Abstract
Skin keratinocytes provide a first line of defense against invading microorganisms in two ways: (i) by acting as a physical barrier to pathogen entry and (ii) by initiating a vigorous innate immune response upon sensing danger signals. How keratinocytes detect virus infections and generate antiviral immune responses is not well understood. Orthopoxviruses are dermatotropic DNA viruses that cause lethal disease in humans. Virulence in animal models depends on the virus-encoded bifunctional Z-DNA/double-stranded RNA (dsRNA)-binding protein E3. Here, we report that infection of mouse primary keratinocytes with a vaccinia ΔE3L mutant virus triggers the production of beta interferon (IFN-β), interleukin-6 (IL-6), CCL4, and CCL5. None of these immune mediators is produced by keratinocytes infected with wild-type vaccinia virus. The dsRNA-binding domain of E3 suffices to prevent activation of the innate immune response. ΔE3L induction of IFN-β, IL-6, CCL4, and CCL5 secretion requires mitochondrial antiviral signaling protein (MAVS; an adaptor for the cytoplasmic viral RNA sensors RIG-I and MDA5) and the transcription factor IRF3. IRF3 phosphorylation is induced in keratinocytes infected with ΔE3L, an event that depends on MAVS. The response of keratinocytes to ΔE3L is unaffected by genetic ablation of Toll-like receptor 3 (TLR3), TRIF, TLR9, and MyD88.
The interactions of viruses with the skin immune system are not well understood. The interface of poxviruses with skin is especially intriguing, given that (i) skin infection and disfigurement are prominent features of smallpox disease; (ii) successful smallpox immunization is achieved by intentional skin infection with vaccinia virus; and (iii) eczema vaccinatum is a major severe complication of vaccination in people with atopic dermatitis (AD), a condition associated with defects in skin barrier function and antiviral innate immunity (25, 26, 69). Concerns about smallpox as a bioterrorism threat against an unvaccinated population, the persistence of monkeypox in Africa and episodes of its extracontinental spread, and the imperative to improve smallpox vaccination strategies all focus attention on poxvirus-host dynamics, particularly with skin. A recent case of life-threatening eczema vaccinatum in a child with AD who became infected by mere household contact with a smallpox vaccinee and then passed the infection on to a third party (68) highlights just how delicate the balance is between poxvirus virulence and skin immunity.
Keratinocytes comprise the predominant cell type in the epidermis. Liu et al. (43) reported that vaccinia virus had limited replicative capacity in human keratinocytes and that infection induced keratinocytes to produce the Th2 cytokines transforming growth factor β, interleukin-10 (IL-10), and IL-13, which suggested that vaccinia virus might downregulate skin immune responses. Human keratinocytes express Toll-like receptors (TLRs) that initiate innate immune signaling by binding to ligands referred to as pathogen-associated molecular patterns (30, 40, 47, 49). Human keratinocytes produce a repertoire of cytokines, chemokines, and antimicrobial peptides in response to TLR stimulation (6, 40, 66). Such cytokines and chemokines augment innate and acquired immunity mediated by dendritic cells, neutrophils, macrophages, NK cells, and T cells, which reside in the skin or are recruited to the skin in the setting of infection or inflammation (39).
The type I interferons (IFN-α and IFN-β) are key mediators of antiviral innate immunity (65). Double-stranded RNA (dsRNA) introduced during virus infection is a potent inducer of the type I IFN response. dsRNA can trigger distinct signaling pathways by engaging either (i) the endosomal membrane-bound receptor TLR3 (42), (ii) the soluble cytoplasmic receptors RIG-I and MDA5 (28, 29, 31, 73), or (iii) the dsRNA-dependent protein kinase PKR (16). Signaling through TLR3 leads to activation of the transcription factors IFN regulatory factor 3 (IRF3) and NF-κB via the adaptor molecule TRIF. As a consequence, IRF3 is phosphorylated and then moves to the nucleus to activate IFN-β expression (59). In contrast, cytoplasmic dsRNA produced during replication of RNA viruses binds to RIG-I or MDA5 and triggers activation of IRF3 and NF-κB through the mitochondrial antiviral signaling protein, MAVS (58), also known as IPS-1 (32), VISA (71), or Cardif (48). Cytoplasmic 5′-triphosphate-terminated single-stranded RNA is also able to activate the RIG-I and PKR pathways (24, 52). Relatively little is known about these pathways in skin cells. Dai et al. (13) reported that treatment of human keratinocytes with exogenous poly(I:C), a TLR3 agonist, induced phosphorylation and nuclear translocation of IRF3. To our knowledge, there is no report of the status of cytoplasmic viral RNA sensing in keratinocytes or during a poxvirus infection.
Poxviruses are extraordinarily adept at evading and antagonizing multiple innate immune signaling pathways by encoding proteins that interdict the extracellular and intracellular components of those pathways (57). Chief among the poxvirus antagonists of intracellular innate immune signaling is the vaccinia virus dsRNA-binding protein E3, which can inhibit the PKR and NF-κB pathways (10, 14, 38) that would otherwise be activated by vaccinia virus infection. A mutant vaccinia virus lacking the E3L gene (ΔE3L) has a restricted host range, is highly sensitive to IFN, and has greatly reduced virulence in animal models of lethal poxvirus infection (5, 7, 8). Recent studies have shown that infection of cultured cell lines with ΔE3L virus elicits proinflammatory responses that are masked during infection with wild-type vaccinia virus (14, 38). We reported that infection of a mouse epidermal dendritic cell line with wild-type vaccinia virus attenuated proinflammatory responses to the TLR agonists lipopolysaccharide (LPS) and poly(I:C), an effect that was diminished by deletion of E3L. Moreover, infection of the dendritic cells with ΔE3L virus triggered NF-κB activation in the absence of exogenous agonists (14). These results suggested that E3L might play a role in actively suppressing skin innate immunity during poxvirus infection.
Here, we explore this theme by studying the responsiveness of primary murine keratinocytes to TLR agonists and to infection by wild-type and ΔE3L vaccinia viruses. The instructive finding is that sensing of poxvirus infection by keratinocytes is blocked by E3L, deletion of which unmasks a proinflammatory response entailing secretion of IFN-β, IL-6, CCL4, and CCL5. Using primary keratinocytes from knockout mice, we determined that the innate immune response to ΔE3L infection is independent of the TLR pathway components TLR3, TRIF, TLR9, and MyD88. Rather, the ΔE3L response is completely dependent on MAVS and IRF3. This is, to our knowledge, the first report of the activation of the MAVS-driven cytoplasmic RNA-sensing pathway during infection by a poxvirus.
MATERIALS AND METHODS
Cell lines.
BSC40 cells (African green monkey kidney cells) were maintained in Dulbecco's modified Eagle's medium supplemented with 5% fetal bovine serum (FBS). BHK-21 (baby hamster kidney) and RK13 (rabbit kidney) cells were cultured in Dulbecco's modified Eagle's medium containing 10% FBS, 0.1 mM nonessential amino acids, and 50 μg/ml gentamicin. All cells were grown at 37°C in a 5% CO2 incubator.
Viruses.
The WR strain of vaccinia virus was propagated, and virus titers were determined in BSC40 monolayers at 37°C. The ΔE3L and E3LΔ83N viruses were kindly provided by B. L. Jacobs (Arizona State University). ΔE3L was propagated in BHK-21 cells, and virus titers were determined on RK13 cells. E3LΔ83N was propagated, and virus titers were determined BSC40 cells. UV-inactivation of ΔE3L was performed with a UV Stratalinker 2400 by exposing the virus stock to three cycles of radiation with 0.36 J of energy in the auto-cross-link mode.
Mice.
Female BALB/c and C57B/6 mice between 8 and 12 weeks of age were purchased from the Jackson Laboratory and were used for the preparation of epidermal cells and primary keratinocytes. These mice were maintained in the animal facility at the Sloan-Kettering Cancer Institute. All procedures were performed with the consent of the Institutional Animal Use and Care Committee. IRF3−/−, TLR3−/−, MyD88−/−, TLR9−/−, and TRIF−/− (TRIFLPS2/LPS2) mice were generated in the laboratories of Tadatsugu Taniguchi (University of Tokyo), Richard Flavell (Yale University), Shizuro Akira (Osaka University), and Bruce Beutler (Scripps Research Institute). Mice deficient for IFN α/β receptor (IFNAR−/−) were provided by Eric Pamer (Sloan-Kettering Institute); the mice were purchased from B & K Universal and were backcrossed with C57BL/6 mice for five generations. The MAVS−/− mice were maintained at the animal facilities at the University of Texas Southwestern Medical Center.
