Abstract
The herpes simplex virus type 1 (HSV-1) UL37 gene encodes a 120-kDa polypeptide which resides in the tegument structure of the virion and is important for morphogenesis. The goal of this study was to use green fluorescent protein (GFP) to follow the fate of UL37 within cells during the normal course of virus replication. GFP was inserted in frame at the C terminus of UL37 to generate a fluorescent-protein-tagged UL37 polypeptide. A virus designated K37eGFP, which replicated normally on Vero cells, was isolated and was shown to express the fusion polypeptide. When cells infected with this virus were examined by confocal microscopy, the fluorescence was observed to be predominantly cytoplasmic. As the infection progressed, fluorescence began to accumulate in a juxtanuclear structure. Mannosidase II and giantin were observed to colocalize with UL37eGFP at these structures, as judged by immunofluorescence assays. Therefore, UL37 traffics to the Golgi complex during infection. A VP26mRFP marker (red fluorescent protein fused to VP26) was recombined into K37eGFP, and when cells infected with this “dual-color” virus were examined, colocalization of the red (capsid) and green (UL37) fluorescence in the Golgi structure was observed. Null mutations in VP5 (ΔVP5), which abolished capsid assembly, and in UL36 (Δ36) were recombined into the K37eGFP virus genome. In cells infected with K37eGFP/ΔVP5, localization of UL37eGFP to the Golgi complex was similar to that for the parental virus (K37eGFP), indicating that trafficking of UL37eGFP to the Golgi complex did not require capsid structures. Confocal analysis of cells infected with K37eGFP/Δ36 showed that, in the absence of UL36, accumulation of UL37eGFP at the Golgi complex was not evident. This indicates an interaction between these two proteins that is important for localization of UL37 in the Golgi complex and thus possibly for cytoplasmic envelopment of the capsid. This is the first demonstration of a functional role for UL36:UL37 interaction in HSV-1-infected cells.
The herpes simplex virus type 1 (HSV-1) virion is composed of four structural elements: a large, double-stranded DNA molecule in the central space; an icosahedral capsid, which encloses the genome; a proteinaceous layer that is attached to the capsid, termed the tegument; and an outer envelope, which encloses the whole structure and in which the viral glycoproteins are embedded (31, 40). The tegument is one of the most complex and diverse structures of the virion in terms of both protein composition and the functions encoded by the constituents of this structure.
The morphogenetic life cycle of herpesviruses is complex and involves the participation of many gene products, which are required at different times during infection and within different structures of the cell. Capsid assembly for herpesviruses is a nuclear event resulting in the production of an icosahedral protein coat whose components are the major capsid protein VP5, the two triplex stabilizing proteins VP19C and VP23, and the small capsid decoration protein VP26 (reviewed in references 16, 30, and 35). Packaging of viral DNA into capsid shells is a complex process requiring the functions of several gene products, some of which remain capsid associated (reviewed in reference 16). Initial envelopment of the virion takes place at the inner nuclear membrane. These primary enveloped virions then fuse with the outer nuclear membrane, depositing a naked (nonenveloped) particle into the cytoplasm. These capsids are transported to the Golgi structure or Golgi-derived organelle for final envelopment (15, 24, 25, 34). This cytoplasmic site must accumulate all the various tegument proteins that are incorporated into the mature or secondary enveloped virion.
One of the most intriguing aspects of this morphogenetic pathway is the role of the tegument proteins in this dual envelopment process. Many studies have shown a clear distinction in polypeptide composition of the primary enveloped particles (enveloped by the inner nuclear envelop) and the secondary enveloped particles (enveloped by a cytoplasmic membrane). These differences are in the composition of the tegument (24, 26). The questions that still remain unanswered are the cellular locations and movement of tegument proteins prior to their incorporation into the maturing virus and the virus factors/signals that traffic particles to the maturation compartment. The multitude of tegument proteins have different locations within the cell; some are exclusively cytoplasmic and others exclusively nuclear, and yet others are detected in both compartments. Thus, as the virus particle progresses on its way to the surface it has to ensure that all the appropriate tegument proteins become incorporated into the final, mature virion.
The UL37 gene encodes a 120-kDa phosphorylated polypeptide which is expressed late in the virus replication cycle. It is a component of both mature virions and light particles, and detergent extraction studies show that it is a resident of the tegument structure (23, 33). Immunofluorescence assays of HSV-1-infected cells revealed that the UL37 polypeptide is distributed throughout the infected cell but is predominantly localized to the cytoplasm (22, 23, 33). This diffuse cytoplasmic distribution was not dependent on the presence of another HSV-1 gene product, because it was the same in cells infected with a UL37-expressing vaccinia virus vector (33). Studies have also identified a nuclear export signal (NES), which may be responsible for this distribution (39). Both HSV-1 UL37 and pseudorabies virus (PRV) UL37 have been shown to interact with HSV-1 UL36 and PRV UL36, respectively, in yeast two-hybrid assays (17, 27, 38). The human cytomegalovirus homolog of UL37 (UL47) has similarly been shown to interact with the UL36 homolog (UL48) both in coimmunoprecipitation assays and in the mature virion (13). The function of this bimolecular interaction is not known. A null mutation in the HSV-1 UL37 gene results in the abrogation of infectious virus production; specifically, there is a marked defect in the egress of DNA-filled capsids from the nucleus (8). In addition, cytoplasmic nucleocapsids fail to acquire an envelope and thus are noninfectious (8). Similar analysis of a PRV UL37 mutant virus revealed a marked reduction in the production of infectious progeny (18). Thus, the herpesvirus UL37 gene specifies an important function for virion morphogenesis.
