Abstract
Hepatitis C virus (HCV) nonstructural protein 4B (NS4B), a poorly characterized integral membrane protein, is thought to function as a scaffold for replication complex assembly; however, functional interactions with the other HCV nonstructural proteins within this complex have not been defined. We report that a Con1 chimeric subgenomic replicon containing the NS4B gene from the closely related H77 isolate is defective for RNA replication in a transient assay, suggesting that H77 NS4B is unable to productively interact with the Con1 replication machinery. The H77 NS4B sequences that proved detrimental for Con1 RNA replication resided in the predicted N- and C-terminal cytoplasmic domains as well as the central transmembrane region. Selection for Con1 derivatives that could utilize the entire H77 NS4B or hybrid Con1-H77 NS4B proteins yielded mutants containing single amino acid substitutions in NS3 and NS4A. The second-site mutations in NS3 partially restored the replication of Con1 chimeras containing the N-terminal or transmembrane domains of H77 NS4B. In contrast, the deleterious H77-specific sequences in the C terminus of NS4B, which mapped to a cluster of four amino acids, were completely suppressed by second-site substitutions in NS3. Collectively, these results provide the first evidence for a genetic interaction between NS4B and NS3 important for productive HCV RNA replication.
Hepatitis C virus (HCV) is an enveloped positive-sense RNA virus belonging to the genus Hepacivirus of the Flaviviridae family. The 9.6-kb HCV genome encodes a polyprotein of ∼3,000 amino acids which is translated via an internal ribosome entry site within the 5′ nontranslated region (NTR). Cleavage of the polyprotein by host enzymes and two viral proteases produces three structural proteins (core, E1, and E2), a small hydrophobic polypeptide named p7, and six nonstructural proteins (NS2, NS3, NS4A, NS4B, NS5A, and NS5B).
The HCV proteins NS3, NS4A, NS4B, NS5A, and NS5B are necessary and sufficient for RNA replication. These proteins form a membrane-associated replication complex responsible for synthesizing the positive-strand RNA genome via negative-strand RNA intermediates (9, 14, 33, 38). Although many questions pertaining to the replication complex remain, specific functions for NS3, NS4A, and NS5B have been defined (reviewed in references 1 and 4). NS3 is a multifunctional protein with a serine protease domain spanning the 180 N-terminal amino acids and a RNA helicase/NTPase domain in the remaining C-terminal portion of the protein. The NS4A protein forms a stable complex with the N terminus of NS3 and is required to tether the NS3-4A complex to intracellular membranes and completely fold and activate the NS3 protease domain for cleavages at the NS3/4A, NS4A/4B, NS4B/5A, and NS5A/5B junctions of the viral polyprotein. The NS5B RNA-dependent RNA polymerase is the catalytic subunit of the viral replication complex.
In contrast to NS3, NS4A, and NS5B, NS4B and the differentially phosphorylated NS5A protein play essential, but as yet undefined, roles in RNA replication. NS4B is an endoplasmic reticulum (ER)-associated integral membrane protein predicted to contain four transmembrane segments flanked by cytoplasmic N- and C-terminal regions (TMHMM; www.cbs.dtu.dk). Although membrane association appears to be mediated by the transmembrane sequences, the N-terminal region can also interact with membranes via an amphipathic helix (8) and has been reported to partially translocate into the ER lumen by a posttranslational mechanism (30, 31). NS4B induces the formation of the membranous web, a specific membrane alteration that harbors HCV replication complexes in cell culture (11, 35), and is therefore theorized to play a critical role in organizing the membrane-bound replication complex. Previous work has shown that the specific NS4B sequence is critical for efficient RNA replication (2, 26), and cell culture-adaptive mutations that enhance genotype 1 replication have been identified in NS4B (13, 21, 28), supporting the notion that NS4B is an essential component of the replication complex. There is also evidence that NS4B encodes a GTPase activity (7), modulates the activity of NS3 in vitro (37), alters the phosphorylation status of the NS5A protein (16), and forms oligomers (46). However, the importance of these properties for HCV replication remains to be determined.
The protein-protein interactions required to form a functional replication complex are poorly defined. Structure predictions suggest that NS4B possesses a modular domain architecture (43), and if NS4B provides a scaffold for replication complex formation, it is likely that NS4B is involved in multiple interactions within this complex. Although interactions between NS4B and the other nonstructural proteins NS3, NS4A, NS5A, and NS5B have been described (6, 10, 24, 37), it is not clear if these interactions are necessary for productive RNA replication. In this study, we show that genotype 1b Con1 chimeric subgenomic replicons containing the entire or specific domains of NS4B derived from the genotype 1a H77 strain are defective for RNA replication compared to the parental Con1 strain. Genetic analysis of adapted Con1 variants that can utilize H77 NS4B or hybrid Con1-H77 NS4B proteins identified single amino acid substitutions in both NS3 and NS4A critical for efficient RNA replication of Con1 chimeras, suggesting that direct or indirect interactions between NS4B and NS3-4A are critical for productive HCV RNA replication.
MATERIALS AND METHODS
Cell culture.
Huh-7.5 cells were grown in Dulbecco's modified Eagle medium (DMEM; Mediatech) supplemented with 10% heat-inactivated fetal bovine serum (FBS) and 0.1 mM nonessential amino acids (HyClone) (DMEM-10%FBS).
Construction of chimeric and mutant plasmids.
The subgenomic replicon plasmids pH/ΔE1-p7(L+I)/NB, pC1/ΔE1-p7(I), pC1/ΔE1-p7(I)/NB, pC1/ΔE1-p7(I)/NBX (C1/WT), pC1/SG-Neo(I) (C1Neo/WT), and pGEM3Zf(+)/C1_4A-5A/NB have been described previously (2). All Con1-derived subgenomic replicon plasmids contain the adaptive mutation S2204I in NS5A and a ScaI site for linearization prior to RNA transcription. Residues in NS3 and NS5A are numbered according to their positions within the Con1 polyprotein commencing with the core-coding region; however, the amino acids in NS4B are numbered relative to the first amino acid residue of the NS4B protein. Standard molecular biology techniques were used throughout. Primed DNA synthesis was performed with KlenTaqLA DNA polymerase (Wayne Barnes, Washington University, St. Louis, MO), and regions amplified by PCR were verified by sequencing. Primers used in this study are shown in Table 1.
TABLE 1.
Oligonucleotides used for constructing chimeric and mutant Con1 repliconsa
| Name | Sequence (5′ → 3′) |
|---|---|
| 41 | (+) CATCATGGCATGCATGTCGG |
| 46 | (−) CCACGGATCCGGAGCATGGCGTGGAGC |
| 48 | (−) GTGGTGTACGCGTTAATGGG |
| 69 | (−) CCATCTGCATTCCCTGTTCGATGTAAGGGAGGTGTTGGGAGCACTCTTCCATCTCATCGA |
| 76 | (−) GAGCTGCTCAGCGAGCATCATCCCTTGCTCGATGTACGGTAAGTGCTGAGAGCACTCTTCCATCTCATCGA |
| 77 | (+) TCATGGCATGCATGTCGGCT |
| 99 | (+) CGACCCTCGAGGCCTTCTGGGCGAAGCATAT |
| 112 | (−) ACACGTGCGGCCGCGTCGCTCTCAGGCACATAGT (creates a NotI site) |
| 113 | (+) GCGATGCGGCCGCCCGCGTCACTGCCATACTCAG (creates a NotI site) |
| 115 | (+) GCGACGCGGCCGCACGTGTCACTCAGATCCTCTC (creates a NotI site) |
| 148 | (−) GCTTGGACTGGAGCCAGGTC |
| 185 | (−) CCAAGGATCCGGAGCATGGCGTGGAGCAGTCCTCGTTGATCCACTGGTGAAGCCTCCTCAGCAGCTGAGTGATGGTAA |
| 186 | (+) CTAAGCGGCCGCCCGCGTCACTGCCATCCTCTCTAGTCTTACCAT (creates a NotI site) |
| 190 | (−) CACACTCGAGGGTCCGCCACTTGGATTCCACCACGGGAGCAGCAGCCTCTGCTTGGCGGGACGCG |
| 237 | (+) GCGACGCGGCCGCACGTGTCACTCAGATCCTCTCTAGTCTTACCGTAACTCAGCTGCTGAAGAGG (creates a NotI site) |
| 238 | (−) CCACGGATCCGGAGCATGGCGTGGTGCACTCCGAGCTGATCCACTGGTGAAGCC |
| 260 | (−) CCACGCGCGCCTGCCACCCCTGCTCCATAACCTGCCAAAATATCCACAAGCACCTTCCCAAGGCCTATGCTGCCGATAGCCGCTCCAGCGATGCC |
| 261 | (−) CCACGCGCGCCTGCCACCCCTGCTCCATAACCTGCCAAAATATCCACAAGCACCTTCCCAAGGCCAACGCTGCCAACAGCCGCTCC |
| 262 | (−) CCACGCGCGCCTGCCACCCCTGCTCCATAACCTGCCAAAATATCCACAAGCACCTTCCCAAGGCCAACGCTGCCGATAGCCGCTCCAGCGATGCC |
Restriction sites used for cloning are underlined and the polarity of each oligonucleotide is indicated as either genome RNA (+) or its complement (−).
The replication-defective plasmid pC1/ΔE1-p7(I)/pol− (C1/pol−), containing a lethal mutation in the GDD motif of the NS5B RNA polymerase, was constructed by subcloning the EcoRI-SpeI fragment from pC1/SG-Neo(I)/pol− (3) into similarly digested pC1/ΔE1-p7(I).
