Abstract
Previously, combination DNA/nonreplicating adenovirus (Ad)- or poxvirus-vectored vaccines have strongly protected against SHIV89.6P, DNAs expressing cytokines have modulated immunity elicited by DNA vaccines, and replication-competent Ad-recombinant priming and protein boosting has strongly protected against simian immunodeficiency virus (SIV) challenge. Here we evaluated a vaccine strategy composed of these promising components. Seven rhesus macaques per group were primed twice with multigenic SIV plasmid DNA with or without interleukin-12 (IL-12) DNA or IL-15 DNA. After a multigenic replicating Ad-SIV immunization, all groups received two booster immunizations with SIV gp140 and SIV Nef protein. Four control macaques received control DNA plasmids, empty Ad vector, and adjuvant. All vaccine components were immunogenic, but the cytokine DNAs had little effect. Macaques that received IL-15-DNA exhibited higher peak anti-Nef titers, a more rapid anti-Nef anamnestic response postchallenge, and expanded CD8CM T cells 2 weeks postchallenge compared to the DNA-only group. Other immune responses were indistinguishable between groups. Overall, no protection against intrarectal challenge with SIVmac251 was observed, although immunized non-Mamu-A*01 macaques as a group exhibited a statistically significant 1-log decline in acute viremia compared to non-Mamu-A*01 controls. Possible factors contributing to the poor outcome include administration of cytokine DNAs to sites different from the Ad recombinants (intramuscular and intratracheal, respectively), too few DNA priming immunizations, a suboptimal DNA delivery method, failure to ensure delivery of SIV and cytokine plasmids to the same cell, and instability and short half-life of the IL-15 component. Future experiments should address these issues to determine if this combination approach is able to control a virulent SIV challenge.
AIDS is one of the greatest pandemics of our time, affecting the health and the social and economic foundations of countries worldwide. A potent human immunodeficiency virus (HIV) vaccine offers the best hope for controlling the spread of the virus. While a single immune correlate has not been identified, both antibodies and CD8 T-cell responses contribute to control of infection with HIV or the related simian immunodeficiency virus (SIV) and disease progression (5, 17, 23, 28, 35-39, 43, 46, 58). Appropriately designed envelope immunogens able to induce broad, potent neutralizing antibodies have not yet been achieved, but vaccine-elicited virus-specific cellular immune responses have been more readily elicited. Both DNA and recombinant viral vectors have emerged as prominent candidate vaccines for this purpose. Although DNA vaccines are not as immunogenic as other vectored vaccines, a variety of approaches can enhance their potency (20). Further, the striking observations that DNA priming followed by boosting with an adenovirus (Ad) or modified vaccinia virus Ankara (MVA)-vectored vaccine elicits enhanced immunity and protective efficacy (3, 59) suggest that among many available vectors (27) other DNA-vector combinations might be equally or more potent.
Increasing knowledge of cytokine networks and their influences on the immune system has provided new opportunities for vaccine design and propelled the field toward tailored immune responses. IL-12, first described as natural killer (NK) cell stimulatory factor (25), and IL-15 (18) are among promising candidate cytokine adjuvants for directing such tailored immune responses. Both interleukins have strong effects on NK cells and T cells, influencing the magnitude and quality of cellular responses (1, 4, 15, 30, 63). IL-12 acts as adjuvant for both CD4 and CD8 T-cell responses. When administered as a DNA expression vector in combination with other DNA vaccines, it increased cellular immunity in mice (24) and enhanced both humoral and cellular immune responses in rhesus macaques (11, 13, 57, 61). Recently, the addition of IL-12 DNA plasmids to a SIVgag DNA vaccine regimen and to a prime/boost DNA/vesicular stomatitis virus-SIVgag regimen increased protective efficacy against a SHIV89.6P challenge (11, 13). IL-15, in contrast, primarily increases cellular immunity and is important for development of memory T cells (44). IL-15 may enhance central memory cells, while IL-12 may lead to greater terminal differentiation and development of effector memory cells (1). In nonhuman primates, IL-15 enhances both CD4 and CD8 effector memory T cells (41, 52) and, depending on the timing of administration, production of long-lived CD4 and CD8 memory T cells (62). However, potent, polyfunctional cytokines such as IL-15 must be administered cautiously. In macaques, for example, IL-15 prevented vaccine-induced control of viral replication (22), and in SIV-infected macaques it increased viral load and the rate of disease progression (40).
We are developing a replication-competent Ad recombinant vaccine approach (33), having demonstrated in chimpanzees that at the same or lower dose, priming with replicating Ad-HIVenv followed by envelope protein boosting elicited better cellular and humoral immune responses than a similar regimen using a nonreplicating Ad-HIVenv vaccine (50). A contribution of the HIV envelope immunogens to protection has been established using the SHIV89.6P model, in which prechallenge antibody titers elicited by a combination prime-boost regimen incorporating HIV Env and Tat in comparison to a multigenic regimen were associated with significantly stronger protection against the viral challenge (12). Furthermore, the value of an envelope protein component in the vaccine strategy was confirmed in a study showing better protection against SHIV89.6P elicited by an Ad-prime/protein boosting regimen in comparison to Ad priming alone (47). Importantly, in the rigorous SIVmac251 challenge model, priming with multigenic Ad-SIV recombinants and boosting with envelope subunits potently protected 39% of rhesus macaques (49). The protection was durable, as shown in a subsequent rechallenge study 1 year later with no intervening booster immunization (32).
Priming by first-generation, unmodified DNA plasmids followed by boosting with a replication-competent Ad type 5 host range mutant (Ad5hr)-SIV recombinant did not enhance subsequent immunogenicity in a small pilot study (34). Here we revisited this question, using improved multigenic SIV DNA plasmid vaccines as priming vehicles, with and without additional plasmid DNAs encoding rhesus IL-12 and IL-15, to elicit stronger, long-lived immune responses. We structured the study around our standard immunization regimen (two mucosal administrations of replication-competent Ad recombinants followed by two boosts with envelope protein), which has elicited strong immunogenicity and long-lasting protection against SIVmac251 (31, 32, 48, 49). We asked if two sequential DNA priming immunizations could substitute for the initial Ad recombinant priming. These were followed by a single boost with replicating multigenic Ad5hr-SIV recombinants and two boosts with SIV gp140 and SIV Nef proteins. The macaques were subsequently challenged intrarectally with pathogenic SIVmac251.
MATERIALS AND METHODS
Immunization and challenge of macaques.
Twenty-five Indian rhesus macaques were housed at Advanced BioScience Laboratories, Inc. (ABL; Kensington, MD). The care and maintenance of the animals were in compliance with established guidelines, and the animal protocol received approval from the ABL Animal Care and Use Committee prior to study initiation. The macaques were immunized as outlined in Table 1. The three experimental immunization groups contained seven monkeys each, and the control group contained four. Eight Mamu-A*01-positive macaques were assigned to groups, two animals per group. Peripheral blood and tissue samples were obtained prior to immunization and periodically over the course of immunization and following challenge. At week 48 the macaques were challenged intrarectally with 10 50% monkey infectious doses of a rhesus peripheral blood mononuclear cell (PBMC)-grown SIVmac251 challenge stock kindly provided by Ronald C. Desrosiers, New England National Primate Research Center, and made available by Nancy Miller, Division of AIDS, NIAID.
TABLE 1.
Immunization and challenge protocol
| Immunogen | Challenge, by groupa
|
|||
|---|---|---|---|---|
| Group 1, DNA | Group 2, DNA/ IL-12 | Group 3, DNA/ IL-15 | Group 4, control | |
| DNA,b wks 0 and 4, intramuscular | SIVmac239env | SIVmac239env | SIVmac239env | Control DNA |
| SIVmac239gag | SIVmac239gag | SIVmac239gag | ||
| SIVmac239rev/nef | SIVmac239rev/nef | SIVmac239rev/nef | ||
| Control DNA | DNA/IL-12 | DNA/IL-15 | ||
| Ad5hr-SIV,c wk 12, intratracheal | SIVsmH4env/rev | SIVsmH4env/rev | SIVsmH4env/rev | Ad5hr-ΔE3 vector |
| SIVmac239gag | SIVmac239gag | SIVmac239gag | ||
| SIVmac239nefΔ1-13 | SIVmac239nefΔ1-13 | SIVmac239nefΔ1-13 | ||
| Proteins,d wk 24 and 36, intramuscular | SIVmac251 gp140 | SIVmac251 gp140 | SIVmac251 gp140 | MPL-SE |
| SIVmac239 Nef | SIVmac239 Nef | SIVmac239 Nef | Adjuvant | |
| Challenge,e wk 48, intrarectal | SIVmac251 | SIVmac251 | SIVmac251 | SIVmac251 |
Groups 1 to 3 had seven macaques per group; group 4 had four macaques.
At 2.5 mg DNA per dose, mixed together and administered at multiple sites in both thighs.
At 5 × 108 PFU/recombinant; total Ad dose was 1.5 × 109 PFU.
SIV gp140, 100 μg/dose; SIV Nef, 50 μg/dose.
At 10 50% monkey infectious doses.
Immunogens.
