Abstract
Foot-and-mouth disease virus (FMDV), a member of the Picornaviridae, is a pathogen of cloven-hoofed animals and causes a disease of major economic importance. Picornavirus-infected cells show changes in cell morphology and rearrangement of cytoplasmic membranes, which are a consequence of virus replication. We show here, by confocal immunofluorescence and electron microscopy, that the changes in morphology of FMDV-infected cells involve changes in the distribution of microtubule and intermediate filament components during infection. Despite the continued presence of centrosomes in infected cells, there is a loss of tethering of microtubules to the microtubule organizing center (MTOC) region. Loss of labeling for γ-tubulin, but not pericentrin, from the MTOC suggests a targeting of γ-tubulin (or associated proteins) rather than a total breakdown in MTOC structure. The identity of the FMDV protein(s) responsible was determined by the expression of individual viral nonstructural proteins and their precursors in uninfected cells. We report that the only viral nonstructural protein able to reproduce the loss of γ-tubulin from the MTOC and the loss of integrity of the microtubule system is FMDV 3Cpro. In contrast, infection of cells with another picornavirus, bovine enterovirus, did not affect γ-tubulin distribution, and the microtubule network remained relatively unaffected.
Infection of cells with most picornaviruses leads to a cytopathic effect (CPE) characterized by dramatic changes in cell shape and the production of large numbers of membrane vesicles within the cytoplasm. Early studies focused on the CPE produced by poliovirus (PV) and coxsackievirus (16, 33, 70) and revealed crystalline arrays of virus in the cytoplasm and viruses associated with, and contained within, membrane vesicles (7, 10, 43). The origin of vesicles induced by PV has been the subject of several studies with various conclusions. High-pressure freezing preparation for electron microscopy showed double-membrane vesicles in cells infected with PV (71). The vesicles are thought to be derived from the endoplasmic reticulum (ER), arising from either COPII-coated vesicles or autophagic vesicles (6, 32, 63, 66, 71, 73, 74). However, the presence of other organelle markers associated with PV vesicles, at later time points of infection, suggests that the ER is not the sole contributor of these membranes (66).
In a previous publication (51), we reported that while foot-and-mouth disease virus (FMDV) induced the formation of membrane vesicles in infected cells, as observed in PV-infected cells, there were a number of differences between the effects observed with these two viruses. FMDV-induced vesicles predominantly had a single membrane and were not found in clusters as reported for PV. Further differences between PV and FMDV replication are seen in studies of virus sensitivity to the fungal metabolite, brefeldin A, which inhibits the function of Arf1-GTPase. Brefeldin A completely inhibits PV replication, whereas FMDV replication appears unaffected (15, 22, 30, 48, 51, 58). Differences between PV and FMDV are also seen in some of the functions of the nonstructural proteins. Studies examining the effects of FMDV, and its nonstructural proteins, on the host-cell protein secretory pathway revealed that, unlike the PV 3A protein, FMDV 3A was unable to slow protein secretion (12, 49). Interestingly, although both FMDV 2BC and PV 2BC are able to slow protein trafficking though the secretory pathway, they appear to act at different sites. PV 2BC is thought to slow the processing of secretory proteins at the Golgi body, whereas FMDV 2BC expression leads to retention of secretory proteins within the ER (2, 19, 49, 50, 64). Taken together, these observations indicate significant differences in the virus-host cell interactions of PV (an enterovirus) and FMDV (an aphthovirus).
In contrast to the work on membrane vesicles, relatively little work has been reported on the effect of picornavirus infection on the cytoskeleton. However, it is becoming increasingly clear that many of these viruses use and modify the cytoskeleton for cell entry, replication, and egress. For example, Theiler's virus (a cardiovirus) has been reported to associate with intermediate filaments (55). PV has been reported to associate with the cytoskeleton and intermediate filaments (42, 75), and the 3Cpro protein of PV cleaves microtubule-associated protein 4 (34, 35). Coxsackievirus B4 infection induces cleavage of the intermediate filament cytokeratin 8 via the 2A protease, and this occurs during the onset of CPE (67). In addition, it has been reported that FMDV proteins may associate with intermediate filaments (23). Rearrangement of intermediate filaments has been reported in cells infected with other viruses around sites of virus assembly and also actin projections have been implicated in virus release and cell-to-cell spread (28, 36-38, 54, 59, 61, 83).
We have now sought to determine the changes in the cytoskeleton that underlie the FMDV-induced modifications in cell morphology. Most interesting is the observation that FMDV infection leads to a lack of tethering of the microtubules to the microtubule organizing center (MTOC) within infected cells. This appeared to result from virus-induced loss of γ-tubulin (but not pericentrin) from the MTOC. We sought to identify the viral protein(s) responsible through the expression of individual FMDV nonstructural proteins within uninfected cells and assessing their effect upon the γ-tubulin located at the MTOC. The FMDV nonstructural protein 3Cpro was identified as the causative protein from this screening process. Comparison, within the same cell type, of the FMDV-induced changes to those induced by bovine enterovirus (BEV) which (like PV), is an enterovirus, showed substantial differences between the cellular effects of these two virus infections.
MATERIALS AND METHODS
Cells and viruses.
BHK-38 and BHK-21 (ECACC 85011433) cells were obtained from ECACC, and BSR T7/5 cells (which express the T7 RNA polymerase) were kindly provided by K. Conzelmann (8). Cells were cultured in growth medium (Dulbecco modified Eagle medium supplemented with 10% fetal calf serum [FCS], 2 mM glutamine, penicillin [100 SI U/ml], and streptomycin [100 μg/ml]) at 37°C in 5% CO2. BSR cells were supplemented with G418 (0.5 mg/ml) (Sigma). Cells were seeded for 16 h before use onto 13-mm glass coverslips (Agar Scientific, Ltd., Stansted, United Kingdom) for immunofluorescence and onto 13-mm Thermanox coverslips (Science Services, London, United Kingdom) for electron microscopy.
Stocks of FMDV O1BFS and BEV were prepared, and virus titers were determined using BHK-38 cells as described previously (31) (except that for BEV, horse serum was used in place of FCS). In all subsequent experiments involving BEV, pig serum was used in place of FCS. The multiplicity of infection (MOI) was based on the virus titer on BHK-38 cells. The modified vaccinia virus Ankara (MVA-T7), a modified, host-range-restricted, vaccinia virus strain that expresses the bacteriophage T7 RNA polymerase gene (72), was kindly provided by Gerd Sutter (Institut für Molekulare Virologie, Oberschleissrheim, Germany).
Virus infections.
Cells prepared on coverslips as described above were washed with cold phosphate-buffered saline (PBS) containing 1 mM CaCl2 and 1 mM MgCl2 and then incubated with virus at an MOI of between 10 and 100 PFU/cell for 30 min at 4°C. The cells were washed with PBS to remove unattached virus, cell culture medium was added, and the cells were incubated at 37°C.
Antibodies.