The goal of the present study was to investigate the cytoplasmic distribution and trafficking of UL37 in infected cells. The use of a live-cell reporter, such as green fluorescent protein (GFP), allows one to follow the fates of individual proteins (fused to GFP) within cells and subsequently virions (incorporating the GFP-tagged structural protein) during egress to the cell surface. This paper describes the analysis of such a fusion protein, UL37eGFP. This protein is transported and accumulates in the Golgi complex, presumably to await the capsids that are independently translocated here. The trafficking of UL37eGFP to the Golgi complex is dependent on the presence of its interacting partner, UL36. Thus, we have identified one function of this bimolecular interaction, that is, cytoplasmic localization of UL37 to budding sites at the Golgi complex.
MATERIALS AND METHODS
Cells and viruses.
Vero cells, transformed Vero cell lines, and human foreskin fibroblasts (HFT) were grown in minimum essential medium-alpha medium supplemented with 10% fetal calf serum (Gibco-Invitrogen) and passaged as described by Desai et al. (6). HFT is an immortalized cell line that is transduced with a retrovirus expressing human telomerase (14). Telomerase-immortalized endothelial (TIME) cells (19) were obtained from Martin McMahon and Don Ganem (University of California—San Francisco) and passaged using endothelial cell basal medium 2 (EBM-2) supplemented with the EGM-2 MV SingleQuots (Lonza) plus 5% fetal bovine serum. Stocks of the parental wild-type virus strain KOS (HSV-1) and the recombinant viruses were prepared as previously described (6).
Plasmids.
For this study, a plasmid designated pKBD was used (8). This plasmid carries the BamHI-DraI (79,441- to 84,369-bp) fragment of HSV-1 strain KOS, which includes the UL37 gene. The enhanced GFP (eGFP) sequence was PCR amplified with PFU turbo (Stratagene) polymerase, using pEGFPN1 (Clontech) as a template. The primers used were GGACTAGTATGGTGAGCAAGGGCGAGGAGCTG (forward) and GGACTAGTCTTGTACAGCTCGTCCATGCCGAG (reverse). The PCR product was digested with SpeI (underlined sequence) and cloned in the correct orientation into the pKBD plasmid, also cut with SpeI. This plasmid was designated pKUL37eGFP. The monomeric red fluorescent protein (mRFP1) gene was amplified from the pREST-mRFP1 template (forward primer, CCTGTACACCATGGCCTCCTCCGAGGACGTCATC, and reverse primer, CCCTCGAGGCGGCGCCGGTGGAGTGGCGGCCCTC), digested with BsrGI and XhoI (underlined sequences), and cloned into pKEXNBr, which is a repaired version of the original plasmid pKEXNB (7; E. Huang and P. Desai, unpublished data). All PCR-amplified genes were sequenced to check for authentic synthesis.
Antibodies.
Antibody to UL37 (UL37C) was raised in rabbits by using a C-terminal peptide (CDTVAPPTDLPLTSYQ) as an immunogen, and antibody 780 was a generous gift from Frank Jenkins (University of Pittsburgh) (1). The GFP and giantin rabbit polyclonal antibodies were purchased from Molecular Probes. Rabbit anti-human mannosidase II was obtained from Serotec. Mouse antibody to Golgin 97 and GM130 was purchased from BD Biosciences.
Marker transfer assays.
Marker transfer of the gene cassettes was done using the methods described by Person and Desai (29). Vero or BD45 cell monolayers (1 × 106) in 60-mm dishes were cotransfected with 25 μl of infected cell DNA and 0.1 to 0.05 μg linearized plasmid DNA. When plaques began to appear (48 to 72 h after transfection), the cell monolayers were harvested, frozen/thawed once, and sonicated and the titer of the total virus progeny was determined. Single-plaque isolates were screened with a fluorescence microscope for green or red fluorescence. Isolates that exhibited a green or red fluorescence phenotype were plaque purified three times prior to further characterization.
Western blot analysis.
Infected cell extracts were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to Immobilon P membranes (Millipore) in Tris-glycine buffer by using a Bio-Rad mini-transblot apparatus. SDS-PAGE analysis was performed as described by Person and Desai (29). The transfer buffer and procedures were used according to the manufacturer's protocol. Western blots were carried out using 125I-labeled protein A for detection as described in Okoye et al. (28).
Confocal analysis.
HFT or TIME cells in LabTek (no. 1.5 borosilicate glass) four-well chamber slides (6 × 105 cells) were infected at a multiplicity of infection (MOI) of 10 PFU/cell. The cells were overlaid with Leibowitz L12 media (Gibco-Invitrogen) for live confocal analysis. The infected cells were analyzed with a Zeiss LSM 510 confocal microscope. Most images were collected with a pinhole set at 1 Airy unit. The optical slices (Z slice) ranged from 0.7 to 1.2 μm. For dually labeled images, the optical slices were identical.
Immunofluorescence assays.