DNA plasmids used for vaccination were cytomegalovirus promoter-driven codon-optimized sequences of SIVmac239env, C-terminally truncated SIVmac239gag (p37), and SIVmac239rev/nef. A dual promoter expression vector encoding the rhesus IL-12 p35 and p40 genes (13) and a rhesus IL-15 expression plasmid (11) contained codon-optimized genes for high expression in mammalian cells. The plasmids were manufactured and purified by Puresyn (Malvern, PA) and formulated in 0.15 M citrate buffer and 0.25% bupivicaine for intramuscular administration. The replication-competent Ad5hr recombinants encoding SIVsmH4env/rev, SIVmac239gag, and SIVmac239nefΔ1-13 have been described previously (10, 48, 64) and were administered intratracheally in phosphate-buffered saline (PBS) at a dose of 5 × 108 PFU/recombinant. Empty Ad5hrΔE3 vector (1.5 × 109 total dose) served as a control. Protein boosts consisted of SIVmac251 gp140 and SIVmac239 Nef (ABL) mixed with a 1/10 volume of monophosphoryl lipid A-stable emulsion (Corixa, Hamilton, MT) and administered intramuscularly, 100 μg and 50 μg per dose, respectively.
Sample collection.
PBMCs were isolated using Ficoll-Paque Plus (GE Healthcare) and used fresh in all assays unless otherwise stated. Surplus cells were frozen in 90% fetal bovine serum (Invitrogen) and 10% dimethyl sulfoxide (Sigma-Aldrich, St. Louis, MO) and stored in liquid nitrogen until use. Lymph node biopsies and bronchoalveolar lavages (BAL) were collected periodically during the study. Lymph nodes were minced and passed through a 70-μm cell strainer (BD Pharmingen). The isolated cells were pelleted at 550 × g for 7 min and washed twice with PBS before use. BAL cells were obtained by flushing one bronchus with PBS and separating out the lymphocytes on a discontinuous Percoll (Sigma-Aldrich) gradient as described previously (47). The cells were maintained overnight at 37°C and 5% CO2 in R10 (RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum, 100 U/ml penicillin, 100 μg/ml streptomycin, and 1 mM l-glutamine) and used the next day.
Cellular immune responses.
For the evaluation of virus-specific gamma interferon (IFN-γ)-secreting cells, the monkey IFN-γ enzyme-linked immunospot (ELISPOT) kit from U-CyTech Biosciences (Utrecht, The Netherlands) was used according to the manufacturer's protocol. PBMCs were distributed in triplicate wells of 96-well plates (100 μl at dilutions of 1 × 106 and 5 × 105 cells/ml) and stimulated by adding a single pool of Gag, Nef, or Env peptides at a final concentration of 1 μg/ml for each peptide. Concanavalin A (5 μg/ml) served as a positive control and R10 or R10 plus dimethyl sulfoxide (final concentration, 0.7%) as negative controls. Results are reported as spot-forming cells (SFC)/million PBMCs following subtraction of spots in negative control wells. SIVsmH4 Env and SIVmac251 Nef peptides (ABL) and SIVmac239 Gag peptides (AIDS Research and Reference Reagent Program, NIAID, NIH) were 15-mers overlapping by 11 amino acids.
A similar ELISPOT was conducted using the Mabtech anti-rhesus IFN-γ antibody (Mabtech, Sweden) at a concentration of 15 μg/ml in 0.1 M carbonate-bicarbonate solution (pH 9.6) in 96-well nitrocellulose membrane plates (Millipore, MA). Each sample was set up in triplicate at 2 × 105 cells per well. The samples were stimulated for 24 h with four separate pools of SIVmac239 Env peptides (AIDS Research and Reference Reagent Program, NIAID, NIH) and three separate pools of SIVmac239 Gag peptides. The responses were detected with a biotinylated anti-IFN-γ antibody followed by streptavidin-alkaline phosphatase. Spots were visualized with 5-bromo-4-chloro-3-indolylphospate-nitroblue tetrazolium substrate (Sigma-Aldrich). A positive response was defined as 2 SFC/2 × 105 PBMCs above the control as well as background levels assessed at week zero. ELISPOT responses to SIVmac239 Gag were also evaluated following CD8 depletion of PBMCs using anti-human CD8 antibody, cross-reactive with rhesus CD8, that was conjugated to magnetic Dynal beads according to the manufacturer's protocol (Dynal, Invitrogen, CA).
Intracellular cytokine staining.
Intracellular cytokine staining for detection of SIV Gag-, Env (smH4)-, and Nef-specific IFN-γ-, IL-2-, and tumor necrosis factor alpha (TNF-α)-secreting CD8+ and CD4+ central and effector memory T cells was performed on freshly isolated PBMCs, BAL, and lymph node (LN) cells. Cells (1 × 106) in 1 ml of R10 were either not stimulated or incubated with pools of Env, Nef, or Gag peptides (1 μg/ml each peptide) or concanavalin A (positive control) for 6 h at 37°C and 5% CO2. One hour into the incubation, Golgi-Stop (BD Pharmingen) was added to all tubes. Poststimulation the cells were transferred into fluorescence-activated cell sorter tubes (BD) and washed twice with PBS (Invitrogen). A cocktail of the following surface antibodies was added: CD4-peridinin chlorphyll protein (clone L200; BD Pharmingen) or CD8β-R-phycoerythrin-Texas Red (clone 2ST8.5H7; Beckman-Coulter), CD95-phycoerythin (clone DX2; BD Pharmingen), and CD28-fluorescein isothiocyanate (clone CD28.2; BD Pharmingen). The cells were incubated in the dark for 25 min at room temperature, washed with PBS, and fixed in 125 μl of Fix and Perm solution A (Invitrogen) for 15 min. After further washing the cells were incubated in 125 μl Fix and Perm solution B containing a cocktail of anti-IFN-γ (clone B27), anti-IL-2 (clone MQ1-17H12), and anti-TNF-α (clone MAb11) antibodies (all from BD Pharmingen) coupled to allophycocyanin as described above. The cocktail approach was used since experiments were performed using a four-color flow cytometer. Cell numbers were also limited, precluding multiple staining reactions. The cells were washed in PBS and stored in PBS containing 3.7% formaldehyde solution at 4°C until analysis. Analysis was performed on a BD FACSCalibur using CellQuest software. A minimum of 50,000 events in the lymphocytic gate, based on forward and side scatter, were acquired. A positive response was defined as an increase in the percentage of IFN-γ-positive cells in stimulated PBMCs over unstimulated PBMCs that was significant at the two-tailed α level of 0.05 by the continuity-adjusted chi-squared test. The response comparison was excluded from the analysis if the harmonic mean of the gated central or effector memory event numbers in the comparison were less than 300, due to the substantial loss of power for detecting a response.
Humoral immune responses.
Binding antibodies to SIV gp140 and SIV Nef were assessed by enzyme-linked immunosorbent assay as described previously (48). The antibody titer was defined as the reciprocal of the serum dilution at which the optical density of the test serum was two times greater than that of a naïve control macaque serum diluted 1:50.
ADCC assay.
The antibody-dependent cellular cytotoxicity (ADCC) assay was performed as described in detail elsewhere (16) using heat-inactivated serum or plasma samples and human PBMCs as effectors. The target cells were CEM-NKr coated with 15 μg/ml of recombinant SIVmac251 gp140. The ADCC assay results were acquired on a BD FACSCalibur machine and analyzed with WinMDI version 2.8.
Virologic assays.
For the assessment of viral loads, the enhanced chemiluminescence-based nucleic acid sequence-based amplification assay with a sensitivity of 2,000 copies/input volume was used (55). To evaluate plasma samples consistently below this detection limit, a real-time nucleic acid sequence-based amplification assay with a sensitivity of <50 copies/input volume was used (32).
Statistical analyses.
Analyses of ELISPOT responses, antibody titers, and viral loads used the exact Wilcoxon rank sum test for simple two-group comparisons, the exact Kruskal-Wallis test for comparisons across the three immunization groups or all four groups at once, and the exact Wei-Johnson test for two-group comparisons over multiple times. P values reported have been corrected for the multiple comparisons between groups, except as noted below for Fig. 6E.
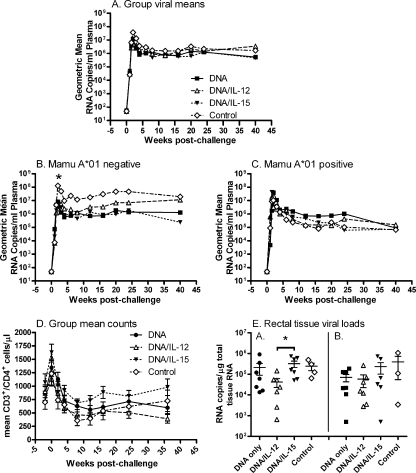
FIG. 6.
Evaluation of protective efficacy following intrarectal challenge with SIVmac251. (A) Viral loads by immunization group over time, including all macaques. (B and C) Viral loads by immunization group for Mamu-A*01-negative (B) and Mamu-A*01-positive (C) macaques only. The asterisk in panel B denotes a 1-log reduction in acute viremia of combined immunized macaques compared to controls (P = 0.037). (D) Mean CD4 counts by immunization group. Error bars indicate the standard errors of the means (SEM). (E) Viral loads in rectal biopsies 2 and 8 weeks postchallenge (graphs A and B, respectively). Viral loads are plotted for individual animals in each group. Group means (large bars) and SEM (small bars) are indicated. The DNA/IL-12 group showed a lower viral load compared to the DNA/IL-15 group at week 2 postchallenge (marked by the asterisk), which was marginally nonsignificant after correction for multiple comparisons (P = 0.065).
RESULTS
Prechallenge ELISPOT responses.
