Abstract
The latency-related (LR) RNA encoded by bovine herpesvirus 1 is abundantly expressed in the trigeminal ganglia of latently infected calves. Expression of LR proteins is necessary for reactivation from latency and the protection of infected neurons from apoptosis. In this study, we demonstrated that an LR-encoded protein, open reading frame 2 (ORF-2), or ORF-2 fusion proteins encoded by alternatively spliced LR transcripts inhibit cold shock or Fas ligand-induced apoptosis in mouse neuroblastoma (neuro-2A) cells. Frameshift mutants of ORF-2 do not inhibit apoptosis, which suggests that protein expression, not LR RNA expression, mediates the antiapoptotic activity of the LR gene in transfected neuro-2A cells.
Bovine herpesvirus 1 (BHV-1) is a Herpesviridae family member that causes significant economical losses to the cattle industry (47). The primary sites for BHV-1 latency are the sensory neurons within the trigeminal ganglia (TG). Viral gene expression (43) and infectious virus (22) can be detected in TG from 1 to 6 days after infection but are then extinguished (establishment of latency). Administration of dexamethasone to calves or rabbits latently infected with BHV-1 leads to reproducible reactivation from latency, as judged by virus shedding from ocular or nasal cavities (22, 27-30, 42).
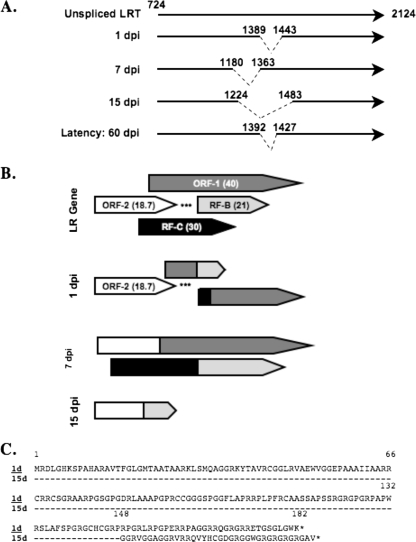
The latency-related (LR) gene has two open reading frames (ORFs), ORF-1 and ORF-2, and two reading frames without an initial ATG sequence, RF-B and RF-C (Fig. 1B). An antibody directed against ORF-2 recognizes a protein encoded by the LR gene (20, 23, 24). LR gene products inhibit cell proliferation, bICP0 RNA expression (5, 13, 44), and apoptosis (9). A mutant BHV-1 strain with three stop codons at the N terminus of ORF-2 does not express ORF-2 or RF-C (24) or reactivate from latency (22), which suggests that expression of LR proteins regulates the latency reactivation cycle.
FIG. 1.
Schematic of LR alternatively spliced transcripts and LR ORFs. (A) Schematic of LR transcript (LRT) and the various alternatively spliced LR transcripts previously identified (11). The nucleotide position of the start site in a productive infection, the 3′ end of LR transcription (12, 20), and the respective splice junction sites are provided for reference. (B) Schematic of ORFs in the LR gene and the effect of splicing on the organization of these ORFs. ORF-1 and ORF-2 are the largest protein-coding domains present in the LR gene (33). Reading frames B and C (RF-B and RF-C) each contain an ORF that lacks an initiating Met. The numbers in parentheses are the approximate sizes of the ORFs present in the LR gene. The putative ORFs present in alternatively spliced LR transcripts are shown as comparisons to those in the LR gene. (C) Amino acid sequence of ORF-2 and the ORF-2 fusion protein encoded by cDNA at 15 dpi. The asterisks (*) in panel B denote the positions of stop codons that are in-frame with the respective ORF. The dashed lines (-) denote identical amino acids shared by cDNA at 1 and 15 dpi.
The LR mutant virus induces higher levels of apoptosis in the TG neurons of infected calves during the establishment of latency (35), and plasmids with the same stop codon mutations exhibit little or no antiapoptotic activity in transfected cells (9). When the LR gene is inserted into a herpes simplex virus type 1 latency-associated transcript-null mutant, activation of caspase 3 or caspase 9 is reduced during productive infection (18). The antiapoptotic functions of the LR gene are not required during productive infection because the LR mutant virus and wild-type BHV-1 inhibit UV-induced apoptosis with similar levels of efficiency during productive infection (14). LR RNA is alternatively spliced in TG during acute infection versus during latency (12) (Fig. 1 A and B), which complicates the identification of LR-coding sequences that have antiapoptotic activities. We hypothesized that ORF-2 may have antiapoptotic functions because the LR mutant virus contains stop codons adjacent to ORF-2 and most of the ORF-2 coding sequences are retained in all LR transcripts (Fig. 1B).
