Abstract
For many envisioned applications of lentivirus vectors as tools in respiratory biology and therapeutic gene delivery, the efficiency of gene transfer must be improved. We previously demonstrated stable, persistent (>11 months) in vivo expression following a single application of a feline immunodeficiency virus (FIV)-based lentivirus vector (GP64-FIV) to murine nasal epithelia. Here we investigate the efficacy of repeated administration of lentivirus vectors to the airways. Using quantitative bioluminescent imaging, we found that consecutive daily dosing achieved a linear increase in gene expression and greatly increased the number of epithelial cells targeted. Surprisingly, reporter gene expression also increased additively following each of seven doses of FIV delivered over consecutive weeks (1 dose/week), without the development of systemic or local neutralizing antibodies. This approach enhanced expression of both reporter and therapeutic transgenes. Transduction efficiency achieved following a single dose of FIV expressing mouse erythropoietin was insufficient to increase hematocrit, whereas seven consecutive daily doses significantly increased hematocrit. These unexpected results contrast strikingly with findings reported for adenovirus vectors. Prolonged gene expression has been observed in vivo following a single dose of virus vector; however, depending on the application, repeated administration of vector may be necessary to achieve stable, therapeutic gene expression.
Several issues limit the application of gene transfer as a useful tool for pulmonary cell biology studies and impede its translational utility for treating diseases of respiratory epithelia. A limitation for many vector systems is the inability to readminister the vector as transgene expression wanes. Mucosal innate and adaptive immune responses against the vector or vector-encoded proteins represent a significant impediment to clinical applications and are well documented for virus vectors such as adenovirus (Ad) (15, 16) and adeno-associated virus (AAV) (14). Indeed, a driving force behind the development of helper-dependent Ad vectors (24) and the search for alternative AAV vector capsids (12, 28) has been avoidance of adaptive immune responses. An alternative strategy is the use of integrating virus vectors of the retrovirus family (13, 39).
Little knowledge exists regarding the potential for readministration of retro- or lentivirus vectors to the airways. In a previous study we showed that a single administration of a vesicular stomatitis virus glycoprotein (VSV-G)-pseudotyped feline immunodeficiency virus (FIV) lentivirus vector with a formulation designed to disrupt epithelial tight junctions achieved a gene transfer efficiency of 1 to 14% in rabbit lower airways (43). Subsequently, we demonstrated that pseudotyping FIV with the envelope glycoprotein from Autographa californica multicapsid nucleopolyhedrovirus (GP64-FIV) conferred novel apical entry properties for transduction of polarized primary cultures of human airway epithelia (37). Furthermore, using a luciferase (Luc) reporter and bioluminescence imaging, we observed persistent in vivo gene expression following delivery of a single dose of GP64-FIV to mouse nasal epithelia. Longitudinal bioluminescence analysis documented expression in nasal epithelia for >11 months without significant decline, suggesting targeting of a population of progenitor cells. Other studies performed with retroviruses in mouse models were of short duration (7, 19, 20, 23, 27, 35) and thus did not address long-term persistence of expression. Furthermore, the transduction efficiency from a single vector application may be insufficient for envisioned applications.
Here we asked whether it is possible to repeat lentivirus vector administration to the respiratory tract and increase gene transfer. A GP64-pseudotyped FIV was repeatedly delivered to murine nasal epithelia. Transduction efficiency, persistence of expression, and host responses were investigated. We report the successful readministration of reporter and therapeutic transgenes to respiratory epithelia without the development of mucosal inhibitory antibodies. These novel findings in the nasal epithelia have implications for the development of gene transfer strategies to study airway biology and to treat genetic and acquired disorders of the respiratory system.
MATERIALS AND METHODS
Vector production.
The FIV vector system utilized in this study (21, 43) expressed either mouse erythropoietin (mEPO), nucleus-targeted β-galactosidase (β-Gal), or firefly Luc. Pseudotyped FIV vector particles were generated by transient transfection and concentrated 250-fold by centrifugation, and their titers were determined by real-time PCR as previously described (38). mEPO cDNA was obtained from Open Biosystems (clone identification no., 8734014; accession no., BC119265), the sequence was confirmed, and the cDNA was cloned into the FIV3.3RSV(Rous sarcoma virus) backbone (38).
In vivo virus vector administration.
Female, 6- to 10-week-old, 18- to 22-g BALB/c mice were used in this study (Harlan, Indianapolis, IN). Approximately 1.25 × 107 transducing units (TU) of FIV vector in a 50-μl volume was delivered to the nasal epithelia via direct instillation. Adenovirus vector was delivered at 1.25 × 107 PFU in a 50-μl volume. This dose was constant for each vector administration and for each protocol. Vector was formulated with 1% methylcellulose as previously described (40). An endotoxin assay revealed detectable levels of endotoxin (<100 endotoxin units) in the delivered volume of vehicle (data not shown). This study was approved by the University of Iowa Institutional Animal Care and Use Committee.
Bioluminescence imaging.
At the time points indicated, animals were injected intraperitoneally (i.p.) with d-luciferin at 100 μl/10 g of body weight of (15 mg/ml in phosphate-buffered saline [PBS]; Xenogen, Alameda, CA) using a 25-gauge needle. Approximately 5 min after luciferin injection, mice were placed in the imaging cabinet, anesthetized with 1 to 3% isoflurane, and imaged using the Xenogen IVIS charge-coupled device camera. Imaging data were analyzed and signal intensity was quantified using Xenogen Living Image software.
GP64 sandwich ELISA.
For the GP64 sandwich enzyme-linked immunosorbent assay (ELISA), a 96-well microtiter plate was coated with GP64 capture antibody (470 μg/μl) and incubated overnight at 4°C. Blocking solution (1× PBS, 5% fetal bovine serum) was applied for 3 h at room temperature without removing the capture antibody. The plate was washed with 1× PBS to remove unbound capture antibody. Standard antigen dilutions were made using purified anti-GP64 monoclonal antibody (eBioscience, catalog no. 14-6995-81). Serum samples and antigen standard dilutions were prepared using blocking buffer and 0.0005% Tween 20. Dilutions for the sample serum and the standard antigen were added to their respective wells in 100-μl aliquots. The plate was again incubated overnight at 4°C and washed with 1× PBS. Goat anti-mouse immunoglobulin G (IgG), horseradish peroxidase (HRP)-conjugated secondary antibody (no. 31432; Pierce) or goat anti-mouse IgA, HRP-conjugated secondary antibody (no. IGA-90P; Innovative Research, Inc.) was added, and the plate was incubated at room temperature for 90 min, washed once again with 1× PBS, and dried. HRP substrate solution was added, and A405 was measured. Recombinant GP64 (a kind gift from Gary Blissard) was used to generate a standard curve. The GP64 envelope glycoprotein is the primary protein displayed on the surface of pseudotyped virions.
Ad ELISA (Indirect ELISA).
A 96-well microtiter plate was coated with Ad serotype 5 (Ad5) vector and incubated overnight at 4°C. Sample dilutions were made with PBS-0.01% Tween 20. The plate was washed, and appropriate standard and sample dilutions were added. The plate was again incubated at 4°C overnight. Blocking solution was added, and the mixture was incubated for 3 h. Unbound capture antibody was removed by washing with PBS-0.01% Tween 20. Plasma and standard dilutions were added, and the plate was washed once again with wash buffer (PBS, 0.01% Tween 20). Secondary antibody, HRP-conjugated goat anti-mouse IgG or IgA, was added at a predetermined dilution and incubated for 1 h at room temperature followed by a final rinse and addition of the substrate for visualization. A405 was measured.
