Abstract
The identity and functionality of biological membranes are determined by cooperative interaction between their lipid and protein constituents. Cholesterol is an important structural lipid that modulates fluidity of biological membranes favoring the formation of detergent-resistant microdomains. In the present study, we evaluated the functional role of cholesterol and lipid rafts for entry of hepatitis B viruses into hepatocytes. We show that the duck hepatitis B virus (DHBV) attaches predominantly to detergent-soluble domains on the plasma membrane. Cholesterol depletion from host membranes and thus disruption of rafts does not affect DHBV infection. In contrast, depletion of cholesterol from the envelope of both DHBV and human HBV strongly reduces virus infectivity. Cholesterol depletion increases the density of viral particles and leads to changes in the ultrastructural appearance of the virus envelope. However, the dual topology of the viral envelope protein L is not significantly impaired. Infectivity and density of viral particles are partially restored upon cholesterol replenishment. Binding and entry of cholesterol-deficient DHBV into hepatocytes are not significantly impaired, in contrast to their release from endosomes. We therefore conclude that viral but not host cholesterol is required for endosomal escape of DHBV.
Enveloped animal viruses must overcome membrane barriers posed by host cells to deliver their genomes to the respective replication site. While some viruses achieve this directly at the plasma membrane, for the majority of viruses, fusion/penetration occurs in the cell interior (30). Evidence for endocytosis as the entry mode of hepatitis B viruses (HBVs) is growing (11, 14, 36). For duck HBV (DHBV) and primary duck hepatocytes (PDHs), it has been shown that virus binding is species and cell type specific, that the preS region of the large viral envelope protein governs viral entry, that a small number of virions bind and enter host cells, that viral uptake requires energy and proceeds with a remarkably slow kinetics, and that virus trafficking requires dynamic microtubules (11, 14, 36). In addition, binding of human HBV to host cells is facilitated by proteoglycans (25, 37). However, major issues such as the identity and distribution of cellular factors/receptors mediating virus entry and mode of entry are still unknown (11, 14, 36).
Membrane trafficking and sorting processes are governed by the lipid composition and organization within membranes. Membranes of eukaryotic cells contain specialized microdomains called lipid rafts enriched in cholesterol and sphingolipids, with caveolae being the prototype (28, 30). The functional role of cholesterol and lipid rafts has been studied by cholesterol depletion or sequestration experiments (29, 31). These studies revealed that lipid rafts represent specialized platforms for cellular processes and are exploited for the entry of pathogens (30, 39). In addition, it has been shown that viruses not only depend on cholesterol in the host membranes but can also rely on the presence of cholesterol in the viral membrane to overcome host membranes during entry (4).
The envelope of animal viruses is derived from host membranes. The lipid composition of the envelope of paramyxo- or togaviruses resembles that of the host cell membrane from which they bud (20, 21). In contrast, other viruses such as influenza virus or Semliki Forest virus seem to enrich certain lipids during morphogenesis, as indicated by a lipid composition that diverges from that of the donor host membranes (26, 33). Hepadnaviruses acquire their envelope at the endoplasmic reticulum (ER) and are then presumably secreted through the constitutive secretion pathway (12). A closer look at the membrane of HBV reveals that its cholesterol content is relatively high compared to the contents of other incorporated lipids (7, 13, 35). The lipid composition thus clearly diverges from that of ER membranes (24) and suggests an important function for cholesterol in the viral membrane.
In the present study, we investigated the role of cholesterol and lipid rafts in membrane-membrane/protein interactions during hepadnaviral entry. Our data show that the cholesterol content of the hepadnaviral envelope, but not of host membranes, is a key determinant of the virus infectivity.
MATERIALS AND METHODS
Cell culture.
Fetal PDHs were prepared and cultivated as described previously (8). HBV-infectible HepaRG cells were cultivated as published (15).
Antibodies and drugs.
Methyl-β-cyclodextrin (CD), water-soluble cholesterol, and mevinolin were obtained from Sigma, reconstituted in H2O or dimethyl sulfoxide, and stored at −20°C. Cholera toxin subunit B (CTB) (Sigma) was reconstituted in H2O and stored at 4°C. We used polyclonal rabbit anti-DHBV L serum as described previously (8), rabbit anti-DHBV core serum (kindly provided by L. Cova, France), mouse anti-actin and anti-α-tubulin (Sigma), rabbit anti-caveolin (BD Transduction Laboratories, United Kingdom), rabbit anti-CTB (Sigma), rabbit anti-herpes simplex virus type 1 (HSV-1 [kindly provided by J. Kühn]), and polyclonal goat anti-vimentin (kindly provided by G. Tolstonog, Germany). Secondary antibodies conjugated with horseradish peroxidase were from Dianova (Germany); secondary antibodies conjugated with Alexa-488 were from Molecular Probes (United Kingdom).
Virus infections.
PDHs were infected with DHBV as described previously (8). HepaRG cells were infected with polyethylene glycol (PEG)-precipitated HBV from HepG2.2.15 cells as described previously (15). Cells were infected with HSV and recombinant adenovirus overnight.
Binding of DHBV and CTB to cell surface domains.
Cultures were mock treated or treated with 10 mM CD for 1 h. Then PDHs were washed with cold medium, drug was readded, and viremic serum was applied. Virus was attached to the cell surface for 2 h at 4°C. As controls for binding specificity and domain purification, 10 μg/ml CTB or preS peptide-encompassing amino acids 1 to 165 of viral L protein (kindly provided by S. Urban) were used. Cells were either washed extensively with ice-cold phosphate-buffered saline and harvested or shifted to 37°C for 30 min. For harvest, cells were scraped in 1 ml ice-cold TNE buffer (25 mM Tris [pH 7.5], 50 mM NaCl, 5 mM EDTA [pH 7.4]) containing 1% Triton X-100. Biochemical isolation of lipid rafts was performed as described previously (6).
For detection of CTB binding on the cellular level, PDHs were treated or not with 10 mM CD for 1 h at 37°C. Then, 10 μg/ml fluorescein isothiocyanate-labeled CTB was added for 30 min at 37°C. Cells were either fixed with 3.7% paraformaldehyde or washed and further incubated to study the reversibility of CD treatment. Pictures were acquired using a confocal laser-scanning microscope (LSM 510 META; Zeiss).
Cholesterol depletion of cells with mevinolin and virus infection.