The plasmid pC1/ΔE1-p7(I)/H_4B (C1/H4B) (Fig. 1), containing the H77 NS4B gene, was constructed in multiple steps. First, an intermediate plasmid was constructed by subcloning the NheI-BamHI fragment from pH/ΔE1-p7(L+I)/NB into similarly digested pGEM3Zf(+)/C1_4A-5A/NB to produce pGEM3Zf(+)/C1(4A)_H(4B)_C1(5A). Second, the BstEII-BlpI and NsiI-BstEII fragments from pGEM3ZF(+)/C1(4A)_H(4B)_C1(5A) were ligated with the BlpI-NsiI fragment from a PCR product amplified from pH/ΔE1-p7(L+I) (3) using primers 76 and 77 to create pGEM3Zf(+)/C1(4A)_H(4B Nhe−). Finally, in a three-piece ligation reaction, the NsiI-MluI fragment from pGEM3Zf(+)/C1(4A)_H(4BNhe−) was combined with the BsrGI-NsiI and MluI-BsrGI fragments from pC1/ΔE1-p7(I)/NBX.
FIG. 1.
Schematic diagram of the Con1 subgenomic replicons used to measure transient RNA replication efficiency in the Huh-7.5 cell line. The Con1 wild-type replicon (C1/WT) is shown at the top with the 5′ and 3′ NTR structures illustrated. The truncated C1/WT open reading frame, lacking the E1-p7 coding region, is depicted as a shaded box with the polyprotein cleavage products indicated along with the position of the cell culture-adaptive mutation S2204I in NS5A. Shown below are H77 NS4B and hybrid Con1-H77 NS4B proteins that replace Con1 NS4B in chimeric replicons. The adopted nomenclature for each subgenomic replicon is indicated on the left and the three NS4B domains, the N terminus (residues 1 to 47), the central membrane-spanning region (residues 48 to 227), and the 34 C-terminal amino acids (residues 228 to 261) are shown. Shaded regions represent Con1 NS4B sequences (C1) and open boxes denote H77-derived NS4B sequences (H).
The Con1 plasmid, containing the H77-specific amino acids Ser and Gln at the P1′ and P2′ sites of the NS4A-NS4B cleavage junction, was constructed in a two-step process. First, the SphI-EcoRI, EcoRI-BglI, and BglI-BsmI fragments from pGEM3Zf(+)/C1_4A-5A/NB were ligated with the BsmI-SphI portion of a PCR product amplified from C1/ΔE1-p7(I)/NB using primers 69 and 41 to generate the intermediate plasmid pGEM3Zf(+)/C1_4A-5A/4B_A1S+S2Q. Second, the SphI-MluI fragment from pGEM3Zf(+)/C1_4A-5A/4B_A1S+S2Q was ligated with the MluI-XbaI, XbaI-BstEII, and BstEII-SphI fragments from C1/ΔE1-p7(I)/NB to create the Con1 mutant plasmid pC1/ΔE1-p7(I)/4B_A1S + S2Q (C1/AS→SQ).
The plasmid pC1/ΔE1-p7(I)/H_4BN47 (C1/HN47) (Fig. 1), containing the first 47 amino acids of H77 NS4B, was created by cloning the BsrGI-NsiI, XhoI-EcoRI, and EcoRI-BsrGI fragments from pC1/ΔE1-p7(I)/NBX together with the NsiI-XhoI fragment from pGEM3Zf(+)/C1(4A)_H(4BNhe−).
The plasmid pC1/ΔE1-p7(I)/H_4BTm (C1/HTm) (Fig. 1), containing amino acids 48 to 227 from H77 NS4B, was created by ligating the BsrGI-XhoI fragment from pC1/ΔE1-p7(I)/NBX, the XhoI-NotI fragment from pH/ΔE1-p7(L+I)/C1_4BC34 (K. J. Blight, unpublished data), and the NotI-MluI fragment of a PCR product amplified using primers 115 and 48 and the template pC1/ΔE1-p7(I) into pC1/ΔE1-p7(I) digested with BsrGI and MluI.
The plasmid pC1/ΔE1-p7(I)/H_4BC214 (C1/HTmC34) (Fig. 1), containing the 214 C-terminal amino acids from H77 NS4B, was generated in a four-piece ligation reaction containing the BsrGI-XhoI, BamHI-EcoRI, and EcoRI-BsrGI fragments from pC1/ΔE1-p7(I)/NBX and the XhoI-BamHI fragment from pH/ΔE1-p7(L+I)/NB.
The plasmid pC1/ΔE1-p7(I)/H_4BC34 (C/HC34) (Fig. 1), containing the 34 C-terminal amino acids of H77 NS4B, was constructed in a four-piece ligation reaction containing the MluI-BsrGI and BsrGI-XhoI fragments from pC1/ΔE1-p7(I)/H_4BC214, the XhoI-NotI portion of a PCR product amplified from pC1/ΔE1-p7(I)/NBX using primers 99 and 112, and the NotI-MluI fragment of a second PCR product using primers 48 and 113 with pC1/ΔE1-p7(I)/H_4BC214.
The replicon plasmid pC1/ΔE1-p7(I)/H_4BN47+C34 (C1/HN47C34) (Fig. 1), containing the 47 N-terminal amino acids and 34 C-terminal amino acids from H77 NS4B, was constructed by ligating the BsrGI-XhoI fragment from pC1/ΔE1-p7(I)/H_4BN47 and the XhoI-EcoRI fragment from pC1/ΔE1-p7(I)/H_4BC34 into pC1/ΔE1-p7(I)/NBX digested with BsrGI and EcoRI.
The Con1 replicon plasmid, containing the first 227 amino acids of H77 NS4B, was called pC1/ΔE1-p7(I)/H_4BN47+Tm (C1/HN47Tm) (Fig. 1) and was constructed by cloning the BsrGI-XhoI fragment from pC1/ΔE1-p7(I)/H_4B together with the XhoI-NotI fragment from pH/ΔE1-p7(L+I)/C1_4BC34 and the NotI-MluI portion of a PCR product amplified from pC1/ΔE1-p7(I) by use of primers 115 and 48 into pC1/ΔE1-p7(I) digested with BsrGI and MluI.
The chimeric plasmids pC1/SG-Neo(I)/H_4B (C1Neo/H4B), pC1/SG-Neo(I)/H_4BN47Tm (C1Neo/HN47Tm), pC1/SG-Neo(I)/H_4BN47C34 (C1Neo/HN47C34), and pC1/SG-Neo(I)/H_4BC214 (C1Neo/HTmC34) were constructed by replacing the BsrGI-EcoRI fragment of pC1/SG-Neo(I) with the corresponding fragment from pC1/ΔE1-p7(I)/H_4B, pC1/ΔE1-p7(I)/H_4BN47+Tm, pC1/ΔE1-p7(I)/H_4BN47+C34, and pC1/ΔE1-p7(I)/H_4BC214, respectively.
The Con1 replicons containing single H77-specific amino acid substitutions in the 34 C-terminal amino acids of NS4B, namely, pC1/ΔE1-p7(I)/4B_Q235A (C1/Q235A), pC1/ΔE1-p7(I)/4B_I242V (C1/I242V), and pC1/ΔE1-p7(I)/4B_K247R (C1/K247R), were constructed by ligating the EcoRI-NotI and BamHI-EcoRI fragments from pC1/ΔE1-p7(I)/H_4BC34 with the NotI-BamHI fragment from PCR products generated using the template pC1/ΔE1-p7(I)/NBX and primers 186 and 148 for C1/Q235A, primers 237 and 46 for C1/I242V, and primers 115 and 185 for C1/K247R. The same strategy was used to construct pC1/ΔE1-p7(I)/4B_Q235A+ I242V+K247R (C1/QA+IV+KR) and pC1/ΔE1-p7(I)/4B_N254S+E255S+D256E+S258T (C1/NEDCS→SSECT), which encode three and four C-terminal amino acid substitutions, respectively, except that PCR products were generated using primers 113 and 46 with template pC1/ΔE1-p7(I)/C14B1-10-H4B11-241-C14B242-251 (Blight, unpublished) for C1/QA+IV+KR and primers 115 and 238 with template pC1/ΔE1-p7(I)/NBX for C1/NEDCS→SSECT.
Con1 replicons containing either the 33 N-terminal amino acids or amino acids 34 to 47 of H77 NS4B were constructed in four-piece ligation reactions. The plasmid pC1/ΔE1-p7(I)/H_4BN33 (C1/HN33) was created by ligating the EcoRI-BsrGI, BsrGI-NsiI, and XhoI-EcoRI fragments from pC1/ΔE1-p7(I)/NBX with an NsiI-XhoI-digested PCR fragment amplified using primers 77 and 190 with the template pC1/ΔE1-p7(I)/H_4BN47. The plasmid pC1/ΔE1-p7(I)/H_4BN34-47 (C1/HN34-47) was created by ligating the EcoRI-BsrGI, BsrGI-NheI, and XhoI-EcoRI fragments from pC1/ΔE1-p7(I)/NBX with the NheI-XhoI fragment from plasmid pH/ΔE1-p7(L+I)/C1_4BN33 (2).
Con1 replicons pC1/ΔE1-p7(I)/4B_V128I (C1/V128I), pC1/ΔE1-p7(I)/4B_I131V (C1/I131V), and pC1/ΔE1-p7(I)/4B_V128I+I131V (C1/V128I+I131V), encoding H77-specific amino acid substitutions in the predicted cytoplasmic loop connecting transmembrane domains 2 and 3 of NS4B, were constructed in four-piece ligation reactions. The EcoRI-BsrGI, BsrGI-XhoI, and BssHII-EcoRI fragments of pC1/ΔE1-p7(I)/NBX were ligated with the XhoI-BssHII-digested PCR product amplified from pC1/ΔE1-p7(I)/NBX using primer 99 with one of the following reverse primers: 260 (C1/V128I), 261 (C1/I131V), or 262 (C1/V128I+I131V).
