Abstract
The human cytomegalovirus (HCMV) 72-kDa immediate-early 1 (IE1) protein is thought to modulate cellular antiviral functions impacting on promyelocytic leukemia (PML) nuclear bodies and signal transducer and activator of transcription (STAT) signaling. IE1 consists of four distinct regions: an amino-terminal region required for nuclear localization, a large central hydrophobic region responsible for PML targeting and transactivation activity, an acidic domain, and a carboxyl-terminal chromatin tethering domain. We found that the acidic domain of IE1 is required for binding to STAT2. A mutant HCMV encoding IE1(Δ421-475) with the acidic domain deleted was generated. In mutant virus-infected cells, IE1(Δ421-475) failed to bind to STAT2. The growth of mutant virus was only slightly delayed at a high multiplicity of infection (MOI) but was severely impaired at a low MOI with low-level accumulation of viral proteins. When cells were pretreated with beta interferon, the mutant virus showed an additional 1,000-fold reduction in viral growth, even at a high MOI, compared to the wild type. The inhibition of STAT2 loading on the target promoter upon infection was markedly reduced with mutant virus. Furthermore, sumoylation of IE1 at this acidic domain was found to abolish the activity of IE1 to bind to STAT2 and repress the interferon-stimulated genes. Our results provide genetic evidence that IE1 binding to STAT2 requires the 55-amino-acid acidic domain and promotes viral growth by interfering with interferon signaling and demonstrate that this viral activity is negatively regulated by a cellular sumoylation pathway.
Human cytomegalovirus (HCMV) infection of newborns and immunocompromised individuals, such as organ transplant recipients and patients with AIDS, and reactivation from latency cause severe disease (20). During lytic cycle infection, HCMV gene expression occurs in a three-step sequential fashion with immediate-early (IE), early, and late kinetics. IE gene expression is activated by virion-associated factors, and IE gene products are responsible for the expression of all downstream viral genes. Among the IE proteins, 72-kDa IE1 (also called IE72 or IE1491aa) is the first and most abundantly expressed viral protein that localizes in the nucleus and associates with metaphase chromosomes in infected cells. IE1 also plays an important role in triggering the lytic cycle, especially at a low multiplicity of infection (MOI). The function of IE1 in augmenting the IE2-mediated transactivation of viral early genes and in activating its own promoter, as well as some cellular promoters, have largely been studied from transient assays (reference 20 and references therein). Genetic studies using a mutant virus with IE1 deleted, CR208, have demonstrated that IE1 is required for virus growth at a low MOI in cell culture (11, 19). Further studies have revealed that the growth defect of the CR208 virus results from a failure or delay in the accumulation of early gene products (3, 10).
Other important functions of IE1 appear to be related to the modulation of host cells. The roles of IE1 in stimulating host cells toward an S-phage-like state and in regulating cellular apoptosis and cell cycle have been suggested (7-9, 33, 34). In addition, IE1 has been shown to modulate cellular antiviral functions that are exerted by promyelocytic leukemia (PML) protein or PML-associated nuclear bodies (NBs) and signal transducer and activator of transcription (STAT) signaling. IE1 binds to PML and disrupts PML NBs (also know as nuclear domain 10 and PML oncogenic domains), leading to displacement of PML-NB-associated proteins such as PML, Sp100, and Daxx (2, 4, 14, 31). Studies on the mechanism underlying this event have shown that IE1 induces the loss of the sumoylated forms of PML (17, 21), which are required for the formation of PML NBs. Our in vitro study also suggested that IE1 does not have intrinsic SUMO protease activity, although this process may involve inhibition of PML oligomerization by IE1 (13). This activity of IE1 is believed to promote viral growth based on the findings that PML overexpression in semipermissive U373 cells confers resistance to HCMV infection (3), and that silencing PML using small interfering RNA in permissive human foreskin fibroblast (HF) cells enhances viral IE gene expression and production of progeny virions (27). In addition, IE1 has been shown to directly bind to STAT2 and, to a lesser extent, to STAT1 (23). That study suggested that IE1 may modulate type I interferon (IFN) responses by inhibiting the activation of the IFN-inducible promoters containing IFN-stimulated regulatory elements (ISREs). Whether the interaction of IE1 with STAT proteins is indeed necessary to inhibit IFN signaling and promote viral growth has not been addressed using a mutant virus.
IE1 consists of four distinct regions: a short amino-terminal region that is required for nuclear localization (16, 31), a large central hydrophobic region that is responsible for PML targeting and transactivation activity (2, 17), a near carboxyl-terminal acidic domain containing a lysine residue that is covalently modified by small ubiquitin-like modifier (SUMO) (26, 32), and a carboxyl-terminal chromatin tethering domain (15, 31). A recent study demonstrated that the glutamic acid-rich acidic domain (amino acids 421 to 475) is needed for efficient complementation of the growth defect of the mutant virus with IE1 deleted, suggesting an important role for this domain in promoting viral replication (24). However, the role of this domain has not been addressed in the context of virus, and the mechanism by which this domain promotes viral replication is not known. In the present study, we demonstrate that the acidic domain of IE1 is required for binding to STAT2. We generated a mutant HCMV encoding an IE1 with the acidic domain deleted: IE1(Δ421-475). By characterizing this mutant virus, we provide genetic evidence that the acidic domain of IE1 is necessary for binding to STAT2 and that IE1-STAT2 interaction is required for the efficient viral growth and inhibition of STAT signaling in the context of virus-infected cells. We also show that sumoylation of IE1 at this acidic domain negatively regulates STAT2 binding and its repressive effect on IFN-regulated gene expression.
MATERIALS AND METHODS
Cell culture and virus.
The procedures for primary HF cell culture and preparation of the stocks for wild-type Towne virus and CR208 mutant virus with IE1 deleted were described previously (17). The virus titers were determined as infectious units (IFU) after the measurement of the IE1-positive cells in the infectious center assays using HF cells. The U373-SUMO-1 cell line that constitutively expressed flag-tagged SUMO-1 was described previously (32) and maintained in the medium containing G418 (0.4 mg per ml).
Plasmids.
Mammalian expression plasmids for wild-type IE1, IE1(1-420), IE1(290-320), and IE1(K450R) were generated on a pSG5 (12) background (17). Plasmids expressing IE1(1-475) and IE1(Δ421-475) were also generated on the same background by using PCR methods. A plasmid expressing the IE1-SUMO-1 fusion was generated by the in-frame insertion of SUMO-1 at the carboxyl terminus of IE1. Similarly, a bacterial expression plasmid for the glutathione S-transferase (GST)-IE1-SUMO-1 fusion was generated on a background of the GST-IE1 construct (13). For in vitro transcription/translation reactions or expression in cultured cells by transfection, the STAT2, HDAC3, or UL44 cDNA was cloned into pSPUTK (without tag) (Stratagene) or pCS3-MT (with 6myc tag) plasmids (29) using the Gateway technology (Invitrogen). pT-E1E2S1, which encodes for the E1 and E2 enzymes of SUMO conjugation, as well as an active form of SUMO-1, was used to introduce a synthetic SUMO-1 conjugation pathway into E. coli (30). A plasmid containing the IGS54 ISRE-luciferase gene (25) was used for reporter assays.
Infectious center assays.
The diluted samples were used to inoculate a monolayer of 105 HF cells in a 24-well plate. At 24 h postinfection, cells were fixed with 500 μl of cold methanol for 10 min. The cells were then washed three times in phosphate-buffered saline (PBS) and incubated with anti-IE1 rabbit polyclonal antibody (PAb) in PBS at 37°C for 1 h, followed by incubation with phosphatase-conjugated anti-rabbit immunoglobulin G (IgG) antibody in phosphate-buffered saline (PBS) at 37°C for 1 h. Finally, the cells were gently washed in PBS and treated with 200 μl of developing solution (nitroblue tetrazolium/BCIP [5-bromo-4-chloro-3-indolylphosphate]:AP buffer, 1:1) at room temperature for 1 h. The positively stained cells were counted for at least three to five separate fields per well under a light microscope (×200 magnification).
