Abstract
Nef is an accessory protein of human immunodeficiency virus type 1 (HIV-1) that enhances the infectivity of progeny virions when expressed in virus-producing cells. The requirement for Nef for optimal infectivity is, at least in part, determined by the envelope (Env) glycoprotein, because it can be eliminated by pseudotyping HIV-1 particles with pH-dependent Env proteins. To investigate the role of Env in the function of Nef, we have examined the effect of Nef on the infectivity of Env-deficient HIV-1 particles pseudotyped with viral receptors for cells expressing cognate Env proteins. We found that Nef significantly enhances the infectivity of CD4-chemokine receptor pseudotypes for cells expressing HIV-1 Env. Nef also increased the infectivity of HIV-1 particles pseudotyped with Tva, the receptor for subgroup A Rous sarcoma virus (RSV-A), even though Nef had no effect if the pH-dependent Env protein of RSV-A was used for pseudotyping. However, Nef does not always enhance viral infectivity if the normal orientation of the Env-receptor interaction is reversed, because the entry of Env-deficient HIV-1 into cells expressing the vesicular stomatitis virus G protein was unaffected by Nef. Together, our results demonstrate that the presence of a viral Env protein during virus production is not required for the ability of Nef to increase viral infectivity. Furthermore, since the infectivity of Tva pseudotypes was blocked by inhibitors of endosomal acidification, we conclude that low-pH-dependent entry does not always bypass the requirement for Nef.
Nef is an accessory gene product unique to primate immunodeficiency viruses that is required for high-titer virus replication and AIDS pathogenesis in a monkey model (38). Furthermore, infection with nef-defective strains of human immunodeficiency virus type 1 (HIV-1) has been associated with the absence of disease progression in humans (28, 39, 45). Numerous studies have shown that Nef downmodulates the viral receptor CD4 from the cell surface, possibly to prevent interference with the function of the viral envelope (Env) glycoproteins (4, 8, 12, 34, 35, 37, 40, 41, 46, 59, 63, 64, 66). Nef also induces the selective downregulation of specific major histocompatibility complex class I molecules from the cell surface and thereby protects infected cells against recognition by cytotoxic T cells (60). Additionally, Nef affects the surface expression of multiple other cellular proteins (60) and modulates the activation state of T cells and macrophages (33).
Although not essential for HIV-1 replication in cell culture, Nef can significantly stimulate virus replication in primary peripheral blood mononuclear cells that are infected prior to activation with mitogens (50, 70). Nef has been shown to increase virus release and infectivity by removing CD4 from the surface of virus-producing cells (40, 63). However, Nef also enhances the infectivity of HIV-1 if CD4-negative cells are used to produce progeny virions (5, 24, 25). Nef-defective HIV-1 can be rescued by providing Nef in trans in virus producer cells but not in target cells (5, 51), suggesting that Nef in some way alters the molecular makeup of the virion. While small quantities of Nef are incorporated into viral particles (18, 56, 78), Nef otherwise has no evident effect on the protein composition of virions and does not noticeably affect virion morphology or core stability (32). Virion-associated HIV-1 Nef is largely cleaved by the viral protease, but this processing event is not required for the ability of Nef to stimulate virion infectivity (22). Whether Nef exerts its effect as a component of the virion remains unclear, because a dominant-negative version of the Nef binding partner dynamin 2 inhibited the ability of Nef to enhance infectivity without affecting the incorporation of Nef into virions (57).
HIV-1 virions produced in the absence of Nef are impaired in their ability to reverse transcribe the viral RNA genome (5, 24, 68), indicating that Nef facilitates an early step of the viral replication cycle. It has been proposed that the effect of Nef becomes manifest at the level of virus entry, based on the observation that Nef enhances the delivery of HIV-1 capsid (CA) protein into the cytosol of target cells (67). However, subsequent studies have shown that Nef does not affect the initial steps of HIV-1 fusion with target cells up to the formation of a fusion pore (19, 20, 72). Nevertheless, the effect of Nef on viral infectivity appears to depend on the route of entry, because Nef was no longer required for maximal infectivity after pseudotyping with pH-dependent Env proteins that mediate entry through endocytic compartments (3, 21, 42). Interestingly, the infectivity defect of Nef-deficient HIV-1 can also be complemented by disrupting the actin cytoskeleton of target cells (19). Together, these findings have been interpreted as evidence for a role of Nef in facilitating viral penetration through the cortical actin barrier, a function that would become dispensable if entry occurs through endocytosis (19).
Zhou and Aiken have shown that the infectivity enhancement by Nef does not require its presence during viral core formation (81). In that study, the authors used an elegant “virion transcomplementation” approach, which involved the fusion of Env-bearing donor HIV-1 virions harboring defective cores with Env-defective target virions pseudotyped with CD4 and CXCR4. Surprisingly, in this transcomplementation assay, Nef enhanced infection only when present in the Env-bearing donor virions. In contrast, Nef had no effect on infection resulting from virion transcomplementation when expressed during production of the receptor-pseudotyped target virions (81). Pseudotyping per se could not account for this observation, since it is well documented that Nef enhances the infectivity of HIV-1 particles pseudotyped with amphotropic murine leukemia virus Env (5, 51, 68). Together, these observations raised the possibility that the effect of Nef on infectivity depends on its coexpression with a viral Env protein.
In the present study, we show that Nef can significantly enhance the infectivity of receptor pseudotypes, ruling out that the presence of a viral Env protein during virus production is required for the Nef phenotype. We also show that Nef can increase the infectivity of receptor-pseudotyped virions even if entry is pH dependent, indicating that entry though endosomes does not always bypass the stage of infection where Nef exerts its effect.
MATERIALS AND METHODS
Plasmids.
The Env-deficient HIV-1 proviruses HXB/Env−/Nef+ and HXB/Env−/Nef− have been described previously (30). HXB/Env−/Nef+ harbors the intact nef gene of HIV-1LAI, whereas HXB/Env−/Nef− encodes only the first 35 residues of Nef because of a frameshift mutation. HXB/Gag− is a HIV-1HXB2-based provirus that is unable to express Gag because of a premature termination codon and a frameshift mutation in the gag gene (29). The pEnvHXB plasmid is a pBJ5-based expression vector for HIV-1HXB2 Env (58), and pEnvHXBΔCT is a derivative of pEnvHXB that encodes a truncated HIV-1 Env that lacks 144 residues of the cytoplasmic domain (58).
pCHGFPW is a HIV-1 vector encoding green fluorescent protein (GFP) (74), and pMD.G is an expression vector for the vesicular stomatitis virus G (VSV G) protein (54). The pCD4ΔCT plasmid, which expresses a C-terminally truncated human CD4, was obtained by inserting a PCR product encoding CD4 residues 1 through 408 followed by a premature termination codon into pBJ5. A PCR product encoding full-length human CXCR4 with a C-terminal FLAG epitope was inserted into a pcDNA6 expression vector (Invitrogen) to yield pcDNA6/CXCR4-FLAG. The pKZ261 expression vector for hemagglutinin (HA)-tagged Tva and the pAB6 expression vector for the Env glycoprotein (EnvA) of subgroup A Rous sarcoma virus (RSV-A) were donated by John Young (15, 82).
Transfections.
To produce pseudotyped HIV-1 particles, 293T or Jurkat TAg cells (4 × 106) were transfected using Lipofectamine 2000 (Invitrogen) with HXB/Env−/Nef+ or HXB/Env−/Nef−, along with vectors expressing viral glycoproteins or receptors. Where indicated, a vector expressing Dyn2(K44A) and/or pCHGFPW was also cotransfected. To obtain target cells susceptible to infection with the pseudotypes, HeLa or TZM-bl indicator cells (3 × 105) were transfected with expression vectors encoding the cognate viral glycoproteins or receptors, as appropriate, using Fugene 6 (Roche).
RT assay.