In vitro RNA transcription.
Plasmid DNA was linearized with ScaI and purified by phenol-chloroform extraction and ethanol precipitation. One microgram of linearized template DNA was transcribed in a 50-μl reaction mixture containing 40 mM Tris-HCl (pH 7.5), 10 mM NaCl, 12 mM MgCl2, 2 mM spermidine, 10 mM dithiothreitol, 0.035 U inorganic pyrophosphatase (Fermentas), 50 U RNasin (Promega), 3 mM each ATP, CTP, GTP, and UTP, and 100 U T7 RNA polymerase (Epicentre Biotechnologies). Reactions were incubated for 1.5 h at 37°C before the addition of 5 U RNase-free DNase I (Roche) for 20 min at 37°C to remove template DNA. After extraction with phenol and chloroform, HCV RNA was precipitated with isopropanol and dissolved in RNase-free water. Remaining template DNA was degraded by two serial DNase I digestions for 20 min at 37°C followed by extraction with phenol and chloroform and precipitation with ethanol. After the RNA pellets were washed in 80% ethanol, the RNA transcripts were dissolved in RNase-free water. To ensure that equal amounts of each HCV replicon RNA were used in transfection experiments, the concentration of RNA transcripts was quantified by measuring the absorbance at 260 nm and by agarose gel electrophoresis.
HCV RNA transfection.
Huh-7.5 cells were electroporated with RNA transcripts as described previously (2). In brief, 1 μg of purified replicon RNA was mixed with 5 × 106 Huh-7.5 cells in 0.4 ml of ice-cold phosphate-buffered saline (PBS) and immediately pulsed in an ElectroSquarePorator (BTX). Cells were left to recover for 10 min at room temperature before being diluted to 10 ml in DMEM-10%FBS. Cells were seeded into six-well plates and subsequently harvested for HCV RNA quantification at 24 h, 48 h, 72 h, and 96 h posttransfection and for protein analysis at 96 h posttransfection. For G418 selection, electroporated cells were divided equally between three 150-mm-diameter dishes. After 48 h, the medium was replaced with DMEM-10%FBS supplemented with 0.8 mg G418 per ml (Gibco) and the medium was changed every 3 to 4 days thereafter. Individual colonies visible after ∼3 weeks of G418 selection were isolated and trypsinized before being expanded in DMEM-10%FBS containing 0.7 mg/ml G418.
Quantification of HCV RNA.
To determine RNA replication efficiency, total cellular RNA was extracted from transfected Huh-7.5 cell monolayers with TRIzol reagent (Invitrogen) in accordance with the manufacturer's protocol and precipitated with isopropanol. The RNA pellet was washed in 80% ethanol and resuspended in RNase-free water. HCV RNA was quantified in an ABI Prism 7000 sequence detection system using the TaqMan EZ reverse transcription-PCR (RT-PCR) core reagents (Applied Biosystems) and a primer pair and a probe specific for the 5′ NTR as previously described (2). Synthetic HCV RNA standards of known concentrations were amplified in parallel reactions and used to calculate the number of HCV RNA molecules in 1 μg of total cellular RNA.
Viral protein analysis.
Transfected cells in six-well plates were washed with PBS and lysed by the addition of sodium dodecyl sulfate (SDS) lysis buffer (0.1 M NaPO4, pH 7.0, 1% SDS, 80 μg/ml phenylmethylsulfonyl fluoride [PMSF], 2× Complete protease inhibitor cocktail [Roche]). The total protein concentration was determined using the bicinchoninic acid protein assay (Pierce) according to the manufacturer's directions. Ten to twelve micrograms of total protein was separated by SDS-8% polyacrylamide gel electrophoresis and analyzed by immunoblotting with a polyclonal rabbit antiserum specific for NS5A (GSK#308; kindly provided by Robert Sarisky, GlaxoSmithKline) followed by horseradish peroxidase-conjugated anti-rabbit immunoglobulin G (Pierce). Proteins were visualized by chemiluminescence using reagents from the Supersignal West Pico kit (Pierce).
Metabolic labeling and immunoprecipitation.
Subconfluent Huh-7.5 cell monolayers were infected for 1 h at room temperature with a recombinant vaccinia virus expressing T7 RNA polymerase. After being washed with DMEM-10%FBS, 1 μg of T7-driven plasmid DNA was transfected using the LTI transfection reagent (Mirus Corporation). Twenty-four hours later, cells were starved for 1 h in cysteine- and methionine-deficient MEM containing 5% dialyzed FBS (dMEM) and then labeled with 200 μCi/ml Express 35S protein labeling mix (Perkin-Elmer) in dMEM. After 45 min, cells were washed with ice-cold PBS and harvested in SDS lysis buffer before the total protein concentration was determined as described above. Eighty micrograms of the protein lysate was brought up to 0.5 ml with TNA (50 mM Tris-HCl, pH 7.5, 1 mM EDTA, 150 mM NaCl, 0.34% Triton X-100, 0.67% SDS, 80 μg/ml PMSF, 1.5× Complete protease inhibitor cocktail) and mixed with 1 μl of HCV-positive patient serum (kindly provided by Charles Rice, The Rockefeller University, New York, NY). After overnight rocking at 4°C, antigen-antibody complexes were captured by adding 50 μl of Pansorbin cells (Calbiochem) and rocking for 1.5 h at 4°C. Complexes were collected by centrifugation and washed four times in TNA containing 0.125% SDS and once in TNE (50 mM Tris-HCl, pH 7.5, 1 mM EDTA, 150 mM NaCl, 80 μg/ml PMSF, 1× Complete protease inhibitor cocktail). The final washed pellet was resuspended in sample buffer (100 mM Tris-HCl, pH 6.8, 20% glycerol, 4% SDS, 20 mM dithiothreitol, 4% 2-mercaptoethanol, 0.1% bromophenol blue) and heated for 20 min at 80°C. Labeled proteins were separated on an SDS-9% polyacrylamide gel, visualized by autoradiography, and quantified using a phosphorimager with a FLA-5000 scanner (Fuji).
Identification of second-site mutations.
Total cellular RNA, extracted from G418-resistant Huh-7.5 cells supporting persistent HCV replication with TRIzol reagent, was converted to cDNA and amplified by PCR using a series of oligonucleotides spanning the NS3-5B polyprotein. Cellular RNA (150 ng) was mixed with 5 pmol of HCV-specific primer and the primer was extended at 53°C for 1 h in a 5-μl reaction mixture containing 50 mM Tris-HCl, pH 8.3, 75 mM KCl, 3 mM MgCl2, 5 mM dithiothreitol, 0.5 mM each dATP, dCTP, dGTP, and dTTP, 4 U RNasin (Promega), and 80 U of SuperScript III reverse transcriptase (Invitrogen). The reverse transcriptase was inactivated by heating at 96°C for 10 min prior to PCR amplification using KlenTaqLA DNA polymerase and 35 cycles of 95°C for 30 s, 55 to 60°C for 30 s, and 68°C for 4 min. PCR products were purified from preparative low-melting-point agarose by repeated phenol extraction and second-site mutations identified by directly sequencing ∼45 ng of purified PCR product by use of primers spanning the NS3-5B coding region. PCR products harboring second-site mutations were subsequently cloned into C1/WT and Con1-H77 NS4B chimeras and PCR-amplified regions confirmed by sequencing.
RESULTS
H77 NS4B is not functional for Con1 RNA replication.
NS4B is one of the more divergent nonstructural proteins, and the NS4B proteins from the closely related genotype 1 strains H77 and Con1 share approximately 87% amino acid identity. Previously, we showed that the specific Con1 NS4B sequence significantly enhanced H77 RNA replication in cell culture (2). In this study, we examined the ability of H77 NS4B to support Con1 RNA replication by determining the replication ability of a Con1 chimeric subgenomic replicon in which the Con1 NS4B sequence was replaced with the coding region from H77 NS4B (C1/H4B) (Fig. 1). Huh-7.5 cells were transfected with in vitro-transcribed replicon RNA, HCV RNA levels were quantified over time by RT-PCR, and NS5A protein expression was monitored by immunoblotting at 96 h following RNA transfection. As controls, the polymerase-defective replicon (C1/pol−) and the parental Con1 replicon (C1/WT) were electroporated in parallel. Ninety-six hours posttransfection, HCV RNA levels for C1/H4B were 500- to 1,000-fold lower than those for C1/WT, and the NS5A protein was not detected, results similar to those obtained for C1/pol− (Fig. 2), indicating that H77 NS4B cannot function in the Con1 background. This replication defect was not due to differences in the cellular localization of NS4B as immunofluorescence staining of transiently expressed HCV proteins in Huh-7.5 cells showed that H77 and Con1 NS4B similarly colocalized with both Con1 NS5A and the ER marker calnexin (data not shown).
FIG. 2.
H77 NS4B does not support Con1 replication. (A) Huh-7.5 cells were electroporated with 1 μg of replicon RNA and at 24, 48, 72, and 96 h posttransfection total cellular RNA was harvested. HCV RNA was quantified by real-time RT-PCR and the number of HCV RNA molecules was normalized to 1 μg of total cellular RNA (HCV RNA/μg total cell RNA). (B) At 96 h postelectroporation, Huh-7.5 cell monolayers were lysed and the relative levels of NS5A expression were visualized by Western blotting. The migration of NS5A is shown on the left. Similar results were obtained in six independent experiments.
