Abstract
The herpes simplex virus type 1 tegument protein known as VP13/14, or hUL47, localizes to the nucleus and binds RNA. Using fluorescence loss in photobleaching analysis, we show that hUL47 undergoes nucleocytoplasmic shuttling during infection. We identify the hUL47 nuclear export signal (NES) as a C-terminal 10-residue hydrophobic peptide and measure its efficiency relative to that of the classical human immunodeficiency virus type 1 Rev NES. Finally, we show that the hUL47 NES is sensitive to the inhibitor of CRM1-mediated nuclear export leptomycin B. Hence, hUL47 joins a growing list of virus-encoded RNA-binding proteins that use CRM1 to exit the nucleus.
The alphaherpesvirus UL47 gene encodes a major structural protein that is assembled into the tegument of the virus particle (1, 9, 11, 20). Although the role of UL47 has not yet been defined, herpes simplex virus type 1 (HSV-1) variants unable to express the UL47 protein (VP13/14 or hUL47) display defects at the early stages of virus growth (21, 22). In addition, all the UL47 proteins encoded by HSV-1, bovine herpesvirus 1 (BHV-1), varicella-zoster virus, equine herpesvirus 1, and pseudorabies virus exhibit a nuclear localization, suggesting a role for these proteins in that compartment of the infected cell (3, 4, 9, 16-18, 23). An important characteristic of the UL47 proteins encoded by HSV-1 (hUL47) and BHV-1 (bUL47) is that they both undergo nucleocytoplasmic trafficking when expressed alone (4, 17, 18, 23). We and others previously identified nonclassical arginine-rich nuclear localization signals (NLSs) in the N termini of hUL47 and bUL47 (see Fig. 2A) (17, 23), and we went on to show that the NLS of hUL47 also functions as an RNA binding domain (5). In the case of bUL47, a leucine-rich nuclear export signal (NES) was first identified in the middle of the protein by Zheng and coworkers (see Fig. 2A, NES1) (23), suggesting that the bUL47 export signal is similar to the classical HIV-1 Rev protein NES, which uses the CRM1 receptor to exit the nucleus (6, 7, 15, 19). Subsequently, we identified a second, and in our hands more potent, NES at the N terminus of bUL47 that contained no hydrophobic residues and was resistant to treatment with the CRM1 inhibitor leptomycin B (LMB) (see Fig. 2A, NES2) (17). The export receptor for this second NES has yet to be identified.
FIG. 2.
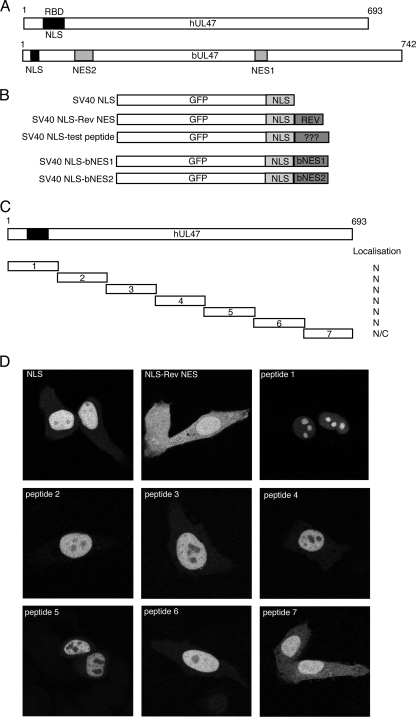
The C-terminal 100 amino acids of hUL47 can direct a nuclear protein into the cytoplasm. (A) Line diagram of hUL47 and its homologue from BHV-1, bUL47, showing previously identified NLS, RNA binding (RBD), and NES domains. (B) Line drawing of GFP fusion proteins used in this study. (C) Line drawing of hUL47 and the corresponding seven peptides that were fused to GFP-SV40-NLS. (D) Plasmids expressing the fusion proteins were transfected into Hep-2 cells grown on coverslips. Twenty hours later, the cells were fixed with paraformaldehyde, examined by confocal microscopy, and scored for relative nuclear/cytoplasmic localization.