Infected cells were washed two times with Dulbecco's phosphate-buffered saline (DPBS), fixed with 4% paraformaldehyde in DPBS for 25 min, washed two times with DPBS, and permeabilized with 0.25% Triton X-100 in DPBS for 30 min. After permeabilization, the cells were washed two times with 3% bovine serum albumin (BSA) in DPBS, and nonspecific reactivity was blocked for 30 min in the same buffer. Primary antibody was diluted in 3% BSA-DPBS and 250 μl added to each chamber well for 60 min (room temperature). Subsequently, the cells were washed three times with 3% BSA-DPBS and then incubated with secondary antibody for 45 min (room temperature). The cells were then washed three times with 3% BSA-DPBS and then incubated in Fluoromount G (Electron Microscopy Sciences) prior to being imaged.
Data and figure preparation.
For figure preparation, autoradiographs were scanned at 300 dots per inch with Adobe Photoshop. Figures were compiled using Adobe Photoshop. Confocal images were saved as 12-bit TIFF files and imported into Adobe Photoshop for figure preparation.
RESULTS
Isolation of viruses expressing GFP-tagged UL37.
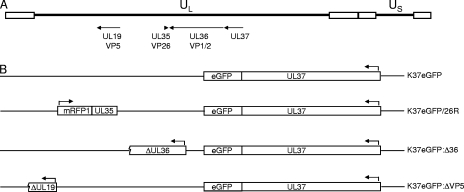
Previously, McLauchlan (22, 23) had shown that insertion of an epitope sequence at a unique SpeI site at the end of the UL37 gene did not affect the function of the protein, in that a virus specifying this insertion was easily isolated and displayed replication properties similar to those of the wild-type virus. We took advantage of this finding to introduce the eGFP open reading frame (ORF) (Clontech) in frame at the C terminus of UL37. The eGFP fusion cassette was introduced into the wild-type parental KOS genome by homologous recombination methods following cotransfection of Vero cells, and green-fluorescing plaques were picked and purified. Two independent isolates from this experiment were analyzed, and they were called K37eGFP1 and K37eGFP2 (Fig. 1B). Both viruses plaqued normally on Vero cells, indicating that the insertion did not affect UL37 function. The viruses were also analyzed by Southern blot hybridization to confirm the correct genotype both for the presence of the eGFP ORF at the unique SpeI site and for the insertion into the correct UL37 genomic region (data not shown).
FIG. 1.
The genetic organization of HSV-1 genes and viruses. (A) Locations of the UL37, UL36, UL35, and UL19 genes on the HSV-1 genome, their directions of transcription, and their approximate sizes. (B) Genotypes of the viruses made in this study. K37eGFP is a virus in which the eGFP is fused to the C terminus of UL37. K37eGFP/26R is a virus that also carries a VP26mRFP1 fusion gene (mRFP is fused at the N terminus of UL35). K37eGFP:Δ36 and K37eGFP:ΔVP5 are viruses in which the UL36 and UL19 genes, respectively, specify null mutations (5, 9). Arrows indicate directions of transcription. ORF blocks are of approximate sizes.
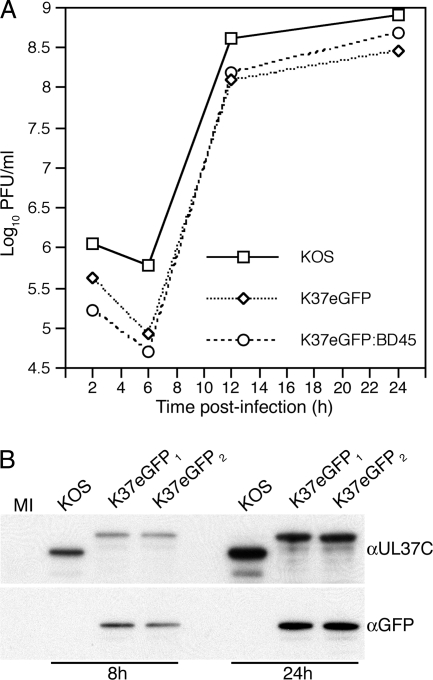
To determine the growth properties of these fluorescent-protein-tagged viruses, single-step growth curves were carried out on Vero cells and on the UL37-complementing BD45 cells (Fig. 2A). Both viruses replicated with similar kinetics on Vero cells; the data for K37eGFP2 are shown in Fig. 2A. There was an approximately threefold reduction in yield at late times (24 h) of infection. This decrease was partially restored in the helper BD45 cell line.
FIG. 2.
(A) Single-step growth of the UL37 recombinant virus. Vero or BD45 cells (5 × 105) were infected with the indicated viruses at an MOI of 10 PFU/cell and the infected cells harvested at 2, 6, 12, and 24 h postinfection (1-ml final volume). K37eGFP2 was used in this experiment. Virus yields were determined by titration on Vero or BD45 cells. The data are averages for two experiments. (B) UL37eGFP polypeptide accumulation in infected cells. Vero cells (1 × 106) were infected with KOS and K37eGFP1/2 or mock infected (MI) at an MOI of 10 PFU/cell and the cells harvested at 8 and 24 h postinfection. The lysates prepared were analyzed by SDS-PAGE (12% acrylamide) and the proteins transferred to a membrane for Western blot analysis. Membranes were probed with anti-UL37C (αUL37C) and αGFP antibodies.