Mouse anti-FMDV 2C (1C8; 1:1,000) and mouse anti-FMDV 3C (4E9, isotype immunoglobulin G2b [IgG2b]; 1:10) were kindly provided by E. Brocchi (IZSLE, Brescia, Italy). Rabbit anti-FMDV capsid (1:100) was provided by IAH, and rabbit anti-BEV capsid antibody (1:100) was from the The Queen's University of Belfast. Mouse anti-V5 (isotype IgG2a; 1:1,000) was purchased from Invitrogen (Paisley, United Kingdom). Mouse anti-α-tubulin (1:1,000; or 1:4,000 for Western blot), mouse anti-vimentin (1:200), and mouse anti-γ-tubulin (isotype IgG1) (1:200; or 1:5,000 for Western blot) were purchased from Sigma (Poole, United Kingdom). Mouse anti-pericentrin (1:200) was purchased from Covance (Cambridge Bioscience, Cambridge, United Kingdom). Actin was detected with phalloidin-Alexa488 conjugate diluted 1:25 in PBS (Invitrogen).
Plasmids.
Plasmids, based on the vector pcDNA3.1 (Invitrogen), which express the FMDV nonstructural proteins 2BV5 (which contains a V5 C-terminal tag), 2BC, 2C, 3A, and 3AB have been described previously (49). The plasmid pSKRH3C (5) expresses the FMDV 3Cpro under the control of the T7 RNA polymerase promoter; therefore, some experiments used BSR T7/5 cells, which constitutively express the T7 RNA polymerase (8).
Transfection.
BHK-21 cells or BSR T7/5 were prepared as described above and then transfected with plasmid DNA (0.8 μg/well in 24-well plates) by using Lipofectamine 2000 (Invitrogen) in HEPES-buffered Dulbecco modified Eagle medium for 3 h at 37°C. Cells were incubated for a further 16 h, at 37°C, in growth medium essentially as described previously (49).
Characterization of changes in cell morphology observed with live-cell imaging.
Cells were seeded onto tissue culture dishes with an optically clear glass coverslip cemented into the base (Iwaki; SLS, Nottingham, United Kingdom). After 16 h the cells were infected with FMDV as described above and then transferred to a climate-controlled chamber on a Leica SP2 confocal microscope. Cells were imaged every 2 min for 3 h postinfection (hpi) by using differential interference contrast (DIC) optics.
Indirect immunofluorescence.
Coverslips with infected and/or transfected cells plus uninfected control cells were fixed for 30 min in 4% paraformaldehyde in PBS at 2 hpi for FMDV, 4 hpi for BEV; time points were selected as the estimated “midpoint” in infection (51) or 20 h posttransfection with FMDV plasmids. Fixed coverslips were washed in PBS and stored at 4°C. Immediately before γ-tubulin staining, cells were incubated in methanol for 4 min and washed in PBS. Cells used for immunolabeling were permeabilized in 0.05% Triton X-100 in PBS for 15 min, followed by a wash in PBS. Nonspecific labeling was blocked with a 30-min incubation in PBS with 0.5% bovine serum albumin (PBS/BSA; Sigma). Primary antibodies were diluted in PBS/BSA and incubated for 1 h at room temperature. After three 5-min PBS washes, the primary antibodies were detected with Alexa488- or Alexa568-conjugated species-specific antibodies (Molecular Probes/Invitrogen) diluted 1:200 in PBS/BSA for 1 h. After three 5-min washes, the coverslips were incubated in DAPI (Sigma) diluted 1:10,000 in water for 5 min to label the cellular DNA. Labeled coverslips were mounted in Vectashield (Vector Laboratories, Peterborough, United Kingdom) and sealed with clear nail varnish. Control uninfected cells were labeled as for infected cells. The cells were imaged with a Leica TCS SP2 confocal microscope. Most images are from single confocal image planes, but in order to illustrate MTOC labeling in several cells, some images are Z-stacks. This means that in some images the MTOC will appear to be “in” the nucleus, whereas it is actually above (or below) the nucleus.
Metabolic labeling and immunoprecipitation.
BSR cells grown to ca. 90% confluence in 175-cm2 flasks were incubated for 0, 1, 2, 3, and 4 h with cycloheximide at 100 μg/ml. Cells were treated with trypsin, starved in methionine and cysteine-free Eagle medium for 10 min, and then labeled with 10 MBq of 35S-Promix (Amersham Life Sciences, Chalfont St Giles, United Kingdom) per ml in the same medium for 10 min. Cells were washed and lysed on ice in immunoprecipitation buffer (10 mM Tris [pH 7.5]; 150 mM NaCl; 10 mM iodoacetamide; 1 mM EDTA; 1 mM phenylmethylsulfonyl fluoride; and 1 mg/ml each of leupeptin, pepstatin, chymostatin, and antipain) containing 1% Nonidet P-40. Alpha-tubulin was immunoprecipitated by overnight incubation with antibodies immobilized with protein G coupled to Sepharose 4B. Proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and detected by autoradiography.
Western blotting.
Proteins were resolved by SDS-PAGE and transferred onto a Protran BA85 cellulose nitrate membrane (Schleicher & Schuell, Dassel, Germany). The membrane was blocked for 1 h in 5% Marvel, 10% normal goat serum, and PBS-Tween 20; incubated with the primary mouse antibody; washed in PBS-Tween 20; and further incubated with goat anti-mouse antibody conjugated to horseradish peroxidase (Southern Biotechnology Associates, Inc., Birmingham, AL); antibodies were diluted in 10% normal goat serum-5% Marvel PBS-Tween 20. After the washes, detection was achieved by using enhanced chemiluminescence reagents (Amersham Life Sciences, Buckinghamshire, United Kingdom).
Electron microscopy.
At 2 hpi, control uninfected and FMDV-infected cells growing on Thermanox coverslips cultured as for the glass coverslips were fixed in situ in 2% glutaraldehyde in 0.05 M phosphate buffer (pH 7.2 to 7.4, osmotic pressure adjusted to 350 mosmol with sucrose) for 2 h. Cells were postfixed in 1% osmium tetroxide in phosphate buffer for 4 h. They were dehydrated through an ethanol series and embedded in Agar 100 resin (Agar Scientific, Stansted, United Kingdom). The resin was polymerized at 60°C, but before the resin had fully polymerized, the Thermanox coverslips were peeled from the resin block, leaving the cells embedded in the resin. Sections were cut parallel to the coverslip surface with a Diatome diamond knife (Leica Microsystems, Milton Keynes, United Kingdom) and contrasted with uranyl acetate and lead citrate (EMStain; Leica). They were imaged in a FEI Tecnai 12 transmission electron microscope at 100 kV.
RESULTS
FMDV-induced CPE.
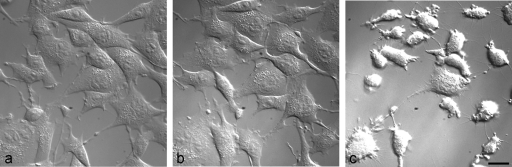
Infection by positive-strand RNA viruses results in CPE, which is characterized by cell rounding and subsequent detachment from the substratum. Major changes in cell shape are generally associated with changes in the cytoskeleton. Here we have undertaken a detailed investigation of the effects of FMDV and BEV on the cytoskeleton by immunofluorescence microscopy. These viruses were chosen because they are representatives of the aphthovirus and enterovirus genera of the Picornaviridae which replicate in the same cell types. In Fig. 1, a series of images are shown from a live-cell recording at increasing times after infection of cells with FMDV (see supplemental information at http://www.iah.ac.uk/research/bioimaging/media/bioimg_vid1.shtml). The cells were viewed by DIC optics to monitor their outline and surface properties. During the first hour after infection the cells remained flat, indicating secure attachment to the coverslip, and many cells were motile and produced cellular extensions. Changes in cell shape were observed from between 1 and 2 h after infection. After this time, the number of cellular extensions produced was greatly reduced, and the cells began to round up and detach from the coverslip. CPE was also seen in BEV-infected cells except that the cell rounding occurred between 2.5 and 4 hpi (data not shown).