Virus-containing supernatants were harvested at 48 h posttransfection, clarified by low-speed centrifugation, and filtered through 0.45-μm-pore filters. Reverse transcriptase (RT) activity in the supernatants was quantified using a Sybr green I-based real-time PCR enhanced RT assay that possess both high sensitivity and an extraordinary dynamic range (6). Briefly, virions in cell-free supernatants were disrupted by adding an equal volume of a solution containing 0.25% Triton X-100, 50 mM KCl, 100 mM Tris-HCl (pH 7.4), 0.4 U/μl RNase inhibitor (RiboLock; MBI Fermentas), and 0.2 mM dithiothreitol. Lysed virions were used for reverse transcription of a brome mosaic virus RNA template (Promega) as previously described (69). Quantification of reverse-transcribed products was carried out in a Light Cycler (Roche) using Sybr green I, hot-start Taq and reaction buffer (Fermentas), and the primer set described previously (69). A standard curve was obtained using known concentrations (expressed in functional units) of recombinant HIV-1 RT (Ambion).
Infectivity assays.
HIV-1 particle concentrations in different virus samples were normalized to 5 U of recombinant HIV-1 RT per ml. Target cells (2 × 104) were seeded into 48-well plates and challenged in triplicate with 10-fold serial dilutions of each virus sample. Infectivities were evaluated at 48 h postinfection by staining infected TZM-bl cells with X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) and by counting blue-stained foci under a light microscope. The infectivities of virions packaging the vector encoding GFP were evaluated either by counting GFP-positive foci at 48 h postinfection under a fluorescence microscope or by flow cytometry.
Western blot analysis.
To examine the association of viral receptors with HIV-1 particles, viral particles present in filtered culture supernatants were pelleted through sucrose cushions as described previously (2). Pelletable material and the cell lysates were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting, using a mouse monoclonal antibody to HIV-1 p55/p24 and a sheep antiserum to CD4 (both from NIBSC, Centre for AIDS Reagents), anti-HA antibody HA.11 (Covance), anti-FLAG M2 antibody (Sigma-Aldrich), or a rabbit antiserum against Nef (18) as appropriate.
Cell surface biotinylation.
Labeling was performed in suspension. After several washes in phosphate-buffered saline (pH 8.0), the cells were incubated on ice for 1 h in 2 mM sulfo-NHS-LC-biotin (Pierce), followed by three washes in phosphate-buffered saline with 100 mM glycine to quench the biotin reagent. The cells were then lysed, and biotinylated proteins were collected on streptavidin beads and analyzed by Western blotting.
RESULTS
Nef enhances the infectivity of CD4-chemokine receptor pseudotypes.
While the study by Zhou and Aiken suggested that Nef does not act on receptor pseudotypes (81), the fact that both virus-virus and virus-cell fusion events were required for infection complicates the interpretation of the results. We therefore examined whether Nef affects the ability of receptor-pseudotyped HIV-1 to directly infect target cells expressing HIV-1 Env.
To avoid the downregulation of cell surface CD4 molecules by Nef, we pseudotyped HIV-1 particles with a truncated CD4 molecule (CD4ΔCT) that lacks the 25 C-terminal residues of the cytoplasmic domain. In particular, the CD4ΔCT molecule lacks a dileucine motif that is critical for Nef-induced downregulation (4). To provide a coreceptor for incorporation into HIV-1 particles, we used a FLAG-tagged version of CXCR4 to facilitate its detection in viral particle preparations. To obtain receptor pseudotypes, 293T cells were transfected with Env-deficient proviral constructs that differed only in the presence or absence of an intact nef gene, along with vectors expressing CD4ΔCT and CXCR4-FLAG. To facilitate the quantification of infectious events, a HIV-1-based vector capable of expressing enhanced GFP upon transduction of target cells was also cotransfected. Viral particles released by the transfected cells were normalized for RT activity and used to infect HeLa cells that had been transiently transfected with a plasmid expressing HIV-1 Env. Since it has been shown that the amount of Env expression on the surface of target cells influences the efficiency of entry of receptor pseudotypes (31), we used a C-terminally truncated Env called EnvHXBΔCT (44) in order to maximize the surface levels of Env on the target cells. EnvHXBΔCT lacks the 144 C-terminal amino acids of the cytoplasmic domain of the transmembrane glycoprotein and thus lacks dileucine- and tyrosine-based signals known to promote the internalization of Env (13, 14, 16, 55, 79). For comparison, we also complemented the Env-deficient proviruses with HIV-1 Env expressed in trans and used the resulting recombinant viruses to infect HeLa cells transfected with vectors expressing CD4 and CXCR4.
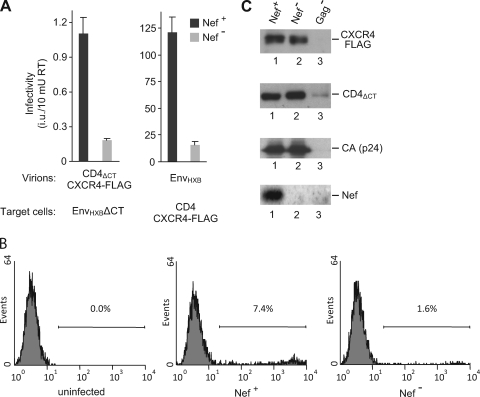
Consistent with a previous report (31), Env-deficient HIV-1 particles pseudotyped with CD4 and CXCR4 were capable of infecting cells expressing HIV-1 Env (Fig. 1A). However, the titers normalized for RT activity were about 100-fold lower than those obtained with particles complemented with HIV-1 Env (Fig. 1A). Pseudotyping with CD4ΔCT alone did not result in detectable levels of infectivity, indicating that the endogenous levels of CXCR4 expressed by 293T cells were not sufficient (data not shown). As expected, infection mediated by the CD4-chemokine receptor pseudotypes depended on Env-receptor interactions, since cells not expressing Env were refractory to infection (data not shown). In repeated experiments, CD4-chemokine receptor pseudotypes encoding Nef were about four-to sixfold more infectious for cells expressing HIV-1 EnvΔCT than pseudotypes that lacked an open nef gene (Fig. 1A and B). This effect of Nef on the receptor pseudotypes was similar in magnitude to the effect of Nef on the single-cycle infectivity of Env-deficient virus complemented with HIV-1 Env (Fig. 1A).
FIG. 1.
Nef enhances the infectivity but not the formation of CD4-chemokine receptor pseudotypes. (A) 293T cells were transfected with HXB/Env−/Nef+ or HXB/Env−/Nef−, along with a HIV-1 vector encoding GFP and either pCD4ΔCT together with pcDNA6/CXCR4-FLAG (left) or pEnvHXB (right). Progeny virions were normalized for RT activity and used to infect HeLa cells that had been transfected with vectors expressing either EnvHXBΔCT (left), or CD4 together with CXCR4-FLAG (right). Infectivities were evaluated by counting GFP-positive foci. Values represent the average from three parallel infections, and error bars correspond to one standard deviation. (B) HeLa cells expressing EnvHXB were infected with Nef+ and Nef− CD4ΔCT/CXCR4 receptor pseudotypes produced as described for panel A, and GFP-positive infected cells were quantified by flow cytometry. The percentage of infected cells in each panel is indicated. (C) Nef does not affect the incorporation of CD4ΔCT or of CXCR4-FLAG into Env-deficient HIV-1 particles. Aliquots of the receptor-pseudotyped viruses used for the infections shown in panel A were pelleted through sucrose and analyzed by Western blotting with anti-FLAG, anti-CD4, anti-HIV-1 CA, and anti-Nef antibodies. A sample derived from cells cotransfected with pCD4ΔCT, pcDNA6/CXCR4-FLAG, and a Gag-deficient HIV-1 provirus was included as a background control.