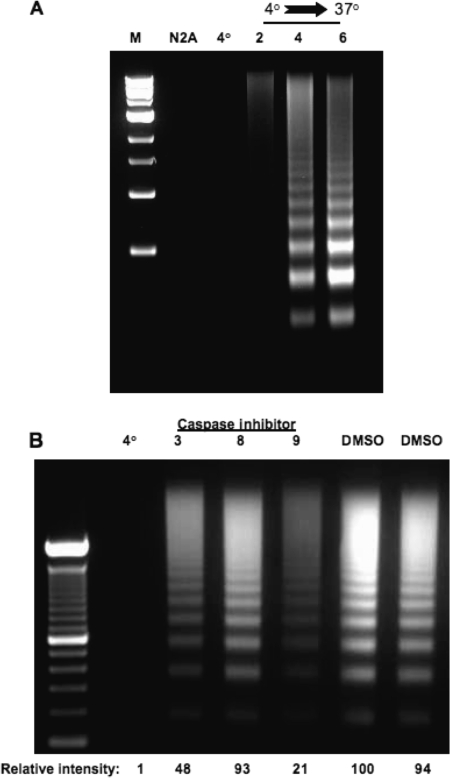
ORF-2 sequences encoded by cDNA 1 or 15 days postinfection (dpi) were synthesized by GenScript Corporation (Piscataway, NJ). The entire ORF-2 protein is present in cDNA at 1 dpi, whereas at 7 or 15 dpi, cDNA contains ORF-2 fused to ORF-1 or RF-B, respectively (Fig. 1C). Plasmids expressing these sequences were transfected into mouse neuroblastoma cells (neuro-2A), and their antiapoptotic activities were analyzed by cold shock-induced apoptosis. Although several previous studies demonstrated that cold shock treatment induces apoptosis in various cell types (7, 15, 16, 32, 39, 41), it was necessary to prove that neuro-2A cells undergo apoptosis following cold shock. Neuro-2A cells (Fig. 2A, lane N2A) or neuro-2A cells placed on ice for 2 h (Fig. 2, lane 4) contained no detectable DNA laddering. When cells were returned to 37°C for 4 or 6 h, extensive DNA laddering was observed, demonstrating that apoptosis occurred (37, 51).
FIG. 2.
Analysis of cold shock-induced apoptosis in neuro-2A cells. (A) Neuro-2A cells were transfected with the designated plasmids, using TransIT neural transfection reagent as described by the manufacturer (Mirus, Madison, WI). Approximately 75% of the cells were transfected when this protocol was performed with a green fluorescent protein expression plasmid. Approximately 24 h prior to transfection, cells were plated in complete growth medium at a density of 3 × 105 cells per 60-mm2 plastic dish, which was 50% to 70% confluent the following day. After being transfected for 24 h, one-half of the cells were reseeded into a T25 plastic flask and incubated in a humidified 5% CO2 atmosphere at 37°C for 24 h. Normal Earle's minimal essential medium was replaced by fresh medium containing 2% serum. After 12 h of incubation, cells containing 2% serum were incubated on ice (4°C) for 60 min with the caps of the flasks sealed by Parafilm. At the end of 2 h on ice, the caps were loosened and the flasks were incubated at 37°C for 2, 4, or 6 h. Cells were collected, and apoptotic DNA was prepared as previously described (14, 25). Apoptotic DNA from 3 × 105 cells was loaded onto a 2% agarose gel. Following staining with ethidium bromide, a photograph of the gel was taken. These results are representative of at least 10 independent experiments. (B) Neuro-2A cells were treated with 25 μM of a caspase 3 inhibitor (DEVD-CHO, catalog number 235422; Calbiochem), caspase 8 inhibitor (IETD-CHO, catalog number 218773; Calbiochem), or a caspase 9 inhibitor (LEHD-CHO, catalog number 218776; Calbiochem) for 12 h prior to cold shock induction of apoptosis as described for panel A. All caspase inhibitors are cell permeable. Apoptotic DNA was analyzed on an agarose gel as for panel A. The relative amounts of apoptotic DNA in the lanes of panel B were measured using a Bio-Rad molecular imager FX. The results are representative of three independent experiments. DMSO, dimethyl sulfoxide.
To further examine cold shock-induced apoptosis, neuro-2A cells were treated with specific caspase inhibitors. Caspase 8 and caspase 9 regulate the two major apoptotic pathways, the extrinsic and intrinsic pathways, respectively (45, 49), and thus were examined. A caspase 3 inhibitor was also chosen because the intrinsic or extrinsic pathway activates caspase 3 (8, 49). Inhibiting caspase 9 reduced the levels of cold shock-induced apoptosis nearly fivefold relative to that for neuro-2A cells treated with the solvent dimethyl sulfoxide (Fig. 2B). The caspase 3 inhibitor also reduced apoptosis approximately twofold, which is consistent with the findings of a previous independent study (7). Conversely, the caspase 8 inhibitor had no effect on cold shock-induced apoptosis in neuro-2A cells (Fig. 2B).