NS4B sequences act synergistically for efficient HCV RNA replication.
To determine which region of the H77 NS4B protein was responsible for the lethal replication phenotype of the C1/H4B chimera, we divided the 261-amino-acid NS4B protein into three domains based on both sequence identity and predicted topology; the poorly conserved 47 amino acids at the N terminus (∼68% identity), the central, predominantly membrane-spanning region of 180 amino acids (93% identity), and the 34 C-terminal residues (79% identity). We tested three new Con1 chimeras containing one of these domains derived from the H77 NS4B sequence. The first contained the 47 N-terminal amino acids of H77 NS4B fused to the remaining Con1 NS4B sequence (C1/HN47), the second contained the transmembrane region of H77 NS4B flanked by the N- and C-terminal portions from Con1 NS4B (C1/HTm), and the third contained the N-terminal and transmembrane regions of Con1 NS4B followed by the last 34 amino acids of H77 NS4B (C1/HC34) (Fig. 1). In transient RNA replication assays, similar decreases in HCV RNA levels were observed for these chimeras compared to C1/WT (∼3- to 4-fold) (Fig. 3) and NS5A protein expression was also consistently lower than that for C1/WT (Fig. 3B), indicating that the specific sequences of all three NS4B domains are important for efficient Con1 replication. However, unlike the C1/H4B chimera containing the entire H77 NS4B sequence, a severe drop in RNA replication was not observed, indicating that multiple NS4B domains contribute to the severe replication defect of the C1/H4B chimera.
FIG. 3.
RNA replication efficiencies of Con1 subgenomic replicons encoding hybrid Con1-H77 NS4B proteins. (A) The number of HCV RNA molecules in 1 μg of total cellular RNA was quantified by real-time RT-PCR at 24, 48, 72, and 96 h posttransfection. (B) At the 96-h time point NS5A expression was analyzed by Western blotting. Percentages below the blot (% RNA) represent the quantified HCV RNA levels at the 96-h time point relative to that for C1/WT, which has been set at 100%. The results are representative of three independent experiments.
To investigate the importance of interactions between these NS4B domains, two domains of Con1 NS4B were replaced with the corresponding H77-derived NS4B sequences. These Con1-H77 NS4B chimeras, designated C1/HN47Tm, C1/HTmC34, and C1/HN47C34, contain the first 227 amino acids, the 214 C-terminal amino acids, and the N- and C-terminal regions from H77 NS4B, respectively (Fig. 1). Ninety-six hours following RNA transfection, HCV RNA levels for all three chimeras were down ≥25-fold compared to the parental C1/WT replicon and ∼10-fold lower than the levels for the chimeras containing a single domain from H77 NS4B (Fig. 3). The relative amounts of NS5A expression paralleled the HCV RNA levels (Fig. 3B). The RNA replication defects of these chimeras were not due to changes in the stability of the chimeric NS4B proteins (data not shown). Thus, the 25- to 50-fold decrease in RNA replication efficiency when two domains of H77 NS4B were combined compared to the 3- to 4-fold decrease caused by any one single domain of H77 NS4B supports the hypothesis that the three NS4B domains are important and act synergistically for optimal RNA replication.
NS4B biogenesis was not altered in Con1-H77 NS4B chimeras.
Replacing the N and C termini of Con1 NS4B with the corresponding sequence from H77 NS4B changed the residues at the P1′ and P2′ positions of the NS4A/4B junction and the P4, P6, P7, and P8 positions of the NS4B/5A junction. It was possible that the decreased replication efficiency of Con1-H77 NS4B chimeras was a consequence of defective NS3-4A serine protease-mediated polyprotein processing due to these modified cleavage sites or changes in protein conformation important for efficient processing. Given that Con1 chimeras containing either two or three domains from H77 NS4B replicated poorly, the processing of the chimeric and parental Con1 polyproteins was examined independent of HCV replication by transfecting replicon DNA into Huh-7.5 cells infected with a recombinant vaccinia virus expressing T7 RNA polymerase. In addition to the chimeras mentioned thus far, we also tested Con1 mutants that contained the H77-specific residues at the P1′ and P2′ positions of the NS4A/4B cleavage junction (C1/AS→SQ) and at the P4 and P6-P8 positions of the NS4B/5A junction (C1/NEDCS→SSECT). Small differences in NS4B expression and biogenesis were observed between the mutant, chimeric, and parental replicons (Fig. 4), but these differences did not correlate with replication efficiency. A band where uncleaved NS4A-4B was expected to migrate was visible for all the Con1 derivatives tested. Given that this same product was also observed in C1/WT (Fig. 4), it was doubtful that incomplete cleavage at the NS4A/4B junction was responsible for the replication defect of Con1-H77 chimeras. A contributing factor for the slight differences in relative NS4B expression most likely is the patient serum used for immunoprecipitation, which has a greater reactivity to H77 NS4B than to Con1 NS4B (Fig. 4; compare the percentages of NS4B expressed by H/WT [123%] and C1/WT [100%]; also see reference 2). The fact that the NS4B signal was greatest for every Con1-H77 NS4B chimera containing the N-terminal domain from H77 NS4B suggests that the epitope(s) recognized by this antiserum resides within this region of NS4B. Additionally, the C1/AS→SQ derivative did not exhibit a replication defect in our transient RNA replication assay, and restoring both the Con1-specific NS4A/4B and NS4B/5A cleavage junctions in the C1/H4B chimera did not rescue the lethal replication phenotype (data not shown). Collectively, these results suggest that the decrease in RNA replication efficiency of the Con1-H77 NS4B chimeras was not due to altered polyprotein processing at the NS4A/4B and NS4B/5A junctions but rather to the specific sequence of the H77 NS4B protein.
FIG. 4.
Foreign H77 NS4B sequences do not affect polyprotein processing. Huh-7.5 cells infected with a recombinant vaccinia virus expressing T7 RNA polymerase were transfected with the indicated plasmid DNA and labeled with [35S]methionine-cysteine. Labeled NS3, NS4B, and NS5A was immunoprecipitated using patient serum before separation on an SDS-9% polyacrylamide gel. Proteins were visualized using autoradiography and quantified by phosphorimaging. The NS4B signal in each sample was normalized to the corresponding NS3 signal to account for differences in transfection efficiencies. Shown below the gel is the percentage of normalized NS4B expressed in each sample relative to the NS4B level expressed from C1/WT, which has been set at 100%. As controls, Huh-7.5 cells were transfected with the expression plasmid pcDNA (Invitrogen) or the H77 replicon plasmid (H/WT) (2) and treated as described above. The molecular mass standard in kilodaltons is labeled on the left and the mobilities of the various HCV proteins are indicated on the right. The difference in migration of Con1 NS4B compared to the migration of H77 NS4B may be attributed to differences in amino acid sequence.
Identification of suppressor mutations facilitating efficient RNA replication of Con1-H77 NS4B chimeras.
We hypothesized that NS4B function requires interactions with other components within the replication complex and that H77 NS4B was incompatible in the Con1 background because it lacked sequences essential for interactions with the Con1 replication machinery. If the H77 NS4B sequence perturbed an essential interaction, then identifying second-site mutations that overcome the replication defect could reveal novel interactions with NS4B required for RNA replication. To test this hypothesis, the entire H77 NS4B gene or the hybrid NS4B sequences from the severely impaired chimeras, C1/HN47Tm, C1/HN47C34, and C1/HTmC34, were cloned into the G418-selectable Con1 replicon (C1Neo/WT) and subjected to G418 selective pressure following RNA transfection of Huh-7.5 cells. G418-resistant colonies arose for each of the transfected chimeras; however, consistent with the transient RNA replication data, fewer colonies were observed for the Con1 replicon encoding the entire H77 NS4B protein than for the replicons C1Neo/HN47Tm, C1Neo/HN47C34, and C1Neo/HTmC34, which exhibited similar colony-forming efficiencies. Independent G418-resistant colonies were isolated from each selection, and HCV replication was verified by detecting NS3 and NS5A protein expression (data not shown). The consensus sequence of the NS3-5B coding region was determined for three clones supporting C1Neo/H4B replication, one clone supporting C1Neo/HTmC34 replication, and a single clone harboring replication of the C1Neo/HN47C34 chimera by directly sequencing overlapping uncloned RT-PCR products. Four clones contained a single amino acid substitution in NS3; Q1112R or E1202G in the protease domain were identified in two clones, while the other two clones contained either S1369R or D1431Y in the helicase domain (Table 2). In the clone supporting C1Neo/HN47C34 replication, K1691R in NS4A was detected instead of an NS3 mutation (Table 2). Mutations were not found in the NS4B, NS5A, or NS5B genes of these clones. Given that the majority of these clones contained an NS3 mutation, we sequenced the NS3 coding region from the remaining 21 cell clones. Single amino acid substitutions in NS3 were identified in each of these cell clones with the exception of one mutant derivative of C1Neo/H4B, which carried both P1115R in the protease domain and S1560G in the helicase domain (Table 2). In summary, 11 different second-site mutations mapping to the protease or helicase domains of NS3 and a single mutation near the C terminus of NS4A were identified.
TABLE 2.