In a recent study, we obtained no evidence for an effect of Nef on the incorporation of HIV-1 Env into virions (57). However, it has also been reported that Nef can enhance the incorporation of other retroviral Env proteins into HIV-1 by increasing their localization in late endosomes (65). It thus seemed possible that a related mechanism accounted for the effect of Nef on CD4-chemokine receptor pseudotypes. To examine this possibility, we compared the amounts of CD4ΔCT and CXCR4-FLAG that were incorporated into viral particles in the presence and absence of Nef. Viral particles released from transfected 293T cells were partially purified by ultracentrifugation through 20% sucrose, and pelleted material was analyzed by Western blotting. As a control, we also analyzed particulate material released from cells that had been transfected with a HIV-1 provirus unable to express Gag, together with vectors expressing CD4ΔCT and CXCR4-FLAG. As shown in Fig. 1C, CD4ΔCT and CXCR4-FLAG were both incorporated into HIV-1 particles, and Nef had no significant effect on the amounts of particle-associated CD4ΔCT or CXCR4-FLAG. As expected, Nef was detectable in virions produced by the nef-positive provirus (Fig. 1C). We conclude that the increased infectivity of the receptor pseudotypes expressing Nef was not due to an increase in the incorporation of CD4 or CXCR4.
We also used the CD4-chemokine receptor pseudotypes as “target” virions in the virion transcomplementation assay described by Zhou and Aiken (81). In this assay, infectious virus results from the fusion of Env-deficient target virions bearing CD4-chemokine receptors with donor virions that carry Env but contain a defective core (81). To produce defective donor virions, we used the CA1/M4I Gag cleavage site mutant (1), which is equivalent to the CA5 proviral clone used by Zhou and Aiken (81). As previously reported (81), CA1/M4I particles were poorly infectious for target cells expressing CD4. When nef-negative CA1/M4I particles were mixed with Env-deficient CD4-chemokine receptor pseudotypes, we observed a four- to fivefold increase in infectivity for CD4-positive TZM-bl cells, irrespective of whether the receptor pseudotypes were nef positive or nef negative (data not shown). As expected, the Env-deficient receptor pseudotypes by themselves lacked any infectivity for TZM-bl cells. These results are in agreement with the observation by Zhou and Aiken that Nef had no effect in the virion transcomplementation assay when present in the target virus (81). Nevertheless, the present study reveals that Nef can enhance the infectivity of receptor pseudotypes if target cells are infected directly and thus demonstrates that the ability of Nef to enhance infectivity does not depend on the expression of a viral Env protein during virus production.
Roles of dynamin 2 and of the actin cytoskeleton in the effect of Nef on receptor pseudotypes.
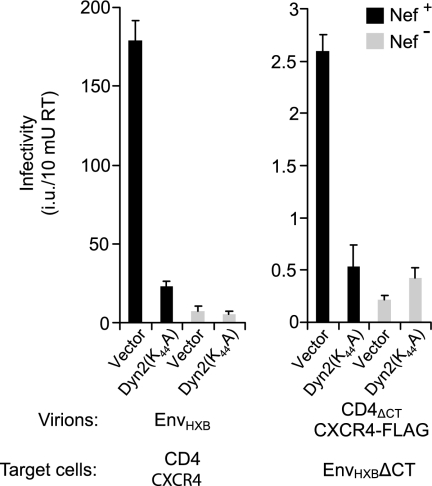
We recently showed that Nef interacts with dynamin 2, a GTPase required for clathrin-mediated endocytosis (57). We also reported that the infectivity enhancement function of HIV-1 Nef depends on dynamin 2 and is inhibited by Dyn2(K44A), a dominant-negative mutant of dynamin 2 that blocks endocytosis (57). We therefore examined whether the effect of Nef on the infectivity of CD4-chemokine receptor pseudotypes for target cells expressing HIV-1 Env is sensitive to Dyn2(K44A). As previously reported (57), Dyn2(K44A) expressed in virus producer cells inhibited the single-cycle infectivity of Env-deficient, nef-positive progeny virions that were complemented with HIV-1 Env in trans (Fig. 2, left). In contrast, Dyn2(K44A) did not reduce the residual infectivity of nef-negative virions complemented with HIV-1 Env, confirming that Dyn2(K44A) selectively inhibits the effect of Nef on HIV-1 infectivity. Similarly, Dyn2(K44A) expressed in producer cells reduced the infectivity of nef-positive but not of nef-negative CD4-chemokine receptor pseudotypes (Fig. 2, right).
FIG. 2.
Dominant-negative Dyn2(K44A) expressed in virus-producing cells selectively inhibits the effect of Nef on the single-round infectivity of Env-deficient virions complemented with HIV-1 Env in trans (left) and of CD4-chemokine receptor pseudotypes (right). Pseudotypes were produced in the presence of a HIV-1 vector encoding GFP and used to infect TZM-bl cells or HeLa cells transiently expressing EnvHXBΔCT, as appropriate. Infectivities were evaluated by counting GFP-positive foci. Error bars indicate standard deviations.
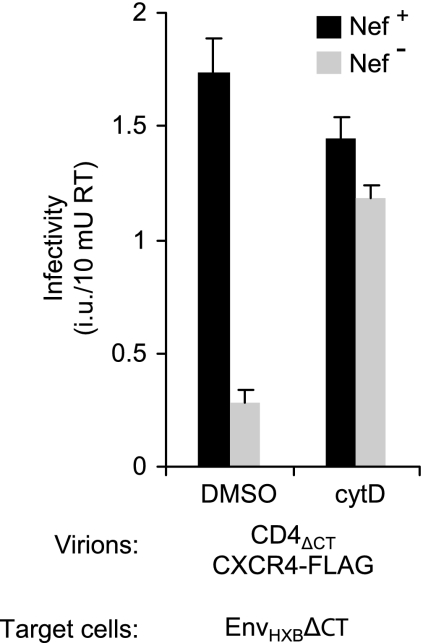
It has been shown that the treatment of target cells with compounds known to disrupt the actin cytoskeleton can relieve the infectivity defect of HIV-1 virions lacking Nef (19). For instance, when single-cycle infections were performed in the presence of cytochalasin D (cytD), the infectivity of a Δnef derivative of HIV-1LAI for a HeLa-derived indicator cell line became comparable to that of the wild-type virus (19). Based on these findings, we determined whether disruption of the actin cytoskeleton in target cells stimulates the infectivity of receptor pseudotypes lacking Nef. As shown in Fig. 3, the infectivity of nef-positive CD4-chemokine receptor pseudotypes for HeLa cells expressing HIV-1 Env was slightly reduced when infections were carried out in the presence of cytD. In contrast, the infectivity of nef-negative CD4-chemokine receptor pseudotypes was enhanced in the presence of cytD and reached levels close to those obtained with the nef-positive receptor pseudotypes under the same conditions. Taken together, these observations indicate that the mechanisms by which Nef enhances the infectivities of authentic HIV-1 virions and of receptor pseudotypes are related.
FIG. 3.
Actin depolymerization in target cells selectively enhances the infectivity of nef-negative CD4-chemokine receptor pseudotypes. HeLa cells transiently expressing EnvHXBΔCT were pretreated for 1 h with 3 μM cytD and then infected in the continued presence of the drug with nef-positive or nef-negative CD4-chemokine receptor pseudotypes produced as described in the legend to Fig. 1. The drug was removed at 18 h postinfection, and infectivities were scored at 48 h postinfection. As a control, identical infections were carried out in the absence of cytD. Error bars indicate standard deviations.
Nef enhances infectivity of HIV-1 pseudotyped with the receptor for an unrelated retrovirus.
A recent study suggests that HIV-1 Nef can reduce the cell surface levels of CXCR4, in addition to its well-documented effect on the surface expression of CD4 (76). While our results demonstrate that Nef did not affect the virion association of overexpressed CXCR4, it remained possible that the effect of Nef on the infectivity of receptor pseudotypes was related to its effect on the trafficking of CXCR4. We therefore wished to determine whether Nef can also enhance the infectivity of HIV-1 after pseudotyping with an unrelated viral receptor. To this end, we examined whether HIV-1 can be pseudotyped with Tva, the receptor for RSV-A. Tva was chosen because it has been shown that murine leukemia virus particles bearing Tva can infect cells expressing the cognate Env protein (7). Tva is a small type I membrane glycoprotein that is unrelated to CD4 or to other retroviral receptors and binds tightly to the RSV-A glycoprotein EnvA (10, 27). Furthermore, in contrast to HIV-1, RSV-A does not require a coreceptor to mediate entry.