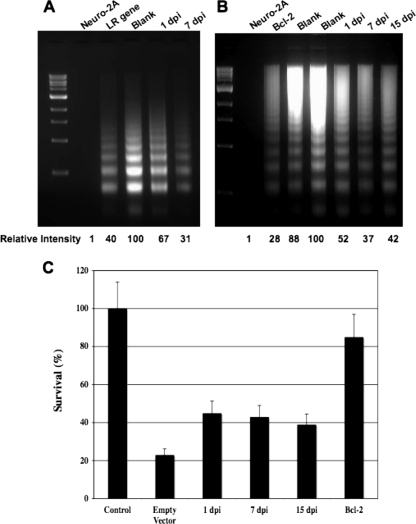
Experiments were then performed to determine if the LR gene or plasmids expressing sequences derived from the respective cDNAs had an effect on cold shock-induced apoptosis. Neuro-2A cells were transfected with the designated plasmids as described in the legend to Fig. 2. Cold shock-induced apoptosis was performed at approximately 36 h after transfection, and the level of apoptotic DNA was measured by agarose gel electrophoresis. Compared to that in neuro-2A cells transfected with the blank expression vector, DNA laddering was reduced more than twofold in cells transfected with the LR gene (Fig. 3A). When neuro-2A cells were transfected with an expression plasmid containing ORF-2 encoded by cDNA at 1, 7, or 15 dpi, the amount of apoptosis was also reduced (Fig. 3A and B). In most experiments, the cDNA from 1 dpi did not inhibit apoptosis as efficiently as the intact LR gene. The antiapoptotic gene Bcl-2 reduced cold shock-induced apoptosis (Fig. 3B) more efficiently than the LR gene or the ORF-2 clones. The LR gene or ORF-2-expressing plasmids also increased the number of surviving cells and reduced the percentage of cells containing condensed chromatin after cold shock-induced apoptosis (data not shown), which is consistent with the DNA-laddering results. These results suggest that the LR gene and ORF-2 are capable of inhibiting the intrinsic pathway of apoptosis, because cold shock-induced apoptosis was effectively inhibited by a caspase 9 inhibitor (Fig. 2B). Furthermore, these results were consistent with previous findings that demonstrated that LR gene products can inhibit caspase 3 or caspase 9 activation (18).
FIG. 3.
The LR gene and ORF-2, encoded by the LR gene, inhibit cold shock-induced apoptosis in neuro-2A cells. (A) Neuro-2A cells were transfected with cytomegalovirus (CMV) expression plasmids (6 μg DNA) containing the LR gene, ORF-2 sequences in cDNA at 1 dpi, or cDNA at 7 dpi containing ORF-2 fused to a portion of ORF-1. Cold shock-induced apoptosis, collection of apoptotic DNA, and analysis of apoptotic DNA were performed as described for Fig. 2. (B) Neuro-2A cells were transfected with CMV expression plasmids (6 μg DNA) containing the Bcl-2 gene, ORF-2 sequences in cDNA at 1 and 7 dpi, or the ORF-2/RF-B sequences derived from cDNA at 15 dpi. Following cold shock-induced apoptosis, apoptotic DNA was collected and analyzed. The results shown in panels A and B are representative of at least 10 independent experiments. (C) Neuro-2A cells were cotransfected with a plasmid that overexpresses the Fas ligand (FasL; 2 μg DNA), the designated LR gene constructs (3 μg DNA), and a β-Gal expression vector (pCMV-β-Gal; 1 μg DNA). The FasL expression vector was purchased from GeneCopoeica, Germantown, MD. The levels of cell survival were determined as previously described (9, 10, 21, 26, 38). In brief, the number of β-Gal+ cells was quantified, and the number in the control lane was set at 100% survival. The control lane was transfected with pCMV-β-Gal (1 μg DNA) and an expression vector (pcDNA3.1, 5 μg DNA). The empty vector lane (control for the efficiency of FasL-induced cell death) contained pCMV-β-Gal (1 μg DNA), the FasL plasmid (2 μg DNA), and 5 μg of the empty expression vector. These results are representative of three individual experiments.
