Abstract
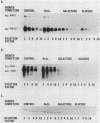
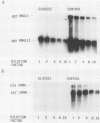
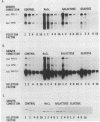
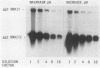
The effect of glucose on accessory gene regulator (agr) expression in Staphylococcus aureus was examined. agr is a global regulator that affects the expression of numerous genes, including those for some factors implicated in virulence, such as toxic shock syndrome toxin 1, alpha-hemolysin, and protein A. The agr locus determines two divergent transcripts, designated RNAII and RNAIII. RNAII contains four open reading frames (agrABCD), and RNAIII encodes delta-hemolysin. The mechanisms responsible for agr-mediated regulation are not well understood, but it appears that the RNAIII transcript plays a central role in the regulation of a number of target genes, including those for alpha-hemolysin (hla), beta-hemolysin (hlb), protein A (spa), and staphylococcal enterotoxin B (seb+). In this study, S. aureus cultures were grown either in a shake flask system with a complex medium or in a fermentor system with a completely defined medium in which the pH and glucose concentration were maintained. Northern (RNA) blot analysis revealed that a dramatic reduction in agr expression was apparent only when the cultures contained glucose and when the pH was 5.5 or was not maintained. The effect of glucose on two agr target genes, sec+ and hla, was also studied. Glucose-containing cultures produced less sec+ and hla mRNAs at maintained pH (6.5). In addition, the glucose effect on sec+ and hla was enhanced under conditions that inhibited agr expression (i.e., pH 5.5 or a nonmaintained pH).
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betley M. J., Mekalanos J. J. Nucleotide sequence of the type A staphylococcal enterotoxin gene. J Bacteriol. 1988 Jan;170(1):34–41. doi: 10.1128/jb.170.1.34-41.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compagnone-Post P., Malyankar U., Khan S. A. Role of host factors in the regulation of the enterotoxin B gene. J Bacteriol. 1991 Mar;173(5):1827–1830. doi: 10.1128/jb.173.5.1827-1830.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couch J. L., Betley M. J. Nucleotide sequence of the type C3 staphylococcal enterotoxin gene suggests that intergenic recombination causes antigenic variation. J Bacteriol. 1989 Aug;171(8):4507–4510. doi: 10.1128/jb.171.8.4507-4510.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csonka L. N. Physiological and genetic responses of bacteria to osmotic stress. Microbiol Rev. 1989 Mar;53(1):121–147. doi: 10.1128/mr.53.1.121-147.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J. L., Cho G. J. Production of staphylococcal alpha toxin. II. Glucose repression of toxin formation. Infect Immun. 1972 Nov;6(5):689–694. doi: 10.1128/iai.6.5.689-694.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iandolo J. J., Shafer W. M. Regulation of staphylococcal enterotoxin B. Infect Immun. 1977 May;16(2):610–616. doi: 10.1128/iai.16.2.610-616.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janzon L., Arvidson S. The role of the delta-lysin gene (hld) in the regulation of virulence genes by the accessory gene regulator (agr) in Staphylococcus aureus. EMBO J. 1990 May;9(5):1391–1399. doi: 10.1002/j.1460-2075.1990.tb08254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janzon L., Löfdahl S., Arvidson S. Identification and nucleotide sequence of the delta-lysin gene, hld, adjacent to the accessory gene regulator (agr) of Staphylococcus aureus. Mol Gen Genet. 1989 Nov;219(3):480–485. doi: 10.1007/BF00259623. [DOI] [PubMed] [Google Scholar]
- Jarvis A. W., Lawrence R. C., Pritchard G. G. Glucose repression of enterotoxins A, B and C and other extracellular proteins in staphlyococci in batch and continuous culture. J Gen Microbiol. 1975 Jan;86(1):75–87. doi: 10.1099/00221287-86-1-75. [DOI] [PubMed] [Google Scholar]
- Kehoe M., Duncan J., Foster T., Fairweather N., Dougan G. Cloning, expression, and mapping of the Staphylococcus aureus alpha-hemolysin determinant in Escherichia coli K-12. Infect Immun. 1983 Sep;41(3):1105–1111. doi: 10.1128/iai.41.3.1105-1111.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiswirth B. N., Löfdahl S., Betley M. J., O'Reilly M., Schlievert P. M., Bergdoll M. S., Novick R. P. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature. 1983 Oct 20;305(5936):709–712. doi: 10.1038/305709a0. [DOI] [PubMed] [Google Scholar]
- Morfeldt E., Janzon L., Arvidson S., Löfdahl S. Cloning of a chromosomal locus (exp) which regulates the expression of several exoprotein genes in Staphylococcus aureus. Mol Gen Genet. 1988 Mar;211(3):435–440. doi: 10.1007/BF00425697. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Brodsky R. Studies on plasmid replication. I. Plasmid incompatibility and establishment in Staphylococcus aureus. J Mol Biol. 1972 Jul 21;68(2):285–302. doi: 10.1016/0022-2836(72)90214-8. [DOI] [PubMed] [Google Scholar]
- Peng H. L., Novick R. P., Kreiswirth B., Kornblum J., Schlievert P. Cloning, characterization, and sequencing of an accessory gene regulator (agr) in Staphylococcus aureus. J Bacteriol. 1988 Sep;170(9):4365–4372. doi: 10.1128/jb.170.9.4365-4372.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recsei P., Kreiswirth B., O'Reilly M., Schlievert P., Gruss A., Novick R. P. Regulation of exoprotein gene expression in Staphylococcus aureus by agar. Mol Gen Genet. 1986 Jan;202(1):58–61. doi: 10.1007/BF00330517. [DOI] [PubMed] [Google Scholar]
- Regassa L. B., Couch J. L., Betley M. J. Steady-state staphylococcal enterotoxin type C mRNA is affected by a product of the accessory gene regulator (agr) and by glucose. Infect Immun. 1991 Mar;59(3):955–962. doi: 10.1128/iai.59.3.955-962.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler P., Weisblum B. Erythromycin-induced stabilization of ermA messenger RNA in Staphylococcus aureus and Bacillus subtilis. J Mol Biol. 1988 Oct 20;203(4):905–915. doi: 10.1016/0022-2836(88)90116-7. [DOI] [PubMed] [Google Scholar]
- Schlievert P. M., Blomster D. A. Production of staphylococcal pyrogenic exotoxin type C: influence of physical and chemical factors. J Infect Dis. 1983 Feb;147(2):236–242. doi: 10.1093/infdis/147.2.236. [DOI] [PubMed] [Google Scholar]
- Smith J. L., Bencivengo M. M., Buchanan R. L., Kunsch C. A. Enterotoxin A production in Staphylococcus aureus: inhibition by glucose. Arch Microbiol. 1986 Mar;144(2):131–136. doi: 10.1007/BF00414722. [DOI] [PubMed] [Google Scholar]