Keratinocyte preparation.
Female BALB/c and C57BL/6 mice at 8 to 12 weeks of age were used. Mice were shaved and chemically depilated. Truncal skins were removed and depleted of subcutaneous fat. The skin samples were floated, dermis side down, on a solution containing 0.5 U/ml of dispase (Boehringer Mannheim) and 0.38% trypsin (Sigma) in phosphate-buffered saline (PBS) for 40 min at 37°C. Epidermal sheets were collected and dissociated in Hanks balanced salt solution with 2% heat-inactivated FBS for 20 min at room temperature under gentle agitation. The cells were then filtered through a 40-μm-pore-size cell strainer (BD Biosciences) and washed in Hanks balanced salt solution-2% FBS. To remove Langerhans cells, the epidermal cells were incubated with anti-Ia antibody (BD PharMingen), followed by washing and incubation with Dynabeads M-450 coated with goat anti-mouse immunoglobulin (Dynal AS) (14).
Materials.
LPS and poly(I:C) were purchased from Sigma. An LPS stock solution (1 mg/ml) in PBS was stored at −80°C. A poly(I:C) stock solution (1 mg/ml) in PBS was stored at −20°C. CpG oligodeoxynucleotide ODN2395 was purchased from Alexis Biochemicals. Its stock solution (1 mg/ml) was stored at −20°C. Cytosine arabinoside (araC) was from Sigma. Concentrations of cytokines and chemokines were determined by enzyme-linked immunosorbent assay (ELISA) with assay kits purchased from PBL Biomedical Laboratories and R & D Systems. The assays were performed as specified by the kit vendor; the ELISA data were converted to cytokine/chemokine concentrations by interpolation to standard curves generated with cytokine/chemokine standards provided with the kits.
RESULTS
Responsiveness of mouse keratinocytes to TLR agonists and vaccinia virus infection.
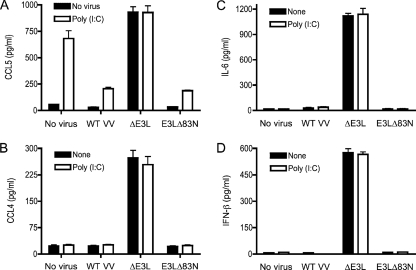
Epidermal keratinocytes isolated freshly from mouse skin were exposed to the TLR agonists LPS (specific for TLR4), poly(I:C) (which acts via TLR3), and CpG (which stimulates TLR9). Cell supernatants were collected after 18 h and tested by ELISA for secreted cytokines and chemokines. Treatment of keratinocytes with 1 μg/ml LPS or CpG failed to induce production of TNF-α, IL-6, IL-1β, IFN-α, IFN-β, IFN-γ, CCL3, CCL4, CCL5, CXCL9, or CXCL10. Incubation of keratinocytes with 10 μg/ml poly(I:C) uniquely induced production of CCL5 (Fig. 1A) but not of IFN-β, IL-6, or CCL4 (Fig. 1A) or any of the other aforementioned cytokines and chemokines (data not shown). Thus, mouse keratinocytes display a narrow response to exogenous TLR agonists. The positive response to poly(I:C) by secretion of CCL5 attests to the presence of a TLR3-driven signaling pathway. CCL5 is a proinflammatory chemokine that has antiviral activities (51). CCL5 expression is controlled by both NF-κB and IRFs (18).
FIG. 1.
Keratinocyte responses to poly(I:C) and vaccinia virus infection. Keratinocytes were prepared from female BALB/c mouse epidermal cells depleted of Langerhans cells. Cells (4 × 106) were infected with wild-type vaccinia virus (WT VV), ΔE3L, or E3LΔ83N virus at a multiplicity of 10 for 1 h. Infected cells and mock-infected controls (No virus) were washed to remove unabsorbed viruses and then incubated in fresh medium with no additive (none) or medium containing 10 μg/ml poly(I:C). Supernatants were collected 18 h later, and the concentrations of IFN-β, IL-6, CCL4, and CCL5 were determined by ELISA with assay kits from PBL Biomedical Laboratories and R&D Systems. Error bars represent standard deviations from the means of triplicate experiments.
Infection of keratinocytes with wild-type vaccinia virus (strain WR) at a multiplicity of 3 was nonproductive; i.e., only a twofold increase in viral titer was observed at 2 days postinfection (not shown). Pulse labeling of vaccinia virus-infected keratinocytes with [35S]methionine highlighted the appearance of new, labeled polypeptides at 4 to 12 h postinfection that were not evident in uninfected control cells; a subset of virus-specific labeled species appeared at 8 to 12 h that was not evident at 4 h, consistent with a transition from early to intermediate/late viral protein synthesis (data not shown). We have not attempted here to define the restriction point(s) that prevents vaccinia virus from replicating productively in primary keratinocytes. Culture supernatants collected from keratinocytes at 18 h postinfection revealed no secretion of TNF-α, IL-6, IL-1β, IFN-α, IFN-β, IFN-γ, CCL3, CCL4, CCL5, CXCL9, or CXCL10 compared to uninfected controls (Fig. 1 and data not shown). Infection of keratinocytes with wild-type vaccinia virus attenuated the production of CCL5 in response to poly(I:C) (Fig. 1A). The metabolic labeling experiment indicated that absence of a CCL5 response in vaccinia virus-infected keratinocytes is not caused by a global shutoff of host cell protein synthesis (data not shown). These findings suggest that vaccinia virus actively suppresses proinflammatory signaling in keratinocytes.
ΔE3L virus infection in murine primary keratinocytes triggers innate immune responses.
Infection of mouse keratinocytes with ΔE3L vaccinia virus induced the production of IFN-β, IL-6, CCL4, and CCL5 (Fig. 1), implying that keratinocytes sense the poxvirus infection but are impeded from responding by the viral E3 protein. The pattern of protein synthesis in ΔE3L-infected keratinocytes over 12 h postinfection resembled that seen for wild-type vaccinia virus, except for the absence in the ΔE3L-infected cells of a labeled 27-kDa polypeptide that we presume corresponds to E3 (data not shown). E3 is composed of two structural modules: an N-terminal Z-DNA binding domain (19) and a C-terminal dsRNA binding domain (9, 21). The dsRNA binding domain is required for IFN resistance, interdiction of PKR signaling, broad host range in cultured cells, and virulence in animals (10, 11, 70). A mutant vaccinia virus in which only the Z-DNA binding domain is deleted (E3LΔ83N) displays a wild-type host range in cultured cells but has attenuated virulence (33, 34, 37, 51). Langland et al. (38) reported upregulation of a broad spectrum of proinflammatory and apoptotic genes in ΔE3L-infected HeLa cells, while noting that infection with the E3LΔ83N virus induced a smaller subset of such genes. Here, we find that E3LΔ83N phenocopied wild-type vaccinia virus with respect to (i) its failure to induce keratinocytes to produce IFN-β, IL-6, CCL4, or CCL5 (Fig. 1) and (ii) its attenuation of CCL5 production by keratinocytes exposed to poly(I:C) (Fig. 1A). These results indicate that the E3 dsRNA binding domain suffices to suppress the innate immune response of mouse keratinocytes to vaccinia virus infection.
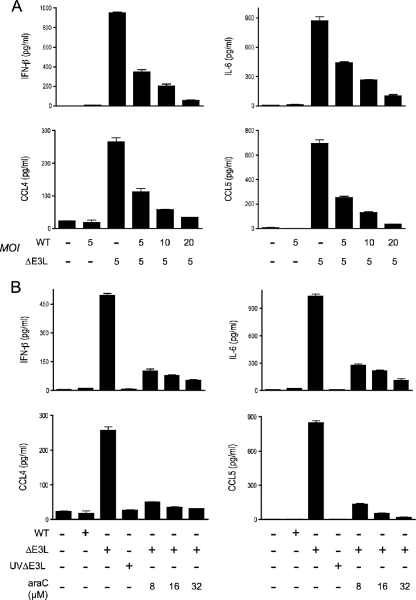
We envision that viral dsRNA produced in ΔE3L-infected keratinocytes is the immediate trigger of the antiviral immune response. If this is the case, then one predicts that coinfection of keratinocytes with wild-type vaccinia virus and ΔE3L would dampen the output of immune signaling compared to cells infected with ΔE3L alone. Indeed, we found that coinfection of both viruses at a multiplicity of 5 reduced the production of IFN-β, IL-6, CCL4, and CCL5 by 64%, 50%, 57%, and 64%, respectively (Fig. 2A). Increasing the wild-type vaccinia virus multiplicity to 10 or 20 further reduced IFN-β, IL-6, CCL4, and CCL5 secretion (Fig. 2A).