Over the immunization course, IFN-γ ELISPOT responses to SIV Env, Gag, and Nef were elicited (Fig. 1). Responses to Rev were low throughout and are not reported here. Two sets of Env peptides were used for stimulating the PBMCs as shown (Fig. 1A and B): one matched the SIVsmH4env encoded in the Ad5hr recombinant and the other matched the SIVmac239env DNA plasmid and was closely related to the SIVmac251 Env protein boost. Only weak responses were observed following stimulation with either peptide pool following the two DNA immunizations, but a boosting effect was observed after administration of Ad5hr-SIVsmH4env (Fig. 1A and B). Despite the high background of the control animals at week 14 and the variability among animals as shown by the error bars (Fig. 1B), responses to both SIVmac239 and SIVsmH4 Env peptides were observed, reflecting a priming effect by the SIVmac239env plasmid inoculations. Following the first SIVmac251 envelope immunization, SIVmac239 Env responses were boosted, reflecting the initial SIVmac239env DNA priming. PBMCs obtained after the second envelope boost were only available for assay with SIVsmH4 Env peptides. Overall, no significant differences in Env-specific IFN-γ secretion were seen between the immunization groups.
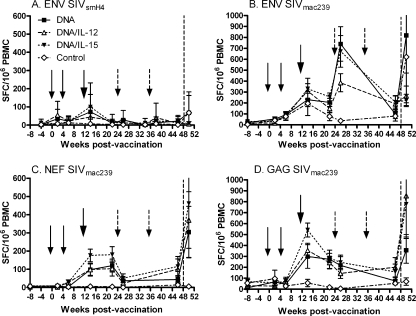
FIG. 1.
IFN-γ ELISPOT responses to SIV Env, Nef, and Gag peptide pools. Mean SFC ± the standard errors of the means for each immunization group are shown for each immunogen. Env-specific responses were evaluated using both SIVsmH4 and SIVmac239 Env peptide pools (A and B). Small arrows indicate times of DNA administration (weeks 0 and 4), the large arrow shows the time of Ad5hr-SIV recombinant immunization at week 12, and the broken arrows mark Env and Nef protein boosts at weeks 24 and 36. The vertical dashed lines indicate the time of challenge.
Responses to Nef and Gag exhibited similar patterns (Fig. 1C and D). High levels of ELISPOT responses were observed following the Ad5hr-SIV administrations at week 12, but administration of Nef protein at weeks 24 and 36 did not boost the number of Nef-specific IFN-γ-secreting cells. Both Nef- and Gag-specific responses declined prior to challenge. As with the Env responses, no significant differences were seen between the three immunization groups.
CD8 depletion ELISPOT.
SIV Gag-specific IFN-γ-secreting PBMCs were further analyzed after depletion of CD8-positive cells. As shown in Table 2, DNA immunization elicited a predominantly CD4 response, which shifted to include more CD8 T-cell responses following the Ad5hr-SIV immunizations. By week 46, 2 weeks prior to challenge, the ELISPOT responses were again predominantly CD4. Overall, there were no significant differences in levels of CD4 or CD8 responses between immunization groups.
TABLE 2.
Proportion of SIV Gag-specific CD4+ IFN-γ-secreting T cells in peripheral blood over time as evaluated by ELISPOT, with and without CD8+ T-cell depletion
| Wk | ELISPOT response to SIV Gaga
|
CD3+ CD4+ countb
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group 1, DNA
|
Group 2, DNA/IL-12
|
Group 3, DNA/IL-15
|
||||||||||
| PBMC | CD8− PBMC | % CD4+ PBMC | PBMC | CD8− PBMC | % CD4+ PBMC | PBMC | CD8− PBMC | % CD4+ PBMC | Group 1, DNA | Group 2, DNA/IL-12 | Group 3, DNA/IL-15 | |
| Postvaccination | ||||||||||||
| 2 | 66 ± 29 | 49 ± 25 | 74 | 28 ± 16 | 34 ± 10 | 122 | 59 ± 27 | 58 ± 28 | 98 | |||
| 14 | 299 ± 107 | 223 ± 80 | 75 | 354 ± 65 | 205 ± 50 | 58 | 541 ± 64 | 417 ± 69 | 77 | |||
| 22 | 274 ± 83 | 160 ± 53 | 59 | 242 ± 55 | 125 ± 41 | 52 | 285 ± 42 | 170 ± 28 | 60 | |||
| 26 | 218 ± 79 | 126 ± 36 | 58 | 144 ± 45 | 103 ± 28 | 71 | 245 ± 66 | 133 ± 20 | 54 | |||
| 46 | 74 ± 33 | 88 ± 39 | 118 | 158 ± 88 | 165 ± 89 | 104 | 158 ± 72 | 195 ± 70 | 124 | 863 ± 128 | 904 ± 119 | 1,069 ± 161 |
| Postchallenge | ||||||||||||
| 2 | 357 ± 120 | 59 ± 33 | 16 | 854 ± 390 | 83 ± 27 | 10 | 778 ± 279 | 43 ± 15 | 6 | 1,132 ± 127 | 870 ± 171 | 1,006 ± 85 |
The PBMC and CD8− PBMC data are reported as mean SFC ± the standard error of the mean; the percent CD4+ PBMCs was calculated as follows: (mean CD8− PBMC SFC)/(mean PBMC SFC) × 100.
Group mean CD4 cell counts ± standard errors of the means.
Analysis of prechallenge memory T cells.
SIV Env-, Nef-, and Gag-specific effector and central memory CD4 and CD8 T cells in PBMCs and LN were examined by intracellular cytokine staining for production of IL-2, IFN-γ, and TNF-α in combination and in BAL cells for production of IFN-γ. Rectal pinch biopsies were obtained as well but yielded too few cells, precluding collection of meaningful data. While the ELISPOT results indicated a preponderance of vaccine-elicited IFN-γ-secreting SIV Gag-specific CD4 T cells in peripheral blood (Table 2), SIV Env-specific CD8CM T cells made up the greatest proportion of memory cells (Fig. 2C versus A, B, and D). This may have been due to the majority of effector memory cells homing to mucosal effector sites. Previously, we reported the presence of gut homing receptors on CD8+ T cells induced by our vaccine regimen, as well as central and effector memory cells in BAL, an effector site (65). Here, the strongest responses prior to challenge (weeks 2 to 38) were elicited by SIVsmH4 Env peptides. In view of the weak Env-specific IFN-γ ELISPOT responses following the two DNA immunizations (Fig. 1A and B), one must assume these DNA vaccine-elicited CD8CM T cells were producing primarily IL-2 and/or TNF-α. Overall, none of the memory cell compartments displayed differences among immunization groups prior to challenge.
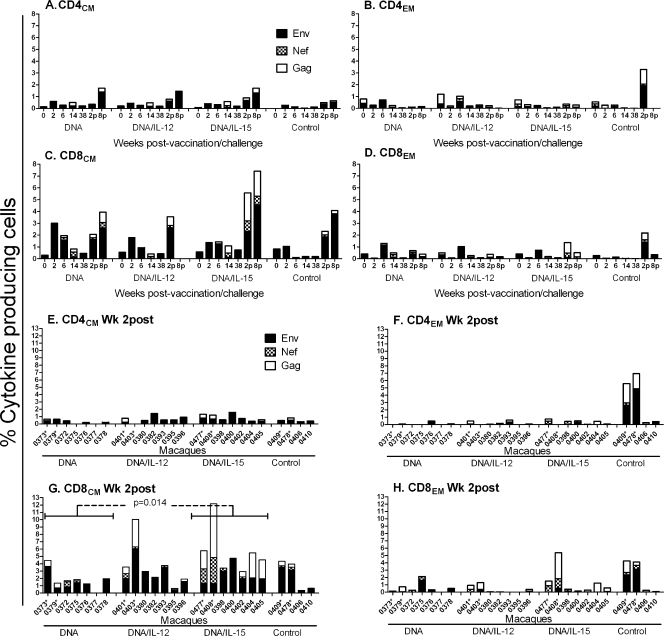
FIG. 2.
Intracellular cytokine staining for SIV-specific CD4 and CD8 memory T cells secreting IFN-γ, IL-2, and TNF-α in PBMCs. (A to D) Stacked responses to SIV Env, Nef, and Gag peptide pools by CD4 and CD8 central and effector memory cells over time. Weeks 2 and 8 postchallenge are designated 2p and 8p, respectively. Mean responses for each group at each time point are plotted. (E to H) Stacked responses observed 2 weeks postchallenge for individual animals in each group. The asterisks denote Mamu-A*01-positive macaques. Macaque 0377 was not assayed at week 2 postchallenge.
LN biopsies obtained at weeks 6 and 14 following the DNA and Ad5hr recombinant immunizations revealed low and sporadic SIV-specific CD4 and CD8 memory T-cell responses with no significant differences among groups (data not shown). In contrast, BAL cells, tested only for secretion of IFN-γ, exhibited strong CD8CM and CD8EM responses as expected at week 14 after the Ad5hr-SIV immunizations, which persisted through week 38 prior to challenge. The responses were mainly against Gag and Nef, reflecting the priming by the matched DNA immunizations (Fig. 3A and B). Once again, no differences were observed between immunization groups.
