Abstract
The promotion of membrane fusion by Newcastle disease virus (NDV) requires an interaction between the viral hemagglutinin-neuraminidase (HN) and fusion (F) proteins, although the mechanism by which this interaction regulates fusion is not clear. The NDV HN protein exists as a tetramer composed of a pair of dimers. Based on X-ray crystallographic studies of the NDV HN globular domain (S. Crennell et al., Nat. Struct. Biol. 7:1068-1074, 2000), it was proposed that the protein undergoes a significant conformational change from an initial structure having minimal intermonomeric contacts to a structure with a much more extensive dimer interface. This conformational change was predicted to be integral to fusion promotion with the minimal interface form required to maintain F in its prefusion state until HN binds receptors. However, no evidence for such a conformational change exists for any other paramyxovirus attachment protein. To test the NDV model, we have engineered a pair of intermonomeric disulfide bonds across the dimer interface in the globular domain of an otherwise non-disulfide-linked NDV HN protein by the introduction of cysteine substitutions for residues T216 and D230. The disulfide-linked dimer is formed both intracellularly and in the absence of receptor binding and is efficiently expressed at the cell surface. The disulfide bonds preclude formation of the minimal interface form of the protein and yet enhance both receptor-binding activity at 37°C and fusion promotion. These results confirm that neither the minimal interface form of HN nor the proposed drastic conformational change in the protein is required for fusion.
The Paramyxoviridae are a family of enveloped, negative-strand RNA viruses, which includes several important pathogens, such as measles virus, mumps virus, Sendai virus, respiratory syncytial virus, the various parainfluenza viruses, and Newcastle disease virus (NDV) (26). Although predominantly recognized as an avian pathogen, NDV has recently gained added importance for its ability to selectively kill tumor cells and its use as an oncolytic agent (12, 25, 45), as well as its potential as a vaccine vector (11, 14, 16, 29, 37).
The surfaces of paramyxovirions and infected cells possess two types of spikes, composed of the attachment and fusion proteins. For paramyxoviruses that recognize sialic acid-containing receptors, the hemagglutinin-neuraminidase (HN) protein mediates receptor binding and also possesses sialidase or neuraminidase (NA) activity, the ability to cleave sialic acid (39). The fusion (F) protein mediates virus-cell and cell-cell fusion for all paramyxoviruses (2), following the proteolytic generation of a “fusion peptide” (39).
For most paramyxoviruses, including NDV, the F protein is incapable of promoting fusion by itself (reviewed in reference 26). It requires a virus-specific contribution from the homologous attachment protein, which is mediated by a direct interaction between the two protein spikes. A complex between NDV HN and F can be detected at the surface of both infected and transfected cells (9, 28, 30-32). By the analysis of chimeric HN proteins, it has been shown for several viruses in the family, including NDV, that the stalk region of HN determines its specificity for the homologous F protein (7, 10, 42, 44, 46). Moreover, we have shown that the introduction of N-linked glycans and point mutations in the HN stalk severely impairs, or completely eliminates, fusion and that this correlates with a proportionate decrease in the extent of HN-F complex formation at the cell surface (31, 32).
Like other paramyxovirus HN proteins (3, 36, 43), NDV HN is a type II membrane glycoprotein that exists on the virion and infected-cell surface as a tetramer comprised of a pair of dimers (34). The ectodomain consists of a long stalk supporting a terminal globular domain, in which reside the attachment, NA and antigenic sites (17-19, 23, 34, 43).
The X-ray crystal structures of the ligand-bound and unliganded dimer of the globular domain of the NDV HN protein have been determined (6). Each monomer has a β-sheet propeller motif with an NA active site at its center. Based on these structures, it was postulated that the NA site mediates both attachment and NA activity via a quite drastic conformational change from a structure having a minimal dimer interface to one with a much more extended interface (6). Further, it was postulated that this drastic conformational change is integral to HN′s role in the fusion process. Hydrophobic residues exposed in the minimal interface form of the protein were proposed to interact with complementary residues in the F protein, thus maintaining the latter in its prefusion conformation. Upon receptor binding, the protein was proposed to switch to a markedly different structure, in the process sequestering the purported F-interactive hydrophobic residues in a much more extensive interface and releasing F to assume its fusion-active form (6). This was supported by the claim that mutations of some of the hydrophobic residues abolished fusion with no effect on attachment (41). Subsequently, a second sialic acid binding site was identified (48); it is positioned at the membrane-distal end of the dimer interface, is composed of residues from both monomers, and lacks NA activity. This second site was proposed to maintain the interaction of HN with receptors as fusion proceeds (1).
However, this mechanism for fusion promotion is inconsistent with the evidence cited above indicating that it is the stalk region of NDV HN that determines its F specificity and may directly mediate the interaction with F. The model was also subsequently called into question by our demonstration that at least some of the interface mutations that decrease fusion do significantly affect attachment at 37°C, the temperature at which fusion is assayed (5). Finally, the mechanism proposed for the role of the NDV HN protein in fusion is not supported by studies of two other paramyxoviruses. The structures of the HN from human parainfluenza virus 3 (hPIV3) both unliganded and with several ligands were determined at pH 7.5 (27). Although the structure is similar to that of NDV HN, there is a single flexible site that mediates both receptor binding and NA by a structural change that is limited to the active site. Similarly, the structure of the parainfluenza virus 5 (PIV5) HN protein also identifies a single site with only a single flexible tyrosine residue (47). There is no crystallographic evidence in hPIV3 or PIV5 HN for either a second site or a conformational change upon ligand binding that is anything like that predicted for NDV HN.
To address the issue of the requirement for the minimal interface form of HN for fusion, we took the approach of precluding its formation by locking the protein into the extensive interface form via the introduction of a pair of cysteine mutations that form two disulfide bonds across the dimer interface. As proof of principle, we first produced and characterized a form of hPIV3 HN with monomers in each dimer linked by a single intermonomeric disulfide bond and showed that it retains fusion-promoting activity. An NDV HN protein carrying T216C and D230C mutations also forms disulfide-linked homodimers, which are formed both intracellularly and in the absence of receptor binding and are efficiently expressed at the cell surface. Most importantly, the mutated protein promotes fusion more efficiently than the parental wild-type (wt) protein. With the caveat that the disulfide-linked form of the protein could be promoting fusion via a radically different mechanism than the parent protein, these findings question both the minimal interface structure for NDV HN and its role in fusion.
MATERIALS AND METHODS
Cells.
BHK-21F cells (provided by Rebecca Dutch) were maintained in Dulbecco modified Eagle medium with high glucose, supplemented with 5% fetal calf serum, 20 mM l-glutamine, 4 U of penicillin/ml, and 4 μg of streptomycin/ml. All tissue culture reagents were obtained from Invitrogen (Carlsbad, CA).
Recombinant plasmids and site-directed mutagenesis.
The preparation of the HN gene from the L-Kansas (L) strain of NDV in the pBluescript SK(+) (Stratagene Cloning Systems, La Jolla, CA) was described previously (28), as were the preparations of the HN and F genes from the Australia-Victoria (AV) strain of NDV (8, 33) and the HN and F genes of hPIV3 (10).