FIG. 2.
Viral requirements for innate immune signaling in ΔE3L-infected keratinocytes. (A) Keratinocytes prepared from C57B/6 mice were infected with wild-type vaccinia virus and ΔE3L at the indicated multiplicity (MOI; shown as PFU/cell). Infected cells and mock-infected controls were washed after 1 h and then incubated in fresh medium. Supernatants were collected at 18 h postinfection. (B) Keratinocytes prepared from C57B/6 mice were infected with wild-type vaccinia virus, ΔE3L, or UV-inactivated ΔE3L (UVΔE3L). After a washing step to remove unadsorbed virus, the cells were incubated in fresh medium with no additive or in medium containing araC (8, 16, or 32 μM). Supernatants were collected at 18 h postinfection. The concentrations of IFN-β, IL-6, CCL4, and CCL5 were determined by ELISA. Error bars represent standard deviations from the means of triplicate experiments. WT, wild type.
A prediction of this model is that viral RNA synthesis ought to be required for ΔE3L infection to elicit an innate immune response in keratinocytes. UV treatment of vaccinia virus particles damages the DNA genome and attenuates viral mRNA synthesis by the virion-encapsidated transcription machinery. If it is the case that UV-inactivated ΔE3L retains its ability to trigger an innate immune response, then one can conclude that viral protein synthesis is not required and that the inciting factor is either a virion component or a short abortive transcript from an early viral gene. To perform this experiment, we exposed ΔE3L virus to a UV dose that reduced infectivity by 10,000-fold. Keratinocytes were infected with UV-treated ΔE3L in parallel with an equal aliquot of untreated control ΔE3L virus. The instructive finding was that UV treatment of ΔE3L abolished its ability to induce secretion of IFN-β, IL-6, CCL4, and CCL5 (Fig. 2B), signifying that virion components are not sufficient for the keratinocyte antiviral response.
araC inhibits poxvirus DNA replication and the transcription of viral intermediate and late genes, without affecting the synthesis of viral early mRNAs and early proteins. Exposure of ΔE3L-infected keratinocytes to araC concentrations sufficient to block vaccinia virus replication in a permissive epidermal cell line (14) caused a progressive ablation of IFN-β, IL-6, CCL4, and CCL5 production (Fig. 2B). Thus, viral DNA replication and/or postreplicative mRNA synthesis is necessary for ΔE3L-induced antiviral signaling. Postreplicative transcription of convergently oriented vaccinia virus genes is a likely source of the dsRNA signal (45) that is masked by E3 when keratinocytes are infected with wild-type vaccinia virus.
The antiviral response of ΔE3L-infected keratinocytes is independent of TLR3 and TRIF.
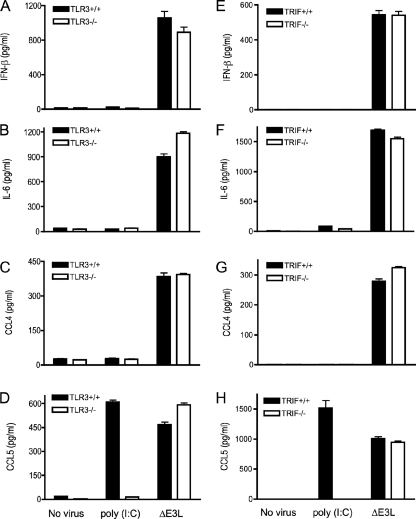
TLR3 recognizes dsRNA and activates the NF-κB and IRF3 signaling pathways (3, 15). The observed keratinocyte response to ΔE3L infection, entailing production of IFN-β, IL-6, CCL4, and CCL5, is clearly distinct from the response to the TLR3 agonist poly(I:C), which induces only CCL5. These results imply either (i) that ΔE3L triggers two dsRNA-dependent pathways, one involving CCL5 induction via TLR3 and another leading to IFN-β, IL-6, and CCL4 secretion or (ii) that ΔE3L infection stimulates production of all four secreted proteins by TLR3-independent pathway(s). To address this issue, we isolated primary keratinocytes from homozygous TLR3−/− knockout mice and from age-matched wild-type littermate controls. The keratinocytes were treated with poly(I:C) or infected with ΔE3L. Whereas poly(I:C) induced secretion of CCL5 by wild-type keratinocytes, this response was abolished in cells lacking TLR3 (Fig. 3D), verifying that poly(I:C) signals exclusively through TLR3. In contrast, ΔE3L infection elicited similar levels of IFN-β, IL-6, CCL4, and CCL5 production in keratinocytes derived from wild-type and TLR3−/− knockout animals (Fig. 3A to D). The cytoplasmic adaptor protein TRIF is essential for TLR3-mediated induction of IFN-β and activation of IRF3 (53, 72). Primary keratinocytes from homozygous TRIF−/− mice and from age-matched wild-type littermate controls were treated with poly(I:C) or infected with ΔE3L. As expected, poly(I:C)-induced secretion of CCL5 was abolished in cells devoid of TRIF (Fig. 3H). However, ΔE3L infection resulted in similar levels of IFN-β, IL-6, CCL4, and CCL5 production in wild-type and TRIF−/− keratinocytes (Fig. 3E to H). We conclude that the keratinocyte response to poxvirus infection is independent of TLR3 and TRIF.
FIG. 3.
Innate immune response of ΔE3L-infected keratinocytes is independent of TLR3 and TRIF. Keratinocytes were prepared from the skin of female TLR3−/− mice or TRIF−/− mice and their wild-type littermate controls. Cells (4 × 106) were infected with ΔE3L at a multiplicity of 10 for 1 h. Infected cells were washed to remove unabsorbed viruses and then incubated in fresh medium. Uninfected cells were processed in parallel and either incubated in fresh medium (No virus) or medium supplemented with 10 μg/ml poly(I:C). Supernatants were collected 18 h later, and the concentrations of IL-6, CCL4, CCL5, and IFN-β were determined by ELISA. Error bars represent standard deviations from the means of triplicate experiments.
Sensing ΔE3L virus infection in keratinocytes is independent of TLR9 and MyD88.
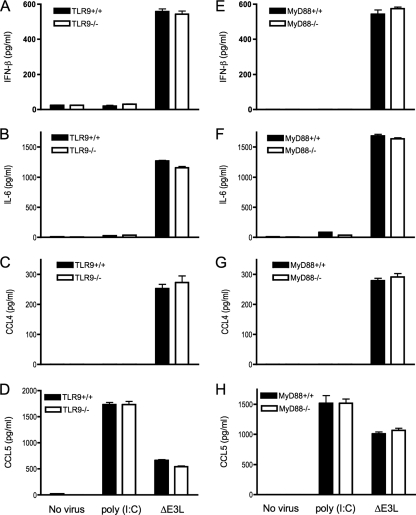
Large DNA viruses such as herpes simplex and cytomegalovirus induce production of type I interferon partly through activation of TLR9 by CpG motifs in viral DNA (22, 36, 46, 63). Although human keratinocytes express TLR9, the functional relevance of TLR9 in skin innate immune defense against bacterial or viral pathogens has not been defined. To evaluate whether TLR9 is involved in mouse keratinocyte innate immune responsiveness, we isolated primary keratinocytes from homozygous TLR9−/− knockout mice and from age-matched wild-type littermates and exposed them to poly(I:C) or infected them with ΔE3L. The induction of CCL5 by poly(I:C) was unaffected by loss of TLR9, as expected (Fig. 4D). ΔE3L infection induced similar levels of induction of IFN-β, IL-6, CCL4, and CCL5 production in wild-type and TRL9−/− knockout keratinocytes (Fig. 4A to D).
FIG. 4.
