
- 44.Starczynowski, D. T., J. G. Reynolds, and T. D. Gilmore. 2005. Mutations of tumor necrosis factor alpha-responsive serine residues within the C-terminal transactivation domain of human transcription factor REL enhance its in vitro transforming ability. Oncogene 247355-7368. [DOI] [PubMed] [Google Scholar]
- 45.Starczynowski, D. T., H. Trautmann, C. Pott, L. Harder, N. Arnold, J. A. Africa, J. R. Leeman, R. Siebert, and T. D. Gilmore. 2007. Mutation of an IKK phosphorylation site within the transactivation domain of REL in two patients with B-cell lymphoma enhances REL's in vitro transforming activity. Oncogene 262685-2694. [DOI] [PubMed] [Google Scholar]
- 46.Stein, B., A. S. Baldwin, Jr., D. W. Ballard, W. C. Greene, P. Angel, and P. Herrlich. 1993. Cross-coupling of the NF-kappa B p65 and Fos/Jun transcription factors produces potentiated biological function. EMBO J. 123879-3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suh, J., F. Payvandi, L. C. Edelstein, P. S. Amenta, W.-X. Zong, C. Gélinas, and A. B. Rabson. 2002. Mechanisms of constitutive NF-kB activation in human prostate cancer cells. Prostate 52183-200. [DOI] [PubMed] [Google Scholar]
- 48.Ventura, A., A. Meissner, C. P. Dillon, M. McManus, P. A. Sharp, L. Van Parijs, R. Jaenisch, and T. Jacks. 2004. Cre-lox-regulated conditional RNA interference from transgenes. Proc. Natl. Acad. Sci. USA 10110380-10385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weniger, M. A., S. Gesk, S. Ehrlich, J. I. Martin-Subero, M. J. Dyer, R. Siebert, P. Moller, and T. F. Barth. 2007. Gains of REL in primary mediastinal B-cell lymphoma coincide with nuclear accumulation of REL protein. Genes Chromosomes Cancer 46406-415. [DOI] [PubMed] [Google Scholar]
- 50.Xu, X., C. Prorock, H. Ishikawa, E. Maldonado, Y. Ito, and C. Gelinas. 1993. Functional interaction of the v-Rel and c-Rel oncoproteins with the TATA-binding protein and association with transcription factor IIB. Mol. Cell. Biol. 136733-6741. [DOI] [PMC free article] [PubMed] [Google Scholar]