BAC mutagenesis.
The T-BAC clone was used as a template for mutagenesis (18). To create a transfer vector to mutate IE1, a 4.1-kb SalI-PvuII restriction fragment containing the UL123 allele was cloned into GS284, a derivative of the positive suicide selection vector, pCV442 (ampicillin resistant). To transfer DNA sequences in pGS284 to T-BAC, the Escherichia coli S17-pir (GS111) strain containing the GS284 donor plasmid was conjugated with a RecA+ derivative of E. coli DH10B (GS243 strain) harboring the wild-type T-BAC DNA (chloramphenicol resistant) by cross-streaking on an LB plate at 37°C. To select the exoconjugates, the cells from the intersection region were streaked onto an LB plate containing ampicillin plus chloramphenicol, followed by incubation at 37°C. On the next day, several colonies were inoculated into 5 ml of LB broth containing chloramphenicol and incubated for 6 h at 37°C. The culture was serially diluted (1:1,000, 1:10,000, and 1:100,000) and spread onto LB plates containing chloramphenicol and LB plates (without NaCl) containing chloramphenicol plus 5% sucrose. These plates were then incubated at 30°C. pGS284 contains the SacB gene, which encodes levansucrase. This enzyme metabolizes sucrose to levan, which is toxic to E. coli. Therefore, enrichment for E. coli lacking the SacB gene was achieved by incubating the cells at 30°C on LB plates lacking NaCl but containing 5% sucrose. Isolated colonies from sucrose plates were replicated on LB plates containing chloramphenicol and LB plates containing ampicillin. Colonies that grew on an LB plate containing chloramphenicol, but not on an LB plate containing ampicillin, were further examined by DNA sequencing and restriction enzyme digestion analysis.
Pulsed-field gel electrophoresis.
The restriction enzyme-digested DNA fragments were electrophoresed in a 1% agarose (Bio-Rad) gel with a CHEF-DRII apparatus (Bio-Rad) for 5 h at 6 V/cm with switching times ramped at 0.1 s.
Electroporation.
Electroporation of HF cells was conducted by using a Microporator MP-100 (Digital Bio). For each reaction, 5 × 106 HF cells were suspended in 45 μl of resuspension buffer and mixed with expression plasmids (up to 4 μg) in a 1.5-ml tube. After electroporation at 1,400 V and 30 ms, the cells were plated in T-25 plates. When the surviving cells became confluent, the cells were split into new plates at a ratio of 1:2.
Southern blot analysis.
The restriction enzyme-digested DNAs were loaded on the 1% agarose gel and separated by pulse-field gel electrophoresis. DNA fragments were denatured and transferred to uncharged nylon membranes (Hybond-N). DNA probes were radiolabeled with [α-32P]dCTP (Amersham) by use of the random-primed DNA labeling kit (Roche, Mannheim, Germany). Standard prehybridization and hybridization procedures were used as described previously (17).
Antibodies and IFA.
Mouse monoclonal antibody (MAb) 810R, which detects epitopes present in both IE1 and IE2, and MAb 6E1 specific to IE1 were purchased from Chemicon and Vancouver Biotech, respectively. Mouse MAbs against IE1 (CH443) and UL44 (p52) were obtained from Virusys. Mouse MAb against β-actin was purchased from Sigma. The anti-STAT2 rabbit PAb N17 was obtained from Santa Cruz Biotechnology. Rabbit PAb raised against purified IE1 was used for infectious center assays with 1/1,000 dilution. The fluorescein isothiocyanate-labeled donkey anti-mouse IgG and rhodamine/redX-coupled donkey anti-rabbit IgG were obtained from Jackson Immunoresearch Laboratories, Inc. The dilution ratios of antibodies were as follows: for immunoblotting, 1/3,000 for 810R and 6E1, 1/40,000 for anti-UL44, 1/20,000 for anti-β-actin, 1/3,000 for N17, and 1/5,000 for PAb against IE1 and for indirect immunofluorescence assay (IFA), 1/50 for 6E1 and 1/300 for N17.
For IFA, cells were fixed by either the methanol or the paraformaldehyde procedure, and the previously described procedures were followed (17).
Immunoblot analysis.
Samples were prepared by boiling in loading buffer and were then separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a nitrocellulose membrane (Schleicher & Schuell, Dassel, Germany). The membrane was blocked for 1 h in PBS-T (PBS plus 0.1% Tween 20 [Sigma]) containing 5% skim milk and was then washed with PBS-T. After incubation with the appropriate antibody, the proteins were visualized by the standard procedure using the enhanced chemiluminescence system (Roche).
CoIP.
293T (8 × 105 in 100-mm dish) or HF (2 × 106 in 150-mm dish) cells were harvested and sonicated in 1 ml of coimmunoprecipitation (CoIP) buffer (50 mM Tris-Cl [pH 7.4], 50 mM NaF, 5 mM sodium phosphate, 0.1% Triton X-100, containing protease inhibitors [Sigma]) by using a Microtip probe (Vibra cell; Sonics and Materials, Inc.) for 10 s (pulse on, l s; pulse off, 3 s). The clarified cell lysates were incubated with appropriate antibodies for 16 h at 4°C. A total of 60 μl of a 50% slurry of protein A- and protein G-Sepharose (Amersham) was then added. After 2 h of incubation at 4°C, the mixture was pelleted and washed seven times with CoIP buffer. The beads were resuspended and boiled for 5 min in loading buffer. Each sample was analyzed by SDS-PAGE, and immunoblotting was then performed.
In vitro binding assays.
The GST fusion proteins were generated in E. coli. In vitro translation products were produced by using the TNT Quick-Coupled transcription/translation system (Promega). CoIP assays using these proteins were conducted as described above. The standard procedure for the GST pull-down assays used was described previously (13).
ChIP assay and quantitative real-time PCR.
Chromatin immunoprecipitation (ChIP) assays were performed using a kit purchased from Upstate Biotechnology, Inc., with minor modification. In brief, HF cells (2 × 106) were fixed with 1% formaldehyde for 10 min at 12 h after infection and were then lysed with a lysis buffer provided in the kit. ChIP assays were performed with 4 μg of anti-STAT2 antibody or control IgG. One-third of the lysate was kept to facilitate quantitation of the amount of DNA present in different samples prior to immunoprecipitation. Relative changes in coprecipitated DNA were calculated by using quantitative real-time PCR with the Applied Biosystems AIB prism SDS software. The PCR-amplified region comprised a 200-bp sequence covering the ISRE element and the transcription initiation site of the human ISG54 promoter. The primer sequences were 5′-GGAGGAAAAAGAGTCCTCTA-3 (for ISG54P forward) and 5-AGCTGCACTCTTCAGAAA-3′ (for ISG54P reverse). A portion (5 μl) from a total 50 μl of the precipitated DNA samples was used for PCR, and the program used consisted of 95°C for 10 min, followed by 40 amplification cycles (94°C for 30 s, 48°C for 1 min, and 68°C for 30 s), and a final step at 72°C for 10 min.
Reverse transcription-PCR (RT-PCR) and quantitative real-time PCR.