Innate immune response of ΔE3L-infected keratinocytes independent of TLR9 and MyD88. Keratinocytes were prepared from the skin of female TLR9−/− mice or MyD88−/− mice and their wild-type littermate controls. Cells (4 × 106) were infected with ΔE3L at a multiplicity of 10 for 1 h. Infected cells were washed to remove unabsorbed viruses and then incubated in fresh medium. Uninfected cells were processed in parallel and either incubated in fresh medium (No virus) or medium supplemented with 10 μg/ml poly(I:C). Supernatants were collected 18 h later, and the concentrations of IL-6, CCL4, CCL5, and IFN-β were determined by ELISA. Error bars represent standard deviations from the means of triplicate experiments.
MyD88 is an essential cytoplasmic adaptor molecule for signaling by all TLRs except TLR3 (1). Keratinocytes from homozygous MyD88−/− knockout mice and from age-matched wild-type littermates were exposed to poly(I:C) or infected with ΔE3L. The induction of CCL5 by poly(I:C) was unaffected by loss of MyD88, again, as expected (Fig. 4H). ΔE3L infection induced similar levels of induction of IFN-β, IL-6, CCL4, and CCL5 production in wild-type and MyD88−/− knockout keratinocytes (Fig. 4E to H). We surmise that the keratinocyte response to poxvirus infection is independent of TLR9 and MyD88 and, by inference, independent of other TLRs that rely on MyD88.
MAVS/IRF3 pathway is required for ΔE3L-induced antiviral innate immune responses.
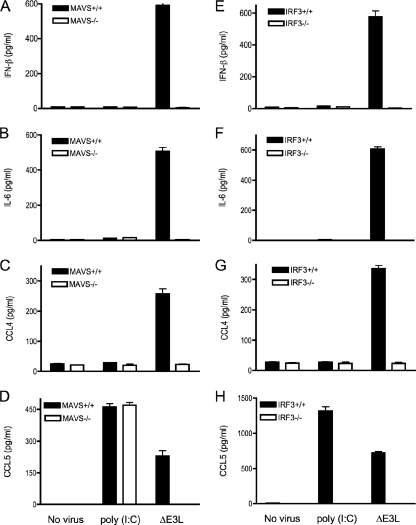
Cytoplasmic RNA (either dsRNA or 5′ triphosphate-terminated RNA) produced during viral replication binds to RIG-I/MDA-5 and triggers activation of IRF3 and NF-κB through the mitochondrial adaptor molecule MAVS (32, 48, 58, 71). MAVS is required for innate immune responses to Sendai virus in embryonic fibroblasts, macrophages, and conventional dendritic cells but not in plasmacytoid dendritic cells (62). MAVS+/− and MAVS−/− mice are more susceptible to lethal infection with vesicular stomatitis virus than wild-type littermate controls (62). Whether MAVS is involved in antiviral innate immunity in keratinocytes is unclear, as is the role of MAVS during a poxvirus infection. To address these questions, we infected primary keratinocytes from MAVS−/− mice and wild-type littermates with ΔE3L virus and then determined the levels of IFN-β and other proinflammatory cytokines/chemokines by ELISA. We found that ΔE3L-induced production of IFN-β, IL-6, CCL4, and CCL5 was completely abolished in MAVS-deficient keratinocytes (Fig. 5A to D). In contrast, poly(I:C)-induced production of CCL5 was unaffected by ablation of MAVS (Fig. 5D).
FIG. 5.
MAVS and IRF3 are required for the ΔE3L-induced innate immune response. Keratinocytes were prepared from the skin of female MAVS−/− mice or IRF3−/− mice and their wild-type littermate controls. Cells (4 × 106) were infected with wild-type ΔE3L at a multiplicity of 10 for 1 h. Infected cells were washed to remove unabsorbed viruses and then incubated in fresh medium. Uninfected cells were processed in parallel and either incubated in fresh medium (No virus) or medium supplemented with 10 μg/ml poly(I:C). Supernatants were collected 18 h later, and the concentrations of IL-6, CCL4, CCL5, and IFN-β were determined by ELISA. Error bars represent standard deviations from the means of triplicate experiments.
IRF3 is an essential regulator of IFN-β expression and plays an important role in antiviral innate immunity (56). IRF3 also directly activates the expression of CCL5 (41). To address whether IRF3 might be involved in ΔE3L-induced antiviral innate immunity in keratinocytes, we compared the production of IFN-β, IL-6, CCL4, and CCL5 in virus-infected keratinocytes from IRF3+/+ and IRF3−/− mice. We found that ΔE3L-induced production of IFN-β, IL-6, CCL4, and CCL5 was completely abolished in IRF3−/− keratinocytes (Fig. 5E to H). Poly(I:C)-induced production of CCL5 was also eliminated in IRF3−/− keratinocytes (Fig. 5H). Thus, IRF3 is essential for sensing ΔE3L infection and for responding to poly(I:C) via TLR3 and TRIF.
ΔE3L virus infection of keratinocytes induces MAVS-dependent activation of IRF3.
Western blot analyses of whole-cell lysates of primary keratinocytes infected with wild-type vaccinia or ΔE3L virus were performed with antibodies directed against the active Ser396-phosphorylated form of IRF3 (P∼IRF3). An immunoreactive P∼IRF3 protein was detected at 8, 12, and 22 h postinfection of keratinocytes with ΔE3L but not in uninfected cells or cells infected with wild-type vaccinia virus (Fig. 6A). The designation of the immunoreactive protein as P∼IRF3 was verified by its absence in ΔE3L-infected IRF3−/− keratinocytes (Fig. 6B). A second keratinocyte polypeptide detected by Western blotting was clearly not IRF3, insofar as it persisted in IRF3−/− cells. ΔE3L induction of IRF3 phosphorylation was abolished in MAVS−/− keratinocytes (Fig. 6C).
FIG. 6.
ΔE3L virus infection of keratinocytes induces MAVS-dependent activation of IRF3. (A) Keratinocytes from the skin of female C57BL/6 mice (4 × 106 cells) were infected with wild-type (WT) vaccinia virus or ΔE3L at a multiplicity of 10. Whole-cell lysates of infected cells or mock-infected controls were prepared at 1, 4, 8, 14, and 22 h postinfection. Aliquots (20 μl) of the lysates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and the polypeptides were transferred electrophoretically to a nitrocellulose membrane. Phosphorylation of IRF3 was determined using a rabbit polyclonal antibody specific for phosphoserine-396 of IRF3 (Cell Signaling). The immunoreactive polypeptides were visualized with a chemiluminescence detection kit (Amersham). The polypeptide corresponding to P-IRF3 is indicated by the arrow at right. The positions of 50-kDa and 40-kDa size markers are indicated at left. (B) Keratinocytes (4 × 106 cells) from the skin of IRF3−/− mice and their littermate controls were infected with ΔE3L virus at a multiplicity of 10. Whole-cell lysates were prepared at 22 h postinfection. Phosphorylation of IRF3 was gauged by Western blot analysis as described in panel A. (C) Keratinocytes from the skin of MAVS−/− mice and their littermate controls (4 × 106 cells) were infected with ΔE3L virus at a multiplicity of 10. Whole-cell lysates of infected cells and mock-infected controls were prepared at 3, 8, and 22 h postinfection. P-IRF3 was detected by Western blot analysis. hpi, hours postinfection.
Keratinocyte response to ΔE3L does not require the IFN-α/β receptor.
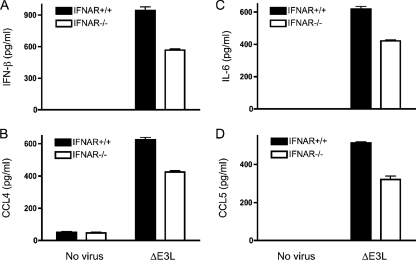
Secreted type I IFNs boost cellular responses to virus infection by binding a cell surface receptor and activating a signaling pathway that results in transcription of many cellular genes involved in innate and acquired immunity (65). IFNs can exert an autocrine effect on the innate immune response in virus-infected cells or a paracrine effect on uninfected bystander cells. The observation that keratinocytes respond to ΔE3L by secreting IFN-β raises the issue of whether IL-6, CCL4, and CCL5 are produced as part of a coordinated response to the poxvirus signal or as a secondary consequence of IFN-β signaling. To answer this question, we exploited primary keratinocytes from homozygous IFNAR−/− knockout mice that lack a functional IFN-α/β receptor and are therefore incapable of mounting a response to IFN-β (27, 50). We found that ΔE3L-induced production of IFN-β, IL-6, CCL4, and CCL5 was only modestly diminished in IFNAR−/− keratinocytes (by 32 to 40%) compared to wild-type control cells (Fig. 7). We surmise that most of the cytokine response to ΔE3L infection is generated directly by the infected keratinocytes, with at most a small boost from feedback signaling by secreted IFN-β.