Site-directed mutagenesis was performed as described previously (4). Briefly, single-stranded DNA template was rescued by R408 helper phage (Stratagene) in CJ236 cells. Mutagenesis primers (Integrated DNA Technologies, Coralville, IA) were annealed to the template and extended with T4 DNA polymerase, and the ends were ligated with T4 DNA ligase. The mutagenesis reactions were transformed into DH5α cells that were then selected for ampicillin resistance. Identification of colonies carrying mutated genes was facilitated by screening for the presence of a unique restriction site introduced by each mutagenic primer. Multiple clones were characterized for each mutated DNA, and the presence of the desired mutation(s) was confirmed by DNA sequencing.
Transient-expression system.
Wt and mutated HN proteins were expressed using the vaccinia virus T7 RNA polymerase expression system (13), as described previously using 1 μg of each plasmid (5). All experiments were performed in six-well plates seeded a day earlier at 4 × 105 cells per well.
Functional assays.
Cell surface expression was quantitated by flow cytometric analysis using a mixture of monoclonal antibodies (MAbs) for NDV HN, including HN1b, HN2a, HN3c, HN4a, HN14f, and HN23a (17, 18, 23, 24), and a MAb specific for hPIV3 HN (American Type Culture Collection, Manassas, VA). Hemadsorption (HAd) activity was determined by the abilities of the expressed HN proteins to adsorb guinea pig erythrocytes (Bio-Link, Inc., Liverpool, NY) and quantitated as described previously (31). The NA activity of cell surface HN was determined colorimetrically using sialyllactose (Sigma Chemical Co., St. Louis, MO) as a substrate. For NDV HN, cell monolayers were incubated with substrate for 20 min in 0.1 M sodium acetate (pH 6) as described previously (31). To determine the NA activity of hPIV3 HN, the assay was modified such that cell monolayers were incubated for 1 h at pH 4.8. For fusion pictures, transfected monolayers were fixed with methanol and stained with Giemsa stain (Sigma). The abilities of the mutated HN proteins to complement the homologous F protein in the promotion of fusion were quantitated by using a content-mixing assay, which measures β-galactosidase activity in target cells after fusion induced by HN-F-expressing effector cells (31).
Immunoprecipitation.
The immunoprecipitation protocol has been described previously (32). Briefly, at 20 to 22 h posttransfection, BHK cells were starved for 1 h at 37°C in medium lacking cysteine and methionine. The cells were labeled with 1 ml of medium containing 100 μCi of Expre35S[35S] cysteine-methionine labeling mix (Perkin-Elmer, Boston, MA) and chased for 90 min with growth medium. The cells were lysed in lysis buffer (phosphate-buffered saline containing 1% Triton X-100, 0.5% deoxycholate, 30 mM N-ethylmaleimide, and 1 mM phenylmethylsulfonylfluoride), and the NDV HN proteins were immunoprecipitated either with a cocktail of MAbs or with individual MAbs. Immunoprecipitation of hPIV3 HN was performed according to the same protocol except using a polyclonal guinea pig antiserum prepared against the whole virus. The antigen-antibody complexes were collected with Ultralink-Immobilized Protein A Plus (Pierce, Rockford, IL) and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in the presence (10% gel) or absence (7.5%) of β-mercaptoethanol (BME).
RESULTS
The introduction of a disulfide bond across the dimer interface of hPIV3 HN does not impair its fusion-promoting activity.
X-ray crystallographic studies of the hPIV3 HN protein indicate that its role in fusion requires only a minor change in the structure of the dimer interface (27). Based on this, it is reasonable to expect that the introduction of an intermonomeric disulfide bond across the dimer interface of the protein may not interfere with its ability to complement the homologous F protein in the promotion of fusion.
To test this hypothesis, we sought to identify residues directly apposed across the dimer interface at which cysteine mutations could be introduced, which would have the potential to form intermonomeric disulfide bonds. Figure 1A shows that the S554 residues on the two monomers in the hPIV3 HN dimer are very close across the dimer interface. In this position, it seemed possible that the introduction of an S554C mutation would result in the formation of an intermonomeric disulfide bond. Thus, S554C-mutated hPIV3 HN was prepared, and its oligomeric structure was evaluated by SDS-PAGE under both nonreducing and reducing conditions (Fig. 1B). In the absence of BME, the wt L HN protein migrates as a monomer of ∼70 kDa. However, the S554C-mutated protein migrates at a much slower rate as two distinct bands under nonreducing conditions; there is minimal monomer present. The slower-migrating form has an estimated molecular mass of 147 kDa, which is only slightly greater than that expected for a dimer. The more diffuse band, estimated to be 128 kDa, is smaller than expected for a dimer. In the presence of BME (Fig. 1B), the S554C-mutated protein comigrates exclusively with the wt monomer. This confirms that the monomers in each dimer are disulfide linked. As a control, an S554A-mutated protein migrates as a monomer in both gels.
FIG. 1.
hPIV3 HN S554 residues in the two monomers are in close contact across the dimer interface and mediate an intermonomeric disulfide bond, which does not impair fusion. (A) Top view of the hPIV3 HN homodimer with the S554 residue on each monomer shown in space-fill mode in blue on one monomer and yellow in the other. The figure was generated with Swiss PDB-Viewer using the structure of Lawrence et al. (27). (B) BHK cells were transfected with vector, wt hPIV3 HN, or hPIV3 HN carrying either a S554C or S554A (as a control) mutation, starved for cysteine and methionine for 1 h, labeled for 3 h, and chased with complete medium for 90 min. The cells were lysed in the presence of N-ethylmaleimide, the hPIV3 HN protein was immunoprecipitated, and the samples were run either with or without BME. The numbers in the lanes marked “M” indicate the migration rates of markers in kilodaltons. (C) The HAd activities of wt and S554C-mutated HN expressed at the surface of BHK cells were assayed at 4 and 37°C. The error bars represent the standard deviations of a minimum of five determinations.
The effect of the intermonomeric disulfide bond on the functions of the mutated protein relative to those of wt hPIV3 HN was determined. The disulfide-linked protein is efficiently expressed at the cell surface, as detected by flow cytometry (90.5% ± 8.6% of wt) and exhibits greater-than-wt NA activity (127.9% ± 19.9% of wt). The HAd activity of the disulfide-linked molecule was determined at both 4 and 37°C. As shown in Fig. 1C, the HAd activity of the wt protein is reduced significantly at 37°C relative to that at 4°C. This is to be expected, given that NA is functional at the higher, but not the lower, temperature. However, the disulfide-linked protein exhibits a different profile. Its HAd activity is more than one-third greater at 37°C than it is at the lower temperature, suggesting that the disulfide bond may stabilize receptor binding at the higher temperature. Finally, the fusion-promoting activity of the mutated hPIV3 HN protein was compared to that of the wt protein. Quantitation of the extent of fusion of the mutated protein in the content mixing assay reveals that it promotes fusion 94.6% ± 13.1% as efficiently as the wt protein. Thus, the presence of a disulfide bond across the dimer interface of hPIV3 HN does not impair the ability of the protein to complement the F protein in the promotion of fusion. This is consistent with the prediction from the crystallographic data that the role of hPIV3 HN in the promotion of membrane fusion involves no change in its dimer interface.
Residues 216 and 230 are juxtaposed across the dimer interface in the extensive interface form of NDV HN but far apart in the minimal interface form.