Locations and frequencies of NS3-4A second-site mutations identified in G418-resistant Huh-7.5 clones supporting replication of Con1-H77 NS4B chimeras
| Location | Mutation | No. of indicated clones selecteda
|
|||
|---|---|---|---|---|---|
| C1Neo/H4B | C1Neo/HN47Tm | C1Neo/HN47C34 | C1Neo/HTmC34 | ||
| NS3 protease | Q1112R | 2 | 1 | ND | 1 |
| P1115R | ND | ND | ND | 1 | |
| E1202G | 1 | ND | 1 | 1 | |
| M1205K | ND | 1 | ND | ND | |
| NS3 helicase | A1218D | ND | ND | 1 | ND |
| R1283G | ND | 1 | ND | ND | |
| T1287K | ND | ND | 1 | ND | |
| S1369R | 3 | 1 | 1 | 1 | |
| D1431N | ND | ND | 1 | 2 | |
| D1431Y | 1 | ND | 1 | ND | |
| S1560G | 1 | ND | ND | ND | |
| NS3 protease + helicase | P1115R + S1560G | 1 | ND | ND | ND |
| NS4A | K1691R | ND | ND | 1 | ND |
ND, not detected.
To determine if these second-site mutations in NS3 could suppress the replication defect caused by the H77 NS4B sequence, eight of the NS3 mutations plus the double mutation, P1115R and S1560G, were engineered into the C1/H4B replicon containing the entire H77 NS4B gene and RNA replication efficiency was examined in Huh-7.5 cells. In contrast to the lethal replication phenotype of the C1/H4B parent, each amino acid substitution in NS3 significantly enhanced C1/H4B RNA and NS5A protein levels (Fig. 5). Slight differences in replication efficiencies were observed depending on the NS3 mutation present; however, none of the NS3 mutations completely suppressed the defect, with HCV RNA levels remaining three- to eightfold below that of the C1/WT replicon (Fig. 5). When P1115R or S1560G was present, the efficiency of C1/H4B replication was 31% or 19% of that of C1/WT, respectively, but when both mutations were present (PR+SG) the levels of C1/H4B RNA increased to 45%, only twofold lower than that of C1/WT (Fig. 5), indicating that these two mutations have an additive effect. Importantly, replication of the parental C1/WT replicon was only slightly enhanced (<1.5-fold) by the introduction of these second-site mutations in NS3, including the double mutation P1115R and S1560G (data not shown and Fig. 6B and 7C). Thus, these amino acid substitutions in NS3 were not simply adaptive for Con1 replication but compensated for the foreign H77 NS4B sequence.
FIG. 5.
Second-site mutations in NS3 suppress the replication defect of the Con1 chimera encoding the entire H77 NS4B protein. At 96 h postelectroporation, NS5A expression was analyzed by immunoblotting. The numbers of HCV RNA molecules in 1 μg of total cellular RNA were quantified at 96 h posttransfection by real-time RT-PCR and are shown below the NS5A blot (% RNA) as percentages of the C1/WT level, which has been set at 100%. PR+SG refers to the chimera containing both P1115R and S1560G in NS3. Similar results were obtained in three independent experiments and the same trends in RNA replication efficiency were also observed at 24 h, 48 h, and 72 h posttransfection, except that the difference in HCV RNA levels between experimental RNAs and the C1/pol− control was greater at later time points.
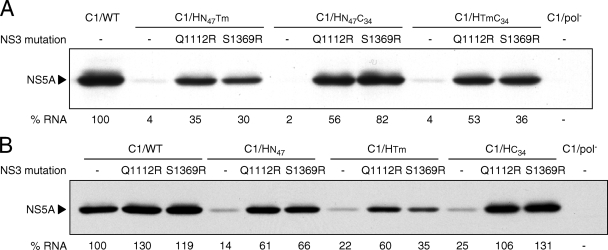
FIG. 6.
Second-site mutations, Q1112R in the protease domain and S1369R in the helicase domain of NS3, enhance RNA replication of Con1-H77 NS4B chimeras. At 96 h posttransfection, NS5A protein expression was analyzed by immunoblotting and the number of HCV RNA molecules in 1 μg of total cellular RNA was quantified by real-time RT-PCR. Below the blot for NS5A, HCV RNA levels (% RNA) are shown as percentages of the C1/WT level, which has been set at 100%. (A) Replication abilities of the Con1 chimeras C1/HN47Tm, C1/HN47C34, and C1/HTmC34 (Fig. 1), containing two domains from H77 NS4B. (B) Replication abilities of the single domain Con1 chimeras C1/HN47, C1/HTm, and C1/HC34 (Fig. 1). NS5A protein expression and HCV RNA levels are representative of at least two independent experiments.
FIG. 7.
The replication defect caused by the C-terminal residues of H77 NS4B is completely suppressed by the NS3 mutations Q1112R and S1369R. (A) Schematic representation of NS4B showing the alignment of the 34 C-terminal amino acids of Con1 and H77 NS4B. Divergent residues are shown in boldface, and the arrows indicate the mutations tested. (B and C) At 96 h posttransfection, NS5A protein expression was analyzed by immunoblotting and the number of HCV RNA molecules in 1 μg of total cellular RNA was quantified by real-time RT-PCR. Below the NS5A blots, HCV RNA levels (% RNA) are shown as percentages of the C1/WT level, which has been set at 100%. NS5A protein expression and HCV RNA levels are representative of at least three independent experiments, except for C1/Q235A, C1/I242V, and C1/K247R, which were tested only once.
Identification of H77 NS4B sequences suppressed by second-site mutations in NS3.
Second-site mutations in NS3 did not restore C1/H4B replication to C1/WT levels (Fig. 5), indicating that not all deleterious H77 NS4B sequences were suppressed by these NS3 mutations. To determine if second-site mutations in NS3 could completely rescue the severe RNA replication defects of Con1 chimeras containing two domains from H77 NS4B (Fig. 3), Q1112R in the protease domain and S1369R in the helicase domain were individually engineered into C1/HN47Tm, C1/HN47C34, and C1/HTmC34. These NS3 mutations were chosen because S1369R was selected in all four severely impaired chimeras, while Q1112R was identified in three of these four selected chimeras (Table 2). Both Q1112R and S1369R independently enhanced C1/HN47Tm, C1/HN47C34, and C1/HTmC34 replication; however, HCV RNA levels and NS5A protein expression were not restored to those of C1/WT (Fig. 6A). Additionally, the relative increase in replication depended on the specific H77 NS4B sequences present in chimeric replicons. Specifically, the greatest level of suppression by Q1112R or S1369R was observed for the chimera containing the N- and C-terminal sequences from H77 NS4B (C1/HN47C34), with increases in RNA replication of 25- to 40-fold compared to the C1/HN47C34 parent (Fig. 6A). Similar increases in C1/HN47C34 replication were observed when we tested five additional NS3 mutations (E1202G, A1218D, T1287K, D1431N, and D1431Y) and the K1691R mutation in NS4A (data not shown). In comparison, NS3 mutations enhanced C1/HN47Tm and C1/HTmC34 replication by only ∼8- to 12-fold compared to the respective parental chimeras (Fig. 6A). These data suggest that the 47 N-terminal amino acids, the 34 C-terminal amino acids, or both play a major role in the NS3-mediated rescue of Con1-H77 NS4B chimeras.
To define the domains of NS4B that harbor the sequence(s) suppressed by NS3 mutations, we tested the ability of Q1112R and S1369R to rescue replication of the chimeras containing a single domain from H77 NS4B (C1/HN47, C1/HTm, and C1/HC34) (Fig. 1). Both Q1112R and S1369R increased the level of C1/HN47 replication ∼3- to 4-fold above the level for the parental chimera (Fig. 6B). Replication of the Con1 chimera containing the transmembrane domain from H77 NS4B (C1/HTm) was enhanced by almost 3-fold by Q1112R, while only a subtle increase (∼1.5-fold) was observed in the presence of S1369R (Fig. 6B). The slight increase in RNA replication efficiency of C1/HTm and C1/WT when S1369R was present was too similar to conclude that this NS3 mutation suppresses the defect caused by the transmembrane sequence derived from H77 NS4B. Regardless, the replication efficiency of C1/HN47 and C1/HTm containing Q1112R or S1369R remained consistently below that of the C1/WT replicon (Fig. 6B). In contrast, Q1112R or S1369R increased replication of the C1/HC34 chimera to levels greater than that of C1/WT (Fig. 6B). Five additional NS3 mutations were also tested (P1115R, E1202G, D1431N, S1560G, and P1115R plus S1560G) (Table 2) and shown to rescue C1/HN47, C1/HTm, and C1/HC34 replication in a manner similar to that seen for Q1112R (data not shown). Given that the level of suppression observed for these NS3 mutations depended on the specific foreign H77 NS4B sequence, there is further evidence that these mutations are not simply enhancing Con1 RNA replication but suppress the defects caused by specific NS4B sequences. Thus, second-site mutations in NS3 can rescue replication defects caused by all three domains derived from H77 NS4B; however, only the deleterious H77-specific sequences in the C terminus could be completely suppressed by NS3 mutations.
C-terminal amino acids of NS4B are essential for efficient Con1 RNA replication.
Comparison of the 34-amino-acid C termini of Con1 and H77 NS4B revealed seven divergent amino acids (Fig. 7A) that could be responsible for the replication defect of the C1/HC34 chimera. H77-specific amino acid substitutions at these seven positions were engineered into the C1/WT replicon and the replication efficiencies of these Con1 mutants in Huh-7.5 cells were determined. Mutants with the individual amino acid substitutions Q235A, I242V, and K247R and a mutant combining all three substitutions (C1/QA+IV+KR) replicated to levels comparable to C1/WT (Fig. 7B), indicating that these three amino acid differences are not detrimental for Con1 RNA replication. In contrast, a mutant containing the four downstream amino acid substitutions (N254S, E255S, D256E, and S258T; C1/NEDCS→SSECT) exhibited replication kinetics similar to those of C1/HC34 (Fig. 7B). Thus, one or more of these divergent amino acids at the C terminus are responsible for the defect in C1/HC34 replication. Furthermore, the NS3 mutations Q1112R and S1369R completely suppressed the replication defect of the C1/NEDCS→SSECT mutant replicon (Fig. 7C), providing additional support for a genetic interaction between these C-terminal residues and NS3 during HCV replication.