Neutralizing antibody assay.
The neutralizing antibody assay was adapted from a previously described protocol (41). Briefly, HT1080 cells were seeded at a density of 3 × 106 cells/well in a 12-well plate. Dilutions were prepared for each serum or bronchoalveolar lavage (BAL) fluid specimen ranging from 1:10 to 1:160 in serum-free media. Each plate included a serum-free, medium-only control. GP64-FIV-Luc or Ad-Luc was incubated with sample dilutions prior to delivery to cells at a multiplicity of infection of ∼1. Medium was changed after 24 h, and lysates were collected after 96 h. The dilution of sera or BAL sufficient to neutralize 50% of virus vector-mediated Luc expression was determined by Luc assay (Promega E-1501).
Histochemical analysis.
Three weeks after vector delivery, heads were removed, fixed, stained with X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside), decalcified (32), embedded in paraffin, sectioned, and counterstained with nuclear fast red per standard techniques. Eight-micrometer-thick coronal sections were collected at 200-mm intervals. Sections were collected at arbitrary levels of 100, 125, 150, 175, and 200 (1 level = 30 μm). Stereology was performed using basic methods as previously described (8). The respiratory and olfactory epithelial areas were examined by capturing all microscopic images at a ×20 magnification with an Olympus DP70 digital camera and analyzed using Image-Pro Plus version 4.1 computer software (Media Cybernetics, Inc., Silver Spring, MD). Areas of epithelium where the apical membrane reached the airway lumen were traced, and the number of β-Gal-positive cells was calculated and compared to the total number of cells within the traced area. Calculations were derived from images taken from four mice, and four epithelial areas were examined from each animal. The epithelial areas were collected ∼3.0 to 3.4 mm caudal from the nose. Images were coded and counted by an observer blinded to this code. Measurements were counted twice independently with reproducible results.
Cytokine assays.
At the appropriate end point of each experiment, mice were euthanized by carbon dioxide overdose. After euthanasia, the nasal cavity was washed with 200 μl saline (in 20-μl increments; 100 μl per nares) as previously described (31). Saline was administered in the nares, and the nasal wash fluid was collected with a pipetter fitted with a 20-μl plastic tip, pooled, and centrifuged in a microcentrifuge at 2,300 × g for 10 min to pellet cells and debris. The nasal wash fluid supernatant was removed and stored at −80°C without inhibitors. As described previously (30, 31), we determined the concentrations of 14 cytokines using a multiplex fluorescent bead-based immunoassay (kit 48-004, Upstate Biotechnology, Lake Placid, NY). Samples were incubated with anti-mouse multicytokine beads at 4°C for 18 h. Unbound material was removed by filtration. Anti-mouse multicytokine biotin reporter was added, and reaction mixtures were incubated at room temperature for 1.5 h in the dark. Streptavidin-phycoerythrin was then added, and plates were incubated at room temperature for 30 min. Stop solution was added, and the plates were read in the plate reader (model 100 IS; Luminex, Austin, TX).
Hematocrit measurements.
Blood was collected in heparinized capillary tubes following facial vein puncture using Goldenrod lancets (MEDIpoint, Inc). The collected blood was spun in a microhematocrit centrifuge (no. 15401-628; VWR Scientific, Buffalo Grove, IL) for 2 min to separate plasma from red blood cells. The percentage of red blood cells was measured with a metric ruler.
Statistics.
Unless otherwise noted, all numerical data are presented as means ± standard deviations. Statistical analyses were performed under consultation with Kathryn Chaloner of the University of Iowa College of Public Health Department of Biostatistics.
RESULTS
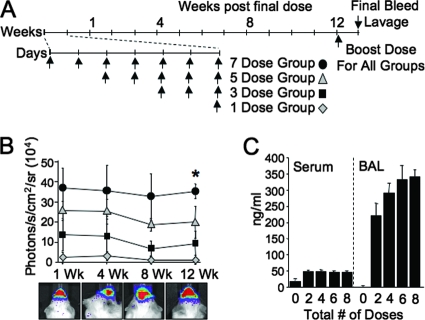
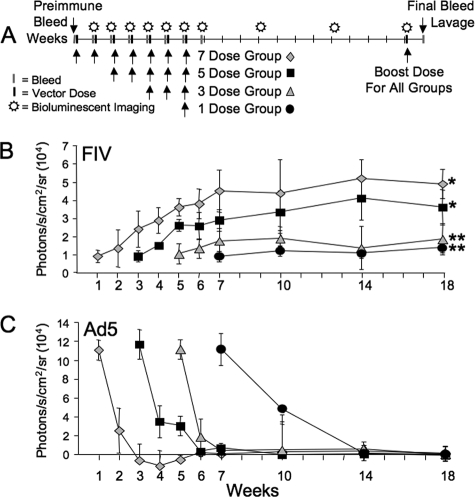
To investigate expression-enhancing benefits of acute lentivirus vector readministration to nasal epithelia, we used Luc as a sensitive and easily quantified reporter gene. In this experimental protocol (Fig. 1A), four groups of mice received one, three, five, or seven total doses of GP64-FIV-Luc over the same number of consecutive days. A fifth group received no treatment and served as a baseline of background luminescence (not shown). Background luminescence may vary slightly from day to day; thus, naïve mice were included in every imaging session and baseline values were always subtracted from the experimental values. One week following the final dose, mice underwent bioluminescence imaging and animals were imaged again 4, 8, and 12 weeks following the final dose. After the final imaging, mice received a vector booster dose and sera and BAL fluid were collected. As shown in Fig. 1B, there was a linear increase in Luc expression from one to seven doses observed at 1 week postdelivery and expression remained stable over the duration of the experiment. These data confirm that acute repeat administration is an effective means to improve the gene transfer efficiency.
FIG. 1.
Acute repeat administration of lentivirus vector expressing Luc. GP64-pseudotyped FIV expressing Luc was delivered to mice following the protocol shown (A). At the indicated time points, mice were given luciferin via i.p. injection and photographed by bioluminescent imaging (B). At the conclusion of the experimental protocol, mice received a booster dose of vector. One week later, serum and BAL fluid were collected. Total serum and BAL IgG antibodies against GP64 were determined by ELISA (C). n = 5; *, P < 0.0001 compared to naïve control by analysis of variance, using the Tukey-Kramer adjustment for multiple comparisons.
The total concentrations of anti-GP64 IgG antibodies in sera and BAL fluid were determined 1 week following a booster dose. Low-level IgG anti-GP64 antibodies (∼45 ng/ml) were observed in the sera for all groups (Fig. 1C), and higher levels of IgG anti-GP64 antibodies (∼200 to 350 ng/ml) were observed in the BAL specimen (Fig. 1C). Importantly, the levels of inactivating antibodies in BAL fluid were below the limit of detection (data not shown). These data indicate that while an adaptive immune response is mounted against the vector, it is insufficient to block gene transfer; however, we cannot rule out the presence of low-level inhibitory antibodies to GP64 or other vector proteins.
