Abstract
During untreated human immunodeficiency virus type 1 (HIV-1) infection, virus-specific CD8+ T cells partially control HIV replication in peripheral lymphoid tissues, but host mechanisms of HIV control in the central nervous system (CNS) are incompletely understood. We characterized HIV-specific CD8+ T cells in cerebrospinal fluid (CSF) and peripheral blood among seven HIV-positive antiretroviral therapy-naïve subjects. All had grossly normal brain magnetic resonance imaging and spectroscopy and normal neuropsychometric testing. Frequencies of epitope-specific CD8+ T cells by direct tetramer staining were on average 2.4-fold higher in CSF than in blood (P = 0.0004), while HIV RNA concentrations were lower. Cells from CSF were readily expanded ex vivo and responded to a broader range of HIV-specific human leukocyte antigen class I restricted optimal peptides than did expanded cells from blood. HIV-specific CD8+ T cells, in contrast to total CD8+ T cells, in CSF and blood were at comparable maturation states, as assessed by CD45RO and CCR7 staining. The strong relationship between higher T-cell frequencies and lower levels of viral antigen in CSF could be the result of increased migration to and/or preferential expansion of HIV-specific T cells within the CNS. This suggests an important role for HIV-specific CD8+ T cells in control of intrathecal viral replication.
Human immunodeficiency virus type 1 (HIV-1) invades the central nervous system (CNS) early during primary infection (21, 30, 35), and proviral DNA persists in the brain throughout the course of HIV-1 disease (7, 25, 29, 47, 77, 83). Limited data from human and nonhuman primate studies suggest that little or no viral replication occurs in the brain during chronic, asymptomatic infection, based on the absence of demonstrable viral RNA or proteins (8, 85). In contrast, cognitive impairment affects approximately 40% of patients who progress to advanced AIDS without highly active antiretroviral therapy (21, 30, 35, 65). During HIV-associated dementia, there is active HIV-1 replication in the brain (23, 52, 61, 81), and viral sequence differences between cerebrospinal fluid (CSF) and peripheral tissues suggest distinct anatomic compartments of replication (18, 19, 22, 53, 75, 76, 78). Host mechanisms that control viral replication in the CNS during chronic, asymptomatic HIV-1 infection are incompletely understood.
Anti-HIV CD8+ T cells are present in blood and peripheral tissues throughout the course of chronic HIV-1 infection (2, 14). Multiple lines of evidence support a critical role for these cells in controlling HIV-1 replication. During acute HIV-1 infection, the appearance of CD8+ T-cell responses correlates temporally with a decline in viremia (11, 43), and a greater proliferative capacity of peripheral blood HIV-specific CD8+ T cells correlates with better control of viremia (36, 54). In addition, the presence of certain major histocompatibility complex class I human leukocyte antigen (HLA) alleles, notably HLA-B*57, predicts slower progression to AIDS and death during chronic, untreated HIV-1 infection (55, 62). Finally, in the simian immunodeficiency virus (SIV) model, macaques depleted of CD8+ T cells experience increased viremia and rapid disease progression (39, 51, 67).
Little is known regarding the role of intrathecal anti-HIV CD8+ T cells in HIV neuropathogenesis. Nonhuman primate studies have identified SIV-specific CD8+ T cells in the CNS early after infection (16, 80). Increased infiltration of SIV antigen-specific CD8+ T cells and cytotoxic T lymphocytes has been detected only in CSF of slow progressors without neurological symptoms (72). In chronically infected macaques with little or no SIV replication in the brain, the frequency of HIV-specific T cells was higher in CSF than in peripheral blood but did not correlate with the level of plasma viremia or CD4+ T-cell counts (56). Although intrathecal anti-HIV CD8+ T cells may help control viral replication, a detrimental role in the neuropathogenesis of HIV-1 has also been postulated (38). Immune responses contribute to neuropathogenesis in models of other infectious diseases, and during other viral infections cytotoxic T lymphocytes can worsen disease through direct cytotoxicity or release of inflammatory cytokines such as gamma interferon (IFN-γ) (3, 17, 31, 37, 42, 44, 71).
We tested the hypothesis that quantitative and/or qualitative differences in HIV-specific CD8+ T-cell responses are present in CSF compared to blood during chronic, untreated HIV-1 infection. We characterized HIV-specific CD8+ T-cell responses in CSF among seven antiretroviral therapy-naïve adults with chronic HIV-1 infection, relatively high peripheral blood CD4+ T-cell counts, and low plasma HIV-1 RNA concentrations. We show that among these HIV-positive individuals with no neurological symptoms and with little or no HIV-1 RNA in CSF, frequencies of HIV-specific T cells are significantly higher in CSF than in blood. These CSF cells are at a state of differentiation similar to that of T cells in blood and are functionally competent for expansion and IFN-γ production. The higher frequency of functional HIV-specific CD8+ T cells in CSF, in the context of low or undetectable virus in CSF, suggests that these cells play a role in the control of intrathecal viral replication.
MATERIALS AND METHODS
Study participants.
Individuals with chronic HIV-1 infection were recruited for study participation from the Vanderbilt-affiliated Comprehensive Care Center (Nashville, TN). Participants were antiretroviral therapy naïve with relatively high CD4+ T-cell counts and low plasma HIV-1 RNA concentrations. Additional eligibility criteria included at least 100,000 platelets/mm3 within 1 week prior to lumbar puncture, no history of significant CNS abnormality such as trauma, congenital malformation or genetic disorder, and no evidence of CNS mass lesion upon physical examination or magnetic resonance imaging (MRI). Typing of HLA class-I alleles was performed by sequence-specific primer PCR (DCI, Nashville, TN). Plasma and CSF HIV-1 RNA were quantified by Cobas Amplicor monitor HIV-1 version 1.5 (Roche Diagnostic Systems, Branchburg, NJ) with a lower limit of quantitation of 50 copies/ml. The Vanderbilt Institutional Review Board approved this study, and all participants provided written informed consent.
Collection and processing of CSF and peripheral blood.
With the participant in the lateral decubitus position, lumbar CSF was obtained through a 20-gauge spinal needle by gravity drainage. A Whittaker tip needle was used if possible to minimize postdural headache. During each procedure approximately 40 ml of CSF was collected directly into a 50-ml plastic conical tube on ice. Mononuclear cells from CSF (CSFMCs) were pelleted by centrifugation at 350 × g for 15 min at 4°C. Approximately 100 ml of peripheral blood was obtained immediately before lumbar puncture and processed in parallel. Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque density gradient centrifugation.
Flow cytometry.
CSFMCs and PBMCs were labeled ex vivo with a panel of fluorochrome-labeled antibodies. Freshly isolated cells were incubated sequentially with HIV-specific HLA class I tetramers (phycoerythrin [PE] or allophycocyanin Beckman Coulter or NIH tetramer facility), an anti-CCR7 (mouse anti-human CCR7 antibody; R&D Systems) at room temperature for 10 min, and goat anti-mouse immunoglobulin G (Pacific Blue; secondary antibody for CCR7 antibody; Molecular Probes) for 30 min on ice, followed by a cocktail of antibodies to CD3 (PE-Cy7; BD Biosciences), CD8 (Pacific Orange; BD Biosciences), CD4 (allophycocyanin-Cy7; BD Biosciences), CD45RO (PE-Texas Red; Invitrogen), and CD14, CD19, and CD56 in a dump channel (all PE-Cy5; BD Biosciences) on ice for 30 min. The cell viability dye 7-amino actinomycin D (Viaprobe; BD Biosciences) was added prior to analysis. Stained samples were acquired on a FACSAria cell sorter (BD Biosciences) and analyzed with FACSDiva software (BD Biosciences). The choice of tetramers for CSF staining was informed by previous analyses of tetramer responses measured in blood from each participant. Since CCR7 staining involved a two-step process with an additional two washes, CCR7 stains were done for only three participants from whom we had >1.0 × 105 CSFMCs.
Expansions of CSFMCs and PBMCs ex vivo.
Approximately 0.5 × 103 to 1.0 × 103 CSFMCs and PBMCs were expanded for 15 days with 1 μg/ml phytohemagglutinin (PHA) in the presence of 2 × 106 irradiated autologous feeder PBMCs and 10 U/ml of interleukin 2. Culture medium was changed on days 7 and 10. Cells were rested in R10 culture medium without interleukin 2 on day 14, prior to the harvesting of cells on day 15.
ELISPOT assay.