Interpretation of the structural data for liganded and unliganded NDV HN has led to the prediction that the protein undergoes a drastic conformational change during fusion that converts it from a form having a minimal dimer interface to one with a much more extensive interface (6). To test this hypothesis, we reasoned that the introduction of a disulfide bond in the appropriate position across the dimer interface of the extensive interface form would preclude formation of the minimal interface form of the protein. If this disulfide bond impaired fusion, it would argue in favor of the model. However, if an HN protein disulfide linked in this way were to retain significant fusion-promoting activity, this would argue that the role of NDV HN in the promotion of fusion does not require a drastic rearrangement of the dimer interface.
Thus, as we did for hPIV3 HN, we looked for residues that are in close apposition across the dimer interface in the extensive interface conformation of NDV-L HN. We could find no single residue that meets this requirement. However, residues T216 and D230 from opposite monomers are in close contact across the dimer interface in the extensive interface form (Fig. 2A). The introduction of cysteine mutations at these positions has the potential to introduce two disulfide bonds at opposite ends of the dimer interface. As shown in Fig. 2B, residues 216 and 230 are approximately 40 Å apart in the minimal interface form of the protein, making it impossible for cysteines introduced at these two positions to take part in a disulfide bond while maintaining this structure.
FIG. 2.
NDV HN residues 216 and 230 from different monomers are closely apposed across the dimer interface in the extensive interface form but far apart in the minimal interface form. Membrane views of the NDV HN dimer in the extensive interface form (A) and in the minimal interface form (B), showing residues T216 and D230 from one monomer in blue and from the other monomer in yellow, are presented. The figures were generated with Swiss PDB-Viewer, using the structures of Crennell et al. (6).
T216C-D230C-mutated NDV-L HN is disulfide linked.
NDV-L HN carrying T216C and D230C mutations was prepared by site-directed mutagenesis, and its oligomeric structure was analyzed by SDS-PAGE under nonreducing (Fig. 3A) and reducing (Fig. 3B) conditions. wt L HN migrates as a monomer (74 kDa) under both reducing and nonreducing conditions. This is expected, as the protein does not possess intermonomeric disulfide bonds. As a control, the HN from the AV strain of NDV migrates as a protein of 135 kDa in the absence of BME, similar to the rate expected for a dimer. This is to be expected, since its monomers are linked by an intermonomeric disulfide bond mediated by a cysteine at position 123 in the stalk region (40). The mutated form of L HN migrates at a much slower rate under nonreducing conditions and significantly slower than the AV HN dimer, predominantly at the rate expected for a protein of 161 kDa. The 161-kDa protein is slightly larger than expected for a dimer (148 kDa) but certainly not large enough to be either a trimer or tetramer. A small amount of the protein migrates as a monomer and there are also two minor bands migrating at 106 and 128 kDa. All of the mutated L HN protein comigrates with the wt protein under reducing conditions (Fig. 3B). These results confirm that the monomers in the T216C-D230C-mutated L HN are linked by intermonomeric disulfide bond(s). In addition, the presence of N-ethylmaleimide in the lysis buffer eliminates the possibility that these bonds are formed only upon cell lysis.
FIG. 3.
T216C-D230C-mutated NDV-L HN is a disulfide-linked dimer, which is expressed at the cell surface. BHK cells were transfected with vector alone, AV HN, L HN, or T216C-D230C-mutated L HN. At 22 h posttransfection, the monolayers were starved for cysteine and methionine for 1 h, labeled for 3 h, and chased for 90 min. The cells were lysed in the presence of N-ethylmaleimide, and the HN protein was immunoprecipitated with a mixture of anti-HN-specific MAbs. The samples were subjected to SDS-PAGE either without (A) or with BME (B). The numbers in the lanes marked “M” indicate the migration rates of markers in kilodaltons.
The disulfide-linked form of NDV-L HN is efficiently expressed at the cell surface and promotes fusion more efficiently than wt HN.
The effect of the intermonomeric disulfide bonds on the structure and function of the NDV-L HN protein was determined. The T216C-D230C-mutated protein is efficiently expressed, as detected by flow cytometry using a mixture of HN-specific MAbs (118.0% ± 8.6% of the wt). However, flow cytometry does not distinguish between covalently and noncovalently linked oligomers at the cell surface. To confirm that the engineered, covalently linked dimers are, indeed, fully surface expressed, we immunoprecipitated HN from the surface of cells expressing the mutated protein. Figure 4 shows that the disulfide-linked form of the mutated protein can be immunoprecipitated from the surface of transfected cells, confirming that the disulfide-linked form is efficiently transported to the cell surface. Also, noteworthy is the fact that the 106- and 128-kDa forms are not detectable at the cell surface. This confirms that the 161-kDa form of the disulfide-linked mutated protein is by far the predominant one at the cell surface.
FIG. 4.
The disulfide-linked form of the T216C-D230C-mutated NDV-L HN is expressed at the cell surface. Cells were transfected as indicated and labeled for 3 h with 300 μCi of Expre35S[35S] cysteine-methionine labeling mix. The cells were then placed on ice and washed twice with ice-cold phosphate-buffered saline containing 0.1 mM CaCl2 and 1 mM MgCl2 (PBS-CM). The monolayers were incubated for 1 h in the cold with 2 ml of a mixture of anti-HN hybridoma supernatants, washed five times with cold PBS-CM, and lysed, and HN was immunoprecipitated as described above, followed by SDS-PAGE without BME. The numbers in the lane marked M indicate the migration rates of markers in kilodaltons.
To determine the effect of the intermonomeric disulfide bonds on the fusion-helper function of the protein, we compared the abilities of wt and disulfide-linked HN to complement the NDV F protein in the promotion of fusion. As shown in Fig. 5, the presence of the intermonomeric disulfide bonds does not impair fusion (compare Fig. 5C to B). Indeed, quantitation of the extent of fusion in the content mixing assay reveals a ca. 50% increase in fusion by the mutated protein relative to the wt (Fig. 5D). Thus, prevention of formation of the minimal interface form of HN clearly does not impair fusion but rather appears to enhance it.
FIG. 5.
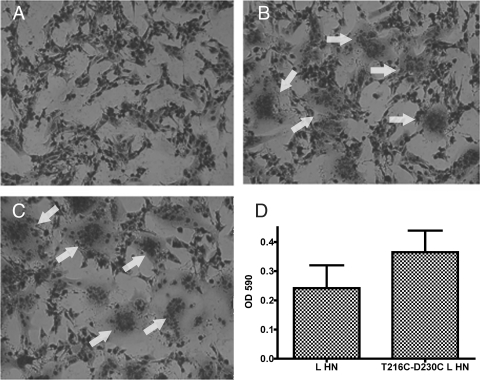
T216C-D230C-mutated NDV-L HN promotes fusion more effectively than the wt protein. BHK cell monolayers were transfected with vector alone (A), wt HN and F (B), or T216C-D230C-mutated L HN and wt F (C) and stained for fusion. Arrows indicate syncytia. (D) The promotion of fusion by wt F complemented with either wt L HN or T216C-D230C-mutated L HN was quantitated in the content-mixing assay. The error bars represent the standard deviations of a minimum of five determinations.
The intermonomeric disulfide bonds alter the receptor-binding properties of NDV HN.