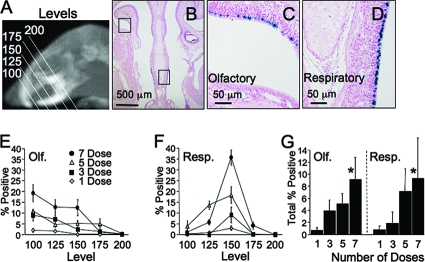
In parallel experiments, we delivered GP64-FIV expressing nucleus-targeted β-Gal to a separate cohort of mice following the same experimental timeline described in Fig. 1A. One month following vector delivery, coronal sections through the mouse muzzle were obtained as shown schematically (Fig. 2A). Tissue section levels were arbitrarily assigned and were matched between mice based on morphological landmarks. Low-power digital photographs (Fig. 2B) were taken of the olfactory (Fig. 2C) and respiratory (Fig. 2D) epithelia, and the percentage of transduced cells was determined. Within the olfactory epithelia, we observed a greater percentage of β-Gal-positive cells rostrally (level 100) compared to caudally (level 200) (Fig. 2E). For respiratory epithelia, the greatest percentage of β-Gal-positive cells was detected at a midpoint (level 150) (Fig. 2F). By averaging the total percentages of positive cells across all levels, we observed a dose-dependent increase in expression from <1% for a single dose to ∼10% for seven doses (Fig. 2G). Furthermore, we observed no preferential GP64-mediated transduction of respiratory or olfactory epithelium.
FIG. 2.
Acute repeat administration of lentivirus vector expressing β-Gal. GP64-pseudotyped FIV expressing nucleus-targeted β-Gal was delivered to mice following the same protocol as Fig. 1A. Four weeks following the final dose, sections were collected from five standardized levels based on anatomical landmarks, as shown schematically (A). One level is ∼30 μm. Low-power photographs (B) of both olfactory (C) and respiratory (D) epithelia were collected from each level. Using ImagePro software, the percentages of β-Gal-positive surface epithelial cells were determined for both olfactory (Olf. [E]) and respiratory (Resp. [F]) epithelia. In addition, the total percent positive was determined as a function of number of doses (G). n = 3; *, P < 1 × 10−5, using the F test for dependence on dose response.
The innate immune response following delivery of a single dose of GP64-FIV, Ad5, or vehicle was examined by measuring levels of 14 cytokines in nasal lavage at 4, 24, and 72 h after delivery (Table 1). Most cytokines increased slightly (<50 pg/ml) at 4 h and returned to naïve levels by 24 or 72 h. Two cytokines, KC and interleukin-6 (IL-6), increased the most at 4 h (2,000 to 3,000 pg/ml), but returned to naïve levels by 24 h. In addition, no differences were observed between GP64-FIV, Ad5, or vehicle. These data suggest that vehicle administration, in combination with the simple act of mucosal stimulation, is sufficient to elicit a response.
TABLE 1.
Nasal lavage concentrations of 14 cytokines after single-dose administration of GP64-FIV, Ad5, or vehicle
| Sample type and treatment group | Time postdose (h) | Cytokine concn (pg/ml)a
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IL-12 (p40) | KC | MCP-1 | RANTES | GM-CSFb | IFN-γ | IL-10 | IL-12 (p70) | IL-6 | IL-1β | IL-2 | IL-4 | IL-5 | TNF-α | ||
| Nasal wash fluid | |||||||||||||||
| FIV | |||||||||||||||
| 4 | <3 | 2,963 ± 835 | 43 ± 3 | 11 ± 1 | 36 ± 15 | 15 ± 2 | 10 ± 6 | 49 ± 10 | 1,764 ± 671 | 21 ± 4 | 12 ± 2 | 14 ± 2 | 15 ± 2 | 43 ± 15 | |
| 24 | <3 | 357 ± 70 | 20 ± 11 | 11 ± 1 | 9 ± 5 | 7 ± 2 | <3 | <3 | 49 ± 19 | <3 | 7 ± 1 | 10 ± 1 | 11 ± 1 | 11 ± 1 | |
| 72 | <3 | 261 ± 24 | 16 ± 9 | 10 ± 1 | 12 ± 4 | 8 ± 1 | <3 | <3 | 42 ± 10 | <3 | 8 ± 12 | 10 ± 1 | 9 ± 3 | 12 ± 1 | |
| Ad5 | |||||||||||||||
| 4 | <3 | 3,318 ± 630 | 26 ± 8 | 11 ± 1 | 36 ± 4 | 14 ± 1 | 5 ± 4 | 49 ± 7 | 2,355 ± 572 | 21 ± 5 | 10 ± 1 | 13 ± 1 | 14 ± 1 | 36 ± 6 | |
| 24 | <3 | 365 ± 27 | 35 ± 3 | 10 ± 1 | 7 ± 3 | 9 ± 1 | <3 | <3 | 60 ± 16 | <3 | 8 ± 1 | 10 ± 1 | 5 ± 2 | 13 ± 1 | |
| 72 | <3 | 254 ± 39 | 27 ± 9 | 10 ± 1 | 7 ± 4 | 10 ± 1 | <3 | 9 ± 5 | 42 ± 3 | <3 | 5 ± 2 | 10 ± 1 | 12 ± 2 | 15 ± 2 | |
| Vehiclec | |||||||||||||||
| 4 | <3 | 3,243 ± 288 | 37 ± 4 | 10 ± 1 | 32 ± 8 | 13 ± 1 | 6 ± 2 | 45 ± 6 | 1,800 ± 487 | 22 ± 4 | 11 ± 1 | 13 ± 1 | 15 ± 1 | 36 ± 4 | |
| 24 | <3 | 403 ± 41 | 32 ± 4 | 12 ± 1 | 11 ± 3 | 9 ± 1 | <3 | 5 ± 3 | 162 ± 44 | 5 ± 2 | 10 ± 1 | 9 ± 1 | 8 ± 4 | 17 ± 1 | |
| 72 | <3 | 326 ± 23 | 26 ± 12 | 12 ± 1 | 20 ± 4 | 11 ± 1 | <3 | 5 ± 3 | 86 ± 24 | 11 ± 1 | 9 ± 1 | 10 ± 1 | 11 ± 3 | 22 ± 2 | |
| Naive | |||||||||||||||
| 4 | <3 | 707 ± 47 | 28 ± 9 | 13 ± 1 | 14 ± 4 | 10 ± 1 | <3 | 6 ± 5 | 141 ± 54 | 13 ± 1 | 9 ± 1 | 11 ± 1 | 12 ± 1 | 25 ± 2 | |
| 24 | <3 | 448 ± 67 | 12 ± 7 | 12 ± 1 | 10 ± 4 | 9 ± 1 | <3 | <3 | 42 ± 8 | 5 ± 2 | 9 ± 1 | 10 ± 1 | 8 ± 2 | 16 ± 1 | |
| 72 | <3 | 217 ± 42 | 12 ± 10 | 10 ± 1 | 9 ± 4 | 9 ± 1 | <3 | <3 | 34 ± 9 | <3 | 8 ± 1 | 9 ± 1 | 12 ± 2 | 13 ± 1 | |
| Serum | |||||||||||||||
| FIV | 24 | 6 ± 1 | 100 ± 11 | 15 ± 4 | 20 ± 1 | 15 ± 5 | 13 ± 9 | 8 ± 5 | 28 ± 17 | 11 ± 7 | 42 ± 24 | 7 ± 4 | 4 ± 3 | 5 ± 3 | 2 ± 1 |
| Ad5 | 24 | 7 ± 2 | 91 ± 12 | 11 ± 1 | 20 ± 1 | 16 ± 6 | 15 ± 6 | 12 ± 6 | 36 ± 19 | 11 ± 4 | 40 ± 20 | 6 ± 3 | 5 ± 3 | 4 ± 1 | 2 ± 1 |
| Vehiclec | 24 | 29 ± 24 | 65 ± 14 | 8 ± 1 | 18 ± 2 | 10 ± 1 | 6 ± 2 | 4 ± 1 | 11 ± 3 | 4 ± 1 | 14 ± 5 | 3 ± 1 | 1 ± 1 | 3 ± 1 | 2 ± 1 |
| Naive | 24 | 5 ± 1 | 41 ± 11 | 13 ± 3 | 21 ± 3 | 11 ± 2 | 12 ± 5 | 7 ± 2 | 17 ± 4 | 9 ± 3 | 25 ± 9 | 2 ± 1 | 3 ± 1 | 3 ± 1 | 2 ± 1 |
All numerical data are shown as the average ± standard deviation (n = 4 animals/time point).