Congenitally DHBV-infected PDHs were treated with 100 μM mevinolin for 24 h and subsequently treated with 10 μM mevinolin for a further 48 h. Then supernatants were harvested and the infectivity of progeny virus was tested. To test the role of host cholesterol for DHBV entry, PDHs were treated for 24 h with 100 μM and subsequently for 48 h with 10 μM mevinolin to inhibit cholesterol biosynthesis prior to DHBV infection in the presence of the drug. To control the efficacy of cholesterol depletion, PDHs were infected with HSV 24 h and 48 h after the commencement of mevinolin treatment.
Add-in assay.
Cells were exposed to DHBV at 4°C. After 2 h of binding, cells were washed three times with phosphate-buffered saline to remove unbound virus. The plate was transferred to 37°C to allow virus internalization. CD was added before or after 30 min, 1 h, or 2 h of incubation at 37°C. After 16 h of further incubation, cells were washed to remove drug and unbound virus. Three days later, cells were harvested and immunoblotted for L protein.
Cholesterol depletion and replenishment of hepadnaviral particles.
Twenty microliters of DHBV viremic serum or 100 μl PEG-precipitated HBV was incubated for 1 h at 37°C with different concentrations of the drug or H2O in medium. Viral particles were precipitated with 10% PEG after treatment. Then PDHs or HepaRG cells were infected with treated virus and productive infection was tested as described below. For cholesterol replenishment, viral particles were treated as described above. Then 50 μg/ml water-soluble cholesterol was added and incubation continued for 1 h. Alternatively, CD and cholesterol were added simultaneously. Particles were precipitated, and their infectivity was tested.
Subcellular fractionation, immunoblotting, immunofluorescence staining, and DHBV DNA-specific PCR.
The subcellular fractionation, immunoblotting, immunofluorescence staining, and DHBV DNA-specific PCR assays were done as described previously (8, 38). Signals were quantified after acquisition with a Fluor-S MultiImager (Bio-Rad, Germany) using Quantity One software.
HBV infection of HepaRG cells.
Cells were infected with the above-mentioned inoculum as described previously (15). Cell culture supernatant was harvested 9 days after inoculation and was tested by enzyme-linked immunosorbent assay for HBe antigen using a commercial kit (Abbot, United Kingdom).
Electron microscopy.
Viral particles were immunoprecipitated with anti-L antibodies, treated as indicated for 1 h at 37°C, and processed for electron microscopy as described previously (9, 32).
Density-based gradient ultracentrifugation and DNA-dot blot analysis.
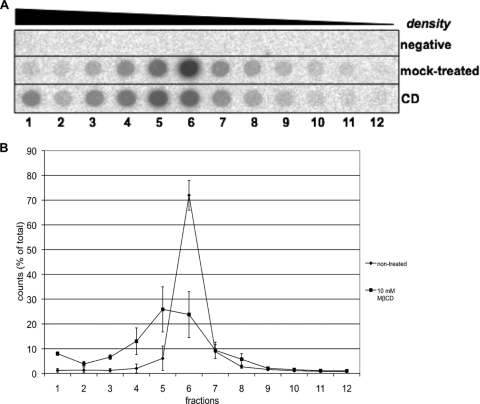
An aliquot of mock-treated or treated viral particles was loaded onto a cesium chloride gradient ranging from 1.4 g/cm3 to 1.2 g/cm3. Fractions of about 200 μl were collected from the bottom. The fractions were dotted onto a membrane and hybridized with a DHBV DNA-specific radioactive probe. Signals were acquired and quantified using a PhosphorImager and AIDA software.
Protease protection assay.
Mock- and CD-treated viral particles were digested with trypsin as described previously (22, 23).
Determination of cholesterol content.
The amount of cholesterol was determined using the Amplex red cholesterol assay kit (Invitrogen) according to the manufacturer's instructions. The amount of cholesterol was normalized to the protein content of the sample.
RESULTS
DHBV preferentially binds to detergent-soluble domains on hepatocellular membranes.
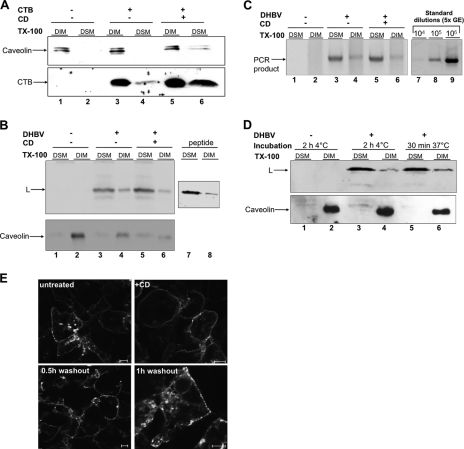
We have previously shown that endocytic internalization of DHBV requires an unusually long time period (9, 10) and diverges from that of viruses known to be taken up by clathrin-mediated endocytosis. Since the time requirement resembles more closely that of polyomaviruses, which enter the cells via lipid rafts (2), we assumed that DHBV uses an analogous entry route. To test this, we first determined the fraction of DHBV particles binding to lipid rafts on hepatocellular membranes. Prior to virus inoculation, cells were treated or not with 10 mM CD to deplete cholesterol from the plasma membrane and disrupt lipid rafts. This treatment reduced the cholesterol content of the cells by an average of 37% and led to redistribution of caveolin-1 (marker protein for lipid rafts) and CTB (known to bind lipid rafts) from the detergent-insoluble membrane (DIM) fraction to the detergent-soluble membrane (DSM) fraction (Fig. 1A, lanes 5 and 6). In addition, disruption of lipid rafts upon cholesterol depletion was visualized using FITC-labeled CTB and fluorescence microscopy (Fig. 1E). CD-treated cells exhibited a smooth and regular pattern of CTB distribution on the cell membrane, in marked contrast to the patchy one in untreated cells. Also CTB uptake into the cells was strongly reduced. Removal of the drug resulted in partial reformation of the lipid rafts after about 1 h (Fig. 1E).
FIG. 1.
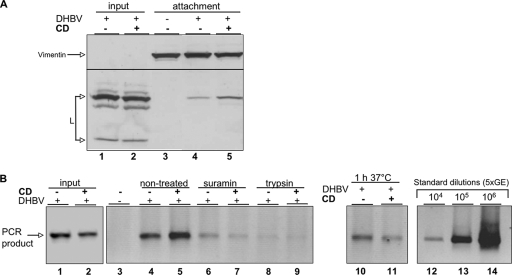
DHBV preferentially binds to DSM domains on hepatocytes. (A) CTB was attached to PDHs. DIMs and DSMs were isolated, and fractions were analyzed for caveolin-1 and CTB by immunoblotting. (B) DHBV or recombinant preS protein (peptide) was attached to PDHs. DIMs and DSMs were isolated and analyzed by immunoblotting for L protein and caveolin. (C) PCR analysis of the same fractions for DHBV DNA. PCR was standardized using serial dilutions of a viremic serum with known GE. (D) After DHBV attachment at 4°C, unbound inoculum was removed and cultures were warmed to 37°C. DIMs and DSMs were isolated and subjected to immunoblot analysis for L and caveolin protein. (E) PDHs were treated with CD for 1 h at 37°C and then incubated with 10 μg/ml FITC-CTB for 30 min at 37°C. Cells were either fixed or washed to remove unbound ligand and further cultivated for the indicated time periods and then fixed. Cells were analyzed by confocal scanning microscopy. Bars correspond to 5 μm. TX-100, Triton X-100.