To try to understand the basis for the increased fusion promotion activity of the disulfide-linked protein, its receptor-binding properties were compared to those of the wt protein. This was done by comparing the HAd activities of the two proteins at both 4 and 37°C. The wt HN protein hemadsorbs significantly more efficiently in the cold (optical density at 540 nm [OD540] of 0.133) than it does at 37°C (OD540 of 0.088) (Fig. 6A). The NA activity of HN is not functional in the cold, while it acts to dissociate the HN-receptor complex at 37°C. However, the disulfide-linked protein has the opposite phenotype, hemadsorbing more than twofold better at 37°C (OD540 of 0.172) than it does in the cold (OD540 of 0.075) (Fig. 5A). This is similar to the profile exhibited by disulfide-linked hPIV3 HN (Fig. 1C). Indeed, the disulfide-linked NDV-L HN hemadsorbs better at the elevated temperature than does the wt protein in the cold. This could be related to the sharp decrease in NA activity exhibited by the mutated protein (25.2% of wt NDV-L HN) but could also be due, in part, to a stabilization of the second sialic acid binding site at the membrane-distal end of the dimer interface.
FIG. 6.
The presence of the intermonomeric disulfide bonds in NDV-L HN alters the receptor recognition profile of the protein. (A) The ability of wt and T216C-D230C-mutated L HN expressed at the surface of BHK cells to HAd guinea pig erythrocytes was assayed at 4 and 37°C. (B) The HAd activity of wt HN from the AV strain was compared at 4 and 37°C. The error bars represent standard deviations of a minimum of five determinations.
The data shown in Fig. 6B illustrate an additional point. This figure compares the HAd activity of wt NDV-AV HN at 4 and 37°C. As discussed previously, monomers of this HN protein are linked by intermonomeric disulfide bonds between cysteines in the stalk region. This protein exhibits a profile similar to that of wt L HN, i.e., slightly decreased HAd at 37°C relative to that in the cold. Thus, HN carrying a naturally occurring intermonomeric disulfide bond in its stalk does not exhibit the same HAd phenotype as a protein with intermonomeric disulfide bonds in the globular domain. The altered HAd profile is a function of the presence of two intermonomeric disulfide bonds and/or their placement in the globular head.
Binding to receptors is not required for formation of the intermonomeric disulfide bond in T216C-D230C-mutated NDV HN.
It was originally proposed that the conversion of NDV HN from the minimal interface form to the extensive interface conformation is triggered at the cell surface by its binding to receptors (6). Assuming that the intermonomeric disulfide bonds between residues 216 and 230 cannot exist in the minimal interface form of the protein, this predicts that the disulfide-linked protein will not be present prior to HN reaching the cell surface and binding to receptors.
We took two approaches to test this hypothesis. First, we looked for the presence of the disulfide-linked dimer inside the cell. In the experiments shown in Fig. 3, designed to detect cell surface HN, we expressed the mutated protein, radiolabeled for 3 h and incubated in chase medium for 90 min. Here, in order to determine whether the disulfide-linked, T216C-D230C-mutated HN is present intracellularly, we labeled for only 30 min and did not use a chase. Since the half time for NDV HN to reach the surface has been determined to be 78 min (35), we should be dealing exclusively with intracellular HN. As shown in Fig. 7A, the disulfide-linked dimer of the mutated protein can be detected in significant amounts under these conditions, indicating that the intermonomeric disulfide bond forms before the protein has reached the surface. As a control, the same can be said for the HN from the AV strain, which is linked via a disulfide bond in the stalk (40). Again, the presence of N-ethylmaleimide in the lysis buffer ensures that the dimer is formed intracellularly. These results call into question the idea that the extensive interface conformation of NDV HN is formed at the cell surface triggered by the binding of HN to cellular receptors. Note that the amounts of the 106- and 128-kDa forms of the mutated L HN protein are minimal compared to the 161-kDa form.
FIG. 7.
The intermonomeric disulfide bonds form in NDV-L HN both intracellularly and in the absence of receptor binding. At 22 h posttransfection, BHK cells expressing either vector alone or the HN protein shown were starved for cysteine and methionine and labeled for 30 min without a subsequent chase (A) or labeled for 3 h followed by a 90-min chase (B). The cells were then lysed, HN was immunoprecipitated using a mixture of HN-specific MAbs, and HN was displayed by SDS-PAGE in the absence of BME. The numbers in the lane marked “M” indicate the migration rates of markers in kilodaltons.
As another approach to the question of the relationship between formation of the extensive interface conformation and receptor binding, we have taken advantage of a mutation previously identified in NDV HN that completely eliminates the ability of the protein to mediate receptor binding. A D198R mutation in the NA active site of NDV-AV HN results in a totally a functional protein, i.e., one that lacks NA, attachment, and fusion-promoting activities, despite efficient expression at the cell surface (9). If HN does not assume the extensive interface form until receptor binding, HN carrying a D198R mutation should remain in the minimal interface form and D198R-mutated HN carrying T216C and D230C mutations should not be capable of forming the intermonomeric disulfide bond.
To test this, we prepared D198R-mutated NDV-L HN both with or without the two cysteine mutations and confirmed that both proteins are expressed and lack appreciable HAd activity. Indeed, no HAd activity at all can be detected for D198R-mutated wt L HN at 4°C, and it exhibits only 5.5% ± 0.3% of the activity of the wt protein at 37°C. No HAd activity can be detected for the D198R-T216C-D230C-mutated protein at either temperature. Similarly, the NA activities of the D198R-mutated proteins are reduced to very minimal levels. D198R-mutated L HN and D198R-T216C-D230C-mutated L HN exhibit only 2.4% ± 2.0% and 1.5% ± 0.6% of the NA activity of the wt protein, respectively.
The D198R-mutated proteins were expressed in BHK cells, labeled, and chased to the surface. The cells were lysed in the presence of N-ethylmaleimide, and HN was immunoprecipitated and analyzed by SDS-PAGE (Fig. 7B). The presence of the D198R mutation and the resulting loss, or near loss, of receptor-binding activity have no discernible effect on the amount of the protein that migrates as a disulfide-linked dimer. Interestingly, the amounts of the minor bands that migrate ahead of the dimer are decreased in the D198R-mutated, disulfide-linked protein relative to the D198R-mutated wt protein (compare lanes 4 and 5 in Fig. 7B). These results are consistent with the conclusion that receptor binding is not required for the formation of the intermolecular disulfide bond, suggesting that it is also not required for the formation of the extensive interface form of the protein. The relatively more diffuse migration patterns for the D198R-mutated wt and disulfide-linked proteins are consistent with our previous results (9) and are due to their lack of detectable NA activity, which results in molecules with various levels of sialylation.
The presence of the intermonomeric disulfide bonds increases the avidity with which MAbs to two antigenic sites recognize NDV-L HN.
We initially identified four antigenic sites (sites 1 to 4) in the globular domain of the AV strain of NDV HN (18). Subsequently, three additional sites, which overlap two of the original ones in competition antibody binding and additive neutralization assays, were identified and named site 12 (overlaps site 1 and 2), site 14 (overlaps sites 1 and 4), and site 23 (overlaps sites 2 and 3) (17, 20, 23).