To test whether the LR gene has the potential to inhibit the extrinsic pathway of apoptosis, neuro-2A cells were cotransfected with a plasmid that overexpresses the Fas ligand (FasL), LR gene constructs, and a β-galactosidase (β-Gal) expression vector (pCMV-β-Gal). This sensitive assay has been used previously to identify genes that have antiapoptotic activity (9, 10, 21, 26, 38). In brief, the assay is based on the observation that when a proapoptotic gene (in this case the plasmid expressing FasL) is transfected into cells, the number of β-Gal-positive (β-Gal+) cells is reduced compared to that of cells transfected with an empty expression vector. When an antiapoptotic gene (in this case one of the plasmids expressing the LR gene or ORF-2 derived from an LR cDNA) is cotransfected with the FasL expression plasmid, the number of β-Gal+ cells is reduced if that plasmid has antiapoptotic activity. As expected, overexpression of the proapoptotic FasL gene consistently reduced the number of β-Gal+ neuro-2A cells relative to that of control cells transfected with an empty vector (pcDNA3.1−) and the β-Gal expression vector. Cotransfection of cells with the FasL plasmid, the β-Gal plasmid, and expression plasmids from cDNA at 1, 7, or 15 dpi also consistently reduced the number of β-Gal+ cells relative to that of cells transfected with just the FasL and β-Gal plasmids (Fig. 3C). When Bcl-2 was cotransfected with the FasL plasmid and the β-Gal plasmid, the number of β-Gal+ cells was also reduced, which was expected. In summary, this study provided evidence that LR ORF-2 expression plasmids inhibit FasL-induced apoptosis. Furthermore, this study indicated that Bcl-2 inhibited apoptosis more efficiently than any of the LR gene constructs, which was consistent with the findings of previous studies using cold shock-induced apoptosis (Fig. 3A and B).
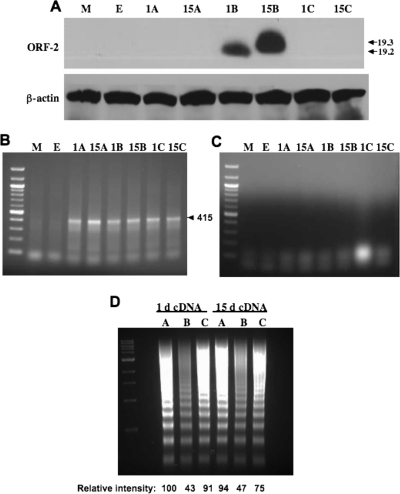
To test whether ORF-2 expression was necessary for inhibiting apoptosis, the ORF-2 fragments were cloned into pCMV-Tag2 vectors in all three reading frames (A to C). The LR expression plasmids used for Fig. 3 were cloned into pcDNA3.1−, and thus it was expected that ORF-2 would be expressed. The ORF-2 clones constructed in pCMV-Tag2 vectors were transfected into neuro-2A cells, and the expression of a Flag fusion protein was examined by Western blot analysis by using a monoclonal antibody directed against Flag. For constructs with cDNA at 1 or 15 dpi, a Flag fusion protein migrating between 19 and 20 kDa was detected at 40 h after transfection in cells transfected with pCMV-Tag2B (Fig. 4A). The Flag-tagged ORF-2 protein encoded by cDNA at 1 dpi was predicted to have a mass of 18.7 kDa and that encoded by cDNA at 15 dpi to have a mass of 18.8 kDa. The difference in size was expected because the ORF-2 protein encoded by cDNA at 1 dpi is 182 amino acids, whereas the ORF-2 fusion protein encoded by cDNA at 15 dpi is 185 amino acids (Fig. 1C). A Flag fusion protein was not detected in cells transfected with pCMV-Tag2A or pCMV-Tag2C constructs, regardless of whether ORF-2 was encoded by cDNA at 1 or 15 dpi. As expected, all pCMV-Tag2 constructs expressed similar levels of LR RNA (Fig. 4B). Omitting reverse transcriptase from the mixture for reverse transcription (RT)-PCR eliminated the LR-specific band, which indicated that the RNA preparations were not contaminated with DNA (Fig. 4C).
FIG. 4.