To extend our understanding of how and why these proteins may shuttle between the nucleus and cytoplasm, we investigated the nature of hUL47 nuclear export. We first wished to determine if hUL47 shuttled between the nucleus and cytoplasm of HSV-1-infected cells. Vero cells grown on glass-bottom culture dishes (MatTek) were infected at a multiplicity of infection of 5 with recombinant HSV-1 (strain 17) expressing yellow fluorescent protein (YFP)-tagged hUL47, which was described previously and behaves as wild-type virus (3). Six hours later, at a time at which hUL47 is predominantly nuclear (as shown in our previous studies), the infected cells were examined in the temperature-controlled chamber of a Zeiss LSM 510 confocal microscope for the presence of nuclear YFP-hUL47 (Fig. 1A). To determine if this population of hUL47 shuttled between the nucleus and cytoplasm, fluorescence loss in photobleaching (FLIP) analysis was carried out. Cells were subjected to repeated photobleaching in a specific area of their cytoplasms (Fig. 1A) using 20 iterations of the 488-nm laser line of the LSM-510 at 50% power. Bleaching was repeated every 10 s, with images being acquired immediately after bleaching, for a total of 100 repetitions. Representative images from a typical FLIP experiment are presented in Fig. 1A, where it can be seen that the fluorescence intensity of the nucleus in the photobleached cell decreases with time compared to that of the nucleus of a control, unbleached cell. Quantification of the fluorescence intensity of these nuclei was carried out using LSM software (Zeiss), and relative intensity was plotted against time in the graph shown in Fig. 1C. To confirm that hUL47 nucleocytoplasmic shuttling occurred in the absence of other virus proteins, a similar FLIP experiment was carried out on Hep-2 cells that had been transfected 20 h earlier with a plasmid expressing green fluorescent protein (GFP)-tagged hUL47 (4). Although the hUL47 protein appears to be entirely nuclear (Fig. 1B), photobleaching of the relevant area in the cytoplasm resulted in a loss of nuclear fluorescence similar to that seen in infected cells (Fig. 1B and C).
FIG. 1.
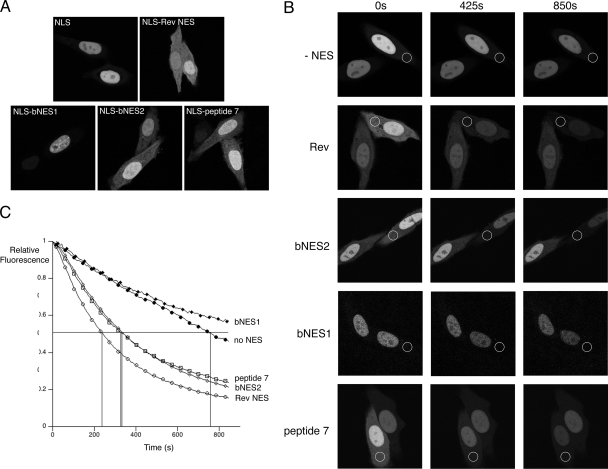
hUL47 undergoes nuclear export in HSV-1-infected cells. (A) Vero cells grown in a glass-bottom dish were infected with recombinant HSV-1 expressing YFP-UL47 at a multiplicity of infection of 5. Six hours later, the cells were examined with a Zeiss-LSM 510 confocal microscope, and individual cells were chosen for FLIP analysis. The 488-nm laser line was used to carry out sequential photobleaching events of the area indicated by the white circle. This area was exposed to 20 iterations of 50% laser power every 10 s for 100 repeats. An image of the field was acquired after each bleaching event to determine the loss of fluorescence in the nucleus of the cell. (B) Hep-2 cells were transfected with plasmid pMD10 expressing GFP-hUL47 and 20 h later were subjected to FLIP studies as described for panel A. (C) For both the bleached and unbleached cells, the fluorescence intensity of the nuclei from panels A and B was quantified for each time point using LSM software, and relative fluorescence was calculated and plotted against time using Kaleidagraph software.