IFN-γ enzyme-linked immunospot (ELISPOT) analyses were performed using expanded CSFMCs and PBMCs incubated with HIV peptides representing optimal HLA class I-restricted epitopes (10 μg/ml of each peptide). The peptides were selected for evaluation on the basis of each participant's HLA class I alleles as previously described (70). A complete description of HIV peptide/epitopes and accompanying criteria for determining HLA restriction are provided in the HIV molecular immunology database (http://www.hiv.lanl.gov/content/immunology/index.html). CSFMCs and PBMCs were plated at 50,000 cells/well in 96-well polyvinylidene plates (Millipore, Bedford, MA) precoated with 1 μg/ml anti-IFN-γ monoclonal antibody (1-DIK-1; Mabtech, Stockholm, Sweden). Plates were incubated overnight at 37°C under 5% CO2 and developed by sequentially adding 1 μg/ml biotinylated-anti-IFN-γ monoclonal antibody (clone 7-B6-1; Mabtech), streptavidin, and 5-bromo-4-chloro-3-indolyl phosphate (BCIP)-Nitro Blue Tetrazolium substrate (Bio-Rad, Hercules, CA). Spot-forming cells (SFC) were quantified with an ELISPOT plate counter (CTL, Cleveland, OH). Values are expressed as SFC/million cells after subtracting the average background in two negative control wells. To reduce background, expanded cells were rested in R10 medium without interleukin 2 for 1 day prior to ELISPOT assays as previously described (10). The background ranged from zero to five SFC per well. Peptides that stimulated IFN-γ responses to at least three SFC above background were considered positive.
Neurological and neuropsychological evaluations.
Prior to lumbar puncture, each participant underwent a neurological examination and a battery of neuropsychological tests adapted from that used in the Multicenter AIDS Cohort Study. The battery included the Wechsler test of adult reading; the Wechsler abbreviated scale of intelligence; the Wechsler memory scale (third edition); the Hopkins verbal learning test, revised version; the Stroop test; Trails A and B; the symbol-digit modalities test; the Ruff 2 and Ruff 7 tests of selective attention; grooved pegboard, timed gait, and finger Tapping tests; CalCAP, standard edition; the Center for Epidemiological Studies’ depression scale; and Symptom Checklist 90, revised. The battery took as long as 4 hours to administer.
Neuroimaging and 1H-MRS.
Prior to lumbar puncture, each participant underwent standard structural MRI on a 3.0 T Achieva MR scanner (Philips Medical Systems) with an eight-channel SENSE head coil to rule out significant structural brain abnormalities. Structural MRIs consisted of standard T1-weighted spin echo (TR/TE = 550/14 ms), PD/T2-weighted fast spin echo (TR/TE1/TE2 = 4,000/20/17), FLAIR-TSE (TI/TR/TE = 860/2,000/17 ms), and T1-weighted (inversion-prepared) three-dimensional FFE (TI/TR/TE = 900/9.8/4.6 ms). Volume-localized short TE 1H-magnetic resonance (MR) spectra (point-resolved spectroscopy) were collected. The structural images guided placement of volumes of interest (VOI) in deep gray, frontal white, and frontal cortical gray matter for spectroscopy. These structural images were used for retrospective assessment of tissue composition in each VOI. 1H-MR spectroscopy (MRS) spectra were collected from VOI placed in the left putamen, the left frontal white matter, and the medial frontal cortex bilaterally by use of the standard short TE point-resolved spectroscopy sequence with the following parameters: TR/TE of 2,000/31 ms; NS of 128; VOI, adjustable location between subjects and fixed within subject. Reference water spectra were collected from each VOI to monitor shim quality and to make eddy current corrections. A water T2 series was collected for estimates of absolute metabolite levels. MRS data were first inspected to ensure adequate shimming and water suppression and metabolites were subsequently quantified with LCModel (64).
Statistical analysis.
All comparisons between CSFMCs and PBMCs, including numbers of epitopes recognized, were performed using two-tailed Wilcoxon matched-pairs test with GraphPad Prism 5. A P value of less than 0.05 was considered significant.
RESULTS
Comparison of viral loads and T-cell numbers in peripheral blood and CSF.
We compared CSF and blood samples from seven asymptomatic antiretroviral therapy-naïve HIV-infected adults. These individuals were selected for the study because they had previously been identified as having robust HIV-specific CD8+ T-cell responses in peripheral blood in the absence of antiretroviral therapy. Their durations of HIV-1 infection ranged from 3 to 16 years. Neurological examinations and neuropsychological test results were normal in all participants, as were MRIs of the brain. In addition, MRS markers of glial activation and neuronal dropout were grossly normal (data not shown). Basic parameters from CSF and peripheral blood are listed in Table 1. Peripheral blood CD4+ T cells exceeded 500 cells/mm3 for all but one individual, who was also the only participant with quantifiable HIV-1 RNA in CSF. HIV-1 RNA levels were lower in CSF than in blood for all participants. Ratios of CD4+ to CD8+ T cells were similar in CSF (median, 1.3) and blood (median, 1.2) (P > 0.05). All but one participant had CSF total protein of <30 mg/dl (participant 10027, 66 mg/dl). Differential counting of CSF cells identified neither neutrophils nor evidence of erythrocyte contamination in any individual, indicating that CSF lymphocytes were not contaminated by peripheral blood lymphocytes introduced during lumbar puncture.
TABLE 1.
Characteristics of study participants
| Subject ID | HIV-1 RNA (copies/ml)
|
Blood CD4+ T cells (per mm3) | CD4/CD8 ratio
|
CSFMCs collecteda | ||
|---|---|---|---|---|---|---|
| Plasma | CSF | Blood | CSF | |||
| 10024 | 60 | <50 | 841 | 0.8 | 0.8 | 200,000 |
| 10027 | 16,800 | 1,210 | 491 | 0.7 | 0.9 | 156,000 |
| 10031 | 7,950 | <50 | 612 | 0.7 | 1.3 | 110,000 |
| 10040 | 79 | <50 | 864 | 2.0 | 2.6 | 49,000 |
| 10060 | 720 | <50 | 647 | 1.2 | 1.2 | 43,000 |
| 10067 | 745 | <50 | 704 | 3.7 | 2.0 | 30,000 |
| 20018 | 608 | <50 | 1,185 | 2.2 | 1.8 | 20,000 |
Approximate number of CSFMCs collected from 40 ml of CSF.
Frequencies of tetramer-positive CD8 T cells in CSF by direct ex vivo staining.
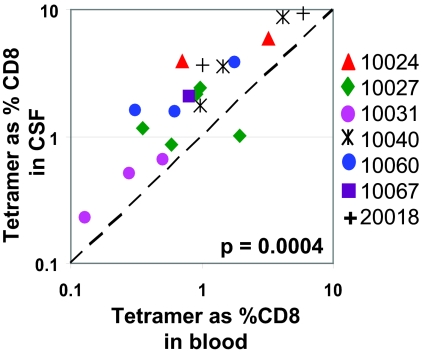
To determine frequencies of HIV-specific CD8+ T cells in CSF, at least 5,000 freshly isolated cells were stained with HIV-specific HLA class I tetramers (listed in Table 2). Although participants were not specifically recruited based on HLA alleles, these subjects were selected from a cohort of healthy antiretroviral therapy-naïve subjects with robust HIV-specific CD8+ T-cell responses in peripheral blood, and all participants carried the HLA B*57 allele, which is associated with slower HIV disease progression (55, 62). In addition to HLA-B*57 tetramers, available A*02, A*03, and B*08 tetramers were first screened for responses in peripheral blood. We subsequently tested for as many of these tetramer responses as possible in matched CSF and blood samples depending on the total yield of CSF cells. Tetramer frequencies as low as 0.2% of CD8+ T cells could be directly enumerated with as few as 5,000 CSFMCs. Tetramer responses detected in blood were always present in CSF and responses to as many as five tetramers were detected in CSF (Fig. 1A and B). Comparing 19 tetramer responses for seven study participants, frequencies of HIV-specific tetramer-positive cells were on an average 2.4-fold higher in CSF (median, 2.1%) than in peripheral blood (median, 0.9%) (P = 0.0004) (Fig. 2). Since multiple tetramer responses were tested per individual, and to ensure that a single individual did not spuriously skew these results, we determined the total percentages of tetramer-positive cells in peripheral blood and CSF for each subject. In all seven participants, total frequencies of tetramer-positive cells still remained significantly higher in CSF (median, 7.9%) than in blood (median, 3.8%) (P = 0.016). The higher frequency of tetramer-positive cells in CSF was not limited to HLA-B57-restricted epitopes. Subjects 10027 and 10031 had tetramer responses to HLA-B*08 and HLA-A*03 epitopes, respectively, and frequencies of these tetramer-positive cells were also higher in CSF than in peripheral blood (Fig. 1B).