Construction of ORF-2 frameshift mutations in PCMV-Tag2. ORF-2 sequences derived from cDNA at 1 or 15 dpi were cloned into a unique BamHI-HindIII site, pCMV-Tag2A, pCMV-Tag2B, or pCMV-Tag2C (Stratagene, La Jolla, CA), in order to obtain the respective ORF-2 sequences in all three reading frames. ORF-2 sequences from cDNA at 1 or 15 dpi are in-frame with the Flag epitope when cloned into plasmid pCMV-Tag2B. The respective plasmids were transfected into neuro-2A cells, and at 40 h after transfection, a cell lysate was prepared. Western blot analysis was performed, using 1,000 μg protein. (A) ORF-2 protein expression was detected by using a Flag-specific monoclonal antibody (top). β-actin protein levels were determined to ensure that similar levels of protein were loaded for sodium dodecyl sulfate-12% polyacrylamide gel electrophoresis (bottom). (B) Neuro-2A cells were transfected with the respective ORF-2 expression plasmids, and total RNA was prepared at 40 h after transfection. RT-PCR was performed to demonstrate that similar levels of LR RNA were expressed regardless of the reading frame ORF-2. The primers used to amplify a 415-bp fragment from ORF-2 were 5′-GGATCCATGCGCGACCTGGGCCATAAAAGC-3′ and (reverse primer) 5′-GAGAGAAGGCCAGCGAGCGCCATGGCGC-3′. PCR products were analyzed on a 2% Tris-borate-EDTA agarose gel. (C) RT-PCRs performed without reverse transcriptase verified that the RNA samples were not contaminated with plasmid DNA. Lane M, mock-transfected cells; lane E, neuro-2A cells transfected with the empty PCMV-Tag2 plasmid; lanes 1A to C, neuro-2A cells transfected with pCMV-Tag2A, -2B, or -2C containing ORF-2 sequences from cDNA at 1 dpi; lanes 15A to C, neuro-2A cells transfected with pCMV-Tag2A, -2B, or -2C containing ORF-2 sequences from cDNA at 15 dpi. (D) The respective ORF-2 expression clones were transfected into neuro-2A cells, and cold shock-induced apoptosis was performed as for Fig. 2. Lanes 1A to C, neuro-2A cells transfected with pCMV-Tag2A, -2B, or -2C containing ORF-2 derived from cDNA at 1 dpi; lanes A to C, neuro-2A cells transfected with pCMV-Tag2A, -2B, or -2C containing ORF-2 derived from cDNA at 1 or 15 dpi. The relative intensity of apoptotic DNA was measured by using a Bio-Rad molecular imager FX, and the numbers were normalized relative to the value for ORF-2 from cDNA at 1 dpi cloned into pCMV-Tag2A. These results are representative of those of three independent experiments.
The pCMV-Tag2B constructs that contained the coding sequences of ORF-2 encoded by cDNA at 1 or 15 dpi reduced the levels of DNA laddering in transiently transfected neuro-2A cells after cold shock-induced apoptosis (Fig. 4D, lanes 1B and 15B). In contrast, the same fragments cloned into pCMV-Tag2A or pCMV-Tag2C had higher levels of DNA laddering, which was similar to that of empty pCMV-Tag2 vectors (data not shown). In summary, this study suggested that expression of the ORF-2 protein, not LR RNA, was sufficient and necessary for inhibiting cold shock-induced apoptosis in neuro-2A cells.
The results from this study suggest that expression of ORF-2 or an ORF-2 fusion protein inhibits the intrinsic or extrinsic pathway of apoptosis. The ORF-2 sequences encoded by cDNA at 1, 7, or 15 dpi inhibited cold shock-induced apoptosis with a level of efficiency similar to that of the entire LR gene (Fig. 3A and B). A two-hybrid screen indicated that an ORF-2/ORF-1 fusion protein encoded by cDNA at 7 dpi has the potential to interact with two proteins that can induce apoptosis (Bid and Cdc42) (36). Bid links the extrinsic pathway of apoptosis to the intrinsic pathway, in part because caspase 8 cleaves Bid (34). Truncated Bid then interacts with mitochondria, resulting in the release of cytochrome c and Smac/Diablo (49). Bid can also be cleaved by granzyme B (3, 4, 40), suggesting that the LR gene can impair cytotoxic-T-lymphocyte-induced neuronal death. Neuronal stress, including the removal of nerve growth factor from neuronal cultures (31, 52), induces the activity of Cdc42, a Rho GTPase family member, and apoptosis (17, 31, 52). Dexamethasone is a potent stressor and induces reactivation from latency (1, 2, 6, 19, 29, 42, 46, 50), suggesting that Cdc42 is activated in TG. It will be of interest to test whether ORF-2 alone interacts with Bid or Cdc42. Conversely, ORF-2 may interact with and inhibit a novel cellular protein that activates the intrinsic and extrinsic pathways of apoptosis.
Cold shock may have relevance to BHV-1-induced reactivation from latency because cold stress can induce recurrent herpetic keratitis in squirrel monkeys (48). In the context of the BHV-1 latency reactivation cycle, we suggest that the antiapoptotic functions of the LR gene promote survival of infected neurons that can support reactivation from latency. Although the LR mutant virus establishes latency in a subset of neurons, these neurons do not support dexamethasone-induced reaction from latency, as judged by the shedding of infectious virus (22, 35). Since LR RNA levels are reduced dramatically during dexamethasone-induced reactivation from latency (42), the ability of the LR gene to inhibit apoptosis may be more important during the establishment and maintenance of latency than during reactivation from latency.