GM-CSF, granulocyte-macrophage colony-stimulating factor.
Vehicle was composed of a 50:50 mix of 1% methylcellulose and tris-lactose buffer.
In addition to acute readministration to nasal epithelia, we delivered successive doses of GP64-FIV-Luc to the intrapulmonary airways via tracheal instillation (see Fig. S1A in the supplemental material). As with nasal instillation, Luc expression increased following repeat dosing. Interestingly, we observed both lung and nasal Luc expression following direct tracheal instillation (see Fig. S1B and C in the supplemental material), possibly due to mucociliary clearance of vector. Total serum IgG anti-GP64 antibodies increased slightly following tracheal delivery compared to nasal instillation (see Fig. S1D in the supplemental material); however, inactivating antibodies were below the limit of detection (see Fig. S1E in the supplemental material).
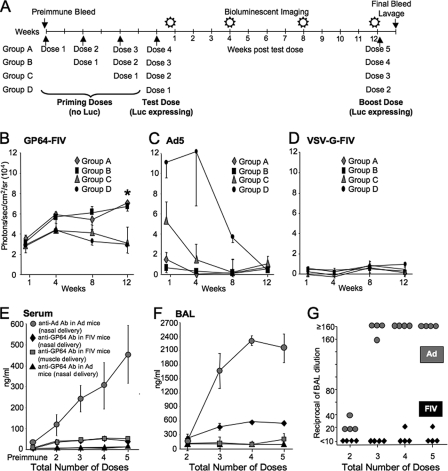
To investigate the possibility of increasing the time interval between vector doses, we delivered priming doses of GP64-FIV, Ad5, or VSV-G-pseudotyped FIV vectors to nasal epithelia followed by a test dose. The priming dose(s) of FIV carried a firefly Luc transgene without a promoter. No Luc expression was observed following the delivery of this vector either in vitro (data not shown) or in vivo (38). The test dose was the identical lentivirus vector, with an RSV promoter driving Luc. Mice received three, two, one, or zero priming dose at 2-week intervals (groups A, B, C, and D, respectively) followed by a test dose (Fig. 3A). Two weeks was chosen as sufficient time for the development of adaptive immune responses. Following the test dose, mice were imaged at 4-day, 4-week, 8-week, and 12-week time points (Fig. 3A). At the 12-week time point, a boost dose was given. One week later, serum and BAL fluid were collected. Importantly, priming doses of GP64-FIV did not result in a loss of expression from the test dose (Fig. 3B). Unexpectedly, animals that received two or three priming doses had higher Luc expression after 12 weeks than animals receiving zero or one priming dose (Fig. 3B). As expected, in mice that received Ad-Empty (priming dose) followed by Ad-Luc (test dose), expression from the test dose was significantly attenuated after a single priming dose (Fig. 3C). No expression was observed in mice that received VSV-G-pseudotyped FIV (Fig. 3D), consistent with previous observations that this vector poorly transduces polarized epithelia in the absence of agents that disrupt tight junctions (37).
FIG. 3.
Priming vector doses followed by a test dose. Priming or test doses of GP64-pseudotyped FIV, Ad5, or VSV-G-pseudotyped FIV was delivered to mice at 2-week intervals following the protocol as shown (A). At the indicated time points, mice that received GP64-FIV (B), Ad5 (C), or VSV-G-FIV (D) were given luciferin via i.p. injection and photographed by bioluminescent imaging. Mice were imaged and bled between each dose of vector as indicated. At the conclusion of the experimental protocol, mice received a booster dose of vector. One week later, serum and BAL fluid were collected. Total IgG GP64 or Ad antibodies (Ab) were quantified in sera (E) and BAL fluid (F) by ELISA. n = 4; *, P < 0.001 compared to naïve control by analysis of variance, using the Tukey-Kramer adjustment for multiple comparisons. Inactivating antibodies against Ad (gray circles) or GP64 (black diamonds) were measured in the BAL fluid (G). Equal TU of vector (1.25 × 107 TU, as determined by real-time PCR) were delivered for priming and test doses.
We measured IgG antibodies against Ad or GP64 in serum (Fig. 3E) or BAL fluid (Fig. 3F) collected 1 week after the booster dose at the end of the delivery protocol (Fig. 3A). As expected, we observed a dose-dependent increase in anti-Ad antibodies in sera following Ad vector intranasal (i.n.) instillation (Fig. 3E). In contrast, serum levels of total anti-GP64 IgG antibodies following i.n. or intramuscular (i.m.) GP64-FIV administration plateaued after three doses (Fig. 3E). The findings for total anti-Ad or GP64 IgG antibody production in BAL mirrored the sera, with the exception that no antibodies were observed in BAL following i.m. GP64-FIV (Fig. 3F). In mice that received Ad vector, no detectable anti-GP64 IgG antibodies were found in sera (Fig. 3E) or BAL fluid (Fig. 3F). The attenuated expression of Ad correlated with production of neutralizing antibodies following repeated dosing (Fig. 3G). In contrast, only low-level neutralizing antibody production was observed following lentivirus vector delivery and neutralizing antibody levels did not increase significantly with repeated doses (Fig. 3G).
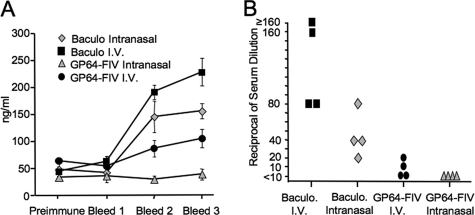
The GP64 envelope glycoprotein used to pseudotype FIV is identical in amino acid sequence to the Autographa californica baculovirus envelope, which is known to elicit a strong immune response. Indeed, baculovirus-expressed GP64 fusion proteins provide tools for antibody generation (29, 42). However, baculovirus envelope glycoprotein produced in insect cells will display glycosylation patterns different from GP64-FIV produced in human cells (18). We delivered three doses of baculovirus or GP64-FIV via the i.n. or tail vein (intravenous [i.v.]) route at 2-week intervals. The delivered doses (∼9.1 × 106 TU of GP64-FIV or ∼3.0 × 106 PFU of baculovirus) were matched for total GP64 protein as determined by immunoblotting (data not shown). The greatest serum IgG anti-GP64 antibody levels were observed in mice receiving i.v. or i.n. baculovirus delivery (Fig. 4A). The prevalence of neutralizing antibodies (Fig. 4B) was consistent with the total serum anti-GP64 antibodies. These data suggest that baculovirus-associated immunogenicity is likely due to a combination of the delivery route, the GP64 glycosylation pattern, and other adjuvants missing from lentivirus vector preparations.
FIG. 4.
Readministration of lentivirus vector versus baculovirus vector. Three doses of GP64-pseudotyped FIV or baculovirus (Baculo.) were delivered to mice at 2-week intervals. Mice received either i.n. or i.v. (tail vein) vector. Total IgG anti-GP64 antibodies were quantified in sera collected 1 week following the final dose by ELISA (A). Inactivating antibodies against GP64-FIV-Luc in the sera from each experimental group were determined (B). n = 4.