Although we have demonstrated that a cocktail containing a mixture of these MAbs efficiently recognizes T216C-D230C-mutated NDV-L HN protein, we wanted to explore the possibility that the presence of the intermonomeric disulfide bonds might alter the antigenic structure of HN in a site-specific way. Thus, lysates from cells expressing the HN from the AV strain (against which the MAbs were made), the L strain, and the disulfide-linked mutated form of L HN were individually immunoprecipitated with MAbs to six of the seven sites (Fig. 8A). (MAbs to site 12 were not tested as they immunoprecipitate HN only weakly.) The site 14 MAb is a positive control; it recognizes a linear epitope (24) that is highly conserved in all NDV strains tested (17). (All of the other MAbs recognize conformational epitopes.) As expected, this MAb efficiently immunoprecipitates all three proteins (Fig. 8A). The site 23 MAb serves as a negative control, as it is highly specific for the AV strain due to a mutation at position 193 (23). As expected, this MAb efficiently immunoprecipitates AV HN, but neither wt nor mutated L HN (Fig. 8A). A similar result was obtained with the site 2 MAb, which we have previously shown recognizes L HN very weakly and apparently not with high enough avidity to immunoprecipitate it (22).
FIG. 8.
The presence of the intermonomeric disulfide bonds in NDV-L HN renders the protein capable of being immunoprecipitated with MAbs to antigenic sites 3 and 4. (A) BHK cells expressing wt AV HN, wt L HN, and the disulfide-linked mutated form of L HN were starved, labeled, and chased as described in Fig. 3. The cells were then lysed and HN immunoprecipitated with individual MAbs to the antigenic sites shown, and the immunoprecipitates were displayed on SDS-PAGE in the presence of BME. (B) BHK cells expressing vector alone, wt AV or L HN, or L HN carrying the mutation(s) shown were treated as in panel A. HN was immunoprecipitated with MAb to either site 3 or 14, and the immunoprecipitates were analyzed by SDS-PAGE in the presence of BME. (C) BHK cells expressing wt AV-HN, L HN, or T216C-D230C-mutated L HN were immunoprecipitated with MAbs to site 3, 4, or 14, and the immunoprecipitates were displayed on SDS-PAGE in the absence of BME. The numbers in the lane marked “M” indicate the migration rates of markers in kilodaltons.
However, interesting results were obtained with the MAbs to the three remaining antigenic sites. Whereas the site 3 and 4 MAbs fail to immunoprecipitate the parental L HN protein, they do immunoprecipitate the disulfide-linked mutated form of the protein quite efficiently. This is especially true of the site 3 MAb (Fig. 8A). Thus, the introduction of the disulfide bonds in L HN renders the protein capable of being immunoprecipitated by two MAbs (prepared against another strain of the virus) that do not bring down the parental wt protein. In addition, while the site 1 MAb immunoprecipitates L HN weakly, it loses this ability with the mutated protein (Fig. 8A), indicating that this site is also altered by the introduction of the disulfide bonds, although apparently to a lesser extent.
As a negative control, we generated mutated forms of wt and disulfide-linked L HN carrying a D287N mutation and analyzed them by immunoprecipitation and SDS-PAGE (Fig. 8B). The D287N mutation was identified in a variant of the AV strain that escapes neutralization by the site 3 MAb by the addition of an N-glycan at this position (21). As a control, AV HN, L HN and all its mutated forms are efficiently immunoprecipitated by the site 14 MAb. The slower migration rate of the wt L HN and T216C-D230C-mutated L HN proteins, carrying the additional D287N mutation, relative to the respective parental wt proteins, confirms the presence of the supernumerary N-glycan. As expected, neither of the D287N-mutated L HN proteins was efficiently immunoprecipitated by the site 3 MAb (Fig. 8B). This confirms that the immunoprecipitation of the disulfide-linked mutated protein by the MAb is specific. Thus, linking the monomers in the L HN dimer by intermolecular disulfide bonds alters the antigenic structure of the protein in a site-specific way.
Having shown that the T216C and D230C mutations render the L HN protein capable of being immunoprecipitated by MAbs to sites 3 and 4, we wanted to confirm that it is actually the dimer form of the protein that is immunoprecipitated by these antibodies. To do so, we compared immunoprecipitates obtained with MAbs to sites 3, 4, and 14 from cells expressing the HN proteins from strain AV, L, and the double-cysteine-mutated form of the latter on SDS-PAGE under nonreducing conditions (Fig. 8C). The immunoprecipitates of the mutated protein obtained with MAbs to both sites 3 and 4 exhibit a band that comigrates with the dimer form present in the site 14 immunoprecipitate. This confirms that it is, indeed, the disulfide-linked dimer that is immunoprecipitated by the MAbs to sites 3 and 4.
Attempts to disulfide link NDV-L HN dimers.
The NDV HN protein is a tetramer, composed of a pair of dimers. Although our results with the mutated protein in which the two monomers in each dimer are linked by intermonomeric disulfide bonds argue against a drastic rearrangement of the dimer interface during fusion, they do not address the possibility that fusion involves a dissociation of the dimers in each tetramer. We have tried to address this possibility by introducing disulfide bonds between dimers across the tetramer interface. As we did for the dimer interface, we identified pairs of residues that are closely apposed across the tetramer interface, mutated them to cysteines, and looked for the formation of disulfide-linked tetramers in SDS-PAGE. We made a total of three doubly mutated NDV-L HN proteins: G134C-T232C, T216C-K536C, and T255C-R480C. All three combinations have the potential to form two disulfide bonds between each pair of dimers in the tetramer. While the mutated proteins are expressed, hemadsorb, and promote fusion comparable to the wt L HN protein (data not shown), disulfide-linked tetramer formation was inefficient. Thus, we cannot say yet whether the role of HN in fusion involves an alteration of the tetramer interface.
DISCUSSION
X-ray crystallographic studies of unliganded and ligand-bound forms of the HN proteins of hPIV3 (27) and PIV5 (47) indicate that the dimer interfaces of both proteins undergo only minor conformational changes after interaction with receptors. This is in stark contrast to the results from similar studies of NDV HN, which led to the conclusion that, during fusion, it undergoes a drastic conformational rearrangement triggered by receptor binding that results in its conversion from a form with minimal dimer interface contacts to one with a much more extensive interface (6). Thus, these studies led to the conclusion that the mechanism by which NDV HN contributes fusion-helper function to its homologous F protein may be quite different from those of the other two viruses.
However, the model for the role of the minimal interface conformation of HN in fusion is confounded by the fact that, in this structure, residue G124 from one monomer of the dimer is 62 Å away from the same residue on the other monomer. This is difficult to reconcile with the fact that the two G124 residues, which are at the top of the stalk, would be expected to be closely aligned in the monomers in each dimer, especially in strains in which cysteines at position 123 mediate an intermonomeric disulfide bond between monomers in the same dimer (40). This led even the original authors to question the physiological significance of the minimal interface form (6).
We have prevented the formation of the minimal interface form of the protein by the introduction of intermonomeric disulfide bond(s) across the dimer interface in the globular domain. The presence of the disulfide bond(s) does not impair fusion, as would be expected if the minimal interface form of the protein were required for fusion. These results argue very strongly that neither the minimal interface form of NDV HN nor a gross conformational change in the protein is required for NDV-L HN to complement the F protein in the promotion of fusion. However, we cannot rule out the possibility that the HN protein may undergo a change within the constraints imposed by the intermonomeric disulfide bonds, i.e., similar to those identified for hPIV3 HN. Indeed, such a change might be instrumental to the formation of the second site at the dimer interface. Furthermore, we also cannot rule out the possibility that the disulfide-linked NDV-L HN protein may promote fusion by a totally different mechanism than does the wt protein. However, this seems unlikely since the two ostensibly different mechanisms do not require any changes in the complementary fusion protein.