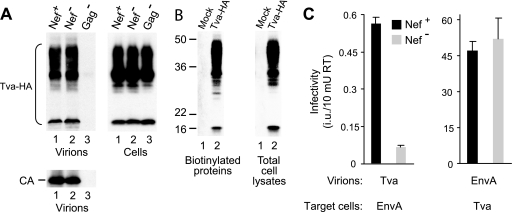
HIV-1(Tva) pseudotypes were produced by cotransfecting 293T cells with Env-deficient HIV-1 proviruses along with plasmid pKZ261, which encodes an HA-tagged transmembrane form of Tva (11, 82). As previously described, pKZ261 produced a heterogeneous array of products as a result of extensive posttranslational modification by N- and O-linked sugars (11, 62). All forms of Tva were incorporated into HIV-1 particles irrespective of the presence or absence of Nef (Fig. 4A, lanes 1 and 2). The appearance of Tva in the particulate fraction was dependent on the coexpression of HIV-1 Gag, confirming that Tva was incorporated into viral particles (Fig. 4A, lane 3). Although a previous study had suggested that only the higher-molecular-weight forms of Tva are present at the cell surface (11), we found that all forms of Tva could be recovered from cell lysates on streptavidin-agarose beads after cell surface biotinylation (Fig. 4B).
FIG. 4.
Nef enhances the infectivity of HIV-1 particles pseudotyped with the receptor for an avian retrovirus. (A) Nef does not affect the incorporation of Tva into Env-deficient HIV-1 particles. 293T cells were transfected with HXB/Env−/Nef+, HXB/Env−/Nef−, or HXB/Gag−, along with a vector expressing HA-tagged Tva. Progeny virions were pelleted and analyzed by Western blotting with anti-HA antibody to detect Tva and with anti-HIV-1 CA antibody to monitor particle production. The expression levels of Tva in the virus producer cells were also examined by Western blotting. (B) All forms of Tva-HA can be detected at the cell surface. Control and Tva-HA-expressing 293T cells were surface biotinylated with the membrane-impermeative reagent sulfo-NHS-LC-biotin, and proteins recovered on streptavidin beads from cell lysates and the unfractionated cell lysates were analyzed by Western blotting with anti-HA. (C) Effect of Nef on the infectivities of HIV-1(Tva) and HIV-1(EnvA) pseudotypes. To produce pseudotyped viral particles, 293T cells were transfected with HXB/Env−/Nef+ or HXB/Env−/Nef−, along with vectors expressing HA-tagged Tva or EnvA as indicated. Supernatants were normalized for RT activity and used to infect TZM-bl indicator cells expressing either EnvA or Tva, as appropriate. Infectious events were quantified by counting blue foci after staining with X-Gal. Error bars indicate standard deviations.
To examine the infectivity of HIV-1(Tva) pseudotypes, a plasmid expressing EnvA was transfected into HeLa-derived TZM-bl indicator cells, which express β-galactosidase upon infection with HIV-1 (77). Additionally, to obtain target cells permissive for HIV-1(EnvA) pseudotypes, TZM-bl cells were transfected with the Tva expression vector pKZ261. nef-positive HIV-1(Tva) pseudotypes were infectious for cells expressing EnvA, although the titers normalized for RT activity were about 80-fold lower than those obtained with HIV-1(EnvA) on cells expressing Tva (Fig. 4C). Interestingly, nef-negative HIV-1(Tva) pseudotypes were nearly 10-fold less infectious for EnvA-expressing cells than nef-positive HIV-1(Tva) pseudotypes (Fig. 4C). This result demonstrated that Nef can enhance the infectivity of HIV-1 particles pseudotyped with a receptor that is unrelated to those normally used by HIV-1.
Entry of HIV-1(Tva) receptor pseudotypes is pH dependent.
As shown in Fig. 4C, Nef had no effect on the infectivity of HIV-1(EnvA) pseudotypes. Since entry mediated by EnvA depends on a critical low-pH step and is inhibited by dominant-negative dynamin (52), this result was consistent with the notion that targeting virus entry to an endocytic pathway circumvents the requirement for Nef (3, 21, 42). However, since Nef clearly enhanced the infectivity of the Tva receptor pseudotypes, it remained possible that in this case entry occurred through a pH-independent route.
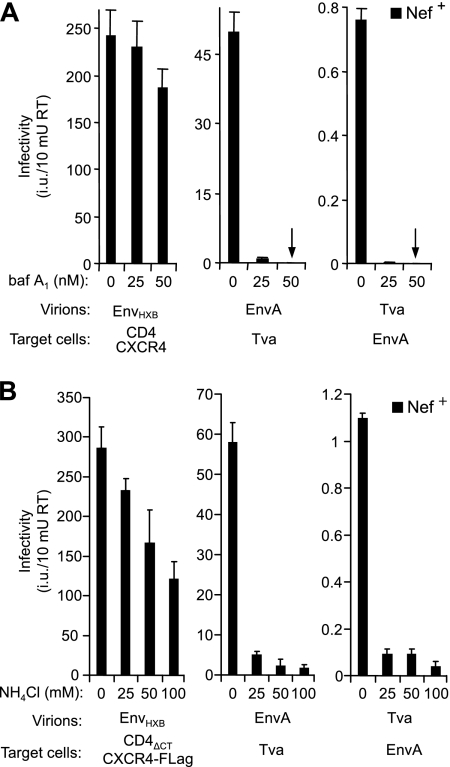
To examine this possibility, infections were carried out in the absence or presence of bafilomycin A1 (baf A1), an inhibitor of the vacuolar H+/ATPase that prevents endosomal acidification and blocks the entry of pseudotypes bearing EnvA (52). As shown in Fig. 5A, baf A1 at 25 or 50 nM nearly blocked the infectivities both of nef-positive HIV-1(EnvA) and of nef-positive HIV-1(Tva) pseudotypes. Similarly, even 25 nM of baf A1 was sufficient to completely prevent the infection of cells expressing EnvA with nef-negative HIV-1(Tva) pseudotypes (data not shown). In contrast, the infectivity of HIV-1 particles complemented with HIV-1 Env was only modestly affected, as expected since HIV-1 entry is thought to occur at the plasma membrane and is pH independent (47, 71).
FIG. 5.
Infection with HIV-1(Tva) pseudotypes is pH dependent. To produce recombinant virions, 293T (A) or Jurkat TAg (B) cells were transfected with HXB/Env−/Nef+, along with vectors expressing HIV-1 Env (pEnvHXB), EnvA (pAB6), or Tva (pKZ261). To obtain indicator cells susceptible to EnvA and Tva pseudotypes, TZM-bl cells were transfected with pKZ261 and pAB6, respectively. Target cells were pretreated with the indicated concentrations of baf A1 (A) or NH4Cl (B), and infections were carried out for 12 h (A) or 6 h (B) in the continued presence of drug. The medium was then replaced, and infectivities were evaluated at 48 h postinfection after staining with X-Gal. Error bars indicate standard deviations.
The pH dependence of virus entry has also been studied using the acidotropic weak base NH4Cl, which neutralizes the pH of acidic intracellular organelles such as endosomes (36, 48). Consistent with previous studies (47, 48, 71), we observed that the titer of HIV-1 particles bearing HIV-1 Env proteins was only moderately reduced if infections were carried out in the presence of 25 mM NH4Cl, and even at 100 mM NH4Cl titers were reduced only slightly more than twofold (Fig. 5B). In contrast, 25 mM NH4Cl was sufficient to largely prevent the infection of susceptible target cells with HIV-1(EnvA) and HIV-1(Tva) pseudotypes (Fig. 5B). Collectively, these results imply that Nef enhances the infectivity of HIV-1(Tva) pseudotypes even though their entry depends on a low-pH step.