Total RNA was isolated from 2 × 105 cells using TRIzol reagent (Invitrogen) and MaXtract High Density (Qiagen). First-strand cDNA was synthesized by using the random hexamer primers in the SuperScript III system (Invitrogen). Quantitative real-time PCR was performed using the Applied Biosystems ABI Prism SDS software and the following primers: 5′-GACATCCCTGAGATTAAG-3′(IFN-β forward), 5′-ATGTTCCTGGAGCATCTCG-3′ (IFN-β reverse), 5′-TCTTCATGCTCCAGACGAAC-3′ (MxA forward), 5′ CCAGCTGTAGGTGTCCTTG-3′ (MxA reverse), 5′-GCTTAGACATATTCTGAGCCTAC-3′ (CXCL10 forward), 5′-AGCTGATTTCCTGACCATCATTG-3′ (CXCL10 reverse), 5′-AGAAATCAAGGGAGAAAGAA-3′ (ISG54 forward), 5′-AAGGTGACTAAGCAAATGGT-3′ (ISG54 reverse), 5′-AGCGGGAAATCGTGCGTG-3′ (β-actin forward), and 5′-CAGGGTACATGGTGGTGCC-3′ (β-actin reverse).
Luciferase reporter assay.
Cells were collected and lysed by using three freeze-thaw steps in 200 μl of 0.25 M Tris-HCl (pH 7.9) plus 1 mM dithiothreitol. Subsequent procedures were performed as described previously (17). A TD-20/20 luminometer (Turner Designs) was used for the 10-s assay of the photons produced (measured in relative light units).
Retrovirus transduction and selection of stably transduced cells.
Recombinant retroviruses were prepared by cotransfecting 293T cells with pMIN plasmid (16) encoding wild-type IE1 or IE1-SUMO1ΔGG (without double glycine residues) fusion and the packaging plasmids pHIP60 (Gag-Pol) and pMD-G (VSV-G) (27) using Metafectene reagents (Biontex). Viral supernatants were harvested at 48 h after transfection. Low passage of HF cells was transduced by retroviruses in the medium supplemented with 0.4 mg of G418 (Calbiochem) per ml to select a stably transduced cell population.
RESULTS
An acidic domain near the carboxyl-terminal of IE1 is required for binding to STAT2.
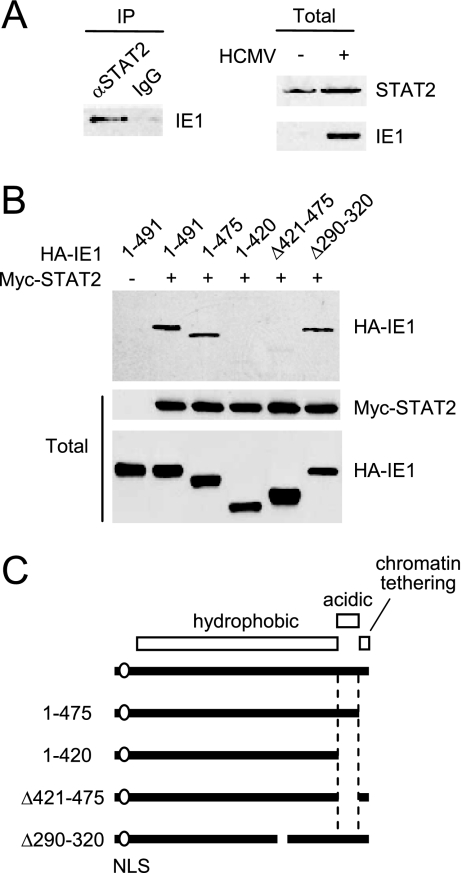
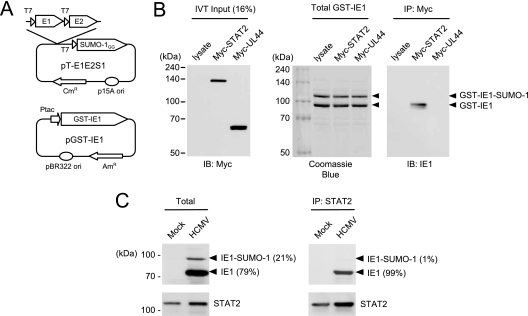
We previously demonstrated that the correlated activities of IE1 in upregulating cellular and viral promoters and in inducing PML desumylation in transient assays do not require its C-terminal region, which harbors both the acidic and chromatin tethering domains (17). Since IE1 has been shown to interact with STAT2 and, to a lesser extent, STAT1 (23), we investigated whether the C-terminal region may be involved in binding to STAT proteins. First, we confirmed the interaction of IE1 with STAT proteins in HCMV-infected cells. The results of CoIP assays showed that IE1 bound to STAT2 in virus-infected HF cells at 24 h after infection (Fig. 1A). We did not detect a reliable interaction between IE1 and STAT1 under similar conditions (data not shown). This is generally consistent with the previous report that IE1 binds to STAT2 but only binds weakly, if at all, to STAT1 (23).
FIG. 1.
The acidic domain of IE1 is required for STAT2 binding. (A) Interaction of IE1 with STAT2 in HCMV-infected cells. HF cells were mock infected or infected with HCMV at an MOI of 2 (IFU/cell). (Left panel) At 24 h, immunoprecipitation was carried out with anti-STAT2 rabbit PAb or control IgG, followed by immunoblotting with anti-IE1 mouse MAb. (Right panels) The protein levels of STAT2 and IE1 in total cell lysates of infected and uninfected cells were measured by immunoblotting. (B) CoIP of IE1 with STAT2 in cotransfected cells. 293T cells were cotransfected with myc-STAT2 and wild-type or mutant HA-IE1 expression plasmids. (Upper panel) At 48 h, total cell lysates were immunoprecipitated with anti-Myc antibody, followed by immunoblotting with anti-HA antibody. (Lower panels) Input protein levels of STAT2 and IE1 proteins in total cell lysates as measured by immunoblotting. (C) Summary of the structure of IE1 proteins used in cotransfection assays. The amino-terminal region required for the NLS function (an open circle), the large central hydrophobic region, and the carboxyl-terminal acidic plus chromatin tethering domains (open boxes) within IE1 are indicated.
To investigate the potential role of the glutamic acid-rich acidic domain of IE1 in support of the interaction with STAT2, CoIP assays were performed after the 293T cells had been cotransfected with plasmids expressing myc-STAT2 and wild-type or mutant HA-IE1. The results showed that both IE1(1-420) and IE1(Δ421-475), which lack the acidic domain from 421 to 475, failed to bind to STAT2, whereas wild-type IE1(1-491) and IE1(1-475) lacking only the carboxyl-terminal chromatin tethering domain both efficiently bound to STAT2 under the same conditions (Fig. 1B and C). IE1(Δ290-320), which contains a small deletion within the central hydrophobic region and is defective in all PML-associated effects (17), still bound to STAT2 (Fig. 1B and C). These results strongly suggest that the 55-amino-acid acidic domain of IE1 is required for STAT2 binding.
Construction of the recombinant HCMV Towne-BAC clones and reconstitution of the recombinant viruses.
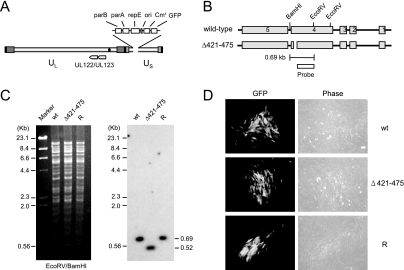
To address the role of the 55-amino-acid acidic domain of IE1 in the context of virus, we generated a recombinant virus encoding a mutant IE1 protein with the acidic domain deleted, IE1(Δ421-475). The HCMV Towne strain-based bacterial artificial chromosome (T-BAC) clone, which has a 9-kb deletion from the dispensable portion of the US region from US1 to US12, and instead contains the BAC sequences and a green fluorescent protein (GFP) expression cassette in the deleted region, was used as a template for mutagenesis (18) (Fig. 2A). The IE1(Δ421-475) mutant gene was introduced into the T-BAC clone in place of the wild-type IE1 gene (see Materials and Methods). The recombinant T-BAC genome containing the IE1(Δ421-475) gene was selected and isolated after direct DNA sequencing screening for PCR-amplified DNA fragments that contained the mutated allele (data not shown). To confirm the deletion of the IE1 region encoding residues 421 to 475, the restriction fragments of the viral genome were separated by pulse-field gel electrophoresis, and Southern blot analysis was then performed. The results showed that the appropriate DNA hybridization probe detected a smaller 0.52-kb EcoRV-BamHI fragment rather than the wild-type 0.69-kb version, as would be expected from a mutant HCMV-BAC clone containing the 165-bp deletion (Fig. 2B and C). In addition, the lack of any other apparent alterations in the viral genomes was also checked by comparing the restriction fragment patterns of the viral genomes of wild-type and mutant T-BACs (Fig. 2C). A revertant T-BAC clone was also generated by allelic exchange of the mutated allele with the wild-type DNA fragment cloned in pGS284. The correct conversion of the mutant allele back to the wild-type allele was also confirmed by sequencing (data not shown) and Southern blot analysis (Fig. 2C).