TABLE 2.
HLA class I types of subjects and tetramers tested
| Subject ID | HLA types | Tetramers (HLA-epitope) | Epitope sequence |
|---|---|---|---|
| 10024 | A*02 A*3002 | B57-KF11 | KAFSPEVIPMF |
| B*5703 B*5802 | B57-IW9 | ISPRTLNAW | |
| 10027 | A*0101 A*0201 | B57-KF11 | KAFSPEVIPMF |
| B*0801 B*5701 | B57-IW9 | ISPRTLNAW | |
| B57-QW9 | QASQEVKNW | ||
| B8-EI8 | EIYKRWII | ||
| B8-FL8 | FLKEKGGL | ||
| A2-SL9a | SLYNTVATL | ||
| 10031 | A*0301 | B57-KF11a | KAFSPEVIPMF |
| B*1401 B*5704 | B57-IW9a | ISPRTLNAW | |
| B57-QW9 | QASQEVKNW | ||
| A3-RK9 | RLRPGGKKK | ||
| A3-QK10 | QVPLRPMTYK | ||
| A3-KK9a | KIRLRFPGGK | ||
| 10040 | A*0101 A*3101 | B57-KF11 | KAFSPEVIPMF |
| B*4402 B*5701 | B57-IW9 | ISPRTLNAW | |
| B57-QW9 | QASQEVKNW | ||
| 10060 | A*0101 A*3301 | B57-KF11 | KAFSPEVIPMF |
| B*4201 B*5701 | B57-IW9 | ISPRTLNAW | |
| B57-QW9 | QASQEVKNW | ||
| 10067 | A*3002 A*3301 | B57-KF11 | KAFSPEVIPMF |
| B*1302 B*5703 | B57-IW9a | ISPRTLNAW | |
| B57-QW9a | QASQEVKNW | ||
| 20018 | A*0201 A*2601 | B57-KF11 | KAFSPEVIPMF |
| B*4001 B*5701 | B57-IW9 | ISPRTLNAW |
Staining for these tetramers was negative in both CSF and blood. All other tetramers were detected in blood and CSF.
FIG. 1.
Frequency of HIV-specific tetramer-positive cells among freshly isolated cells from CSF and blood. (A) Flow cytometric plots of all HLA-B57 tetramer-positive (IW9, KF11, and QW9) stains among seven asymptomatic HIV-infected individuals. (B) Flow cytometric plots from two HIV-infected individuals (10027 and 10031) with non-HLA-B*57 (HLA-B*08 and HLA*A*03, respectively) tetramer responses are shown. Each tetramer stain was performed with 1.0 × 106 freshly isolated cells from blood and 0.5 × 104 to 3.0 × 104 freshly isolated cells from CSF. Numbers in plots represent frequencies of HIV-specific T cells as percentages of CD8+ T cells from CSF and blood.
FIG. 2.
Relationship between frequencies of tetramer-positive CD8+ T cells among freshly isolated cells from CSF and blood. Frequencies of HIV-specific tetramer-positive CD8+ T cells among seven asymptomatic HIV-infected individuals are derived from plots shown in Fig. 1. Each data point represents an individual tetramer response for each participant studied. The diagonal dashed line represents the line of unity. The P value is derived from a two-tailed Wilcoxon matched-pairs t test.
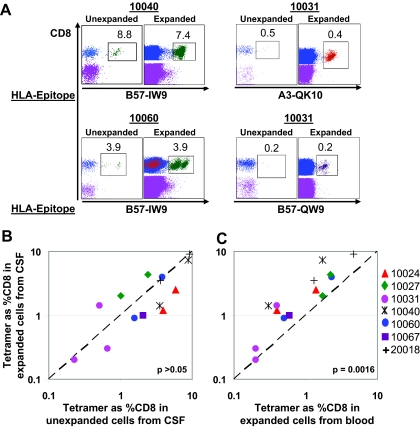
Ex vivo expansion and tetramer staining of T cells from CSF and blood.
The above analyses involved direct labeling of freshly isolated cells. To evaluate the expansion potential of HIV-specific T cells in CSF and to obtain more cells for analyses, we expanded cells from CSF and blood in parallel for 2 weeks with PHA. Starting with 0.5 × 104 to 1.0 × 104 freshly isolated cells, we obtained 2 × 106 to 3 × 106 cells after 2 weeks of expansion. From each participant, 1.0 × 106 expanded cells were evaluated for tetramer frequencies. We tested 14 of the 19 tetramer responses previously identified with fresh cells. In every case, tetramer responses detected with freshly isolated cells were also present in expanded cells (representative results shown in Fig. 3A). This confirms our ability to reliably detect tetramer-positive CD8+ T cells from relatively few cells in CSF. There was a strong correlation between tetramer frequencies of unexpanded cells from CSF and ex vivo expanded cells from CSF (R2 = 0.7468, P < 0.0001), and the magnitudes of the responses did not differ significantly, as shown in Fig. 3B (medians, 3.0% in unexpanded cells from CSF and 1.7% in expanded cells from CSF; P > 0.05). This indicates that HIV-specific T cells in CSF were capable of expansion in culture. We also compared the expansion potential of HIV-specific T cells in CSF to that of those in peripheral blood. The relatively higher frequency of HIV-specific CD8+ T cells in CSF (median, 1.7%) than in blood (median, 0.95%) was maintained in expanded cell populations (P = 0.0017) (Fig. 3C). To confirm the equivalent expansion potentials of these cell populations, we calculated the ratio of tetramer frequencies of CD8+ T cells expanded from CSF to the ratio of those of unexpanded cells from CSF (median, 0.85) and the ratio of tetramer frequencies of CD8+ T cells expanded from peripheral blood to the ratio of those of unexpanded cells from peripheral blood (median, 0.81). There was no significant difference between these values (P > 0.05). We therefore conclude that HIV-specific CD8+ T cells in CSF and peripheral blood have similar expansion potentials.
FIG. 3.
Frequencies of HIV-specific tetramer-positive cells among ex vivo expanded cells. CSFMCs and PBMCs were expanded ex vivo for 2 weeks with PHA and then assessed for frequencies of HIV-specific tetramer-positive CD8+ cells. (A) Representative flow cytometric plots show tetramer frequencies among unexpanded and expanded cells from CSF. (B) Relationship between tetramer frequencies among ex vivo expanded CD8+ cells from CSF and freshly isolated cells from CSF. (C) Relationship between tetramer frequencies among ex vivo expanded CD8+ cells from CSF and ex vivo expanded CD8+ cells from blood. The diagonal dashed line represents the line of unity. The P value is derived from a two-tailed Wilcoxon matched-pairs t test.
Functionality of HIV-specific T cells in CSF.
In addition to tetramer frequencies, expanded cells were also tested for their capacity to produce IFN-γ by ELISPOT assay. Ex vivo expanded cells from CSF, ex vivo expanded cells from blood, and unexpanded cells from blood were stimulated with individual HIV-specific optimal class I peptides selected based on each subject's HLA type, which included epitopes evaluated above by HLA class I tetramers. Expanded cells from CSF from each participant produced IFN-γ in response to at least 6 and to as many as 16 peptides (Table 3). In all but one individual, expanded cells from CSF responded to more peptides (median, 9) than did expanded cells from blood (median, 8) (P = 0.042) or unexpanded cells from blood (median, 7) (P = 0.035). Frequencies of IFN-γ SFC among expanded cells from CSF were also higher than among expanded cells from blood (median difference, 3.2-fold) (P < 0.0001) (Fig. 4A) or unexpanded cells from blood (median difference, 2.5-fold) (P = 0.0007) (Fig. 4B). When we totaled the magnitudes of the multiple peptide responses within each individual, the total frequencies of IFN-γ SFC among expanded cells from CSF (median, 4,624 SFC/106 cells) were higher than those of expanded cells from blood (median, 1,765 SFC/106 cells) and were also higher than those of unexpanded cells from blood (median, 3,483 SFC/106 cells). However, these differences in magnitude were not statistically significant. These data suggest that CSF contains expansion-competent, functional IFN-γ-producing CD8+ T cells that potentially recognize a broader range of HIV-1 epitopes than do CD8+ T cells from peripheral blood.
TABLE 3.