FIG. 7.
ΔE3L infection of keratinocytes lacking the IFN receptor. Keratinocytes were prepared from the skin of female IFNAR−/− mice and their wild-type littermate controls. Cells (4 × 106) were infected with ΔE3L at a multiplicity of 10 for 1 h. Infected cells were washed to remove unabsorbed viruses and then incubated in fresh medium Uninfected cells were processed in parallel. Supernatants were collected 18 h later, and the concentrations of IL-6, CCL4, CCL5, and IFN-β were determined by ELISA. Error bars represent standard deviations from the means of triplicate experiments.
DISCUSSION
The present study provides new insights to the interaction of poxviruses with the skin immune system. Prior analyses focused on understanding the etiology of eczema vaccinatum, a fulminant vaccinia virus skin infection that occurs in the setting of AD. Howell et al. (25) showed that cathelicidin, an antimicrobial peptide produced by injured or infected skin, inactivates vaccinia virus infectivity when the peptide is incubated with virions prior to inoculation of cell monolayers. Cathelicidin-deficient mice developed larger and more numerous skin lesions when infected by scarification with vaccinia virus (25). Cathelicidin production rises in response to vaccinia virus infection of skin biopsies; notably, this response is attenuated in vaccinia virus-infected AD skin (26). Cathelicidin induction in vaccinia virus-infected skin was apparently mediated via TLR3 and could be elicited in uninfected skin biopsies by the TLR3 agonist poly(I:C) (26).
As shown here, antimicrobial peptides and TLR3 signaling are not the full story with respect to the poxvirus-skin dynamic. Indeed, we find that murine keratinocytes can mount a vigorous antiviral cytokine response when they sense a vaccinia virus infection. However, this response is thwarted completely by the virus-encoded RNA-binding protein E3, which acts in keratinocytes as an inhibitor of a signaling pathway through MAVS and IRF3. Little information was available previously concerning the capacity of primary mouse keratinocytes to mount an innate immune response. We find here that they do not respond to agonists of TLR4 and TLR9, as gauged by secretion of any of the proinflammatory mediator proteins assayed. However, they are stimulated by exogenous poly(I:C) to produce CCL5 via a signaling pathway requiring TLR3, TRIF, and IRF3.
Vaccinia ΔE3L infection of keratinocytes results in secretion of IFN-β, IL-6, and two CC chemokines: CCL4 (MIP1-β) and CCL5 (RANTES). The CC chemokines attract and activate macrophages and T cells. There are obvious advantages to a dermatotrophic virus to suppress this arm of the host's innate immune response in the skin. A remarkable feature of poxviruses is that they evade the CC chemokines at multiple levels: (i) by E3-mediated inhibition of CCL4 and CCL5 production in the infected skin cell (see above) and (ii) by encoding a secreted 35-kDa CC chemokine inhibitor protein (vCCI) that binds with high affinity to CC-type chemokines (4, 74) and blocks their productive interaction with chemokine receptors on the surface of leukocytes (2). Most poxviruses that infect mammalian species (including variola, cowpox, and the Lister strain of vaccinia) produce vCCI, the exception being the vaccinia WR strain used here and in most other studies of viral replication. Reading et al. (54) showed that introducing the vaccinia virus Lister vCCI gene into vaccinia virus WR attenuated lung inflammatory responses in a mouse model of lethal intranasal infection. Thus, poxviruses do not only interdict production of CC chemokines by virus-infected cells (at least in skin cells) but also impede the action of CC chemokines elaborated by proinflammatory bystander cells in other organs such as lung (54).
IL-6 is a cytokine with multiple immune functions that plays a critical role in defending mice against vaccinia virus infection (35). We find that IL-6 is secreted by ΔE3L-infected mouse keratinocytes but not by cells infected with wild-type vaccinia virus. These results are consistent with a prior report that deletion of E3L leads to increased levels of IL-6 mRNA in HeLa cells infected with modified vaccinia Ankara (44).
IFN-β is a key mediator of innate antiviral immunity that exerts many diverse effects on both poxvirus-infected cells and uninfected immune responder cells; hence, poxviruses take pains to disrupt IFN signaling pathways at multiple levels, both extracellular and intracellular (57, 65). Vaccinia virus interdicts this process at the earliest possible step during infection of mouse keratinocytes by suppressing the very production of IFN-β. Our finding that ΔE3L-infected keratinocytes secrete IFN-β is in accord with earlier reports that (i) ΔE3L infection of cultured human HT10180 cells induced the accumulation of IFN-β mRNA, whereas no mRNA response was seen in cell infected with wild-type vaccinia virus (70); and (ii) chicken embryo fibroblasts secreted type I IFNs when abortively infected with modified vaccinia Ankara-ΔE3L virus (23).
By exploiting keratinocytes derived from knockout mice, we have identified the pathway components that sense vaccinia virus infection and are blocked by E3 (MAVS and IRF3) as well as those that are irrelevant to the keratinocytes’ antipoxviral response (TLR3, TRIF, TLR9, and MyD88). We see that IRF3 phosphorylation, a marker for its transcriptionally active state, is triggered in ΔE3L-infected primary keratinocytes but not in cells infected with wild-type vaccinia virus. E3L blockade of IRF3 phosphorylation and/or IRF3-mediated transcriptional responses was noted previously in a variety of continuous cell lines (38, 60, 70). Although consideration of E3's effects on virus host dynamics and cell physiology have most often focused on PKR as the major target, this view has been challenged by studies showing (i) that E3 interdicts a PKR-independent IRF3 activation pathway (60) and (ii) that the avirulent vaccinia ΔE3L mutant remains avirulent in mice knocked out for PKR and RNase L (70). A more nuanced view is emerging whereby E3's blockade of PKR is critical for a productive cellular infection by virtue of preventing PKR-induced apoptosis and PKR-induced inhibition of viral protein synthesis (75), but another cellular pathway is targeted by E3 to suppress the innate immune response to poxvirus infection.
Here, we identified MAVS as an essential component of the poxvirus-sensing pathway in murine keratinocytes, acting upstream of IRF3. MAVS is an obligate mediator of signaling through the cytoplasmic viral RNA sensors RIG-I/MDA5 that drive the innate immune responses to many RNA viruses. Ours is the first demonstration that the MAVS-dependent RNA-sensing pathway is triggered by infection with a poxvirus (a DNA virus) and then actively suppressed by a poxvirus gene product. RIG-I binds and responds to either dsRNA or 5′-triphosphate single-stranded RNA (12, 17, 64). It is most likely that the agonist for innate immune signaling in ΔE3L-infected cells is dsRNA formed by vaccinia virus intermediate mRNAs (45). Single-stranded viral RNA is an unlikely agonist in this setting because the 5′-triphosphate ends of nascent vaccinia virus mRNAs are quickly converted to a 5′-diphosphate and then further modified to m7GpppRNA by the vaccinia virus mRNA capping enzyme.
Our finding that the C-terminal dsRNA binding domain of E3 suffices to suppress the keratinocyte response to vaccinia virus infection further implicates dsRNA as the agonist, insofar as this domain binds avidly to dsRNA duplexes but not appreciably to duplex DNA or an RNA/DNA hybrid (21). A single K167A mutation in the E3 C-terminal domain that disrupts dsRNA binding (20) abolishes the ability of E3 to inhibit PKR-independent IRF phosphorylation in cultured cells (60). Moreover, E3 point mutations that disrupt dsRNA binding also eliminate the ability of E3 to block the RIG-I-dependent activation of the IFN-β promoter triggered in mouse fibroblasts infected with Sendai viruses that were engineered to produce cytoplasmic dsRNA with capped 5′ ends (61). The latter scenario, entailing viral transcription of capped mRNAs from complementary sense/antisense templates, is analogous to the mechanism by which dsRNA is formed in poxvirus-infected cells. A simplistic view is that E3 blocks the MAVS-dependent cytoplasmic viral RNA sensing pathway in poxvirus-infected cells by competing with RIG-I and/or MDA5 for binding to dsRNA. We do not rule out more complex scenarios in which E3 engages in inhibitory protein-protein interactions (55) with one or more of the components of the MAVS-dependent RNA sensing pathway.