CD-treated cells were incubated with viremic serum or a recombinant peptide encompassing amino acids 1 to 165 of the viral envelope protein L. After 2 h of incubation at 4°C, cells were harvested and lysed in a Triton X-100-containing buffer. DIM were separated from DSM and analyzed for the presence of viral L protein and DNA by immunoblotting and PCR, respectively. In both mock- and CD-treated cells, L protein was enriched to about 60% in DSM fractions, although a small amount could be detected in the DIM fraction (Fig. 1B, lanes 3 and 4). This slightly increased after cholesterol depletion to about 75% (Fig. 1B, lanes 5 and 6). Thus, cholesterol depletion from cellular membranes did not significantly change the membrane partitioning of bound viral particles. The partitioning of recombinant peptide on hepatocellular membranes was indistinguishable from that of viral particles (Fig. 1B, lanes 7 and 8). PCR analysis of the same fractions for viral DNA revealed that the partitioning profile of virions paralleled that of L protein with about 70% of total viral DNA present in the DSM fraction (Fig. 1C, lanes 3 and 4). These data show that binding of subviral particles and virions as well as nonparticulate L protein involves sites preferentially located in nonlipid raft regions of the hepatocellular plasma membrane.
In order to investigate whether viral particles preattached to hepatocellular membranes redistribute from DIMs to DSMs or vice versa upon initiation of membrane mobility, cultures were warmed for 30 min to 37°C. This warming did not result in a significant redistribution of attached viral particles (Fig. 1D, lanes 5 and 6). The lipid raft marker protein caveolin-1 remained in the DIM fraction during the temperature shift, indicating domain stability and excluding a significant mixing of domains by lateral diffusion (Fig. 1D, lanes 5 and 6). The data indicate that viral particles do not first bind to DSMs and then shift to DIMs or vice versa.
DHBV infection does not depend on cellular cholesterol.
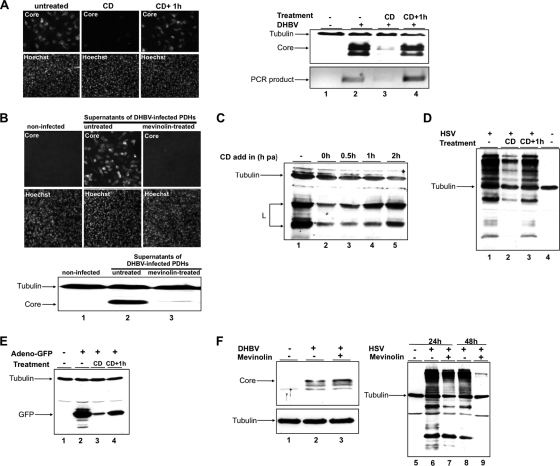
To test the requirement for cellular cholesterol only during DHBV entry, PDHs were pretreated for 1 h with 10 mM CD. Afterwards, cells were washed and incubated for 1 h without the drug before inoculation with DHBV. This washout assay was performed to ensure that cholesterol was depleted only from cellular, but not viral, membranes prior to DHBV infection. Alternatively, CD was present during the inoculation period overnight, thus acting on both virus and cellular membranes. Infection efficiency was strongly reduced when CD was present in the medium at the time of inoculation, while it was not reduced when CD was absent (Fig. 2A). This indicated that cellular cholesterol is dispensable for virus infection and that CD presumably acts on the virus itself. To obtain additional evidence for the cholesterol dependence of infection, we used viral particles produced from congenitally DHBV-infected cells treated with mevinolin. This drug inhibits cholesterol biosynthesis in the cells, and this should lead to the production of progeny virus with a reduced cholesterol content (1). Treatment of DHBV-infected PDHs with mevinolin led to a moderate decrease in secreted viral particles as determined by PCR and immunoblotting (see Fig. S1 in the supplemental material). This decrease was taken into account when cells were infected and the multiplicity of genome equivalents (GE) was consequently adjusted. Anticore immunoblotting and fluorescence of PDHs infected with viral particles from mevinolin-treated cells show a strong reduction in infectivity of the progeny virus (Fig. 2B), thus indicating that cholesterol in the virus membrane is required for infectivity of DHBV. However, we cannot exclude at this stage that mevinolin treatment resulted in conformational changes of the viral particle which reduced infectivity.
FIG. 2.
Cellular cholesterol is dispensable for DHBV infection. (A) PDHs were pretreated with CD. Thereafter, cultures were infected with DHBV in the presence (CD) or 1 h after removal of the drug (CD+1h). Cells were maintained for a further 3 days in medium devoid of drugs and virus, harvested, and subjected to immunofluorescence (left panel) and immunoblot analysis for core protein and to PCR for viral DNA (right panel). (B) Congenitally DHBV-infected PDHs were treated or not with mevinolin, and supernatants were harvested. Infectivity of virus progeny in the supernatants was tested by immunofluorescence (upper panel) and immunoblot (lower panel) analysis for core protein. (C) After DHBV attachment at 4°C and removal of unbound inoculum, PDHs were treated with CD at indicated time points at 37°C. The lysates were analyzed by immunoblotting for L; tubulin served as a loading control. (D and E) PDHs were treated, infected with HSV or a recombinant adenovirus encoding GFP, harvested, and analyzed by immunoblotting for HSV and GFP, respectively. Tubulin served as a loading control. (F) PDHs were treated with mevinolin to inhibit cholesterol biosynthesis prior to DHBV infection. To control the extent of cholesterol depletion by mevinolin treatment, PDHs were infected with HSV 24 h and 48 h after inhibition of cholesterol biosynthesis. Immunoblots for DHBV core (left panel) and HSV (right panel) were performed. Tubulin served as a loading control. h pa, hour postattachment.