IFN-γ responses to HIV-specific HLA class I optimal epitopes
| Subject ID | No. of epitopes tested | No. of epitopes with IFN-γ response
|
||
|---|---|---|---|---|
| Unexpanded cells from blood | Expanded cells from blood | Expanded cells from CSF | ||
| 10024 | 28 | 7 | 8 | 7 |
| 10027 | 33 | 7 | 8 | 11 |
| 10031 | 25 | 8 | 8 | 9 |
| 10040 | 19 | 11 | 10 | 16 |
| 10060 | 12 | 8 | 2 | 11 |
| 10067 | 15 | 0 | 2 | 9 |
| 20018 | 28 | 1 | 3 | 6 |
FIG. 4.
Frequencies of IFN-γ-secreting cells, as assessed by ELISPOT assay. CSFMCs and PBMCs were expanded for 2 weeks with PHA and then assessed for frequency of IFN-γ SFC to HIV-specific optimal class I peptides by ELISPOT assay. Numbers of peptides tested and numbers with IFN-γ responses are listed in Table 3. (A) Relationship between SFC frequencies among ex vivo expanded CD8+ cells from CSF and ex vivo expanded CD8+ cells from blood. (B) Relationship between SFC frequencies among ex vivo expanded CD8+ cells from CSF and unexpanded CD8+ cells from blood. The diagonal dashed line represents the line of unity. The vertical and horizontal dashed lines represent lower limits of quantitation. The P values are derived from a two-tailed Wilcoxon matched-pairs t test.
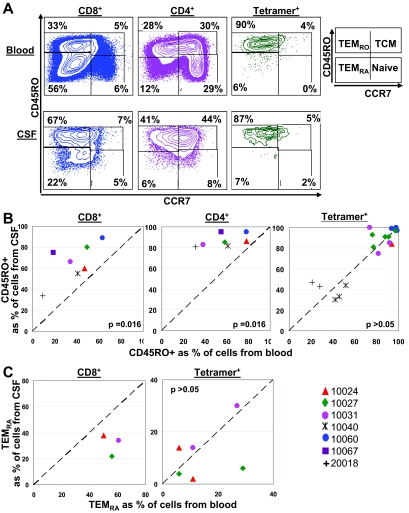
Distribution of memory T cells in CSF and blood.
To evaluate the maturation states of HIV-specific CD8+ T cells, we evaluated expression of CD45RO and CCR7 on freshly isolated cells from CSF and blood. CCR7 and CD45RO distinguish central memory (TCM), effector memory (TEMRO), and terminally differentiated (TEMRA) T cells (Fig. 5A). Because substantial cellular expression of CD45RA and CD45RO is mutually exclusive (reference 33 and unpublished data), CD45RO− CCR7− T cells were considered to be TEMRA T cells. The frequency of CD45RO+ expression (TEMRO and TCM) on CD8+ and CD4+ T cells was higher in CSF (medians, 66% for CD8+ and 85% for CD4+ T cells) than in blood (medians, 41% for CD8+ and 58% for CD4+ T cells) (the P value was 0.016 for each comparison) (Fig. 5B). Since CCR7 staining involved a two-step process with two additional washes, CCR7 stains were done for only three participants from whom we had >1.0 × 105 cells from CSF. Among these three participants, frequencies of CD8+ TEMRA cells were lower in CSF (median, 34%) than in blood (median, 56%), although this difference was not statistically significant (Wilcoxon matched-pair test P value, >0.05; paired t test P value, 0.064) (Fig. 5C), and frequencies of naïve and TCM CD8+ T cells were similar in CSF (medians, 5% for naïve and 4% for TCM) and blood (medians, 5% for naïve and 3% for TCM). Although we would need to evaluate more subjects to achieve statistical significance, this trend suggests an increased migration to or expansion of TEMRO CD8+ cells in CSF and a relative absence of TEMRA CD8+ cells in CSF.
FIG. 5.
Distribution of memory markers. Cells from CSF and blood were assessed for distribution of CD45RO and CCR7 T-cell differentiation markers on CD4+, CD8+, and HIV-specific tetramer-positive cells by flow cytometry. (A) Flow cytometric plots of CD45RO and CCR7 on CD8+, CD4+, and HIV-specific tetramer-positive (B57-KF11) cells from CSF and blood of one representative participant (10027). Numbers in plots represent frequencies of cells in the quadrant as percentages of CD8+, CD4+, and tetramer-positive T cells. TCM, CD45RO+ CCR7+; TEMRO, CD45RO+ CCR7−; TEMRA, CD45RO− CCR7−; naïve T cells, CD45RO− CCR7+. (B) Relationship between the frequencies of CD45RO+ cells among CD8+, CD4+, and tetramer-positive cells from CSF and blood. (C) Relationship between the frequencies of TEMRA (CD45RO− CCR7− T cells) among tetramer-positive cells from CSF and blood. CCR7 stains were done for only three participants from whom we had a sufficient number of cells (>1.0 × 105) from CSF. The diagonal dashed line represents the line of unity. The P values are derived from a two-tailed Wilcoxon matched-pairs t test.
Evaluation of the memory marker status of tetramer-positive cells revealed that the frequencies of CD45RO+ expression for HIV-specific CD8+ T cells were similar in CSF (median, 85%) and blood (median, 82%) (P > 0.05) (Fig. 5B). CCR7 and CD45RO staining for the three participants described above (six tetramers) further indicated that the proportions of HIV-specific TEMRA cells was similar for CSF (median, 11%) and blood (median, 10%) (P > 0.05) (Fig. 5C). These data suggest that HIV-specific CD8+ T cells in CSF are in a differentiation state similar to that seen for blood and that HIV-specific TEMRA CD8+ cells, in contrast to total TEMRA CD8+ cells, are capable of migration to and/or expansion in CSF.
DISCUSSION
The present study demonstrates that among HIV-infected individuals with relatively good immune control of plasma viremia and without apparent brain injury based on neuropsychological testing and neuroimaging, the relative frequencies of HIV-specific CD8+ T cells are greater in CSF than in peripheral blood. Collecting large volumes of CSF allowed us to characterize, by direct staining, HIV-specific tetramer-positive CD8+ T cells in individuals without apparent CSF pleocytosis. Our data also suggest that HIV-specific CD8+ T cells in CSF may have good expansion potential and a functional capacity greater than seen for those in peripheral blood. To confirm our findings generated with fresh cells, CSFMCs were expanded ex vivo. We show that expanded cells from CSF recognize not only dominant epitopes, based on tetramer staining, but also several other nondominant epitopes, based on IFN-γ ELISPOT assay.
Cross-sectional studies evaluating the relationship of peripheral HIV-specific CD8+ T-cell responses and control of viremia have shown either no relationship (1) or even a positive association between the magnitude of responses and the level of viremia (9). In this study we explored the hypothesis that the level of epitope-specific T cells in CSF were related to the level of control of viral replication. We were able to uniquely quantitate immune responses within two discrete anatomical compartments. We hypothesized that if the level of HIV antigen were driving the frequency of epitope-specific T cells, the frequency of these cells would be lower in CSF than in blood, and that if the migration of T cells between peripheral blood and CSF were random, then the frequencies in CSF would reflect those in the peripheral blood. In fact, we found a consistently higher frequency of HIV-specific T cells in CSF and consistently lower levels of virus in CSF, suggesting that at least in HIV patients with no apparent neurological symptoms, HIV-specific T cells contribute to the control of viral replication in the CNS.
At least two mechanisms could explain the relatively increased frequency of HIV-specific CD8+ T cells in CSF: the preferential migration of CD8+/CD45RO+ memory T cells from the periphery into the CNS and/or the intrathecal persistence or expansion of HIV-specific T cells after migration into the CNS. These mechanisms are not mutually exclusive, and each may be driven by the presence of HIV-1 antigens in the CNS. In macaques, activated T cells preferentially enter the intrathecal compartment and increase in frequency early after acute SIV infection (41, 50). In humans, studies showing activated T cells in CSF among both HIV-infected and HIV-uninfected individuals (50, 58, 66) support homing of activated T cells to the CNS. The increased expression of adhesion molecules and chemokine receptors on HIV-specific CD8+ T cells in CSF during HIV infection also suggests that the trafficking of antigen-specific T cells to the brain may be due to a combination of the systemic activation of T cells and response to intrathecal inflammation (69). Our finding may not be unique to the brain, since in SIV-infected macaques high frequencies of virus-specific T cells have been demonstrated in the gastrointestinal tract, urogenital tissue, spleen, bone marrow, and liver compared to what is seen for peripheral blood (32, 46, 68, 73). Recent data from a rodent model suggest that the expression of viral antigens in the brain may upregulate endothelial cell major histocompatibility complex class I expression, which in turn favors CD8+ T-cell migration into the brain (27). However, it is likely that very little HIV-1 antigen was being produced intrathecally among our study participants, based on the lack of quantifiable CSF HIV-1 RNA in all but one subject and the normal neuropsychological performance of study participants. Limited data from human and nonhuman primate studies also suggest the absence of viral proteins in the brain during chronic, asymptomatic infection (8, 85). However, we cannot exclude viral replication in basal ganglia and deep white matter structures that are typically infected during HIV-associated dementia (6, 13, 15, 26, 45, 59, 82), since such replication may not be apparent in CSF or by neuropsychological testing or neuroimaging.