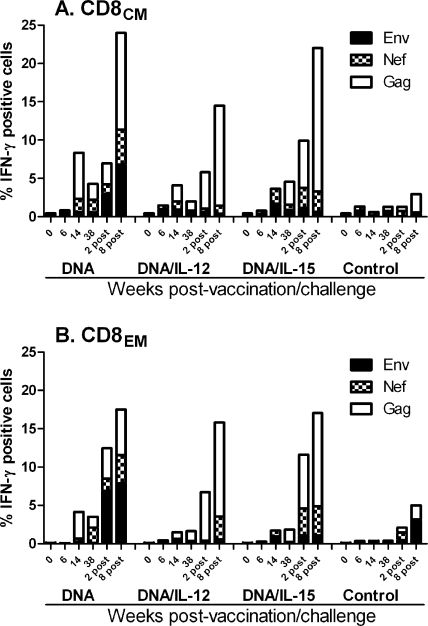
FIG. 3.
Intracellular cytokine staining for SIV-specific CD8CM (A) and CD8EM (B) T cells secreting IFN-γ in bronchoalveolar lavage fluid. Stacked responses to SIV Env, Nef, and Gag peptide pools are shown over time. Mean responses for each group at each time point are plotted.
Prechallenge humoral immune responses.
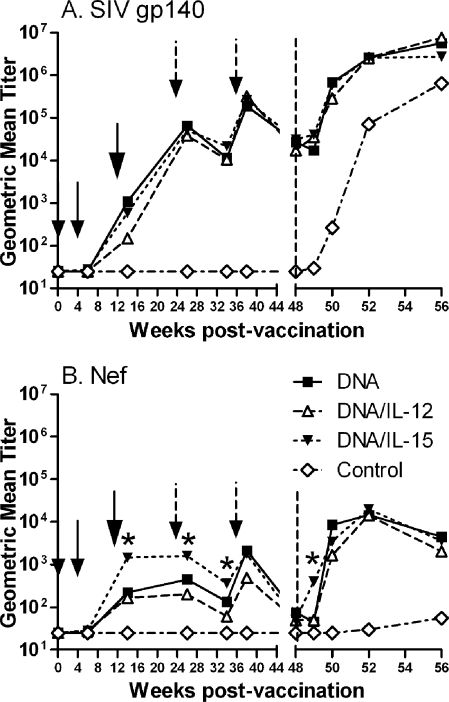
SIV gp140-specific binding antibodies were induced in all immunization groups following the Ad5hr-SIV immunizations at week 12 and were boosted to similar high titers following the envelope protein immunizations (Fig. 4A). Antibodies to Nef also appeared following the Ad5hr recombinant immunizations in all three vaccinated groups (Fig. 4B); however, in contrast to envelope-specific antibodies, macaques in group 3 primed in the presence of DNA encoding IL-15 exhibited anti-Nef titers significantly higher than for groups 1 and 2 at weeks 14, 26, and 34 (P values of 0.04, 0.044, and 0.029, respectively). By the time of challenge, both envelope and Nef antibodies had declined to comparable values in all immunization groups.
FIG. 4.
Vaccine-induced antibody responses. Geometric mean antibody binding titers against SIV gp140 (A) and SIV Nef (B) are plotted over time. Small arrows indicate times of DNA administration (weeks 0 and 4), the large arrows show the time of Ad5hr-SIV recombinant immunization at week 12, and the broken arrows mark Env and Nef protein boosts at weeks 24 and 36. The vertical dashed lines indicate the time of challenge, and stars indicate significantly higher titers of the DNA/IL-15 group compared to groups 1 and 2 (P = 0.04, 0.044, 0.029, and 0.014 at weeks 14, 26, 34, and 49, respectively).
ADCC.
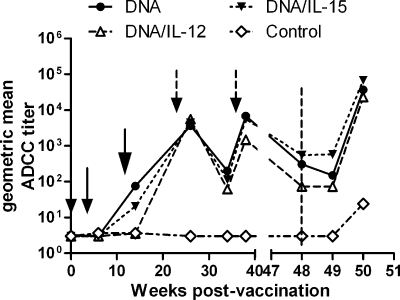
Previously, anti-envelope antibodies with binding titers of approximately 104 at the time of challenge were not able to neutralize the primary SIVmac251 challenge virus (49) but mediated ADCC activity, which is significantly correlated with reduced acute-phase viremia (17). Therefore, macaque sera were tested for the ability to mediate ADCC activity using SIVmac251 gp140-coated target cells (Fig. 5). Sera from macaques in group 1 (DNA) and group 3 (DNA/IL-15) first showed ADCC activity after administration of the Ad5hr recombinants. While activity in all immunized macaque sera was strongly enhanced by the first protein boosts, ADCC titers remained low subsequently, with macaques in group 2 (DNA/IL-12) exhibiting the lowest titers. Overall, there were no significant differences between the three immunization groups.
FIG. 5.
Vaccine-induced ADCC. ADCC antibody titers were evaluated over the course of the study using target cells coated with SIV gp140. Small arrows indicate times of DNA administration (weeks 0 and 4), the large arrow shows the time of Ad5hr-SIV recombinant immunization at week 12, and the broken arrows mark Env and Nef protein boosts at weeks 24 and 36. The vertical dashed line indicates the time of challenge.
SIVmac251 challenge outcome.
Following intrarectal challenge with SIVmac251, all animals became infected. No protection was observed in the immunized macaques compared to controls (Fig. 6A). The majority of Mamu-A*01-positive animals controlled viremia better than their Mamu-A*01-negative counterparts, as expected (data not shown). However, when Mamu-A*01-negative and -positive animals were analyzed separately (Fig. 6B and C), the combined immunized Mamu-A*01-negative animals showed a clear protective effect, with a 1-log reduction in acute viremia (weeks 1 to 4) compared to the controls (P = 0.037) (Fig. 6B). No difference was observed between immunization groups. Although the control non-Mamu-A*01 macaques continued to display viral loads approximately 1.5 logs higher than macaques in the DNA and DNA/IL-15 groups during the chronic phase, these differences were not statistically significant. In contrast, the strong effect of Mamu-A*01 in the two control and six immunized macaques carrying this allele obscured any protective effect of the vaccine regimen, and the immunization groups were indistinguishable from the controls at all time points (Fig. 6C). Patterns of CD3+ CD4+ T-cell decline similarly revealed no differences in protective efficacy between the immunized and control macaques (Fig. 6D) regardless of whether the macaques were separated into Mamu-A*01-negative or -positive groups (data not shown).
Rectal pinch biopsies were obtained postchallenge for analysis of viral RNA (Fig. 6E). All samples tested were positive. At week 2 postchallenge the immunized groups taken together were not different from the controls. The IL-12-primed animals (group 2) exhibited a lower viral load than the IL-15-primed animals (group 3) (P = 0.011) and the controls (P = 0.024) (Fig. 6E), but when corrected for multiple comparisons, the differences became only borderline significant (group 2 versus group 3, P = 0.065; group 2 versus controls, P = 0.073). Group 2 macaques maintained the lowest rectal tissue viral load at week 8 postchallenge (Fig. 6E), but overall no significant differences among groups were observed at this later time point.
Postchallenge cellular immune responses.
Env-specific recall responses in PBMCs were not observed at week 2 postchallenge by ELISPOT assay using either SIVsmH4 or SIVmac239 Env peptides (Fig. 1A and B), perhaps reflective of the mixed immunizations, including SIVmac239env encoded in DNA, SIVsmH4env encoded in Ad5hr-SIV, and SIVmac251 gp140 envelope protein. However, strong recall responses were observed against both Nef and Gag peptides (Fig. 1C and D), but with no differences between the three immunization groups.
A strong expansion of SIV-specific CD8CM cells in PBMCs was also observed 2 and 8 weeks postchallenge (Fig. 2C and G), whereas little change was seen in the CD8EM or CD4 memory populations (Fig. 2A, B, D to F, and H). Further, 2 weeks postchallenge the macaques of group 3 (DNA/IL-15) exhibited significantly higher total (Env, Gag, Nef) SIV-specific CD8CM T-cell responses compared to macaques that received DNA without any cytokine (Fig. 2G) (P = 0.014). Across all the groups, the Mamu-A*01 macaques tended to develop the strongest recall CD8CM SIV-specific responses (Fig. 2G).
The expansion of memory CD8 T cells postchallenge was also implied by the sudden decrease in the percentage of SIV Gag-specific CD4 T cells detected by ELISPOT to only 6 to 16% of the overall ELISPOT response (Table 2). This decrease was not due to a loss of CD4 cells in blood, since the mean CD4 counts remained unchanged 2 weeks postchallenge compared to prechallenge values at week 46 (Table 2).
Recall responses were observed even more strongly in the BAL compartment (Fig. 3A and B). As BAL is an effector site, expansion of CD8EM as well as CD8CM cells was observed. In macaques that received only DNA priming, a homogeneous response to all three antigens, Env, Gag, and Nef, was observed. In contrast, the macaques that received DNA plus either IL-12 or IL-15 showed recall responses primarily to Gag and Nef. In BAL, as in peripheral blood, the strongest responses were among CD8CM rather than CD8EM cells. There were no significant differences between the immunized groups at weeks 2 and 8 postchallenge.
Postchallenge humoral immune responses.
Postchallenge anamnestic antibody responses to Env and Nef rapidly developed in all three immunization groups (Fig. 4A and B). Elevated anti-Nef titers first appeared at week 49, 1 week postchallenge, in macaques of group 3 immunized with IL-15 DNA compared to all other macaques (P = 0.014) (Fig. 4B). By week 50, however, no differences among immunization groups were observed in either anti-Env or anti-Nef titers.