Vaccinia viruses purposefully deleted of genes that affect replication fitness in mammalian cells are considered plausible candidates for the next generation of safer live vaccines. Vaccination of mice with a relatively modest dose of ΔE3L by the intranasal route resulted in limited replication in the nasal mucosa (with no spread to lung or brain) and elicited protective immunity against subsequent challenge with a lethal dose of wild-type vaccinia virus (67). Our observations concerning how ΔE3L infection activates innate immunity in skin epithelial cells suggests that the MAVS pathway could be a brake on viral spread in nasal epithelium, and they raise the prospect that scarification with ΔE3L might be an effective immunization strategy with lower risk of unchecked spread in the skin than wild-type vaccinia virus.
Acknowledgments
This work was supported by NIH grant K-08 AI073736 (L.D.) and a Northeast Biodefense Center Targeted Project Award (S.S. and L.D.) supported by NIH grant U54-AI057158. L.D. is the recipient of a Dermatologist Investigator Research Fellowship and a Physician Scientist Career Development Award from the Dermatology Foundation. T.P. is the recipient of a Research Training Fellowship for Medical Students from the Howard Hughes Medical Institute. S.S. is an American Cancer Society Research Professor.
We thank Eric Pamer and Edward Niles for helpful discussions.
We have no conflicting financial interests.
Footnotes
Published ahead of print on 20 August 2008.
REFERENCES
- 1.Akira, S., S. Uematsu, and O. Takeuchi. 2006. Pathogen recognition and innate immunity. Cell 124783-801. [DOI] [PubMed] [Google Scholar]
- 2.Alcamí, A., J. A. Symons, P. D. Collins, T. J. Williams, and G. L. Smith. 1998. Blockade of chemokine activity by a soluble chemokine blocking protein from vaccinia virus. J. Immunol. 160624-633. [PubMed] [Google Scholar]
- 3.Alexopoulou, L., A. C. Holt, R. Medzhitov, and R. A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 413732-738. [DOI] [PubMed] [Google Scholar]
- 4.Arnold, P. L., and D. H. Fremont. 2006. Structural determinants of chemokine binding by an ectromelia virus-encoded decoy receptor. J. Virol. 807439-7449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beattie, E., E. B. Kauffman, H. Martinez, M. E. Perkus, B. L. Jacobs, E. Paoletti, and J. Tartaglia. 1996. Host-range restriction of vaccinia virus E3L-specific deletion mutants. Virus Genes. 1289-94. [DOI] [PubMed] [Google Scholar]
- 6.Braff, M. H., and R. L. Gallo. 2006. Antimicrobial peptides: an essential component of the skin defensive barrier. Curr. Top. Microbiol. Immunol. 30691-110. [DOI] [PubMed] [Google Scholar]
- 7.Brandt, T., M. C. Heck, S. Vijaysri, G. M. Jentarra, J. M. Cameron, and B. L. Jacobs. 2005. The N-terminal domain of the vaccinia virus E3L-protein is required for neurovirulence, but not induction of a protective immune response. Virology 333263-270. [DOI] [PubMed] [Google Scholar]
- 8.Brandt, T. A., and B. L. Jacobs. 2001. Both carboxy- and amino-terminal domains of the vaccinia virus interferon resistance gene, E3L, are required for pathogenesis in a mouse model. J. Virol. 75850-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang, H. W., and B. L. Jacobs. 1993. Identification of a conserved motif that is necessary for binding of the vaccinia virus E3L gene products to double-stranded RNA. Virology 194537-547. [DOI] [PubMed] [Google Scholar]
- 10.Chang, H. W., J. C. Watson, and B. L. Jacobs. 1992. The E3L gene of vaccinia virus encodes an inhibitor of the interferon-induced, double-stranded RNA-dependent protein kinase. Proc. Natl. Acad. Sci. USA 894825-4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang, H. W., L. H. Uribe, and B. L. Jacobs. 1995. Rescue of vaccinia virus lacking the E3L gene by mutants of E3L. J. Virol. 696605-6608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cui, S., K. Eisenächer, A. Kirchhofer, K. Brzózka, A. Lammens, K. Lammens, T. Fujita, K. K. Conzelmann, A. Krug, and K. P. Hopfner. 2008. The C-terminal regulatory domain is the RNA 5′-triphosphate sensor of RIG-I. Mol. Cell 29169-179. [DOI] [PubMed] [Google Scholar]
- 13.Dai, X., K. Sayama, K. Yamasaki, M. Tohyama, Y. Shirakata, Y. Hanakawa, S. Tokumaru, Y. Yahata, L. Yang, A. Yoshimura, and K. Hashimoto. 2006. SOCS1-negative feedback of STAT1 activation is a key pathway in the dsRNA-induced innate immune response of human keratinocytes. J. Investig. Dermatol. 1261574-1581. [DOI] [PubMed] [Google Scholar]
- 14.Deng, L., P. Dai, W. Ding, R. D. Granstein, and S. Shuman. 2006. Vaccinia virus infection attenuates innate immune responses and antigen presentation by epidermal dendritic cells. J. Virol. 809977-9987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doyle, S., S. Vaidya, R. O'Connell, H. Dadgostar, P. Dempsey, T. Wu, G. Rao, R. Sun, M. Haberland, R. Modlin, and G. Cheng. 2002. IRF3 mediates a TLR3/TLR4-specific antiviral gene program. Immunity 17251-263. [DOI] [PubMed] [Google Scholar]
- 16.García, M. A., J. Gil, I. Ventoso, S. Guerra, E. Domingo, C. Rivas, and M. Esteban. 2006. Impact of protein kinase PKR in cell biology: from antiviral to antiproliferative action. Microbiol. Mol. Biol. Rev. 701032-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gee, P., P. K. Chua, J. Gevorkyan, K. Klumpp, I. Najera, D. C. Swinney, and J. Deval. 2008. Essential role of the N-terminal domain in the regulation of RIG-I ATPase activity. J. Biol. Chem. 2839488-9496. [DOI] [PubMed] [Google Scholar]
- 18.Génin, P., M. Algarté, P. Roof, R. Lin, and J. Hiscott. 2000. Regulation of RANTES chemokine gene expression requires cooperativity between NF-κB and IFN-regulatory factor transcription factors. J. Immunol. 1645352-5361. [DOI] [PubMed] [Google Scholar]
- 19.Ha, S. C., N. K. Lokanath, D. Van Quyen, C. A. Wu, K. Lowenhaupt, A. Rich, Y. G. Kim, and K. K. Kim. 2004. A poxvirus protein forms a complex with left-handed Z-DNA: crystal structure of a Yatapoxvirus Zα bound to DNA. Proc. Natl. Acad. Sci. USA 10114367-14372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho, C. K., and S. Shuman. 1996. Mutational analysis of the vaccinia virus E3 protein defines amino acid residues involved in E3 binding to double-stranded RNA. J. Virol. 702611-2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ho, C. K., and S. Shuman. 1996. Physical and functional characterization of the double-stranded RNA binding protein encoded by the vaccinia virus E3 gene. Virology 217272-284. [DOI] [PubMed] [Google Scholar]
- 22.Hochrein, H., B. Schlatter, M. O'Keeffe, C. Wagner, F. Schmitz, M. Schiemann, S. Bauer, M. Suter, and H. Wagner. 2004. Herpes simplex virus type-1 induces IFN-α production via Toll-like receptor 9-dependent and -independent pathways. Proc. Natl. Acad. Sci. USA 10111416-11421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hornemann, S., O. Harlin, C. Staib, S. Kisling, V. Erfle, B. Kaspers, G. Häcker, and G. Sutter. 2003. Replication of modified vaccinia virus Ankara in primary chicken embryo fibroblasts requires expression of the interferon resistance gene E3L. J. Virol. 778394-8407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hornung, V., J. Ellegast, S. Kim, K. Brzózka, A. Jung, H. Kato, H. Poeck, S. Akira, K. K. Conzelmann, M. Schlee, S. Endres, and G. Hartmannn. 2006. 5′-Triphosphate RNA is the ligand for RIG-I. Science 314994-997. [DOI] [PubMed] [Google Scholar]