Other groups have noted selective recruitment of memory cells to the brain and higher proportions of CD8+ and CD4+ memory T cells in the brains of both HIV-infected and healthy individuals under both normal and neuropathological conditions (58, 74, 79). Our results also indicate higher frequencies of CD45RO+ memory CD8+ and CD4+ T cells and lower frequencies of TEMRA CD8+ cells in CSF than in blood of relatively healthy HIV-positive subjects with little or no detectable intrathecal virus. HIV-specific CD8+ T cells in CSF are at the same differentiation state as in blood, as assessed by CD45RO and CCR7 T-cell differentiation markers. This, together with the expansion potential and functional capacity of the HIV-specific T cells in CSF, suggests that these HIV-specific T cells are as capable as cells in peripheral blood of controlling viral replication. Further, higher frequencies of HIV-specific T cells in CSF may reflect an increased migration of CD45RO+ memory CD8+ T cells to the CSF and also an increased migration and/or expansion of TEMRA HIV-specific CD8+ T cells in CSF.
The present study has potential implications for HIV-1 neuropathogenesis. Normal uninfected brain has multiple intrinsic mechanisms to maintain its state of relative immune quiescence. These include tight junctions and low levels of adhesion molecule expression by capillary endothelial cells; neuronal expression of CD200, which reduces microglial cell activation; local production of neurotrophins; and transforming growth factor β secretion by astrocytes and meningeal cells (31). In addition, the brain is a potential anatomic reservoir for HIV-1 replication (24, 63). While inflammatory and innate immune mechanisms that involve trafficking of monocyte/macrophages are central to the pathogenesis of HIV-associated dementia (28, 84-86), viral replication also plays a major role, with viral proteins gp120 (4, 5, 20, 34) and tat (12, 49, 57, 60) being directly neurotoxic. Control of HIV replication by HIV-specific CD8+ T cells may therefore be critical in preventing this complication, although direct evidence for this in the CNS during HIV infection is scant. To our knowledge, there is only a single report which described unexpanded HIV-specific CD8+ T cells in CSF. Tetramer staining for a single gag epitope was tested in two HLA-A2-positive individuals, both of whom had substantial viremia in plasma (HIV-1 RNA of >40,000 copies/ml) and CSF (>2,500 copies/ml). Tetramer-positive cells were somewhat more frequent in CSF than in blood (69), consistent with our findings. Previous studies of nonhuman primates have identified the presence and/or enrichment of SIV-specific CD8+ T cells in the CNS soon after primary infection (16, 80), and increased numbers of CD8+ T cells and virus-specific cytotoxic T cells were detected in CSF of macaques with slow disease progression and fewer neurological findings (72). In chronically infected macaques with little or no viral replication in the brain, the frequency of SIV-specific T cells was higher in CSF than that in peripheral blood (56).
A detrimental effect for T cells in the pathogenesis of HIV-associated dementia has also been postulated (38), based on the observation that immune responses contribute to neuropathogenesis in models of other infectious diseases through direct cytotoxicity or the release of inflammatory cytokines (3, 17, 31, 37, 42, 44, 71). Therefore, while virus-specific T cells could contribute to neuropathogenesis, the data we present here suggest that subjects with stable nonprogressive HIV disease, with very low levels of virus in CSF and no clinically detectable neuropathology, have higher breadths and magnitudes of HIV-specific CD8+ T cells in CSF than in peripheral blood. The limited ability of many antiretroviral drugs to cross the blood-brain barrier (40, 48) heightens the reliance on host mechanisms for controlling HIV replication in the CNS.
Our study was limited to antiretroviral therapy-naïve HIV-infected individuals with good immune control of viral replication and no apparent neurological symptoms. Further, since this was a cross-sectional study, it is not known whether these HIV-specific responses change over time, nor was the effect of subsequent antiretroviral therapy known. Characterization of HIV-specific T cells for HIV-positive individuals with advanced AIDS, particularly for those with neuropsychological impairment, and for those on antiretroviral therapy will further our understanding of the role of CD8+ T cells in the control of HIV replication and neuropathogenesis. Studies on a larger cohort may also help confirm the apparent distribution of memory differentiation markers on T cells in CSF.
In summary, the relative frequencies of HIV-specific CD8+ T cells are greater in CSF than in peripheral blood among asymptomatic HIV-infected individuals with good immune control of plasma viremia. These responses in CSF have broad epitope specificity and may have expansion and functional capacity greater than those in peripheral blood. These results point toward preferential migration to and/or expansion of HIV-specific CD8+ T cells within CSF and suggest that HIV-specific CD8+ T cells mediate the control of intrathecal viral replication.
Acknowledgments
This study was supported by NIH grants MH071205 (D.W.H.) and AI39966 (S.A.K.) and by Vanderbilt CTSA grant RR024975. S.A.K. is a Scientist of the Elizabeth Glaser Pediatric AIDS Foundation. Flow cytometry was performed at the Vanderbilt-Meharry Center for AIDS Research Immunopathogenesis Core, an NIH-funded program (P30 AIS4999).
We gratefully acknowledge all the study volunteers. We are thankful for helpful statistical advice provided by Ayumi Shintani.
Footnotes
Published ahead of print on 20 August 2008.
REFERENCES
- 1.Addo, M. M., X. G. Yu, A. Rathod, D. Cohen, R. L. Eldridge, D. Strick, M. N. Johnston, C. Corcoran, A. G. Wurcel, C. A. Fitzpatrick, M. E. Feeney, W. R. Rodriguez, N. Basgoz, R. Draenert, D. R. Stone, C. Brander, P. J. Goulder, E. S. Rosenberg, M. Altfeld, and B. D. Walker. 2003. Comprehensive epitope analysis of human immunodeficiency virus type 1 (HIV-1)-specific T-cell responses directed against the entire expressed HIV-1 genome demonstrate broadly directed responses, but no correlation to viral load. J. Virol. 772081-2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altfeld, M., J. van Lunzen, N. Frahm, X. G. Yu, C. Schneider, R. L. Eldridge, M. E. Feeney, D. Meyer-Olson, H. J. Stellbrink, and B. D. Walker. 2002. Expansion of pre-existing, lymph node-localized CD8+ T cells during supervised treatment interruptions in chronic HIV-1 infection. J. Clin. Investig. 109837-843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baenziger, J., H. Hengartner, R. M. Zinkernagel, and G. A. Cole. 1986. Induction or prevention of immunopathological disease by cloned cytotoxic T cell lines specific for lymphocytic choriomeningitis virus. Eur. J. Immunol. 16387-393. [DOI] [PubMed] [Google Scholar]
- 4.Bagetta, G., M. T. Corasaniti, A. M. Paoletti, L. Berliocchi, R. Nistico, A. M. Giammarioli, W. Malorni, and A. Finazzi-Agro. 1998. HIV-1 gp120-induced apoptosis in the rat neocortex involves enhanced expression of cyclo-oxygenase type 2 (COX-2). Biochem. Biophys. Res. Commun. 244819-824. [DOI] [PubMed] [Google Scholar]
- 5.Barks, J. D., X. H. Liu, R. Sun, and F. S. Silverstein. 1997. gp120, a human immunodeficiency virus-1 coat protein, augments excitotoxic hippocampal injury in perinatal rats. Neuroscience 76397-409. [DOI] [PubMed] [Google Scholar]