Entry of Env-deficient HIV-1 into cells expressing VSV G is unaffected by Nef.
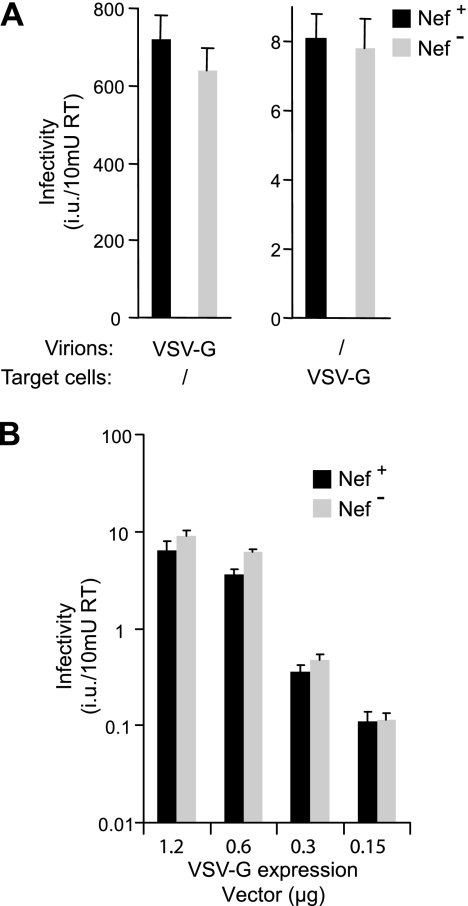
The results described above raised the possibility of a relationship between the differential effects of Nef on HIV-1(Tva) versus HIV-1(EnvA) and the considerably lower baseline infectivity of receptor pseudotypes. To further investigate this possibility, we examined whether Env-deficient HIV-1 particles can infect cells expressing VSV G. Although a specific receptor for VSV has yet to be identified (26), the pantropism of VSV implies that any specific molecules required for entry are widely expressed. If expressed from a self-replicating RNA, VSV G can propagate in an infectious manner in the absence of any other viral protein (61), implying that VSV G-mediated infection is extremely efficient. We therefore speculated that the putative receptor for VSV G may be incorporated into naked HIV-1 particles in sufficient quantities to support detectable levels of entry into cells expressing VSV G. As shown in Fig. 6A, Env-deficient HIV-1 produced in 293T cells was indeed able to infect TZM-bl cells that had been transfected with an expression vector for VSV G. However, the titers obtained with the naked particles were nearly 100-fold lower than those obtained with HIV-1(VSV G). Nevertheless, as in the case of HIV-1(VSV G), Nef had no effect on the infectivity of naked HIV-1 particles measured on VSV G-expressing cells (Fig. 6A). To reduce the VSV G-mediated infectivity even further, we titrated down the amount of VSV G expression vector that was transfected into the target cells. As expected, this led to a corresponding reduction in the number of infectious events obtained with naked HIV-1 particles (Fig. 6B). However, their infectivity remained unaffected by Nef, even at titers that were lower than those obtained with CD4/CXCR4 or Tva receptor pseudotypes. Together, these observations demonstrate that there is no strict correlation between baseline infectivity and the effect of Nef on infectivity.
FIG. 6.
Nef does not enhance the infectivity of naked HIV-1 virions for cells expressing VSV G. (A) Naked virions are capable of infecting target cells expressing VSV G. To obtain viral particles, 293T cells were transfected with HXB/Env−/Nef+ or HXB/Env−/Nef− in the presence or absence of a vector expressing VSV G. Supernatants normalized for RT activity were used to inoculate either untransfected TZM-bl indicator cells or TZM-bl cells transfected with the VSV G expression vector, as indicated. Infectivities were evaluated after staining with X-Gal. (B) Nef does not affect VSV G-mediated entry even if the number of infectious events is lowered by titrating down the amount of VSV G expression vector transfected into the target cells, as indicated. Error bars indicate standard deviations.
DISCUSSION
HIV-1 strains differ significantly in their dependence on Nef for optimal infectivity (43), and in the case of HIV-1SF2 it has been shown that the relatively high Nef dependency of this particular isolate is determined by its env gene (73). A role for Env in the function of Nef is also suggested by the observation that pseudotyping of HIV-1 particles with the glycoproteins of some unrelated viruses largely suppresses the requirement for Nef (3, 21, 42, 65). In particular, Nef was no longer required after pseudotyping with glycoproteins that fuse at low pH. Based on these findings, it has been proposed that the effect of Nef on infectivity depends on whether entry occurs at the cell surface or through endocytosis (3, 21, 42). However, it is noteworthy that a substantial effect of Nef on infectivity has been observed only when retroviral glycoproteins were used to complement HIV-1 particles (21, 42, 65). Thus, it has not been ruled out that the dependency on Nef is a specific property of retroviral Env proteins. Consistent with this possibility, it was recently reported that Nef stimulates the formation of pseudotypes with the glycoproteins of certain retroviruses but not with those of other viruses (65).
In a study by Zhou and Aiken, Nef differentially affected the infectivity resulting from intervirion fusion after mixing donor HIV-1 particles containing defective cores with target virions pseudotyped with the HIV-1 receptors (81). Intriguingly, Nef enhanced infection only when present in the donor virus. This finding supported the hypothesis that Nef affects the function of HIV-1 Env during virus production and therefore does not act on receptor pseudotypes. However, our results now show that Nef can increase the infectivity of receptor pseudotypes for Env-expressing target cells, and they thus rule out the possibility that the presence of a viral glycoprotein during virus production is required for the function of Nef.
While Nef enhanced the infectivity of CD4/CXCR4 and Tva receptor pseudotypes, it had no effect on pseudotype formation, because the incorporation of viral receptors into Env-deficient HIV-1 particles was unaffected. This observation contrasts with a recently described effect of simian immunodeficiency virus Nef on the incorporation of glycoproteins derived from distantly related retroviruses, which appeared to be due to increased colocalization of Gag and Env proteins in late endosomes (65). However, it is perhaps worth noting in this regard that we have not observed any effects of Nef on the incorporation of HIV-1 Env protein into HIV-1 particles (57).
Interestingly, the effect of Nef on CD4-chemokine receptor pseudotypes was inhibited by dominant-negative dynamin 2, which also inhibits the effect of Nef on the infectivity of authentic HIV-1 virions (57). Furthermore, as has been shown for HIV-1 virions lacking Nef (19), the infectivity of nef-negative receptor pseudotypes could be enhanced to levels close to those obtained with nef-positive pseudotypes by disrupting the actin cytoskeleton in target cells. These parallels suggest that Nef enhances the infectivity of authentic virions and of receptor pseudotypes by a similar mechanism.
It has been suggested that Nef enhances viral infectivity by increasing the levels of newly synthesized cholesterol in progeny virions (80). However, we recently observed that dominant-negative dynamin 2 counteracts the effect of Nef on infectivity without decreasing overall virion cholesterol levels (57). More recently, Brugger et al. have analyzed the lipidome of HIV-1 particles produced in T lymphocytes by a highly sensitive and quantitative mass spectrometry approach, and they failed to detect any appreciable impact of Nef on the overall cholesterol content of HIV-1 virions (17). Thus, although Nef affects cholesterol homeostasis (53, 75), the study by Brugger et al. clearly demonstrates that Nef can enhance virion infectivity without affecting overall virion cholesterol levels.
Nef did not enhance the infectivity of HIV-1 particles pseudotyped with EnvA, a retroviral glycoprotein that requires exposure to low pH to elicit full fusion and to mediate viral penetration into the cytosol (9, 52). While this result appeared consistent with the model that entry through an endosomal compartment circumvents the requirement for Nef, we also observed that Nef did significantly enhance viral infectivity if the orientation of the EnvA-Tva interaction was reversed. Previous work has suggested that Tva pseudotypes are duplicating the events of normal viral entry, because the functional requirements for Tva and EnvA are the same whether they are on the virion or on the target cell surface (7). Furthermore, our results show that the infectivity of HIV-1(Tva) pseudotypes for cells expressing EnvA is profoundly inhibited by baf A1 and NH4Cl, implying that Tva pseudotypes maintain the requirement for exposure to low pH in an acidic intracellular compartment. We thus infer that an entry pathway that involves fusion at the plasma membrane is not an absolute requirement for the enhancement of infectivity by Nef.