To investigate further the relationship between dosing interval and sustained increases in transgene expression, we delivered one, three, five, or seven doses of vector over the same number of consecutive weeks (1 dose/week) (Fig. 5A). Between each dose, mice were imaged and sera were collected. Of note, with weekly administration of GP64-FIV-Luc, a linear increase in reporter gene expression was again observed (Fig. 5B). Furthermore, expression was stable for the duration of the experiment. In contrast, with weekly administration of Ad5-Luc, expression quickly dropped to naïve levels (Fig. 5C).
FIG. 5.
Chronic repeat administration of lentivirus vector. GP64-pseudotyped FIV or Ad5 expressing Luc was delivered at 1-week intervals to mice following the protocol shown (A). At the indicated time points, mice that received GP64-FIV-Luc (B) or Ad5-Luc (C) were given luciferin via i.p. injection and photographed by bioluminescent imaging. Between each dose of vector, mice were imaged and bled as indicated. n = 5; *, P < 0.0001, and **, P < 0.001, compared to the naïve control by analysis of variance, using the Tukey-Kramer adjustment for multiple comparisons.
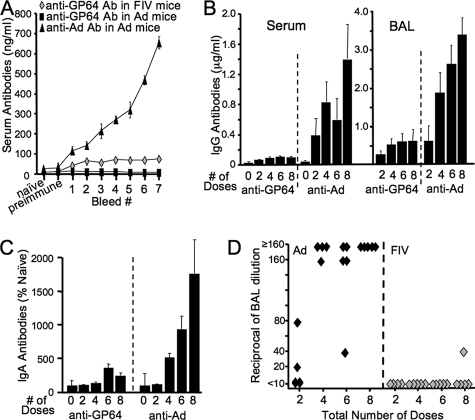
We quantified total IgG serum antibodies generated against Ad5 or GP64 by ELISA following each weekly vector dose (Fig. 6A). As expected, anti-Ad5 antibodies increased with successive doses. In contrast, mice receiving repeated administration of GP64-FIV-Luc generated anti-GP64 antibodies that plateaued after the second dose. As a negative control, we measured anti-GP64 antibodies following Ad5-Luc and observed no antibodies. The total serum and BAL (Fig. 6B) IgG antibodies against Ad5 or GP64 were quantified 1 week following a booster dose of the appropriate vector at the end of the 19-week experiment. For Ad5-Luc, we observed a dose-dependent increase in IgG antibody production in both sera and BAL fluid (Fig. 6B). In contrast, mice that received GP64-FIV-Luc repeatedly generated low levels of anti-GP64 antibodies that reached a plateau following four doses in both sera and BAL fluid (Fig. 6B). Again, no anti-GP64 antibodies were detected in either the sera or BAL fluid following repeated doses of Ad5-Luc (data not shown). Similarly, little evidence of anti-GP64 IgA antibody response was present in BAL fluid after single or multiple doses (Fig. 6C). This contrasted with the robust IgA responses elicted by Ad (Fig. 6C). Neutralizing antibodies against Ad5-Luc were detected in the BAL in 2 of 4 mice after two doses and all (14 of 14 total) mice after four, six, or eight doses (Fig. 6D). Conversely, no inactivating antibodies to GP64-FIV-Luc were detected, with the exception of 1 of 5 mice after eight doses (Fig. 6D). These encouraging results suggest that GP64-FIV-based vectors are well suited for repeat administration to nasal epithelia. Additional study is required to determine the therapeutic utility of vector readministration to the lower respiratory tract.
FIG. 6.
Adaptive immune response following chronic repeat administration of lentivirus vector. Mice were bled between each weekly dose during the chronic repeat administration experimental protocol. Total IgG serum antibodies (Ab) against GP64 or Ad were determined by ELISA (A). At the conclusion of the chronic repeat administration experimental protocol, mice received a booster dose of vector. One week later, serum and BAL fluid were collected. Total serum and BAL IgG antibodies (B) or BAL IgA antibodies (C) against GP64 or Ad were determined by ELISA. The presence of neutralizing antibodies against GP64-FIV-Luc or Ad5-Luc in the BAL fluid was determined (D). n = 4 or 5.
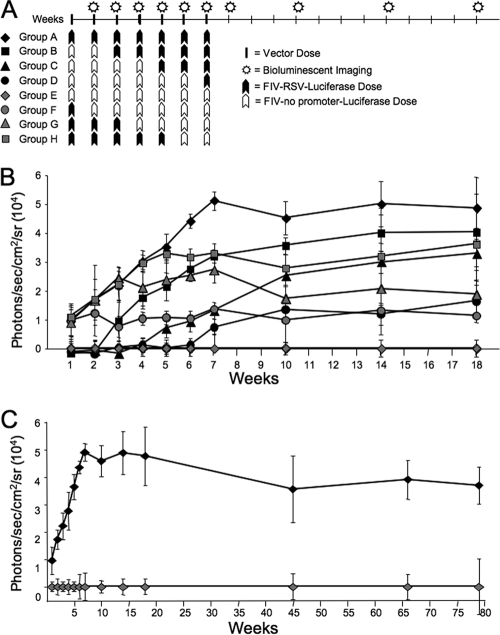
We performed an additional experiment to investigate how previous vector exposure affects the success of repeat administration with longer dosing intervals. Eight groups of mice (5 per group) received seven doses of GP64-FIV-Luc (Fig. 7A). However, the vector either contained an RSV internal promoter driving Luc or lacked an internal promoter. Again, mice that received seven doses of FIV-RSV-Luc displayed a linear increase in reporter gene expression (Fig. 7B). Animals receiving seven doses of FIV-no promoter-Luc displayed no expression above naïve controls (not shown), while those pretreated with two or four doses of FIV-no promoter-Luc displayed linear increases in expression with each successive dose of FIV-RSV-Luc. Likewise, subsequent applications of six, four, or two doses of FIV-no promoter-Luc conferred stable Luc expression over the 3 months following the final dose. We noted a trend for mice predosed with FIV-no promoter-Luc to exhibit higher Luc expression at the conclusion of the experiment than animals receiving postdosing of FIV-no promoter-Luc. Long-term stable expression lasting >78 weeks (1.5 years) was documented in mice that received seven doses of GP64-FIV-Luc (Fig. 7C).
FIG. 7.
Chronic repeat administration of lentivirus vector with or without an internal promoter. GP64-FIV-Luc was delivered at 1-week intervals to mice following the protocol shown (A). Between each dose of vector, mice were imaged. At the indicated time points, mice were given luciferin via i.p. injection and photographed by bioluminescent imaging (B). Long-term persistence (∼1.5 years) was monitored in two cohorts of mice, group A and group E (C). n = 5.
To investigate the potential for enhancing secreted protein expression following topical administration of lentivirus vector to nasal epithelia, we selected mEPO as a transgene. Recombinant EPO is used clinically to treat anemia from chronic disease. Airway-delivered mEPO expressed from an Ad vector was previously demonstrated to increase hematocrit (9). Here we compared seven doses of GP64-FIV-mEPO delivered over 7 consecutive days (1 dose/day) to a single dose (Fig. 8). By 2 weeks postdelivery, functional mEPO expression was observed from the seven-dose group, as evidenced by a significant increase in hematocrit. The hematocrit increase persisted for 13 weeks, the last time point tested. We note that the hematocrit of the one-dose group was not statistically different from that of the naïve control group. These results further demonstrate that repeated vector administration is feasible and may be required to achieve desired expression levels for some transgenes.
FIG. 8.