FIG. 1.
CPE in FMDV-infected cells. Still images from a live-cell experiment in which BHK-21 cells were infected with FMDV and imaged in a confocal microscope by DIC optics are shown. The three still images are from the cells at 0 (a), 76 (b), and 152 min (c) postinfection. Scale bar (for all three panels), 20 μm.
Infections by FMDV and BEV have different effects on the cytoskeleton.
In the following experiments (unless otherwise stated) FMDV infections are shown at 2 hpi and BEV at 4 hpi due to the BEV replication cycle being slower than that of FMDV. At these times, the cells were still adherent to the coverslip but were beginning to show changes in morphology. Other time points were also examined for both viruses, and the observations reported here were consistent and not specific to any particular time point. FMDV-infected cells were detected by labeling with the rabbit anti-capsid antibody, which identifies the virus replication site generally located in a perinuclear region. BEV-infected cells were detected with a rabbit antiserum raised against whole virus. The cells were infected at an MOI that allowed infected and uninfected cells to be seen in the same image. Antibodies against α-tubulin and vimentin were used to identify microtubules and intermediate filaments, respectively, while phalloidin-Alexa488 was used to image filamentous actin (F-actin).
The effects of viral infection on vimentin were examined to compare the distribution of intermediate filaments in cells infected with FMDV (Fig. 2a to c) or BEV (Fig. 2d to f) to that seen in uninfected cells (Fig. 2g). Although the levels of vimentin expression in uninfected cells appeared to vary between cells, it was arranged in a filamentous network running throughout the cytoplasm (Fig. 2g). In cells infected with FMDV or BEV, vimentin was found redistributed to sites of viral replication. Interestingly, FMDV and BEV had different effects on vimentin distribution. In FMDV-infected cells, vimentin was rearranged into a ring surrounding the area of labeling for viral proteins (Fig. 2a to c), while BEV caused a striking concentration of the majority of the cellular vimentin into a ball of filaments in the same area of the cell occupied by BEV proteins (Fig. 2d to f).
FIG. 2.
Effect of FMDV and BEV infection on host cell vimentin. BHK-21 cells were infected with FMDV and fixed with paraformaldehyde at 2 hpi (panels a and b, merged in panel c) and BEV fixed at 4 hpi (panels d and e, merged in panel f). An uninfected cell is shown in panel g. The cells were immunolabeled for the presence of viral structural proteins detected with anti-rabbit antibody-Alexa488 (green) and mouse anti-vimentin detected with anti-mouse antibody-Alexa568 conjugate (red). Nuclei were labeled with DAPI (blue). Scale bars: a to c, 10 μm; d to f, 20 μm; g, 10 μm.
Examining the appearance of microtubules in infected cells also revealed differences between the effects of FMDV and BEV (Fig. 3). In control cells, the microtubules (visualized with antibodies recognizing α-tubulin) were arranged in a filamentous network running throughout the cytoplasm. However, the tubulin pattern differed from the distribution of vimentin in that it showed a radial distribution, with microtubules originating from the MTOC (Fig. 3g). The microtubules in FMDV-infected cells (Fig. 3a to c) still extended to the plasma membrane; however, the peripheral microtubules were disorganized and lacked the radial distribution seen in control cells. Most interesting was the exclusion of most of the microtubules from the area of the cell containing FMDV proteins (Fig. 3a and c). In contrast, in BEV-infected cells the microtubule distribution retained a radial pattern (Fig. 3d to f).
FIG. 3.
Effect of FMDV and BEV infection on host cell microtubules. BHK-21 cells were infected with FMDV and fixed with paraformaldehyde at 2 hpi (panels a and b, merged in panel c) and BEV fixed at 4 hpi (panels d and e, merged in panel f). An uninfected cell is shown in panel g. The cells were immunolabeled for the presence of FMDV and BEV structural proteins, which were detected with anti-rabbit antibody-Alexa488 conjugate (green), and the microtubule cytoskeleton was immunolabeled with mouse anti-α-tubulin, which was detected with anti-mouse antibody-Alexa568 conjugate (red). Nuclei were labeled with DAPI (blue). Scale bars: a to f, 10 μm; g, 10 μm.
The effects of viral infection on F-actin in cells were examined using fluorescently labeled phalloidin (data not shown). In control cells, F-actin was predominantly found in “stress fibers” in the region of the cell adjacent to the coverslip surface. FMDV infection led to a loss of actin stress fibers; however, some actin labeling was still visible at the plasma membrane. This suggested that FMDV infection leads to depolymerization of F-actin. BEV infection also induced the loss of F-actin bundles from the basal region of the cell. However, in contrast to FMDV, actin labeling at the plasma membrane was not detectable in BEV-infected cells.
FMDV infection disrupts γ-tubulin localization but not pericentrin or centrioles.
As shown above, FMDV infection leads to microtubule disruption throughout the cell. The centrosome consists of pericentrin and a matrix of large protein complexes containing γ-tubulin and associated proteins. This matrix is thought to be the site of microtubule nucleation. FMDV-induced disorganization of microtubules (comprised of heterodimers of α and β tubulin) suggested that the virus may be affecting the function of the centrosome. In control cells, we found that 99 of 100 cells examined showed strong staining at the MTOC for γ-tubulin and pericentrin. We therefore examined the distribution of γ-tubulin in cells infected with FMDV. Figure 4a to c shows the analysis of cells at 3 hpi with FMDV. Labeling for FMDV was with an antibody recognizing nonstructural FMDV 3A protein. In these images, two cells showed strong labeling for FMDV, while a third cell was uninfected. Significantly, γ-tubulin staining (red) was absent from the cells with high levels of FMDV protein, while it remained present in uninfected cells (Fig. 4a to c). The image is representative of 100 FMDV-infected cells examined at 3 hpi. We found that in 85% of cases FMDV infection led to a loss of γ-tubulin at the MTOC. In the remaining 15% of cases, γ-tubulin at the MTOC was detectable, but staining was weaker than that observed in control cells. Interestingly, this corresponded with a lower level of FMDV infection. Our results indicated that FMDV infection led to a loss of γ-tubulin from the MTOC with a concomitant untethering of the microtubules. This is consistent with the disorganization of microtubules observed in previous experiments (see Fig. 3). This effect was also seen at earlier time points. Figure 4d to f shows that BEV infection had no noticeable effect on γ-tubulin distribution in cells, which remained similar to that observed in control cells. Labeling for γ-tubulin was clearly visible in cells expressing high levels of viral proteins.
FIG. 4.
FMDV, but not BEV, induces loss of γ-tubulin from the MTOC. BHK-21 cells were infected with FMDV and fixed with paraformaldehyde at 2 hpi (panels a and b, merged in panel c) and BEV fixed at 4 hpi (panels d and e merged in panel f). The cells were immunolabeled for the presence of viral structural proteins, which were detected with anti-rabbit antibody-Alexa488 conjugate (green), and γ-tubulin, which was detected with anti-mouse antibody-Alexa568 conjugate to show the location of the MTOC (red). Nuclei were labeled with DAPI (blue). Scale bar, 10 μm.
