Abstract
Innate defenses help to eliminate infection, but some of them also play a major role in shaping the magnitude and efficacy of the adaptive immune response. With regard to influencing subsequent adaptive immunity, NK cells aided by dendritic cells may be the most relevant components of the innate reaction to herpes simplex virus (HSV) infection. We confirm that mice lacking or depleted of NK cells are susceptible to HSV-induced lesions. The quantity and quality of CD8+ cytotoxic T lymphocytes generated in the absence of NK cells were diminished, thereby contributing to susceptibility to HSV-induced encephalitis. We demonstrate a novel helper role for NK cells, in that NK cells compensate for the loss of CD4 helper T cells and NK cell supplementation enhances the function of wild type anti-HSV CD8 T cells. In addition, NK cells were able to partially rescue the dysfunctional CD8+ T cells generated in the absence of CD4 T helper cells, thereby performing a novel rescue function. Hence, NK cells may well be exploited for enhancing and rescuing the T-cell response in situations where the CD4 helper response is affected.
We have known since the pioneering studies of Bloomfield and Lopez that NK cells influence susceptibility to herpes simplex virus (HSV) infection (4). Most notably, susceptibility to severe herpes infection occurs in humans with genetic defects in the NK cell response (3), and in mice genetic susceptibility differences may be explained by NK cell functional differences (39), but details of this genetic control remain confused (28). We know that the NK cell response and perhaps also NK-T cells (15) help to prevent initial infection, but for HSV it is not clear if the virus-NK cell interaction affects the pattern of the subsequent adaptive immune response. It has become quite evident that the NK cell system is diverse and that several phenotypic and some functional subsets exist (41). The basis of the phenotypic diversity is explained by the multiple receptors expressed on NK cells, some of which are expressed on all NK cells and others of which are limited to different subsets (41). Some of them are also expressed on CD8+ αβ T cells (33).
The NK cell receptors fall into two major functional groups: inhibitory receptors and activating receptors. The inhibitory receptors mainly recognize major histocompatibility complex (MHC) proteins, and when such ligands are diminished, as can occur on a virus-infected or tumor cell, the inferior signal stimulation results in NK cell activation (31). This “missing self” hypothesis could explain why HSV-infected cells, which downregulate MHC molecules (37), result in NK cell activation. NK cells additionally express many activating receptors which may be engaged directly by viral proteins, as has been observed with murine cytomegalovirus (CMV). It was shown that the CMV M157 protein binds to the activating receptor Ly-49H and that mouse strains that encode Ly-49H are resistant to infection (1). It is not known if other herpesviruses, such as HSV, encode proteins that engage NK activating receptors, but doubtless the search for such molecules is ongoing. Since HSV expresses both Toll-like receptor 9 (TLR9) and TLR2 ligand activities (23, 27), conceivably HSV could also interact with NK cells by acting as a ligand for TLR2 or TLR9, both of which are present on NK cells (45). Additionally, the ability of HSV to induce interleukin-12 (IL-12) or other proinflammatory cytokines could act as means of NK cell activation. These NK promoting activities may in turn promote skewing of the adaptive immune response to a protective Th1 phenotype. Furthermore, activated NK products may contribute toward avidity maturation of responding T cells, such as by inducing them to become multiple-cytokine producers (9). Evidence from both human and mouse studies suggests that NK cells may also contribute to adaptive immune responses by modulating dendritic cell (DC) function or by producing effector cytokines (7, 32). In mice, NK cells were shown to be important for inducing Th1 responses and for early resistance to infection (50). Depletion of NK cells inhibited the generation of Toxoplasma-specific CD8+ T-cell immunity in CD4−/− mice, and adoptive transfer of NK cells restored their CD8+ T-cell response (6). However, it is not clear if a similar series of events occurs with HSV infection.
The enhanced NK activities after HSV-1 infection have been well documented both in vitro and in vivo (10, 12, 16, 26), but the molecular mechanism(s) responsible for this innate immune response has not been fully investigated. Moreover, NK cell functional biology is much more complex than previously thought (reviewed in reference 18). For example, NK cells are highly interactive with several cell types, including DCs, B cells, and perhaps T cells. Accordingly, in CMV infection NK cells were shown to differentially modulate different type of DCs (42). While the function of plasmacytoid DCs was limited by the NK cells, the conventional DC compartment was promoted or preserved, leading to accelerated antiviral CD8 T-cell responses (42). Our study extends these findings to the direct helper role of NK cells that skews the immune response to Th1 during priming and also highlights their ability to compensate for the loss of CD4 helper T-cell-mediated grooming of CD8 T cells.
Our results presented in this article suggest that the resistance pattern observed in C57BL/6 mice can be altered if NK cells are depleted. In addition, we demonstrate a novel helper role for NK cells in that cytokines produced by NK cells (gamma interferon [IFN-γ] and/or IL-15 and IL-12) contribute to effective adaptive immune response generation and help in CD8+ cytotoxic T-lymphocyte (CTL) avidity maturation. Although the site of interaction and the actual viral protein ligand that activate the NK cell are not known, the data strongly support the HSV-induced productive interaction of DCs and NK cells as the probable mechanism. The increase in NK cell number at the site of immune induction and its additional novel noncytotoxic helper role during immune induction and memory recall augment the anti-HSV immune response.
MATERIALS AND METHODS
Mice.
C57BL/6 mice were purchased from Harlan Sprague Dawley, Indianapolis, IN, and maintained according to the Guide for the Care and Use of Laboratory Animals (34). Animals were kept in specific-pathogen-free conditions in the Division of Laboratory Animal Resources, College of Medicine, East Tennessee State University, which is fully accredited by the American Association for Accreditation of Laboratory Animal Care.
Viruses.
The HSV type 1 (HSV-1) KOS and HSV-1 17 strains were titrated after growth on Vero cells (CCL81; American Type Culture Collection, Manassas, VA). The virus was stored in aliquots at −80°C until required.
Cell lines.
The Vero and YAC cell lines were obtained from ATCC. The Vero cells were maintained in Dulbecco modified Eagle medium supplemented with 10% heat-inactivated fetal bovine serum, 100 U of penicillin G/ml, 100 μg of streptomycin sulfate/ml, and 2 mM l-glutamine. The YAC cells were maintained in RPMI supplemented with 10% fetal bovine serum, HEPES, sodium bicarbonate, and 2.25 g glucose/liter.
Infection of mice.
The zosteriform infection was performed as described earlier (20). Briefly, hair was depleted from the skin dorsal to the posterior tip of the spleen, corresponding to the 10th thoracic dermatome, by using Veet hair removal gel cream after anesthetizing the mice using avertin. The skin was then scarified using a Dremel variable-speed rotary tool, and 20 μl of HSV-1 17 containing 105 PFU of virus was applied to hair-depleted area of the skin and massaged. Another route of infection used was the tail vein, where a mouse restrainer was used. The footpad and intraperitoneal routes of infection were also used based on the needs of the experiment. All animal experiments were performed in agreement with the American Association for Accreditation of Laboratory Animal Care.
HSV- and peptide-specific lymphoproliferation.
Splenocytes from experimental mice were restimulated in vitro for assessing proliferative ability as described earlier (22). In brief, responders were stimulated with either peptide-pulsed or virus-infected antigen-presenting cells (APCs) for 3 days. The controls included stimulators with anti-CD3 and naïve stimulators. The last 18 h of the incubation was done in the presence of [3H]thymidine. After incubation, plates were harvested and read using the Inotech cell harvester and reader (Inotech, Biosystems International, Lansing, MI). Proliferative responses, tested in quadruplicate wells, were expressed as mean cpm ± standard deviation (SD).
In vitro CTL assay.
The CTL assay was performed as described previously (21). In brief, the splenocytes were serially diluted and added to 96-well U-bottomed plates containing 51Cr-labeled SSIEFARL-pulsed MHC-matched MC-38 cells (H-2b), which served as the target cells. Uninfected MC-38 cells and HSV-infected EMT6 cells (H-2d) were used as syngeneic uninfected and allogeneic infected controls. The effecter/target ratios were 90:1, 30:1, 10:1, and 3:1. After incubation for 4 h at 37°C, the culture supernatant was collected and analyzed for radioactivity with a scintillation counter. Spontaneous release of 51Cr was determined by incubating the target cells with medium alone, and maximum release was determined by adding Triton X-100 to a final concentration of 5%. The percentage of specific lysis was calculated as 100 × [(experimental release − spontaneous release)/(maximum release − spontaneous release)].
Tetramer staining and flow cytometry.