To confirm synthesis and accumulation of the fusion proteins, Vero cells were infected with K37eGFP (Fig. 2B), and Western blot methods were employed using antisera specific for the C terminus of UL37 (αUL37C) and GFP. As shown in Fig. 2B, the fusion protein was detected in infected cells and it had a slower mobility in the gel than wild-type UL37 due to the fusion of the 27-kDa fluorescent protein. There do appear to be reductions in accumulation of the fusion proteins at both 8 and 24 h postinfection. This antibody also reacts to a degraded product of UL37 in Vero cells. A UL37 breakdown product was also seen in BHK cells by McLauchlan (22). When the same extracts were probed with the GFP antibody, only the fusion proteins were detected in the blot.
Cellular trafficking of UL37eGFP.
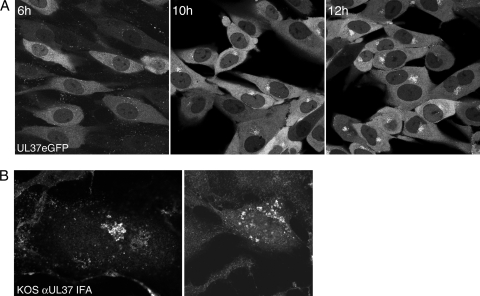
The movement of the UL37-tagged protein in infected cells was visualized using confocal microscopy of live cultures. Initially, Vero cell monolayers were used for this analysis, but we discovered that they were not ideal for confocal analysis, because they round up and become larger due to the cytopathic effects of the virus replication. Instead, HFT were used because they are less susceptible to the cytopathic effects of the virus and also they are more flat, which is suited to this analysis. Another advantage of this cell line was the reduced Golgi fragmentation upon infection relative to that for Vero cells (3), which was a fortuitous property, as shown in subsequent analyses. Using this culture system, confocal analyses were performed on infected cells. Early after infection, the fluorescence observed was predominantly cytoplasmic, with lesser amounts in the nucleus (Fig. 3A, left), similar to that observed by immunofluorescence methods using UL37 antibodies (23, 33). At 10 h postinfection, there was significant localization of fluorescence at a site adjacent to the nucleus in these cells, and this was more pronounced in cells visualized 12 h after infection. The fluorescence accumulation at this asymmetric juxtanuclear site is likely at the Golgi structure. Using immunofluorescence assays, we have observed a similar perinuclear localization of wild-type UL37 in KOS-infected TIME cells by using the UL37 rabbit antibody (1) (Fig. 3B). The staining visualized was localized on tubular structures characteristic of the Golgi complex. The TIME cell line is an immortalized endothelial cell line that in our hands is also suitable for analyzing localization of UL37eGFP in the Golgi complex. In addition, for immunostaining experiments, the UL37 antibody (780 antisera) showed less nonspecific reactivity in this cell line than in HFT cells. In the right panel of Fig. 3B, the Golgi structure was fragmented, and this has also been observed with this cell line for K37eGFP virus. Watanabe et al. detected HSV-2 UL37 at a perinuclear site in Vero cells early in the infectious cycle (39). Thus, using the eGFP live reporter, a more detailed visualization of UL37 trafficking was obtained.
FIG. 3.
UL37eGFP localizes to a juxtanuclear site. (A) HFT cell monolayers (6 × 105 cells per chamber slide) were infected with K37eGFP2 at an MOI of 10 PFU/cell and the cells visualized with a Zeiss LSM 510 Meta confocal microscope at 6, 10, and 12 h postinfection. The objective lens was 63×. (B) TIME cell monolayers were infected with KOS at an MOI of 10 PFU/cell. The cells were fixed at 15 h postinfection, stained with antibody to UL37 (780), and imaged by confocal analysis.
UL37 GFP localizes to the Golgi structure.
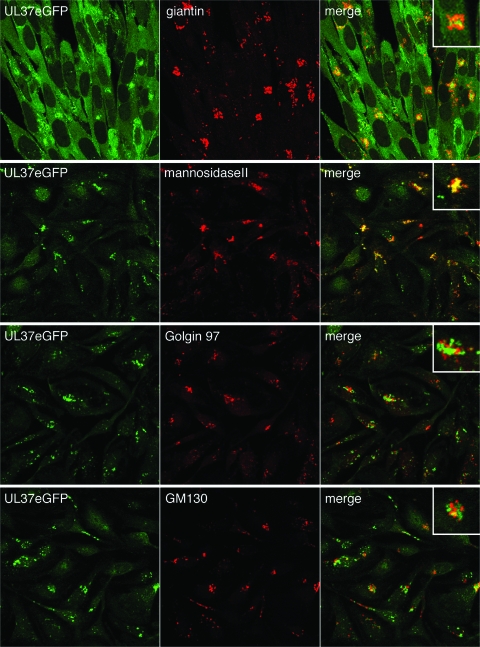
The accumulation of fluorescence at the juxtanuclear site indicated the possibility that this cytoplasmic structure was the Golgi complex. To confirm this, immunofluorescence assays were carried out with K37eGFP-infected cells by using antibodies to cellular proteins. For this experiment, the cells were fixed 12 or 16 h after infection. The data show the individual images for UL37eGFP (pseudocolored green), those of the cellular protein (pseudocolored red), and the merged image (Fig. 4). For both gaintin and mannosidase II, there were clear areas of colocalization (see insets); however, for either Golgin 97 or GM130, although both fluorescence signals were observed in the same larger structure, there was very little or no overlap of the two proteins, as judged by the fluorescence signals. One explanation for this observation is that the peripheral markers (GM130 and Golgin 97) dissociate from the membranes at sites where UL37 localizes whereas resident markers do not. Similar relocalization of Golgi peripheral markers was observed by Harley et al. (15) in virus-organelle binding assays. These data indicate that UL37eGFP is restricted to different structures or membranes of the Golgi organelle, presumably sites for virus budding.