NS4B sequences within the N-terminal and transmembrane domains required for Con1 RNA replication.
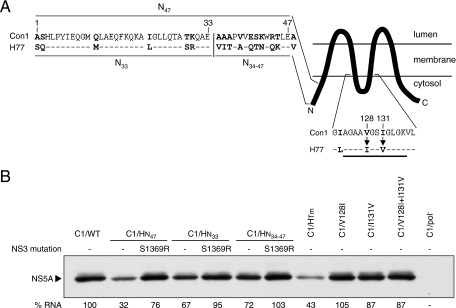
To define the sequences within the N terminus of H77 NS4B responsible for the replication defect of the C1/HN47 chimera, two additional Con1 chimeras were constructed. The first contained the 33 N-terminal amino acids from H77 NS4B and the remaining sequence from Con1 NS4B (C1/HN33) (Fig. 8A). In the second, amino acids 34 to 47 were replaced with the corresponding sequence from H77 NS4B (C1/HN34-47) (Fig. 8A). At 96 h posttransfection, HCV RNA levels and NS5A protein expression for both C1/HN33 and C1/HN34-47 were slightly lower than the C1/WT replicon, indicating that amino acid differences both in the 33 N-terminal amino acids and between residues 34 to 47 contribute to the replication defect observed for the C1/HN47 chimera (Fig. 8B). The addition of the NS3 helicase mutation S1369R enhanced the replication of both the C1/HN33 and the C1/HN34-47 chimeras to levels comparable to that for C1/WT (Fig. 8B). Given that these chimeras without S1369R were only slightly impaired compared to C1/WT, it was difficult to gauge whether S1369R increased replication by suppressing specific defects in one or both of the N-terminal regions or by acting as a general enhancer of Con1 replication.
FIG. 8.
Dissection of the H77-derived sequences in the N-terminal and transmembrane domains of NS4B that are detrimental for Con1 RNA replication. (A) A cartoon schematic of the predicted topology of NS4B (TMHMM; www.cbs.dtu.dk), showing the N- and C-terminal cytoplasmic domains flanking the larger transmembrane domain. The first 48 amino acids of H77 and Con1 NS4B are aligned on the upper left with divergent residues in boldface. The vertical line shows the division of the N terminus into the first 33 amino acids (C1/HN33) and residues 34 to 47 (C1/HN34-47). Shown below is a comparison of H77 and Con1 NS4B sequences between residues 122 and 137 of NS4B. The proposed cytosolic loop is underlined, amino acid differences are shown in boldface, and arrows denote the amino acid substitutions tested. (B) At 96 h posttransfection, NS5A protein expression was visualized by Western blotting and the number of HCV RNA molecules in 1 μg of total cellular RNA was quantified by real-time RT-PCR. Below the NS5A blot, HCV RNA levels (% RNA) are shown as percentages of the C1/WT level, which has been set at 100%. The HCV RNA levels are presented as the mean RNA level from two independent experiments with an average standard deviation of ±5%.
The Con1 chimera containing the predominantly membrane-spanning domain from H77 NS4B (C1/HTm) (Fig. 1) replicated to levels ∼3- to 4-fold lower than that of the Con1 parental replicon (Fig. 6B). Based on our predicted topology of NS4B, the transmembrane domain contains four membrane-spanning segments with two loops in the lumen and a small loop exposed to the cytoplasm (TMHMM; www.cbs.dtu.dk) (Fig. 8A). Since most NS3 mutations tested partially suppressed the defect caused by the transmembrane sequence of H77 NS4B (Fig. 6B and data not shown), we hypothesized that divergent residues in cytosol-exposed regions may be partly responsible for the replication defect of C1/HTm. Three amino acid differences between the Con1 and H77 transmembrane domains are predicted to reside in the cytosol: A48V, located at the start of our transmembrane domain, and V128I and I131V, residing in the loop between the second and third transmembrane segments of NS4B (Fig. 8A). Therefore, Con1 mutants C1/A48V, C1/V128I, and C1/I131V and a double mutant containing both V128I and I131V (C1/V128I+I131V) were tested (Fig. 8A). The mutations V128I, I131V, and V128I plus I131V had little or no effect on Con1 replication (Fig. 8B). Similarly, only a slight decrease in RNA replication was observed for the A48V mutation (∼1.5-fold; data not shown). Thus, these three amino acid differences were not responsible for the major replication defect of the C1/HTm chimera, suggesting that Con1-derived sequences within the transmembrane segments or the predicted lumenal loops are important for efficient NS4B function.
DISCUSSION
In this study, we demonstrate that a Con1 subgenomic chimera containing the H77 NS4B gene does not replicate in transient RNA replication assays. Since the H77 NS4B protein can function in the context of the H77 replicon, the lethal replication phenotype of the C1/H4B chimera containing the entire H77 NS4B gene suggests that H77 NS4B does not productively interact with the Con1 replication machinery. Therefore, we reasoned that compensatory second-site mutations may provide insight into the interactions taking place between NS4B and the other components of the replication complex. In support of this hypothesis, we isolated single amino acid substitutions in the Con1 NS3-4A complex that were sufficient to suppress, at least in part, the replication defects caused by the foreign H77 NS4B sequences. RNA replication of the C1/H4B chimera is not only rescued by second-site mutations in Con1 NS3 but also can be restored by replacing Con1 NS3 with the NS3 sequence from the H77 replicon (data not shown). Thus, we have identified a genetic interaction between NS3 and NS4B important for HCV RNA replication in cell culture.
The second-site mutations identified in G418-selected Con1-H77 NS4B chimeric derivatives (Table 2) reside on the surface of NS3 and far away from the serine protease and helicase/NTPase active sites. The residues in the NS3 protease domain (Q1112, P1115, E1202, and M1205) clustered together, adjacent to where K1691 in NS4A is expected to reside. In contrast, the other NS3 residues lie on different surfaces of the helicase domain. Mutational, biochemical, and structural studies suggest that NS3 forms multimers and in particular dimers (22, 23, 27, 32, 39, 44). When NS3 substitutions were mapped on the dimeric NS3-4A complex, most localized on two distinct faces of the dimeric NS3-4A complex (Fig. 9A), with the exception of S1560, which resided on the opposite side of the protein (Fig. 9B). Position 1369 in the helicase domain maps to the dimer interface and if NS3-4A forms dimers during HCV replication, then an Arg substitution at this locus may have the potential to alter the NS3-4A dimer conformation. This is particularly interesting considering that S1369R was the only mutation tested that did not partially rescue the replication defect caused by the transmembrane domain from H77 NS4B, even though S1369R suppresses the N- and C-terminal defects in a manner similar to that seen for the other NS3 mutations tested. In fact, S1369R was the least efficient suppressor whenever the H77-derived transmembrane domain was present in Con1-H77 NS4B chimeras (Fig. 6). While speculative, NS3-mediated suppression of the defect conferred by the transmembrane domain of H77 NS4B may require a specific NS3-4A dimer conformation that is perturbed by the S1369R substitution.
FIG. 9.
Location of second-site mutations identified in Con1 NS3. (A) Space-filling view of the NS3-4A dimer derived from HCV genotype 1 (Protein Data Bank accession number, 1CU1) (44). The cofactor peptide of NS4A (residues 1678 to 1690 in the polyprotein) is shown in purple and the location of the second-site mutations is shown in red on chain A and yellow on chain B. (B) Flipped view displaying the back side of the NS3-4A dimer. Images were generated using Pymol (pymol.sourceforge.net).
Eight of the second-site mutations targeted residues that were conserved between Con1 and H77 NS3-4A; however, it is worth noting that H77 NS3 encodes P1112, Q1115, T1369, and G1560. Only the Ser at position 1560 in Con1 NS3 mutated to the H77-specific Gly residue, and it is possible that the S1560G substitution restores a native interaction, whether direct or indirect, with H77 NS4B. We found that the ability of both P1115R and S1560G to suppress the replication defect of the C1/H4B chimera was additive, suggesting that these residues are involved in multiple interactions within the replication complex. In contrast, the level of suppression was not additive when two protease mutations were combined (E1202G and M1205K; data not shown), suggesting that protease mutations that cluster together have a common or redundant function. Given these observations, it would be interesting to test how other combinations of second-site mutations in NS3-4A impact the replication of Con1-H77 NS4B chimeras.
Although all the second-site mutations in NS3 that we tested enhanced the replication of the Con1 chimera encoding the entire H77 NS4B sequence (C1/H4B), they did not restore replication to the levels of the wild-type Con1 replicon (Fig. 5). Moreover, NS3 mutations only partially rescued the defective H77-derived sequences residing in the N-terminal and transmembrane domains of NS4B. These observations suggest that the foreign H77 sequences in these NS4B domains disrupt functions that cannot be completely restored by mutations in NS3. Since NS4B has been proposed to serve as the scaffold for replication complex assembly and thus is likely to be involved in multiple protein-protein interactions, one possibility is that the N terminus of NS4B, which is predicted to lie within the cytoplasm, functions as a platform for interactions with other HCV proteins or with host factors required for efficient RNA replication in cell culture. In support of this hypothesis, the N-terminal domain of NS4B has been shown to harbor a putative amphipathic helix that appears to be important for proper localization of the HCV nonstructural proteins (8), to contain sequences important for NS4B oligomerization (46), and to interact with the transcription factor ATF6, which is activated upon ER stress (40). While it is not yet known whether these or additional interactions with the N terminus are important for efficient RNA replication, it is possible that the decrease in RNA replication of the Con1-H77 NS4B chimeras is the result of suboptimal protein-protein interactions with the N terminus of H77 NS4B.