MHC class I (H-2b) tetramers to measure SSIEFARL-specific T cells were provided by the NIAID MHC Tetramer Core Facility (Atlanta, GA). A total of 106 cells obtained from various mice were stained with a mixture of appropriate fluorescein isothiocyanate (FITC)- and PerCp-labeled markers as needed in combination with phycoerythrin (PE)-labeled tetramers for 45 min at 4°C. The controls included an isotype control, stained cells, and unstained cells. They were then analyzed by using a FACScalibur machine and FCS software. The cells were gated on HSV gB tetramer-positive cells and analyzed for various markers to determine HSV-specific events.
Flow cytometric analysis.
A total of 106 splenocytes/ml were incubated with antibody (Ab) to lysosome-associated membrane proteins CD107a and CD107b (FITC labeled) for 1 hour, followed by activation with the SIEFFARL peptide along with addition of the secretion inhibitor monensin (Golgistop), followed by incubation for another 4 to 5 h. After incubation, the cells were stained for surface markers using anti-CD8 (FITC, PerCP, or APC), anti-CD4 (FITC), anti-CD3 (FITC), anti-NK1.1 (PE), anti-Granzyme (Alexafluor 647), and anti-CD69 (FITC). The cells were then fixed and permeabilized using Perm-fix and Perm wash, followed by two washes. The cells were then stained for intracellular markers with anti-IFN-γ (PE), anti-IL-2 (PE), anti-tumor necrosis factor alpha (TNF-α) (FITC), and anti-IL-15 receptor alpha (IL-15Rα) (PE). The samples were analyzed using a FACScalibur four-color flow cytometer, and the data were analyzed using Cellquest or FCS Express software.
NK cell purification for adoptive transfer.
Splenocytes of C57BL/6 mice were red blood cell lysed, washed with RPMI, and plated on a petri dish for 1 h at 37°C. The nonadherent cells were collected and further purified for NK cells using magnetic bead columns (BD Biosciences) by the negative-selection principle. The fraction eluted out of the column was >95% pure for NK cells (CD3− NK1.1+ cells). The NK cells were exposed to 10 μl (1 × 106 cells) of recombinant IL-2, washed thoroughly, and then adoptively transferred to B6 mice via the tail vein.
In vivo CD4 T-cell and NK cell depletion.
The CD4 T-cell depletion was performed as described earlier (13). To deplete CD4 T cells, mice received 250 μg of clone GK1.5 monoclonal Ab (MAb) (GK 1.5 hybridoma; ATCC TIB207, Manassas, VA) on days 0, 12, and 15. Ascitic fluid containing rat immunoglobulin G2b (IgG2b) Ab served as an isotype control. Fluorescence-activated cell sorter (FACS) staining was performed to determine the efficacy of CD4 T-cell depletion. The NK cells were depleted using a polyclonal Ab against asialo-ganglio-N-tetraosylceramide (asialo-GM1) (Wako), expressed at high levels on NK cells, or anti-NK1.1 MAb. The mice were given a 40-μl dose of the Ab intraperitoneally at 5 days postinfection (p.i.), and the ability of the Ab to deplete the NK cells was measured by assessing NK cell functional activity on YAC cells.
In vivo assay for CTL activity measure.
Splenocytes from the different groups of mice were stained with CFSE (Molecular Probes, Eugene, OR), and 5 × 106 cells of each population were adoptively transferred via the tail vein route into the corresponding groups of mice. The spleens were isolated from the mice after 5 h, and the lymphocytes were isolated from the spleens as previously described (20). Target cells were distinguished from recipient cells based on CFSE staining. Histogram plots were used to demonstrate the difference in separation pattern based on intensity of CFSE staining. The recovery and percent killing of the various CFSE-labeled peptide-pulsed targets were calculated as follows: 100 − ([(percentage of peptide pulsed in immunized mice/percentage of peptide unpulsed in immunized mice)/(percentage of peptide pulsed in unimmunized mice/percentage of peptide unpulsed in unimmunized mice)] × 100.
Statistical analysis.
The statistical analysis was performed using the SPSS package version 1.5 and Graphpad Prism 4. The Mann-Whitney U test and analysis of variance were used to arrive at the statistical significance. The P values are provided in each figure and table.
RESULTS
Effect of NK cell depletion on susceptibility to HSV.
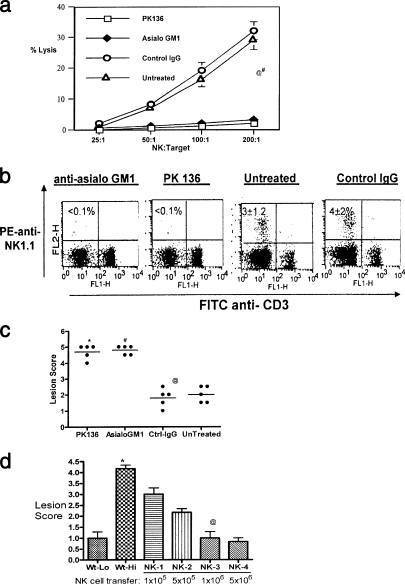
NK cells have been known as killers of virus-infected cells, but their ability to assist the T-cell responses, especially those mediated by CD8+ T cells, has not been evaluated in detail. To measure the influence of NK cells on shaping the primary T-cell responses to HSV, we compared normal B6 mice with those depleted of NK cells using the anti-NK1.1 Ab (PK136) or anti-asialo-GM1 Ab. The efficacy of depletion was measured both by FACS staining (FITC-anti-CD3 and PE-NK1.1 antibodies) and killing assay to measure NK cell-mediated cytolytic activity. As shown in Fig. 1a, the NK cell activity, as measured by its ability to lyse YAC cells, was significantly diminished in both the anti-asialo-GM1 MAb- and PK136-treated groups compared to controls given IgG or untreated control animals. In addition, flow cytometric analysis of the FACS-stained cells revealed that CD3− NK1.1+ cells, which are indicative of NK cells, were below detection limits in anti-asialo-GM1- and PK136-treated mice (Fig. 1b). In contrast, the NK (CD3− NK1.1+) cell numbers were 3% ± 1.5% and 4% ± 2% in the untreated mice and control IgG recipients, respectively. The data presented above were observed 48 h after administration of 50 μl of anti-asialo-GM1 Ab (Wako) or 1 mg of PK136, which were the optimal time and dose as established by dose response and kinetics of depletion studies. Both antibodies were effective in depleting NK cells; however, we used the PK136 MAb in experiments involving CD8+ T-cell responses, since some of these cells are known to express GM1 on their surface (14, 46). In addition, wherever applicable and meaningful, PK136 transgenic mice (PK136 Tg mice), which are chronically depleted of NK cells, were used in parallel in support of our findings.
FIG. 1.
Lack of NK cells contributes to susceptibility, while supplementation enhances resistance. (a) Mice given anti-asialo-GM1 and anti-NK 1.1 antibodies lack detectable NK cell lytic activity. Sample animals from groups administered anti-asialo-GM1 or control IgG were sacrificed 48 h later. Spleen cells were analyzed for in vitro NK cell cytotoxic activity. A suspension of 107/ml of spleen cells was serially diluted with 100 ml in each triplicate well of a 96-well round-bottomed plate. Target 51Cr-labeled YAC-1 mouse lymphoma cells were added to give a effector/target cell ratio of 200, 100, 50, or 25. After 4 h of incubation at 37°C, supernatants containing released 51Cr were collected and counted with an automatic scintillation counter. Specific lysis was calculated as (experimental release − spontaneous release)/(total release − spontaneous release) × 100%, where spontaneous release was derived from wells without effectors and total release from wells with 3% Triton-X added to it. @, asialo-GM1 versus control IgG or untreated, P < 0.0001; #, PK136 versus control IgG and untreated controls, P < 0.0001. (b) Depletion of NK cells by anti-NK1.1 and asialo-GM1 antibodies. B6 mice given anti-asialo-GM1 and PK136 were analyzed for the absence of NK cells by flow cytometry. Single-cell suspensions of spleens were stained with anti-NK1.1 (PE) and anti-CD3 (FITC). The plots represent data obtained from one mouse in each group. The numbers within the plot are the means ± SDs for five mice in each group. The controls included untreated mice and mice given control IgG. Cells that are CD3− NK1.1+ are taken to be the NK cells. (c) Early onset of lesions upon zosteriform challenge in NK-depleted mice. Zoster challenge experiments were performed as described elsewhere (20). Before challenge, the left flank area was depilated by a combination of hair clipping and use of the chemical Nair (Carter-Wallace, New York, NY). The animals were anesthetized with avertin, and scarifications were made in a ∼4-mm2 area. To such scarifications, 10 μl containing 104 PFU of HSV-1 (strain 17) was added and gently massaged. Animals were inspected daily for the development of zosteriform ipsilateral lesions, general behavior changes, encephalitis, and mortality. The severity of the lesions was scored as follows: 1+, vesicle formation; 2+, local erosion and ulceration of the local lesion; 3+, mild to moderate ulceration; 4+, severe ulceration, hind limb paralysis, and encephalitis; and 5+, ultimate death (*, mice that were moribund and hence euthanized). The experiments were repeated three times with five mice in each group, and the outcomes were similar. The lesion scores of all mice within a group at day 10 postchallenge from one such experiment are shown. @, Asialo-GM1 versus control IgG and untreated, P < 0.0001; #, PK136 versus control IgG and untreated, P < 0.0001. (d) NK cell transfer augments protection against HSV. Wild-type B6 mice were divided into six groups of three mice each. Groups 1 and 2 were challenged (zosteriform) with low (5 × 103) (Wt-Lo) and high (1 × 105) (Wt-Hi) doses of virus, respectively. Groups 3 to 6 (NK-1, -2, -3, and -4, respectively) were adoptively transferred with increasing numbers of purified NK cells as indicated 15 h before challenge with the high dose of virus. The animals were handled as described for panel c and lesion scores recorded. The experiment was repeated three times with three mice per group. Data collected on day 10 postchallenge in one such experiment are shown. *, animals in this group were moribund and hence sacrificed. @, statistically significant difference between NK-3 (1 million NK cells) and Wt-Hi (P < 0.05).