The disulfide-linked mutated form of L HN exhibits a slower migration rate (161 kDa) relative to the AV HN dimer (135 kDa). This may be a reflection of its having an additional intermonomeric disulfide bond relative to AV HN and/or the fact that the two intermonomeric disulfide bonds in the mutated L HN protein are located in the globular head, while the single one in AV HN is located in the stalk. There are also two minor bands present in the immunoprecipitate with estimated molecular masses of 106 and 128 kDa. The latter migrates at a rate similar to that of the AV HN dimer. We postulate that this band may be made up of L HN linked by only a single intermonomeric disulfide bond. Most importantly, neither of these minor forms is detectable at the cell surface. Also, they are immunoprecipitated by the site 14, but not by the site 3 and 4 MAbs (Fig. 8C). Since, of the three MAbs, only the site 14 antibody recognizes a linear epitope, this could mean that the minor forms represent misfolded forms of the protein.
The model further contends that the minimal interface form of NDV HN serves to hold F in its prefusion conformation through contacts mediated by residues exposed in the minimal interface form and later sequestered in the extensive interface conformation with this conversion triggered by receptor binding (41). We tested this by examining the relationship between receptor binding and formation of the extensive interface form of HN. We showed that the disulfide-linked dimer forms both intracellularly and in the absence of receptor-binding activity. These results confirm that, in contrast to the predictions of the model, the extensive interface form of HN is formed before the protein binds receptors.
Our results are also inconsistent with a peptide-based study which claimed that the interaction with F is mediated by a domain defined by residues 124 to 152 at the stalk-globular head interface in NDV HN and it is the shift from the minimal interface conformation to the extensive interface form that leads to release of HN from a preformed complex with F and, in turn, fusion (15). This is not surprising in light of our finding that the introduction of a N-glycan within this domain does not significantly reduce the level of fusion (32), and the overwhelming body of evidence indicates that the F-interactive site in NDV HN resides in its stalk region (10, 31, 32, 46). Taking all this into consideration, it appears that, if HN does control fusion by maintaining F in its prefusion state, this interaction is most likely mediated by residues in the stalk.
The presence of the disulfide bonds across the dimer interface of L HN not only enhances its fusion-promoting activity but also leads to a remarkable shift in its receptor-binding profile. While the mutations reduce the ability of the protein to bind receptors in the cold, they significantly enhance it at 37°C. Indeed, enhanced fusion may be causally related to increases in receptor binding at 37°C. Our interpretation of this is that the disulfide bonds stabilize the second sialic acid binding site at the dimer interface. We postulate that this is the obverse of our demonstration that the elimination of hydrogen bonds across the dimer interface decreases binding at 37°C, in turn sharply decreasing fusion (5). In retrospect, this destabilization of the dimer interface may disrupt the second sialic acid binding site, although it cannot be ruled out that an intact dimer interface is required for NDV HN′s role in fusion.
Disulfide-linked hPIV3 HN exhibits a receptor-binding profile similar to that of the disulfide-linked NDV-L HN mutated protein. Its HAd activity is greater at 37°C than it is at 4°C. Although not identified in the crystal structure of the protein (27), it has been proposed that hPIV3 HN, like NDV HN, also possesses a second sialic acid binding at its dimer interface (38). The slight increase in NA activity in the mutated hPIV3 protein supports the idea that its increased HAd at the higher temperature is not related to changes in NA. Thus, the increased HAd at 37°C relative to 4°C exhibited by the disulfide-linked hPIV3 HN protein could be due to stabilization of a second sialic acid binding site, if one exists in this protein. Conclusive proof of the existence of a second site on hPIV3 HN awaits the detection of ligand bound to it.
Although the introduction of the intermonomeric disulfide bonds in NDV-L HN does not grossly alter the conformation of the protein, the immunoprecipitation assays with individual MAbs indicate that it does alter the structure of some antigenic sites. Most important is the demonstration that linking the globular heads by intermonomeric disulfide bonds makes it possible for MAbs to sites 3 and 4 to immunoprecipitate the dimer form of the L HN mutated protein, whereas the parental protein is not immunoprecipitated in appreciable amounts by these MAbs. The failure of the site 3 MAb to immunoprecipitate wt L HN is consistent with our previous observation that this MAb binds NDV-L HN at very low avidity, as evidenced by its failure to neutralize the NDV-L virus unless rabbit anti-mouse immunoglobulin is added (17, 22). Thus, the intermonomeric disulfide bonds apparently alter the globular domain such that the avidity by which it is recognized by site 3 and 4 MAbs is increased sufficiently to make immunoprecipitation of the protein possible.
Whereas MAbs to sites 1, 2, 12, 14, and 23 inhibit both attachment and fusion (20), MAbs to sites 3 and 4 exhibit unique functional inhibition profiles. Although they do not inhibit viral attachment (20), both MAbs efficiently block fusion-from-without (FFWO), a model for virus entry, while MAbs to site 3, but not those to site 4, block fusion-from-within (FFWI) (21). Thus, MAbs to these sites are capable of blocking virus-cell and/or cell-cell fusion at a step subsequent to receptor binding. The acquisition of the ability to immunoprecipitate the disulfide-linked form of L HN suggests that site 3 and 4 MAbs recognize this form of the protein with increased avidity.
Amino acid residues in the globular domain of HN that contribute to each of the seven sites have been identified by the isolation and sequencing of antigenic variants (21, 23, 24). The antigenic structure of the NDV HN globular domain generated in this way is shown in Fig. 9. The inhibition of attachment by MAbs to sites 1, 2, 12, 14, and 23 is consistent with their localization either overlapping the NA active site (site 23), in close proximity to the second sialic acid binding site (sites 2 and 12) or on the top surface of the globular domain (sites 1 and 14). However, both site 3 and site 4 are located on the lateral surface of the globular head, which is quite a distance from the receptor binding sites; this likely accounts for their inability to inhibit attachment.
FIG. 9.
Location of antigenic sites in the globular head of the NDV HN dimer. View from the top of the extensive interface structure of the NDV HN dimer. Antigenic sites on both monomers in the dimer identified by the isolation and sequencing of antigenic variants (21, 23, 24) are shown in space-fill mode. The location of the NA sites and the second sialic acid binding sites are shown as blue and green ovals, respectively. The figures were generated with Swiss PDB-viewer, using the extensive interface structure of Crennell et al. (6).
Thus, the introduction of disulfide bonds across the dimer interface increases the avidity with which two MAbs, which bind to the lateral surface of the globular domain and specifically block fusion, interact with the HN from a heterotypic strain of NDV. These results raise the possibility that disulfide-linked L HN represents a form of the protein more relevant to fusion and argues for a role for the lateral surface of the globular domain of HN in fusion promotion, as proposed by Yuan et al. (47), although not necessarily in mediating the interaction with F. Alternatively, one could envision how the binding of an antibody to the lateral surface of the globular domain could sterically block the HN-F interaction, even if it is mediated by the stalk of HN. Thus, as stated before, although our data are consistent with the minimal interface form being an artifact, they do not rule out the possibility that NDV-L HN may undergo a much less drastic conformational change during fusion from a form that is not recognized by MAbs to sites 3 and 4 to one that is recognized and is similar to the disulfide-linked protein we have created by mutagenesis.