To identify the NES in hUL47, we next investigated which region(s) of the protein was able to relocalize a nuclear protein into the cytoplasm of the cell. To do this, we first constructed a nuclear reporter that consisted of GFP fused to the simian virus 40 (SV40) NLS (Fig. 2B). We then fused the classical 10-residue NES from the human immunodeficiency virus type 1 (HIV-1) Rev protein downstream of the SV40 NLS to act as a positive control for nuclear export (Fig. 2B); to locate the hUL47 NES, seven peptides of hUL47 in approximately 100 amino acid blocks were fused in the same position (Fig. 2C). To assess the subcellular localization of these proteins, we transfected each construct into Hep-2 cells grown on coverslips in 12-well plates, as described previously (17). Twenty hours later, the cells were fixed for 20 min in 4% paraformaldehyde, coverslips were mounted in Vectashield (Vector Labs), and the localization of the GFP-tagged fusion proteins was examined by confocal microscopy. As expected, the GFP-SV40 NLS fusion protein was predominantly nuclear in Hep-2 cells (Fig. 2D), while the fusion containing the Rev NES was nuclear and cytoplasmic, indicative of export of this fusion protein from the nucleus to the cytoplasm (Fig. 2D). In the case of the hUL47 fusion proteins, five of the seven were predominantly nuclear and exhibited a localization pattern indistinguishable from that of the NLS alone (Fig. 2D, peptides 2 to 6). Notably, peptide 1 of hUL47 conferred a striking nucleolar localization on GFP, suggesting that this sequence, which contains the hUL47 NLS and RNA binding domain, may also contain a nucleolar targeting or retention signal (Fig. 2D, peptide 1). In contrast, the final 100 residues of hUL47 resulted in the appearance of the GFP-NLS protein in the cytoplasm of the cell (Fig. 2D, peptide 7), suggesting that the amino acids in peptide 7, residues 597 to 693 of hUL47, could behave in a manner similar to that of the Rev NES and direct a nuclear protein into the cytoplasm.
We next wished to confirm that peptide 7 truly functions as an NES and to compare the efficiency of export by this peptide to that of Rev and the two NESs previously characterized in bUL47 (NES1 and NES2 in Fig. 2B). We first compared the relative nuclear/cytoplasmic localization of these fusion proteins in live Hep-2 cells (Fig. 3A). Representative images show that the fusions of Rev, peptide 7, and bNES2 to GFP-NLS all result in a cytoplasmic distribution of the protein, with the Rev fusion protein being consistently more intense in the cytoplasm than the other two fusions (Fig. 3A). Interestingly, however, bNES1, the original NES to be identified in bUL47, appears to be mainly nuclear in this assay and cannot be distinguished from the reporter protein alone (Fig. 3A, compare NLS and NLS-bNES1). To determine if the relative cytoplasmic localizations of these proteins correlate with relative rates of nuclear export, we carried out FLIP analysis of all the proteins shown in Fig. 3A as described for Fig. 1 (Fig. 3B). Quantification of the relative fluorescence intensity of nuclei in bleached cells revealed that cells expressing the reporter alone showed a consistently slow loss of nuclear fluorescence over the course of the assay, which we interpret to be due to the passive diffusion of this small fusion protein from the nucleus to the cytoplasm (Fig. 3B and C). However, cells expressing the Rev NES fusion protein showed rapid and significant loss of nuclear fluorescence, with 50% of fluorescence lost in around 220 s, confirming the efficient nuclear export of this protein (Fig. 3B and C, compare no-NES results with Rev results). Cells expressing the hUL47 peptide 7 and bNES2 fusions also showed significant reduction in nuclear fluorescence, with their relative rate of export (50% loss occurring for both proteins after 350 s) approaching that of the Rev fusion protein (Fig. 3B and C). Finally, cells expressing the fusion protein containing the previously characterized LMB-sensitive NES in bUL47 (bNES1) showed a reduction in nuclear fluorescence during photobleaching that was similar to that observed for the reporter alone, suggesting that, in this assay at least, this NES is nonfunctional (Fig. 3B and C).
FIG. 3.
hUL47 contains a discrete NES at its extreme C terminus. (A) Hep-2 cells grown in glass-bottomed dishes were transfected with plasmids encoding GFP-NLS fused to the NES sequences from HIV-1 Rev, bUL47 (bNES1 and bNES2), and hUL47 (peptide 7). Representative images were acquired 20 h later using confocal microscopy. (B) Hep-2 cells transfected with the plasmids used for panel A were subjected to FLIP analysis as described for Fig. 1A, except that bleaching repeats were performed every 8 s. Photobleached areas are indicated by white circles. (C) The fluorescence intensity in the nuclei of bleached cells was quantified for each time point using LSM software, and relative fluorescence was calculated and plotted against time using Kaleidagraph software. The data are averages of three FLIP analyses for each fusion protein.