Why did the effect of Nef on infectivity differ depending on the direction of the EnvA-Tva interaction? It is possible that this difference is related to the markedly different baseline infectivities of the EnvA and Tva pseudotypes. Consistent with this notion, the magnitude of the effect of Nef on HIV-1 infectivity appears to correlate inversely with the baseline infectivity observed in the absence of Nef (73). Also, the requirement for Nef for optimal HIV-1 infectivity can be reduced by increasing the amount of Env on the virion or the receptor density on the target cells (73). Thus, Nef may become more important if the number or affinity of functional Env-receptor interactions is relatively low, which we assume tends to be the case if the normal direction of the interaction is reversed. However, the effect of Nef is not strictly linked to baseline infectivity, because Nef did not enhance the relatively low infectivity of naked HIV-1 virions for cells expressing VSV G.
It has been demonstrated that Nef does not affect the transfer of a β-lactamase-Vpr protein chimera from virions into target cells as a result of Env-mediated fusion (20, 72). However, this observation does not rule out that Nef facilitates an entry step subsequent to the formation of an initial fusion pore, such as fusion pore expansion, which is necessary to deliver the relatively bulky viral core into the cytosol. Indeed, a study which monitored individual retroviral fusion events by fluorescence microscopy indicates that pore expansion to allow the cytosolic delivery of viral cores occurs only slowly after Env-induced lipid mixing (49). Also, it is thought that fusion pore expansion is the most energy-demanding fusion stage that requires the participation of the largest number of activated fusion proteins (23). As has been pointed out previously (20), a role of Nef in facilitating this apparently rate-limiting step would be consistent with the finding that Nef enhances the cytoplasmic delivery of HIV-1 cores (67) and could also explain the dependence of the effect of Nef on the Env-receptor interaction. However, by showing that receptor pseudotypes remain responsive to Nef, the present study argues against a simple model in which Nef would act directly on Env.
Acknowledgments
We thank Walther Mothes and John A. T. Young for providing pKZ261 and pAB6; the Centre for AIDS Reagents (CFAR), NIBSC, United Kingdom, for providing antibodies; and Myra O. McClure for suggestions and support to M.P. TZM-bl was obtained from John C. Kappes, Xiaoyun Wu, and Tranzyme Inc. through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH.
This work was supported by grants AI029873 and AI077412 from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health.
Footnotes
Published ahead of print on 20 August 2008.
REFERENCES
- 1.Accola, M. A., S. Hoglund, and H. G. Gottlinger. 1998. A putative alpha-helical structure which overlaps the capsid-p2 boundary in the human immunodeficiency virus type 1 Gag precursor is crucial for viral particle assembly. J. Virol. 722072-2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Accola, M. A., B. Strack, and H. G. Gottlinger. 2000. Efficient particle production by minimal Gag constructs which retain the carboxy-terminal domain of human immunodeficiency virus type 1 capsid-p2 and a late assembly domain. J. Virol. 745395-5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aiken, C. 1997. Pseudotyping human immunodeficiency virus type 1 (HIV-1) by the glycoprotein of vesicular stomatitis virus targets HIV-1 entry to an endocytic pathway and suppresses both the requirement for Nef and the sensitivity to cyclosporin A. J. Virol. 715871-5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aiken, C., J. Konner, N. R. Landau, M. E. Lenburg, and D. Trono. 1994. Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell 76853-864. [DOI] [PubMed] [Google Scholar]
- 5.Aiken, C., and D. Trono. 1995. Nef stimulates human immunodeficiency virus type 1 proviral DNA synthesis. J. Virol. 695048-5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arnold, B. A., R. W. Hepler, and P. M. Keller. 1998. One-step fluorescent probe product-enhanced reverse transcriptase assay. BioTechniques 2598-106. [DOI] [PubMed] [Google Scholar]
- 7.Balliet, J. W., and P. Bates. 1998. Efficient infection mediated by viral receptors incorporated into retroviral particles. J. Virol. 72671-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bandres, J. C., A. S. Shaw, and L. Ratner. 1995. HIV-1 Nef protein downregulation of CD4 surface expression: relevance of the lck binding domain of CD4. Virology 207338-341. [DOI] [PubMed] [Google Scholar]
- 9.Barnard, R. J., S. Narayan, G. Dornadula, M. D. Miller, and J. A. Young. 2004. Low pH is required for avian sarcoma and leukosis virus Env-dependent viral penetration into the cytosol and not for viral uncoating. J. Virol. 7810433-10441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bates, P., J. A. Young, and H. E. Varmus. 1993. A receptor for subgroup A Rous sarcoma virus is related to the low density lipoprotein receptor. Cell 741043-1051. [DOI] [PubMed] [Google Scholar]
- 11.Belanger, C., K. Zingler, and J. A. Young. 1995. Importance of cysteines in the LDLR-related domain of the subgroup A avian leukosis and sarcoma virus receptor for viral entry. J. Virol. 691019-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benson, R. E., A. Sanfridson, J. S. Ottinger, C. Doyle, and B. R. Cullen. 1993. Downregulation of cell-surface CD4 expression by simian immunodeficiency virus Nef prevents viral super infection. J. Exp. Med. 1771561-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berlioz-Torrent, C., B. L. Shacklett, L. Erdtmann, L. Delamarre, I. Bouchaert, P. Sonigo, M. C. Dokhelar, and R. Benarous. 1999. Interactions of the cytoplasmic domains of human and simian retroviral transmembrane proteins with components of the clathrin adaptor complexes modulate intracellular and cell surface expression of envelope glycoproteins. J. Virol. 731350-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blot, G., K. Janvier, S. Le Panse, R. Benarous, and C. Berlioz-Torrent. 2003. Targeting of the human immunodeficiency virus type 1 envelope to the trans-Golgi network through binding to TIP47 is required for Env incorporation into virions and infectivity. J. Virol. 776931-6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boerger, A. L., S. Snitkovsky, and J. A. Young. 1999. Retroviral vectors preloaded with a viral receptor-ligand bridge protein are targeted to specific cell types. Proc. Natl. Acad. Sci. USA 969867-9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boge, M., S. Wyss, J. S. Bonifacino, and M. Thali. 1998. A membrane-proximal tyrosine-based signal mediates internalization of the HIV-1 envelope glycoprotein via interaction with the AP-2 clathrin adaptor. J. Biol. Chem. 27315773-15778. [DOI] [PubMed] [Google Scholar]
- 17.Brugger, B., E. Krautkramer, N. Tibroni, C. E. Munte, S. Rauch, I. Leibrecht, B. Glass, S. Breuer, M. Geyer, H. G. Krausslich, H. R. Kalbitzer, F. T. Wieland, and O. T. Fackler. 2007. Human immunodeficiency virus type 1 Nef protein modulates the lipid composition of virions and host cell membrane microdomains. Retrovirology 470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bukovsky, A. A., T. Dorfman, A. Weimann, and H. G. Gottlinger. 1997. Nef association with human immunodeficiency virus type 1 virions and cleavage by the viral protease. J. Virol. 711013-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campbell, E. M., R. Nunez, and T. J. Hope. 2004. Disruption of the actin cytoskeleton can complement the ability of Nef to enhance human immunodeficiency virus type 1 infectivity. J. Virol. 785745-5755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cavrois, M., J. Neidleman, W. Yonemoto, D. Fenard, and W. C. Greene. 2004. HIV-1 virion fusion assay: uncoating not required and no effect of Nef on fusion. Virology 32836-44. [DOI] [PubMed] [Google Scholar]