The loss of γ-tubulin labeling from the MTOC of cells infected with FMDV could either reflect a specific loss of γ-tubulin from the centrosomes or a more generalized disruption of the organelle. Either scenario could lead to microtubule disorganization. In order to determine which of these possibilities was occurring, we analyzed FMDV-infected cells for the presence of another centrosome component: pericentrin. This protein is a structural component of the centrosome and interacts with γ-tubulin (17) to form a lattice required for the regulation of microtubule formation. Pericentrin also associates with the motor protein dynein and this enables pericentrin-γ-tubulin complexes to be transported along microtubules to the centrosome (60, 82). Using an antibody specific for pericentrin, we examined the effects of FMDV infection on the centrosomal structure. In uninfected cells, the pattern of γ-tubulin (Fig. 5a) and pericentrin (Fig. 5b) labeling is similar, with small dots often close to the nucleus (Fig. 5a and b). In cells infected with FMDV the characteristic γ-tubulin labeling seen at the perinuclear location in control cells was lost (Fig. 5d), whereas pericentrin labeling was unaffected (Fig. 5e). These results suggested that the MTOC may remain intact, but its inability to organize microtubules in cells infected with FMDV was caused by loss of the tethering protein, γ-tubulin. Cells infected with FMDV were therefore examined by electron microscopy for evidence of intact centrioles. Infection of cells with FMDV led to an accumulation of membrane vesicles in the cytoplasm, and these were used to identify infected cells in electron micrographs. Figure 5c shows a control cell with the MTOC present in the center of the image, and Fig. 5f shows a cell infected with FMDV at 2 hpi. The cytoplasm contains FMDV-induced vesicles, and the main structural component of the centrioles of the MTOC was still present and is located in the center of the image. Centrioles consist of two sets of short microtubules at 90° to each other and may have different appearances depending on the angle of sectioning.
FIG. 5.
Effect of FMDV infection or nonstructural protein 3Cpro expression on the MTOC. (a to c) Uninfected BHK-21 cells fixed in paraformaldehyde and labeled for γ-tubulin (a) and pericentrin (b) (green; arrows) or for TEM showing centrioles (c). (d and e) BHK21 cells infected with FMDV and fixed in paraformaldehyde at 2 hpi and labeled for FMDV structural proteins detected with anti-rabbit antibody-Alexa568 conjugate (red) and either γ-tubulin (d) or pericentrin (e) (green). (f) BHK-21 cells infected with FMDV and fixed for TEM at 2 hpi. The cell cytoplasm contains typical FMDV-induced vesicles, but the centrioles are visible in the center of the image. (g) BHK-21 cells transfected with 2BV5 and immunolabeled with anti-V5 tag detected by anti-mouse IgG2a-Alexa568 (red) and γ-tubulin detected by anti-mouse IgG1-Alexa488 (green). (h and i) BSR cells transfected with pSKRH3C, which expresses the FMDV 3Cpro, and immunolabeled for 3Cpro (antibody 4E9) detected by anti-mouse IgG2b-Alexa568 (red) and with mouse anti-γ-tubulin (green). Nuclei were labeled with DAPI (blue). Scale bars: a, b, d, e, g, h, and i, 10 μm; c, 500 nm; f, 400 nm.
FMDV nonstructural protein 3Cpro induces loss of γ-tubulin.
We then sought to identify the cause of FMDV-induced γ-tubulin loss from the MTOC and hypothesized that a viral nonstructural protein or proteins (rather than structural proteins) would be a likely candidate. We undertook a series of transfections of BHK cells with plasmids that express the FMDV nonstructural proteins or their precursors. Plasmids, transfected into cells, encoded FMDV 2B, 2BC 2C, 3A, 3AB, or 3C. Transiently transfected cells were fixed after 20 h and labeled for expression of the V5 tag (FMDV 2B) or FMDV nonstructural protein (2BC 2C, 3A, 3AB, and 3Cpro) using specific antibodies and for the presence of γ-tubulin. In all cases, except for cells that expressed FMDV 3Cpro (see below), γ-tubulin was unaffected. As an example, Fig. 5g shows a cell expressing FMDV 2B, where the viral protein is labeled red and γ-tubulin is green.
Transient expression of FMDV 3Cpro can be achieved from plasmid pSKRH3C in BHK cells after infection with the recombinant vaccinia viruses vTF7-3 or MVA-T7, which express the T7 RNA polymerase (44). Preliminary experiments using the MVA-T7 system, to drive transcription of the plasmid, indicated that the expression of 3Cpro disrupted the γ-tubulin labeling pattern in a similar way to FMDV infection. However, a previous study with vaccinia virus has shown a loss of γ-tubulin (59) during virus infection. Although changes in γ-tubulin distribution in the MVA-T7-infected cells were not observed, we performed transfection experiments with BSR T7/5 cells to exclude totally the possibility that the virus infection was modifying the result. These cells constitutively express T7 RNA polymerase (8), thus removing the need for the vaccinia virus to drive FMDV 3Cpro expression. The results clearly showed that transient expression of FMDV 3Cpro alone in cells (in the absence of vaccinia virus) led to a depletion of γ-tubulin labeling at the MTOC (Fig. 5h and i). This image is representative of 50 3C transfected cells examined. In 90% of transfected cells examined, the γ-tubulin at the MTOC was not detectable. The remaining 10% of transfected cells showed some γ-tubulin labeling, and this staining was weaker that that seen in control cells. These cells expressed much lower quantities of the FMDV 3C protease. Similarly, as with FMDV infection, transient expression of FMDV 3Cpro in cells did not affect the presence of pericentrin at the MTOC. Of 50 cells examined, 96% showed labeling for pericentrin.
To determine whether FMDV was targeting γ-tubulin for degradation, the steady-state level of γ-tubulin was examined over the course of an FMDV infection. Infected cells were lysed at 1-h intervals starting from 2 hpi and analyzed by Western blotting (Fig. 6a). FMDV protein expression was detectable (using an antibody against the nonstructural protein 2C) by 3 hpi, and the levels of 2C increased with time. With a longer exposure of the blot, virus infection was also clearly detectable at 2 hpi (data not shown). Both γ- and α-tubulin were detectable throughout the virus infection at constant levels, indicating that while γ-tubulin was being displaced from the MTOC, it was not targeted for degradation.
FIG. 6.
Effects of FMDV and cycloheximide on γ- and α-tubulin. (a) BHK-21 cells were infected with FMDV 01BFS strain. Samples were lysed at 0, 2, 3, and 4 hpi. Proteins were separated by SDS-PAGE, blotted, and analyzed for the presence of FMDV 2C, α-tubulin, and γ-tubulin. (b) Cycloheximide was added to cells at a concentration of 100 μg/ml. Cells were examined over the subsequent 4 h. The level of α-tubulin synthesis in cells was examined at 0, 1, 2, 3, and 4 h after the addition of cycloheximide, by radiolabeling with 35S followed by immunoprecipitation. (c) γ-Tubulin levels at the MTOC were examined by confocal microscopy at the same time points as in panel b. (d) BSR cells transfected with pSKRH3C, which expresses the FMDV 3Cpro, and immunolabeled for 3Cpro (antibody 4E9) detected by anti-mouse IgG2b-Alexa488 (green) and with mouse anti-α-tubulin detected with Alexa568 (red). Scale bars, 10 μm.
