Abstract
The conservation of the core structure and diversification of the external features among the turreted reoviruses appear to be relevant to structural evolution in facilitating the infection of diverse host species. The structure of Rice ragged stunt virus (RRSV), in the genus Oryzavirus of the family Reoviridae, is determined to show a core composed of capsid shell, clamps, and long turrets. The RRSV core structure is equivalent to the core structure of Orthoreovirus and the virion structure of Cytoplasmic polyhedrosis virus (CPV). In RRSV, five peripheral trimers surround each long turret and sit at the Q trimer position in the T=13l icosahedral symmetry, a structural feature unique to turreted reoviruses. That is, the core of RRSV is partially covered by 60 copies of the peripheral trimer. In contrast, the core of Orthoreovirus is covered by 200 copies of the trimer that sit at the Q, R, S, and T trimer positions. Our results suggest that among the three viruses, RRSV has a structure intermediate between that of Orthoreovirus and the CPV virion. This conclusion coincides with the results of the phylogenetic analysis of amino acid sequences of RNA-dependent RNA polymerases.
Reoviridae is the largest and most diverse family of double-stranded RNA (dsRNA) viruses. It includes 12 established genera, namely Aquareovirus, Coltivirus, Cypovirus, Fijivirus, Idnoreovirus, Mycoreovirus, Orbivirus, Orthoreovirus, Oryzavirus, Phytoreovirus, Rotavirus, and Seadornavirus. The hosts of these viruses include plants, vertebrates, insects, and fungi (22). All known viruses in this family are 600 to 800 Å in diameter and consist of an inner core that is surrounded by a few layers of protein, with the exception of the single-layered Cytoplasmic polyhedrosis virus (CPV), which encapsidates 9 to 12 segments of dsRNA and the enzymes involved in transcription. Whereas the precise morphology varies among genera, the morphologies of the innermost capsid shells are similar in spite of the absence of significant sequence homology among component proteins. The conserved innermost capsid of reoviruses is composed of 120 copies of thin crescent-shaped proteins, and the respective subunits exhibit similar overall folding, with substantial modifications that appear to have developed during viral evolution (11, 24, 28, 37, 42). Except in CPV, the innermost capsid shell is covered by additional outer layers, the organization and structure of which vary among the genera in the family. The outer capsid shell appears to play important roles in maintaining the stability of the thin innermost capsid shell and sequestering the dsRNA genome, as well as in conferring host specificity and mediating entry into host cells.
Reoviruses can be divided structurally into two subgroups, the turreted and nonturreted reoviruses, on the basis of a critical structural feature. Members of the seven genera Aquareovirus, Cypovirus, Fijivirus, Idnoreovirus, Mycoreovirus, Orthoreovirus, and Oryzavirus are classified as turreted reoviruses (22), with distinctive pentameric turrets that sit on the outside of the innermost capsid at each fivefold axis. Reoviruses are capable of the endogenous transcription of mRNA within the intact viral particle, exploiting virus-encoded enzymes for the initiation of transcription, elongation, and 5′ capping prior to the release of mRNA from the capsid shell. The turrets of orthoreoviruses (28), aquareoviruses (9, 25, 30), and cypoviruses (16, 37, 42) have been shown or, in some cases, appear to mediate the guanylyltransferase (GTase) and methyltransferase reactions in the 5′ capping of the viral plus-strand RNA transcripts (4-5, 10, 35-36, 38). The capping reaction starts with the transfer of a guanosine to the 5′ terminus of the newly synthesized transcript near the base of a turret, at the site where the transcript enters the cavity of the turret. A methyl group then is transferred both to the N7 of the added guanosine and to the 2′O of the first template-encoded nucleotide (28). The spatial distribution of functional domains within the turret corresponds to the order of these reactions. The cores of these viruses also are distinctive, in that each has either 120 or 150 copies of a so-called clamp protein that sit on the capsid shell protein and contribute to the stability of the capsid shell (28, 42). RNA-dependent RNA polymerases (RdRps) are encapsidated within the core and are located just beneath the innermost capsid shell at each icosahedral fivefold axis. In the case of Orthoreovirus, the atomic structure of the RdRp has been fitted into the cryoelectron microscopy (cryo-EM) density map at a 7.6-Å resolution, and this has revealed the mechanism of transcription and the exit pathway for newly synthesized plus-strand RNA transcripts from the RdRp through the capsid shell to the central cavity of the turret (40). Thus, the transcription and posttranscriptional processing of mRNA occur in a series of coordinated steps, which begin with the transcription of mRNA at the complex of transcriptional enzymes within the inner shell and are followed by 5′-terminal capping of the mRNA and the release of the capped mRNA through the multifunctional turret.