In parallel with the strong anamnestic response in anti-Env binding antibody, the ability of sera of the immunized macaques to mediate ADCC also increased sharply, compared to controls, by 2 weeks postchallenge (Fig. 5).
DISCUSSION
Both IL-12 and IL-15 have been shown to modulate immune responses and augment the immunogenicity of vectored vaccines. Previously, for example, a 10-fold increase in Gag-specific antibodies and an approximate 5-fold increase in SIV Gag-specific IFN-γ-secreting cells followed administration of IL-12 DNA together with an SIVgag plasmid vaccine (57). IL-12 also has an activating function on murine and human B cells, resulting in their differentiation into immunoglobulin M-producing cells (2). Similarly, IL-15 may modulate antibody responses in addition to its well-known effects on T cells and NK cells, as it enhances germinal center B-cell proliferation (45). Therefore, we anticipated that the cytokine groups compared to the DNA-only group would exhibit both enhanced SIV-specific immunity as well as protective efficacy. Unexpectedly, with few exceptions, similar immune responses were seen in all immunization groups, and all three immunization regimens failed to achieve significant protection. Although regrouping the macaques according to their Mamu-A*01 status revealed a modest statistically significant 1-log reduction in acute viremia in immunized Mamu-A*01-negative macaques compared as a group with Mamu-A*01-negative control macaques, this effect was transient. The observation suggests the major histocompatibility complex class I haplotype effect was stronger than any vaccine-induced control.
The lack of protective efficacy in this study is puzzling, as all regimens were immunogenic, eliciting systemic and mucosal cellular responses and serum antibodies to Env and Nef. While low-level immune responses were observed following the DNA immunizations, as expected, the Ad5hr-SIV immunization boosted both cellular and humoral immunity. The humoral responses after the single Ad5hr-SIV immunization were higher than previously observed after one administration (12, 47), highlighting the good priming features of the two DNA immunizations. Of note, the mismatched Ad5hr-SIVsmH4env/rev recombinant boosted SIVmac239 Env-specific IFN-γ SFC, primed by DNA encoding SIVmac239 env and rev genes (Fig. 1B). This response was further boosted by the Env protein immunization. However, functional ADCC-mediating antibody titers remained lower than those elicited by two sequential Ad5hr-SIV recombinant immunizations and SIV gp120 boosting (49), which had been previously associated with control of acute-phase viremia (17). Overall, the cytokine DNA priming immunizations did not enhance immune responses compared to priming with DNA only, in contrast to previous reports (8, 11). We did observe higher anti-Nef titers in the DNA/IL-15 group prior to challenge (Fig. 4B) and a more rapid anti-Nef anamnestic response compared to the other immunization groups. However, these responses had no apparent effect on protective efficacy. Postchallenge CD8CM responses were also higher in the DNA/IL-15 group compared to DNA alone (Fig. 2G) but again did not improve the challenge outcome. Otherwise, immune responses were indistinguishable between groups. The lack of enhancement of immune responses in the DNA/IL-12 and DNA/IL-15 groups cannot be attributed to the quality or expression levels of the plasmids encoding the cytokines, since the same plasmids were used previously in other studies and were shown to increase IFN-γ-secreting cells and/or T-cell proliferative responses (7, 8, 11, 54). Rather, the number of immunizations was likely important, as effects in these earlier studies were seen after three or more immunizations.
The lack of protection was unexpected, in view of earlier results showing that priming rhesus macaques with DNA vaccines enhanced the protective efficacy of nonreplicating Ad- and MVA-vectored SIV vaccines (3, 59). As two sequential immunizations with replicating Ad5hr-SIV recombinants followed by envelope protein boosting elicits potent, durable protection against intrarectal SIVmac251 challenge of rhesus macaques (32, 49), we anticipated even better protection when combining the two vaccine modalities, even without additional cytokines. In retrospect, however, the ability of DNA priming to strongly enhance protection was established using the SHIV89.6P challenge model. Few comparable studies have been performed in rhesus monkeys by using pathogenic SIV challenge models, and in these cases, results using DNA alone or in combination with other vectored vaccines have been less impressive. Nine sequential SIV DNA intramuscular immunizations resulted in reduced viral loads following intrarectal challenge with SIVmac251 (42), while seven SIV DNA gene gun immunizations reduced initial viral loads in four of seven rhesus macaques challenged with SIVΔB670 (14). Using combination approaches, 10 DNA immunizations followed by 2 immunizations with MVA, both encoding multiple SIV genes, led to a transient reduction in acute viremia after intrarectal challenge with SIVmac239 (21). Similarly, four SIV DNA intramuscular immunizations followed by three oral administrations of Listeria monocytogenes encoding SIV genes resulted in an initial reduction in viral burden after intrarectal SIVmac239 challenge that was not sustained (9). Overall, the outcome for non-Mamu-A*01 macaques in this study, in which fewer immunizations were used, is in line with these results.
Evaluation of cytokine DNAs as vaccine adjuvants in rhesus macaques has also used primarily the SHIV89.6P challenge model. While a vaccine regimen comprised of six SIV DNA immunizations plus IL-15 DNA better controlled peak SHIV89.6P viremia than that in either control macaques or macaques that received the DNA vaccines without DNA/IL-15 (8), in general, comparative studies of IL-12 and IL-15 plasmids have shown vaccines incorporating IL-12 to be most effective. After four immunizations with a SIVgag plasmid, macaques that also received an IL-12 plasmid exhibited the greatest reductions in peak and set point viremia following SHIV89.6P challenge (11). Similarly, rhesus macaques that were primed with SIV plasmid DNA, including IL-12 DNA, prior to recombinant vesicular stomatitis virus-SIV administration exhibited the best protective outcome after SHIV89.6P challenge (13). With regard to a pathogenic SIV challenge model, priming three times with a multigenic SIV plasmid DNA plus IL-12 DNA followed by boosting with a multigenic nonreplicating Ad recombinant without IL-12 led to reduced viral burdens after intrarectal SIVmac251 challenge (60). This study was promising although difficult to evaluate, as few animals were studied and rapid progressors, but not Mamu-A*01 macaques, were eliminated from statistical analysis. An experimental arm lacking IL-12 was not included. Administration of the DNA and Ad-SIV vaccines to the same intramuscular sites may have enhanced synergism between the different modalities. Here, following the intramuscular DNA immunizations, the Ad5hr-SIV vaccine was administered intratracheally, a more permissive site for replication of the host range mutant vector in macaques compared to the intramuscular or even the alternate mucosal intranasal route. This may have diminished a potential enhancing effect. It has been reported that IL-12 DNA needs to be administered at the same site as antigen for elicitation of an augmented cellular, but not humoral, response (57). Administration of Ad5hr-SIV vaccine intratracheally resulted in strong CD8EM and CD8CM responses in BAL fluid, an effector site that mirrors the small intestine (51), but this might have been at the expense of an IL-12 effect. It would be of interest to investigate administration of DNA with or without cytokine DNAs to the same site, followed by the replicating Ad5hr recombinants.
In addition to maintaining the same immunization route, other alterations in the vaccine strategy might lead to improved protection. Here, the DNA delivery method was not optimal. In comparison to intramuscular immunization, administration of DNA by electroporation greatly enhances cellular and humoral immune responses (19, 29). Further, since SIV env, gag, rev/nef, IL-12, and IL-15 were all encoded in different plasmids, there might have been poor cotransfection or coexpression of antigens and cytokines. Use of bicistronic vectors to provide simultaneous expression of cytokine and antigen in the same transfected cell might be optimal.
Further, although the goal of this study was to determine if two sequential DNA priming immunizations could substitute for an initial prime with replicating Ad-SIV recombinants, simply increasing the number of DNA immunizations, as mentioned above, might have improved the experimental outcome. Three immunizations with SIVgag DNA plus IL-12 DNA elicited a significant increase in IFN-γ-secreting cells compared to SIVgag DNA only; however, only two vaccinations were ineffective (7), in accord with our findings (Fig. 1).
The increasing understanding of the complex biology of IL-15 suggests that DNA expression plasmids should also express the IL-15 receptor, IL-15Rα, in order to achieve desired adjuvant effects. IL-15 is more stable and has a longer half-life when it is bound to IL-15Rα or is membrane associated and most effectively stimulates strong, durable CD8 memory responses if it is produced by cells that also present antigen and IL-15Rα (6, 56). Muscle cells express IL-15Rα (53) and could, following IL-15 DNA immunization, present de novo-synthesized IL-15 to lymphocytes according to the trans-presentation model of Ma et al. (30). Upon first priming, naïve T cells encounter expressed DNA; however, IL-15 has the most profound effect on differentiated effector and central memory T cells (52), which benefit most from IL-15 stimulation. Therefore, as memory cells home to lymph nodes, if IL-15 is to have an effect upon subsequent immunizations, it should be expressed with IL-15Rα for stability and trafficking to sites where memory cells reside. However, achieving the proper balance of IL-15 expression is also critical, since with constant dosing, the ability of T cells to respond to IL-15 wanes due to feedback inhibition (26) and overall is not beneficial (52, 62).
In summary, two DNA plasmid immunizations followed by a single administration of replication-competent Ad5hr-SIV recombinant vaccine did not substitute for two sequential Ad5hr-SIV recombinant immunizations with regard to protective efficacy against virulent SIVmac251. Although the DNA/Ad regimen was immunogenic, the addition of DNAs encoding cytokine adjuvants did not augment immune responses to any appreciable extent. A number of factors might have contributed to the ineffectiveness of this vaccine regimen. In view of the advantages of focusing the immune response on gene inserts instead of vector components by the use of DNA priming, further exploration of vaccine strategies combining DNA and replicating Ad recombinant vaccines should proceed. The potential of cytokine adjuvants for tailored modulation of vaccine-elicited immune responses remains strong and should be exploited in future vaccine approaches.