To determine at which time point upon viral entry cholesterol became dispensable for infection, we performed an “add-in” assay. Cells were exposed to DHBV at 4°C. Following removal of the inoculum, internalization of attached virus was initiated at 37°C and cells were treated at indicated time points with CD. This assay revealed strong inhibition of infection when cells were treated during inoculation and up to 30 min postinternalization compared to the nontreated control (Fig. 2C, lanes 1 to 3). Then L protein amounts gradually increase, suggesting protection of the virus from the CD treatment. Cholesterol depletion 2 h after virus internalization only led to a minor reduction in virus infection (Fig. 2C, lanes 4 to 5). These findings indicate that internalized DHBV are largely protected from the antiviral effect of CD, and thus virus entry depends on a cholesterol-sensitive step very early in infection.
The efficiency of cholesterol depletion from PDHs was controlled by infection experiments using HSV and a recombinant adenovirus encoding green fluorescent protein (GFP). As expected, cholesterol-depleted hepatocytes were inefficiently infected by HSV and adenovirus (3, 18) (Fig. 2D and E).
To assess whether DHBV entry depends on host cell cholesterol, PDHs were pretreated with mevinolin for up to 48 h and subsequently inoculated with DHBV and HSV as a control. After about 16 h of incubation, the drug and inoculum were removed. Cells were harvested 3 days later, and lysates were subjected to immunoblot analysis for core protein. Mevinolin treatment had no negative effect on DHBV infection since the core signal in treated cells was comparable to that of nontreated cells (Fig. 2F, lanes 2 and 3). In contrast, the permissiveness of PDHs for HSV was strongly reduced 24 and 48 h after inhibition of cholesterol biosynthesis in PDHs (Fig. 2F, lanes 6 to 9).
Taken together, our data show that host cell cholesterol is dispensable for productive entry of DHBV in PDHs. These findings are consistent with the notion that DHBV preferentially binds to DSMs. Thus, DHBV entry into hepatocytes is presumably not mediated to an infection-relevant extent by lipid rafts.
The cholesterol content of the envelope governs infectivity of hepatitis B viruses.
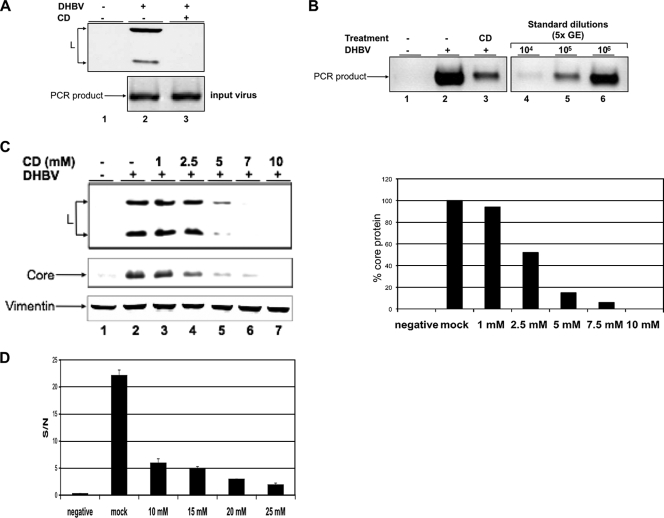
The data described above indicate that CD acts on the virus itself and reduces its infectivity. CD selectively binds cholesterol and depletes it very efficiently from membranes without disturbing other lipids (19). If the envelope-mediated entry of DHBV is strictly dependent on cholesterol, CD-treated viral particles should exhibit an altered infectivity. To investigate this, DHBV-containing serum was treated with 10 mM CD, which reduced the cholesterol content by about 20% or an equivalent amount of H2O as the mock control. Thereafter, viral particles were pelleted to remove CD and used for infection experiments. The amount of input virus was controlled by PCR in this (Fig. 3A) and all following experiments (data not shown). All assays showed an almost complete loss of virus infectivity upon cholesterol depletion (Fig. 3A to C). The L immunoblot showed no signal in the case of cultures inoculated with CD-treated DHBV, in contrast to cultures inoculated with mock-treated virus, although the amount of input virus was comparable (Fig. 3A, lanes 2 and 3). The PCR analysis corroborated these results and showed strongly reduced signals in cells infected with cholesterol-depleted viral particles (Fig. 3B, lanes 2 and 3).
FIG. 3.
Cholesterol depletion strongly reduces infectivity of both DHBV and HBV. DHBV viremic serum was treated with CD; viral particles were pelleted and used as the inoculum. (A) PDHs were harvested 3 days after DHBV infection, and lysates were subjected to immunoblot analysis for L (upper panel). The amount of input virus was controlled by PCR (lower panel). (B) Replicative intermediates were extracted 3 days after DHBV infection and assayed by PCR. PCR was standardized using serial dilutions of a viremic serum with known GE. (C) The antiviral effect of CD is dose dependent. PDHs were infected with DHBV treated with CD at the indicated concentrations. Three days after infection, cells were harvested and lysates were subjected to immunoblot analysis for viral L and core protein. Vimentin served as a loading control. Quantification of the core blot signals is shown in the right panel. (D) Cell culture supernatants of HepaRG cells infected with CD-treated HBV were harvested 9 days after infection and analyzed for HBe antigen. The standard deviation from two experiments is shown.
The antiviral effect of CD was dose dependent, as determined by dose escalation experiments and observed with doses as low as 2.5 mM (Fig. 3C, lanes 4 to 7). As judged from the quantification of the core immunoblot, the 50% effective concentration of CD for DHBV was about 2.5 mM (Fig. 3C, right panel). When higher concentrations were used, infection efficiency decreased to less than 1% at 10 mM compared to that of the mock-treated control. This indicates that a threshold cholesterol concentration in the viral membrane is required for virus infectivity. When a ratio of five CD molecules binding one cholesterol molecule is assumed (40), CD may deplete up to 106 cholesterol molecules per viral particle at the highest concentration used.
To corroborate the results obtained for DHBV, we performed a proof-of-principle experiment using HBV and HepaRG cells. Nine days after infection with CD-treated HBV particles, cell culture supernatants were analyzed for HBe antigen by enzyme-linked immunosorbent assay. This assay showed that cholesterol depletion strongly and concentration dependently reduced infectivity of HBV (Fig. 3D).
Taken together, these data show that cholesterol depletion of hepadnaviral particles leads to a strong reduction of their infectivity. The cholesterol requirement for virus infectivity seems to be a conserved feature of at least human HBV and DHBV.
Cholesterol depletion alters the morphology of the viral envelope and buoyant density of viral particles.