Rice ragged stunt virus (RRSV), which belongs to the genus Oryzavirus and is a turreted reovirus, infects plants in the family Graminae and is transmitted in a persistent manner by brown plant hoppers after proliferation in the vector insect. RRSV causes serious damage to rice plants and affects the production of rice (19). RRSV has an icosahedral capsid of approximately 700 Å in diameter, which consists of a polyhedral core particle of about 500 Å in diameter to which spikes of approximately 200 Å in diameter and 100 Å in height are attached (15). The turrets of RRSV are larger than those of Orthoreovirus (28) and CPV (approximately 150 Å in diameter and 100 Å in height in both cases) (42). RRSV contains at least six structural proteins, namely P1, P2, P3, P4A, P8B, and P9, with molecular weights of 138,000 (138K), 133K, 131K, 141K, 42K, and 39K, respectively (13, 15, 32-34), that are encoded by 10 segments of the dsRNA genome (26). The capsid of RRSV, encapsidating RdRp (P4A protein) (33) and 10 segments of dsRNA, appears to consist of at least four kinds of protein: P2, a capping enzyme (31); P3, a capsid shell protein (13); P8, a major capsid protein that is cleaved to yield P8A and P8B (32); and P9, a spike protein involved in transmission via the insect vector (6a, 34, 41). The P3, P8, and P9 proteins react most strongly with polyclonal antiserum raised against RRSV particles. However, the organization and three-dimensional (3D) structure of RRSV remain unknown. We present here the structure of RRSV, as determined by cryo-EM and 3D reconstruction, and a comparison of RRSV to other reoviruses. Our observations reveal striking similarities among the turreted reoviruses as well as several unique features, and they suggest details of the structural evolution of the turreted reoviruses.
MATERIALS AND METHODS
Preparation of virus.
RRSV was purified as described by Omura et al. (26). Infected rice leaves were macerated with a meat chopper in 0.1 M potassium phosphate buffer, pH 7.0, that contained 0.01 M MgCl2. After differential centrifugations of the slurry of chopped leaves, viral particles were purified by sucrose density gradient centrifugation on 10 to 40% sucrose and then on 40 to 60% sucrose. The banded viral particles were pelleted by high-speed centrifugation and resuspended in a solution of 0.1 M histidine, 0.01 M MgCl2, pH 6.2. The purified virus was able to infect rice plants when inoculated via vector insects into which the virus had been injected (26). Purified particles were negatively stained with 2% uranyl acetate and examined under an electron microscope (H-7000; Hitachi, Japan) (Fig. 1A), and the purity of the virus sample was checked by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (Fig. 1B).
FIG. 1.
(A) Electron micrograph of uranyl acetate-stained RRSV (upper) and projection images calculated from the 3D reconstruction (lower). The bar represents 100 nm. (B) Sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis of proteins from particles of RRSV (lane 1) and RDV (lane 2). Positions of RDV protein species are shown. (C) Electron micrograph of RRSV embedded in vitreous ice. The image was recorded at an applied underfocus value of 3.0 μm with a cryoelectron micrograph (JEM-2100F) operated at 200 kV and a nominal magnification of ×30,000. Empty particles are indicated by the arrows. The bar represents 100 nm. (D) Resolution assessment. The Fourier shell correlation coefficient is plotted.
Cryo-EM and imaging procedures.
Samples of RRSV were embedded in vitreous ice and examined at ∼100 K with a cryoelectron microscope (JEM-2100F; JEOL, Japan) operated at 200 kV and at a nominal magnification of ×30,000. Images were recorded with a 4,000- by 4,000-pixel charge-coupled device (CCD) at applied underfocus values ranging from 0.9 to 3.0 μm. Approximately 20 particles per electron micrograph were boxed out, and the individual images were corrected for the contrast transfer function. Small amounts of the empty particles were observed in the micrographs (Fig. 1C) and were not used for the reconstruction. The initial origins and orientations of selected images of particles were obtained by polar Fourier transform procedures (2) in which the density map of the reovirus core was used as the initial model. These calculations were followed by the refinement of interparticle orientation by cross-common lines procedures. The total amount of the purified RRSV material was very limited due to the low productivity of the virus, and long-term storage was avoided. The final reconstruction of RRSV was computed from 721 particles, and the resolution was restricted to 21 Å (Fig. 1D). The resolution was assessed with a 0.5 threshold in the Fourier shell correlation between two reconstructions, which was calculated from two halves of each data set.
Structural analysis.
To compare the structure of RRSV to the structure of the CPV core, we calculated electron density maps at a 20-Å resolution from atomic coordinates. The correct handedness for RRSV was determined by reference to the core structure of Orthoreovirus.
RESULTS AND DISCUSSION
Overall structure and organization of RRSV.