- 25.Howell, M. D., J. F. Jones, K. O. Kisich, J. E. Streib, R. L. Gallo, and D. Y. Leung. 2004. Selective killing of vaccinia virus by LL-37: implications for eczema vaccinatum. J. Immunol. 1721763-1767. [DOI] [PubMed] [Google Scholar]
- 26.Howell, M. D., R. L. Gallo, M. Boguniewicz, J. F. Jones, C. Wong, J. E. Streib, and D. Y. Leung. 2006. Cytokine milieu of atopic dermatitis skin subverts the innate immune response to vaccinia virus. Immunity 24341-348. [DOI] [PubMed] [Google Scholar]
- 27.Hwang, S. Y., P. J. Hertzog, K. A. Holland, S. H. Sumarsono, M. J. Tymms, J. A. Hamilton, G. Whitty, I. Bertoncello, and I. Kola. 1995. A null mutation in the gene encoding a type I interferon receptor component eliminates antiproliferative and antiviral responses to interferons alpha and beta and alters macrophage responses. Proc. Natl. Acad. Sci. USA 9211284-11288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kato, H., O. Takeuchi, S. Sato, M. Yoneyama, M. Yamamoto, K. Matsui, S. Uematsu, A. Jung, T. Kawai, K. J. Ishii, O. Yamaguchi, K. Otsu, T. Tsujimura, C. S. Koh, C. Reis e Sousa, Y. Matsuura, T. Fujita, and S. Akira. 2006. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441101-105. [DOI] [PubMed] [Google Scholar]
- 29.Kato, H., S. Sato, M. Yoneyama, M. Yamamoto, S. Uematsu, K. Matsui, T. Tsujimura, K. Takeda, T. Fujita, O. Takeuchi, and S. Akira. 2005. Cell type-specific involvement of RIG-I in antiviral response. Immunity 2319-28. [DOI] [PubMed] [Google Scholar]
- 30.Kawai, K., H. Shimura, M. Minagawa, A. Ito, K. Tomiyama, and M. Ito. 2002. Expression of functional Toll-like receptor 2 on human epidermal keratinocytes. J. Dermatol. Sci. 30185-194. [DOI] [PubMed] [Google Scholar]
- 31.Kawai, T., and S. Akira. 2006. Innate immune recognition of viral infection. Nat. Immunol. 7131-137. [DOI] [PubMed] [Google Scholar]
- 32.Kawai, T., K. Takahashi, S. Sato, C. Coban, H. Kumar, H. Kato, K. J. Ishii, O. Takeuchi, and S. Akira. 2005. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat. Immunol. 6981-988. [DOI] [PubMed] [Google Scholar]
- 33.Kim, Y. G., K. Lowenhaupt, D. B. Oh, K. K. Kim, and A. Rich. 2004. Evidence that vaccinia virulence factor E3L binds to Z-DNA in vivo: Implications for development of a therapy for poxvirus infection. Proc. Natl. Acad. Sci. USA 1011514-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim, Y. G., M. Muralinath, T. Brandt, M. Pearcy, K. Hauns, K. Lowenhaupt, B. L. Jacobs, and A. Rich. 2003. A role for Z-DNA binding in vaccinia virus pathogenesis. Proc. Natl. Acad. Sci. USA 1006974-6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kopf, M., H. Baumann, G. Freer, M. Freudenberg, M. Lamers, T. Kishimoto, R. Zinkernagel, H. Bluethmann, and G. Köhler. 1994. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature 368339-342. [DOI] [PubMed] [Google Scholar]
- 36.Krug, A., G. D. Luker, W. Barchet, D. A. Leib, S. Akira, and M. Colonna. 2004. Herpes simplex virus type 1 activates murine natural interferon-producing cells through Toll-like receptor 9. Blood 1031433-1437. [DOI] [PubMed] [Google Scholar]
- 37.Langland, J. O., and B. L. Jacobs. 2002. The role of the PKR-inhibitory genes, E3L and K3L, in determining vaccinia virus host range. Virology 299133-141. [DOI] [PubMed] [Google Scholar]
- 38.Langland, J. O., J. C. Kash, V. Carter, M. J. Thomas, M. G. Katze, and B. L. Jacobs. 2006. Suppression of proinflammatory signal transduction and gene expression by the dual nucleic acid binding domains of the vaccinia virus E3L proteins. J. Virol. 8010083-10095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lebre, M. C., A. M. van der Aar, L. van Baarsen, T. M. van Capel, J. H. Schuitemaker, M. L. Kapsenberg, and E. C. de Jong. 2007. Human keratinocytes express functional Toll-like receptor 3, 4, 5, and 9. J. Investig. Dermatol. 127331-341. [DOI] [PubMed] [Google Scholar]
- 40.Lebre, M. C., J. C. Antons, P. Kalinski, J. H. Schuitemaker, T. M. van Capel, M. L. Kapsenberg, and E. C. De Jong. 2003. Double-stranded RNA-exposed human keratinocytes promote Th1 response by inducing a type-1 polarized phenotype in dendritic cells: role of keratinocyte-derived tumor necrosis factor alpha, type I interferons, and interleukin-18. J. Investig. Dermatol. 120990-997. [DOI] [PubMed] [Google Scholar]
- 41.Lin, R., R. S. Noyce, S. E. Collins, R. D. Everett, and K. L. Mossman. 2004. The herpes simplex virus ICP0 RING finger domain inhibits IRF3- and IRF7-mediated activation of interferon-stimulated genes. J. Virol. 781675-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu, L., I. Botos, Y. Wang, J. N. Leonard, J. Shiloach, D. M. Segal, and D. R. Davies. 2008. Structural basis of Toll-like receptor 3 signaling with double-stranded RNA. Science 320379-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu, L., Z. Xu, R. C. Fuhlbrigge, V. Peña-Cruz, J. Lieberman, and T. S. Kupper. 2005. Vaccinia virus induces strong immunoregulatory cytokine production in healthy human epidermal keratinocytes: a novel strategy for immune evasion. J. Virol. 797363-7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ludwig, H., J. Mages, C. Staib, M. H. Lehmann, R. Lang, and G. Sutter. 2005. Role of viral factor E3L in modified vaccinia virus Ankara infection of human HeLa cells: regulation of the virus life cycle and identification of differentially expressed host genes. J. Virol. 792584-2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ludwig, H., Y. Suezer, Z. Waibler, U. Kalinke, B. S. Schnierle, and G. Sutter. 2006. Double-stranded RNA-binding protein E3 controls translation of viral intermediate RNA, marking an essential step in the life cycle of modified vaccinia virus Ankara. J. Gen. Virol. 871145-1155. [DOI] [PubMed] [Google Scholar]
- 46.Lund, J., A. Sato, S. Akira, R. Medzhitov, and A. Iwasaki. 2003. Toll-like receptor 9-mediated recognition of herpes simplex virus-2 by plasmacytoid dendritic cells. J. Exp. Med. 198513-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mempel, M., V. Voelcker, G. Köllisch, C. Plank, R. Rad, M. Gerhard, C. Schnopp, P. Franuberger, A. K. Walli, J. Ring, D. Abeck, and M. Ollert. 2003. Toll-like receptor expression in human keratinocytes: nuclear factor kappaB controlled gene activation by Staphylococcus aureus is Toll-like receptor 2 but not Toll-like receptor 4 or platelet activating factor receptor dependent. J. Investig. Dermatol. 1211389-1396. [DOI] [PubMed] [Google Scholar]
- 48.Meylan, E., J. Curran, K. Hofmann, D. Moradpour, M. Binder, R. Bartenschlager, and J. Tschopp. 2005. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature 4371167-1172. [DOI] [PubMed] [Google Scholar]