FMDV 3Cpro has been shown to modify the host cell protein synthesis machinery and induces cleavage of eIF4A and eIF4GI (5). This may contribute to the loss of protein synthesis at late times within infected cells (but the initial loss of host protein synthesis is mediated through the action of the FMDV leader [L] protease). To examine whether host protein synthesis shutoff alone leads to disruption of γ-tubulin labeling at the MTOC, BSR cells were incubated with cycloheximide (cycloheximide blocks peptidyl transferase activity in eukaryotic ribosomes, leading to a block in protein synthesis), and cells were examined at hourly intervals over 4 h. To detect protein synthesis, a portion of the cells were labeled with [35S]methionine for 10 min. Immunoprecipitation of the cellular host protein α-tubulin revealed that cycloheximide had shut off host protein synthesis within the first hour, as expected. [35S]methionine-labeled α-tubulin was not detectable after the addition of cycloheximide (Fig. 6b). The remaining cells were labeled for the presence of γ-tubulin (Fig. 6c). The levels and location of γ-tubulin in cycloheximide treated cells were similar to those of untreated cells, indicating that the effects triggered by host protein synthesis shutoff did not lead to disruption of the γ-tubulin at the MTOC.
FMDV nonstructural protein 3Cpro induces dissociation of microtubules from the MTOC.
BSR cells transiently expressing FMDV 3Cpro were further examined to determine the effects of the loss of γ-tubulin on the microtubule network. Staining for α-tubulin in cells expressing FMDV 3Cpro (Fig. 6d) revealed that the majority of α-tubulin has relocated to the edges of the cell, thus closely mimicking the effect of FMDV infection and confirming that a major contributor to the change in cell morphology is due to the dissociation of microtubules from the MTOC.
DISCUSSION
FMDV.
Infection of BHK cells with FMDV causes dramatic changes in cell morphology.
The cytoskeleton shows dramatic rearrangements in many of its components. The network of actin filaments is disrupted from the early stage of infection, and this is reported to be a common response in cells to viral infection (4, 68). However, taking the other observations reported here it is likely that changes in other components of the cytoskeleton, and microtubules in particular, may play a key role in this process. The changes in the microtubule network are also clear from the early stage in FMDV infection and result in the FMDV replication area being devoid of microtubules. Early signs of FMDV infection (as detected by labeling for nonstructural proteins) were seen in the area of the Golgi body (39), and this organelle is often closely associated with the MTOC. Changes have also been detected in the buoyant density of Golgi membranes during FMDV infection (50), suggesting Golgi disruption or modification. The protein γ-tubulin forms a ring around the centrosome and acts in anchoring the “+” ends of microtubules to the MTOC. The loss of γ-tubulin labeling in FMDV-infected cells gave a possible mechanism for the disruption of the microtubule structure. However, surprisingly, the MTOC protein pericentrin appeared to be unaffected, as did the basic physical structure of the centrioles as seen in FMDV-infected cells by TEM. Thus, a specific targeting of the tethering of microtubules to the MTOC seemed possible.
We hypothesized that a nonstructural (rather than a structural) FMDV protein was most likely to be responsible for this effect. The expression of individual and precursor forms of nonstructural FMDV proteins in uninfected cells showed that 3Cpro alone induced the loss of γ-tubulin labeling from the MTOC region of the cell. This effect was not seen in cells that expressed any of the other nonstructural proteins or their precursors. Furthermore, the microtubule pattern in cells expressing 3Cpro alone closely resembled that seen in FMDV-infected cells, in that the microtubules accumulated in a peripheral location. Exactly how FMDV 3Cpro exerts its effect on γ-tubulin remains unclear since the γ-tubulin does not appear to be targeted for degradation, and we did not detect any cleavage of it during virus infection (Fig. 6). Microtubule nucleation at the centrosome is mediated by the γ-tubulin ring complex, and in human cells this complex is made up of γ-tubulin and the γ-tubulin complex proteins (GCP): 2-6 (21, 52, 53) and GCP-WD (or NEDD1) (24, 45). Interestingly, depletion of GCP-WD (a γ-tubulin targeting factor) results in a depletion of γ-tubulin, at the MTOC, but not pericentrin (45), and the levels of γ-tubulin in the cell detected by Western blotting remained constant. Since γ-tubulin does not appear to be a direct target of the FMDV 3Cpro, it may be that FMDV 3Cpro targets a different member of the γ-tubulin ring complex, which indirectly leads to γ-tubulin depletion at the centrosome.
The effects on the host cell MTOC that we have detected and attributed to FMDV protein 3Cpro have not been reported for any other picornavirus. The functions of the PV nonstructural proteins on host cells have been investigated by transfection of each protein individually and in combination. These experiments indicate that PV 2C and 2BC induce the formation of smooth membrane vesicles (11) and that 2B induces disassembly of the Golgi apparatus (64) and causes plasma membrane permeabilization (1, 19, 41, 46). PV 3A slows protein movement through the secretory pathway (12, 18, 19) and causes swelling of ER membranes (18). The PV 3Cpro has a number of reported functions on host cells, and these include inhibition of host cell RNA synthesis through proteolytic cleavage, and thus the inactivation, of specific transcription factors (13, 14, 76, 79, 80), induction of apoptosis (3, 9), suppression of NF-κβ through proteolytic cleavage of the p65-RelA component (57), and contribution to translation shutoff via inactivation of ribosome-associated PABP (40). However, no direct effect on the host cell microtubules or the MTOC has been reported, although cleavage of microtubule-associated protein 4 (MAP4) by 3Cpro has been demonstrated (35).
The redistribution of microtubules may well explain the pattern of vimentin seen around the FMDV replication area. The mechanisms that control the expression and distribution of vimentin filaments in uninfected cells are not clear. In uninfected cells vimentin forms a network spread through the cytoplasm with no clear focal point. In FMDV-infected cells the vimentin filaments are rearranged into a network surrounding the replication area. The formation of a vimentin network around areas of viral replication has recently been reported for other viruses (25, 26, 56, 62, 65, 69, 77, 78), and the similarity between this pattern and the structure of aggresomes is tantalizing. It is possible to speculate that the vimentin “cage” around the FMDV area of replication is actually a host cell response to the infection rather than a virus induced-change that enhances viral replication.
Comparison of cells infected by FMDV and BEV.
Although there is some consistency in the overall pattern of cellular responses to infection by FMDV and BEV, there are a number of key differences. Infection of cells with BEV does not lead to the loss of γ-tubulin or pericentrin labeling at the MTOC, and there was no resultant dramatic change in the overall pattern of microtubules. From its major role in anchoring components in normal cells, it could be predicted that there should be less disruption of the cellular components in BEV-infected cells. This was not actually the case. Although vimentin filaments are drawn around the FMDV replication area, in BEV-infected cells, the vimentin is drawn into the virus replication area and may play a similar role for other viruses (56, 77, 78). Interestingly, the 3Cpro protein of PV has been shown to cleave MAP4 (35), and also observed in PV-infected cells is the rearrangement of intermediate filaments into a perinuclear region (42). MAP4 is thought to bind microtubules and stimulate their polymerization and increase their stability (27, 47). A truncated form of MAP4 has also been shown to increase microtubule stability (81); however, MAP4 detachment from microtubules leads to an increased instability of the microtubule network (29). A breakdown of the vimentin network has also been observed with detachment of MAP4 from the microtubules (20). It may be that in BEV-infected cells, cleavage of MAP4 could leave a truncated version on the microtubules, retaining microtubule stability, while also leading to vimentin detachment from the microtubules and allowing it to move into the virus replication complex.