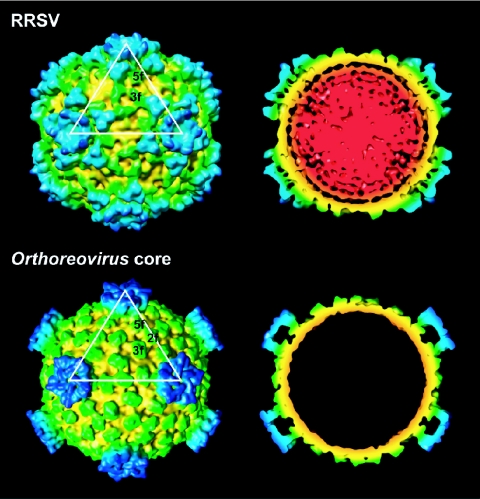
The 3D structure of the RRSV virion is shown in Fig. 2. The projection images calculated from the 3D reconstruction are consistent with the appearances of the particles in the electron micrograph of uranyl acetate-stained RRSV (Fig. 1A), and the protrusions of the particle (corresponding to the turret) are observed in the micrograph. The diameter of the core capsid, excluding the turrets, was ∼540 Å (Fig. 2B). The turrets were composed of two parts: a long turret of ∼150 Å in width and ∼90 Å in height and peripheral trimers with sides of ∼70 Å in length and ∼55 Å in height. Such trimers are unique to RRSV among known reoviruses, and each binds three clamps. The clamps protrude as 120 blobs from the smooth continuous layer of the core capsid protein and are thought to stabilize the capsid shell in Orthoreovirus (28). The core capsid protein forms a smooth and thin continuous layer, a structure commonly found in all of the viruses analyzed in the family Reoviridae to date. The molecular weight of the P3 protein of RRSV (131K) is similar to that of the major capsid shell VP1 protein of CPV (148K) and the λ1 protein of Orthoreovirus (142K). The P3 protein of RRSV has partial homologies to the major capsid shell VP1 protein of CPV (12, 13), which has conserved holding and protein organization among capsid shell proteins of the reoviruses (37, 42). Indeed, the secondary-structure prediction showed that the P3 protein of RRSV contains many α-helical elements in addition to the β-strand elements, which suggests that the P3 protein of RRSV has α + β holds, a structure similar to those of other members of Reoviridae (3). Moreover, the atomic structures of the λ1 protein of the Orthoreovirus genus are well accommodated into the capsid shell region of the cryo-EM map of RRSV without any positional modifications. Thus, the capsid shell P3 protein of RRSV is considered to have structure and protein organization similar to those of viruses that belong to the family Reoviridae.
FIG. 2.
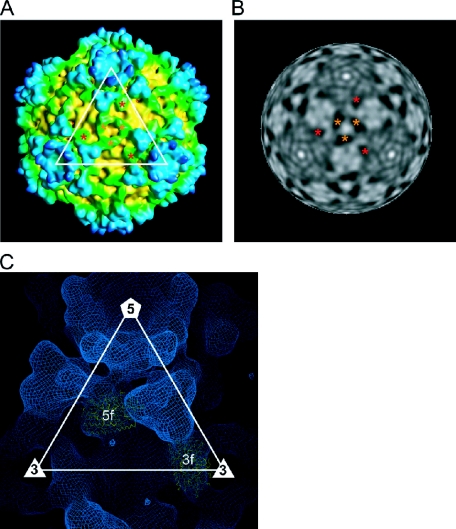
Overall structure of RRSV. (A) Surface representation of RRSV. (B) Central cross-section (40 Å thick). The map is colored according to the distance from the center of the viral particle, for which color coding is indicated. Local structures are visible as follows: core capsid, yellow; clamp proteins, green; long turret, blue structures located at the fivefold axes; and peripheral trimers, blue trimers located around turrets.
Fivefold symmetric capping turrets.
Turreted CPV and the Orthoreovirus core have similar architectures. Each pentameric turret at icosahedral fivefold axes is a hollow cylinder and includes polypeptides with GTase and methylase activities. Nascent mRNA is processed at the 5′ terminus via coordinated steps as it passes along the central channel. In Orthoreovirus virions, the turret protein (λ2 protein; 144K) has a series of seven domains that includes GTase and two methylase domains (methylase-1 and -2) (28). The sequences of domains from the base of the turret correspond to the order of reactions along the pathway. The long turret of RRSV is also a hollow cylinder of ∼150 Å in width and ∼90 Å in height (Fig. 3A). Radially cued density revealed the molecular boundaries of each monomer within a pentamer (Fig. 3B). The long turret of RRSV has two types of globular domains in its outermost region (Fig. 3A). Molecular docking pentameric λ2 of Orthoreovirus, a protein homologous to P2 of RRSV and a protein that has been putatively designated a component of the long turret based on the fact that the proteins have a conserved Hx8H motif of the type found in the turrets of Mycoreovirus (31), suggests that these two globular domains in RRSV correspond to the two methylase domains mentioned above (Fig. 3C). Furthermore, molecular docking showed that P2 of RRSV lacks the carboxy-terminal immunoglobulin domains (the carboxy-terminal 250 residues) of λ2 of Orthoreovirus that make the flap at the top of the turret (Fig. 3C) (28). The λ2 flap has been shown to be involved in the association with the cell attachment protein σ1, and it can undergo major conformational changes at different stages of viral infection and maturation (6, 8). Activation for the production of mRNA requires the removal of the outer capsid protein, including the σ1 protein, and the opening up of the turret like a flower (8). The P2 protein of RRSV lacks this domain and the spike protein, which corresponds to the σ1 protein on the turret that is involved in cell attachment; thus, RRSV has a depression in the middle of the top of its turrets. The absence of a flap domain in RRSV suggests that mechanisms that confer host specificity differ. Orthoreovirus might have acquired a flap domain to exploit the spike protein at the top of the turret for attachment to host cells. However, the organization of functional domains is strongly conserved among the turreted reoviruses, implying that the mechanism for mRNA capping is shared among the turreted reoviruses.
FIG. 3.