FIG. 2.
Generation of the recombinant T-BAC clone. (A) Genome structure of the T-BAC clone used in the present study. The F plasmid sequences containing the partition and replication functions (parA, parB, and repE), replication origin (ori), chloramphenicol resistance marker (Cmr), and the GFP eukaryotic expression cassette are indicated. (B) Genome structures of the T-BAC containing the wild-type or IE1(Δ421-475) gene. The region encompassing the MIE locus and the locations of the restriction enzyme sites used for mapping by Southern blot analysis are shown. The location for the 400-bp probe used for Southern blot analysis is shown. (C) Southern blot analysis. (Left) Ethidium bromide-stained total restriction DNA fragment patterns after EcoRV/BamHI digestion of three T-BAC DNAs (wild type [wt], Δ421-475, and revertant [R]) obtained by pulsed-field gel electrophoresis. (Right) Results of autoradiography after Southern blotting with 32P-labeled probe. Marker, λ-HindIII/EcoRI. (D) Infectivity of the transfected T-BAC DNAs in permissive HF cells. HF cells were electroporated with 2 μg of wild-type [wt], IE1(Δ421-475) mutant, or revertant [R] T-BAC DNAs. Each reaction also included 1 μg of plasmid pCMV71 encoding pp71 and 1 μg of plasmid pEGFP-C1. The cells were monitored for the spreading of the GFP signals. The GFP images and phase-contrast images were taken at 12 days after electroporation.
To generate the recombinant viruses, we electroporated permissive HF cells with the purified T-BAC DNAs of the wild type, IE1(Δ421-475), or the revertant. For each electroporation process, expression plasmids encoding HCMV pp71 and GFP were cotransfected with BAC plasmid DNA in order to enhance the activation of the MIE promoter (5) and to monitor electroporation efficiency, respectively. The electroporation efficiency, as judged by the initial transient GFP signals from the transfected cells, was measured to be 30 to 50% in all experiments. This transient GFP signal disappeared within the first week after electroporation. When the HF cells also received T-BAC DNAs, the GFP signals (from the GFP cassette within the viral genome) began to reappear in all cells at around 10 days after electroporation. The wild-type, IE1(Δ421-475), and revertant viruses all spread to the surrounding cells with similar efficiency (Fig. 2D). This result demonstrates that the acidic domain of IE1 is not essential for reconstitution of virus from the input naked DNA genome in cultured HF cells.
IE1(Δ421-475) does not bind to STAT2 in mutant virus-infected cells.
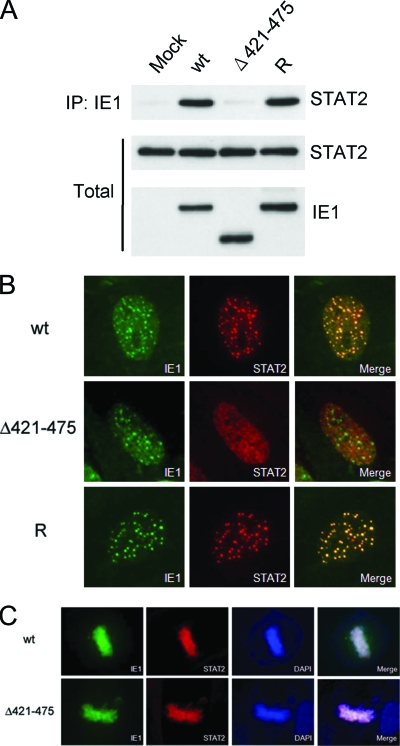
To investigate whether the acidic domain of IE1 is required for STAT2 binding in the context of virus infection, HF cells were mock infected or infected with the recombinant wild-type, IE1(Δ421-475), and revertant viruses. At 2 days postinfection, the cells were lysed, and the proteins were immunoprecipitated with MAb against IE1, followed by immunoblotting with anti-STAT2 antibody. The result showed that STAT2 was coimmunoprecipitated with IE1 in wild-type or revertant virus-infected cells, but not in mutant virus-infected cells. These findings demonstrate that the acidic domain of IE1 is necessary for STAT2 binding in the context of the virus genome (Fig. 3A).
FIG. 3.
The acidic domain of IE1 is required for STAT2 binding in virus-infected cells. (A) HF cells were mock infected or infected with the wild-type, IE1(Δ421-475), or revertant viruses at an MOI of 2 IFU per cell. (Upper panel) At 24 h, total cell lysates were immunoprecipitated with anti-IE1 MAb CH443 (10 μg per 15 mg of total proteins), followed by immunoblotting with anti-STAT2 antibody. (Lower panels) The total protein levels of STAT2 and IE1 proteins in the cell extracts are also shown after immunoblotting. (B) HF cells were infected with recombinant viruses at an MOI of 1 IFU per cell. At 3 h postinfection, the cells were fixed with methanol, followed by double-label IFA with anti-IE1 and anti-STAT2 antibodies. (C) Metaphase association of wild-type or Δ421-475 mutant IE1. HF cells were infected with wild-type or IE1(Δ421-475) mutant at an MOI of 1 IFU per cell. At 72 h postinfection, the cells were fixed with methanol, followed by double-label IFA with anti-IE1 and anti-STAT2 antibodies. DAPI was used to stain DNA.
When HF cells expressing IE1 were treated with IFN-α, STAT2 was shown to be colocalized with IE1 as punctate forms in a minority of interphase nuclei and as metaphase-associated forms in cells undergoing mitosis, whereas both proteins were diffusely distributed in most interphase nuclei (23). This colocalization of STAT2-IE1 appears to reflect the interaction between STAT2 and IE1. When HF cells were infected with wild-type, IE1(Δ421-475) mutant, and revertant viruses and were stained with IE1 and STAT2 antibodies at 2 to 3 h, we found that STAT2 was completely colocalized with punctate forms of IE1, which indicates transient targeting of IE1 to PML NBs (2, 4, 14, 31), in both wild-type and revertant virus-infected cells (Fig. 3B). However, in mutant virus-infected cells, STAT2 remained primarily as a diffuse form in the nucleus, although some were also distributed as foci, which appear to be different from normal PML NBs, in a majority (85%) of IE1-positive cells (Fig. 3B). In uninfected cells, STAT2 was stained in the cytoplasm (data not shown). Therefore, translocation of STAT2 to the nucleus in infected cells is independent of presence of IE1 binding. These results suggest that STAT2 interacts with and is colocalized with IE1 in PML NBs in virus-infected cells and that the acidic domain of IE1 is required for this colocalization interaction with STAT2. At later times after infection, both STAT2 and IE1 were displaced into the nuclear diffuse forms as PML NBs were disrupted, and this displacement occurred in both wild-type and IE1(Δ421-475) mutant virus-infected cells (data not shown).
In HCMV-infected cells, STAT2, like IE1, has been found to be associated with metaphase chromosomes in cells undergoing mitosis (23). Interestingly, we found that both wild-type and Δ421-475 mutant IE1 proteins were associated with metaphase chromosomes in mitotic cells, which suggests that the deposition of STAT2 in metaphase chromosomes may be independent of IE1 binding (Fig. 3C).