Acknowledgments
This study was supported by two USDA grants (2005-01554 and 2006-01627). In addition, a grant from the NIAID (R21AI069176) and a PHS grant (1P20RR15635) to the Nebraska Center for Virology have also supported the laboratory of C.J.
Footnotes
Published ahead of print on 20 August 2008.
REFERENCES
- 1.Ackermann, M., E. Peterhans, and R. Wyler. 1982. DNA of bovine herpesvirus type 1 in the trigeminal ganglia of latently infected calves. Am. J. Vet. Res. 4336-40. [PubMed] [Google Scholar]
- 2.Ackermann, M., and R. Wyler. 1984. The DNA of an IPV strain of bovid herpesvirus 1 in sacral ganglia during latency after intravaginal infection. Vet. Microbiol. 953-63. [DOI] [PubMed] [Google Scholar]
- 3.Alimonti, J. B., L. Shi, P. K. Baijal, and A. H. Greenberg. 2001. Granzyme B induces BID-mediated cytochrome c release and mitochondrial permeability transition. J. Biol. Chem. 2766974-6982. [DOI] [PubMed] [Google Scholar]
- 4.Barry, M., J. A. Heibein, M. J. Pinkoski, S. F. Lee, R. W. Moyer, D. R. Green, and R. C. Bleackley. 2000. Granzyme B short-circuits the need for caspase 8 activity during granule-mediated cytotoxic T-lymphocyte killing by directly cleaving Bid. Mol. Cell. Biol. 203781-3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bratanich, A. C., N. D. Hanson, and C. Jones. 1992. The latency-related gene of bovine herpesvirus 1 inhibits the activity of immediate-early transcription unit 1. Virology 191988-991. [DOI] [PubMed] [Google Scholar]
- 6.Brown, G. A., and H. J. Field. 1990. Experimental reactivation of bovine herpesvirus 1 (BHV-1) by means of corticosteroids in an intranasal rabbit model. Arch. Virol. 11281-101. [DOI] [PubMed] [Google Scholar]
- 7.Carpenter, D., C. Hsiang, L. Jin, N. Osorio, L. BenMohamed, C. Jones, and S. L. Wechsler. 2007. Stable cell lines expressing high levels of the herpes simplex virus type 1 LAT are refractory to caspase 3 activation and DNA laddering following cold shock induced apoptosis. Virology 36912-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang, H. Y., and X. Yang. 2000. Proteases for cell suicide: functions and regulation of caspases. Microbiol. Mol. Biol. Rev. 64821-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ciacci-Zanella, J., M. Stone, G. Henderson, and C. Jones. 1999. The latency-related gene of bovine herpesvirus 1 inhibits programmed cell death. J. Virol. 739734-9740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ciacci-Zanella, J. R., and C. Jones. 1999. Fumonisin B1, a mycotoxin contaminant of cereal grains, and inducer of apoptosis via the tumour necrosis factor pathway and caspase activation. Food Chem. Toxicol. 37703-712. [DOI] [PubMed] [Google Scholar]
- 11.Devireddy, L., Y. Zhang, and C. Jones. 2003. Cloning and initial characterization of an alternatively spliced transcript encoded by the bovine herpes virus 1 latency related gene. J. Neurovirol. 9612-622. [DOI] [PubMed] [Google Scholar]
- 12.Devireddy, L. R., and C. Jones. 1998. Alternative splicing of the latency-related transcript of bovine herpesvirus 1 yields RNAs containing unique open reading frames. J. Virol. 727294-7301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geiser, V., M. Inman, Y. Zhang, and C. Jones. 2002. The latency related gene of bovine herpes virus 1 can inhibit the ability of bICP0 to activate productive infection. J. Gen. Virol. 832965-2971. [DOI] [PubMed] [Google Scholar]
- 14.Geiser, V., S. Rose, and C. Jones. 2007. Bovine herpesvirus type 1 induces cell death by a cell-type-dependent fashion. Mol. Pathol. 44459-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gregory, C. D., and A. E. Milner. 2006. Regulation of cell survival in Burkitt lymphoma: implications from studies of apoptosis following cold-shock treatment. Int. J. Cancer 57419-426. [DOI] [PubMed] [Google Scholar]
- 16.Guedez, L., W. G. Stetler-Stevenson, L. Wolff, J. Wang, P. Fukushima, A. Mansoor, and M. Stetler-Stevenson. 1998. In vitro suppression of programmed cell death of B cells by tissue inhibitor of metalloproteinases-1. J. Clin. Investig. 1022002-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harwood, A., and V. M. M. Braga. 2003. Cdc42 and GSK-3: signals at the crossroads. Nat. Cell Biol. 5275-277. [DOI] [PubMed] [Google Scholar]