The role of a putative nucleotide-binding motif located in the predicted cytosolic loop of the transmembrane domain of NS4B has not been resolved, although specific amino acid substitutions at I131 and other residues within this region abolish Con1 RNA replication (7). A comparison of NS4B between Con1 and H77 revealed that Con1 encodes an Ile at position 131, whereas H77 encodes Val. While it was doubtful that this conservative substitution would impact nucleotide binding and HCV RNA replication efficiency, we confirmed that I131V and an upstream substitution, V128I, did not significantly affect Con1 RNA replication. These data as well as the observation that the A48V substitution at the start of the transmembrane domain was not completely responsible for the replication defect of the C1/HTm chimera suggest that sequences within the transmembrane segments or the proposed lumenal loops influence HCV RNA replication efficiency. It is conceivable that the membrane-spanning segments of H77 NS4B could significantly alter membrane association or protein-protein interactions within the membrane important for organizing a higher-order structure within the replication complex, either of which could explain the decrease in replication ability of Con1-H77 NS4B chimeras harboring the transmembrane domain of H77 NS4B. Consistent with the latter possibility, specific residues within the transmembrane region of NS5B and the N-terminal amphipathic helix of NS5A play critical, but as yet undefined, roles in HCV replication beyond those of membrane anchoring (12, 15, 20, 34, 36). Alternatively, sequences that reside in the ER lumen may be important for NS4B function. Indeed, amino acid substitutions within the two proposed lumenal loops of NS4B can attenuate HCV replication (26). Experiments to determine if amino acid differences between Con1 and H77 NS4B within the lumenal loops contribute to replication efficiency are in progress.
In contrast to the partial suppression of replication defects caused by the first 227 amino acids of H77 NS4B, mutations in either the protease or the helicase domain of Con1 NS3 were sufficient to completely suppress the RNA replication defect caused by the C-terminal 34 amino acids, providing evidence for a functional interaction between NS3 and the C terminus of NS4B. This result is particularly interesting, since a function has not been assigned to the C terminus of NS4B, although it has been reported that overexpressed NS4B is palmitoylated on conserved cysteine residues at positions 257 and 261 (46). However, it is not clear whether this posttranslational modification occurs or is important during HCV RNA replication.
In an effort to further characterize the C terminus of NS4B, we identified four divergent amino acids residing between positions 254 and 258 of NS4B (Con1 [Asn-Glu-Asp-Cys-Ser] versus H77 [Ser-Ser-Glu-Cys-Thr]; Fig. 8A) that were entirely responsible for the replication defect caused by the 34 C-terminal amino acids of H77 NS4B. A comparison of the NS4B gene from 277 genotype 1b isolates in the European Hepatitis C Virus database (ehHCVdb) (5) identified 11 isolates that diverged from the consensus genotype 1b sequence between residues 254 and 258 (Asn-Glu-Asp-Cys-Ser); four isolates contained N254S, a single isolate harbored N254D, two isolates contained a Phe instead of the highly conserved Cys at position 257, and four isolates carried S258T. Similarly, N254S or S258T were the only substitutions found in 2 out of 42 interferon-treated patients infected with HCV genotype 1b (42), suggesting that the N254S and S258T substitutions are tolerated. Importantly, none of the isolates that we analyzed contained changes at either E255 or D256, and preliminary experimental evidence suggests that both the E255S and D256E substitutions are the major contributors to the RNA replication defect of the Con1-H77 NS4B chimeras containing the 34 C-terminal amino acids of H77 NS4B (data not shown). Thus, these two residues appear to be critical for efficient HCV genotype 1b replication in both patients and cell culture.
Although we provide genetic evidence for an interaction between NS4B and NS3 important for HCV RNA replication, the nature of this interaction is unknown. For example, it is unknown whether the NS3-NS4B interaction involves direct or indirect binding of these two proteins. If these proteins do bind, does this interaction modulate NS3 or NS4B function in the replication complex? Coimmunoprecipitation and yeast two-hybrid assays have shown that NS4B interacts with both NS3 and NS4A (6, 24), and NS4B from HCV and the related dengue virus appears to directly modulate NS3 function in vitro (37, 41). Based on these previous observations, one possible hypothesis is that the genetic interaction identified in this study may partially correlate with direct protein-protein interactions between NS3 and NS4B.
An alternate hypothesis is that the foreign H77 NS4B sequences disrupt the higher-order structure of an active Con1 replication complex and NS3 mutations function by restoring disrupted or suboptimal direct or indirect interactions among the various proteins that comprise the replication complex. There are several observations that provide support for this model. First, the NS3 suppressor mutations map to different regions on the surfaces of both the protease and helicase domains, and the mutations that we tested rescue the replication defects caused by all three domains of H77 NS4B, at least to some extent. Second, the double mutation, P1115R plus S1560G, was more efficient at rescuing replication of the C1/H4B chimera than the single substitutions. Finally, some of the NS3-4A mutations described in this study have also been found to function either as adaptive mutations for genotype 1 replication when combined with specific amino acid substitutions in NS4B, NS5A, or NS5B (17-19, 28, 29, 45) or as suppressors of replication defects caused by mutations that disrupt the acidic C terminus of Con1 NS4A (25). Taken together, the preferential selection of NS3-4A mutations on discontinuous surfaces of the NS3-4A complex and their ability to both suppress different deleterious mutations in NS4A and NS4B and enhance genotype 1 replication under certain conditions not only suggest that NS3 has a higher tolerance for amino acid substitutions but also point to a central role for the NS3-4A complex and NS4B in the higher-order structure of the replication complex. Clearly, further experimentation is required to define the higher-order interactions within an active replication complex.
In conclusion, this genetic approach highlights the utility of chimeric replicons for mapping interactions required for efficient HCV RNA replication in cell culture, in particular the genetic interaction between NS3 and NS4B. Further studies are needed to directly examine the nature of this interaction and to determine the exact role of NS4B in RNA replication.
Acknowledgments
We thank Suzanne Scheaffer for expert technical assistance and helpful discussions and Henry Huang, Sondra Schlesinger, and Dong Yu for critical review of the manuscript. We are also grateful to Charles Rice for providing Huh-7.5 cells and the plasmids pC1/SG-Neo(I), pC1/SG-Neo(I)/pol−, and pC1/ΔE1-p7(I) and to Robert Sarisky for supplying HCV-specific antisera.
This work was funded by the Ellison Medical Foundation New Scholars in Global Infectious Disease (ID-NS-119-03) and NIH grant AI065985. K.J.B. and Washington University may receive income based on a license of related technology by the University to Apath, LLC. Apath, LLC, did not support this work.
Footnotes
Published ahead of print on 20 August 2008.
REFERENCES
- 1.Bartenschlager, R., M. Frese, and T. Pietschmann. 2004. Novel insights into hepatitis C virus replication and persistence. Adv. Virus Res. 6371-180. [DOI] [PubMed] [Google Scholar]
- 2.Blight, K. J. 2007. Allelic variation in the hepatitis C virus NS4B protein dramatically influences RNA replication. J. Virol. 815724-5736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blight, K. J., J. A. McKeating, J. Marcotrigiano, and C. M. Rice. 2003. Efficient replication of hepatitis C virus genotype 1a RNAs in cell culture. J. Virol. 773181-3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blight, K. J., and E. A. Norgard. 2006. Recent advances in hepatitis C virus replication, p. 45-90. In K. L. Hefferon (ed.), Recent advances in RNA virus replication. Transworld Research Network, Kerala, India.