Growth defect of the IE1(Δ421-475) mutant virus at a low MOI.
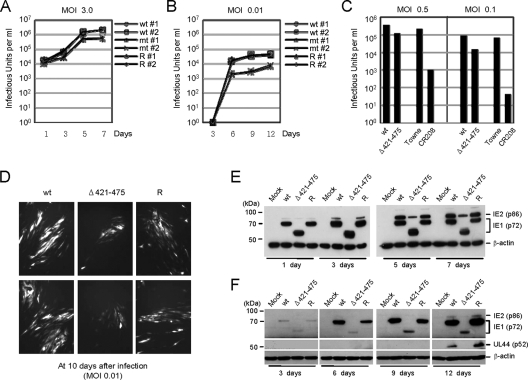
We then examined the growth kinetics of the mutant virus at high and low MOIs. HF cells were infected with wild-type, IE1(Δ421-475) mutant, and revertant viruses at 3 or 0.01 IFU per cell. The viruses released in the culture supernatants and associated with the cells were collected and pooled at various time points after infection, and the titers of the infectious progeny virions were determined by infectious center assays. At high MOIs (3 IFU/cell), the growth kinetics of the mutant virus were only slightly delayed relative to the wild-type and revertant viruses (Fig. 4A). However, in the case of a low MOI (0.01 IFU/cell), the growth kinetics of the IE1(Δ421-475) mutant virus was 10-fold lower than those of the wild-type and revertant viruses (Fig. 4B). This 10-fold growth defect of the IE1(Δ421-475) virus at a low MOI appeared to be less than that of CR208, which has a deletion of the whole of exon-4. Indeed, when we directly compared the virus titers produced in HF cells infected with the IE1(Δ421-475) or CR208 viruses at low MOIs, CR208 showed much more severe growth defects at low MOIs.
FIG. 4.
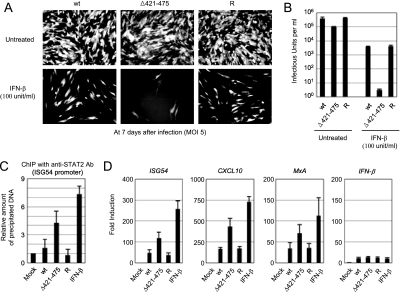
Analysis of the growth of recombinant viruses. (A and B) Analysis of growth curves in recombinant virus-infected cells. HF cells in 12-well plates were infected with wild-type, IE1(Δ421-475) mutant, or revertant virus at an MOI of 3 (A) or 0.01 (B) IFU/cell. The time course results shown represent the total infectious center units of infectious virus present in 2 ml of culture supernatant at the indicated sampling times. Note that the growth curves of wild-type and revertant viruses are indistinguishable, whereas those of mutant viruses (both 1 and 2) show different patterns (but almost identical) compared to those of wild-type and revertant viruses. (C) Comparison of viral growth between the IE1(Δ421-475) and CR208 viruses. HF cells were infected with the IE1(Δ421-475) and its parent viruses (reconstituted from the T-BAC clone), or the CR208 and its parent Towne viruses at an MOI of 0.5 or 0.1. At 5 days after infection, the total progeny virus titers were determined by measuring the numbers of IE2-positive infectious centers. (D) The images of GFP signals in infected cell cultures were taken at 10 days after infection at an MOI of 0.01 (IFU/cell). (E and F) Comparison of the accumulation of viral proteins in recombinant virus-infected cells. HF cells in six-well plates were infected with the recombinant viruses at an MOI of 3 (E) or 0.01 (F) IFU/cell. At the indicated time points, the total cell lysates were prepared and subjected to SDS-PAGE (8%), and immunoblotting was then performed with antibodies specific for IE1/IE2 and delayed-early p52(UL44) proteins. β-Actin was detected on the same blot as a loading control. wt, Wild type; R, revertant.
This growth defect of the IE1(Δ421-475) mutant virus at a low MOI was also confirmed by the observed delayed spreading of the GFP signals in mutant virus-infected cells (Fig. 4D). We also examined the accumulation of viral major IE proteins (IE1 and IE2) in cells infected with these recombinant viruses. When the HF cells were infected at a high MOI (3 IFU/cell), the protein levels of IE1 were similar among wild-type, IE1(Δ421-475) mutant, and revertant virus-infected cells, whereas those of IE2 were only slightly lower in mutant virus-infected cells than in wild-type or revertant virus-infected cells (Fig. 4E). However, when cells were infected at a low MOI (0.01 IFU/cell), the accumulation of both IE1 and IE2 proteins and early protein p52 (UL44) was severely delayed in mutant virus-infected cells (Fig. 4F). These results show that the growth defect of IE1(Δ421-475) mutant virus correlates with the low-level accumulation of viral proteins and that the acidic domain of IE1 is required for efficient viral growth, especially at a low MOI.
The acidic domain of IE1 is necessary for viral growth in IFN-pretreated cells even at a high MOI.
Because the acidic domain of IE1 is required for binding to STAT2, the growth defect of the IE1(Δ421-475) mutant virus may be attributed to the inefficient dysregulation of IFN signaling. To address this question, we examined the growth of this mutant virus in cells pretreated with beta IFN (IFN-β). IFN-β-pretreated or untreated HF cells were infected with wild-type, IE1(Δ421-475) mutant, or revertant virus at an MOI of 5 IFU per cell, and the viral growth was compared by observing the spread of GFP signals. Consistent with our earlier observation, the growth of the mutant virus in untreated cells at this high MOI was comparable to those of wild-type and revertant viruses (Fig. 5A, top panels). However, the growth of the mutant virus in IFN-β-pretreated cells was severely suppressed, even at this high MOI (Fig. 5A, bottom panels). When we measured the yield of progeny virions at 10 days postinfection, the titers of mutant virus were only fourfold lower than those of wild-type and revertant viruses in untreated cells. However, in IFN-β-pretreated cells, the titers of mutant virus were reduced by 105-fold compared to only 102-fold for wild-type and revertant viruses (Fig. 5B). These findings of a drastic 103-fold additional decrease in progeny virus yields strongly suggest that the growth defect of IE1(Δ421-475) virus results from a failure in the level of dysregulation of IFN pathway signaling.
FIG. 5.
Comparison of the growth of recombinant viruses in cells pretreated with IFN-β. HF cells in 12-well plates were untreated or pretreated with 100 U of IFN-β for 24 h and then infected with wild-type, IE1(Δ421-475) mutant, or revertant virus at an MOI of 5 (IFU/cell). (A) The images of GFP signals in infected cells were taken at 7 days after infection. (B) The production of progeny virions at 10 days after infection from untreated and IFN-β-pretreated cells was measured by infectious center assays. The results shown are the mean values and standard errors of four independent experiments. (C) ChIP assays for the loading of STAT2 on the ISG54 promoter in cells infected with recombinant viruses were performed. HF cells were mock infected or infected with wild-type, IE1(Δ421-475), or revertant virus at an MOI of 2 IFU per cell for 12 h. As a positive control, HF cells were treated with IFN-β (1,000 U/ml) for 2 h. ChIP assays were conducted as described in Materials and Methods. The amounts of coprecipitated DNA were quantified by real-time PCR and normalized to input. The results shown are the mean values and standard errors of three independent experiments. (D) Expression of IFN-induced genes in cells infected with recombinant viruses. HF cells were infected with recombinant viruses or treated with IFN-β as in panel C. mRNAs for ISG54, CXCL10, MxA, and IFN-β were quantified by quantitative RT-PCR. The amounts of mRNAs in cells infected with viruses or treated with IFN-β over those in mock-infected cells are indicated as the fold induction. wt, wild type; R, revertant.