- 18.Henderson, G., G.-C. Perng, A. Nesburn, S. Wechsler, and C. Jones. 2004. The latency related gene of bovine herpesvirus 1 can suppress caspase 3 and caspase 9 during productive infection. J. Neurovirol. 1064-70. [DOI] [PubMed] [Google Scholar]
- 19.Homan, E. J., and B. C. Easterday. 1983. Experimental latent and recrudescent bovine herpesvirus-1 infections in calves. Am. J. Vet. Res. 44309-313. [PubMed] [Google Scholar]
- 20.Hossain, A., L. M. Schang, and C. Jones. 1995. Identification of gene products encoded by the latency-related gene of bovine herpesvirus 1. J. Virol. 695345-5352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inman, M., G.-C. Perng, G. Henderson, H. Ghiasi, A. B. Nesburn, S. L. Wechsler, and C. Jones. 2001. Region of herpes simplex virus type 1 latency-associated transcript sufficient for wild-type spontaneous reactivation promotes cell survival in tissue culture. J. Virol. 753636-3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inman, M., L. Lovato, A. Doster, and C. Jones. 2002. A mutation in the latency related gene of bovine herpesvirus 1 interferes with the latency-reactivation cycle of latency in calves. J. Virol. 766771-6779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang, Y., A. Hossain, M. T. Winkler, T. Holt, A. Doster, and C. Jones. 1998. A protein encoded by the latency-related gene of bovine herpesvirus 1 is expressed in trigeminal ganglionic neurons of latently infected cattle and interacts with cyclin-dependent kinase 2 during productive infection. J. Virol. 728133-8142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang, Y., M. Inman, Y. Zhang, N. A. Posadas, and C. Jones. 2004. A mutation in the latency related gene of bovine herpesvirus 1 inhibits protein expression of a protein from open reading frame 2 and an adjacent reading frame during productive infection. J. Virol. 783184-3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin, L., G.-C. Perng, D. J. Brick, J. Naito, A. B. Nesburn, C. Jones, and S. L. Wechsler. 2004. Methods for detecting the HSV-1 LAT antiapoptotic activity in virus infected tissue culture cells. J. Virol. Method. 1189-13. [DOI] [PubMed] [Google Scholar]
- 26.Jin, L., W. Peng, G.-C. Perng, A. B. Nesburn, C. Jones, and S. L. Wechsler. 2003. Identification of herpes simplex virus type 1 latency associated transcript sequences that both inhibit apoptosis and enhance the spontaneous reactivation phenotype. J. Virol. 776556-6561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones, C. 1998. Alphaherpesvirus latency: its role in disease and survival of the virus in nature. Adv. Virus Res. 5181-133. [DOI] [PubMed] [Google Scholar]
- 28.Jones, C. 2003. Herpes simplex virus type 1 and bovine herpesvirus 1 latency. Clin. Microbiol. Rev. 1679-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones, C., T. J. Newby, T. Holt, A. Doster, M. Stone, J. Ciacci-Zanella, C. J. Webster, and M. W. Jackwood. 2000. Analysis of latency in cattle after inoculation with a temperature sensitive mutant of bovine herpesvirus 1 (RLB106). Vaccine 183185-3195. [DOI] [PubMed] [Google Scholar]
- 30.Jones, C., V. Geiser, G. Henderson, Y. Jiang, F. Meyer, S. Perez, and Y. Zhang. 2006. Functional analysis of bovine herpesvirus 1 (BHV-1) genes expressed during latency. Vet. Microbiol. 113199-210. [DOI] [PubMed] [Google Scholar]
- 31.Kanamoto, T., M. Mota, K. Takeda, L. L. Rubin, K. Miyazono, H. Ichijo, and C. E. Bazenet. 2000. Role of apoptosis signal-regulating kinase in regulation of the c-Jun N-terminal kinase pathway and apoptosis in sympathetic neurons. Mol. Cell. Biol. 20196-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kruman, I. I., A. S. Gukovaskaya, V. V. Petrunyaka, I. P. Beletsky, and E. S. Trepakova. 1992. Apoptosis of murine BW 5147 thymoma cells induced by cold shock. J. Cell. Physiol. 153112-117. [DOI] [PubMed] [Google Scholar]
- 33.Kutish, G., T. Mainprize, and D. Rock. 1990. Characterization of the latency-related transcriptionally active region of the bovine herpesvirus 1 genome. J. Virol. 645730-5737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li, H., H. Zhu, C. J. Xu, and J. Yuan. 1998. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94491-501. [DOI] [PubMed] [Google Scholar]