Turret structure. (A) Close-up view of a turret. The two globular domains that are marked by asterisks might correspond to two methylase domains. (B) Radially cued density viewed along an icosahedral fivefold axis at a radius of 343 Å showing the molecular boundaries of the five subunits in a turret. High to low densities are indicated by a gray scale. (C) Atomic structure of the Orthoreovirus λ2 turret. A CPK model of a pentameric turret is shown in the upper panel. Five subunits are shown in different colors. Asterisks indicate methylase domains as described for panel A. The lower panel shows ribbon drawings of the monomer structure of the turret protein. The GTase, methylase-1, methylase-2, and immunoglobulin-like domains are colored in red, yellow, green, and cyan, respectively.
Clamp proteins.
The capsid shell of a turreted reovirus is decorated with 120 or 150 copies of clamp proteins (28, 42). By contrast, nonturreted reoviruses, such as rice dwarf virus (RDV), bluetongue virus (BTV), and rotavirus, lack such clamp proteins. In the Orthoreovirus core, the clamp proteins, namely 150 copies of a 47K σ2 protein, bind at three distinct locations within each icosahedral asymmetric unit, where they act as molecular clamps between the λ1 proteins of the capsid shell below them. Thus, they appear to stabilize the capsid shell. The interactions between the clamp proteins and the capsid proteins are not equivalent, but the bonding is strong and specific. In RRSV, 120 copies of the clamp protein were observed at positions similar to those in members of Orthoreovirus, although no 2f clamp protein, observed at the icosahedral twofold axes in the Orthoreovirus core, was found in RRSV (Fig. 4A and 5). The clamp protein at the twofold axes also was not observed in CPV (42). Two kinds of blobs were found at 3f and 5f clamp positions of turreted reoviruses (28, 42). They were similar in size and shape. Their dimensions also were similar to those of Orthoreovirus. After the positional modification, the atomic structures of the σ2 clamp protein of Orthoreovirus were well accommodated into the cryo-EM map of the RRSV (Fig. 4C). These results suggest that two kinds of blobs are composed of the same protein and correspond to the 3f and 5f clamps of turreted reoviruses; thus, its number is 120 in a particle. The positions of the clamp protein around the threefold axis (3f clamp) in RRSV differed by ∼20 Å from the position in Orthoreovirus, while the positions of the clamp protein around the fivefold axis (5f clamp protein) in RRSV were almost same as those in Orthoreovirus. P8B (42K) of RRSV, a protein similar in size to that of σ2, was tentatively assigned to the clamp protein. Unlike nonturreted reoviruses, which maintain a second capsid layer (T=13 symmetry), turreted reoviruses become single-layered particles during transcription (22). Therefore, the clamp molecules that tie together neighboring capsid shell proteins might be required to increase capsid stability. In fact, the σ2 clamp protein of Orthoreovirus is required for the assembly of λ1 capsid shell proteins into icosahedral particles (28).
FIG. 4.
Clamp proteins. (A and B) Surface representation (A) and radially cued density at a radius of 293 Å (B), viewed along a threefold axis. Two icosahedrally independent 3f and 5f clamp proteins are marked with orange and red asterisks, respectively. (C) The atomic structures of the clamp σ2 protein of Orthoreovirus were fitted into the cryo-EM map of RRSV.
FIG. 5.
Structural comparison between the RRSV virion and the Orthoreovirus core. Surface representations and central cross-sections (40 Å thick) of the RRSV virion and the Orthoreovirus core are shown. The color coding is the same as that described in the legend to Fig. 1.
Peripheral trimers.
Each turret of RRSV is surrounded by five peripheral trimers, and this structural feature is unique among known turreted reoviruses (Fig. 6). Single-shelled CPV and the Orthoreovirus core do not have trimers around their turrets. One trimer of RRSV binds three clamp proteins, as shown in Fig. 6A. Thus, the clamp protein might act as molecular scaffolding for the trimers as well as acting as a molecular clamp in the capsid shell. In the Orthoreovirus virion, the core is coated by a layer of trimers that are composed of μ1 and σ3 proteins. The sizes of the sides of the μ13σ33 trimer (∼80 Å) are similar to those of RRSV, but the μ13σ33 trimer is much taller than the RRSV trimer (Fig. 6C) (18). These proteins form an incomplete T=13l icosahedral lattice interrupted at the fivefold axes by the λ2 turrets. The trimers are designated Q, R, S, and T trimers according to their positions in the incomplete T=13l icosahedral lattice. It is noteworthy in this context that RRSV has only one type of trimer in each icosahedral asymmetric unit, and the position of binding to the capsid shell corresponds to that of the Q trimer in Orthoreovirus (Fig. 6D). In other words, RRSV lacks the R, S, and T trimers, and the innermost capsid layer of RRSV is only partially covered with the trimers. Thus, the clamp proteins at icosahedral twofold axes (2f clamp protein) in Orthoreovirus, which act as scaffolding for trimers as described above, might be required for sitting the R, S, and T trimers on the capsid shell. However, although aquareovirus virions have only 120 copies of the clamp protein (25), they have Q, R, S, and T trimers. Therefore, the 2f clamp proteins are not essential for the assembly of the R, S, and T trimers. The trimer in Orthoreovirus plays important roles in the viral penetration of the cell membrane (18). By analogy, the trimers in RRSV might have a similar function. Indeed, the P9 protein, which has been putatively identified as the trimer protein, plays an important role in transmission via the insect vector (6a, 41). The estimated volume for a P9 trimer (117 kDa) is 1.4 × 105 Å3 (1.23 Å3/Da) (17, 20), which is sufficiently coincident with the segmented volume for a trimer (1.8 × 105 Å3). Based on these data, we have tentatively assigned the P9 protein to a component of the peripheral trimer.