Based on the kinetics and dose ascertained previously, the C57BL/6 mice were either depleted of NK cells (1 mg of anti-NK1.1) or given control IgG 2 days before being challenged with a sublethal dose of HSV using the Zosteriform skin infection model (21). This model, as previously demonstrated (20, 21), depends on all arms of immunity for complete protection. Additional controls included mice that received adoptive transfer of 1 million MACS column-purified NK cells and PK136 Tg mice. Low-dose and high-dose challenges with the virus (HSV-1 17) were performed. The low dose did not cause any fatalities in control mice but may induce mild skin lesions (lesion score of 2). However, the high dose will cause death in naïve control mice but should be resisted by HSV-immune and NK cell-supplemented mice. These doses were chosen based on our previously published dose-response studies (30). The NK cell-depleted mice showed early onset of diseases (skin lesion-pustule-lesion score of <2), and as shown in Fig. 1c, all of them succumbed to encephalitis by day 10. The control group suffered only minor lesions, except for one mouse which developed severe encephalitis and was sacrificed on day 12. The lesion score in NK-depleted animals compared to control animals administered IgG was always significantly higher on any day until day 10 (P < 0.0001).
To further confirm the protective effect, wild-type B6 mice were adoptively transferred with various numbers of magnetic column-purified NK cells (1 × 104 to 5 × 106) and challenged 15 h later with a high dose of HSV-1 17 virus. The mice that did not receive any NK cells succumbed to challenge with the high dose of virus, while animals that received additional NK cells showed a decrease in lesion score with the increase in NK cells transferred (Fig. 1d). One million NK cells provided the optimum protection, and hence this number was routinely used in subsequent experimentation. Taken together, the data indicate that the PK136 MAb effectively depleted NK cells and that the absence of NK cells lowers the threshold of protection. Increasing the number of NK cells by adoptive transfer also increased their innate resistance and possibly also contributed to better or augmented anti-HSV adaptive immune responses.
NK cells orchestrate the anti-HSV adaptive immune response. (i) NK cells stimulated by HSV polarize primary immune responses to a protective Th1 type.
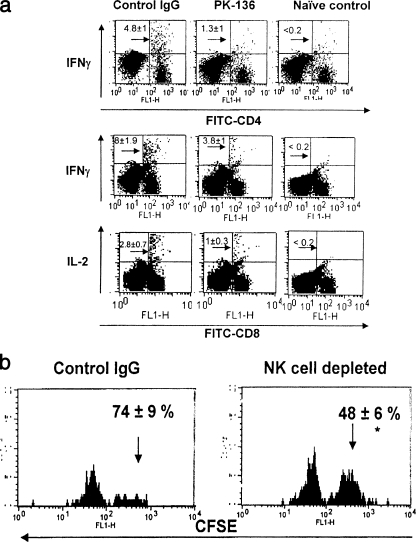
The results accrued from the challenge model described earlier portray the negative impact of NK cell loss on immune protection. To analyze the influence of NK cells on the adaptive immune response, the frequency and function of T cells responding to HSV in intact mice were compared to those in mice depleted of NK cells prior to infection with HSV. Splenocytes from the control or depleted animals were stimulated in vitro with UV-inactivated HSV for 16 h or stimulated for 5 h with HSV gB498-505 peptide (SSIEFARL), respectively, to analyze the HSV-specific CD4+ or CD8+ T-cell phenotype and function. As depicted in Fig. 2a, the frequency of CD4+ IFN-γ+ T cells was reduced about three- to fourfold in NK cell-depleted mice compared to control IgG-treated animals. A similar effect was also observed with the HSV-specific CD8+ T-cell response. While the frequency of CD8+ IFN-γ+ T cells was 8% ± 1.9% in control animals, it was approximately twofold less (3.18% ± 1%) in NK-depleted mice. The frequency of CD8+ IL-2+ T cells was 1% ± 0.3% in the absence of NK cells, compared to 2.8% ± 0.7% in control animals. The frequencies of HSV-specific CD4+ IL-4+ and CD4+ IL-10+ T helper type 2 (Th2) cells were also analyzed in NK cell-depleted and control mice. There was a slight decrease in the frequency of Th2-type cells in NK-depleted mice in response to HSV infection (data not shown). However, the difference was significant only in CD4+ IL-10+ Th2 cells. The frequency of anti-HSV-specific CD4+ IL-10+ cells was 1.13% ± 0.06% in NK-depleted mice, compared to 0.39% ± 0.03% in control animals. It is tempting to suggest a role for these CD4+ IL-10+ cells, but their reduced number in comparison to Th1-type cells may not entirely contribute to the diminished Th1 response in the absence of NK cells. Nevertheless, in all instances Th1 and Tc1 cytokine-secreting cells were significantly less in NK-depleted mice.
FIG. 2.
NK cells contribute to skewing toward the protective Th1 type. (a) Reduced Th1 cytokine production in anti-HSV CD4 and CD8 T cells. C57BL/6 mice were divided into two groups. One group was depleted of NK cells; the other group was administered control IgG and was infected with HSV by the footpad route. At 10 days p.i., their splenocytes were harvested and stimulated with UV-inactivated HSV or SSIEFARL peptide and analyzed for the CD4 and CD8 T-cell activity. Samples were processed individually, and similar patterns were observed within the same group. IFN-γ-producing CD4+ T cells and IFN-γ- and IL-2-producing CD8+ T cells in the control IgG, NK-depleted, and uninfected control animals are shown. The experiment was repeated three times with similar outcomes. The dot plot shows results obtained from one such experiment, and the numbers within the plot are the means ± SDs for five mice. (b) In vivo cytolytic ability is reduced in NK-depleted mice. Splenocytes from wild-type B6 mice were stained with CFSE and loaded with SSIEFARL peptide. A total of 5 × 106 cells were adoptively transferred through the tail vein into control mice and NK-depleted mice. The spleens were isolated from these mice after 5 h and the splenocytes isolated. Target cells were distinguished from recipient cells based on CFSE staining. Histogram plots were used to demonstrate the difference in separation pattern based on intensity of CFSE staining. The recovery and percent killing of the various CFSE-labeled, peptide-pulsed targets were calculated as follows: 100 − ([(percentage of peptide pulsed in immunized mice/percentage of peptide unpulsed in immunized mice)/(percentage of peptide pulsed in unimmunized mice/percentage of peptide unpulsed in unimmunized mice)] × 100). The experiment was repeated two times with three mice in each group. The histogram plot shows the data obtained from one such experiment, and the number within the plot is the mean ± SD of killing ability for three individual mice.
(ii) NK cells contribute to efficacious CD8+ CTL generation.