FIG. 4.
UL37eGFP localizes with giantin and mannosidase II but not with GM130 or Golgin 97. HFT or TIME cell monolayers were infected with K37eGFP2 at an MOI of 10 PFU/cell. The cells were fixed at 12 h (HFT) or 16 h (TIME) after infection and stained with antibodies to giantin (HFT cells), mannosidase II, Golgin 97, and GM130 (TIME cells). Images were collected using a confocal microscope. The GFP signal (pseudocolored green) was merged with the Cy3 signal (pseudocolored red) to determine the colocalization of the two colors and thus the two proteins. The objective lens was 63×.
UL37eGFP localizes to the Golgi complex prior to the arrival of capsids.
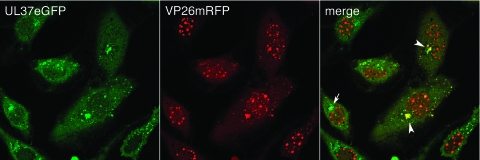
The next experiment was carried out to determine the time and location of the association between capsids and UL37eGFP in infected cells. To address this question, we made use of a dual-fluorescent-protein-tagged virus. Previously, K26GFP, a recombinant HSV-1 virus in which the eGFP ORF was fused at the N terminus of VP26 (7), the small capsid decoration protein, was made. This fluorescent tag was used to visualize capsids as well as mature virions during virus morphogenesis and egress. Using similar methods, a virus in which the monomeric red fluorescent protein (mRFP1) was fused to the N terminus of VP26 was made. This virus, designated K26mRFP, was isolated based on its red fluorescent activity. The VP26mRFP marker was recombined into K37eGFP, and a virus that formed green and red plaques in a fluorescence microscope was isolated, purified, and designated K37eGFP/26R (Fig. 1). This virus was used to infect TIME cells, and the signals from both UL37 (green) and VP26 (red) were visualized using confocal microscopy (Fig. 5). Many red fluorescent nuclear puncta were seen in the infected cells, a pattern typical of the capsid assembly sites where VP26 localizes. Colocalization of both the green and red signals (yellow pseudocolor) was seen in some cells, and this was evident at the Golgi structure in the cytoplasm. In some cells, the accumulation of green fluorescence (UL37eGFP) was evident, but with little or no accumulation of red fluorescence (VP26mRFP), indicating that UL37eGFP localizes to this cytoplasmic structure before the arrival of capsids to this site.
FIG. 5.
UL37eGFP traffics to the Golgi complex before capsids are transported to the same site. TIME cell monolayers in chamber slides were infected with K37eGFP/26R at an MOI of 10 PFU/cell and the cells visualized live with a confocal microscope at 16 h postinfection. White arrowheads indicate the colocalization of the two colors, and the arrow indicates the localization of UL37eGFP in the Golgi complex before transport of capsids to this site. The objective lens was 63×.
UL36 is required for localization of UL37eGFP in the Golgi complex.
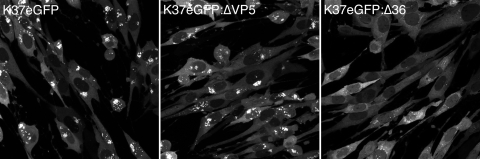
Two additional viruses were made to address genetically the requirement of assembled capsids and the role of UL36, one of the binding partners of UL37, for localization of UL37eGFP in the Golgi complex. The VP5-null mutation encoded by the virus KΔ5Z (5) and the UL36-null mutation encoded by the virus KΔUL36 (9) were recombined into K37eGFP, and viruses encoding both markers were isolated using host range plating and fluorescence methods. These viruses, designated K37eGFP:ΔVP5 and K37eGFP:Δ36 (Fig. 1), were used to infect HFT cells, and the fluorescence localization of UL37eGFP was monitored by confocal microscopy (Fig. 6). Fluorescence observed localized to the Golgi complex in both K37eGFP- and k37eGFP:ΔVP5-infected cells, indicating as shown above (Fig. 5) that accumulation of UL37 in the Golgi complex is not dependent on capsid association or assembly. However, in k37eGFP:Δ36-infected cells, accumulation of UL37eGFP in the Golgi complex was absent. The normal cytoplasmic/nuclear distribution of fluorescence was unaffected in these cells. Thus, these data genetically indicate that UL36 is required for UL37eGFP trafficking to the Golgi complex and/or its accumulation at this site. This is the first demonstration of a functional role for the UL36:UL37 bimolecular interaction.
FIG. 6.