Further, we investigated the effect of IE2-STAT2 binding on IFN signaling in virus-infected cells by measuring the levels of STAT2 protein loaded on the IFN-stimulated gene (ISG) 54 promoter. The results of ChIP assays performed at 12 h postinfection showed that the amount of STAT2 loaded on the ISG15 promoter was threefold higher in cells infected with the IE1(Δ421-475) mutant virus than in cells infected with the wild-type or revertant viruses (Fig. 5C). The amount of STAT2 loaded on the ISG54 promoter in the IE1(Δ421-475) mutant virus-infected cells reached 60% of the level in control IFN-β-treated cells, which was increased by sevenfold compared to mock-infected cells. Our data from the ChIP assays demonstrate that the IE1-STAT2 interaction results in a reduced loading of STAT2 onto its target promoters in the IFN pathway in virus-infected cells.
To relate the results of our ChIP assays to the promoter activities of ISGs, we infected or treated HF cells as described above (Fig. 5C) and measured the mRNA production of ISG54, CXCL10, MxA, and IFN-β (as a control) by quantitative real-time RT-PCR. The results showed that mRNAs of ISG54, CXCL10, and MxA were increased in cells infected with IE1(Δ421-475) virus compared to cells infected with wild-type and revertant viruses, whereas those of IFN-β were comparable among these viruses (Fig. 5D). Therefore, the lack of STAT2 binding in IE1(Δ421-475) virus infection indeed enhanced STAT2 loading on the promoter of ISGs and the subsequent mRNA synthesis.
Sumoylation of IE1 at lysine 450 negatively regulates STAT2 binding and its repressive effect on IFN-regulated gene expression.
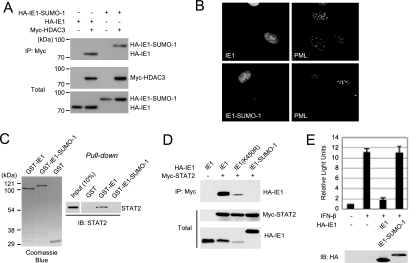
Protein sumoylation regulates protein-protein interactions by providing or masking protein-interacting surfaces. Because a lysine residue at position 450 that is present within the acidic domain is modified by SUMO (26, 32), we investigated the effect of SUMO modification on the IE1-STAT2 interaction. We first tested whether the in vitro-translated STAT2 protein binds to the SUMO-1-modified form of GST-IE1 protein. Both the unmodified and the SUMO-1-modified GST-IE1 proteins were generated in E. coli cells harboring a SUMO system plasmid (pT-E1E2-S1) that expresses the E1-activating and E2-conjugating enzymes for the SUMO pathway, as well as an active form of SUMO-1 and a plasmid expressing GST-IE1 (13) (Fig. 6A). When these proteins were incubated with in vitro-translated myc-STAT2 or myc-UL44 (as a control) and CoIP assays were performed, unmodified GST-IE1 was found to bind efficiently to STAT2, but the SUMO-1-modified GST-IE1 failed to do so (Fig. 6B).
FIG. 6.
Effect of sumoylation of IE1 on STAT2 binding. (A) Structural map of the two plasmids used to produce the sumoylated IE1 protein in E. coli. pT-E1E2S1 expressed E1, E2, and the cleaved active form of SUMO-1. Note that pT-E1E2S1 and pGST-IE1 contain different antibiotic markers and replication origins. (B) CoIP assays using bacterially produced proteins. The GST-IE1 and SUMO-1-modified GST-IE1 fusion proteins were incubated with the control cell lysates or in vitro-translated myc-STAT2 or myc-UL44 proteins. After immunoprecipitation of the samples with anti-myc antibody, the bound proteins were fractionated by SDS-PAGE and visualized by immunoblotting with anti-IE1 antibody (right). One-sixth of the proteins used in the binding reaction were shown by immunoblotting (for myc-tagged proteins, left) and Coomassie blue staining (for GST fusion proteins, middle) as input controls. (C) CoIP assay in virus-infected cells. U373 astrocytoma/glioblastoma cell lines that constitutively overexpress flag-SUMO-1 were mock infected or infected with HCMV at an MOI of 10 (IFU/cell). At 72 h, the total cell lysates were prepared with CoIP buffer containing 0.5 mM N-ethylmaleimide. The expression levels of unmodified IE1, SUMO-modified IE1, and STAT2 in total lysates were shown by immunoblotting with anti-IE1 mouse MAb and anti-STAT2 rabbit PAb (left). Total cell lysates were immunoprecipitated with anti-STAT2 antibody, and immunoblot analysis was conducted with antibodies for IE1 and STAT2 (right). The amounts of unmodified IE1 and SUMO-modified IE1 were measured quantitatively using Scion Image software (Scion Corp., Maryland) and are indicated as a percentage.
We also performed CoIP assays in virus-infected cells. To obtain a high level of IE1 sumoylation, U373 cells that constitutively overexpress SUMO-1 were used for infection. When these U373-SUMO-1 cells were infected with HCMV at a high MOI, 21% of the IE1 produced was modified by SUMO (Fig. 6C, left). When immunoprecipitation was performed with anti-STAT antibody, the coimmunoprecipitated IE1 was almost all unmodified IE1 (99% of total coimmunoprecipitated IE1) (Fig. 6C, right). This result demonstrates that SUMO-modified IE1 fails to bind to STAT2 in virus-infected cells.
GST-IE1 was also expressed as a carboxyl-terminal SUMO-1 fusion in order to mimic SUMO modification at lysine 450. We first investigated whether the C-terminal SUMO-1 fusion affected other known functions of IE1. However, like wild-type IE1, the IE1-SUMO-1 fusion still bound to HDAC3 in cotransfected 293T cells (Fig. 7A) and disrupted PML NBs in retrovirus-transduced HF cells (Fig. 7B), suggesting that the C-terminal SUMO fusion of IE1 still binds to two other proteins that are known to bind to wild-type IE1. We next compared the abilities of bacterially purified GST-IE1 and GST-IE1-SUMO-1 to bind to in vitro-translated STAT2. The result showed that GST-IE1-SUMO-1 again failed to bind to STAT2 in pull-down assays (Fig. 7C). Similarly, when 293T cells were coexpressed with myc-STAT2 and wild-type IE1, IE1(K450R), or IE1-SUMO-1, both the wild-type and the K450R mutant IE1 proteins bound to STAT2, whereas IE1-SUMO-1 again failed to bind to STAT2 (Fig. 7D). Therefore, our binding assays with the SUMO-modified IE1 or IE1-SUMO-fusion proteins demonstrate that sumoylation of IE1 within the acidic domain negatively regulates binding to STAT2.
FIG. 7.
Effect of the carboxyl-terminal SUMO-1 fusion of IE1 on the abilities of IE1 to bind to STAT2 and to repress the IFN-induced gene expression. (A) Interaction of the IE1-SUMO-1 fusion with HDAC3 in cotransfected cells. 293T cells were cotransfected with plasmids encoding myc-tagged HDAC3 and hemagglutinin (HA)-tagged wild-type IE1 or IE1-SUMO1 fusion. The total cell lysates were prepared at 48 h and immunoprecipitated with anti-myc antibody, and SDS-PAGE and immunoblotting with anti-HA antibody were performed (top panel). Immunoblots of the total cell extracts with anti-myc or anti-HA antibody to show the expression levels are shown (bottom panels). (B) Disruption of PML-NBs by IE1-SUMO-1 fusion. HF cells expressing wild-type IE1 or IE1-SUMO1 fusion were fixed in paraformaldehyde, followed by double-label IFA with anti-IE1 (6E1) and anti-PML (PML-C) antibodies. (C) GST pull-down assays. The bacterially purified GST, GST-IE1, and GST-IE1-SUMO-1 proteins immobilized to glutathione-Sepharose beads were incubated with in vitro-translated STAT2 protein. The bound proteins were fractioned by SDS-PAGE and visualized by immunoblotting with anti-STAT2 antibody. One-fifth of the GST or GST fusion proteins used in the binding reaction were shown by Coomassie blue staining, and one-tenth of those in STAT2 were shown by immunoblotting as input controls. (D) CoIP assays using cotransfected cells. 293T cells were cotransfected with myc-STAT2 and the indicated IE1 constructs. At 48 h, CoIP assays were performed as described for Fig. 1B. (E) The reporter assays using the ISG54 ISRE-luciferase construct. 293T cells were cotransfected with 0.5 μg of the ISG54 ISRE-luciferase reporter construct and 0.1 μg of empty vector or plasmid expressing the intact IE1 or IE1-SUMO-1 fusion protein. At 24 h, cells were left untreated or were treated with IFN-β (1,000 U/ml) for 8 h, and luciferase reporter assays were then conducted. The results shown are the mean values and standard errors of three independent experiments. The expression levels of IE1 proteins in the extracts were shown by immunoblotting.