Acknowledgments
We thank Ronald C. Desrosiers, New England National Primate Research Center, and Nancy Miller, Division of AIDS, NIAID, for the SIVmac251 challenge virus, Barbara Felber and George Pavlakis for the DNA plasmids originally provided to the Weiner laboratory, and Ersell Richardson and Kris Aldrich for their technical assistance. We also acknowledge the contributions of Sanjeev Kumar, who sadly passed away during the course of this study. Complete sets of SIVmac239 Gag and SIVmac239 Env peptides were provided by the AIDS Research and Reference Reagent Program, NIAID, NIH.
This work was supported by the Intramural Research Program of the National Institutes of Health National Cancer Institute.
Footnotes
Published ahead of print on 27 August 2008.
REFERENCES
- 1.Ahlers, J. D., I. M. Belyakov, and J. A. Berzofsky. 2003. Cytokine, chemokine, and costimulatory molecule modulation to enhance efficacy of HIV vaccines. Curr. Mol. Med. 3285-301. [DOI] [PubMed] [Google Scholar]
- 2.Airoldi, I., G. Gri, J. D. Marshall, A. Corcione, P. Facchetti, R. Guglielmino, G. Trinchieri, and V. Pistoia. 2000. Expression and function of IL-12 and IL-18 receptors on human tonsillar B cells. J. Immunol. 1656880-6888. [DOI] [PubMed] [Google Scholar]
- 3.Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 29269-74. [DOI] [PubMed] [Google Scholar]
- 4.Anderson, E. J., M. A. McGrath, T. Thalhamer, and I. B. McInnes. 2006. Interleukin-12 to interleukin ‘infinity’: the rationale for future therapeutic cytokine targeting. Springer Semin. Immunopathol. 27425-442. [DOI] [PubMed] [Google Scholar]
- 5.Baba, T. W., V. Liska, R. Hofmann-Lehmann, J. Vlasak, W. Xu, S. Ayehunie, L. A. Cavacini, M. R. Posner, H. Katinger, G. Stiegler, B. J. Bernacky, T. A. Rizvi, R. Schmidt, L. R. Hill, M. E. Keeling, Y. Lu, J. E. Wright, T. C. Chou, and R. M. Ruprecht. 2000. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat. Med. 6200-206. [DOI] [PubMed] [Google Scholar]
- 6.Bergamaschi, C., M. Rosati, R. Jalah, A. Valentin, V. Kulkarni, C. Alicea, G. M. Zhang, V. Patel, B. K. Felber, and G. N. Pavlakis. 2008. Intracellular interaction of interleukin-15 with its receptor α during production leads to mutual stabilization and increased bioactivity. J. Biol. Chem. 2834189-4199. [DOI] [PubMed] [Google Scholar]
- 7.Boyer, J. D., T. M. Robinson, M. A. Kutzler, R. Parkinson, S. A. Calarota, M. K. Sidhu, K. Muthumani, M. Lewis, G. Pavlakis, B. Felber, and D. Weiner. 2005. SIV DNA vaccine co-administered with IL-12 expression plasmid enhances CD8 SIV cellular immune responses in cynomolgus macaques. J. Med. Primatol. 34262-270. [DOI] [PubMed] [Google Scholar]
- 8.Boyer, J. D., T. M. Robinson, M. A. Kutzler, G. Vansant, D. A. Hokey, S. Kumar, R. Parkinson, L. Wu, M. K. Sidhu, G. N. Pavlakis, B. K. Felber, C. Brown, P. Silvera, M. G. Lewis, J. Monforte, T. A. Waldmann, J. Eldridge, and D. B. Weiner. 2007. Protection against simian/human immunodeficiency virus (SHIV) 89.6P in macaques after coimmunization with SHIV antigen and IL-15 plasmid. Proc. Natl. Acad. Sci. USA 10418648-18653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyer, J. D., T. M. Robinson, P. C. Maciag, X. Peng, R. S. Johnson, G. Pavlakis, M. G. Lewis, A. Shen, R. Siliciano, C. R. Brown, D. B. Weiner, and Y. Paterson. 2005. DNA prime Listeria boost induces a cellular immune response to SIV antigens in the rhesus macaque model that is capable of limited suppression of SIV239 viral replication. Virology 33388-101. [DOI] [PubMed] [Google Scholar]
- 10.Cheng, S. M., S. G. Lee, M. Ronchetti-Blume, K. P. Virk, S. Mizutani, J. W. Eichberg, A. Davis, P. P. Hung, V. M. Hirsch, R. M. Chanock, R. H. Purcell, and P. R. Johnson. 1992. Coexpression of the simian immunodeficiency virus Env and Rev proteins by a recombinant human adenovirus host range mutant. J. Virol. 666721-6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chong, S. Y., M. A. Egan, M. A. Kutzler, S. Megati, A. Masood, V. Roopchard, D. Garcia-Hand, D. C. Montefiori, J. Quiroz, M. Rosati, E. B. Schadeck, J. D. Boyer, G. N. Pavlakis, D. B. Weiner, M. Sidhu, J. H. Eldridge, and Z. R. Israel. 2007. Comparative ability of plasmid IL-12 and IL-15 to enhance cellular and humoral immune responses elicited by a SIVgag plasmid DNA vaccine and alter disease progression following SHIV89.6P challenge in rhesus macaques. Vaccine 254967-4982. [DOI] [PubMed] [Google Scholar]
- 12.Demberg, T., R. H. Florese, M. J. Heath, K. Larsen, I. Kalisz, V. S. Kalyanaraman, E. M. Lee, R. Pal, D. Venzon, R. Grant, L. J. Patterson, B. Korioth-Schmitz, A. Buzby, D. Dombagoda, D. C. Montefiori, N. L. Letvin, A. Cafaro, B. Ensoli, and M. Robert-Guroff. 2007. A replication-competent adenovirus-human immunodeficiency virus (Ad-HIV) tat and Ad-HIV env priming/Tat and envelope protein boosting regimen elicits enhanced protective efficacy against simian/human immunodeficiency virus SHIV89.6P challenge in rhesus macaques. J. Virol. 813414-3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Egan, M. A., S. Y. Chong, S. Megati, D. C. Montefiori, N. F. Rose, J. D. Boyer, M. K. Sidhu, J. Quiroz, M. Rosati, E. B. Schadeck, G. N. Pavlakis, D. B. Weiner, J. K. Rose, Z. R. Israel, S. A. Udem, and J. H. Eldridge. 2005. Priming with plasmid DNAs expressing interleukin-12 and simian immunodeficiency virus gag enhances the immunogenicity and efficacy of an experimental AIDS vaccine based on recombinant vesicular stomatitis virus. AIDS Res. Hum. Retrovir. 21629-643. [DOI] [PubMed] [Google Scholar]
- 14.Fuller, D. H., P. A. Rajakumar, L. A. Wilson, A. M. Trichel, J. T. Fuller, T. Shipley, M. S. Wu, K. Weis, C. R. Rinaldo, J. R. Haynes, and M. Murphey-Corb. 2002. Induction of mucosal protection against primary, heterologous simian immunodeficiency virus by a DNA vaccine. J. Virol. 763309-3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geginat, J., F. Sallusto, and A. Lanzavecchia. 2001. Cytokine-driven proliferation and differentiation of human naive, central memory, and effector memory CD4+ T cells. J. Exp. Med. 1941711-1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gomez-Roman, V. R., R. H. Florese, L. J. Patterson, B. Peng, D. Venzon, K. Aldrich, and M. Robert-Guroff. 2006. A simplified method for the rapid fluorometric assessment of antibody-dependent cell-mediated cytotoxicity. J. Immunol. Methods 30853-67. [DOI] [PubMed] [Google Scholar]
- 17.Gomez-Roman, V. R., L. J. Patterson, D. Venzon, D. Liewehr, K. Aldrich, R. Florese, and M. Robert-Guroff. 2005. Vaccine-elicited antibodies mediate antibody-dependent cellular cytotoxicity correlated with significantly reduced acute viremia in rhesus macaques challenged with SIVmac251. J. Immunol. 1742185-2189. [DOI] [PubMed] [Google Scholar]
- 18.Grabstein, K. H., J. Eisenman, K. Shanebeck, C. Rauch, S. Srinivasan, V. Fung, C. Beers, J. Richardson, M. A. Schoenborn, M. Ahdieh, L. Johnson, M. R. Alderson, J. D. Watson, D. M. Anderson, and J. G. Giri. 1994. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science 264965-968. [DOI] [PubMed] [Google Scholar]
- 19.Hirao, L. A., L. Wu, A. S. Khan, D. A. Hokey, J. Yan, A. Dai, M. R. Betts, R. Draghia-Akli, and D. B. Weiner. 2008. Combined effects of IL-12 and electroporation enhances the potency of DNA vaccination in macaques. Vaccine 263112-3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hokey, D. A., and D. B. Weiner. 2006. DNA vaccines for HIV: challenges and opportunities. Springer Semin. Immunopathol. 28267-279. [DOI] [PubMed] [Google Scholar]