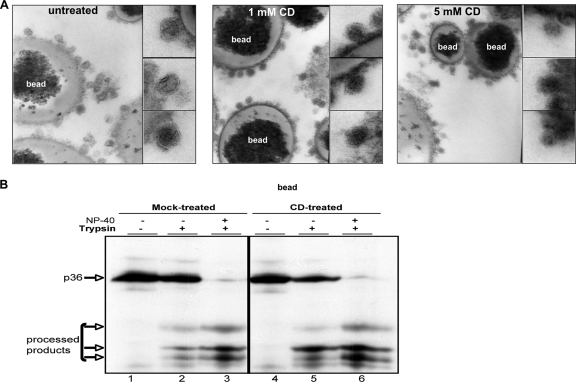
To investigate the impact of cholesterol depletion on the overall structural integrity of viral particles, we performed transmission electron microscopy (TEM) analysis. DHBV particles were immunocaptured from viremic serum using an L-specific antiserum, treated, and analyzed by TEM. In the mock-treated sample, the envelope of the viral particles attached to the beads is clearly visible as two thin lines (Fig. 4A, left panel). After cholesterol depletion with two different CD concentrations, the appearance of the viral envelope was strongly altered and seemed to be reduced to one layer (Fig. 4A, middle and right panels). Particles seem to be collapsed. Otherwise, the overall structures of the viral particles in CD- and mock-treated samples were comparable. These findings show that cholesterol depletion of the viral membrane results in a strong alteration of its ultrastructural appearance.
FIG. 4.
Effect of cholesterol depletion on the ultrastructure of DHBV particles and L topology. (A) Viral particles were immunocaptured to beads from viremic serum, treated with different CD concentrations, and processed for ultrastructural analysis by TEM. The lower magnifications show the decoration of beads with viral particles. The ultrastructure of single viral particles is shown at high magnification in the subpanels. (B) Dual topology of L protein before and after cholesterol depletion of viral particles was determined by protease protection assay. Trypsin-mediated proteolysis of L protein in the membranes of CD- and mock-treated DHBV particles was monitored by immunoblotting before and after addition of NP-40.
As a further control for integrity of the virus envelope, the topology of envelope protein L was determined. DHBV L exists in two topologies: half of the L protein has the amino-terminal preS region located inside the viral particle, and the other half has the region located outside. This results in protection of about half of the viral L molecules from protease digestion. Cholesterol-depleted viral particles did not display significant changes in the dual-L topology, as judged by comparison of tryptic product patterns after limited protease digestion of untreated and CD-treated viral particles (Fig. 4B, lanes 2 and 5, respectively).
We assumed that the observed ultrastructural changes and loss of cholesterol result in an altered density of the viral particles. To test this, cholesterol-depleted viral particles were subjected to isopycnic gradient ultracentrifugation and fractions were analyzed for the presence of viral DNA by dot blot analysis. As judged from the DNA dot blot, cholesterol depletion resulted in an increase of viral particle density (Fig. 5A). The fraction with the highest density presumably contains particles with a severely altered envelope. This fraction is increased after cholesterol depletion of viral particles compared to the mock-treated control from about 1% of total counts to about 8% (Fig. 5B), indicating de-envelopment after cholesterol depletion. Overall, a shift of cholesterol-depleted virions to the denser fractions of the gradient was observed. An L immunoblot of the same fractions showed an increase in signals in the fractions with lower density (data not shown). This is consistent with a partial collapse of subviral particles after cholesterol depletion.
FIG. 5.
Effect of cholesterol depletion on the buoyant density of DHBV particles. Cholesterol-depleted or mock-treated DHBV particles were subjected to CsCl2-based density gradient ultracentrifugation. Fractions were collected and analyzed for viral DNA by dot blotting. (A) Dot blot analysis of fractions for viral DNA. (B) Quantification of the signals from three independent experiments. MβCD, methyl-β-cyclodextrin. Bars show the standard deviation.
In conclusion, cholesterol in the DHBV membrane is essential for the density and structure of viral particles.
Partial recovery of DHBV infectivity upon cholesterol replenishment.
To investigate the reversibility of cholesterol depletion, we incubated cholesterol-depleted viral particles with different amounts of water-soluble cholesterol and determined viral particle density and infectivity. As shown above, viral particle density increased after cholesterol depletion compared to that in the mock-treated control (Fig. 6A). Addition of 50 μg/ml water-soluble cholesterol to mock-treated particles led to a slight decrease in particle density, indicating that mock-treated viral particles were able to incorporate additional cholesterol. Cholesterol replenishment of CD-treated viral particles led to a partial restoration of viral density to levels comparable to that of the mock control (Fig. 6A). A significant fraction of virions shifted to the peak fraction of mock-treated virus. Thus, cholesterol-depleted viral particles were presumably successfully reloaded with cholesterol, as indicated by a decrease in their density. In addition, we determined the cholesterol content of CD- and cholesterol treated particles. These treatments led to a 20% reduction and 5% increase of viral cholesterol content, respectively.
FIG. 6.
Partial recovery of buoyant density, infectivity, and morphology of DHBV upon cholesterol replenishment. (A) Samples were subjected to density gradient centrifugation. Fractions were analyzed by DHBV-DNA dot blotting. Quantification of the signals from three independent experiments is shown. Bars show the standard deviation. (B and C) Viral particles were treated as indicated, PEG precipitated, and used for infection of PDHs. Three days after infection, cells and cell culture supernatants were harvested and analyzed. (B) Semiquantitative PCR analysis of viral DNA in cell culture supernatants. PCR was standardized using serial dilutions of a viremic serum with known GE. (C) Immunoblotting of cellular lysates for viral L and core protein. Tubulin served as a loading control. The right panel shows a quantification of the core immunoblot. “co” indicates that cholesterol and CD were simultaneously added; “succ” indicates that particles were first treated with CD and then with cholesterol. (D) Viral particles were pretreated with CD and cholesterol (Chol.) as indicated and prepared for TEM analysis. The ultrastructure of single viral particles is shown at high magnification.
Infection experiments with the replenished DHBV particles showed a partial restoration of virus infectivity up to 20% (Fig. 6B and C). After cholesterol replenishment, the amount of secreted DNA-containing particles in the cell culture supernatant after infection increased significantly compared to infection with CD-treated particles (Fig. 6B). Immunoblotting of the cellular lysates corroborated these results and showed that cholesterol replenishment of viral particles increased infection efficiency after CD treatment (Fig. 6C). Electron microscopic analysis of replenished particles indicates that viral morphology can be partially restored after cholesterol incubation (Fig. 6D).
Taken together, the results show that cholesterol reloading can at least partially restore density and infectivity of cholesterol-depleted DHBV, indicating that the cholesterol in the viral membrane is indispensable for virus infectivity.