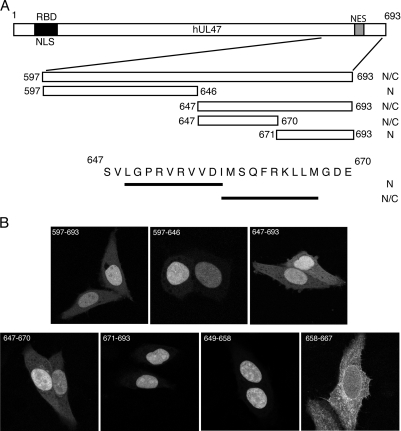
To further refine the NES sequence in hUL47, various subregions of peptide 7 were fused to the C terminus of the GFP-NLS protein (Fig. 4A). The localization of these fusion proteins was examined as before, revealing that the NES of hUL47 was located between residues 647 and 670 (Fig. 4B). Analysis of this peptide revealed two potential hydrophobicity-rich matches for the consensus CRM1-mediated NES (10) (Fig. 4A). To determine if either or both of these sequences were capable of functioning as a discrete NES, each consensus peptide was fused to the GFP-NLS protein and analyzed as before. Fluorescence microscopy demonstrated that while peptide 649-658 was unable to relocalize the GFP-NLS fusion protein, peptide 658-667 very efficiently targeted it to the cytoplasm of the expressing cell (Fig. 4B).
FIG. 4.
The hUL47 NES is a 10-residue hydrophobic peptide. (A) Line drawing of the region of hUL47 covered by peptide 7, together with the smaller peptides used to refine the hUL47 NES. (B) Hep-2 cells grown on coverslips were transfected with plasmids expressing GFP-NLS fused to the regions indicated in panel A. Representative images were acquired 20 h later using confocal microscopy and scored for relative nuclear/cytoplasmic localization.
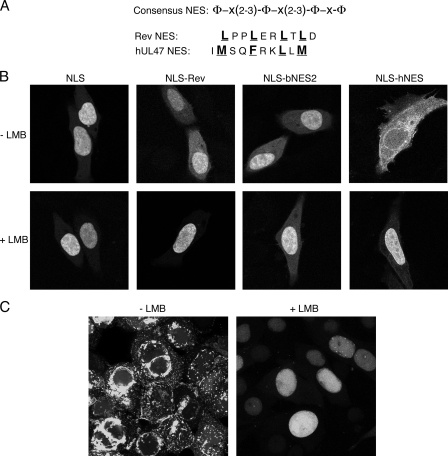
This 10-residue peptide from the C terminus of hUL47 represents a fully transferable NES (hNES) that is similar to the well-characterized 10-residue Rev NES (Fig. 5A). Because the hUL47 NES is a consensus CRM1-dependent NES, we next determined the effect of the CRM1 inhibitor LMB on the localization of the hNES fusion protein in comparison to the other NES fusions. Hep-2 cells expressing these proteins were treated with LMB for 3 h prior to fixation. Fluorescence microscopy revealed that, as expected, LMB had no effect on the localization of the reporter lacking an NES but efficiently inhibited the cytoplasmic localization of GFP-NLS-Rev (Fig. 5B, compare NLS and NLS-Rev). By contrast, LMB had no effect on the cytoplasmic localization of the bNES2 fusion protein, confirming our previous results showing that this NES is resistant to the drug (Fig. 5B). Notably, cytoplasmic localization of the hNES fusion protein was abrogated in the presence of LMB (Fig. 4D), strongly indicating that nuclear export by this hydrophobic NES is mediated by the CRM1 receptor. Finally, we tested the effect of LMB on the localization of hUL47 in HSV-1-infected cells. Vero cells were infected with HSV-1 expressing YFP-tagged hUL47, and LMB was added 8 h later. After a further 8 h, representative images of infected cells were acquired from cells incubated in the absence or presence of LMB (Fig. 5C). As described previously (3), hUL47 localizes in clusters through the cytoplasm and at the cytoplasmic membrane of infected cells grown in the absence of LMB (Fig. 5C). In contrast, in the presence of LMB, hUL47 remains almost entirely nuclear (Fig. 5C). Although we cannot rule out the possibility that this effect on hUL47 localization is an indirect one, these results strongly suggest that hUL47 utilizes CRM-1 to move from the nucleus to the cytoplasm of infected cells.