UL36 is required for localization of UL37eGFP in the Golgi complex. Fibroblast cells were infected with K37eGFP, K37eGFP:ΔVP5, and K37eGFP:Δ36 at an MOI of 10 PFU/cell. The infected cells were analyzed by confocal microscopy 18 h after infection. The objective lens was 63×.
DISCUSSION
One of the goals of studying virion morphogenesis is to derive a visual record of the progression of newly formed virions as they mature and egress from the cell. We have shown the utility of this by using a VP26GFP fusion polypeptide to decorate HSV-1 capsids (7). This tag can and has been used to track virus as it matures from a capsid into a virion and its subsequent egress from the infected cell. This technology has been used to analyze HSV-1 VP22 protein trafficking within infected cells (11) and also that of another HSV-1 tegument protein, VP13/14 (10). The utility of this methodology has advanced to the creation of triple-fluorescent-protein-tagged recombinant viruses so that members of all three structural compartments of the virus can be visualized in the same cell during the maturation process (4, 36).
In this study, the GFP ORF was inserted in frame at the C terminus of UL37 to generate a fluorescent-protein-tagged UL37 polypeptide. Viruses expressing this fusion polypeptide plaqued on Vero cells and replicated with near-wild-type levels of growth in a single cycle. Previous immunofluorescence studies of cells infected with a UL37 expression vector and of infected cells with antisera to UL37 revealed fluorescence that was present throughout the cell, with greater concentrations in the cytoplasm (22, 33). The data shown here reveal a more realistic picture of UL37 trafficking and distribution in the infected cell. When confocal microscopy was used to visualize live-cell cultures during the whole infectious cycle, the dynamic movement of fluorescence, from the diffuse cytoplasmic distribution to accumulation at juxtanuclear sites, was visualized.
Fluorescence accumulation at the juxtanuclear site was shown to be at the Golgi structure; this is the first report of UL37 trafficking to this cytoplasmic site in cultured cells, which was achieved using this fluorescent tag and the fortuitous use of fibroblast and endothelial cells. The accumulation of fluorescence at the Golgi complex was more evident in cells in which this structure is less fragmented (HFT and TIME) than in those cells (Vero) in which this structure is highly fragmented and dispersed especially late in infection. Immunofluorescence assays confirmed the findings from the live cell analysis, that is, colocalization of UL37eGFP with Golgi markers. The data showed colocalization of UL37eGFP with giantin (a Golgi matrix protein) and mannosidase II (a Golgi resident protein) but not with GM130 (a vesicle-tethering protein located in the cis-Golgi) or Golgin 97 (a trans-Golgi network protein). One explanation for the latter observation is that the peripheral Golgi markers (GM130 and Golgin 97) are relocalized or disassociate from membranes where the virus buds. Colocalization of UL37 was also seen with the major capsid protein at this cytoplasmic site and in live cell cultures with capsids, which were decorated with VP26mRFP. These data correlate with those of Turcotte et al. (37), which show that the capsids are transported to the Golgi complex for the final envelopment. Using a similar UL37eGFP-tagged virus, Saksena et al. also showed colocalization of VP5, UL37GFP, and glycoprotein G at budding sites in infected fetal human dorsal root ganglia (32).
The diffuse cytoplasmic fluorescence was also not altered by mutations in either VP5 or UL36, indicating that the cis-acting signals for this localization are different from those responsible for accumulation of UL37 in the Golgi complex. Previous studies by Watanabe et al. (39) identified a NES in HSV-2 UL37. This was a functional signal, as judged by nuclear accumulation in the presence of leptomycin B and mutational analyses (39). We also mutated the NES (leucine-to-alanine substitution) in HSV-1 UL37 and found that this mutation resulted in increased levels of fluorescence in the nucleus, indicating that it may be acting as a NES signal. However, the mutation did not affect virus replication on Vero cells and did not significantly affect accumulation of fluorescence in the Golgi complex (data not shown).
The cytoplasmic translocation of UL37 and its accumulation in the Golgi complex are not dependent on the presence of capsids. This was shown both visually, using a dual-fluorescent-protein-tagged virus, UL37eGFP, which accumulates in the Golgi complex in the absence of capsid transport to this site, and genetically, using UL37eGFP, which accumulates in the Golgi complex in cells infected with a virus that cannot assemble capsid structures. However, UL37 is found associated with both B and C intranuclear capsids (2), and one of the phenotypes of the UL37-null mutant virus was a defect in nuclear egress of DNA-filled capsids (8). These data indicate that, in addition to Golgi complex-associated UL37, there is a nuclear population of UL37 that is associated with capsids and probably important for primary envelopment. The fate of this UL37 population after deenvelopment of capsids at the outer nuclear leaflet is not clear. Furthermore, PRV UL37 is found associated with capsids that are undergoing transport in primary neurons (20), and its presence is required for wild-type kinetics of movement in this culture system (21).
Tegument proteins occupy approximately one-third of the volume of the virion. One of the roles of the tegument can be envisioned as a structure that delivers factors into the cytosol of the infected cell that facilitate the initiation of a successful infection by delivery of the genome into the nucleus. The other role of the tegument is structural. In fact, the tegument proteins appear to be required for the transition of capsids from the site of assembly to the cytoplasmic site for final envelopment. Both UL36 and UL37 specify cytoplasmic functions that mediate virus envelopment (8, 9). These proteins physically interact with each other, and some of their activities may be dependent on this interaction (12, 13, 17, 27, 38). UL37 is transported to the Golgi complex ahead of and independent of capsid translocation to this same site. The cytoplasmic budding sites accumulate UL37 and other tegument proteins in preparation for secondary envelopment of capsids. Data presented here show that UL37 transport to cytoplasmic budding sites in the Golgi structure is dependent on the presence of functional UL36. This is the first demonstration of a functional role for the UL36:UL37 interaction.