FIG. 6.
Peripheral trimers. (A) Close-up view of a trimer. A peripheral trimer is shown bound to three clamps that are marked with asterisks. (B) Radially cued density with fivefold axes at a radius of 333 Å, at which the molecular boundaries of trimers are apparent. (C) Atomic structures of the Orthoreovirus trimers. CPK models of σ33 (left) and μ13σ33 trimers (right) are shown as viewed from the outside of the capsid shell (upper) and after 90° rotation (lower). The σ3 molecules are colored pink, magenta, and orange, and the μ1 molecules are colored cyan, blue, and green. The σ3 molecules are removed from the viral particles during the infection by proteolysis. (D) Surface representation with the icosahedral T=13l net. The positions of trimers that correspond to the Q position in the icosahedral T=13l symmetry are shown and labeled Q.
The possibility of the partial degradation of the outer capsid during the purification, resulting in loss of the R, S, and T trimers, has not been excluded completely. However, the purified viruses are infective to rice plants when inoculated via vector insects into which the virus had been injected (26). Furthermore, we have never observed the double-layered particles in the electron microscopy, and the particle morphology of the dipped materials directly processed from infected plants was similar to those observed in purified preparations. Thus, we considered that the structure analyzed in this study is the RRSV virion.
Relationships among turreted reoviruses and their evolution.
Pentameric turrets at fivefold axes, plus 120 copies of the clamp protein on which peripheral trimers are located, were attached to the thin-shelled core capsid in particles of RRSV. The thin core capsid shell is a structural feature that is common among those reoviruses for which cryo-EM or X-ray crystallographic data are available, namely Aquareovirus (9, 25, 30), Orthoreovirus (28), Rotavirus (27, 39), BTV in Orbivirus (11), RDV in Phytoreovirus (24), CPV in Cypovirus (16, 37, 42), and RRSV in Oryzavirus. Other dsRNA viruses, such as fungal viruses L-A (23) and P4 (7), also have such a structure. Thus, all appear to share a common ancestor in spite of the fact that they have almost no recognizable sequence homologies. Alternatively, this structural feature could be particularly advantageous for viral replication strategies (22). A thin-shelled capsid is advantageous in that it allows the creation of a large cavity in which the virus can package large amounts of dsRNA and proteins required for transcription (14).
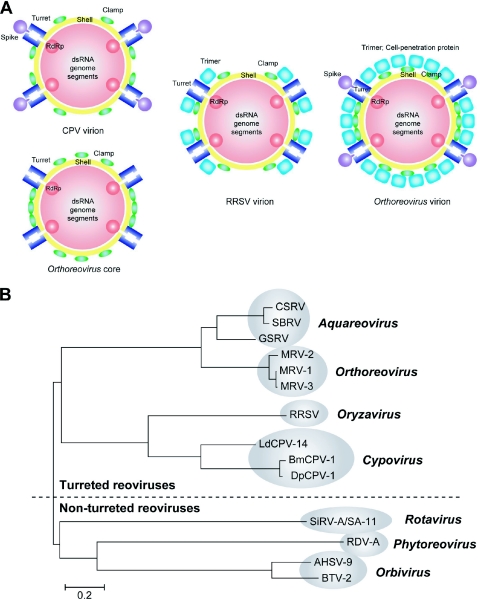
Turreted reoviruses have pentameric turrets that extend from the underlying innermost capsid layer around the fivefold axes. The present study confirmed that RRSV has a typical turret structure, as also reported for the Orthoreovirus core (28) and for CPV (16, 42). Thus, our study also confirmed that these viruses are appropriately grouped together from a structural perspective (16). By contrast, the nonturreted reoviruses, such as Rotavirus, BTV, and RDV, lack turrets, and the innermost capsid shell is fully covered with trimers with T=13l icosahedral symmetry. Thus, the entire innermost capsid is covered with trimers, allowing the grouping of these viruses from a structural perspective as well. These groupings by structure reflect similar groupings based on sequence homologies among RdRps, the only proteins for which significant homologies have been detected among respective proteins of viruses that belong to this family (Fig. 7B). The structural similarities mentioned above, together with available biochemical data, suggest that turreted and nonturreted reoviruses form an evolutionarily related subgroup in the family Reoviridae (1, 21). This grouping does not reflect the host species infected by the viruses. The hosts of turreted reoviruses are plants plus insects, insects, and mammals in the case of RRSV, CPV, and Orthoreovirus, respectively. The hosts of nonturreted reoviruses are plants plus insects for Phytoreovirus, mammals for Rotavirus, and mammals plus insects for BTV. Our observations suggest that reoviruses with a common ancestor separated into turreted and nonturreted groups, and thereafter they separated still further to yield the present genera, possibly via adaptation to their respective hosts.
FIG. 7.