Since, there was an overall dampening effect in the immune response after NK cell depletion, it was presumed that the cytolytic ability of the virus-specific CD8+ T cells would also be affected in vivo. Hence, an in vivo CTL assay was adapted to measure the ability of the HSV-infected mice (NK depleted and NK intact) to clear or lyse peptide-pulsed syngeneic targets that were adoptively transferred. As expected (Fig. 2b), the cytolytic ability was 48% ± 6% in NK cell-depleted mice, compared to 74% ± 9% in control IgG-treated mice. This indicates that the CD8+ CTLs were less efficient in their cytolytic ability in vivo if they were primed in the absence of NK cells. These data further lend support to our hypothesis that NK cells play a pivotal role in setting the stage for the adaptive immune response and possibly also affect the subsequent memory generation.
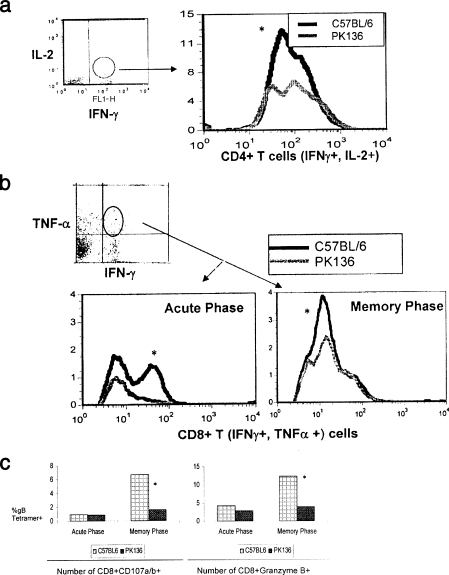
(iii) Diminished response to HSV in PK136 Tg mice.
PK136 Tg mice (49) were infected with HSV, and their subsequent acute and memory CD8+ T-cell responses were compared to those of control animals. The mice were sacrificed on day 8 for acute-phase analyses, and for memory recall studies primed animal were given a low recall dose of virus at day 40 and analyzed 5 days later (day 45). Flow cytometry-based assays were performed on single-cell suspensions. As shown in Fig. 3, significant differences in the frequency of HSV-specific CD4+ and CD8+ T cells were observed between the wild-type and PK136 Tg mice, indicating that the expansion of T cells was not optimal in the absence of NK cells (PK136 Tg mice). Similar difference could be observed when the quality of the T cells was analyzed. Accordingly, 50% more CD4+ T cells responded by producing both IL-2 and IFN-γ upon in vitro stimulation with UV-inactivated HSV in the wild-type mice compared to PK136 Tg mice (Fig. 3a).
FIG. 3.
Reduced frequency and diminished quality of anti-HSV specific CD4+ T helper and CD8+ T-cell numbers in PK136 Tg mice. C57BL/6 mice and PK136 mice were infected with HSV-1 strain 17 by the skin route and analyzed for CD4+ and CD8+ T-cell activity at 7 days p.i. to study the primary immune response and at 45 days p.i. to study the memory response. Splenocytes were harvested at the indicated time points and analyzed for polyfunctional CD4+ T cells (IFN-γ+ and IL-2+) and CD8+ T cells (IFN-γ+ and TNF-α+). In addition, the cytolytic ability of CD8+ T cells was assessed by measuring granzymes and degranulation (CD107a/b). The experiment was done multiple times with similar outcomes. The data shown are representation of one such experiment. (a) Reduced number of polyfunctional CD4+ T cells during the memory phase of infection. Single-cell suspensions were processed as described in Materials and Methods. The sample was gated on IL-2 and IFN-γ double-positive cells and analyzed for CD4 expression. The histogram plot shows the number of CD4+ T cells that are IFN-γ+ and IL-2+ in C57BL/6 and PK136 Tg mice. The patterns were similar in all of the mice within the group. *, the difference is statistically significant. (b) Reduced number of anti-HSV polyfunctional CD8+ T cells during acute and memory infection in PK136 Tg mice. Single-cell suspensions was stained as described above. The cells were gated on IFN-γ+ and TNF-α+ cells as indicated and analyzed for HSV gB tetramer positivity. A representative plot showing the number of gB tetramer-positive CD8+ T cells producing both IFN-γ and TNF-α (polyfunctional CD8+ T cells) during the primary and memory immune responses is shown. *, the difference between the wild-type and PK136 Tg mice was significant. (c) PK136 Tg mice develop reduced CD8+ T cells with cytotoxic ability in HSV infection. Single-cell suspensions were processed as described in Materials and Methods. The analysis for cytolytic activity was done on the CD8+ HSV gB tetramer-positive gated population. A pair of graphs representing the CD107 a/b (left)- and granzyme (right)-expressing HSV-gB tetramer-positive CD8+ T cells in PK136 Tg mice and wild-type mice during primary and recall immune responses is shown. *, although the response was lower in PK136 Tg mice, the difference was statistically significant only during the memory phase analysis.
Even more dramatic effects on the quality of CD8+ T cells could be seen in the PK136 Tg mice compared to wild-type mice. As illustrated in Fig. 3b, the frequency of CD8+ T cells that were induced to produce both IFN-γ and TNF-α was about 40% higher in the wild-type mice (10% CD8+ IFN-γ+ TNF-α+), and it differed significantly from that in the PK136 Tg mice (5.8% CD8+ IFN-γ+ TNF-α+). There was a similar difference in the quality at the acute phase of infection as well, but it was more pronounced during the memory recall response.
The cytolytic ability of the CD8+ T cells as measured by the expression of CD107a and -b and granzyme B was not significantly different between the two groups at the acute phase (Fig. 3c). Nevertheless, a dramatic difference was observed during the memory recall response. Accordingly, 3.5 times more wild-type HSV-specific CD8+ T cells expressed CD107a and -b compared to HSV-specific CD8+ T cells obtained from PK136 Tg mice. Similarly about 4% of HSV-specific PK136 Tg CD8+ T cells expressed granzyme B, compared to about 12% of wild-type CD8+ T cells. This kind of distinct distinction in the diminished quality of memory CD8+ T cells in the PK136 Tg mouse implies the need for NK cell-mediated effects for an optimal memory anti-HSV CD8+ T-cell response.
NK cells play a pertinent role during recall responses and can partially compensate for CD4+ T-cell absence.
The data analyzed at the primary phase suggested that the lack of NK cells affected the T-cell responses, and it is possible that it would have a ripple effect on establishment of memory. In addition, no information exists as to the helper role of NK cells during recall or secondary response or in the event of reactivation from latency. To analyze if NK cell function impinges on secondary recall responses, especially the protective CD8+ T-cell compartment, mice that were primed in the absence of NK cells or supplemented with NK cells (IL-2 activated) were analyzed at the memory phase along with appropriate controls. In addition, the role of NK cells during recall with low-dose (1 × 105 PFU) virus challenge was also assessed in HSV memory mice by depleting NK cells before challenge. As an initial step, the fate of adoptively transferred NK cells was assessed. Accordingly, donor NK cells were labeled with CFSE before transfers into naïve, HSV-infected, and NK-depleted hosts. The recipient animals were sacrificed at different time points and their spleens harvested. Single-cell suspensions were analyzed for CFSE+ NK1.1+ cells. The NK cells expanded (CFSE dilution) depending on “NK space.” Therefore, there was no significant expansion in HSV-infected mice, but they still remained viable and were able to secrete cytokines (data not shown). The behavior was similar to that of a T cell in this regard, as reported earlier (40). The responding CD8+ T cells were analyzed in these animals for their cytolytic activity, cytokine secretion, cytokine receptor expression, and avidity maturation. The cytolytic ability (Table 1), as measured by in vitro CTL assay at an effector cell/target cell ratio of 90:1, was reduced 1.8-fold in NK cell-depleted mice compared to control animals. The percent lysis in the NK-supplemented mice was 1.2-fold higher than that in control animals. The greatest impact was seen in mice that were depleted of CD4+ T cells; it was reduced 1.4-, 2.7-,and 3.1-fold compared to that in NK-depleted, control, and NK-supplemented groups, respectively. The differences between the groups were more pronounced at an effector cell/target cell ratio of 30:1. Besides confirming our previous finding that a lack of concomitant anti-HSV CD4 response affects the quality of HSV-specific CD8+ CTLs (20, 22), this also extends the observation that a similar effect on CD8+ T cells may also be observed with the loss of NK cells. Hence, supplementing or activating NK cells may help during reimmunization of primed animals.
TABLE 1.