Cholesterol in the viral envelope is required for endosomal escape, but not binding and entry of DHBV in PDHs.
The experiments shown above indicate that DHBV entry into hepatocytes depends on the cholesterol content of the virus envelope. To further elucidate this, we investigated the binding and internalization competence of cholesterol-deficient DHBV virions and subviral particles as described previously (8). As a specificity control, cells were pretreated with suramin, which decreases infection-relevant virus binding (8). Comparison of the amounts of viral particles attached to PDHs revealed similar amounts in the case of cells incubated with cholesterol-depleted DHBV compared to the control, indicating that CD treatment of particles did not lead to a significant loss of viral protein or DNA (Fig. 7A and B, lanes 1 and 2). Attached, not-yet-internalized viral particles were almost completely removed by trypsin digestion (Fig. 7B, lanes 8 and 9), as shown previously (8). These results demonstrate that cholesterol in the virus membrane is not required for binding of DHBV to hepatocytes. In addition, analysis of the inoculum revealed that the pattern of viral envelope protein L and DNA did not change significantly after cholesterol depletion (Fig. 7A and B, lanes 1 and 2). Determination of the fraction of internalized viral particles after 1 h and 6 h of entry showed that the amount of internalized DHBV was not strongly reduced in cholesterol-depleted DHBV compared to control virus (Fig. 7B, lanes 10 and 11, and Fig. 8A, lanes 4 and 6, respectively). These data indicate that cholesterol depletion has no detectable influence on DHBV binding and entry and that the infectivity loss of cholesterol-depleted virions is presumably due to a postentry event.
FIG. 7.
Cholesterol-depleted DHBV binds and enters into PDHs. CD- or mock-treated DHBV particles were attached to PDHs. Cells were harvested with or without trypsin or warmed to 37°C for 1 h after removal of the inoculum. Both inoculum and cell lysates were subjected to immunoblotting and PCR analysis for viral L protein (A) and DNA (B), respectively. Vimentin served as a loading control. PCR was standardized with serial dilutions of a viremic serum with known GE.
FIG. 8.
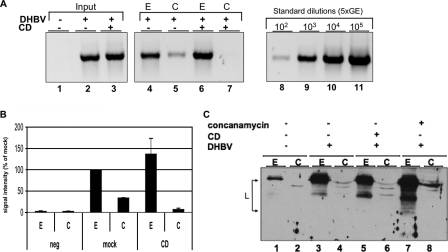
Cholesterol depletion impairs endosomal escape of DHBV. PDHs were inoculated with mock- or CD-treated DHBV. Cells were harvested by trypsin digestion and subjected to subcellular fractionation. The cytosolic (C) and the endosomal (E) fractions were analyzed for DHBV DNA by PCR. A representative agarose gel image of PCR products (A) and quantification of the PCR signals (B) are shown. Bars indicate the standard deviations from two independent experiments. C) Anti-L immunoblot of the same fractions. Subcellular fractions of concanamycin-treated PDHs were included as a control.
Recent work by others and us suggests that DHBV is internalized by endocytosis (5, 9, 38). Therefore, we hypothesized that cholesterol is essential for the membrane-membrane/protein interaction required for release of DHBV from endosomes. To test this, PDHs were inoculated for 6 h with mock- or CD-treated DHBV. Cells were harvested with trypsin to remove cell-surface-bound, not-yet-internalized viral particles, and endosomal and cytosolic fractions were prepared. Purity of the fractions was controlled by immunoblotting fractions for endosomal marker caveolin-1 and cytosolic marker Hsc70 (see Fig. S2 in the supplemental material). Determination of the amount of viral DNA in the respective subcellular fractions revealed that similar amounts of mock- or CD-treated virions were taken up into the endosomal compartments of PDHs. However, in the cytosolic fraction, viral DNA was only detectable in case of cells infected with mock-treated DHBV (Fig. 8A and B, lanes 5 and 7). There was only a barely detectable amount of viral DNA in the cytosolic fraction of cells inoculated with cholesterol-depleted DHBV. In addition, immunoblotting for viral L protein showed that this protein is detectable in the endosomal fraction only (Fig. 8C, lanes 3 and 5). Concanamycin treatment of PDHs prevents the endosomal escape of incoming DHBV and leads to stabilization of L protein, as reported previously (9), and was used as a control.
The data indicate that cholesterol-depleted viral particles were not able to cross the barrier posed by the endosomal membrane. Thus, viral membrane cholesterol is required for endosomal escape of the virus into the cytosol.
DISCUSSION
Enveloped viruses evolved different mechanisms to overcome the barriers posed by host membranes during entry (17). The finding that the endosomal release of incoming cholesterol-depleted virions is significantly impaired together with the strong inhibition of infection suggests that viral membrane cholesterol plays a pivotal role during virus entry. Often, membrane fusion is triggered by the interaction of particular fusogenic (glyco-) proteins in the virus envelope with certain lipids in the membrane. For example, artificial membranes consisting of phosphatidylcholine, phosphatidylethanolamine, sphingomyelin, and cholesterol incubated with the human immunodeficiency virus (HIV) fusion protein gp41 fuse optimally at a 35/30/15/20 molar ratio, respectively (16). An analogous situation might exist during entry of HBVs into host cells. Thus, alteration of the amount of cholesterol in the hepadnaviral envelope may comprise its fusion competence and lead to loss of virus infectivity. In the case of HIV-1, virus-associated cholesterol is essential for the fusion of the virus membrane with the plasma membrane and it has been suggested that virus envelope clustering and restructuring upon virus attachment depend on cholesterol (34). Replenishment of the HIV envelope with cholesterol restored the infectivity of cholesterol-depleted HIV-1 (4). Cholesterol is thus pivotal for structural integrity and infectivity of both hepadnaviruses and HIV, two major human pathogens.
In the case of HIV-1, it has been reported that cholesterol depletion from both cellular and virus membranes substantially reduced the permissiveness of cells and led to loss of viral particle infectivity, respectively (4, 27). In contrast, according to our data, depletion of cholesterol from host membranes does not interfere with the permissiveness of hepatocytes for DHBV. The finding that DHBV preferentially binds to DSMs on the plasma membrane further strengthens this notion. Thus, cholesterol dependence provides a novel example for both conservation of and variation in host factor requirements by different viruses.