One additional aspect of this study is the role played in the CPE by the host cell response to infection. BEV-infected cells show clear signs of apoptosis after around 5 hpi and, by 6 hpi, all of the cells showed peripheral clumping of nuclear heterochromatin visible by both light and electron microscopy, which is highly characteristic of apoptosis. Thus, some of the virus-induced effects may be masked by the cellular apoptosis changes. There were no overt signs of apoptosis in any of the FMDV-infected cells.
Acknowledgments
This study was supported by BBSRC grants 49/CO7867, 49/C14570, and 201/S14654 to T.W., M.R., and G.J.B.
Footnotes
Published ahead of print on 27 August 2008.
REFERENCES
- 1.Aldabe, R., A. Barco, and L. Carrasco. 1996. Membrane permeabilization by poliovirus proteins 2B and 2BC. J. Biol. Chem. 27123134-23137. [DOI] [PubMed] [Google Scholar]
- 2.Barco, A., and L. Carrasco. 1995. A human virus protein, poliovirus protein 2BC, induces membrane proliferation and blocks the exocytic pathway in the yeast Saccharomyces cerevisiae. EMBO J. 143349-3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barco, A., E. Feduchi, and L. Carrasco. 2000. Poliovirus protease 3Cpro kills cells by apoptosis. Virology 266352-360. [DOI] [PubMed] [Google Scholar]
- 4.Bedows, E., K. M. Rao, and M. J. Welsh. 1983. Fate of microfilaments in Vero cells infected with measles virus and herpes simplex virus type 1. Mol. Cell. Biol. 3712-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belsham, G. J., G. M. McInerney, and N. Ross-Smith. 2000. Foot-and-mouth disease virus 3C protease induces cleavage of translation initiation factors eIF4A and eIF4G within infected cells. J. Virol. 74272-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bienz, K., D. Egger, and L. Pasamontes. 1987. Association of polioviral proteins of the P2 genomic region with the viral replication complex and virus-induced membrane synthesis as visualized by electron microscopic immunocytochemistry and autoradiography. Virology 160220-226. [DOI] [PubMed] [Google Scholar]
- 7.Bienz, K., D. Egger, and D. A. Wolff. 1973. Virus replication, cytopathology, and lysosomal enzyme response of mitotic and interphase Hep-2 cells infected with poliovirus. J. Virol. 11565-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchholz, U. J., S. Finke, and K. K. Conzelmann. 1999. Generation of bovine respiratory syncytial virus (BRSV) from cDNA: BRSV NS2 is not essential for virus replication in tissue culture, and the human RSV leader region acts as a functional BRSV genome promoter. J. Virol. 73251-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calandria, C., A. Irurzun, A. Barco, and L. Carrasco. 2004. Individual expression of poliovirus 2Apro and 3Cpro induces activation of caspase-3 and PARP cleavage in HeLa cells. Virus Res. 10439-49. [DOI] [PubMed] [Google Scholar]
- 10.Caliguiri, L. A., and I. Tamm. 1970. Characterization of poliovirus-specific structures associated with cytoplasmic membranes. Virology 42112-122. [DOI] [PubMed] [Google Scholar]
- 11.Cho, M. W., N. Teterina, D. Egger, K. Bienz, and E. Ehrenfeld. 1994. Membrane rearrangement and vesicle induction by recombinant poliovirus 2C and 2BC in human cells. Virology 202129-145. [DOI] [PubMed] [Google Scholar]
- 12.Choe, S. S., D. A. Dodd, and K. Kirkegaard. 2005. Inhibition of cellular protein secretion by picornaviral 3A proteins. Virology 33718-29. [DOI] [PubMed] [Google Scholar]
- 13.Clark, M. E., T. Hammerle, E. Wimmer, and A. Dasgupta. 1991. Poliovirus proteinase 3C converts an active form of transcription factor IIIC to an inactive form: a mechanism for inhibition of host cell polymerase III transcription by poliovirus. EMBO J. 102941-2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clark, M. E., P. M. Lieberman, A. J. Berk, and A. Dasgupta. 1993. Direct cleavage of human TATA-binding protein by poliovirus protease 3C in vivo and in vitro. Mol. Cell. Biol. 131232-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cuconati, A., A. Molla, and E. Wimmer. 1998. Brefeldin A inhibits cell-free, de novo synthesis of poliovirus. J. Virol. 726456-6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dales, S., H. J. Eggers, I. Tamm, and G. E. Palade. 1965. Electron microscopic study of the formation of poliovirus. Virology 26379-389. [DOI] [PubMed] [Google Scholar]
- 17.Dictenberg, J. B., W. Zimmerman, C. A. Sparks, A. Young, C. Vidair, Y. Zheng, W. Carrington, F. S. Fay, and S. J. Doxsey. 1998. Pericentrin and gamma-tubulin form a protein complex and are organized into a novel lattice at the centrosome. J. Cell Biol. 141163-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doedens, J. R., T. H. Giddings, Jr., and K. Kirkegaard. 1997. Inhibition of endoplasmic reticulum-to-Golgi traffic by poliovirus protein 3A: genetic and ultrastructural analysis. J. Virol. 719054-9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doedens, J. R., and K. Kirkegaard. 1995. Inhibition of cellular protein secretion by poliovirus proteins 2B and 3A. EMBO J. 14894-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ebneth, A., G. Drewes, E. M. Mandelkow, and E. Mandelkow. 1999. Phosphorylation of MAP2c and MAP4 by MARK kinases leads to the destabilization of microtubules in cells. Cell Motil. Cytoskelet. 44209-224. [DOI] [PubMed] [Google Scholar]
- 21.Fava, F., B. Raynaud-Messina, J. Leung-Tack, L. Mazzolini, M. Li, J. C. Guillemot, D. Cachot, Y. Tollon, P. Ferrara, and M. Wright. 1999. Human 76p: a new member of the gamma-tubulin-associated protein family. J. Cell Biol. 147857-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gazina, E. V., J. M. Mackenzie, R. J. Gorrell, and D. A. Anderson. 2002. Differential requirements for COPI coats in formation of replication complexes among three genera of Picornaviridae. J. Virol. 7611113-11122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grigera, P. R., and A. Sagedahl. 1986. Cytoskeletal association of an aphthovirus-induced polypeptide derived from the P3ABC region of the viral polyprotein. Virology 154369-380. [DOI] [PubMed] [Google Scholar]
- 24.Haren, L., M. H. Remy, I. Bazin, I. Callebaut, M. Wright, and A. Merdes. 2006. NEDD1-dependent recruitment of the gamma-tubulin ring complex to the centrosome is necessary for centriole duplication and spindle assembly. J. Cell Biol. 172505-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heath, C. M., M. Windsor, and T. Wileman. 2001. Aggresomes resemble sites specialized for virus assembly. J. Cell Biol. 153449-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heath, C. M., M. Windsor, and T. Wileman. 2003. Membrane association facilitates the correct processing of pp220 during production of the major matrix proteins of African swine fever virus. J. Virol. 771682-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirokawa, N. 1994. Microtubule organization and dynamics dependent on microtubule-associated proteins. Curr. Opin. Cell Biol. 674-81. [DOI] [PubMed] [Google Scholar]