In vitro CTL activity is reduced in memory mice that lack NK cells during priminga
| Mouse group | % Lysis (mean ± SD)b with:
|
||
|---|---|---|---|
| MHC-matched MC-38 cells
|
MHC-mismatched EMT6 cells, HSV infected | ||
| HSV infected | Mock infected | ||
| NK depleted | 25 ± 2.8 | 2 ± 1 | <1 |
| Control | 46 ± 4 | 3 ± 1.5 | 1.5 ± 0.5 |
| NK supplemented | 54 ± 5.2 | 4 ± 1 | 2.5 ± 1 |
| CD4 depleted | 17 ± 2 | 1 ± 1 | <1 |
C57BL/6 mice were either depleted of or supplemented with NK cells and primed with HSV. Groups of mice depleted of CD4 T cells and another group of unmanipulated mice served as controls. On day 45, all the mice were infected with a low dose (1 × 105 PFU) of HSV-Kos. The CD8+ T cells from these mice were purified and incubated with MHC-matched HSV-infected or mock-infected, MHC-mismatched HSV-infected, and YAC cell targets at effector/target cell ratios of 90:1, 30:1, 10:1, and 3:1, and an in vitro CTL assay was performed.
Lysis obtained at an effector/target cell ratio of 90:1 from five mice in each group. The data presented are from one of three experiments with similar patterns of results. The YAC cell killing was insignificant. The differences between the four groups of mice are statistically significant (P < 0.0001).
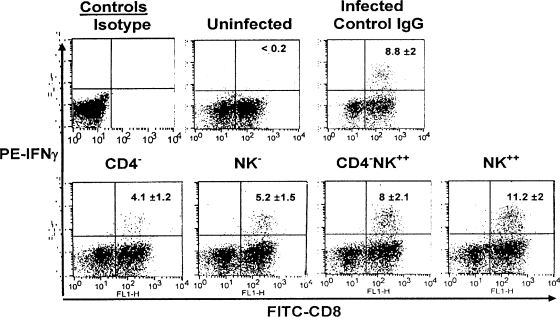
To test this idea, spleen cells isolated from control, NK cell-depleted (NK−), CD4-depleted (CD4−), CD4-depleted and NK-supplemented (CD4− NK++), and normal mice were stimulated with SSIEFARL peptide to measure the outcome in terms of frequency and function of HSV-specific CD8+ T cells. CD8+ T cells that were stimulated in the presence of cognate peptide and IL-2 for 5 h were stained intracellularly for IFN-γ. As seen in Fig. 4, the percentages of CD8+ IFN-γ+ T cells were 4.1 ± 1.2, 5.2 ± 1.5, 8 ± 2.1, and 11.2 ± 2 in the CD4−, NK−, CD4− NK++, and NK++ groups, respectively (NK++ versus CD4− and NK−, P < 0.005; NK++ versus CD4− NK++, P < 0.01). Although the frequency of CD8+ IFN-γ+ was higher in the NK++ group than in the CD4− NK++ group, the difference was not significant. The frequency of CD8+ IFN-γ+ cells was 8.8% ± 2% in animals that received the control IgG. The results suggest that NK cells contribute to the functional efficacy of virus-specific memory CD8+ T cells, that NK cell supplementation has an enhancing effect on memory CD8+ T-cell function, and that NK cells may also contribute to partially compensate for the loss of CD4+ T cells.
FIG. 4.
NK cells affect CD8+ T-cell IFN-γ production, but addition can compensate for the loss of CD4+ T-cell help. C57BL/6 mice with HSV memory were divided into five groups: (i) depleted of NK cells (NK−), (ii) depleted of CD4+ T cells (CD4−), (iii) depleted of CD4+ T cells but supplemented with NK (CD4− NK++), (iv) supplemented with NK cells (NK++), and (v) receiving control IgG. All the mice were given 1 × 105 PFU of HSV and 5 days later were analyzed for IFN-γ production by CD8+ T cells to measure recall responses. The patterns were similar in all three experiments. A representative plot of intracellular IFN-γ staining in peptide-stimulated CD8+ T cells is shown. The data represents one mouse from each of the following groups: uninfected control, control IgG, CD4−, NK−, CD4− NK++, and NK++. The number within the plot is the mean ± SD for five mice in each group. The differences between the NK++ group and the CD4− and NK− groups were statistically significant (P < 0.005). The difference between the NK++ and CD4− NK++ groups was significant (P < 0.01).
NK cell cytokines may contribute to functional improvement and avidity maturation of CD8+ CTLs.
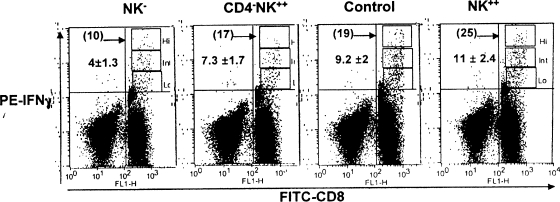
In an attempt to further support this notion, HSV-induced memory mice were divided into four groups: (i) NK cell depleted (NK−), (ii) CD4+ T-cell depleted and NK supplemented (CD4− NK++), (iii) NK cell supplemented (NK++), and (iv) given control IgG. These mice were then given HSV (1 × 105 PFU) to recall CD8+ T cells. Five days later, the spleen cells were harvested and analyzed for their CD8+ T-cell function, including IFN-γ production, cytokine receptor (IL-15Rα) expression, and avidity maturation (CD8β expression). As can be seen in Fig. 5, the percentage of CD8+ IFN-γ+ T cells was 4 ± 1.3 in NK-depleted mice, 7.3 ± 1.7 in CD4-depleted and NK cell-supplemented mice, 11 ± 2.4 in NK-supplemented mice, and 9.2 ± 2 in controls given IgG. In comparison to the control group, there was a 50% reduction in CD8+ T-cell function in NK-depleted mice. However, upon compensation (adoptive transfer) with additional NK cells, the CD4-depleted mice showed an increase of 55% in CD8+ T-cell function, but this was still 20% below the value for the control group. Interestingly, normal memory mice supplemented with NK cells showed an approximate 20% increase in CD8+ T-cell function compared to control mice. Additionally, the CD8+ T cells were separated as high, intermediate, and low producers of IFN-γ in the same FACS dot plot. A quarter of the CD8+ IFN-γ+ T cells were high producers of IFN-γ in the group that received the NK cell supplement (NK++), whereas in the NK-depleted group this segment was less than 10%. The percentages of high producers of IFN-γ are indicated for each group in the representative plots (Fig. 5). Hence, the functional avidity of CD8+ T cells, measured as the capacity to produce IFN-γ (8), was highest in the NK-supplemented groups (both CD4-depleted and normal memory mice). Taken together, the results support our and other previous observations of the importance of CD4+ T cells during recall. In addition, they also highlight the additive or possibly synergistic effect of NK cell supplementation on recall of functionally effective CD8+ T cells.
FIG. 5.
NK cells contribute to higher IFN-γ production by anti-HSV CD8+ T cells. As described for Fig. 4, C57BL/6 HSV memory mice were divided into four groups, in which mice from one group were depleted of NK cells (NK−), another was depleted of CD4 T cells and supplemented with NK cells (CD4− NK++), the third was supplemented with NK cells (NK++), and the fourth served as a control and received control IgG. All the mice were given 1 × 105 PFU of HSV and 5 days later analyzed for IFN-γ production. Representative dot plots show the CD8 T cells producing IFN-γ in the NK-depleted (NK−), CD4 depleted and NK-supplemented (CD4− NK++), control IgG-administered (control), and NK-supplemented (NK++) mice. The FACS plot has been compensated such that the cells are skewed as high, intermediate, and low IFN-γ-producing CD8 T cells. The numbers indicate means ± SDs of the percentage of CD8+ IFN-γ+ T cells from five individual mice. The number in parentheses is the percentage of high IFN-γ producers within the positive population. One-way analysis of variance indicates the difference to be statistically significant (P < 0.0001).