- 21.Chazal, N., G. Singer, C. Aiken, M. L. Hammarskjold, and D. Rekosh. 2001. Human immunodeficiency virus type 1 particles pseudotyped with envelope proteins that fuse at low pH no longer require Nef for optimal infectivity. J. Virol. 754014-4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen, Y. L., D. Trono, and D. Camaur. 1998. The proteolytic cleavage of human immunodeficiency virus type 1 Nef does not correlate with its ability to stimulate virion infectivity. J. Virol. 723178-3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chernomordik, L. V., J. Zimmerberg, and M. M. Kozlov. 2006. Membranes of the world unite! J. Cell Biol. 175201-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chowers, M. Y., M. W. Pandori, C. A. Spina, D. D. Richman, and J. C. Guatelli. 1995. The growth advantage conferred by HIV-1 nef is determined at the level of viral DNA formation and is independent of CD4 downregulation. Virology 212451-457. [DOI] [PubMed] [Google Scholar]
- 25.Chowers, M. Y., C. A. Spina, T. J. Kwoh, N. J. Fitch, D. D. Richman, and J. C. Guatelli. 1994. Optimal infectivity in vitro of human immunodeficiency virus type 1 requires an intact nef gene. J. Virol. 682906-2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coil, D. A., and A. D. Miller. 2004. Phosphatidylserine is not the cell surface receptor for vesicular stomatitis virus. J. Virol. 7810920-10926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Connolly, L., K. Zingler, and J. A. Young. 1994. A soluble form of a receptor for subgroup A avian leukosis and sarcoma viruses (ALSV-A) blocks infection and binds directly to ALSV-A. J. Virol. 682760-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deacon, N. J., A. Tsykin, A. Solomon, K. Smith, M. Ludford-Menting, D. J. Hooker, D. A. McPhee, A. L. Greenway, A. Ellett, C. Chatfield, et al. 1995. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science 270988-991. [DOI] [PubMed] [Google Scholar]
- 29.Dorfman, T., F. Mammano, W. A. Haseltine, and H. G. Gottlinger. 1994. Role of the matrix protein in the virion association of the human immunodeficiency virus type 1 envelope glycoprotein. J. Virol. 681689-1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dorfman, T., E. Popova, M. Pizzato, and H. G. Gottlinger. 2002. Nef enhances human immunodeficiency virus type 1 infectivity in the absence of matrix. J. Virol. 766857-6862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Endres, M. J., S. Jaffer, B. Haggarty, J. D. Turner, B. J. Doranz, P. J. O'Brien, D. L. Kolson, and J. A. Hoxie. 1997. Targeting of HIV- and SIV-infected cells by CD4-chemokine receptor pseudotypes. Science 2781462-1464. [DOI] [PubMed] [Google Scholar]
- 32.Forshey, B. M., and C. Aiken. 2003. Disassembly of human immunodeficiency virus type 1 cores in vitro reveals association of Nef with the subviral ribonucleoprotein complex. J. Virol. 774409-4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Foster, J. L., and J. V. Garcia. 2007. Role of Nef in HIV-1 replication and pathogenesis. Adv. Pharmacol. 55389-409. [DOI] [PubMed] [Google Scholar]
- 34.Garcia, J. V., J. Alfano, and A. D. Miller. 1993. The negative effect of human immunodeficiency virus type 1 Nef on cell surface CD4 expression is not species specific and requires the cytoplasmic domain of CD4. J. Virol. 671511-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garcia, J. V., and A. D. Miller. 1991. Serine phosphorylation-independent downregulation of cell-surface CD4 by nef. Nature 350508-511. [DOI] [PubMed] [Google Scholar]
- 36.Helenius, A., M. Marsh, and J. White. 1982. Inhibition of Semliki forest virus penetration by lysosomotropic weak bases. J. Gen. Virol. 5847-61. [DOI] [PubMed] [Google Scholar]
- 37.Hua, J., and B. R. Cullen. 1997. Human immunodeficiency virus types 1 and 2 and simian immunodeficiency virus Nef use distinct but overlapping target sites for downregulation of cell surface CD4. J. Virol. 716742-6748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kestler, H. W., III, D. J. Ringler, K. Mori, D. L. Panicali, P. K. Sehgal, M. D. Daniel, and R. C. Desrosiers. 1991. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell 65651-662. [DOI] [PubMed] [Google Scholar]
- 39.Kirchhoff, F., T. C. Greenough, D. B. Brettler, J. L. Sullivan, and R. C. Desrosiers. 1995. Absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N. Engl. J. Med. 332228-232. [DOI] [PubMed] [Google Scholar]
- 40.Lama, J., A. Mangasarian, and D. Trono. 1999. Cell-surface expression of CD4 reduces HIV-1 infectivity by blocking Env incorporation in a Nef- and Vpu-inhibitable manner. Curr. Biol. 9622-631. [DOI] [PubMed] [Google Scholar]
- 41.Lundquist, C. A., J. Zhou, and C. Aiken. 2004. Nef stimulates human immunodeficiency virus type 1 replication in primary T cells by enhancing virion-associated gp120 levels: coreceptor-dependent requirement for Nef in viral replication. J. Virol. 786287-6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luo, T., J. L. Douglas, R. L. Livingston, and J. V. Garcia. 1998. Infectivity enhancement by HIV-1 Nef is dependent on the pathway of virus entry: implications for HIV-based gene transfer systems. Virology 241224-233. [DOI] [PubMed] [Google Scholar]
- 43.Luo, T., and J. V. Garcia. 1996. The association of Nef with a cellular serine/threonine kinase and its enhancement of infectivity are viral isolate dependent. J. Virol. 706493-6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mammano, F., E. Kondo, J. Sodroski, A. Bukovsky, and H. G. Gottlinger. 1995. Rescue of human immunodeficiency virus type 1 matrix protein mutants by envelope glycoproteins with short cytoplasmic domains. J. Virol. 693824-3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mariani, R., F. Kirchhoff, T. C. Greenough, J. L. Sullivan, R. C. Desrosiers, and J. Skowronski. 1996. High frequency of defective nef alleles in a long-term survivor with nonprogressive human immunodeficiency virus type 1 infection. J. Virol. 707752-7764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mariani, R., and J. Skowronski. 1993. CD4 down-regulation by nef alleles isolated from human immunodeficiency virus type 1-infected individuals. Proc. Natl. Acad. Sci. USA 905549-5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McClure, M. O., M. Marsh, and R. A. Weiss. 1988. Human immunodeficiency virus infection of CD4-bearing cells occurs by a pH-independent mechanism. EMBO J. 7513-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McClure, M. O., M. A. Sommerfelt, M. Marsh, and R. A. Weiss. 1990. The pH independence of mammalian retrovirus infection. J. Gen. Virol. 71767-773. [DOI] [PubMed] [Google Scholar]
- 49.Melikyan, G. B., R. J. Barnard, L. G. Abrahamyan, W. Mothes, and J. A. Young. 2005. Imaging individual retroviral fusion events: from hemifusion to pore formation and growth. Proc. Natl. Acad. Sci. USA 1028728-8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miller, M. D., M. T. Warmerdam, I. Gaston, W. C. Greene, and M. B. Feinberg. 1994. The human immunodeficiency virus-1 nef gene product: a positive factor for viral infection and replication in primary lymphocytes and macrophages. J. Exp. Med. 179101-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miller, M. D., M. T. Warmerdam, K. A. Page, M. B. Feinberg, and W. C. Greene. 1995. Expression of the human immunodeficiency virus type 1 (HIV-1) nef gene during HIV-1 production increases progeny particle infectivity independently of gp160 or viral entry. J. Virol. 69579-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mothes, W., A. L. Boerger, S. Narayan, J. M. Cunningham, and J. A. Young. 2000. Retroviral entry mediated by receptor priming and low pH triggering of an envelope glycoprotein. Cell 103679-689. [DOI] [PubMed] [Google Scholar]