- 28.Hollinshead, M., G. Rodger, H. Van Eijl, M. Law, R. Hollinshead, D. J. Vaux, and G. L. Smith. 2001. Vaccinia virus utilizes microtubules for movement to the cell surface. J. Cell Biol. 154389-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Illenberger, S., G. Drewes, B. Trinczek, J. Biernat, H. E. Meyer, J. B. Olmsted, E. M. Mandelkow, and E. Mandelkow. 1996. Phosphorylation of microtubule-associated proteins MAP2 and MAP4 by the protein kinase p110markL phosphorylation sites and regulation of microtubule dynamics. J. Biol. Chem. 27110834-10843. [DOI] [PubMed] [Google Scholar]
- 30.Irurzun, A., L. Perez, and L. Carrasco. 1992. Involvement of membrane traffic in the replication of poliovirus genomes: effects of brefeldin A. Virology 191166-175. [DOI] [PubMed] [Google Scholar]
- 31.Jackson, T., F. M. Ellard, R. A. Ghazaleh, S. M. Brookes, W. E. Blakemore, A. H. Corteyn, D. I. Stuart, J. W. Newman, and A. M. King. 1996. Efficient infection of cells in culture by type O foot-and-mouth disease virus requires binding to cell surface heparan sulfate. J. Virol. 705282-5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jackson, W. T., T. H. Giddings, Jr., M. P. Taylor, S. Mulinyawe, M. Rabinovitch, R. R. Kopito, and K. Kirkegaard. 2005. Subversion of cellular autophagosomal machinery by RNA viruses. PLoS Biol. 3e156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jezequel, A. M., and J. W. Steiner. 1966. Some ultrastructural and histochemical aspects of coxsackie virus-cell interactions. Lab. Investig. 151055-1083. [PubMed] [Google Scholar]
- 34.Joachims, M., and D. Etchison. 1992. Poliovirus infection results in structural alteration of a microtubule-associated protein. J. Virol. 665797-5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joachims, M., K. S. Harris, and D. Etchison. 1995. Poliovirus protease 3C mediates cleavage of microtubule-associated protein 4. Virology 211451-461. [DOI] [PubMed] [Google Scholar]
- 36.Jouvenet, N., P. Monaghan, M. Way, and T. Wileman. 2004. Transport of African swine fever virus from assembly sites to the plasma membrane is dependent on microtubules and conventional kinesin. J. Virol. 787990-8001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jouvenet, N., and T. Wileman. 2005. African swine fever virus infection disrupts centrosome assembly and function. J. Gen. Virol. 86589-594. [DOI] [PubMed] [Google Scholar]
- 38.Jouvenet, N., M. Windsor, J. Rietdorf, P. Hawes, P. Monaghan, M. Way, and T. Wileman. 2006. African swine fever virus induces filopodia-like projections at the plasma membrane. Cell Microbiol. 81803-1811. [DOI] [PubMed] [Google Scholar]
- 39.Knox, C., K. Moffat, S. Ali, M. Ryan, and T. Wileman. 2005. Foot-and-mouth disease virus replication sites form next to the nucleus and close to the Golgi apparatus, but exclude marker proteins associated with host membrane compartments. J. Gen. Virol. 86687-696. [DOI] [PubMed] [Google Scholar]
- 40.Kuyumcu-Martinez, N. M., M. Joachims, and R. E. Lloyd. 2002. Efficient cleavage of ribosome-associated poly(A)-binding protein by enterovirus 3C protease. J. Virol. 762062-2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lama, J., and L. Carrasco. 1992. Expression of poliovirus nonstructural proteins in Escherichia coli cells: modification of membrane permeability induced by 2B and 3A. J. Biol. Chem. 26715932-15937. [PubMed] [Google Scholar]
- 42.Lenk, R., and S. Penman. 1979. The cytoskeletal framework and poliovirus metabolism. Cell 16289-301. [DOI] [PubMed] [Google Scholar]
- 43.Levine, R. A., and D. A. Wolff. 1979. Bovine enterovirus CPE at different multiplicities of infection in the absence of viral RNA synthesis. Intervirology 11255-260. [DOI] [PubMed] [Google Scholar]
- 44.Li, W., N. Ross-Smith, C. G. Proud, and G. J. Belsham. 2001. Cleavage of translation initiation factor 4AI (eIF4AI) but not eIF4AII by foot-and-mouth disease virus 3C protease: identification of the eIF4AI cleavage site. FEBS Lett. 5071-5. [DOI] [PubMed] [Google Scholar]
- 45.Luders, J., U. K. Patel, and T. Stearns. 2006. GCP-WD is a gamma-tubulin targeting factor required for centrosomal and chromatin-mediated microtubule nucleation. Nat. Cell Biol. 8137-147. [DOI] [PubMed] [Google Scholar]
- 46.Madan, V., S. Sanchez-Martinez, N. Vedovato, G. Rispoli, L. Carrasco, and J. L. Nieva. 2007. Plasma membrane-porating domain in poliovirus 2B protein: a short peptide mimics viroporin activity. J. Mol. Biol. 374951-964. [DOI] [PubMed] [Google Scholar]
- 47.Mandelkow, E., and E. M. Mandelkow. 1995. Microtubules and microtubule-associated proteins. Curr. Opin. Cell Biol. 772-81. [DOI] [PubMed] [Google Scholar]
- 48.Maynell, L. A., K. Kirkegaard, and M. W. Klymkowsky. 1992. Inhibition of poliovirus RNA synthesis by brefeldin A. J. Virol. 661985-1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moffat, K., G. Howell, C. Knox, G. J. Belsham, P. Monaghan, M. D. Ryan, and T. Wileman. 2005. Effects of foot-and-mouth disease virus nonstructural proteins on the structure and function of the early secretory pathway: 2BC but not 3A blocks endoplasmic reticulum-to-Golgi transport. J. Virol. 794382-4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moffat, K., C. Knox, G. Howell, S. J. Clark, H. Yang, G. J. Belsham, M. Ryan, and T. Wileman. 2007. Inhibition of the secretory pathway by foot-and-mouth disease virus 2BC protein is reproduced by coexpression of 2B with 2C, and the site of inhibition is determined by the subcellular location of 2C. J. Virol. 811129-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Monaghan, P., H. Cook, T. Jackson, M. Ryan, and T. Wileman. 2004. The ultrastructure of the developing replication site in foot-and-mouth disease virus-infected BHK-38 cells. J. Gen. Virol. 85933-946. [DOI] [PubMed] [Google Scholar]
- 52.Murphy, S. M., A. M. Preble, U. K. Patel, K. L. O'Connell, D. P. Dias, M. Moritz, D. Agard, J. T. Stults, and T. Stearns. 2001. GCP5 and GCP6: two new members of the human gamma-tubulin complex. Mol. Biol. Cell 123340-3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murphy, S. M., L. Urbani, and T. Stearns. 1998. The mammalian gamma-tubulin complex contains homologues of the yeast spindle pole body components spc97p and spc98p. J. Cell Biol. 141663-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Murti, K. G., and R. Goorha. 1983. Interaction of frog virus-3 with the cytoskeleton. I. Altered organization of microtubules, intermediate filaments, and microfilaments. J. Cell Biol. 961248-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nédellec, P., P. Vicart, C. Laurent-Winter, C. Martinat, M. C. Prévost, and M. Brahic. 1998. Interaction of Theiler's virus with intermediate filaments of infected cells. J. Virol. 729553-9560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Netherton, C., K. Moffat, E. Brooks, and T. Wileman. 2007. A guide to viral inclusions, membrane rearrangements, factories, and viroplasm produced during virus replication. Adv. Virus Res. 70101-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Neznanov, N., K. M. Chumakov, L. Neznanova, A. Almasan, A. K. Banerjee, and A. V. Gudkov. 2005. Proteolytic cleavage of the p65-RelA subunit of NF-κB during poliovirus infection. J. Biol. Chem. 28024153-24158. [DOI] [PubMed] [Google Scholar]