FIG. 5.
The hUL47 NES is sensitive to LMB. (A) Alignment of the HIV-1 Rev and hUL47 NESs, showing the previously defined consensus sequence for hydrophobic residue-rich export signals. (B) Hep-2 cells grown on coverslips were transfected in duplicate with plasmids expressing GFP-NLS alone or fused to the Rev NES, bUL47 bNES2, and hUL47 hNES. Twenty hours later, LMB was added to one set of transfections at a concentration of 2.5 ng/μl, and the cells were incubated for a further 3 h. All cells were then fixed in 4% paraformaldehyde, and representative images were acquired using confocal microscopy. (C) Vero cells grown in glass-bottom dishes were infected with HSV-1 expressing YFP-tagged hUL47 at a multiplicity of infection of 5. Eight hours later, LMB was added to one dish at a concentration of 2.5 ng/μl, and the infected cells were left for a further 8 h. Cells were examined live using confocal microscopy.
In this study, we have shown for the first time that hUL47 undergoes nuclear export in infected cells in the same way as its BHV-1 homologue, bUL47 (18). However, whereas the predominant NES in bUL47 is N terminal and resistant to LMB (17), the hUL47 NES is C terminal and sensitive to LMB, suggesting that it functions through CRM1. It should be noted that it is possible, though unlikely, that hUL47 has an additional NES(s) present at one of the junctions between our mapping peptides, similar to the situation in bUL47. Further studies will be required to investigate this possibility.
These results extend our previous findings that hUL47 contains an NLS and RNA binding domain at its N terminus and binds viral RNA during infection (4, 5), providing further credence for the idea that UL47 has a role in the nuclear export of RNA. Although HSV-1 encodes ICP27, a well-studied RNA export protein, there is some evidence that ICP27 regulates the export of only a subset of viral transcripts (13). Furthermore, while export of ICP27 is mediated by the TAP mRNA export pathway (2, 8, 12), the export of a range of late mRNAs is known to be sensitive to LMB (14). Hence, it is likely that other LMB-sensitive viral factors are involved in the export of a subset of late mRNAs. Because of its combination of properties, we suggest that hUL47 is a prime candidate for such a viral factor.
Acknowledgments
We are grateful to Michael Hollinshead for assistance with confocal microscopy.
This work was funded by the BBSRC.
Footnotes
Published ahead of print on 20 August 2008.
REFERENCES
- 1.Carpenter, D. E., and V. Misra. 1991. The most abundant protein in bovine herpes 1 virions is a homologue of herpes simplex virus type 1 UL47. J. Gen. Virol. 723077-3084. [DOI] [PubMed] [Google Scholar]
- 2.Chen, I. H., L. Li, L. Silva, and R. M. Sandri-Goldin. 2005. ICP27 recruits Aly/REF but not TAP/NXF1 to herpes simplex virus type 1 transcription sites although TAP/NXF1 is required for ICP27 export. J. Virol. 793949-3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donnelly, M., and G. Elliott. 2001. Fluorescent tagging of herpes simplex virus tegument protein VP13/14 in virus infection. J. Virol. 752575-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donnelly, M., and G. Elliott. 2001. Nuclear localization and shuttling of herpes simplex virus tegument protein VP13/14. J. Virol. 752566-2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donnelly, M., J. Verhagen, and G. Elliott. 2007. RNA binding by the herpes simplex virus type 1 nucleocytoplasmic shuttling protein UL47 is mediated by an N-terminal arginine-rich domain that also functions as its nuclear localization signal. J. Virol. 812283-2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fischer, U., J. Huber, W. C. Boelens, I. W. Mattaj, and R. Luhrmann. 1995. The HIV-1 Rev activation domain is a nuclear export signal that accesses an export pathway used by specific cellular RNAs. Cell 82475-483. [DOI] [PubMed] [Google Scholar]