- 21.Horton, H., T. U. Vogel, D. K. Carter, K. Vielhuber, D. H. Fuller, T. Shipley, J. T. Fuller, K. J. Kunstman, G. Sutter, D. C. Montefiori, V. Erfle, R. C. Desrosiers, N. Wilson, L. J. Picker, S. M. Wolinsky, C. Wang, D. B. Allison, and D. I. Watkins. 2002. Immunization of rhesus macaques with a DNA prime/modified vaccinia virus Ankara boost regimen induces broad simian immunodeficiency virus (SIV)-specific T-cell responses and reduces initial viral replication but does not prevent disease progression following challenge with pathogenic SIVmac239. J. Virol. 767187-7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hryniewicz, A., D. A. Price, M. Moniuszko, A. Boasso, Y. Edghill-Spano, S. M. West, D. Venzon, M. Vaccari, W. P. Tsai, E. Tryniszewska, J. Nacsa, F. Villinger, A. A. Ansari, C. J. Trindade, M. Morre, D. Brooks, P. Arlen, H. J. Brown, C. M. Kitchen, J. A. Zack, D. C. Douek, G. M. Shearer, M. G. Lewis, R. A. Koup, and G. Franchini. 2007. Interleukin-15 but not interleukin-7 abrogates vaccine-induced decrease in virus level in simian immunodeficiency virus mac251-infected macaques. J. Immunol. 1783492-3504. [DOI] [PubMed] [Google Scholar]
- 23.Jin, X., D. E. Bauer, S. E. Tuttleton, S. Lewin, A. Gettie, J. Blanchard, C. E. Irwin, J. T. Safrit, J. Mittler, L. Weinberger, L. G. Kostrikis, L. Zhang, A. S. Perelson, and D. D. Ho. 1999. Dramatic rise in plasma viremia after CD8+ T cell depletion in simian immunodeficiency virus-infected macaques. J. Exp. Med. 189991-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim, J. J., V. Ayyavoo, M. L. Bagarazzi, M. A. Chattergoon, K. Dang, B. Wang, J. D. Boyer, and D. B. Weiner. 1997. In vivo engineering of a cellular immune response by coadministration of IL-12 expression vector with a DNA immunogen. J. Immunol. 158816-826. [PubMed] [Google Scholar]
- 25.Kobayashi, M., L. Fitz, M. Ryan, R. M. Hewick, S. C. Clark, S. Chan, R. Loudon, F. Sherman, B. Perussia, and G. Trinchieri. 1989. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J. Exp. Med. 170827-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kovanen, P. E., and W. J. Leonard. 2004. Cytokines and immunodeficiency diseases: critical roles of the γc-dependent cytokines interleukins 2, 4, 7, 9, 15, and 21, and their signaling pathways. Immunol. Rev. 20267-83. [DOI] [PubMed] [Google Scholar]
- 27.Liniger, M., A. Zuniga, and H. Y. Naim. 2007. Use of viral vectors for the development of vaccines. Expert Rev. Vaccines 6255-266. [DOI] [PubMed] [Google Scholar]
- 28.Loffredo, J. T., J. Maxwell, Y. Qi, C. E. Glidden, G. J. Borchardt, T. Soma, A. T. Bean, D. R. Beal, N. A. Wilson, W. M. Rehrauer, J. D. Lifson, M. Carrington, and D. I. Watkins. 2007. Mamu-B*08-positive macaques control simian immunodeficiency virus replication. J. Virol. 818827-8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luckay, A., M. K. Sidhu, R. Kjeken, S. Megati, S. Y. Chong, V. Roopchand, D. Garcia-Hand, R. Abdullah, R. Braun, D. C. Montefiori, M. Rosati, B. K. Felber, G. N. Pavlakis, I. Mathiesen, Z. R. Israel, J. H. Eldridge, and M. A. Egan. 2007. Effect of plasmid DNA vaccine design and in vivo electroporation on the resulting vaccine-specific immune responses in rhesus macaques. J. Virol. 815257-5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma, A., R. Koka, and P. Burkett. 2006. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu. Rev. Immunol. 24657-679. [DOI] [PubMed] [Google Scholar]
- 31.Malkevitch, N., L. J. Patterson, K. Aldrich, E. Richardson, W. G. Alvord, and M. Robert-Guroff. 2003. A replication competent Ad5hr-SIV recombinant priming/subunit protein boosting vaccine regimen induces broad, persistent SIV-specific cellular immunity to dominant and subdominant epitopes in mamu-A*01 rhesus macaques. J. Immunol. 1704281-4289. [DOI] [PubMed] [Google Scholar]
- 32.Malkevitch, N. V., L. J. Patterson, M. K. Aldrich, Y. Wu, D. Venzon, R. H. Florese, V. S. Kalyanaraman, R. Pal, E. M. Lee, J. Zhao, A. Cristillo, and M. Robert-Guroff. 2006. Durable protection of rhesus macaques immunized with a replicating adenovirus-SIV multigene prime/protein boost vaccine regimen against a second SIVmac251 rectal challenge: role of SIV-specific CD8+ T cell responses. Virology 35383-98. [DOI] [PubMed] [Google Scholar]
- 33.Malkevitch, N. V., and M. Robert-Guroff. 2004. A call for replicating vector prime-protein boost strategies in HIV vaccine design. Expert Rev. Vaccines 3S105-S117. [DOI] [PubMed] [Google Scholar]
- 34.Malkevitch, N., D. Rohne, J. Pinczewski, K. Aldrich, V. S. Kalyanaraman, N. L. Letvin, and M. Robert-Guroff. 2004. Evaluation of combination DNA/replication-competent Ad-SIV recombinant immunization regimens in rhesus macaques. AIDS Res. Hum. Retrovir. 20235-244. [DOI] [PubMed] [Google Scholar]
- 35.Mao, H., B. A. Lafont, T. Igarashi, Y. Nishimura, C. Brown, V. Hirsch, A. Buckler-White, R. Sadjadpour, and M. A. Martin. 2005. CD8+ and CD20+ lymphocytes cooperate to control acute simian immunodeficiency virus/human immunodeficiency virus chimeric virus infections in rhesus monkeys: modulation by major histocompatibility complex genotype. J. Virol. 7914887-14898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mascola, J. R., M. G. Lewis, G. Stiegler, D. Harris, T. C. VanCott, D. Hayes, M. K. Louder, C. R. Brown, C. V. Sapan, S. S. Frankel, Y. Lu, M. L. Robb, H. Katinger, and D. L. Birx. 1999. Protection of macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J. Virol. 734009-4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mascola, J. R., G. Stiegler, T. C. VanCott, H. Katinger, C. B. Carpenter, C. E. Hanson, H. Beary, D. Hayes, S. S. Frankel, D. L. Birx, and M. G. Lewis. 2000. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat. Med. 6207-210. [DOI] [PubMed] [Google Scholar]
- 38.Miller, C. J., M. Genesca, K. Abel, D. Montefiori, D. Forthal, K. Bost, J. Li, D. Favre, and J. M. McCune. 2007. Antiviral antibodies are necessary for control of simian immunodeficiency virus replication. J. Virol. 815024-5035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mothe, B. R., J. Weinfurter, C. Wang, W. Rehrauer, N. Wilson, T. M. Allen, D. B. Allison, and D. I. Watkins. 2003. Expression of the major histocompatibility complex class I molecule Mamu-A*01 is associated with control of simian immunodeficiency virus SIVmac239 replication. J. Virol. 772736-2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mueller, Y. M., D. H. Do, S. R. Altork, C. M. Artlett, E. J. Gracely, C. D. Katsetos, A. Legido, F. Villinger, J. D. Altman, C. R. Brown, M. G. Lewis, and P. D. Katsikis. 2008. IL-15 treatment during acute simian immunodeficiency virus (SIV) infection increases viral set point and accelerates disease progression despite the induction of stronger SIV-specific CD8+ T cell responses. J. Immunol. 180350-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mueller, Y. M., C. Petrovas, P. M. Bojczuk, I. D. Dimitriou, B. Beer, P. Silvera, F. Villinger, J. S. Cairns, E. J. Gracely, M. G. Lewis, and P. D. Katsikis. 2005. Interleukin-15 increases effector memory CD8+ T cells and NK cells in simian immunodeficiency virus-infected macaques. J. Virol. 794877-4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muthumani, K., M. Bagarazzi, D. Conway, D. S. Hwang, K. Manson, R. Ciccarelli, Z. Israel, D. C. Montefiori, K. Ugen, N. Miller, J. Kim, J. Boyer, and D. B. Weiner. 2003. A Gag-Pol/Env-Rev SIV239 DNA vaccine improves CD4 counts, and reduces viral loads after pathogenic intrarectal SIV(mac)251 challenge in rhesus macaques. Vaccine 21629-637. [DOI] [PubMed] [Google Scholar]