- 6.Bell, J. E. 1998. The neuropathology of adult HIV infection. Rev. Neurol. (Paris) 154816-829. [PubMed] [Google Scholar]
- 7.Bell, J. E., R. P. Brettle, A. Chiswick, and P. Simmonds. 1998. HIV encephalitis, proviral load and dementia in drug users and homosexuals with AIDS. Effect of neocortical involvement. Brain 1212043-2052. [DOI] [PubMed] [Google Scholar]
- 8.Bell, J. E., A. Busuttil, J. W. Ironside, S. Rebus, Y. K. Donaldson, P. Simmonds, and J. F. Peutherer. 1993. Human immunodeficiency virus and the brain: investigation of virus load and neuropathologic changes in pre-AIDS subjects. J. Infect. Dis. 168818-824. [DOI] [PubMed] [Google Scholar]
- 9.Betts, M. R., D. R. Ambrozak, D. C. Douek, S. Bonhoeffer, J. M. Brenchley, J. P. Casazza, R. A. Koup, and L. J. Picker. 2001. Analysis of total human immunodeficiency virus (HIV)-specific CD4+ and CD8+ T-cell responses: relationship to viral load in untreated HIV infection. J. Virol. 7511983-11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bihl, F. K., E. Loggi, J. V. Chisholm III, H. S. Hewitt, L. M. Henry, C. Linde, T. J. Suscovich, J. T. Wong, N. Frahm, P. Andreone, and C. Brander. 2005. Simultaneous assessment of cytotoxic T lymphocyte responses against multiple viral infections by combined usage of optimal epitope matrices, anti-CD3 mAb T-cell expansion and “RecycleSpot.” J. Transl. Med. 320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borrow, P., H. Lewicki, B. H. Hahn, G. M. Shaw, and M. B. Oldstone. 1994. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 686103-6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brana, C., T. E. Biggs, C. H. Barton, L. E. Sundstrom, and D. A. Mann. 1999. A soluble factor produced by macrophages mediates the neurotoxic effects of HIV-1 Tat in vitro. AIDS 131443-1452. [DOI] [PubMed] [Google Scholar]
- 13.Brew, B. J., M. Rosenblum, K. Cronin, and R. W. Price. 1995. AIDS dementia complex and HIV-1 brain infection: clinical-virological correlations. Ann. Neurol. 38563-570. [DOI] [PubMed] [Google Scholar]
- 14.Brodie, S. J., B. K. Patterson, D. A. Lewinsohn, K. Diem, D. Spach, P. D. Greenberg, S. R. Riddell, and L. Corey. 2000. HIV-specific cytotoxic T lymphocytes traffic to lymph nodes and localize at sites of HIV replication and cell death. J. Clin. Investig. 1051407-1417. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Budka, H., G. Costanzi, S. Cristina, A. Lechi, C. Parravicini, R. Trabattoni, and L. Vago. 1987. Brain pathology induced by infection with the human immunodeficiency virus (HIV). A histological, immunocytochemical, and electron microscopical study of 100 autopsy cases. Acta Neuropathol. 75185-198. [DOI] [PubMed] [Google Scholar]
- 16.Chakrabarti, L., M. Hurtrel, M. A. Maire, R. Vazeux, D. Dormont, L. Montagnier, and B. Hurtrel. 1991. Early viral replication in the brain of SIV-infected rhesus monkeys. Am. J. Pathol. 1391273-1280. [PMC free article] [PubMed] [Google Scholar]
- 17.Chan, W. L., T. Javanovic, and M. L. Lukic. 1989. Infiltration of immune T cells in the brain of mice with herpes simplex virus-induced encephalitis. J. Neuroimmunol. 23195-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chien, J. W., H. Valdez, G. McComsey, D. McClernon, M. St Clair, and M. M. Lederman. 1999. Presence of mutation conferring resistance to lamivudine in plasma and cerebrospinal fluid of HIV-1-infected patients. J. Acquir. Immune Defic. Syndr. 21277-280. [DOI] [PubMed] [Google Scholar]
- 19.Cinque, P., S. Presi, A. Bestetti, C. Pierotti, S. Racca, E. Boeri, P. Morelli, P. Carrera, M. Ferrari, and A. Lazzarin. 2001. Effect of genotypic resistance on the virological response to highly active antiretroviral therapy in cerebrospinal fluid. AIDS Res. Hum. Retrovir. 17377-383. [DOI] [PubMed] [Google Scholar]
- 20.Corasaniti, M. T., M. C. Strongoli, S. Piccirilli, R. Nistico, A. Costa, A. Bilotta, P. Turano, A. Finazzi-Agro, and G. Bagetta. 2000. Apoptosis induced by gp120 in the neocortex of rat involves enhanced expression of cyclooxygenase type 2 and is prevented by NMDA receptor antagonists and by the 21-aminosteroid U-74389G. Biochem. Biophys. Res. Commun. 274664-669. [DOI] [PubMed] [Google Scholar]
- 21.Davis, L. E., B. L. Hjelle, V. E. Miller, D. L. Palmer, A. L. Llewellyn, T. L. Merlin, S. A. Young, R. G. Mills, W. Wachsman, and C. A. Wiley. 1992. Early viral brain invasion in iatrogenic human immunodeficiency virus infection. Neurology 421736-1739. [DOI] [PubMed] [Google Scholar]
- 22.Di Stefano, M., F. Sabri, T. Leitner, B. Svennerholm, L. Hagberg, G. Norkrans, and F. Chiodi. 1995. Reverse transcriptase sequence of paired isolates of cerebrospinal fluid and blood from patients infected with human immunodeficiency virus type 1 during zidovudine treatment. J. Clin. Microbiol. 33352-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ellis, R. J., K. Hsia, S. A. Spector, J. A. Nelson, R. K. Heaton, M. R. Wallace, I. Abramson, J. H. Atkinson, I. Grant, J. A. McCutchan, et al. 1997. Cerebrospinal fluid human immunodeficiency virus type 1 RNA levels are elevated in neurocognitively impaired individuals with acquired immunodeficiency syndrome. Ann. Neurol. 42679-688. [DOI] [PubMed] [Google Scholar]
- 24.Enting, R. H., R. M. Hoetelmans, J. M. Lange, D. M. Burger, J. H. Beijnen, and P. Portegies. 1998. Antiretroviral drugs and the central nervous system. AIDS 121941-1955. [DOI] [PubMed] [Google Scholar]
- 25.Fujimura, R. K., K. Goodkin, C. K. Petito, R. Douyon, D. J. Feaster, M. Concha, and P. Shapshak. 1997. HIV-1 proviral DNA load across neuroanatomic regions of individuals with evidence for HIV-1-associated dementia. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 16146-152. [DOI] [PubMed] [Google Scholar]
- 26.Gabuzda, D. H., D. D. Ho, S. M. de la Monte, M. S. Hirsch, T. R. Rota, and R. A. Sobel. 1986. Immunohistochemical identification of HTLV-III antigen in brains of patients with AIDS. Ann. Neurol. 20289-295. [DOI] [PubMed] [Google Scholar]
- 27.Galea, I., M. Bernardes-Silva, P. A. Forse, N. van Rooijen, R. S. Liblau, and V. H. Perry. 2007. An antigen-specific pathway for CD8 T cells across the blood-brain barrier. J. Exp. Med. 2042023-2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gartner, S. 2000. HIV infection and dementia. Science 287602-604. [DOI] [PubMed] [Google Scholar]
- 29.Gatanaga, H., S. Oka, S. Ida, T. Wakabayashi, T. Shioda, and A. Iwamoto. 1999. Active HIV-1 redistribution and replication in the brain with HIV encephalitis. Arch. Virol. 14429-43. [DOI] [PubMed] [Google Scholar]
- 30.Goudsmit, J., F. de Wolf, D. A. Paul, L. G. Epstein, J. M. Lange, W. J. Krone, H. Speelman, E. C. Wolters, J. Van der Noordaa, J. M. Oleske, et al. 1986. Expression of human immunodeficiency virus antigen (HIV-Ag) in serum and cerebrospinal fluid during acute and chronic infection. Lancet ii177-180. [DOI] [PubMed] [Google Scholar]