- 35.Lovato, L., M. Inman, G. Henderson, A. Doster, and C. Jones. 2003. Infection of cattle with a bovine herpesvirus 1 strain that contains a mutation in the latency-related gene leads to increased apoptosis in trigeminal ganglia during the transition from acute infection to latency. J. Virol. 774848-4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meyer, F., S. Perez, V. Geiser, M. Sintek, M. Inman, and C. Jones. 2007. A protein encoded by the bovine herpes virus 1 latency-related gene interacts with specific cellular regulatory proteins, including CCAAT enhancer binding protein alpha. J. Virol. 8159-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nicholson, D. W., and N. A. Thornberry. 1997. Caspases: killer proteases. Trends Biochem. Sci. 22299-306. [DOI] [PubMed] [Google Scholar]
- 38.Perng, G.-C., C. Jones, J. Ciacci-Zanella, M. Stone, G. Henderson, A. Yukht, S. M. Slanina, F. M. Hoffman, H. Ghiasi, A. B. Nesburn, and S. L. Wechsler. 2000. Virus-induced neuronal apoptosis blocked by the herpes simplex virus latency-associated transcript. Science 2871500-1503. [DOI] [PubMed] [Google Scholar]
- 39.Perotti, M., F. Todei, F. Mirabelli, M. Vairetti, G. Bellomo, D. J. McConkey, and S. Orrenius. 1990. Calcium-dependent DNA fragmentation in human synovial cells exposed to cold shock. FEBS Lett. 259331-334. [DOI] [PubMed] [Google Scholar]
- 40.Pinkoski, M. J., N. J. Waterhouse, J. A. Heibein, B. B. Wolf, T. Kuwana, J. C. Goldstein, D. D. Newmeyer, R. C. Bleackley, and D. R. Green. 2001. Granzyme B-mediated apoptosis proceeds predominantly through a Bcl-2- inhibitable mitochondrial pathway. J. Biol. Chem. 27612060-12067. [DOI] [PubMed] [Google Scholar]
- 41.Rauen, U., B. Polzar, H. Stephan, H. G. Mannherz, and H. De Groot. 1999. Cold-induced apoptosis in cultured hepatocytes and liver endothelial cells: mediation by reactive oxygen species. FASEB J. 13155-168. [DOI] [PubMed] [Google Scholar]
- 42.Rock, D., J. Lokensgard, T. Lewis, and G. Kutish. 1992. Characterization of dexamethasone-induced reactivation of latent bovine herpesvirus 1. J. Virol. 662484-2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schang, L., and C. Jones. 1997. Analysis of bovine herpesvirus 1 transcripts during a primary infection of trigeminal ganglia of cattle. J. Virol. 716786-6795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schang, L. M., A. Hossain, and C. Jones. 1996. The latency-related gene of bovine herpesvirus 1 encodes a product which inhibits cell cycle progression. J. Virol. 703807-3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmitz, I., S. Kirchhoff, and P. H. Krammer. 2000. Regulation of death receptor-mediated apoptosis pathways. Int. J. Biochem. Cell. Biol. 321123-1136. [DOI] [PubMed] [Google Scholar]
- 46.Sheffy, B. E., and D. H. Davies. 1972. Reactivation of a bovine herpesvirus after corticosteroid treatment. Proc. Soc. Exp. Biol. Med. 140974-976. [DOI] [PubMed] [Google Scholar]
- 47.Turin, L., S. Russo, and G. Poli. 1999. BHV-1: new molecular approaches to control a common and widespread infection. Mol. Med. 5261-284. [PMC free article] [PubMed] [Google Scholar]
- 48.Varnell, E., H. Kaufman, J. Hill, and H. Thompson. 1995. Cold stress-induced recurrences of herpetic keratitis in the squirrel monkeys. Ophthalmol. Vis. Sci. 361181-1183. [PubMed] [Google Scholar]
- 49.Wang, X. 2001. The expanding role of mitochondria in apoptosis. Genes Dev. 152922-2933. [PubMed] [Google Scholar]
- 50.Winkler, M. T., A. Doster, J. H. Sur, and C. Jones. 2002. Analysis of bovine trigeminal ganglia following infection with bovine herpesvirus 1. Vet. Microbiol. 86139-155. [DOI] [PubMed] [Google Scholar]
- 51.Wolf, B. B., and D. R. Green. 1999. Suicidal tendencies: apoptotic cell death by caspase family proteinases. J. Biol. Chem. 27420049-20052. [DOI] [PubMed] [Google Scholar]
- 52.Xu, Z., A. C. Maroney, P. Dobrzanski, N. V. Kukekov, and L. A. Greene. 2001. The MLK family mediates c-Jun N-terminal kinase activation in neuronal apoptosis. Mol. Cell. Biol. 214713-4724. [DOI] [PMC free article] [PubMed] [Google Scholar]