Relationships among turreted reoviruses. (A) Schematic representation of the RRSV virion, the CPV virion, the Orthoreovirus core, and the Orthoreovirus virion. The RdRp, capsid, clamp, trimeric outer capsid, turret, and spike proteins are shown in red, yellow, green, sky blue, blue, and blue-purple, respectively. (B) Neighbor-joining tree constructed from full-length sequences of RdRps of viruses in the family Reoviridae. The genera, strains, abbreviations of names of viruses, and accession numbers of sequences are as follows. For Aquareovirus, Chum salmon reovirus (CSRV; AF418295), Striped bass reovirus (SBRV; AF450318), and Golden shiner reovirus (GSRV; AF403399); for Orthoreovirus, Mammalian orthoreovirus subgroup 1, Lang strain (MRV-1; M24734), Jones strain (MRV-2; M31057), and Dearing strain (MRV-3; M31058); for Oryzavirus, Rice ragged stunt virus (RRSV; U66714); for Cypovirus, Bombyx mori cytoplasmic polyhedrosis virus 1 (BmCPV-1; AF323782), Dendrolimus punctatus cypovirus 1 (DpCPV-1; AAN46860), and Lymantria dispar cypovirus 14 (LdCPV-14; AAK73087); for Rotavirus, Rotavirus A and Simian rotavirus strain SA11 (SiRV-A/SA-11; AF015955); for Phytoreovirus, Rice dwarf virus strain A (RDV-A; D90198); and for Orbivirus, African horse sickness virus serotype 9 (AHSV-9; U94887) and Bluetongue virus serotype 2 (BTV-2; L20508) (1).
A characteristic structural feature of turreted viruses is the presence of clamps that are not found in nonturreted viruses, such as Rotavirus, BTV, and RDV. The number of such clamps, either 120 or 150, on the surface of the core of each viral particle is another criterion for the further grouping of the viruses. The present study showed that RRSV belongs to the group with the 120 copies of the clamp protein, as does CPV.
In the turreted Orthoreovirus, the core is fully covered with trimers with T=13l icosahedral symmetry at the Q, R, S, and T positions, while the RRSV virion has only peripheral trimers that were found only at the Q position around turrets. Orthoreovirus loses all its trimers during the penetration of the cell membrane, and only the core particle remains. The core is transcriptionally active and is quite similar to that of CPV, the structurally simplest member of the Reoviridae, and it lacks an outer capsid layer composed of trimers (16). Thus, RRSV seems to have a novel structure that is architecturally intermediate between that of CPV and that of Orthoreovirus (Fig. 7A). The structure of RRSV suggests that turreted reoviruses have acquired different types of secondary layers during the evolutionary steps that led to the ability to infect a wide variety of host species.
Acknowledgments
Naoyuki Miyazaki and Li Xing were supported by grants from the STINT Foundation and from the NIH HIVRAD program, respectively. R.H.C. was supported by the NIH Discovery program. This study was funded by a grant from the NIH Roadmap Nanomedicine Program and by a grant from the Cancer Research Center of the Swedish Research Council (to R.H.C.); by a grant from the National Project on Protein Structural and Functional Analysis and by a Grant-in-Aid from the 21st Century Centers of Excellence Program (to A.N.); and by a grant from the National Project on Structures of Biological Macromolecular Assemblies and a Grant-in-Aid for Scientific Research on Priority Areas (to T.O.) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Footnotes
Published ahead of print on 10 September 2008.
REFERENCES
- 1.Attoui, H., F. Mohd Jaafar, M. Belhouchet, P. Biagini, J. F. Cantaloube, P. de Micco, and X. de Lamballerie. 2005. Expansion of family Reoviridae to include nine-segmented dsRNA viruses: isolation and characterization of a new virus designated Aedes pseudoscutellaris reovirus assigned to a proposed genus (Dinovernavirus). Virology 343212-223. [DOI] [PubMed] [Google Scholar]
- 2.Baker, T. S., and R. H. Cheng. 1996. A model-based approach for determining orientations of biological macromolecules imaged by cryoelectron microscopy. J. Struct. Biol. 116120-130. [DOI] [PubMed] [Google Scholar]
- 3.Bamford, D. H., J. M. Grimes, and D. I. Stuart. 2005. What does structure tell us about virus evolution? Curr. Opin. Struct. Biol. 15655-663. [DOI] [PubMed] [Google Scholar]
- 4.Bartlett, N., S. Gillies, S. Bullivant, and A. Bellamy. 1974. Electron microscopy study of reovirus reaction cores. J. Virol. 14315-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellamy, A., and J. Harvey. 1976. Biophysical studies of reovirus type 3. III. A laser light-scattering study of the RNA transcriptase reaction. Virology 7028-36. [DOI] [PubMed] [Google Scholar]