- 31.Griffin, D. E. 2003. Immune responses to RNA-virus infections of the CNS. Nat. Rev. Immunol. 3493-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hel, Z., J. Nacsa, B. Kelsall, W. P. Tsai, N. Letvin, R. W. Parks, E. Tryniszewska, L. Picker, M. G. Lewis, Y. Edghill-Smith, M. Moniuszko, R. Pal, L. Stevceva, J. D. Altman, T. M. Allen, D. Watkins, J. V. Torres, J. A. Berzofsky, I. M. Belyakov, W. Strober, and G. Franchini. 2001. Impairment of Gag-specific CD8+ T-cell function in mucosal and systemic compartments of simian immunodeficiency virus mac251- and simian-human immunodeficiency virus KU2-infected macaques. J. Virol. 7511483-11495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Helbert, M. R., J. Walter, J. L'Age, and P. C. Beverley. 1997. HIV infection of CD45RA+ and CD45RO+ CD4+ T cells. Clin. Exp. Immunol. 107300-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hesselgesser, J., D. Taub, P. Baskar, M. Greenberg, J. Hoxie, D. L. Kolson, and R. Horuk. 1998. Neuronal apoptosis induced by HIV-1 gp120 and the chemokine SDF-1 alpha is mediated by the chemokine receptor CXCR4. Curr. Biol. 8595-598. [DOI] [PubMed] [Google Scholar]
- 35.Ho, D. D., M. G. Sarngadharan, L. Resnick, F. Dimarzoveronese, T. R. Rota, and M. S. Hirsch. 1985. Primary human T-lymphotropic virus type III infection. Ann. Intern. Med. 103880-883. [DOI] [PubMed] [Google Scholar]
- 36.Horton, H., I. Frank, R. Baydo, E. Jalbert, J. Penn, S. Wilson, J. P. McNevin, M. D. McSweyn, D. Lee, Y. Huang, S. C. De Rosa, and M. J. McElrath. 2006. Preservation of T cell proliferation restricted by protective HLA alleles is critical for immune control of HIV-1 infection. J. Immunol. 1777406-7415. [DOI] [PubMed] [Google Scholar]
- 37.Jacobson, S., H. Shida, D. E. McFarlin, A. S. Fauci, and S. Koenig. 1990. Circulating CD8+ cytotoxic T lymphocytes specific for HTLV-I pX in patients with HTLV-I associated neurological disease. Nature 348245-248. [DOI] [PubMed] [Google Scholar]
- 38.Jassoy, C., R. P. Johnson, B. A. Navia, J. Worth, and B. D. Walker. 1992. Detection of a vigorous HIV-1-specific cytotoxic T lymphocyte response in cerebrospinal fluid from infected persons with AIDS dementia complex. J. Immunol. 1493113-3119. [PubMed] [Google Scholar]
- 39.Jin, X., D. E. Bauer, S. E. Tuttleton, S. Lewin, A. Gettie, J. Blanchard, C. E. Irwin, J. T. Safrit, J. Mittler, L. Weinberger, L. G. Kostrikis, L. Zhang, A. S. Perelson, and D. D. Ho. 1999. Dramatic rise in plasma viremia after CD8(+) T cell depletion in simian immunodeficiency virus-infected macaques. J. Exp. Med. 189991-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim, R. B., M. F. Fromm, C. Wandel, B. Leake, A. J. Wood, D. M. Roden, and G. R. Wilkinson. 1998. The drug transporter P-glycoprotein limits oral absorption and brain entry of HIV-1 protease inhibitors. J. Clin. Investig. 101289-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim, W. K., S. Corey, G. Chesney, H. Knight, S. Klumpp, C. Wuthrich, N. Letvin, I. Koralnik, A. Lackner, R. Veasey, and K. Williams. 2004. Identification of T lymphocytes in simian immunodeficiency virus encephalitis: distribution of CD8+ T cells in association with central nervous system vessels and virus. J. Neurovirol. 10315-325. [DOI] [PubMed] [Google Scholar]
- 42.Klavinskis, L. S., A. Tishon, and M. B. Oldstone. 1989. Efficiency and effectiveness of cloned virus-specific cytotoxic T lymphocytes in vivo. J. Immunol. 1432013-2016. [PubMed] [Google Scholar]
- 43.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 684650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kreth, H. W., L. Kress, H. G. Kress, H. F. Ott, and G. Eckert. 1982. Demonstration of primary cytotoxic T cells in venous blood and cerebrospinal fluid of children with mumps meningitis. J. Immunol. 1282411-2415. [PubMed] [Google Scholar]
- 45.Kure, K., K. M. Weidenheim, W. D. Lyman, and D. W. Dickson. 1990. Morphology and distribution of HIV-1 gp41-positive microglia in subacute AIDS encephalitis. Pattern of involvement resembling a multisystem degeneration. Acta Neuropathol. 80393-400. [DOI] [PubMed] [Google Scholar]
- 46.Kuroda, M. J., J. E. Schmitz, A. Seth, R. S. Veazey, C. E. Nickerson, M. A. Lifton, P. J. Dailey, M. A. Forman, P. Racz, K. Tenner-Racz, and N. L. Letvin. 2000. Simian immunodeficiency virus-specific cytotoxic T lymphocytes and cell-associated viral RNA levels in distinct lymphoid compartments of SIVmac-infected rhesus monkeys. Blood 961474-1479. [PubMed] [Google Scholar]
- 47.Lazarini, F., D. Seilhean, O. Rosenblum, S. Suarez, L. Conquy, T. Uchihara, V. Sazdovitch, K. Mokhtari, T. Maisonobe, F. Boussin, C. Katlama, F. Bricaire, C. Duyckaerts, and J. J. Hauw. 1997. Human immunodeficiency virus type 1 DNA and RNA load in brains of demented and nondemented patients with acquired immunodeficiency syndrome. J. Neurovirol. 3299-303. [DOI] [PubMed] [Google Scholar]
- 48.Letendre, S. L., J. A. McCutchan, M. E. Childers, S. P. Woods, D. Lazzaretto, R. K. Heaton, I. Grant, and R. J. Ellis. 2004. Enhancing antiretroviral therapy for human immunodeficiency virus cognitive disorders. Ann. Neurol. 56416-423. [DOI] [PubMed] [Google Scholar]
- 49.Ma, M., and A. Nath. 1997. Molecular determinants for cellular uptake of Tat protein of human immunodeficiency virus type 1 in brain cells. J. Virol. 712495-2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marcondes, M. C., E. M. Burudi, S. Huitron-Resendiz, M. Sanchez-Alavez, D. Watry, M. Zandonatti, S. J. Henriksen, and H. S. Fox. 2001. Highly activated CD8(+) T cells in the brain correlate with early central nervous system dysfunction in simian immunodeficiency virus infection. J. Immunol. 1675429-5438. [DOI] [PubMed] [Google Scholar]
- 51.Matano, T., R. Shibata, C. Siemon, M. Connors, H. C. Lane, and M. A. Martin. 1998. Administration of an anti-CD8 monoclonal antibody interferes with the clearance of chimeric simian/human immunodeficiency virus during primary infections of rhesus macaques. J. Virol. 72164-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McArthur, J. C., D. R. McClernon, M. F. Cronin, T. E. Nance-Sproson, A. J. Saah, M. St Clair, and E. R. Lanier. 1997. Relationship between human immunodeficiency virus-associated dementia and viral load in cerebrospinal fluid and brain. Ann. Neurol. 42689-698. [DOI] [PubMed] [Google Scholar]
- 53.McCoig, C., M. M. Castrejon, E. Castano, O. De Suman, C. Baez, W. Redondo, D. McClernon, S. Danehower, E. R. Lanier, C. Richardson, A. Keller, S. Hetherington, X. Saez-Llorens, and O. Ramilo. 2002. Effect of combination antiretroviral therapy on cerebrospinal fluid HIV RNA, HIV resistance, and clinical manifestations of encephalopathy. J. Pediatr. 14136-44. [DOI] [PubMed] [Google Scholar]
- 54.Migueles, S. A., A. C. Laborico, W. L. Shupert, M. S. Sabbaghian, R. Rabin, C. W. Hallahan, D. Van Baarle, S. Kostense, F. Miedema, M. McLaughlin, L. Ehler, J. Metcalf, S. Liu, and M. Connors. 2002. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat. Immunol. 31061-1068. [DOI] [PubMed] [Google Scholar]
- 55.Migueles, S. A., M. S. Sabbaghian, W. L. Shupert, M. P. Bettinotti, F. M. Marincola, L. Martino, C. W. Hallahan, S. M. Selig, D. Schwartz, J. Sullivan, and M. Connors. 2000. HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc. Natl. Acad. Sci. USA 972709-2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moniuszko, M., C. Brown, R. Pal, E. Tryniszewska, W. P. Tsai, V. M. Hirsch, and G. Franchini. 2003. High frequency of virus-specific CD8+ T cells in the central nervous system of macaques chronically infected with simian immunodeficiency virus SIVmac251. J. Virol. 7712346-12351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nath, A., K. Psooy, C. Martin, B. Knudsen, D. S. Magnuson, N. Haughey, and J. D. Geiger. 1996. Identification of a human immunodeficiency virus type 1 Tat epitope that is neuroexcitatory and neurotoxic. J. Virol. 701475-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Neuenburg, J. K., T. A. Cho, A. Nilsson, B. M. Bredt, S. J. Hebert, R. M. Grant, and R. W. Price. 2005. T-cell activation and memory phenotypes in cerebrospinal fluid during HIV infection. J. Acquir. Immune Defic. Syndr. 3916-22. [DOI] [PubMed] [Google Scholar]