- 5.Combet, C., N. Garnier, C. Charavay, D. Grando, D. Crisan, J. Lopez, A. Dehne-Garcia, C. Geourjon, E. Bettler, C. Hulo, P. L. Mercier, R. Bartenschlager, H. Diepolder, D. Moradpour, J. M. Pawlotsky, C. M. Rice, C. Trepo, F. Penin, and G. Deléage. 2007. euHCVdb: the European hepatitis C virus database. Nucleic Acids Res. 35D363-D366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dimitrova, M., I. Imbert, M. P. Kieny, and C. Schuster. 2003. Protein-protein interactions between hepatitis C virus nonstructural proteins. J. Virol. 775401-5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Einav, S., M. Elazar, T. Danieli, and J. S. Glenn. 2004. A nucleotide binding motif in hepatitis C virus (HCV) NS4B mediates HCV RNA replication. J. Virol. 7811288-11295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elazar, M., P. Liu, C. M. Rice, and J. S. Glenn. 2004. An N-terminal amphipathic helix in hepatitis C virus (HCV) NS4B mediates membrane association, correct localization of replication complex proteins, and HCV RNA replication. J. Virol. 7811393-11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El-Hage, N., and G. Luo. 2003. Replication of hepatitis C virus RNA occurs in a membrane-bound replication complex containing nonstructural viral proteins and RNA. J. Gen. Virol. 842761-2769. [DOI] [PubMed] [Google Scholar]
- 10.Gao, L., H. Aizaki, J.-W. He, and M. M. Lai. 2004. Interactions between viral nonstructural proteins and host protein hVAP-33 mediate the formation of hepatitis C virus RNA replication complex on lipid raft. J. Virol. 783480-3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gosert, R., D. Egger, V. Lohmann, R. Bartenschlager, H. E. Blum, K. Bienz, and D. Moradpour. 2003. Identification of the hepatitis C virus RNA replication complex in Huh-7 cells harboring subgenomic replicons. J. Virol. 775487-5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gosert, R., W. Jendrsczok, J. M. Berke, V. Brass, H. E. Blum, and D. Moradpour. 2005. Characterization of nonstructural protein membrane anchor deletion mutants expressed in the context of the hepatitis C virus polyprotein. J. Virol. 797911-7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo, J. T., V. V. Bichko, and C. Seeger. 2001. Effect of alpha interferon on the hepatitis C virus replicon. J. Virol. 758516-8523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardy, R., J. Marcotrigiano, K. J. Blight, J. E. Majors, and C. M. Rice. 2003. Hepatitis C virus RNA synthesis in a cell-free system isolated from replicon-containing hepatoma cells. J. Virol. 772029-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ivashkina, N., B. Wolk, V. Lohmann, R. Bartenschlager, H. E. Blum, F. Penin, and D. Moradpour. 2002. The hepatitis C virus RNA-dependent RNA polymerase membrane insertion sequence is a transmembrane segment. J. Virol. 7613088-13093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koch, J. O., and R. Bartenschlager. 1999. Modulation of hepatitis C virus NS5A hyperphosphorylation by nonstructural proteins NS3, NS4A, and NS4B. J. Virol. 737138-7146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krieger, N., V. Lohmann, and R. Bartenschlager. 2001. Enhancement of hepatitis C virus RNA replication by cell culture-adaptive mutations. J. Virol. 754614-4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lanford, R. E., B. Guerra, and H. Lee. 2006. Hepatitis C virus genotype 1b chimeric replicon containing genotype 3 NS5A domain. Virology 355192-202. [DOI] [PubMed] [Google Scholar]
- 19.Lanford, R. E., B. Guerra, H. Lee, D. R. Averett, B. Pfeiffer, D. Chavez, L. Notvall, and C. Bigger. 2003. Antiviral effect and virus-host interactions in response to alpha interferon, gamma interferon, poly(I)-poly(C), tumor necrosis factor alpha, and ribavirin in hepatitis C virus subgenomic replicons. J. Virol. 771092-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee, K. J., J. Choi, J. H. Ou, and M. M. Lai. 2004. The C-terminal transmembrane domain of hepatitis C virus (HCV) RNA polymerase is essential for HCV replication in vivo. J. Virol. 783797-3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lemm, J. A., M. Liu, R. E. Rose, R. Fridell, D. R. O'Boyle, Jr., R. Colonno, and M. Gao. 2005. Replication-competent chimeric hepatitis C virus subgenomic replicons. Intervirology 48183-191. [DOI] [PubMed] [Google Scholar]
- 22.Levin, M. K., and S. S. Patel. 1999. The helicase from hepatitis C virus is active as an oligomer. J. Biol. Chem. 27431839-31846. [DOI] [PubMed] [Google Scholar]
- 23.Levin, M. K., Y. H. Wang, and S. S. Patel. 2004. The functional interaction of the hepatitis C virus helicase molecules is responsible for unwinding processivity. J. Biol. Chem. 27926005-26012. [DOI] [PubMed] [Google Scholar]
- 24.Lin, C., J. W. Wu, K. Hsiao, and M. S. Su. 1997. The hepatitis C virus NS4A protein: interactions with the NS4B and NS5A proteins. J. Virol. 716465-6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindenbach, B. D., B. M. Pragai, R. Montserret, R. K. F. Beran, A. M. Pyle, F. Penin, and C. M. Rice. 2007. The C terminus of hepatitis C virus NS4A encodes an electrostatic switch that regulates NS5A hyperphosphorylation and viral replication. J. Virol. 818905-8918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindstrom, H., M. Lundin, S. Haggstrom, and M. A. Persson. 2006. Mutations of the Hepatitis C virus protein NS4B on either side of the ER membrane affect the efficiency of subgenomic replicons. Virus Res. 121169-178. [DOI] [PubMed] [Google Scholar]
- 27.Locatelli, G. A., S. Spadari, and G. Maga. 2002. Hepatitis C virus NS3 ATPase/helicase: an ATP switch regulates the cooperativity among the different substrate binding sites. Biochemistry 4110332-10342. [DOI] [PubMed] [Google Scholar]
- 28.Lohmann, V., S. Hoffmann, U. Herian, F. Penin, and R. Bartenschlager. 2003. Viral and cellular determinants of hepatitis C virus RNA replication in cell culture. J. Virol. 773007-3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lohmann, V., F. Korner, A. Dobierzewska, and R. Bartenschlager. 2001. Mutations in hepatitis C virus RNAs conferring cell culture adaptation. J. Virol. 751437-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lundin, M., H. Lindstrom, C. Gronwall, and M. A. Persson. 2006. Dual topology of the processed hepatitis C virus protein NS4B is influenced by the NS5A protein. J. Gen. Virol. 873263-3272. [DOI] [PubMed] [Google Scholar]
- 31.Lundin, M., M. Monne, A. Widell, G. von Heijne, and M. A. A. Persson. 2003. Topology of the membrane-associated hepatitis C virus protein NS4B. J. Virol. 775428-5438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mackintosh, S. G., J. Z. Lu, J. B. Jordan, M. K. Harrison, B. Sikora, S. D. Sharma, C. E. Cameron, K. D. Raney, and J. Sakon. 2006. Structural and biological identification of residues on the surface of NS3 helicase required for optimal replication of the hepatitis C virus. J. Biol. Chem. 2813528-3535. [DOI] [PubMed] [Google Scholar]
- 33.Miyanari, Y., M. Hijikata, M. Yamaji, M. Hosaka, H. Takahashi, and K. Shimotohno. 2003. Hepatitis C virus non-structural proteins in the probable membranous compartment function in viral genome replication. J. Biol. Chem. 27850301-50308. [DOI] [PubMed] [Google Scholar]
- 34.Moradpour, D., V. Brass, E. Bieck, P. Friebe, R. Gosert, H. E. Blum, R. Bartenschlager, F. Penin, and V. Lohmann. 2004. Membrane association of the RNA-dependent RNA polymerase is essential for hepatitis C virus RNA replication. J. Virol. 7813278-13284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moradpour, D., M. J. Evans, R. Gosert, Z. Yuan, H. E. Blum, S. P. Goff, B. D. Lindenbach, and C. M. Rice. 2004. Insertion of green fluorescent protein into nonstructural protein 5A allows direct visualization of functional hepatitis C virus replication complexes. J. Virol. 787400-7409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Penin, F., V. Brass, N. Appel, S. Ramboarina, R. Montserret, D. Ficheux, H. E. Blum, R. Bartenschlager, and D. Moradpour. 2004. Structure and function of the membrane anchor domain of hepatitis C virus nonstructural protein 5A. J. Biol. Chem. 27940835-40843. [DOI] [PubMed] [Google Scholar]
- 37.Piccininni, S., A. Varaklioti, M. Nardelli, B. Dave, K. D. Raney, and J. E. McCarthy. 2002. Modulation of the hepatitis C virus RNA-dependent RNA polymerase activity by the non-structural (NS) 3 helicase and the NS4B membrane protein. J. Biol. Chem. 27745670-45679. [DOI] [PubMed] [Google Scholar]
- 38.Quinkert, D., R. Bartenschlager, and V. Lohmann. 2005. Quantitative analysis of the hepatitis C virus replication complex. J. Virol. 7913594-13605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Serebrov, V., and A. M. Pyle. 2004. Periodic cycles of RNA unwinding and pausing by hepatitis C virus NS3 helicase. Nature 430476-480. [DOI] [PubMed] [Google Scholar]
- 40.Tong, W.-Y., M. Nagano-Fujii, R. Hidajat, L. Deng, Y. Takigawa, and H. Hotta. 2002. Physical interactions between hepatitis C virus NS4B protein and CREB-RP/ATF6B. Biochem. Biophys. Res. Commun. 299366-372. [DOI] [PubMed] [Google Scholar]
- 41.Umareddy, I., A. Chao, A. Sampath, F. Gu, and S. G. Vasudevan. 2006. Dengue virus NS4B interacts with NS3 and dissociates it from single-stranded RNA. J. Gen. Virol. 872605-2614. [DOI] [PubMed] [Google Scholar]
- 42.Welker, M. W., W. P. Hofmann, C. Welsch, M. von Wagner, E. Herrmann, T. Lengauer, S. Zeuzem, and C. Sarrazin. 2007. Correlation of amino acid variations within nonstructural 4B protein with initial viral kinetics during interferon-alpha-based therapy in HCV-1b-infected patients. J. Viral Hepat. 14338-349. [DOI] [PubMed] [Google Scholar]
- 43.Welsch, C., M. Albrecht, J. Maydt, E. Herrmann, M. W. Welker, C. Sarrazin, A. Scheidig, T. Lengauer, and S. Zeuzem. 2007. Structural and functional characterization of the non-structural protein 4B in flaviviridae. J. Mol. Graph. Model. 26546-557. [DOI] [PubMed] [Google Scholar]
- 44.Yao, N., P. Reichert, S. S. Taremi, W. W. Prosise, and P. C. Weber. 1999. Molecular views of viral polyprotein processing revealed by the crystal structure of the hepatitis C virus bifunctional protease-helicase. Struct. Fold Des. 71353-1363. [DOI] [PubMed] [Google Scholar]
- 45.Yi, M., and S. M. Lemon. 2004. Adaptive mutations producing efficient replication of genotype 1a hepatitis C virus RNA in normal Huh7 cells. J. Virol. 787904-7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu, G. Y., K. J. Lee, L. Gao, and M. M. Lai. 2006. Palmitoylation and polymerization of hepatitis C virus NS4B protein. J. Virol. 806013- 6023. [DOI] [PMC free article] [PubMed] [Google Scholar]