- 6.Chandran, K., S. B. Walker, Y. Chen, C. M. Contreras, L. A. Schiff, T. S. Baker, and M. L. Nibert. 1999. In vitro recoating of reovirus cores with baculovirus-expressed outer-capsid proteins μ1 and σ3. J. Virol. 733941-3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6a.Chaogang, S., W. Jianhua, Z. Guoying, S. Gang, P. Baozhen, L. Juanli, J. Dendi, C. Shenxiang, N. M. Upadhyaya, P. Waterhouse, and G. Zuxun. 2003. Ectopic expression of the spike protein of rice ragged stunt Oryzavirus in transgenic rice plants inhibits transmission of the virus to insects. Mol. Breed. 11295-301. [Google Scholar]
- 7.Cheng, R. H., J. R. Caston, G. J. Wang, F. Gu, T. J. Smith, T. S. Baker, R. F. Bozarth, B. L. Trus, N. Cheng, R. B. Wickner, and A. C. Steven. 1994. Fungal virus capsids, cytoplasmic compartments for the replication of double-stranded RNA, formed as icosahedral shells of asymmetric Gag dimers. J. Mol. Biol. 244255-258. [DOI] [PubMed] [Google Scholar]
- 8.Dryden, K. A., G. Wang, M. Yeager, M. L. Nibert, K. M. Coombs, D. B. Furlong, B. N. Fields, and T. S. Baker. 1993. Early steps in reovirus infection are associated with dramatic changes in supramolecular structure and protein conformation: analysis of virions and subviral particles by cryoelectron microscopy and image reconstruction. J. Cell Biol. 1221023-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fang, Q., S. Shah, Y. Liang, and Z. H. Zhou. 2005. 3D reconstruction and capsid protein characterization of grass carp reovirus. Sci. China C Life Sci. 48593-600. [DOI] [PubMed] [Google Scholar]
- 10.Furuichi, Y. 1974. “Methylation-coupled” transcription by virus associated transcriptase of cytoplasmic polyhedrosis virus containing double-stranded RNA. Nucleic Acids Res. 1809-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grimes, J. M., J. N. Burroughs, P. Gouet, J. M. Diprose, R. Malby, S. Zientara, P. P. Mertens, and D. I. Stuart. 1998. The atomic structure of the bluetongue virus core. Nature 395470-478. [DOI] [PubMed] [Google Scholar]
- 12.Hagiwara, K., and H. Naitow. 2003. Assembly into single-shelled virus-like particles by major capsid protein VP1 encoded by genome segment S1 of Bombyx mori cypovirus 1. J. Gen. Virol. 842439-2441. [DOI] [PubMed] [Google Scholar]
- 13.Hagiwara, K., S. Rao, S. W. Scott, and G. R. Carner. 2002. Nucleotide sequences of segments 1, 3 and 4 of the genome of Bombyx mori cypovirus 1 encoding putative capsid proteins VP1, VP3 and VP4, respectively. J. Gen. Virol. 831477-1482. [DOI] [PubMed] [Google Scholar]
- 14.Hagiwara, K., T. Higashi, N. Miyazaki, H. Naitow, R. H. Cheng, A. Nakagawa, H. Mizuno, T. Tsukihara, and T. Omura. 2004. The amino-terminal region of major capsid protein P3 is essential for self-assembly of single-shelled core-like particles of Rice dwarf virus. J. Virol. 783145-3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hagiwara, K., Y. Minobe, Y. Nozu, H. Hibino, I. Kimura, and T. Omura. 1986. Component proteins and structures of Rice ragged stunt virus. J. Gen. Virol. 671711-1715. [Google Scholar]
- 16.Hill, C. L., T. F. Booth, B. V. Prasad, J. M. Grimes, P. P. Mertens, G. C. Sutton, and D. I. Stuart. 1999. The structure of a cypovirus and the functional organization of dsRNA viruses. Nat. Struct. Biol. 6565-568. [DOI] [PubMed] [Google Scholar]
- 17.Kantardjieff, K. A., and B. Rupp. 2003. Matthews coefficient probabilities: improved estimates for unit cell contents of proteins, DNA, and protein-nucleic acid complex crystals. Protein Sci. 121865-1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liemann, S., K. Chandran, T. S. Baker, M. L. Nibert, and S. C. Harrison. 2002. Structure of the reovirus membrane-penetration protein, μ1, in a complex with its protector protein, σ3. Cell 108283-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ling, K. C., E. R. Tiongco, and V. M. Aguiero. 1978. Rice ragged stunt, a new virus disease. Plant Dis. Rep. 62701-705. [Google Scholar]
- 20.Matthews, B. W. 1968. Solvent content of protein crystals. J. Mol. Biol. 33491-497. [DOI] [PubMed] [Google Scholar]
- 21.McQualter, R. B., G. R. Smith, J. L. Dale, and R. M. Harding. 2003. Molecular analysis of Fiji disease Fijivirus genome segments 1 and 3. Virus Genes 26283-289. [DOI] [PubMed] [Google Scholar]
- 22.Mertens, P. P. C., H. Attoui, R. Duncan, and T. S. Dermody. 2005. Reoviridae, p. 447-454. In C. M. Fauquest, M. A. Mayo, J. Maniloff, U. Desselberger, and L. A. Ball (ed.), Virus taxonomy. Eighth report of the International Committee on Taxonomy of Viruses. Elsevier Academic Press, San Diego, CA.