- 59.Neuen-Jacob, E., G. Arendt, B. Wendtland, B. Jacob, M. Schneeweis, and W. Wechsler. 1993. Frequency and topographical distribution of CD68-positive macrophages and HIV-1 core proteins in HIV-associated brain lesions. Clin. Neuropathol. 12315-324. [PubMed] [Google Scholar]
- 60.New, D. R., M. Ma, L. G. Epstein, A. Nath, and H. A. Gelbard. 1997. Human immunodeficiency virus type 1 Tat protein induces death by apoptosis in primary human neuron cultures. J. Neurovirol. 3168-173. [DOI] [PubMed] [Google Scholar]
- 61.Nuovo, G. J., and M. L. Alfieri. 1996. AIDS dementia is associated with massive, activated HIV-1 infection and concomitant expression of several cytokines. Mol. Med. 2358-366. [PMC free article] [PubMed] [Google Scholar]
- 62.O'Brien, S. J., X. Gao, and M. Carrington. 2001. HLA and AIDS: a cautionary tale. Trends Mol. Med. 7379-381. [DOI] [PubMed] [Google Scholar]
- 63.Persidsky, Y., and L. Poluektova. 2006. Immune privilege and HIV-1 persistence in the CNS. Immunol. Rev. 213180-194. [DOI] [PubMed] [Google Scholar]
- 64.Provencher, S. W. 1993. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn. Reson. Med. 30672-679. [DOI] [PubMed] [Google Scholar]
- 65.Resnick, L., J. R. Berger, P. Shapshak, and W. W. Tourtellotte. 1988. Early penetration of the blood-brain-barrier by HIV. Neurology 389-14. [DOI] [PubMed] [Google Scholar]
- 66.Roberts, E. S., S. Huitron-Resendiz, M. A. Taffe, M. C. Marcondes, C. T. Flynn, C. M. Lanigan, J. A. Hammond, S. R. Head, S. J. Henriksen, and H. S. Fox. 2006. Host response and dysfunction in the CNS during chronic simian immunodeficiency virus infection. J. Neurosci. 264577-4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283857-860. [DOI] [PubMed] [Google Scholar]
- 68.Schmitz, J. E., M. J. Kuroda, R. S. Veazey, A. Seth, W. M. Taylor, C. E. Nickerson, M. A. Lifton, P. J. Dailey, M. A. Forman, P. Racz, K. Tenner-Racz, and N. L. Letvin. 2000. Simian immunodeficiency virus (SIV)-specific CTL are present in large numbers in livers of SIV-infected rhesus monkeys. J. Immunol. 1646015-6019. [DOI] [PubMed] [Google Scholar]
- 69.Shacklett, B. L., C. A. Cox, D. T. Wilkens, R. Karl Karlsson, A. Nilsson, D. F. Nixon, and R. W. Price. 2004. Increased adhesion molecule and chemokine receptor expression on CD8+ T cells trafficking to cerebrospinal fluid in HIV-1 infection. J. Infect. Dis. 1892202-2212. [DOI] [PubMed] [Google Scholar]
- 70.Siddique, M. A., K. E. Hartman, E. Dragileva, M. Dondero, T. Gebretsadik, A. Shintani, L. Peiperl, F. Valentine, and S. A. Kalams. 2006. Low CD4+ T cell nadir is an independent predictor of lower HIV-specific immune responses in chronically HIV-1-infected subjects receiving highly active antiretroviral therapy. J. Infect. Dis. 194661-665. [DOI] [PubMed] [Google Scholar]
- 71.Sobel, R. A., A. B. Collins, R. B. Colvin, and A. K. Bhan. 1986. The in situ cellular immune response in acute herpes simplex encephalitis. Am. J. Pathol. 125332-338. [PMC free article] [PubMed] [Google Scholar]
- 72.Sopper, S., U. Sauer, S. Hemm, M. Demuth, J. Muller, C. Stahl-Hennig, G. Hunsmann, V. ter Meulen, and R. Dorries. 1998. Protective role of the virus-specific immune response for development of severe neurologic signs in simian immunodeficiency virus-infected macaques. J. Virol. 729940-9947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stevceva, L., B. Kelsall, J. Nacsa, M. Moniuszko, Z. Hel, E. Tryniszewska, and G. Franchini. 2002. Cervicovaginal lamina propria lymphocytes: phenotypic characterization and their importance in cytotoxic T-lymphocyte responses to simian immunodeficiency virus SIVmac251. J. Virol. 769-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Svenningsson, A., G. K. Hansson, O. Andersen, R. Andersson, M. Patarroyo, and S. Stemme. 1993. Adhesion molecule expression on cerebrospinal fluid T lymphocytes: evidence for common recruitment mechanisms in multiple sclerosis, aseptic meningitis, and normal controls. Ann. Neurol. 34155-161. [DOI] [PubMed] [Google Scholar]
- 75.Tang, Y. W., J. T. Huong, R. M. Lloyd, Jr., P. Spearman, and D. W. Haas. 2000. Comparison of human immunodeficiency virus type 1 RNA sequence heterogeneity in cerebrospinal fluid and plasma. J. Clin. Microbiol. 384637-4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tashima, K. T., T. P. Flanigan, J. Kurpewski, S. M. Melanson, and P. R. Skolnik. 2002. Discordant human immunodeficiency virus type 1 drug resistance mutations, including K103N, observed in cerebrospinal fluid and plasma. Clin. Infect. Dis. 3582-83. [DOI] [PubMed] [Google Scholar]
- 77.van't Wout, A. B., L. J. Ran, C. L. Kuiken, N. A. Kootstra, S. T. Pals, and H. Schuitemaker. 1998. Analysis of the temporal relationship between human immunodeficiency virus type 1 quasispecies in sequential blood samples and various organs obtained at autopsy. J. Virol. 72488-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Venturi, G., M. Catucci, L. Romano, P. Corsi, F. Leoncini, P. E. Valensin, and M. Zazzi. 2000. Antiretroviral resistance mutations in human immunodeficiency virus type 1 reverse transcriptase and protease from paired cerebrospinal fluid and plasma samples. J. Infect. Dis. 181740-745. [DOI] [PubMed] [Google Scholar]
- 79.von Geldern, G., S. Cepok, T. Nolting, Y. Du, V. Grummel, O. Adams, H. P. Hartung, G. Arendt, and B. Hemmer. 2007. CD8 T-cell subsets and viral load in the cerebrospinal fluid of therapy-naive HIV-infected individuals. AIDS 21250-253. [DOI] [PubMed] [Google Scholar]
- 80.von Herrath, M., M. B. Oldstone, and H. S. Fox. 1995. Simian immunodeficiency virus (SIV)-specific CTL in cerebrospinal fluid and brains of SIV-infected rhesus macaques. J. Immunol. 1545582-5589. [PubMed] [Google Scholar]
- 81.Wiley, C. A., and C. Achim. 1994. Human immunodeficiency virus encephalitis is the pathological correlate of dementia in acquired immunodeficiency syndrome. Ann. Neurol. 36673-676. [DOI] [PubMed] [Google Scholar]
- 82.Wiley, C. A., M. Baldwin, and C. L. Achim. 1996. Expression of HIV regulatory and structural mRNA in the central nervous system. AIDS 10843-847. [DOI] [PubMed] [Google Scholar]
- 83.Wiley, C. A., V. Soontornniyomkij, L. Radhakrishnan, E. Masliah, J. Mellors, S. A. Hermann, P. Dailey, and C. L. Achim. 1998. Distribution of brain HIV load in AIDS. Brain Pathol. 8277-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Williams, K., S. Westmoreland, J. Greco, E. Ratai, M. Lentz, W. K. Kim, R. A. Fuller, J. P. Kim, P. Autissier, P. K. Sehgal, R. F. Schinazi, N. Bischofberger, M. Piatak, J. D. Lifson, E. Masliah, and R. G. Gonzalez. 2005. Magnetic resonance spectroscopy reveals that activated monocytes contribute to neuronal injury in SIV neuroAIDS. J. Clin. Investig. 1152534-2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Williams, K. C., S. Corey, S. V. Westmoreland, D. Pauley, H. Knight, C. deBakker, X. Alvarez, and A. A. Lackner. 2001. Perivascular macrophages are the primary cell type productively infected by simian immunodeficiency virus in the brains of macaques: implications for the neuropathogenesis of AIDS. J. Exp. Med. 193905-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Williams, K. C., and W. F. Hickey. 2002. Central nervous system damage, monocytes and macrophages, and neurological disorders in AIDS. Annu. Rev. Neurosci. 25537-562. [DOI] [PubMed] [Google Scholar]