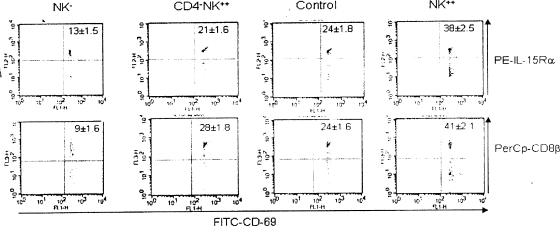
The supportive role of NK cells was more evident upon analysis of IL-15Rα and CD8β expression on SSIEFARL peptide-specific CD8+ T cells. The molecular connection between high IL-15Rα levels and CD8β expression in the context of high-avidity CD8+ CTLs has been addressed earlier (35). Since NK cells are a source of IL-15 and in addition possess the ability to trans-present IL-15 (5), it was hypothesized that NK cells may contribute to enhanced IL-15Rα and CD8β expression on HSV-specific CTLs. To test this, splenocytes harvested from various groups (NK cell depleted [NK−], CD4+ T-cell depleted and NK supplemented [CD4− NK++], NK cell supplemented [NK++], and given control IgG) were stimulated briefly (2 h) with SSIEFARL peptide and immediately analyzed by FACS staining for the expression of IL-15Rα, CD8β, CD69, and CD8α. HSV-specific CD8+ T cells expressed the early-activation marker CD69 as a consequence of brief exposure to the cognate peptide. Thus, peptide-stimulated splenocytes were gated on CD8α+ CD69+ cells and analyzed for the expression of IL-15Rα or CD8β. As shown in Fig. 6, the IL-15R expression was highest on cells that were obtained from mice supplemented with NK cells (38%), followed by the control group administered IgG (24%). The values for the group that was depleted of their CD4+ T cells but given additional NK cells were within the error margin of those for the control group (21%). The lowest expression was found on cells collected from mice that were depleted of NK cells. The difference observed, based on the staining of this receptor, supports the conclusion that NK cell-mediated effects (cytokine IL-15 or IFN-γ) induced high-avidity CTLs that expressed higher levels of IL-15Rα. This enables them to respond to homeostatic proliferation by efficiently capturing IL-15 (5).
FIG. 6.
NK cells mediate functional improvement and avidity maturation of anti-HSV CD8+ T cells. HSV memory C57BL/6 mice were grouped and treated as described in the legend to Fig. 5. The splenocytes were briefly stimulated (2 h) with the HSV immunodominant SSIEFARL (gB 498 to 505) peptide. This was followed by staining with anti-IL-15Rα and anti-CD8β Abs. HSV gB peptide-specific CD8+ T cells were analyzed for the expression of IL-15Rα and CD8β by gating CD69-positive CD8+ T cells. The dot plot represents the IL-15Rα- and CD8β-expressing HSV-specific CD8+ T cells in the four groups mentioned above. The experiment was repeated three times with similar patterns of results. The number within the plot is the mean ± SD from one such experiment. One-way analysis of variance indicates the difference between the groups to be significant (P < 0.0001).
With regard to CD8β expression, the pattern was in line with the IL-15Rα expression. As expected, the HSV-specific CD8+ CTLs isolated from the NK-supplemented mice showed the highest expression of the coreceptor CD8β (41%), and the expression in control group was around 50% reduced (24%). About 28% of the HSV-specific CD8+ T cells in the CD4-depleted NK-supplemented group expressed CD8β, and the least expression (9%) was observed in the NK cell-depleted group (Fig. 6). The expression levels were about twofold higher in the NK-supplemented group than in controls and threefold higher than those in CD8+ CTLs without help.
High-avidity CTLs can kill specific targets even when their coreceptor CD8β is blocked. Hence, to further confirm the above observations, an in vitro CTL assay was performed in the presence of anti-CD8β Ab during the coincubation of CTLs and targets. The blocking of the CD8β did not hamper the lytic ability of the higher-avidity CTLs isolated from NK-supplemented mice, in stark contrast to CTLs isolated from NK-depleted mice, where the cytolysis was reduced around 55% (data not shown).
In conclusion, our results show that NK cells contribute to avidity maturation of CD8+ CTLs by their production and probably trans-presentation of IL-15, and in addition, the IFN-γ produced by them may also be able to partially rescue the CTLs generated in the absence of a helper response.
DISCUSSION
Our results confirm that NK cells contribute to innate resistance of C57BL/6 mice and that depletion of NK cells results in greater susceptibility to HSV infection. In addition, the NK cells also help shape the adaptive immune response, probably by promoting effective interaction between the APCs and T cells by licensing the APCs and lowering the threshold of activation of the responding T cells. Further, in a novel helper role, the NK cell-produced cytokine IFN-γ may contribute toward better differentiation and avidity maturation of CD8+ CTLs either directly or in conjunction with IL-15. NK cells, because of their expression of high IL-15Rα levels, may help in this process by trans-presentation of IL-15. Incidentally, the CD8+ CTLs isolated from NK-supplemented groups showed the highest expression of IL-15Rα and CD8β, which is indicative of their high quality. Although there are multiple studies highlighting the importance of NK cells in the primary antiviral response, we believe that our results show for the first time the critical role that NK cells play during recall of antiviral memory responses. Our experiments with NK supplementation during the recall response to HSV demonstrated their helper role in augmenting the quality of T-cell responses, especially CD8+ T cells. Thus, NK cells, besides performing innate immune functions, could also be exploited during memory recall by specifically targeting them to make key cytokines or even help in direct costimulation.
The general response to viral infections is often an increase in NK activity (2, 43). Our results have confirmed by a number of approaches that NK cell depletion leads to greater susceptibility to HSV infection. The PK136 MAb effectively depleted NK cells in C57BL/6 mice, and the absence of NK cells lowered the threshold of protection. Additionally, PK136 Tg mice (49), which lack NK cells, also mounted an inferior response to HSV infection. Increasing the number of NK cells in wild-type mice by adoptive transfer increased their innate resistance and possibly also contributed to a better or augmented anti-HSV immune response. On detailed analysis it is evident that NK cell depletion resulted in a reduced adaptive immune response to HSV and a partial impairment of T-cell differentiation toward a Th1 or Tc1 phenotype. Furthermore, NK cell depletion had several effects on the quantity and quality of the anti-HSV CD8+ and CD4+ T-cell responses. Evidence from numerous studies with humans and mice has suggested that NK cells play a relevant role in the establishment of adaptive immune responses (6, 7, 32, 48).
Our results further lend support to the finding that NK cells play a pivotal role in setting the stage for the adaptive immune response and possibly also affect the subsequent memory generation as well. In addition, our study for the first time highlights the importance of NK cells as novel helpers in the rescue of CD8+ T cells in the absence of conventional CD4+ T helper cells. NK cell helper function may be manifested in several ways. Evidence from earlier studies indicates that NK cells can be induced to function as noncytotoxic helper cells following stimulation with IL-18 (29). NK cells are also known to costimulate T cells (17), and lastly, NK cells may enable effective antigen presentation by killing antigen-bearing migratory DCs and making it possible for lymphoid resident DCs to cross present to the T cells. Hence, appropriately stimulated NK cells may play a prominent role in the protection and subsequent modulation of the immune responses to HSV during reimmunization or secondary recall.
The advances in our understanding of the mechanisms of activation/inhibition of NK cells have revealed complexity that was not originally expected (24). Thus, while NK cell activation occurred due to a lack of surface MHC class I on targets, the actual functionality and target lysis requires a multitude of signals that include stress proteins, cytokines, and involvement of other sentinel cells (24). Although NK cells were originally thought to act independently, accumulating evidence indicates that NK cells also respond to stimuli from other immune effectors, especially DCs (51). During the preparation of this paper, a study on the role of NK cells in promoting early CD8 T-cell responses against CMV was published by Robbins et al. (42). The mechanism proposed by this group involves the ability of NK cells to limit plasmacytoid DC IFN-α/β production to levels not immunosuppressive to the host, thus permitting promotion of an early CD8 T-cell response. Our preliminary studies also suggest that the reciprocal cross talk between NK cells and DCs that is induced by HSV products not only promotes rapid innate responses against the virus but also favors the generation of appropriate downstream adaptive responses. Detailed studies are under way and may offer better clues as to the site and sequence of events immediately after HSV infection.
Although signaling through activating and inhibitory receptors seems to be important, cytokines also play a critical role in dictating NK cell behavior. Cytokines such as IL-12, IL-15, IL-18, IL-21, and IFNs (IFN-α/β) can induce NK cell survival, proliferation, cytotoxicity, and/or IFN-γ production (25, 47). In fact, IL-12 and IL-18 may also prevent the NK inhibition induced by inhibitory receptor signaling (38). Of all the cytokines, IL-15 may play a dominant role in the NK cell-mediated effects. Strong support for this contention comes from a very recent study by Horng et al. (19). Accordingly, the signaling through an activating NK receptor (NKG2D) and IL-15Rα are tied together, affecting the function and response to each other (19). Additionally, NK cells are also capable of trans-presenting IL-15 (5) efficiently and, as also shown in human studies, could possibly present antigen to stimulate T cells besides producing stimulatory cytokines (17).