- 49.Miller, L. S., O. E. Sørensen, P. T. Liu, H. R. Jalian, D. Eshtiaghpour, B. E. Behmanesh, W. Chung, T. D. Starner, J. Kim, P. A. Sieling, T. Ganz, and R. L. Modlin. 2005. TGF-alpha regulates TLR expression and function on epidermal keratinocytes. J. Immunol. 1746137-6143. [DOI] [PubMed] [Google Scholar]
- 50.Müller, U., U. Steinhoff, L. F. Reis, S. Hemmi, J. Pavlovic, R. M. Zinkernagel, and M. Aguet. 1994. Functional role of type I and type II interferons in antiviral defense. Science 2641918-1921. [DOI] [PubMed] [Google Scholar]
- 51.Nakayama, T., J. Shirane, K. Hieshima, M. Shibano, M. Watanabe, Z. Jin, D. Nagakubo, T. Saito, Y. Shimomura, and O. Yoshie. 2006. Novel antiviral activity of chemokines. Virology 350484-492. [DOI] [PubMed] [Google Scholar]
- 52.Nallagatla, S. R., J. Hwang, R. Toroney, X. Zheng, C. E. Cameron, and P. C. Bevilacqua. 2007. 5′-Triphosphate-dependent activation of PKR by RNAs with short stem-loops. Science 3181455-1458. [DOI] [PubMed] [Google Scholar]
- 53.Oshiumi, H., M. Sasai, K. Shida, T. Fujita, M. Matsumoto, and T. Seya. 2003. TIR-containing adaptor molecule (TICAM)-2, a bridging adaptor recruiting to Toll-like receptor 4 TICAM-1 that induces interferon-beta. J. Biol. Chem. 27849751-49762. [DOI] [PubMed] [Google Scholar]
- 54.Reading, P. C., J. A. Symons, and G. L. Smith. 2003. A soluble chemokine-binding protein from vaccinia virus reduces virulence and the inflammatory response to infection. J. Immunol. 1701435-1442. [DOI] [PubMed] [Google Scholar]
- 55.Romano, P. R., F. Zhang, S. L. Tan, M. T. Garcia-Barrio, M. G. Katze, T. E. Dever, and A. G. Hinnebusch. 1998. Inhibition of double-stranded RNA-dependent protein kinase PKR by vaccinia virus E3: role of complex formation and the E3 N-terminal domain. Mol. Cell. Biol. 187304-7316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sato, M., H. Suemori, N. Hata, M. Asagiri, K. Ogasawara, K. Nakao, T. Nakaya, M. Katsuki, S. Noguchi, N. Tanaka, and T. Taniguchi. 2000. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-α/β gene induction. Immunity 13539-548. [DOI] [PubMed] [Google Scholar]
- 57.Seet, B. T., J. B. Johnston, C. R. Brunetti, J. W. Barrett, H. Everett, C. Cameron, J. Sypula, S. H. Nazarian, A. Lucas, and G. McFadden. 2003. Poxviruses and immune evasion. Annu. Rev. Immunol. 21377-423. [DOI] [PubMed] [Google Scholar]
- 58.Seth, R. B., L. Sun, C. K. Ea, and Z. J. Chen. 2005. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-κB and IRF3. Cell 122669-682. [DOI] [PubMed] [Google Scholar]
- 59.Sharma, S., B. R. tenOever, N. Grandvaux, G. P. Zhou, R. Lin, and J. Hiscott. 2003. Triggering the interferon antiviral response through an IKK-related pathway. Science 3001148-1151. [DOI] [PubMed] [Google Scholar]
- 60.Smith, E. J., I. Marié, A. Prakash, A. García-Sastre, and D. E. Levy. 2001. IRF3 and IRF7 phosphorylation in virus-infected cells does not require double-stranded RNA-dependent protein kinase R or IκB kinase but is blocked by vaccinia virus E3L protein. J. Biol. Chem. 2768951-8957. [DOI] [PubMed] [Google Scholar]
- 61.Strähle, L., J. B. Marq, A. Brini, S. Hausmann, D. Kolakofsky, and D. Garcin. 2007. Activation of the beta interferon promoter by unnatural Sendai virus infection requires RIG-I and is inhibited by viral C proteins. J. Virol. 8112227-12237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sun, Q., L. Sun, H. H. Liu, X. Chen, R. B. Seth, J. Forman, and Z. J. Chen. 2006. The specific and essential role of MAVS in antiviral innate immunity responses. Immunity 24633-642. [DOI] [PubMed] [Google Scholar]
- 63.Tabeta, K., P. Georgel, E. Janssen, X. Du, K. Hoebe, K. Crozat, S. Mudd, L. Shamel, S. Sovath, J. Goode, L. Alexopoulou, R. A. Flavell, and B. Beutler. 2004. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc. Natl. Acad. Sci. USA 1013516-3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Takahasi, K., M. Yoneyama, T. Nishihori, R. Hirai, H. Kumeta, R. Narita, M. Jr. Gale, F. Inagaki, and T. Fujita. 2008. Nonself RNA-sensing mechanism of RIG-I helicase and activation of antiviral immune responses. Mol. Cell 29428-440. [DOI] [PubMed] [Google Scholar]
- 65.Takaoka, A., and H. Yanai. 2006. Interferon signaling network in innate defense. Cell. Microbiol. 8907-922. [DOI] [PubMed] [Google Scholar]
- 66.Tohyama, M., X. Dai, K. Sayama, K. Yamasaki, Y. Shirakata, Y. Hanakawa, S. Tokumaru, Y. Yahata, L. Yang, H. Nagai, A. Takashima, and K. Hashimoto. 2005. dsRNA-mediated innate immunity of epidermal keratinocytes. Biochem. Biophys. Res. Commun. 335505-511. [DOI] [PubMed] [Google Scholar]
- 67.Vijaysri, S., G. Jentarra, M. C. Heck, A. A. Mercer, C. J. McInnes, and B. L. Jacobs. 2008. Vaccinia viruses with mutations in the E3L gene as potential replication-competent, attenuates vaccines: intranasal vaccination. Vaccine 26664-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vora, S., I. Damon, V. Fulginiti, S. G. Weber, M. Kahana, S. L. Stein, S. I. Gerber, S. Garcia-Houchins, E. Lederman, D. Hruby, L. Collins, D. Scott, K. Thompson, J. V. Barson, R. Regnery, C. Hughes, R. S. Daum, Y. Li, H. Zhao, S. Smith, Z. Braden, K. Karem, V. Olson, W. Davidson, G. Trindade, T. Bolken, R. Jordan, D. Tien, and J. Marcinak. 2008. Severe eczema vaccinatum in a household contact of a smallpox vaccinee. Clin. Infect. Dis. 461555-1561. [DOI] [PubMed] [Google Scholar]
- 69.Wollenberg, A., and R. Engler. 2004. Smallpox vaccination and adverse reactions to smallpox vaccine. Curr. Opin. Allergy. Clin. Immunol. 4271-275. [DOI] [PubMed] [Google Scholar]
- 70.Xiang, Y., R. C. Condit, S. Vijaysri, B. L. Jacobs, B. R. Williams, and R. H. Silverman. 2002. Blockade of interferon induction and action by the E3L double-stranded RNA binding proteins of vaccinia virus. J. Virol. 765251-5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu, L. G., Y. Y. Wang, K. J. Han, L. Y. Li, Z. Zhai, and H. B. Shu. 2005. VISA is an adapter protein required for virus-triggered IFN-beta signaling. Mol. Cell 19727-740. [DOI] [PubMed] [Google Scholar]
- 72.Yamamoto, M., S. Sato, H. Hemmi, K. Hoshino, T. Kaisho, H. Sanjo, O. Takeuchi, M. Sugiyama, M. Okabe, K. Takeda, and S. Akira. 2003. Role of adaptor TRIF in the MyD88-independent Toll-like receptor signaling pathway. Science 301640-643. [DOI] [PubMed] [Google Scholar]
- 73.Yoneyama, M., M. Kikuchi, T. Natsukawa, N. Shinobu, T. Imaizumi, M. Miyagishi, K. Taira, S. Akira, and T. Fujita. 2004. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5730-737. [DOI] [PubMed] [Google Scholar]
- 74.Zhang, L., M. Derider, M. A. McCornack, S. C. Jao, N. Isern, T. Ness, R. Moyer, and P. J. LiWang. 2006. Solution structure of the complex between poxvirus-encoded CC chemokine inhibitor vCCI and human MIP-1β. Proc. Natl. Acad. Sci. USA 10313985-13990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang, P., B. L. Jacobs, and C. E. Samuel. 2008. Loss of protein kinase PKR expression in human HeLa cells complements the vaccinia virus E3L deletion mutant phenotype by restoration of viral protein synthesis. J. Virol. 82840-848. [DOI] [PMC free article] [PubMed] [Google Scholar]