Finally, we measured the effects of IE1 and IE1 sumoylation on IFN-induced gene expression by performing reporter assays using the ISG54 ISRE-luciferase reporter construct. The result of these assays showed that IE1 efficiently repressed the IFN-induced activation of the ISRE-containing promoter, whereas the IE1-SUMO-1 fusion did not show this repressive activity (Fig. 7E). These findings suggest that sumoylation abolishes the repressive effect of IE1 on IFN-regulated gene expression.
DISCUSSION
We previously demonstrated that the activities of IE1, in cooperation with IE2, to transactivate the HCMV DNA polymerase promoter and, in the absence of IE2, to transactivate the cellular DNA polymerase promoter and the long terminal repeat of mouse mammary tumor virus all required the large central hydrophobic region, as well as the amino-terminal region harboring the NLS (16, 17). Since the carboxyl-terminal region (421 to 491) was not required for the transactivation functions of IE1 in transient assays (17), both the acidic and the chromatin tethering domains present in this region were not likely to be directly implicated in the activity of IE1 in regulating transcription. In the present study, we generated and characterized a recombinant virus expressing a mutant IE1(Δ421-475) with the acidic domain deleted and provide genetic evidence that the 55-amino-acid acidic domain of IE1 is involved in binding to STAT2. Furthermore, our analysis revealed that, although this acidic domain is not essential for the viral genome to be infectious and is dispensable for viral growth at a high MOI, its presence is necessary for efficient viral growth at a low MOI. Importantly, this mutant virus showed a 1,000-fold-enhanced growth defect even at a high MOI compared to wild-type when cells were pretreated with IFN-β, which demonstrates that the IE1-STAT2 interaction at early time points after infection indeed contributes to antagonizing a host antiviral response through the type I IFN signaling pathway.
The acidic domain of IE1 contains many serine residues that act as phosphorylation sites. It is conceivable that phosphorylation of these serine residues may regulate the interaction of IE1 with STAT2. However, bacterially expressed GST-IE1 was found to be capable of binding to STAT2 in in vitro GST pull-down assays (23; the present study). Therefore, it is unlikely that serine phosphorylation at the acidic domain is required for IE1 to bind to STAT2. Consistent with this notion, serine phosphorylation at these sites was not required for complementation of the growth defect of CR208 (24). This acidic domain also contains a lysine residue at position 450, which acts as a site for sumoylation (26, 32). The exact role of IE1 sumoylation is not yet clear. Although a recent report showed that a recombinant virus encoding a lysine 450-to-arginine substitution mutant IE1(K450R) grew slightly slower than did wild-type virus (22), other reports have demonstrated that the lack of IE1 sumoylation does not affect the abilities of IE1 to transactivate viral genes and to complement the growth defect of the mutant virus with IE1 deleted (17) (26). Moreover, a similar K450R mutant virus that we have constructed grew just as well as the wild-type virus (17; H.-R. Lee and J.-H. Ahn, unpublished data). Therefore, it is unlikely that the growth defect of IE1(Δ421-475) virus is related to the lack of IE1 sumoylation. Intriguingly, our in vitro and in vivo binding assays with the sumoylated IE1 and IE1-SUMO-1 fusion proteins revealed that sumoylation of IE1 blocks the binding of IE1 to STAT2. Therefore, it is conceivable that the mutant virus expressing the sumoylation-deficient IE1(K450R) may grow faster than the wild-type virus. However, we did not observe a significant difference in viral growth between the wild-type and IE1(K450R) mutant viruses, even in HF cells pretreated with IFN-β (data not shown). The fact that only a small fraction of the total IE1 made in infected HF cells is modified by SUMO appears to limit this approach. Alternatively, sumoylation of IE1 may have several distinct countering effects on the progression of viral infection. However, our data revealed a novel cellular mechanism via sumoylation that inhibits a viral strategy to interfere with type I IFN signaling.
Several HCMV proteins released from virions or produced in the cells immediately after infection appear to have a common role in counteracting the host IFN response induced by contact of the incoming virions with Toll-like-receptors at the cell surface and within the cell. Studies using the ΔUL83 mutant virus showed that a viral tegument protein, pp65, inhibits the expression of ISGs and other antiviral genes through pathways that affect IRF1/NF-κB (6) or IRF3 (1). In addition, IE2 has also been shown to antagonize virus-induced IFN-β production, although this was not evaluated in the context of virus infection (28). In the present study, we provide genetic evidence that IE1 plays a role in counteracting STAT-mediated IFN signaling. IE1 has also been considered to regulate PML or PML-NB-mediated antiviral responses (3, 27). We previously found that both the large central hydrophobic region and the NLS-containing amino-terminal region of IE1 were responsible for targeting to PML NBs and inducing desumoylation of PML in transient assays, which are found to correlate with the ability of IE1 to transactivate target promoters (16, 17). The importance of the central hydrophobic region of IE1 in these activities has been further demonstrated by the finding that the IE1(Δ290-320) mutant virus fails to disrupt PML NBs and cannot grow in normal HF cells (17). Since IE1(Δ290-320) was still found to bind to STAT2 in the present study, it is likely that IE1 contains distinct domains that separately target PML and STAT2. These two independent functions of IE1 in antagonizing host repression may be critical to the progression of successful lytic infection.
Acknowledgments
We thank Hisato Saitoh and Curt Horvath for providing us with the pT-E1E2S1 plasmid and the ISRE-luciferase construct.
This study was supported by grants from the Basic Research Program (R01-2006-000-11019-0) and the Ubiquitome Research Program (2008-00983) of the Korea Science and Engineering Foundation to J.-H.A. and an NIH research grant (R01 AI24576) to G.S.H. from the National Institute of Allergy and Infectious Diseases.
Footnotes
Published ahead of print on 13 August 2008.