The molecular connection between high IL-15Rα levels and CD8β expression in the context of high-avidity CD8+ CTLs has been demonstrated earlier in a different context (35). Since NK cells are a potent source of IL-15 and additionally posses the ability to trans-present IL-15, it was hypothesized that they may contribute to enhanced CD8β expression on HSV-specific CTLs. The supportive role of NK cells was more evident upon analysis of IL-15Rα and CD8β expression on SSIEFARL peptide-specific CD8+ T cells. The twofold increase in the staining of this receptor in the NK-supplemented group supports the conclusion that NK cell-mediated effects (cytokine IL-15 or IFN-γ) induced high-avidity CTLs that expressed higher levels of IL-15Rα. This enables them to better respond to homeostatic proliferation by efficiently capturing IL-15. Thus, NK cells probably maintain the frequency of anti-HSV-specific CD8+ IFN-γ+ T cells by producing IFN-γ, which elicits secretion of IL-15 and IL-18 by other cells. These cytokines in turn favor expansion of Tc1 CD8+ T cells. This conclusion has support from earlier studies done with other systems. In a study of persons infected with Mycobacterium tuberculosis (48), it was shown that the frequency of CD8+ IFN-γ+ T cells was restored by activated NK cells. The mechanism for such restoration was mediated by soluble factors such as IFN-γ, IL-15, and IL-18 (anti-IFN-γ abrogated the positive effect) and/or through CD40/CD40 ligand interaction (48). Therefore, NK cells utilize at least two distinct mechanisms to contribute to effective CD8+ T-cell generation.
Our earlier studies on the rescue of defective CD8+ T cells indicated that CD4+ T cells producing IFN-γ were better at restoring the anti-HSV CD8+ T-cell function (20). In addition, IFN-γ has been shown to increase the production of IL-15 in phagocytic cells (11). IL-15, for its part, enhances virus-specific CD8+ T cells (44), and IL-18 also contributes to the development of Tc1 CD8+ T cells (36). We speculate that during the initial interaction at an immune induction site, activated NK cells produce IFN-γ that induces the production of IL-15 in DCs. In turn, the IL-15 is taken up and trans-presented by NK cells, facilitating the expansion of HSV-specific CD8+ T cells that become efficient effector cells. Classically, CD8+ T effector cells that have been optimally activated have been considered to both produce IFN-γ and exhibit CTL activity. Results from assays measuring IFN-γ, CD107a and -b, and granzyme B in HSV-specific CD8+ CTLs indicate the superior quality in NK cell-competent mice.
Thus, we conclude that NK cells activated by the virus directly or by an intermediary cell contribute to the initial reduction in viral load and enhance the stimulatory ability of the DCs by enabling effective antigen processing and presentation. In addition, activated NK cells are able to rescue defective CD8+ T cells that were generated in the absence of cognate help and could compensate for loss of CD4+ T cells. These findings have the following implications: (i) NK cells could help the DCs to increase their stimulatory ability, (ii) the quality of CD8+ T cells is better if NK cells are involved, (iii) NK cells can compensate for the loss in CD4 help, (iv) NK cells can be exploited in situations where CD4+ T cells are depleted and in individuals who are incapable of mounting a strong CD4 response (e.g., elderly individuals who are better responders to a vaccine, such as influenza vaccine, have efficient NK cells). Hence, therapeutic strategies involving immunization protocols specifically exploiting NK cells could be adopted, especially during priming and recall of the adaptive immune response. We plan to selectively expand different subsets of NK cells prior to or early or late in infection to see if this would shape the response. Several drugs, adjuvants, and antibodies do serve to activate and expand NK cells, and it is worthwhile to test some of these for their influence on HSV immunity.
Acknowledgments
Funding for this study was through startup funds from the Department of Microbiology, Quillen College of Medicine, ETSU, and NIH grant AI 106336501 to B.T.R.
Footnotes
Published ahead of print on 20 August 2008.
REFERENCES
- 1.Arase, H., E. S. Mocarski, A. E. Campbell, A. B. Hill, and L. L. Lanier. 2002. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science 2961323-1326. [DOI] [PubMed] [Google Scholar]
- 2.Bancroft, G. J. 1993. The role of natural killer cells in innate resistance to infection. Curr. Opin. Immunol. 5503-510. [DOI] [PubMed] [Google Scholar]
- 3.Biron, C. A., K. S. Byron, and J. L. Sullivan. 1989. Severe herpesvirus infections in an adolescent without natural killer cells. N. Engl. J. Med. 3201731-1735. [DOI] [PubMed] [Google Scholar]
- 4.Bloomfield, S. E., and C. Lopez. 1980. Herpes infections in the immunosuppressed host. Ophthalmology 871226-1235. [DOI] [PubMed] [Google Scholar]
- 5.Burkett, P. R., R. Koka, M. Chien, S. Chai, D. L. Boone, and A. Ma. 2004. Coordinate expression and trans presentation of interleukin (IL)-15Rα and IL-15 supports natural killer cell and memory CD8+ T cell homeostasis. J. Exp. Med. 200825-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Combe, C. L., T. J. Curiel, M. M. Moretto, and I. A. Khan. 2005. NK cells help to induce CD8+-T-cell immunity against Toxoplasma gondii in the absence of CD4+ T cells. Infect. Immun. 734913-4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper, M. A., T. A. Fehniger, A. Fuchs, M. Colonna, and M. A. Caligiuri. 2004. NK cell and DC interactions. Trends Immunol. 2547-52. [DOI] [PubMed] [Google Scholar]
- 8.Cush, S. S., K. M. Anderson, D. H. Ravneberg, J. L. Weslow-Schmidt, and E. Flano. 2007. Memory generation and maintenance of CD8+ T cell function during viral persistence. J. Immunol. 179141-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Darrah, P. A., D. T. Patel, P. M. De Luca, R. W. Lindsay, D. F. Davey, B. J. Flynn, S. T. Hoff, P. Andersen, S. G. Reed, S. L. Morris, M. Roederer, and R. A. Seder. 2007. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat. Med. 13843-850. [DOI] [PubMed] [Google Scholar]
- 10.Djeu, J. Y., N. Stocks, K. Zoon, G. J. Stanton, T. Timonen, and R. B. Herberman. 1982. Positive self regulation of cytotoxicity in human natural killer cells by production of interferon upon exposure to influenza and herpes viruses. J. Exp. Med. 1561222-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doherty, T. M., R. A. Seder, and A. Sher. 1996. Induction and regulation of IL-15 expression in murine macrophages. J. Immunol. 156735-741. [PubMed] [Google Scholar]
- 12.Fitzgerald, P. A., M. Mendelsohn, and C. Lopez. 1985. Human natural killer cells limit replication of herpes simplex virus type 1 in vitro. J. Immunol. 1342666-2672. [PubMed] [Google Scholar]
- 13.Gangappa, S., S. P. Deshpande, and B. T. Rouse. 2000. Bystander activation of CD4+ T cells accounts for herpetic ocular lesions. Investig. Ophthalmol. Vis. Sci. 41453-459. [PubMed] [Google Scholar]
- 14.Ghiasi, H., S. Cai, G. C. Perng, A. B. Nesburn, and S. L. Wechsler. 2000. The role of natural killer cells in protection of mice against death and corneal scarring following ocular HSV-1 infection. Antiviral Res. 4533-45. [DOI] [PubMed] [Google Scholar]
- 15.Grubor-Bauk, B., A. Simmons, G. Mayrhofer, and P. G. Speck. 2003. Impaired clearance of herpes simplex virus type 1 from mice lacking CD1d or NKT cells expressing the semivariant V alpha 14-J.alpha 281 TCR. J. Immunol. 1701430-1434. [DOI] [PubMed] [Google Scholar]
- 16.Habu, S., K. Akamatsu, N. Tamaoki, and K. Okumura. 1984. In vivo significance of NK cell on resistance against virus (HSV-1) infections in mice. J. Immunol. 1332743-2747. [PubMed] [Google Scholar]