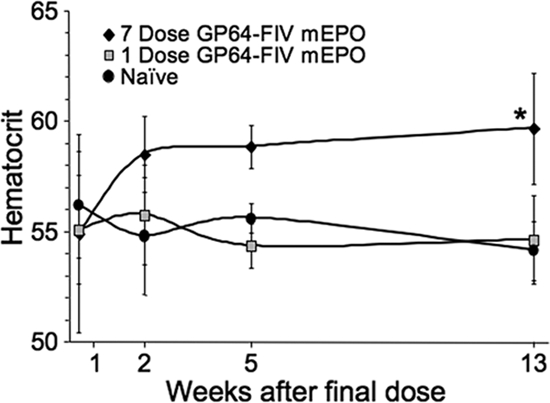
Repeat administration of a lentivirus vector expressing a secreted protein. A single i.n. dose of GP64-FIV-mEPO (squares) was compared to seven doses (1 dose/day) delivered over 7 consecutive days (diamonds). Hematocrit was determined as an indirect measure of mEPO expression. n = 5; *, P < 0.001 compared to naïve control (circles) by analysis of variance, using the Tukey-Kramer adjustment for multiple comparisons.
DISCUSSION
We report here for the first time the successful in vivo readministration of lentivirus vectors to respiratory epithelia to increase expression of both reporter genes and a therapeutic gene, coding for EPO. Unlike the well-characterized and widely recognized local and systemic immune responses to adenovirus vectors that effectively prevent repeat pulmonary administration, little is known regarding host responses to lentivirus vectors. The FIV lentivirus was delivered at intervals of days or weeks with associated stable increases in reporter or therapeutic transgene expression. Despite repeated vector application and low-level antibody production, blocking immune responses failed to develop. To our knowledge, this is the first demonstration of retrovirus vector readministration at a mucosal surface. Although our vector delivery was directed to nasal epithelia, the results have implications for the therapeutic use of lentivirus vectors in pulmonary gene transfer applications.
Innate immune responses to viruses or virus vectors are a first line of defense generated within minutes to hours following virus vector administration and contrast with the slower-to-develop adaptive immunity. These responses have been extensively studied with the Ad and AAV vectors under investigation for pulmonary gene transfer applications. Many preclinical studies and clinical trials with Ad vectors document increases in systemic chemokines and proinflammatory cytokines including RANTES, IP-10, MIP-2, gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α), IL-6, IL-10, and IL-12 in humans (4, 17) and mice (3). A number of studies indicate that a priming dose of an Ad vector will elicit sufficient humoral and cell-mediated immunity to prevent pulmonary readministration without the use of immunosuppressive agents (44, 45) or the masking of vector epitopes with formulations such as pegylation (6). AAV vectors have also been extensively investigated in animal models and in phase I and II clinical trials for cystic fibrosis (1, 36). A number of capsid serotypes have been identified and tested (2, 28). In most cases, a second dose of AAV yields lower levels of pulmonary gene transfer because of neutralizing antibody responses. Preexisting immunity against either Ad or AAV may also present a significant barrier to initial use of these vectors (14, 46). More recently, helper-dependent Ads have shown promise for prolonged expression (22), and perhaps readministration (24). In addition, the ability to switch Ad or AAV capsid serotypes (serotype switching) may offer at least a temporary solution (28, 33).
In contrast to the extensive literature regarding Ad and AAV, relatively little is known regarding the innate and adaptive immune responses to retrovirus-based vectors, and there have been no studies in the airways. McCormack and colleagues detected humoral immune responses to envelope and capsid proteins after i.m. immunization with a Moloney murine leukemia virus (MLV)-based retrovirus vector in mice and monkeys (34). Antibody responses to the MLV vectors were dose dependent and included neutralizing antibodies; however, the antibodies failed to prevent i.m. vector readministration (34). Furthermore, human subjects that received i.m. immunization with an MLV-based vector developed antibodies against vector-related proteins only at very high doses (11). These findings indicated that MLV-based retrovirus vectors pseudotyped with the amphotropic envelope are poorly immunogenic.
Based on the studies described above, one would speculate that lentivirus vectors are less likely to stimulate a neutralizing immune response than encapsidated vectors such as AAV or Ad following topical delivery to the nasal epithelia. We observed minimal innate and adaptive immune responses following topical delivery of GP64-FIV-Luc to murine airway epithelia. However, a significant question regarding our findings is why repeated administration of the FIV vector failed to elicit immune responses that prevent readministration even after seven weekly doses. Based on the performed experiments, we cannot discern if the observed anti-GP64 antibodies are nonneutralizing or if low-level neutralizing antibodies were saturated by the vector dose delivered. It is unlikely that preexisting immunity to the GP64 envelope glycoprotein will present a barrier. We suspect that the GP64 glycoprotein, together with the vector-associated proteins (matrix, capsid, protease, integrase, and reverse transcriptase), is poorly antigenic when presented to the nasal mucosa (25). In this study, only antibodies to the GP64 antigen were measured. It is possible other lentivirus antigens or cell membrane antigens could be immunostimulatory.
A transient early increase in IL-6 and KC (IL-8 ortholog) release occurred following delivery of ∼1 × 107 TU of FIV, titer-matched Ad5, or vehicle alone. These data suggest that vehicle impurities and/or simple physical irritation of the respiratory mucosa are sufficient to elicit transient cytokine release. Importantly, this cytokine response does not preclude persistent transgene expression following lentivirus vector transduction. In comparison to adenovirus and AAV vectors, the protein abundance and diversity of antigens presented by pseudotyped FIV are likely more limited. While not directly related to the present studies, it is interesting to note how difficult it has been to develop successful vaccines using proteins from the human immunodeficiency virus lentivirus (10), again suggesting that lentivirus-derived proteins are poorly antigenic. These findings suggest that repeat administration the airway surface without adverse immune responses is possible.
The GP64-pseudotyped FIV vector efficiently transduced nasal epithelia and persistently expressed a transgene. We demonstrated additive increases in transgene expression with repeat dosing. We speculate that this increase in expression represents both an increase in the percentage of cells expressing a transgene (Fig. 2) and an increase in the number of transgene copies/cell. The route of vector administration may significantly influence the subsequent development of immune responses. Naldini and colleagues recently reported innate and adaptive immune responses to lentivirus vectors administered systemically and targeting hepatocytes in mouse models (5). Our results in the nasal airways suggest that mucosal application of a lentivirus vector is less immunostimulatory than systemic delivery.
For genetic diseases, the expression of a therapeutic protein over the life of the affected individual is a goal; therefore, repeated administration of virus vectors may be necessary, and this presents unique limitations for each vector system. Innate and adaptive immune responses to lentivirus-based vectors likely vary depending on the origin of the vector, the purity of the vector preparation, the dose delivered, the route of delivery, and the number of administrations. The readministration of lentivirus vectors to the respiratory tract offers the possibility to increase the overall transduction efficiency. For example, sequential vector administration to individual lung lobes may be advantageous for practical and safety reasons. In addition, the ability to successfully redose provides a therapeutic option should expression wane over time. Lentivirus vectors are only beginning to make their debut in human clinical trials (26). Our findings in inbred mice are encouraging and have important implications for the translation of lentivirus gene transfer technology into tools for investigating respiratory cell biology and the development of pulmonary disease therapies in large animal models and human trials. It will be important in future studies to investigate these approaches in larger, more genetically diverse animal models.
Supplementary Material
Acknowledgments
We thank Christine Wohlford-Lenane, Erin Burnight, Litao Xie, Lynn Kuchemann, and Kindra Burnell for valuable assistance. Gary Blissard and Jian Zhou provided important reagents and helpful discussions. We thank Beverly Davidson, Michael Welsh, and Joseph Zabner for critical comments on the manuscript.