- 7.Fornerod, M., M. Ohno, M. Yoshida, and I. W. Mattaj. 1997. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell 901051-1060. [DOI] [PubMed] [Google Scholar]
- 8.Koffa, M. D., J. B. Clements, E. Izaurralde, S. Wadd, S. A. Wilson, I. W. Mattaj, and S. Kuersten. 2001. Herpes simplex virus ICP27 protein provides viral mRNAs with access to the cellular mRNA export pathway. EMBO J. 205769-5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kopp, M., B. G. Klupp, H. Granzow, W. Fuchs, and T. C. Mettenleiter. 2002. Identification and characterization of the pseudorabies virus tegument proteins UL46 and UL47: role for UL47 in virion morphogenesis in the cytoplasm. J. Virol. 768820-8833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kutay, U., and S. Guttinger. 2005. Leucine-rich nuclear-export signals: born to be weak. Trends Cell Biol. 15121-124. [DOI] [PubMed] [Google Scholar]
- 11.McLean, G., F. Rixon, N. Langeland, L. Haarr, and H. Marsden. 1990. Identification and characterization of the virion protein products of herpes simplex virus type 1 gene UL47. J. Gen. Virol. 712953-2960. [DOI] [PubMed] [Google Scholar]
- 12.Mears, W. E., and S. A. Rice. 1998. The herpes simplex virus immediate-early protein ICP27 shuttles between nucleus and cytoplasm. Virology 242128-137. [DOI] [PubMed] [Google Scholar]
- 13.Pearson, A., D. M. Knipe, and D. M. Coen. 2004. ICP27 selectively regulates the cytoplasmic localization of a subset of viral transcripts in herpes simplex virus type 1-infected cells. J. Virol. 7823-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soliman, T. M., and S. J. Silverstein. 2000. Herpesvirus mRNAs are sorted for export via Crm1-dependent and -independent pathways. J. Virol. 742814-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stade, K., C. S. Ford, C. Guthrie, and K. Weis. 1997. Exportin 1 (Crm1p) is an essential nuclear export factor. Cell 901041-1050. [DOI] [PubMed] [Google Scholar]
- 16.van Drunen Littel-van den Hurk, S., S. Garzon, J. V. van den Hurk, L. A. Babiuk, and P. Tijssen. 1995. The role of the major tegument protein VP8 of bovine herpesvirus-1 in infection and immunity. Virology 206413-425. [DOI] [PubMed] [Google Scholar]
- 17.Verhagen, J., M. Donnelly, and G. Elliott. 2006. Characterization of a novel transferable CRM-1-independent nuclear export signal in a herpesvirus tegument protein that shuttles between the nucleus and cytoplasm. J. Virol. 8010021-10035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verhagen, J., I. Hutchinson, and G. Elliott. 2006. Nucleocytoplasmic shuttling of bovine herpesvirus 1 UL47 protein in infected cells. J. Virol. 801059-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wen, W., J. L. Meinkoth, R. Y. Tsien, and S. S. Taylor. 1995. Identification of a signal for rapid export of proteins from the nucleus. Cell 82463-473. [DOI] [PubMed] [Google Scholar]
- 20.Whittaker, G. R., M. P. Riggio, I. W. Halliburton, R. A. Killington, G. P. Allen, and D. M. Meredith. 1991. Antigenic and protein sequence homology between VP13/14, a herpes simplex virus type 1 tegument protein, and gp10, a glycoprotein of equine herpesvirus 1 and 4. J. Virol. 652320-2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang, Y., and J. L. McKnight. 1993. Herpes simplex virus type 1 UL46 and UL47 deletion mutants lack VP11 and VP12 or VP13 and VP14, respectively, and exhibit altered viral thymidine kinase expression. J. Virol. 671482-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang, Y., D. A. Sirko, and J. L. McKnight. 1991. Role of herpes simplex virus type 1 UL46 and UL47 in alpha TIF-mediated transcriptional induction: characterization of three viral deletion mutants. J. Virol. 65829-841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng, C., R. Brownlie, L. A. Babiuk, and S. van Drunen Littel-van den Hurk. 2004. Characterization of nuclear localization and export signals of the major tegument protein VP8 of bovine herpesvirus-1. Virology 324327-339. [DOI] [PubMed] [Google Scholar]