- 58.O'Donnell, V. K., J. M. Pacheco, T. M. Henry, and P. W. Mason. 2001. Subcellular distribution of the foot-and-mouth disease virus 3A protein in cells infected with viruses encoding wild-type and bovine-attenuated forms of 3A. Virology 287151-162. [DOI] [PubMed] [Google Scholar]
- 59.Ploubidou, A., V. Moreau, K. Ashman, I. Reckmann, C. Gonzalez, and M. Way. 2000. Vaccinia virus infection disrupts microtubule organization and centrosome function. EMBO J. 193932-3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Purohit, A., S. H. Tynan, R. Vallee, and S. J. Doxsey. 1999. Direct interaction of pericentrin with cytoplasmic dynein light intermediate chain contributes to mitotic spindle organization. J. Cell Biol. 147481-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rietdorf, J., A. Ploubidou, I. Reckmann, A. Holmstrom, F. Frischknecht, M. Zettl, T. Zimmermann, and M. Way. 2001. Kinesin-dependent movement on microtubules precedes actin-based motility of vaccinia virus. Nat. Cell Biol. 3992-1000. [DOI] [PubMed] [Google Scholar]
- 62.Risco, C., J. R. Rodriguez, C. Lopez-Iglesias, J. L. Carrascosa, M. Esteban, and D. Rodriguez. 2002. Endoplasmic reticulum-Golgi intermediate compartment membranes and vimentin filaments participate in vaccinia virus assembly. J. Virol. 761839-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rust, R. C., L. Landmann, R. Gosert, B. L. Tang, W. Hong, H. P. Hauri, D. Egger, and K. Bienz. 2001. Cellular COPII proteins are involved in production of the vesicles that form the poliovirus replication complex. J. Virol. 759808-9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sandoval, I. V., and L. Carrasco. 1997. Poliovirus infection and expression of the poliovirus protein 2B provoke the disassembly of the Golgi complex, the organelle target for the antipoliovirus drug Ro-090179. J. Virol. 714679-4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schepis, A., B. Schramm, C. A. de Haan, and J. K. Locker. 2006. Vaccinia virus-induced microtubule-dependent cellular rearrangements. Traffic 7308-323. [DOI] [PubMed] [Google Scholar]
- 66.Schlegel, A., T. H. Giddings, Jr., M. S. Ladinsky, and K. Kirkegaard. 1996. Cellular origin and ultrastructure of membranes induced during poliovirus infection. J. Virol. 706576-6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Seipelt, J., H. D. Liebig, W. Sommergruber, C. Gerner, and E. Kuechler. 2000. 2A proteinase of human rhinovirus cleaves cytokeratin 8 in infected HeLa cells. J. Biol. Chem. 27520084-20089. [DOI] [PubMed] [Google Scholar]
- 68.Shoeman, R. L., R. Hartig, C. Hauses, and P. Traub. 2002. Organization of focal adhesion plaques is disrupted by action of the HIV-1 protease. Cell Biol. Int. 26529-539. [DOI] [PubMed] [Google Scholar]
- 69.Stefanovic, S., M. Windsor, K. I. Nagata, M. Inagaki, and T. Wileman. 2005. Vimentin rearrangement during African swine fever virus infection involves retrograde transport along microtubules and phosphorylation of vimentin by calcium calmodulin kinase II. J. Virol. 7911766-11775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stuart, J. D. C., and J. Fogh. 1961. Micromorphology of FL cells infected with polio and coxsackie viruses. Virology 13177-190. [Google Scholar]
- 71.Suhy, D. A., T. H. Giddings, Jr., and K. Kirkegaard. 2000. Remodeling the endoplasmic reticulum by poliovirus infection and by individual viral proteins: an autophagy-like origin for virus-induced vesicles. J. Virol. 748953-8965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sutter, G., M. Ohlmann, and V. Erfle. 1995. Non-replicating vaccinia vector efficiently expresses bacteriophage T7 RNA polymerase. FEBS Lett. 3719-12. [DOI] [PubMed] [Google Scholar]
- 73.Taylor, M. P., and K. Kirkegaard. 2007. Modification of cellular autophagy protein LC3 by poliovirus. J. Virol. 8112543-12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Taylor, M. P., and K. Kirkegaard. 2007. Potential subversion of autophagosomal pathway by picornaviruses. Autophagy 4286-289. [DOI] [PubMed] [Google Scholar]
- 75.Weed, H. G., G. Krochmalnic, and S. Penman. 1985. Poliovirus metabolism and the cytoskeletal framework: detergent extraction and resinless section electron microscopy. J. Virol. 56549-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Weidman, M. K., P. Yalamanchili, B. Ng, W. Tsai, and A. Dasgupta. 2001. Poliovirus 3C protease-mediated degradation of transcriptional activator p53 requires a cellular activity. Virology 291260-271. [DOI] [PubMed] [Google Scholar]
- 77.Wileman, T. 2006. Aggresomes and autophagy generate sites for virus replication. Science 312875-878. [DOI] [PubMed] [Google Scholar]
- 78.Wileman, T. 2007. Aggresomes and pericentriolar sites of virus assembly: cellular defense or viral design? Annu. Rev. Microbiol. 61149-167. [DOI] [PubMed] [Google Scholar]
- 79.Yalamanchili, P., U. Datta, and A. Dasgupta. 1997. Inhibition of host cell transcription by poliovirus: cleavage of transcription factor CREB by poliovirus-encoded protease 3Cpro. J. Virol. 711220-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yalamanchili, P., K. Harris, E. Wimmer, and A. Dasgupta. 1996. Inhibition of basal transcription by poliovirus: a virus-encoded protease (3Cpro) inhibits formation of TBP-TATA box complex in vitro. J. Virol. 702922-2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yoshida, T., K. Imanaka-Yoshida, H. Murofushi, J. Tanaka, H. Ito, and M. Inagaki. 1996. Microinjection of intact MAP-4 and fragments induces changes of the cytoskeleton in PtK2 cells. Cell Motil. Cytoskelet. 33252-262. [DOI] [PubMed] [Google Scholar]
- 82.Young, A., J. B. Dictenberg, A. Purohit, R. Tuft, and S. J. Doxsey. 2000. Cytoplasmic dynein-mediated assembly of pericentrin and gamma tubulin onto centrosomes. Mol. Biol. Cell 112047-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhai, Z. H., X. Wang, and X. Y. Qian. 1988. Nuclear matrix-intermediate filament system and its alteration in adenovirus-infected HeLa cell. Cell Biol. Int. Rep. 1299-108. [DOI] [PubMed] [Google Scholar]