This work was supported by NIH grants K01 DK-073367 (P.L.S.), R01 HL-075363 (P.B.M.), and PO1 HL-51670 (P.B.M.) and the Roy J. Carver Charitable Trust (P.B.M.). We also acknowledge the support of the In Vitro Models and Cell Culture Core and Cell Morphology Cores, partially supported by the Center for Gene Therapy for Cystic Fibrosis (NIH P30 DK-54759) and the Cystic Fibrosis Foundation.
Footnotes
Published ahead of print on 3 September 2008.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Aitken, M. L., R. B. Moss, D. A. Waltz, M. E. Dovey, M. R. Tonelli, S. C. McNamara, R. L. Gibson, B. W. Ramsey, B. J. Carter, and T. C. Reynolds. 2001. A phase I study of aerosolized administration of tgAAVCF to cystic fibrosis subjects with mild lung disease. Hum. Gene Ther. 121907-1916. [DOI] [PubMed] [Google Scholar]
- 2.Auricchio, A., E. O'Connor, D. Weiner, G. P. Gao, M. Hildinger, L. Wang, R. Calcedo, and J. M. Wilson. 2002. Noninvasive gene transfer to the lung for systemic delivery of therapeutic proteins. J. Clin. Investig. 110499-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borgland, S. L., G. P. Bowen, N. C. W. Wong, T. A. Libermann, and D. A. Muruve. 2000. Adenovirus vector-induced expression of the C-X-C chemokine IP-10 is mediated through capsid-dependent activation of NF-κB. J. Virol. 743941-3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowen, G. P., S. L. Borgland, M. Lam, T. A. Libermann, N. C. Wong, and D. A. Muruve. 2002. Adenovirus vector-induced inflammation: capsid-dependent induction of the C-C chemokine RANTES requires NF-kappa B. Hum. Gene Ther. 13367-379. [DOI] [PubMed] [Google Scholar]
- 5.Brown, B. D., G. Sitia, A. Annoni, E. Hauben, L. S. Sergi, A. Zingale, M. G. Roncarolo, L. G. Guidotti, and L. Naldini. 2007. In vivo administration of lentiviral vectors triggers a type I interferon response that restricts hepatocyte gene transfer and promotes vector clearance. Blood 1092797-2805. [DOI] [PubMed] [Google Scholar]
- 6.Chillon, M., J. H. Lee, A. Fasbender, and M. J. Welsh. 1998. Adenovirus complexed with polyethylene glycol and cationic lipid is shielded from neutralizing antibodies in vitro. Gene Ther. 5995-1002. [DOI] [PubMed] [Google Scholar]
- 7.Copreni, E., M. Penzo, S. Carrabino, and M. Conese. 2004. Lentivirus-mediated gene transfer to the respiratory epithelium: a promising approach to gene therapy of cystic fibrosis. Gene Ther. 11(Suppl. 1)S67-S75. [DOI] [PubMed] [Google Scholar]
- 8.Cruz-Orive, L. M., and E. R. Weibel. 1981. Sampling designs for stereology. J. Microsc. 122235-257. [DOI] [PubMed] [Google Scholar]
- 9.Davis, B., J. Nguyen, D. Stoltz, D. Depping, K. J. Excoffon, and J. Zabner. 2004. Adenovirus-mediated erythropoietin production by airway epithelia is enhanced by apical localization of the coxsackie-adenovirus receptor in vivo. Mol. Ther. 10500-506. [DOI] [PubMed] [Google Scholar]
- 10.Dropulic, B., and C. H. June. 2006. Gene-based immunotherapy for human immunodeficiency virus infection and acquired immunodeficiency syndrome. Hum. Gene Ther. 17577-588. [DOI] [PubMed] [Google Scholar]
- 11.Fong, T. C., S. L. Sauter, C. E. Ibanez, P. L. Sheridan, and D. J. Jolly. 2000. The use and development of retroviral vectors to deliver cytokine genes for cancer therapy. Crit. Rev. Ther. Drug Carrier Syst. 171-60. [PubMed] [Google Scholar]
- 12.Gao, G., L. H. Vandenberghe, and J. M. Wilson. 2005. New recombinant serotypes of AAV vectors. Curr. Gene Ther. 5285-297. [DOI] [PubMed] [Google Scholar]
- 13.Goldman, M. J., P. S. Lee, J. S. Yang, and J. M. Wilson. 1997. Lentiviral vectors for gene therapy of cystic fibrosis. Hum. Gene Ther. 82261-2268. [DOI] [PubMed] [Google Scholar]
- 14.Halbert, C. L., A. D. Miller, S. McNamara, J. Emerson, R. L. Gibson, B. Ramsey, and M. L. Aitken. 2006. Prevalence of neutralizing antibodies against adeno-associated virus (AAV) types 2, 5, and 6 in cystic fibrosis and normal populations: implications for gene therapy using AAV vectors. Hum. Gene Ther. 17440-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harvey, B. G., N. R. Hackett, S. Ely, and R. G. Crystal. 2001. Host responses and persistence of vector genome following intrabronchial administration of an E1(−)E3(−) adenovirus gene transfer vector to normal individuals. Mol. Ther. 3206-215. [DOI] [PubMed] [Google Scholar]
- 16.Harvey, B. G., P. L. Leopold, N. R. Hackett, T. M. Grasso, P. M. Williams, A. L. Tucker, R. J. Kaner, B. Ferris, I. Gonda, T. D. Sweeney, R. Ramalingam, I. Kovesdi, S. Shak, and R. G. Crystal. 1999. Airway epithelial CFTR mRNA expression in cystic fibrosis patients after repetitive administration of a recombinant adenovirus. J. Clin. Investig. 1041245-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higginbotham, J. N., P. Seth, R. M. Blaese, and W. J. Ramsey. 2002. The release of inflammatory cytokines from human peripheral blood mononuclear cells in vitro following exposure to adenovirus variants and capsid. Hum. Gene Ther. 13129-141. [DOI] [PubMed] [Google Scholar]
- 18.Jarvis, D. L., and E. E. Finn. 1995. Biochemical analysis of the N-glycosylation pathway in baculovirus-infected lepidopteran insect cells. Virology 212500-511. [DOI] [PubMed] [Google Scholar]
- 19.Johnson, L. G., J. P. Mewshaw, H. Ni, T. Friedmann, R. C. Boucher, and J. C. Olsen. 1998. Effect of host modification and age on airway epithelial gene transfer mediated by a murine leukemia virus-derived vector. J. Virol. 728861-8872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson, L. G., J. C. Olsen, L. Naldini, and R. C. Boucher. 2000. Pseudotyped human lentiviral vector-mediated gene transfer to airway epithelia in vivo. Gene Ther. 7568-574. [DOI] [PubMed] [Google Scholar]
- 21.Johnston, J. C., M. Gasmi, L. E. Lim, J. H. Elder, J.-K. Yee, D. J. Jolly, K. P. Campbell, B. L. Davidson, and S. L. Sauter. 1999. Minimum requirements for efficient transduction of dividing and nondividing cells by feline immunodeficiency virus vectors. J. Virol. 734991-5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keriel, A., C. René, C. Galer, J. Zabner, and E. J. Kremer. 2006. Canine adenovirus vectors for lung-directed gene transfer: efficacy, immune response, and duration of transgene expression using helper-dependent vectors. J. Virol. 801487-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kobinger, G. P., D. J. Weiner, Q. C. Yu, and J. M. Wilson. 2001. Filovirus-pseudotyped lentiviral vector can efficiently and stably transduce airway epithelia in vivo. Nat. Biotechnol. 19225-230. [DOI] [PubMed] [Google Scholar]