Acknowledgments
This work was supported by National Institutes of Health PHS grants AI033077 and AI063182.
We acknowledge Ray Ahmed for technical assistance with the Southern blots and Frank Jenkins (University of Pittsburgh) and Jay Brown (University of Virginia) for antibodies. Plasmid pRESTmRFP1 was a kind gift from Roger Tsien (University of California, San Diego). The TIME cells were kindly provided by Martin McMahon and Don Ganem (University of California, San Francisco), and the HFT cells were obtained from Robert Weinberg (Whitehead Institute).
Footnotes
Published ahead of print on 10 September 2008.
REFERENCES
- 1.Albright, A. G., and F. J. Jenkins. 1993. The herpes simplex virus UL37 protein is phosphorylated in infected cells. J. Virol. 674842-4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bucks, M. A., K. J. O'Regan, M. A. Murphy, J. W. Wills, and R. J. Courtney. 2007. Herpes simplex virus type 1 tegument proteins VP1/2 and UL37 are associated with intranuclear capsids. Virology 361316-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campadelli, G., R. Brandimarti, C. Di Lazzaro, P. L. Ward, B. Roizman, and M. R. Torrisi. 1993. Fragmentation and dispersal of Golgi proteins and redistribution of glycoproteins and glycolipids processed through the Golgi apparatus after infection with herpes simplex virus 1. Proc. Natl. Acad. Sci. USA 902798-2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Oliveira, A. P., D. L. Glauser, A. S. Laimbacher, R. Strasser, E. M. Schraner, P. Wild, U. Ziegler, X. O. Breakefield, M. Ackermann, and C. Fraefel. 2008. Live visualization of herpes simplex virus type 1 compartment dynamics. J. Virol. 824974-4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desai, P., N. A. DeLuca, J. C. Glorioso, and S. Person. 1993. Mutations in herpes simplex virus type 1 genes encoding VP5 and VP23 abrogate capsid formation and cleavage of replicated DNA. J. Virol. 671357-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desai, P., N. A. DeLuca, and S. Person. 1998. Herpes simplex virus type 1 VP26 is not essential for replication in cell culture but influences production of infectious virus in the nervous system of infected mice. Virology 247115-124. [DOI] [PubMed] [Google Scholar]
- 7.Desai, P., and S. Person. 1998. Incorporation of the green fluorescent protein into the herpes simplex virus type 1 capsid. J. Virol. 727563-7568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desai, P., G. L. Sexton, J. M. McCaffery, and S. Person. 2001. A null mutation in the gene encoding the herpes simplex virus type 1 UL37 polypeptide abrogates virus maturation. J. Virol. 7510259-10271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desai, P. J. 2000. A null mutation in the UL36 gene of herpes simplex virus type 1 results in accumulation of unenveloped DNA-filled capsids in the cytoplasm of infected cells. J. Virol. 7411608-11618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donnelly, M., and G. Elliott. 2001. Fluorescent tagging of herpes simplex virus tegument protein VP13/14 in virus infection. J. Virol. 752575-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elliott, G., and P. O'Hare. 1999. Live-cell analysis of a green fluorescent protein-tagged herpes simplex virus infection. J. Virol. 734110-4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuchs, W., B. G. Klupp, H. Granzow, and T. C. Mettenleiter. 2004. Essential function of the pseudorabies virus UL36 gene product is independent of its interaction with the UL37 protein. J. Virol. 7811879-11889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gibson, W. 1996. Structure and assembly of the virion. Intervirology 39389-400. [DOI] [PubMed] [Google Scholar]
- 14.Hahn, W. C., C. M. Counter, A. S. Lundberg, R. L. Beijersbergen, M. W. Brooks, and R. A. Weinberg. 1999. Creation of human tumour cells with defined genetic elements. Nature 400464-468. [DOI] [PubMed] [Google Scholar]
- 15.Harley, C. A., A. Dasgupta, and D. W. Wilson. 2001. Characterization of herpes simplex virus-containing organelles by subcellular fractionation: role for organelle acidification in assembly of infectious particles. J. Virol. 751236-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Homa, F. L., and J. C. Brown. 1997. Capsid assembly and DNA packaging in herpes simplex virus. Rev. Med. Virol. 7107-122. [DOI] [PubMed] [Google Scholar]