Acknowledgments
We thank Rebecca Dutch for the BHK-21F cells, Robert Lamb for the NDV-AV F gene, Trudy Morrison for the NDV-AV HN gene, Mark Galinski for the hPIV3 HN and F genes, and Bernard Moss for the recombinant vaccinia virus. We also thank Theodore Jardetzky for the coordinates for the NDV HN tetramer.
This study was supported by grant AI-49268 from the National Institutes of Health.
Footnotes
Published ahead of print on 27 August 2008.
REFERENCES
- 1.Bousse, T. L., G. Taylor, S. Krishnamurthy, A. Portner, S. K. Samal, and T. Takimoto. 2004. Biological significance of the second sialic acid binding site of Newcastle disease virus hemagglutinin-neuraminidase protein. J. Virol. 7813351-13355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choppin, P. W., and A. Scheid. 1980. The role of the viral glycoproteins in adsorption, penetration and pathogenicity of viruses. Rev. Infect. Dis. 240-61. [DOI] [PubMed] [Google Scholar]
- 3.Collins, P. L., and G. Mottet. 1991. Homo-oligomerization of the hemagglutinin-neuraminidase glycoprotein of human parainfluenza virus type 3 occurs before the acquisition of correct intramolecular disulfide bonds and mature immunoreactivity. J. Virol. 652362-2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corey, E. A., and R. M. Iorio. 2007. Mutations in the stalk of the measles virus hemagglutinin protein decrease fusion but do not interfere with virus-specific interaction with the homologous fusion protein. J. Virol. 819900-9910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corey, E. A., A. M. Mirza, E. Levandowsky, and R. M. Iorio. 2003. Fusion deficiency induced by mutations at the dimer interface in the Newcastle disease virus hemagglutinin-neuraminidase is due to a temperature-dependent defect in receptor binding. J. Virol. 776913-6922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crennell, S., T. Takimoto, A. Portner, and G. Taylor. 2000. Crystal structure of the multifunctional paramyxovirus hemagglutinin-neuraminidase. Nat. Struct. Biol. 71068-1074. [DOI] [PubMed] [Google Scholar]
- 7.Deng, R., A. M. Mirza, P. J. Mahon, and R. M. Iorio. 1997. Functional chimeric HN glycoproteins derived from Newcastle disease virus and human parainfluenza virus-3. Arch. Virol. Suppl. 13115-130. [DOI] [PubMed] [Google Scholar]
- 8.Deng, R., Z. Wang, R. L. Glickman, and R. M. Iorio. 1994. Glycosylation within an antigenic site on the HN glycoprotein of Newcastle disease virus interferes with its role in the promotion of membrane fusion. Virology 20417-26. [DOI] [PubMed] [Google Scholar]
- 9.Deng, R., Z. Wang, P. J. Mahon, M. Marinello, A. M. Mirza, and R. M. Iorio. 1999. Mutations in the NDV HN protein that interfere with its ability to interact with the homologous F protein in the promotion of fusion. Virology 25343-54. [DOI] [PubMed] [Google Scholar]
- 10.Deng, R., Z. Wang, A. M. Mirza, and R. M. Iorio. 1995. Localization of a domain on the paramyxovirus attachment protein required for the promotion of cellular fusion by its homologous fusion protein spike. Virology 209457-469. [DOI] [PubMed] [Google Scholar]
- 11.DiNapoli, J. M., A. Kotelkin, L. Yang, S. Elankumaran, B. R. Murphy, S. K. Samal, P. L. Collins, and A. Bukreyev. 2007. Newcastle disease virus, a host-range restricted virus, as a vaccine vector for intranasal immunization against emergin pathogens. Proc. Natl. Acad. Sci. USA 239788-9793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elankumaran, S., D. Rockemann, and S. K. Samal. 2006. Newcastle disease virus exerts oncolysis by both intrinsic and extrinsic caspase-dependent pathways of cell death. J. Virol. 807522-7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuerst, T. R., E. G. Niles, F. W. Studier, and B. Moss. 1986. Eucaryotic transient expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc. Natl. Acad. Sci. USA 838122-8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao, Q., M. S. Park, and P. Palese. 2008. Expression of transgenes from Newcastle disease virus with a segmented genome. J. Virol. 822692-2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gravel, K., and T. G. Morrison. 2003. Interacting domains of the HN and F proteins of Newcastle disease virus. J. Virol. 7711040-11049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang, Z., S. Elankumaran, A. S. Yunus, and S. K. Samal. 2004. A recombinant Newcastle disease virus (NDV) expressing VP2 protein of infectious bursal disease virus (IBDV) protects against NDV and IBDV. J. Virol. 7810054-10063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iorio, R. M., J. B. Borgman, R. L. Glickman, and M. A. Bratt. 1986. Genetic variation within a neutralizing domain on the haemagglutinin-neuraminidase glycoprotein of Newcastle disease virus. J. Gen. Virol. 671393-1403. [DOI] [PubMed] [Google Scholar]
- 18.Iorio, R. M., and M. A. Bratt. 1983. Monoclonal antibodies to Newcastle disease virus: delineation of four epitopes on the HN glycoprotein. J. Virol. 48440-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iorio, R. M., G. M. Field, J. M. Sauvron, A. M. Mirza, R. Deng, P. J. Mahon, and J. Langedijk. 2001. Structural and functional relationship between the receptor recognition and neuraminidase activities of the Newcastle disease virus hemagglutinin-neuraminidase protein: receptor recognition is dependent on neuraminidase activity. J. Virol. 751918-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iorio, R. M., R. L. Glickman, A. M. Riel, J. P. Sheehan, and M. A. Bratt. 1989. Functional and neutralization profile of seven overlapping antigenic sites on the HN glycoprotein of Newcastle disease virus: monoclonal antibodies to some sites prevent viral attachment. Virus Res. 13245-262. [DOI] [PubMed] [Google Scholar]
- 21.Iorio, R. M., R. L. Glickman, and J. P. Sheehan. 1992. Inhibition of fusion by neutralizing monoclonal antibodies to the haemagglutinin-neuraminidase glycoprotein of Newcastle disease virus. J. Gen. Virol. 731167-1176. [DOI] [PubMed] [Google Scholar]
- 22.Iorio, R. M., K. A. Lawton, P. M. Nicholson, and M. A. Bratt. 1984. Monoclonal antibodies identify a strain-specific epitope on the HN glycoprotein of Newcastle disease virus strain Australia-Victoria. Virus Res. 1513-525. [DOI] [PubMed] [Google Scholar]
- 23.Iorio, R. M., R. J. Syddall, R. L. Glickman, A. M. Riel, J. P. Sheehan, and M. A. Bratt. 1989. Identification of amino acid residues important to the neuraminidase activity of the HN glycoprotein of Newcastle disease virus. Virology 173196-204. [DOI] [PubMed] [Google Scholar]
- 24.Iorio, R. M., R. J. Syddall, J. P. Sheehan, M. A. Bratt, R. L. Glickman, and A. M. Riel. 1991. Neutralization map of the HN glycoprotein of Newcastle disease virus: domains recognized by monoclonal antibodies that prevent receptor recognition. J. Virol. 654999-5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krishnamurthy, S., T. Takimoto, R. A. Scoggs, and A. Portner. 2006. Differentially regulated interferon response determines the outcome of Newcastle disease virus infection in normal and tumor cell lines. J. Virol. 805145-5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lamb, R. A., and G. D. Parks. 2007. Paramyxoviridae: the viruses and their replication, p. 1449-1496. In D. M. Knipe and P. M. Howley (ed.), Fundamental virology, 5th ed. Wolters Kluwer/Lippincott, The Williams & Wilkins, Philadelphia, PA.