The amount of viral L protein and its dual topology were not significantly impaired in cholesterol-depleted particles compared to control ones. Thus, it appears that hepadnaviral envelope proteins alone are necessary, but insufficient, to induce membrane fusion and depend on certain lipids, in particular cholesterol, to exert their function. Consistent with this are the changes in the ultrastructural appearance of the DHBV envelope upon cholesterol depletion. Loss of infectivity of DHBV and HBV virions after cholesterol depletion was dose dependent with a 50% effective concentration of about 2.5 mM, indicating that a certain cholesterol concentration is needed for the functionality of the virus membrane. Rearrangements within the entire envelope and/or particular microdomains necessary for infection appear to be increasingly restricted by lowering the membrane cholesterol content.
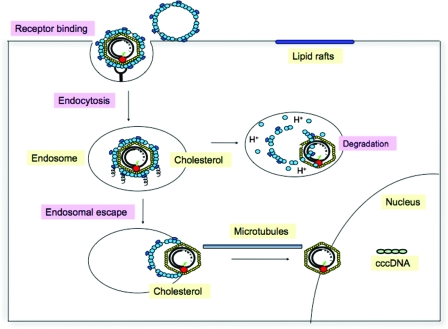
Overall, our data clearly demonstrate that virion-associated cholesterol is required for structural integrity and infectivity of hepatitis B viruses. As depicted in the model in Fig. 9, cholesterol seems to be essential for the membrane-membrane/protein interaction during the endosomal stage of virus entry. If this interaction is disturbed, viral particles are presumably not released from the endosome and degraded. However, the mechanism(s) by which cholesterol controls endosomal escape of the incoming virions is currently not clear. Cholesterol-dependent rearrangements within the virus envelope may be required for fusion of the virus envelope with the endosomal membrane. Mutually not exclusive, cholesterol may be required for the release of capsids out of the endosomal compartment subsequent to fusion pore formation.
FIG. 9.
Model for cholesterol dependence of DHBV entry into hepatocytes. DHBV infection initiates with attachment of viral particles to binding sites preferentially located in detergent-soluble domains of the plasma membrane. Following attachment, viral particles are internalized by endocytosis. Endosomal exit of incoming viral particles strongly depends on the cholesterol content of the virus membrane. Cholesterol depletion from the virus membrane results in their endosomal entrapment and presumably proteolytic clearance leading to abrogation of infection.
Supplementary Material
Acknowledgments
We thank J. Kühn for kindly providing HSV and HSV-1 antibody as well as K. Weber for the pseudotyped lentivirus. S. Urban provided recombinant L protein. We are indebted to L. Cova for providing the anti-DHBV-core antibody. We thank M. Aepfelbacher and S. Polywka for HBe antigen determination. We acknowledge the excellent technical support from B. Holstermann in EM analysis.
This work was supported by grants from the DFG, Studienstiftung des deutschen Volkes, and Stiftung für neurovirale Erkrankungen. The HPI is supported by the Freie und Hansestadt Hamburg and the Bundesministerium für Gesundheit und Soziale Sicherheit.
Footnotes
Published ahead of print on 3 September 2008.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Alberts, A. W., J. Chen, G. Kuron, V. Hunt, J. Huff, C. Hoffman, J. Rothrock, M. Lopez, H. Joshua, E. Harris, A. Patchett, R. Monaghan, S. Currie, E. Stapley, G. Albers-Schonberg, O. Hensens, J. Hirshfield, K. Hoogsteen, J. Liesch, and J. Springer. 1980. Mevinolin: a highly potent competitive inhibitor of hydroxymethylglutaryl-coenzyme A reductase and a cholesterol-lowering agent. Proc. Natl. Acad. Sci. USA 773957-3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, H. A., Y. Chen, and L. C. Norkin. 1996. Bound simian virus 40 translocates to caveolin-enriched membrane domains, and its entry is inhibited by drugs that selectively disrupt caveolae. Mol. Biol. Cell 71825-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bender, F. C., J. C. Whitbeck, M. Ponce de Leon, H. Lou, R. J. Eisenberg, and G. H. Cohen. 2003. Specific association of glycoprotein B with lipid rafts during herpes simplex virus entry. J. Virol. 779542-9552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell, S. M., S. M. Crowe, and J. Mak. 2002. Virion-associated cholesterol is critical for the maintenance of HIV-1 structure and infectivity. AIDS 162253-2261. [DOI] [PubMed] [Google Scholar]
- 5.Chojnacki, J., D. A. Anderson, and E. V. L. Grgacic. 2005. A hydrophobic domain in the large envelope protein is essential for fusion of duck hepatitis B virus at the late endosome. J. Virol. 7914945-14955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Danthi, P., and M. Chow. 2004. Cholesterol removal by methyl-β-cyclodextrin inhibits poliovirus entry. J. Virol. 7833-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diminsky, D., R. Schirmbeck, J. Reimann, and Y. Barenholz. 1997. Comparison between hepatitis B surface antigen (HBsAg) particles derived from mammalian cells (CHO) and yeast cells (Hansenula polymorpha): composition, structure and immunogenicity. Vaccine 15637-647. [DOI] [PubMed] [Google Scholar]
- 8.Funk, A., H. Hohenberg, M. Mhamdi, H. Will, and H. Sirma. 2004. Spread of hepatitis B viruses in vitro requires extracellular progeny and may be codetermined by polarized egress. J. Virol. 783977-3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Funk, A., M. Mhamdi, H. Hohenberg, H. Will, and H. Sirma. 2006. pH-independent entry and sequential endosomal sorting are major determinants of hepadnaviral infection in primary hepatocytes. Hepatology 44685-693. [DOI] [PubMed] [Google Scholar]
- 10.Funk, A., M. Mhamdi, L. Lin, H. Will, and H. Sirma. 2004. Itinerary of hepatitis B viruses: delineation of restriction points critical for infectious entry. J. Virol. 788289-8300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Funk, A., M. Mhamdi, H. Will, and H. Sirma. 2007. Avian hepatitis B viruses: molecular and cellular biology, phylogenesis, and host tropism. World J. Gastroenterol. 1391-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ganem, D., and R. J. Schneider. 2001. Hepadnaviridae: the viruses and their replication, p. 2923-2969. In D. M. Knipe and P. M. Howley (ed.), Fields virology, vol. 2. Lippincott, Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 13.Gavilanes, F., J. M. Gonzalez-Ros, and D. L. Peterson. 1982. Structure of hepatitis B surface antigen. Characterization of the lipid components and their association with the viral proteins. J. Biol. Chem. 2577770-7777. [PubMed] [Google Scholar]