- 17.Hanna, J., T. Gonen-Gross, J. Fitchett, T. Rowe, M. Daniels, T. I. Arnon, R. Gazit, A. Joseph, K. W. Schjetne, A. Steinle, A. Porgador, D. Mevorach, D. Goldman-Wohl, S. Yagel, M. J. LaBarre, J. H. Buckner, and O. Mandelboim. 2004. Novel APC-like properties of human NK cells directly regulate T cell activation. J. Clin. Investig. 1141612-1623. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Hanna, J., and O. Mandelboim. 2007. When killers become helpers. Trends Immunol. 28201-206. [DOI] [PubMed] [Google Scholar]
- 19.Horng, T., J. S. Bezbradica, and R. Medzhitov. 2007. NKG2D signaling is coupled to the interleukin 15 receptor signaling pathway. Nat. Immunol. 81345-1352. [DOI] [PubMed] [Google Scholar]
- 20.Kumaraguru, U., K. Banerjee, and B. T. Rouse. 2005. In vivo rescue of defective memory CD8+ T cells by cognate helper T cells. J. Leukoc. Biol. 78879-887. [DOI] [PubMed] [Google Scholar]
- 21.Kumaraguru, U., I. A. Davis, S. Deshpande, S. S. Tevethia, and B. T. Rouse. 2001. Lymphotoxin alpha−/− mice develop functionally impaired CD8+ T cell responses and fail to contain virus infection of the central nervous system. J. Immunol. 1661066-1074. [DOI] [PubMed] [Google Scholar]
- 22.Kumaraguru, U., S. Suvas, P. S. Biswas, A. K. Azkur, and B. T. Rouse. 2004. Concomitant helper response rescues otherwise low avidity CD8+ memory CTLs to become efficient effectors in vivo. J. Immunol. 1723719-3724. [DOI] [PubMed] [Google Scholar]
- 23.Kurt-Jones, E. A., M. Chan, S. Zhou, J. Wang, G. Reed, R. Bronson, M. M. Arnold, D. M. Knipe, and R. W. Finberg. 2004. Herpes simplex virus 1 interaction with Toll-like receptor 2 contributes to lethal encephalitis. Proc. Natl. Acad. Sci. USA 1011315-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lanier, L. L. 2005. NK cell recognition. Annu. Rev. Immunol. 23225-274. [DOI] [PubMed] [Google Scholar]
- 25.Lauwerys, B. R., J. C. Renauld, and F. A. Houssiau. 1999. Synergistic proliferation and activation of natural killer cells by interleukin 12 and interleukin 18. Cytokine 11822-830. [DOI] [PubMed] [Google Scholar]
- 26.Lopez, C., R. Ryshke, and M. Bennett. 1980. Marrow-dependent cells depleted by 89Sr mediate genetic resistance to herpes simplex virus type 1 infection in mice. Infect. Immun. 281028-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lund, J., A. Sato, S. Akira, R. Medzhitov, and A. Iwasaki. 2003. Toll-like receptor 9-mediated recognition of herpes simplex virus-2 by plasmacytoid dendritic cells. J. Exp. Med. 198513-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lundberg, P., P. Welander, H. Openshaw, C. Nalbandian, C. Edwards, L. Moldawer, and E. Cantin. 2003. A locus on mouse chromosome 6 that determines resistance to herpes simplex virus also influences reactivation, while an unlinked locus augments resistance of female mice. J. Virol. 7711661-11673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mailliard, R. B., S. M. Alber, H. Shen, S. C. Watkins, J. M. Kirkwood, R. B. Herberman, and P. Kalinski. 2005. IL-18-induced CD83+CCR7+ NK helper cells. J. Exp. Med. 202941-953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manickan, E., M. Francotte, N. Kuklin, M. Dewerchin, C. Molitor, D. Gheysen, M. Slaoui, and B. T. Rouse. 1995. Vaccination with recombinant vaccinia viruses expressing ICP27 induces protective immunity against herpes simplex virus through CD4+ Th1+ T cells. J. Virol. 694711-4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mocikat, R., H. Braumuller, A. Gumy, O. Egeter, H. Ziegler, U. Reusch, A. Bubeck, J. Louis, R. Mailhammer, G. Riethmuller, U. Koszinowski, and M. Rocken. 2003. Natural killer cells activated by MHC class I(low) targets prime dendritic cells to induce protective CD8 T cell responses. Immunity 19561-569. [DOI] [PubMed] [Google Scholar]
- 32.Moretta, A. 2002. Natural killer cells and dendritic cells: rendezvous in abused tissues. Nat. Rev. Immunol. 2957-964. [DOI] [PubMed] [Google Scholar]
- 33.Moser, J. M., A. M. Byers, and A. E. Lukacher. 2002. NK cell receptors in antiviral immunity. Curr. Opin. Immunol. 14509-516. [DOI] [PubMed] [Google Scholar]
- 34.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC.
- 35.Oh, S., L. P. Perera, D. S. Burke, T. A. Waldmann, and J. A. Berzofsky. 2004. IL-15/IL-15Ralpha-mediated avidity maturation of memory CD8+ T cells. Proc. Natl. Acad. Sci. USA 10115154-15159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Okamoto, I., K. Kohno, T. Tanimoto, H. Ikegami, and M. Kurimoto. 1999. Development of CD8+ effector T cells is differentially regulated by IL-18 and IL-12. J. Immunol. 1623202-3211. [PubMed] [Google Scholar]
- 37.Orange, J. S., M. S. Fassett, L. A. Koopman, J. E. Boyson, and J. L. Strominger. 2002. Viral evasion of natural killer cells. Nat. Immunol. 31006-1012. [DOI] [PubMed] [Google Scholar]
- 38.Ortaldo, J. R., and H. A. Young. 2003. Expression of IFN-γ upon triggering of activating Ly49D NK receptors in vitro and in vivo: costimulation with IL-12 or IL-18 overrides inhibitory receptors J. Immunol. 1701763-1769. [DOI] [PubMed] [Google Scholar]
- 39.Pereira, R. A., A. Scalzo, and A. Simmons. 2001. A NK complex-linked locus governs acute versus latent herpes simplex virus infection of neurons. J. Immunol. 1665869-5873. [DOI] [PubMed] [Google Scholar]
- 40.Prlic, M., B. R. Blazar, M. A. Farrar, and S. C. Jameson. 2003. In vivo survival and homeostatic proliferation of natural killer cells. J. Exp. Med. 197967-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raulet, D. H. 2004. Interplay of natural killer cells and their receptors with the adaptive immune response. Nat. Immunol. 5996-1002. [DOI] [PubMed] [Google Scholar]
- 42.Robbins, S. H., G. Bessou, A. Cornillon, N. Zucchini, B. Rupp, Z. Ruzsics, T. Sacher, E. Tomasello, E. Vivier, U. H. Koszinowski, and M. Dalod. 2007. Natural killer cells promote early CD8 T cell responses against cytomegalovirus. PLoS Pathog. 3e123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robertson, M. J., and J. Ritz. 1990. Biology and clinical relevance of human natural killer cells. Blood 762421-2438. [PubMed] [Google Scholar]
- 44.Schluns, K. S., K. Williams, A. Ma, X. X. Zheng, and L. Lefrancois. 2002. Requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells. J. Immunol. 1684827-4831. [DOI] [PubMed] [Google Scholar]
- 45.Sivori, S., M. Falco, M. Della Chiesa, S. Carlomagno, M. Vitale, L. Moretta, and A. Moretta. 2004. CpG and double-stranded RNA trigger human NK cells by Toll-like receptors: induction of cytokine release and cytotoxicity against tumors and dendritic cells. Proc. Natl. Acad. Sci. USA 10110116-10121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Slifka, M. K., R. R. Pagarigan, and J. L. Whitton. 2000. NK markers are expressed on a high percentage of virus-specific CD8+ and CD4+ T cells. J. Immunol. 1642009-2015. [DOI] [PubMed] [Google Scholar]
- 47.Smith, P. L., G. Lombardi, and G. R. Foster. 2005. Type I interferons and the innate immune response-more than just antiviral cytokines. Mol. Immunol. 42869-877. [DOI] [PubMed] [Google Scholar]
- 48.Vankayalapati, R., P. Klucar, B. Wizel, S. E. Weis, B. Samten, H. Safi, H. Shams, and P. F. Barnes. 2004. NK cells regulate CD8+ T cell effector function in response to an intracellular pathogen. J. Immunol. 172130-137. [DOI] [PubMed] [Google Scholar]
- 49.Yuan, D., R. Bibi, and T. Dang. 2004. The role of adjuvant on the regulatory effects of NK cells on B cell responses as revealed by a new model of NK cell deficiency. Int. Immunol. 16707-716. [DOI] [PubMed] [Google Scholar]
- 50.Zabaleta, A., L. Arribillaga, D. Llopiz, J. Dotor, J. J. Lasarte, J. Prieto, F. Borras-Cuesta, J. I. Esteban, J. Quer, F. Vayreda, and P. Sarobe. 2007. Induction of potent and long-lasting CD4 and CD8 T-cell responses against hepatitis C virus by immunization with viral antigens plus poly(I:C) and anti-CD40. Antiviral Res. 7425-35. [DOI] [PubMed] [Google Scholar]
- 51.Zitvogel, L. 2002. Dendritic and natural killer cells cooperate in the control/switch of innate immunity. J. Exp. Med. 195F9-F14. [DOI] [PMC free article] [PubMed] [Google Scholar]