- 53.Mujawar, Z., H. Rose, M. P. Morrow, T. Pushkarsky, L. Dubrovsky, N. Mukhamedova, Y. Fu, A. Dart, J. M. Orenstein, Y. V. Bobryshev, M. Bukrinsky, and D. Sviridov. 2006. Human immunodeficiency virus impairs reverse cholesterol transport from macrophages. PLoS Biol. 4e365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Naldini, L., U. Blomer, P. Gallay, D. Ory, R. Mulligan, F. H. Gage, I. M. Verma, and D. Trono. 1996. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 272263-267. [DOI] [PubMed] [Google Scholar]
- 55.Ohno, H., R. C. Aguilar, M. C. Fournier, S. Hennecke, P. Cosson, and J. S. Bonifacino. 1997. Interaction of endocytic signals from the HIV-1 envelope glycoprotein complex with members of the adaptor medium chain family. Virology 238305-315. [DOI] [PubMed] [Google Scholar]
- 56.Pandori, M. W., N. J. Fitch, H. M. Craig, D. D. Richman, C. A. Spina, and J. C. Guatelli. 1996. Producer-cell modification of human immunodeficiency virus type 1: Nef is a virion protein. J. Virol. 704283-4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pizzato, M., A. Helander, E. Popova, A. Calistri, A. Zamborlini, G. Palu, and H. G. Gottlinger. 2007. Dynamin 2 is required for the enhancement of HIV-1 infectivity by Nef. Proc. Natl. Acad. Sci. USA 1046812-6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reil, H., A. A. Bukovsky, H. R. Gelderblom, and H. G. Gottlinger. 1998. Efficient HIV-1 replication can occur in the absence of the viral matrix protein. EMBO J. 172699-2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rhee, S. S., and J. W. Marsh. 1994. Human immunodeficiency virus type 1 Nef-induced down-modulation of CD4 is due to rapid internalization and degradation of surface CD4. J. Virol. 685156-5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roeth, J. F., and K. L. Collins. 2006. Human immunodeficiency virus type 1 Nef: adapting to intracellular trafficking pathways. Microbiol. Mol. Biol. Rev. 70548-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rolls, M. M., P. Webster, N. H. Balba, and J. K. Rose. 1994. Novel infectious particles generated by expression of the vesicular stomatitis virus glycoprotein from a self-replicating RNA. Cell 79497-506. [DOI] [PubMed] [Google Scholar]
- 62.Rong, L., and P. Bates. 1995. Analysis of the subgroup A avian sarcoma and leukosis virus receptor: the 40-residue, cysteine-rich, low-density lipoprotein receptor repeat motif of Tva is sufficient to mediate viral entry. J. Virol. 694847-4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ross, T. M., A. E. Oran, and B. R. Cullen. 1999. Inhibition of HIV-1 progeny virion release by cell-surface CD4 is relieved by expression of the viral Nef protein. Curr. Biol. 9613-621. [DOI] [PubMed] [Google Scholar]
- 64.Salghetti, S., R. Mariani, and J. Skowronski. 1995. Human immunodeficiency virus type 1 Nef and p56lck protein-tyrosine kinase interact with a common element in CD4 cytoplasmic tail. Proc. Natl. Acad. Sci. USA 92349-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sandrin, V., and F. L. Cosset. 2006. Intracellular versus cell surface assembly of retroviral pseudotypes is determined by the cellular localization of the viral glycoprotein, its capacity to interact with Gag, and the expression of the Nef protein. J. Biol. Chem. 281528-542. [DOI] [PubMed] [Google Scholar]
- 66.Sanfridson, A., B. R. Cullen, and C. Doyle. 1994. The simian immunodeficiency virus Nef protein promotes degradation of CD4 in human T cells. J. Biol. Chem. 2693917-3920. [PubMed] [Google Scholar]
- 67.Schaeffer, E., R. Geleziunas, and W. C. Greene. 2001. Human immunodeficiency virus type 1 Nef functions at the level of virus entry by enhancing cytoplasmic delivery of virions. J. Virol. 752993-3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schwartz, O., V. Marechal, O. Danos, and J. M. Heard. 1995. Human immunodeficiency virus type 1 Nef increases the efficiency of reverse transcription in the infected cell. J. Virol. 694053-4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Silver, J., T. Maudru, K. Fujita, and R. Repaske. 1993. An RT-PCR assay for the enzyme activity of reverse transcriptase capable of detecting single virions. Nucleic Acids Res. 213593-3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Spina, C. A., T. J. Kwoh, M. Y. Chowers, J. C. Guatelli, and D. D. Richman. 1994. The importance of nef in the induction of human immunodeficiency virus type 1 replication from primary quiescent CD4 lymphocytes. J. Exp. Med. 179115-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stein, B. S., S. D. Gowda, J. D. Lifson, R. C. Penhallow, K. G. Bensch, and E. G. Engleman. 1987. pH-independent HIV entry into CD4-positive T cells via virus envelope fusion to the plasma membrane. Cell 49659-668. [DOI] [PubMed] [Google Scholar]
- 72.Tobiume, M., J. E. Lineberger, C. A. Lundquist, M. D. Miller, and C. Aiken. 2003. Nef does not affect the efficiency of human immunodeficiency virus type 1 fusion with target cells. J. Virol. 7710645-10650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tobiume, M., K. Tokunaga, E. Kiyokawa, M. Takahoko, N. Mochizuki, M. Tatsumi, and M. Matsuda. 2001. Requirement of nef for HIV-1 infectivity is biased by the expression levels of Env in the virus-producing cells and CD4 in the target cells. Arch. Virol. 1461739-1751. [DOI] [PubMed] [Google Scholar]
- 74.Van Maele, B., J. De Rijck, E. De Clercq, and Z. Debyser. 2003. Impact of the central polypurine tract on the kinetics of human immunodeficiency virus type 1 vector transduction. J. Virol. 774685-4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van 't Wout, A. B., J. V. Swain, M. Schindler, U. Rao, M. S. Pathmajeyan, J. I. Mullins, and F. Kirchhoff. 2005. Nef induces multiple genes involved in cholesterol synthesis and uptake in human immunodeficiency virus type 1-infected T cells. J. Virol. 7910053-10058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Venzke, S., N. Michel, I. Allespach, O. T. Fackler, and O. T. Keppler. 2006. Expression of Nef downregulates CXCR4, the major coreceptor of human immunodeficiency virus, from the surfaces of target cells and thereby enhances resistance to superinfection. J. Virol. 8011141-11152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wei, X., J. M. Decker, H. Liu, Z. Zhang, R. B. Arani, J. M. Kilby, M. S. Saag, X. Wu, G. M. Shaw, and J. C. Kappes. 2002. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob. Agents Chemother. 461896-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Welker, R., H. Kottler, H. R. Kalbitzer, and H. G. Krausslich. 1996. Human immunodeficiency virus type 1 Nef protein is incorporated into virus particles and specifically cleaved by the viral proteinase. Virology 219228-236. [DOI] [PubMed] [Google Scholar]
- 79.Wyss, S., C. Berlioz-Torrent, M. Boge, G. Blot, S. Honing, R. Benarous, and M. Thali. 2001. The highly conserved C-terminal dileucine motif in the cytosolic domain of the human immunodeficiency virus type 1 envelope glycoprotein is critical for its association with the AP-1 clathrin adapter. J. Virol. 752982-2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zheng, Y. H., A. Plemenitas, C. J. Fielding, and B. M. Peterlin. 2003. Nef increases the synthesis of and transports cholesterol to lipid rafts and HIV-1 progeny virions. Proc. Natl. Acad. Sci. USA 1008460-8465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhou, J., and C. Aiken. 2001. Nef enhances human immunodeficiency virus type 1 infectivity resulting from intervirion fusion: evidence supporting a role for Nef at the virion envelope. J. Virol. 755851-5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zingler, K., C. Belanger, R. Peters, E. Agard, and J. A. Young. 1995. Identification and characterization of the viral interaction determinant of the subgroup A avian leukosis virus receptor. J. Virol. 694261-4266. [DOI] [PMC free article] [PubMed] [Google Scholar]