- 14.Glebe, D., and S. Urban. 2007. Viral and cellular determinants involved in hepadnaviral entry. World J. Gastroenterol. 1322-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gripon, P., S. Rumin, S. Urban, J. Le Seyec, D. Glaise, I. Cannie, C. Guyomard, J. Lucas, C. Trepo, and C. Guguen-Guillouzo. 2002. Infection of a human hepatoma cell line by hepatitis B virus. Proc. Natl. Acad. Sci. USA 9915655-15660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haque, M. E., and B. R. Lentz. 2002. Influence of gp41 fusion peptide on the kinetics of poly(ethylene glycol)-mediated model membrane fusion. Biochemistry 4110866-10876. [DOI] [PubMed] [Google Scholar]
- 17.Hernandez, L. D., L. R. Hoffman, T. G. Wolfsberg, and J. M. White. 1996. Virus-cell and cell-cell fusion. Annu. Rev. Cell Dev. Biol. 12627-661. [DOI] [PubMed] [Google Scholar]
- 18.Imelli, N., O. Meier, K. Boucke, S. Hemmi, and U. F. Greber. 2004. Cholesterol is required for endocytosis and endosomal escape of adenovirus type 2. J. Virol. 783089-3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kilsdonk, E. P., P. G. Yancey, G. W. Stoudt, F. W. Bangerter, W. J. Johnson, M. C. Phillips, and G. H. Rothblat. 1995. Cellular cholesterol efflux mediated by cyclodextrins. J. Biol. Chem. 27017250-17256. [DOI] [PubMed] [Google Scholar]
- 20.Klenk, H. D., and P. W. Choppin. 1970. Glycosphingolipids of plasma membranes of cultured cells and an enveloped virus (SV5) grown in these cells. Proc. Natl. Acad. Sci. USA 6657-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laine, R., M. L. Kettunen, C. G. Gahmberg, L. Kääriäinen, and O. Renkonen. 1972. Fatty chains of different lipid classes of Semliki Forest virus and host cell membranes. J. Virol. 10433-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lambert, C., S. Mann, and R. Prange. 2004. Assessment of determinants affecting the dual topology of hepadnaviral large envelope proteins. J. Gen. Virol. 851221-1225. [DOI] [PubMed] [Google Scholar]
- 23.Lambert, C., and R. Prange. 2001. Dual topology of the hepatitis B virus large envelope protein: determinants influencing post-translational pre-S translocation. J. Biol. Chem. 27622265-22272. [DOI] [PubMed] [Google Scholar]
- 24.Lange, Y., and T. L. Steck. 1985. Cholesterol-rich intracellular membranes: a precursor to the plasma membrane. J. Biol. Chem. 26015592-15597. [PubMed] [Google Scholar]
- 25.Leistner, C. M., S. Gruen-Bernhard, and D. Glebe. 2008. Role of glycosaminoglycans for binding and infection of hepatitis B virus. Cell. Microbiol. 10122-133. [DOI] [PubMed] [Google Scholar]
- 26.Lenard, J., and R. W. Compans. 1974. The membrane structure of lipid-containing viruses. Biochim. Biophys. Acta 34451-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manes, S., G. del Real, R. A. Lacalle, P. Lucas, C. Gomez-Mouton, S. Sanchez-Palomino, R. Delgado, J. Alcami, E. Mira, and A. C. Martinez. 2000. Membrane raft microdomains mediate lateral assemblies required for HIV-1 infection. EMBO Rep. 1190-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moffett, S., D. A. Brown, and M. E. Linder. 2000. Lipid-dependent targeting of G proteins into rafts. J. Biol. Chem. 2752191-2198. [DOI] [PubMed] [Google Scholar]
- 29.Nichols, B. J., A. K. Kenworthy, R. S. Polishchuk, R. Lodge, T. H. Roberts, K. Hirschberg, R. D. Phair, and J. Lippincott-Schwartz. 2001. Rapid cycling of lipid raft markers between the cell surface and Golgi complex. J. Cell Biol. 153529-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pelkmans, L. 2005. Viruses as probes for systems analysis of cellular signalling, cytoskeleton reorganization and endocytosis. Curr. Opin. Microbiol. 8331-337. [DOI] [PubMed] [Google Scholar]
- 31.Pralle, A., P. Keller, E. L. Florin, K. Simons, and J. K. Horber. 2000. Sphingolipid-cholesterol rafts diffuse as small entities in the plasma membrane of mammalian cells. J. Cell Biol. 148997-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prassolov, A., H. Hohenberg, T. Kalinina, C. Schneider, L. Cova, O. Krone, K. Frolich, H. Will, and H. Sirma. 2003. New hepatitis B virus of cranes that has an unexpected broad host range. J. Virol. 771964-1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Renkonen, O., L. Kaarainen, K. Simons, and C. G. Gahmberg. 1971. The lipid class composition of Semliki Forest virus and plasma membranes of the host cells. Virology 46318-326. [DOI] [PubMed] [Google Scholar]
- 34.Saez-Cirion, A., S. Nir, M. Lorizate, A. Agirre, A. Cruz, J. Perez-Gil, and J. L. Nieva. 2002. Sphingomyelin and cholesterol promote HIV-1 gp41 pretransmembrane sequence surface aggregation and membrane restructuring. J. Biol. Chem. 27721776-21785. [DOI] [PubMed] [Google Scholar]
- 35.Satoh, O., H. Imai, T. Yoneyama, T. Miyamura, H. Utsumi, K. Inoue, and M. Umeda. 2000. Membrane structure of the hepatitis B virus surface antigen particle. J. Biochem. 127543-550. [DOI] [PubMed] [Google Scholar]
- 36.Schultz, U., E. Grgacic, and M. Nassal. 2004. Duck hepatitis B virus: an invaluable model system for HBV infection. Adv. Virus Res. 631-70. [DOI] [PubMed] [Google Scholar]
- 37.Schulze, A., P. Gripon, and S. Urban. 2007. Hepatitis B virus infection initiates with a large surface protein-dependent binding to heparan sulfate proteoglycans. Hepatology 461759-1768. [DOI] [PubMed] [Google Scholar]
- 38.Stoeckl, L., A. Funk, A. Kopitzki, B. Brandenburg, S. Oess, H. Will, H. Sirma, and E. Hildt. 2006. Identification of a structural motif crucial for infectivity of hepatitis B viruses. Proc. Natl. Acad. Sci. USA 1036730-6734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teissier, E., and E. I. Pecheur. 2007. Lipids as modulators of membrane fusion mediated by viral fusion proteins. Eur. Biophys. J. 36887-899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uekama, K., K. Arimori, A. Sakai, K. Masaki, T. Irie, and M. Otagiri. 1987. Improvement in percutaneous absorption of prednisolone by beta- and gamma-cyclodextrin complexations. Chem. Pharm. Bull. 352910-2913. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.