- 27.Lawrence, M. C., N. A. Borg, V. A. Streltsov, P. A. Pilling, V. C. Epa, J. N. Varghese, J. L. McKimm-Breschkin, and P. M. Colman. 2004. Structure of the haemagglutinin-neuraminidase from human parainfluenza virus type III. J. Mol. Biol. 3351343-1357. [DOI] [PubMed] [Google Scholar]
- 28.Li, J., E. Quinlan, A. Mirza, and R. M. Iorio. 2004. Mutated form of the Newcastle disease virus hemagglutinin-neuraminidase interacts with the homologous fusion protein despite deficiencies in both receptor recognition and fusion promotion. J. Virol. 785299-5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinez-Sobrido, L., N. Gitiban, A. Fernandez-Sesma, J. Cros, S. E. Mertz, N. A. Jewell, S. Hammond, E. Flano, R. K. Durbin, A. Garcia-Sastre, and J. E. Durbin. 2006. Protection against respiratory syncytial virus by a recombinant Newcastle disease virus vector. J. Virol. 801130-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGinnes, L. W., and T. G. Morrison. 2006. Inhibition of receptor binding stabilizes Newcastle disease virus HN and F protein-containing complexes. J. Virol. 802894-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Melanson, V. R., and R. M. Iorio. 2004. Amino acid substitutions in the F-specific domain in the stalk of the Newcastle disease virus HN protein modulate fusion and interfere with its interaction with the F protein. J. Virol. 7813053-13061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Melanson, V. R., and R. M. Iorio. 2006. Addition of N-Glycans in the stalk of the Newcastle disease virus HN protein blocks its interaction with the F protein and prevents fusion. J. Virol. 80623-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mirza, A. M., R. Deng, and R. M. Iorio. 1994. Site-directed mutagenesis of a conserved hexapeptide in the paramyxovirus hemagglutinin-neuraminidase glycoprotein: effects on antigenic structure and function. J. Virol. 685093-5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mirza, A. M., J. P. Sheehan, L. W. Hardy, R. L. Glickman, and R. M. Iorio. 1993. Structure and function of a membrane anchor-less form of the hemagglutinin-neuraminidase glycoprotein of Newcastle disease virus. J. Biol. Chem. 26821425-21431. [PubMed] [Google Scholar]
- 35.Morrison, T. G., and L. J. Ward. 1984. Intracellular processing of the vesicular stomatitis glycoprotein and the Newcastle disease virus hemagglutinin-neuraminidase glycoprotein. Virus. Res. 1225-239. [DOI] [PubMed] [Google Scholar]
- 36.Ng, D. T. W., R. E. Randall, and R. A. Lamb. 1989. Intracellular maturation and transport of the SV5 type II glycoprotein hemagglutinin-neuraminidase: specific and transient association with GRP78-BiP in the endoplasmic reticulum and extensive internalization from the cell surface. J. Cell Biol. 1093273-3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park, M. S., J. Steel, A. Garcia-Sastre, D. Swayne, and P. Palese. 2006. Engineered viral vaccine construct with dual specificity: avian influenza and Newcastle disease. Proc. Natl. Acad. Sci. USA 1038203-8208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Porotto. M., M. Fornabaio, G. E. Kellogg, and A. Moscona. 2007. A second receptor binding site on human parainfluenza virus type 3 hemagglutinin-neuraminidase contributes to activation of the fusion mechanism. J. Virol. 813216-3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scheid, A., and P. W. Choppin. 1974. Identification and biological activities of paramyxovirus glycoproteins: activation of cell fusion, hemolysis and infectivity by proteolytic cleavage of an inactive precursor protein of Sendai virus. Virology 57475-490. [DOI] [PubMed] [Google Scholar]
- 40.Sheehan, J. P., R. M. Iorio, R. J. Syddall, R. L. Glickman, and M. A. Bratt. 1987. Reducing agent-sensitive dimerization of the hemagglutinin-neuraminidase glycoprotein of Newcastle disease virus correlates with the presence of cysteine at residue 123. Virology 161603-606. [DOI] [PubMed] [Google Scholar]
- 41.Takimoto, T., G. L. Taylor, H. C. Connaris, S. J. Crennell, and A. Portner. 2002. Role of the hemagglutinin-neuraminidase protein in the mechanism of paramyxovirus-cell membrane fusion. J. Virol. 7613028-13033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tanabayashi, K., and R. W. Compans. 1996. Functional interaction of paramyxovirus glycoproteins: identification of a domain in Sendai virus HN which promotes cell fusion. J. Virol. 706112-6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thompson, S. D., W. G. Laver, K. G. Murti, and A. Portner. 1988. Isolation of a biologically active soluble form of the hemagglutinin-neuraminidase protein of Sendai virus. J. Virol. 624653-4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsurudome, M., M. Kawano, T. Yuasa, N. Tabata, M. Nishio, H. Komada, and Y. Ito. 1995. Identification of regions on the hemagglutinin-neuraminidase protein of parainfluenza virus type 2 important for promoting cell fusion. Virology 213190-203. [DOI] [PubMed] [Google Scholar]
- 45.Vigil, A., M. S. Park, O. Martinez, M. A. Chua, S. Xioa, J. F. Cros, L. Martinez-Sobrido, S. L. Woo, and A. Garcia-Sastre. 2007. Use of reverse genetics to enhance the oncolytic properties of Newcastle disease virus. Cancer Res. 678285-8292. [DOI] [PubMed] [Google Scholar]
- 46.Wang, Z., A. M. Mirza, J. Li, P. J. Mahon, and R. M. Iorio. 2004. An oligosaccharide at the C terminus of the F-specific domain in the stalk of the human parainfluenza virus 3 hemagglutinin-neuraminidase modulates fusion. Virus Res. 99177-185. [DOI] [PubMed] [Google Scholar]
- 47.Yuan, P., T. B. Thompson, B. A. Wurzburg, R. G. Paterson, R. A. Lamb, and T. S. Jardetzky. 2005. Structural studies of the parainfluenza virus 5 hemagglutinin-neuraminidase tetramer in complex with its receptor, sialyllactose. Structure 13803-815. [DOI] [PubMed] [Google Scholar]
- 48.Zaitsev, V., M. von Itzstein, D. Groves, M. Kiefel, T. Takimoto, A. Portner, and G. Taylor. 2004. Second sialic acid binding site in Newcastle disease virus hemagglutinin-neuraminidase: implications for fusion. J. Virol. 783733-3741. [DOI] [PMC free article] [PubMed] [Google Scholar]