REFERENCES
- 1.Abate, D. A., S. Watanabe, and E. S. Mocarski. 2004. Major human cytomegalovirus structural protein pp65 (ppUL83) prevents interferon response factor 3 activation in the interferon response. J. Virol. 7810995-11006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahn, J. H., E. J. Brignole III, and G. S. Hayward. 1998. Disruption of PML subnuclear domains by the acidic IE1 protein of human cytomegalovirus is mediated through interaction with PML and may modulate a RING finger-dependent cryptic transactivator function of PML. Mol. Cell. Biol. 184899-4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahn, J. H., and G. S. Hayward. 2000. Disruption of PML-associated nuclear bodies by IE1 correlates with efficient early stages of viral gene expression and DNA replication in human cytomegalovirus infection. Virology 27439-55. [DOI] [PubMed] [Google Scholar]
- 4.Ahn, J. H., and G. S. Hayward. 1997. The major immediate-early proteins IE1 and IE2 of human cytomegalovirus colocalize with and disrupt PML-associated nuclear bodies at very early times in infected permissive cells. J. Virol. 714599-4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baldick, C. J., Jr., A. Marchini, C. E. Patterson, and T. Shenk. 1997. Human cytomegalovirus tegument protein pp71 (ppUL82) enhances the infectivity of viral DNA and accelerates the infectious cycle. J. Virol. 714400-4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Browne, E. P., and T. Shenk. 2003. Human cytomegalovirus UL83-coded pp65 virion protein inhibits antiviral gene expression in infected cells. Proc. Natl. Acad. Sci. USA 10011439-11444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castillo, J. P., and T. F. Kowalik. 2004. HCMV infection: modulating the cell cycle and cell death. Int. Rev. Immunol. 23113-139. [DOI] [PubMed] [Google Scholar]
- 8.Castillo, J. P., and T. F. Kowalik. 2002. Human cytomegalovirus immediate-early proteins and cell growth control. Gene 29019-34. [DOI] [PubMed] [Google Scholar]
- 9.Fortunato, E. A., V. Sanchez, J. Y. Yen, and D. H. Spector. 2002. Infection of cells with human cytomegalovirus during S phase results in a blockade to immediate-early gene expression that can be overcome by inhibition of the proteasome. J. Virol. 765369-5379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gawn, J. M., and R. F. Greaves. 2002. Absence of IE1 p72 protein function during low-multiplicity infection by human cytomegalovirus results in a broad block to viral delayed-early gene expression. J. Virol. 764441-4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greaves, R. F., and E. S. Mocarski. 1998. Defective growth correlates with reduced accumulation of a viral DNA replication protein after low-multiplicity infection by a human cytomegalovirus ie1 mutant. J. Virol. 72366-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green, S., I. Issemann, and E. Sheer. 1988. A versatile in vivo and in vitro eukaryotic expression vector for protein engineering. Nucleic Acids Res. 16369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang, H., E. T. Kim, H. R. Lee, J. J. Park, Y. Y. Go, C. Y. Choi, and J. H. Ahn. 2006. Inhibition of SUMO-independent PML oligomerization by the human cytomegalovirus IE1 protein. J. Gen. Virol. 872181-2190. [DOI] [PubMed] [Google Scholar]
- 14.Korioth, F., G. G. Maul, B. Plachter, T. Stamminger, and J. Frey. 1996. The nuclear domain 10 (ND10) is disrupted by the human cytomegalovirus gene product IE1. Exp. Cell Res. 229155-158. [DOI] [PubMed] [Google Scholar]
- 15.Lafemina, R. L., M. C. Pizzorno, J. D. Mosca, and G. S. Hayward. 1989. Expression of the acidic nuclear immediate-early protein (IE1) of human cytomegalovirus in stable cell lines and its preferential association with metaphase chromosomes. Virology 172584-600. [DOI] [PubMed] [Google Scholar]
- 16.Lee, H. R., Y. H. Huh, Y. E. Kim, K. Lee, S. Kim, and J. H. Ahn. 2007. N-terminal determinants of human cytomegalovirus IE1 protein in nuclear targeting and disrupting PML-associated subnuclear structures. Biochem. Biophys. Res. Commun. 356499-504. [DOI] [PubMed] [Google Scholar]
- 17.Lee, H. R., D. J. Kim, J. M. Lee, C. Y. Choi, B. Y. Ahn, G. S. Hayward, and J. H. Ahn. 2004. Ability of the human cytomegalovirus IE1 protein to modulate sumoylation of PML correlates with its functional activities in transcriptional regulation and infectivity in cultured fibroblast cells. J. Virol. 786527-6542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marchini, A., H. Liu, and H. Zhu. 2001. Human cytomegalovirus with IE-2 (UL122) deleted fails to express early lytic genes. J. Virol. 751870-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mocarski, E. S., G. W. Kemble, J. M. Lyle, and R. F. Greaves. 1996. A deletion mutant in the human cytomegalovirus gene encoding IE1(491aa) is replication defective due to a failure in autoregulation. Proc. Natl. Acad. Sci. USA 9311321-11326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mocarski, E. S., T. Shenk, and R. F. Pass. 2007. Cytomegaloviruses, p. 2701-2772. In D. M. Knipe and P. M. Howley (ed.), Fields virology. Lippincott/The Williams & Wilkins Co., Philadelphia, PA.
- 21.Muller, S., and A. Dejean. 1999. Viral immediate-early proteins abrogate the modification by SUMO-1 of PML and Sp100 proteins, correlating with nuclear body disruption. J. Virol. 735137-5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nevels, M., W. Brune, and T. Shenk. 2004. SUMOylation of the human cytomegalovirus 72-kilodalton IE1 protein facilitates expression of the 86-kilodalton IE2 protein and promotes viral replication. J. Virol. 787803-7812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paulus, C., S. Krauss, and M. Nevels. 2006. A human cytomegalovirus antagonist of type I IFN-dependent signal transducer and activator of transcription signaling. Proc. Natl. Acad. Sci. USA 1033840-3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reinhardt, J., G. B. Smith, C. T. Himmelheber, J. Azizkhan-Clifford, and E. S. Mocarski. 2005. The carboxyl-terminal region of human cytomegalovirus IE1491aa contains an acidic domain that plays a regulatory role and a chromatin-tethering domain that is dispensable during viral replication. J. Virol. 79225-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakamoto, S., R. Potla, and A. C. Larner. 2004. Histone deacetylase activity is required to recruit RNA polymerase II to the promoters of selected interferon-stimulated early response genes. J. Biol. Chem. 27940362-40367. [DOI] [PubMed] [Google Scholar]
- 26.Spengler, M. L., K. Kurapatwinski, A. R. Black, and J. Azizkhan-Clifford. 2002. SUMO-1 modification of human cytomegalovirus IE1/IE72. J. Virol. 762990-2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tavalai, N., P. Papior, S. Rechter, M. Leis, and T. Stamminger. 2006. Evidence for a role of the cellular ND10 protein PML in mediating intrinsic immunity against human cytomegalovirus infections. J. Virol. 808006-8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taylor, R. T., and W. A. Bresnahan. 2005. Human cytomegalovirus immediate-early 2 gene expression blocks virus-induced beta interferon production. J. Virol. 793873-3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turner, D. L., and H. Weintraub. 1994. Expression of achaete-scute homolog 3 in Xenopus embryos converts ectodermal cells to a neural fate. Genes Dev. 81434-1447. [DOI] [PubMed] [Google Scholar]
- 30.Uchimura, Y., M. Nakamura, K. Sugasawa, M. Nakao, and H. Saitoh. 2004. Overproduction of eukaryotic SUMO-1- and SUMO-2-conjugated proteins in Escherichia coli. Anal. Biochem. 331204-206. [DOI] [PubMed] [Google Scholar]
- 31.Wilkinson, G. W., C. Kelly, J. H. Sinclair, and C. Rickards. 1998. Disruption of PML-associated nuclear bodies mediated by the human cytomegalovirus major immediate-early gene product. J. Gen. Virol. 79(Pt. 5)1233-1245. [DOI] [PubMed] [Google Scholar]
- 32.Xu, Y., J. H. Ahn, M. Cheng, C. M. apRhys, C. J. Chiou, J. Zong, M. J. Matunis, and G. S. Hayward. 2001. Proteasome-independent disruption of PML oncogenic domains (PODs), but not covalent modification by SUMO-1, is required for human cytomegalovirus immediate-early protein IE1 to inhibit PML-mediated transcriptional repression. J. Virol. 7510683-10695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang, Z., S. M. Huong, X. Wang, D. Y. Huang, and E. S. Huang. 2003. Interactions between human cytomegalovirus IE1-72 and cellular p107: functional domains and mechanisms of upregulation of cyclin E/cdk2 kinase activity. J. Virol. 7712660-12670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu, H., Y. Shen, and T. Shenk. 1995. Human cytomegalovirus IE1 and IE2 proteins block apoptosis. J. Virol. 697960-7970. [DOI] [PMC free article] [PubMed] [Google Scholar]