- 43.O'Connor, D. H., B. R. Mothe, J. T. Weinfurter, S. Fuenger, W. M. Rehrauer, P. Jing, R. R. Rudersdorf, M. E. Liebl, K. Krebs, J. Vasquez, E. Dodds, J. Loffredo, S. Martin, A. B. McDermott, T. M. Allen, C. Wang, G. G. Doxiadis, D. C. Montefiori, A. Hughes, D. R. Burton, D. B. Allison, S. M. Wolinsky, R. Bontrop, L. J. Picker, and D. I. Watkins. 2003. Major histocompatibility complex class I alleles associated with slow simian immunodeficiency virus disease progression bind epitopes recognized by dominant acute-phase cytotoxic-T-lymphocyte responses. J. Virol. 779029-9040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oh, S., J. A. Berzofsky, D. S. Burke, T. A. Waldmann, and L. P. Perera. 2003. Coadministration of HIV vaccine vectors with vaccinia viruses expressing IL-15 but not IL-2 induces long-lasting cellular immunity. Proc. Natl. Acad. Sci. USA 1003392-3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park, C. S., S. O. Yoon, R. J. Armitage, and Y. S. Choi. 2004. Follicular dendritic cells produce IL-15 that enhances germinal center B cell proliferation in membrane-bound form. J. Immunol. 1736676-6683. [DOI] [PubMed] [Google Scholar]
- 46.Parren, P. W., P. A. Marx, A. J. Hessell, A. Luckay, J. Harouse, C. Cheng-Mayer, J. P. Moore, and D. R. Burton. 2001. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J. Virol. 758340-8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patterson, L. J., J. Beal, T. Demberg, R. H. Florese, N. Malkevich, D. Venzon, K. Aldrich, E. Richardson, V. S. Kalyanaraman, I. Kalisz, E. M. Lee, D. C. Montefiori, F. A. Robey, and M. Robert-Guroff. 2008. Replicating adenovirus HIV/SIV recombinant priming alone or in combination with a gp140 protein boost results in significant control of viremia following a SHIV89.6P challenge in Mamu-A01 negative rhesus macaques. Virology 374322-337. [DOI] [PubMed] [Google Scholar]
- 48.Patterson, L. J., N. Malkevitch, J. Pinczewski, D. Venzon, Y. Lou, B. Peng, C. Munch, M. Leonard, E. Richardson, K. Aldrich, V. S. Kalyanaraman, G. N. Pavlakis, and M. Robert-Guroff. 2003. Potent, persistent induction and modulation of cellular immune responses in rhesus macaques primed with Ad5hr-simian immunodeficiency virus (SIV) env/rev, gag, and/or nef vaccines and boosted with SIV gp120. J. Virol. 778607-8620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patterson, L. J., N. Malkevitch, D. Venzon, J. Pinczewski, V. R. Gomez-Roman, L. Wang, V. S. Kalyanaraman, P. D. Markham, F. A. Robey, and M. Robert-Guroff. 2004. Protection against mucosal simian immunodeficiency virus SIVmac251 challenge by using replicating adenovirus-SIV multigene vaccine priming and subunit boosting. J. Virol. 782212-2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peng, B., L. R. Wang, V. R. Gomez-Roman, A. Davis-Warren, D. C. Montefiori, V. S. Kalyanaraman, D. Venzon, J. Zhao, E. Kan, T. J. Rowell, K. K. Murthy, I. Srivastava, S. W. Barnett, and M. Robert-Guroff. 2005. Replicating rather than nonreplicating adenovirus-human immunodeficiency virus recombinant vaccines are better at eliciting potent cellular immunity and priming high-titer antibodies. J. Virol. 7910200-10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Picker, L. J., S. I. Hagen, R. Lum, E. F. Reed-Inderbitzin, L. M. Daly, A. W. Sylwester, J. M. Walker, D. C. Siess, M. Piatak, Jr., C. Wang, D. B. Allison, V. C. Maino, J. D. Lifson, T. Kodama, and M. K. Axthelm. 2004. Insufficient production and tissue delivery of CD4+ memory T cells in rapidly progressive simian immunodeficiency virus infection. J. Exp. Med. 2001299-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Picker, L. J., E. F. Reed-Inderbitzin, S. I. Hagen, J. B. Edgar, S. G. Hansen, A. Legasse, S. Planer, M. Piatak, Jr., J. D. Lifson, V. C. Maino, M. K. Axthelm, and F. Villinger. 2006. IL-15 induces CD4 effector memory T cell production and tissue emigration in nonhuman primates. J. Clin. Investig. 1161514-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Riechman, S. E., G. Balasekaran, S. M. Roth, and R. E. Ferrell. 2004. Association of interleukin-15 protein and interleukin-15 receptor genetic variation with resistance exercise training responses. J. Appl. Physiol. 972214-2219. [DOI] [PubMed] [Google Scholar]
- 54.Robinson, T. M., M. K. Sidhu, G. N. Pavlakis, B. K. Felber, P. Silvera, M. G. Lewis, J. Eldridge, D. B. Weiner, and J. D. Boyer. 2007. Macaques co-immunized with SIVgag/pol-HIVenv and IL-12 plasmid have increased cellular responses. J. Med. Primatol. 36276-284. [DOI] [PubMed] [Google Scholar]
- 55.Romano, J. W., R. N. Shurtliff, E. Dobratz, A. Gibson, K. Hickman, P. D. Markham, and R. Pal. 2000. Quantitative evaluation of simian immunodeficiency virus infection using NASBA technology. J. Virol. Methods 8661-70. [DOI] [PubMed] [Google Scholar]
- 56.Sato, N., H. J. Patel, T. A. Waldmann, and Y. Tagaya. 2007. The IL-15/IL-15Rα on cell surfaces enables sustained IL-15 activity and contributes to the long survival of CD8 memory T cells. Proc. Natl. Acad. Sci. USA 104588-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schadeck, E. B., M. Sidhu, M. A. Egan, S. Y. Chong, P. Piacente, A. Masood, D. Garcia-Hand, S. Cappello, V. Roopchand, S. Megati, J. Quiroz, J. D. Boyer, B. K. Felber, G. N. Pavlakis, D. B. Weiner, J. H. Eldridge, and Z. R. Israel. 2006. A dose sparing effect by plasmid encoded IL-12 adjuvant on a SIVgag-plasmid DNA vaccine in rhesus macaques. Vaccine 244677-4687. [DOI] [PubMed] [Google Scholar]
- 58.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283857-860. [DOI] [PubMed] [Google Scholar]
- 59.Shiver, J. W., T. M. Fu, L. Chen, D. R. Casimiro, M. E. Davies, R. K. Evans, Z. Q. Zhang, A. J. Simon, W. L. Trigona, S. A. Dubey, L. Huang, V. A. Harris, R. S. Long, X. Liang, L. Handt, W. A. Schleif, L. Zhu, D. C. Freed, N. V. Persaud, L. Guan, K. S. Punt, A. Tang, M. Chen, K. A. Wilson, K. B. Collins, G. J. Heidecker, V. R. Fernandez, H. C. Perry, J. G. Joyce, K. M. Grimm, J. C. Cook, P. M. Keller, D. S. Kresock, H. Mach, R. D. Troutman, L. A. Isopi, D. M. Williams, Z. Xu, K. E. Bohannon, D. B. Volkin, D. C. Montefiori, A. Miura, G. R. Krivulka, M. A. Lifton, M. J. Kuroda, J. E. Schmitz, N. L. Letvin, M. J. Caulfield, A. J. Bett, R. Youil, D. C. Kaslow, and E. A. Emini. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415331-335. [DOI] [PubMed] [Google Scholar]
- 60.Suh, Y. S., K. S. Park, U. Sauermann, M. Franz, S. Norley, D. Wilfingseder, H. Stoiber, Z. Fagrouch, J. Heeney, G. Hunsmann, C. Stahl-Hennig, and Y. C. Sung. 2006. Reduction of viral loads by multigenic DNA priming and adenovirus boosting in the SIVmac-macaque model. Vaccine 241811-1820. [DOI] [PubMed] [Google Scholar]
- 61.van der Meide, P. H., F. Villinger, A. A. Ansari, R. J. Groenestein, M. C. de Labie, Y. J. van den Hout, W. H. Koornstra, W. M. Bogers, and J. L. Heeney. 2002. Stimulation of both humoral and cellular immune responses to HIV-1 gp120 by interleukin-12 in rhesus macaques. Vaccine 202296-2302. [DOI] [PubMed] [Google Scholar]
- 62.Villinger, F., R. Miller, K. Mori, A. E. Mayne, P. Bostik, J. B. Sundstrom, C. Sugimoto, and A. A. Ansari. 2004. IL-15 is superior to IL-2 in the generation of long-lived antigen specific memory CD4 and CD8 T cells in rhesus macaques. Vaccine 223510-3521. [DOI] [PubMed] [Google Scholar]
- 63.Waldmann, T. A. 2006. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat. Rev. Immunol. 6595-601. [DOI] [PubMed] [Google Scholar]
- 64.Zhao, J., Y. Lou, J. Pinczewski, N. Malkevitch, K. Aldrich, V. S. Kalyanaraman, D. Venzon, B. Peng, L. J. Patterson, Y. Edghill-Smith, R. Woodward, G. N. Pavlakis, and M. Robert-Guroff. 2003. Boosting of SIV-specific immune responses in rhesus macaques by repeated administration of Ad5hr-SIVenv/rev and Ad5hr-SIVgag recombinants. Vaccine 214022-4035. [DOI] [PubMed] [Google Scholar]
- 65.Zhou, Q., R. Hidajat, B. Peng, D. Venzon, M. K. Aldrich, E. Richardson, E. M. Lee, V. S. Kalyanaraman, G. Grimes, V. R. Gomez-Roman, L. E. Summers, N. Malkevich, and M. Robert-Guroff. 2007. Comparative evaluation of oral and intranasal priming with replication-competent adenovirus 5 host range mutant (Ad5hr)-simian immunodeficiency virus (SIV) recombinant vaccines on immunogenicity and protective efficacy against SIVmac251. Vaccine 258021-8035. [DOI] [PubMed] [Google Scholar]