- 23.Naitow, H., J. Tang, M. Canady, R. B. Wickner, and J. E. Johnson. 2002. L-A virus at 3.4 Å resolution reveals particle architecture and mRNA decapping mechanism. Nat. Struct. Biol. 9725-728. [DOI] [PubMed] [Google Scholar]
- 24.Nakagawa, A., N. Miyazaki, J. Taka, H. Naitow, A. Ogawa, Z. Fujimoto, H. Mizuno, T. Higashi, Y. Watanabe, T. Omura, R. H. Cheng, and T. Tsukihara. 2003. The atomic structure of Rice dwarf virus reveals the self-assembly mechanism of component proteins. Structure 111227-1238. [DOI] [PubMed] [Google Scholar]
- 25.Nason, E. L., S. K. Samal, and B. V. Prasad. 2000. Trypsin-induced structural transformation in aquareovirus. J. Virol. 746546-6555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Omura, T., Y. Minobe, I. Kimura, H. Hibino, T. Tsuchizaki, and Y. Saito. 1983. Improved purification procedure and RNA segments of Rice ragged stunt virus. Ann. Phytopathol. Soc. Jpn. 49670-675. [Google Scholar]
- 27.Prasad, B. V., R. Rothnagel, C. Q. Zeng, J. Jakana, J. A. Lawton, W. Chiu, and M. K. Estes. 1996. Visualization of ordered genomic RNA and localization of transcriptional complexes in rotavirus. Nature 382471-473. [DOI] [PubMed] [Google Scholar]
- 28.Reinisch, K. M., M. L. Nibert, and S. C. Harrison. 2000. Structure of the reovirus core at 3.6 Å resolution. Nature 404960-967. [DOI] [PubMed] [Google Scholar]
- 29.Reference deleted.
- 30.Shaw, A. L., S. K. Samal, K. Subramanian, and B. V. Prasad. 1996. The structure of aquareovirus shows how the different geometries of the two layers of the capsid are reconciled to provide symmetrical interactions and stabilization. Structure 4957-967. [DOI] [PubMed] [Google Scholar]
- 31.Supyani, S., B. I. Hillman, and N. Suzuki. 2007. Baculovirus expression of the 11 mycoreovirus-1 genome segments and identification of the guanylyltransferase-encoding segment. J. Gen. Virol. 88342-350. [DOI] [PubMed] [Google Scholar]
- 32.Upadhyaya, N. M., E. Zinkowsky, W. Kositratana, and P. M. Waterhouse. 1996. The Mr 43K major capsid protein of rice ragged stunt Oryzavirus is a post-translationally processed product of a Mr 67,348 polypeptide encoded by genome segment 8. Arch. Virol. 1411689-1701. [DOI] [PubMed] [Google Scholar]
- 33.Upadhyaya, N. M., K. Ramm, J. A. Gellatly, Z. Li, W. Kositratana, and P. M. Waterhouse. 1998. Rice ragged stunt Oryzavirus genome segment S4 could encode an RNA-dependent RNA polymerase and a second protein of unknown function. Arch. Virol. 1431815-1822. [DOI] [PubMed] [Google Scholar]
- 34.Upadhyaya, N. M., M. Yang, W. Kositratana, A. Ghosh, and P. M. Waterhouse. 1995. Molecular analysis of rice ragged stunt Oryzavirus segment 9 and sequence conservation among isolates from Thailand and India. Arch. Virol. 1401945-1956. [DOI] [PubMed] [Google Scholar]
- 35.White, C., and H. Zweerink. 1976. Studies on the structure of reovirus cores: selective removal of polypeptide lambda 2. Virology 70171-180. [DOI] [PubMed] [Google Scholar]
- 36.Yazaki, K., and K.-I. Miura. 1980. Relation of the structure of cytoplasmic polyhedrosis viruses and the synthesis of its messenger RNA. Virology 105467-479. [DOI] [PubMed] [Google Scholar]
- 37.Yu, X., L. Jin, and Z. H. Zhou. 2008. 3.88 Å structure of cytoplasmic polyhedrosis virus by cryo-electron microscopy. Nature 453415-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang, H., X. K. Yu, X. Y. Lu, J. Q. Zhang, and Z. H. Zhou. 2002. Molecular interactions and viral stability revealed by structural analyses of chemically treated cypovirus capsids. Virology 29845-52. [DOI] [PubMed] [Google Scholar]
- 39.Zhang, X., E. Settembre, C. Xu, P. R. Dormitzer, R. Bellamy, S. C. Harrison, and N. Grigorieff. 2008. Near-atomic resolution using electron cryomicroscopy and single-particle reconstruction. Proc. Natl. Acad. Sci. USA 1051867-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang, X., S. B. Walker, P. R. Chipman, M. L. Nibert, and T. S. Baker. 2003. Reovirus polymerase lambda 3 localized by cryo-electron microscopy of virions at a resolution of 7.6 Å. Nat. Struct. Biol. 101011-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou, G. Y., X. B. Lu, H. J. Lu, J. L. Lei, S. X. Chen, and Z. X. Gong. 1999. Rice ragged stunt Oryzavirus: role of the viral spike protein in transmission by the insect vector. Ann. Appl. Biol. 135573-578. [Google Scholar]
- 42.Zhou, Z. H., H. Zhang, J. Jakana, X. Y. Lu, and J. Q. Zhang. 2003. Cytoplasmic polyhedrosis virus structure at 8 Å by electron cryomicroscopy: structural basis of capsid stability and mRNA-processing regulation. Structure 11651-663. [DOI] [PubMed] [Google Scholar]