- 24.Koehler, D. R., B. Martin, M. Corey, D. Palmer, P. Ng, A. K. Tanswell, and J. Hu. 2006. Readministration of helper-dependent adenovirus to mouse lung. Gene Ther. 13773-780. [DOI] [PubMed] [Google Scholar]
- 25.Leavell, S., B. Wright, L. Scappino, J. Sirriyah, C. Chen, J. D. Clements, and M. J. Burkhard. 2005. Induction of serum and mucosal FIV-specific immune responses by intranasal immunization with p24Gag. Vaccine 231471-1478. [DOI] [PubMed] [Google Scholar]
- 26.Levine, B. L., L. M. Humeau, J. Boyer, R. R. MacGregor, T. Rebello, X. Lu, G. K. Binder, V. Slepushkin, F. Lemiale, J. R. Mascola, F. D. Bushman, B. Dropulic, and C. H. June. 2006. Gene transfer in humans using a conditionally replicating lentiviral vector. Proc. Natl. Acad. Sci. USA 10317372-17377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Limberis, M., D. S. Anson, M. Fuller, and D. W. Parsons. 2002. Recovery of airway cystic fibrosis transmembrane conductance regulator function in mice with cystic fibrosis after single-dose lentivirus-mediated gene transfer. Hum. Gene Ther. 131961-1970. [DOI] [PubMed] [Google Scholar]
- 28.Limberis, M. P., and J. M. Wilson. 2006. Adeno-associated virus serotype 9 vectors transduce murine alveolar and nasal epithelia and can be readministered. Proc. Natl. Acad. Sci. USA 10312993-12998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lindley, K. M., J. L. Su, P. K. Hodges, G. B. Wisely, R. K. Bledsoe, J. P. Condreay, D. A. Winegar, J. T. Hutchins, and T. A. Kost. 2000. Production of monoclonal antibodies using recombinant baculovirus displaying gp64-fusion proteins. J. Immunol. Methods 234123-135. [DOI] [PubMed] [Google Scholar]
- 30.Lu, X., Z. Kurago, and K. A. Brogden. 2006. Effects of polymicrobial communities on host immunity and response. FEMS Microbiol. Lett. 265141-150. [DOI] [PubMed] [Google Scholar]
- 31.Lu, X., L. C. Pingel, K. K. Burnell, J. E. Cavanaugh, and K. A. Brogden. 2007. Carbamoylcholine chloride induces a rapid increase in IL6 in the nasal cavity of C57BL/6 mice. Comp. Med. 57349-354. [PubMed] [Google Scholar]
- 32.Luna, L. G. 1992. Histopathologic methods and color atlas of special stains and tissue artifacts. Johnson Printers, Downers Grove, IL.
- 33.Mastrangeli, A., B. G. Harvey, J. Yao, G. Wolff, I. Kovesdi, R. G. Crystal, and E. Falck-Pedersen. 1996. “Sero-switch” adenovirus-mediated in vivo gene transfer: circumvention of anti-adenovirus humoral immune defenses against repeat adenovirus vector administration by changing the adenovirus serotype. Hum. Gene Ther. 779-87. [DOI] [PubMed] [Google Scholar]
- 34.McCormack, J. E., D. Martineau, N. DePolo, S. Maifert, L. Akabarian, K. Townsend, W. Lee, M. Irwin, N. Sajjadi, D. J. Jolly, and J. Warner. 1997. Anti-vector immunoglobulin induced by retroviral vectors. Hum. Gene Ther. 81263-1273. [DOI] [PubMed] [Google Scholar]
- 35.McKay, T., M. Patel, R. J. Pickles, L. G. Johnson, and J. C. Olsen. 2006. Influenza M2 envelope protein augments avian influenza hemagglutinin pseudotyping of lentiviral vectors. Gene Ther. 13715-724. [DOI] [PubMed] [Google Scholar]
- 36.Moss, R. B., C. Milla, J. Colombo, F. Accurso, P. L. Zeitlin, J. P. Clancy, L. T. Spencer, J. Pilewski, D. A. Waltz, H. L. Dorkin, T. Ferkol, M. Pian, B. Ramsey, B. J. Carter, D. B. Martin, and A. E. Heald. 2007. Repeated aerosolized AAV-CFTR for treatment of cystic fibrosis: a randomized placebo-controlled phase 2B trial. Hum. Gene Ther. 18726-732. [DOI] [PubMed] [Google Scholar]
- 37.Sinn, P. L., E. R. Burnight, M. A. Hickey, G. W. Blissard, and P. B. McCray, Jr. 2005. Persistent gene expression in mouse nasal epithelia following feline immunodeficiency virus-based vector gene transfer. J. Virol. 7912818-12827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sinn, P. L., J. D. Goreham-Voss, A. C. Arias, M. A. Hickey, W. Maury, C. P. Chikkanna-Gowda, and P. B. McCray, Jr. 2007. Enhanced gene expression conferred by stepwise modification of a non-primate lentiviral vector. Hum. Gene Ther. 181244-1252. [DOI] [PubMed] [Google Scholar]
- 39.Sinn, P. L., S. L. Sauter, and P. B. McCray, Jr. 2005. Gene therapy progress and prospects: development of improved lentiviral and retroviral vectors—design, biosafety, and production. Gene Ther. 121089-1098. [DOI] [PubMed] [Google Scholar]
- 40.Sinn, P. L., A. Shah, M. Donovan, and P. B. McCray, Jr. 2005. Viscoelastic gel formulations enhance airway epithelial gene transfer with viral vectors. Am. J. Respir. Cell Mol. Biol. 32404-410. [DOI] [PubMed] [Google Scholar]
- 41.Stein, C. S., J. L. Pemberton, N. van Rooijen, and B. L. Davidson. 1998. Effects of macrophage depletion and anti-CD40 ligand on transgene expression and redosing with recombinant adenovirus. Gene Ther. 5431-439. [DOI] [PubMed] [Google Scholar]
- 42.Tami, C., A. Peralta, R. Barbieri, A. Berinstein, E. Carrillo, and O. Taboga. 2004. Immunological properties of FMDV-gP64 fusion proteins expressed on SF9 cell and baculovirus surfaces. Vaccine 23840-845. [DOI] [PubMed] [Google Scholar]
- 43.Wang, G., V. Slepushkin, J. Zabner, S. Keshavjee, J. C. Johnston, S. L. Sauter, D. J. Jolly, T. W. Dubensky, Jr., B. L. Davidson, and P. B. McCray, Jr. 1999. Feline immunodeficiency virus vectors persistently transduce nondividing airway epithelia and correct the cystic fibrosis defect. J. Clin. Investig. 104R55-R62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang, Y., Q. Li, H. C. J. Ertl, and J. M. Wilson. 1995. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J. Virol. 692004-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang, Y., Q. Su, and J. M. Wilson. 1996. Role of viral antigens in destructive cellular immune responses to adenovirus vector-transduced cells in mouse lungs. J. Virol. 707209-7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zabner, J., B. W. Ramsey, D. P. Meeker, M. L. Aitken, R. P. Balfour, R. L. Gibson, J. Launspach, R. A. Moscicki, S. M. Richards, T. A. Standaert, et al. 1996. Repeat administration of an adenovirus vector encoding cystic fibrosis transmembrane conductance regulator to the nasal epithelium of patients with cystic fibrosis. J. Clin. Investig. 971504-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.