- 17.Klupp, B. G., W. Fuchs, H. Granzow, R. Nixdorf, and T. C. Mettenleiter. 2002. Pseudorabies virus UL36 tegument protein physically interacts with the UL37 protein. J. Virol. 763065-3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klupp, B. G., H. Granzow, E. Mundt, and T. C. Mettenleiter. 2001. Pseudorabies virus UL37 gene product is involved in secondary envelopment. J. Virol. 758927-8936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lagunoff, M., J. Bechtel, E. Venetsanakos, A. M. Roy, N. Abbey, B. Herndier, M. McMahon, and D. Ganem. 2002. De novo infection and serial transmission of Kaposi's sarcoma-associated herpesvirus in cultured endothelial cells. J. Virol. 762440-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luxton, G. W., S. Haverlock, K. E. Coller, S. E. Antinone, A. Pincetic, and G. A. Smith. 2005. Targeting of herpesvirus capsid transport in axons is coupled to association with specific sets of tegument proteins. Proc. Natl. Acad. Sci. USA 1025832-5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luxton, G. W., J. I. Lee, S. Haverlock-Moyns, J. M. Schober, and G. A. Smith. 2006. The pseudorabies virus VP1/2 tegument protein is required for intracellular capsid transport. J. Virol. 80201-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McLauchlan, J. 1997. The abundance of the herpes simplex virus type 1 UL37 tegument protein in virus particles is closely controlled. J. Gen. Virol. 78189-194. [DOI] [PubMed] [Google Scholar]
- 23.McLauchlan, J., K. Liefkens, and N. D. Stow. 1994. The herpes simplex virus type 1 UL37 gene product is a component of virus particles. J. Gen. Virol. 752047-2052. [DOI] [PubMed] [Google Scholar]
- 24.Mettenleiter, T. C. 2004. Budding events in herpesvirus morphogenesis. Virus Res. 106167-180. [DOI] [PubMed] [Google Scholar]
- 25.Mettenleiter, T. C. 2002. Herpesvirus assembly and egress. J. Virol. 761537-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mettenleiter, T. C., B. G. Klupp, and H. Granzow. 2006. Herpesvirus assembly: a tale of two membranes. Curr. Opin. Microbiol. 9423-429. [DOI] [PubMed] [Google Scholar]
- 27.Mijatov, B., A. L. Cunningham, and R. J. Diefenbach. 2007. Residues F593 and E596 of HSV-1 tegument protein pUL36 (VP1/2) mediate binding of tegument protein pUL37. Virology 36826-31. [DOI] [PubMed] [Google Scholar]
- 28.Okoye, M. E., G. L. Sexton, E. Huang, J. M. McCaffery, and P. Desai. 2006. Functional analysis of the triplex proteins (VP19C and VP23) of herpes simplex virus type 1. J. Virol. 80929-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Person, S., and P. Desai. 1998. Capsids are formed in a mutant virus blocked at the maturation site of the UL26 and UL26.5 open reading frames of herpes simplex virus type 1 but are not formed in a null mutant of UL38 (VP19C). Virology 242193-203. [DOI] [PubMed] [Google Scholar]
- 30.Rixon, F. J. 1993. Structure and assembly of herpesviruses. Semin. Virology 4135-144. [Google Scholar]
- 31.Roizman, B., and D. Furlong. 1974. The replication of herpesviruses, p. 11-68, In H. Fraenkel-Conrat and R. R. Wagner (ed.), Comprehensive virology. Plenum Press, New York, NY.
- 32.Saksena, M. M., H. Wakisaka, B. Tijono, R. A. Boadle, F. Rixon, H. Takahashi, and A. L. Cunningham. 2006. Herpes simplex virus type 1 accumulation, envelopment, and exit in growth cones and varicosities in mid-distal regions of axons. J. Virol. 803592-3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmitz, J. B., A. G. Albright, P. R. Kinchington, and F. J. Jenkins. 1995. The UL37 protein of herpes simplex virus type 1 is associated with the tegument of purified virions. Virology 2061055-1065. [DOI] [PubMed] [Google Scholar]
- 34.Skepper, J. N., A. Whiteley, H. Browne, and A. Minson. 2001. Herpes simplex virus nucleocapsids mature to progeny virions by an envelopment → deenvelopment → reenvelopment pathway. J. Virol. 755697-5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steven, A. C., and P. G. Spear. 1996. Herpesvirus capsid assembly and envelopment, p. 312-351. In R. Burnett, W. Chiu, and R. Garcea (ed.), Structural biology of viruses. Oxford University Press, New York, NY.
- 36.Sugimoto, K., M. Uema, H. Sagara, M. Tanaka, T. Sata, Y. Hashimoto, and Y. Kawaguchi. 2008. Simultaneous tracking of capsid, tegument, and envelope protein localization in living cells infected with triple fluorescent herpes simplex virus 1. J. Virol. 825198-5211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turcotte, S., J. Letellier, and R. Lippe. 2005. Herpes simplex virus type 1 capsids transit by the trans-Golgi network, where viral glycoproteins accumulate independently of capsid egress. J. Virol. 798847-8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vittone, V., E. Diefenbach, D. Triffett, M. W. Douglas, A. L. Cunningham, and R. J. Diefenbach. 2005. Determination of interactions between tegument proteins of herpes simplex virus type 1. J. Virol. 799566-9571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watanabe, D., Y. Ushijima, F. Goshima, H. Takakuwa, Y. Tomita, and Y. Nishiyama. 2000. Identification of nuclear export signal in UL37 protein of herpes simplex virus type 2. Biochem. Biophys. Res. Commun. 2761248-1254. [DOI] [PubMed] [Google Scholar]
- 40.Wildy, P., W. C. Russell, and R. W. Horne. 1960. The morphology of herpes virus. Virology 12204-222. [DOI] [PubMed] [Google Scholar]