Abstract
The paramyxovirus simian virus 5 (SV5) is a poor activator of human dendritic cell (DC) maturation pathways in vitro, and infected DC do not upregulate cell surface costimulatory proteins or secretion of immunomodulatory cytokines. We evaluated the hypothesis that activation of SV5-infected DC would be enhanced by engineering SV5 to express a Toll-like-receptor (TLR) ligand. To test this hypothesis, a novel virus was engineered such that the gene encoding an intracellular form of the TLR5 ligand flagellin was expressed from the genome of wild-type (WT) SV5 (SV5-flagellin). Cells infected in vitro with the flagellin-expressing virus released low levels of biologically active flagellin, which was capable of stimulating TLR5 signaling. Infection of human peripheral blood mononuclear cell-derived immature DC with SV5-flagellin resulted in enhanced levels of interleukin-6 (IL-6) and IL-12 compared to infection with DC with the parental virus, WT SV5. In contrast to cytokine induction, the flagellin-expressing virus did not appreciably increase DC surface expression of the costimulatory molecule CD80 or CD86 above the level seen with WT SV5 alone. In mixed-culture assays, DC infected with the flagellin-expressing virus were more effective at activating gamma interferon secretion from both CD8+ and CD4+ allogeneic T cells than DC infected with WT SV5. Our results with SV5-directed intracellular expression of flagellin may be applicable to other vectors or pathogenic viruses where overcoming impairment of DC activation could contribute to the development of safer and more effective vaccines.
Dendritic cells (DC) are important professional antigen-presenting cells that are capable of recognizing microbial products and promoting innate and adaptive immune responses (5, 38). Upon sensing viral components, DC are triggered to undergo a conversion from a phenotype termed immature into a form that is capable of activating naïve-T-cell functions, such as proliferation, cytokine secretion, and cytolytic activity (5, 38). These DC activation events include increased cell surface expression of costimulatory molecules, such as CD40, CD80, and CD86, as well as increased capacity to secrete immunomodulatory cytokines, such as interleukin-12 (IL-12), IL-6, and tumor necrosis factor alpha (TNF-α) (5, 14, 38). As such, the ability of DC to be activated in response to infection has important consequences for development of immunity against viruses. Here we describe engineering the paramyxovirus simian virus 5 (SV5) to be more effective at activating human DC to stimulate T-cell function in vitro.
Many viruses are poor activators of DC, with infected immature DC showing only limited production of cytokines or upregulation of costimulatory molecules (28). Examples include herpes simplex virus, cytomegalovirus (CMV), vaccinia virus, measles virus (MeV), influenza virus, Lassa fever virus, poliovirus, and Ebola virus (4, 9, 17, 18, 20, 36, 43). This lack of activation of infected DC can result from virus-induced suppression of signaling pathways. For example, CMV infection of immature DC fails to induce an increase in maturation markers and makes infected cells refractory to activation induced by external stimuli (36). Likewise, DC infected with respiratory syncytial virus (RSV) or MeV are blocked in their response to single-stranded-RNA and double-stranded-DNA agonists (40).
Like many other paramyxoviruses, SV5 is a poor inducer of DC responses in vitro. SV5 establishes a highly productive infection of human immature DC, but infected cells show very little cytokine secretion or upregulation of maturation markers (2). We previously engineered a recombinant SV5 mutant (P/V-CPI−) (44) to carry six substitutions in the viral P/V gene, which encodes both the phosphoprotein P subunit of the viral polymerase (30) and the V protein, which counteracts antiviral responses (15, 22). While the P/V-CPI− mutant is more effective than wild-type (WT) SV5 at inducing DC activation in vitro, P/V-CPI− replication is restricted, and infected DC show only a small upregulation of costimulatory markers (2). In light of recent work proposing SV5 as a vaccine vector (11, 37, 42), these results raise the question of whether WT SV5 could be engineered to provide stronger signals to drive a more complete activation of DC.
Virus-induced DC activation can occur through recognition of components of the incoming virion particle or newly synthesized viral components (8, 28, 32, 38). A major mechanism of virus-induced DC activation involves signaling through the Toll-like-receptor (TLR) pathways (26, 41). Individual TLRs can recognize a specific class of microbial components and initiate a cascade of DC intracellular signaling events that results in increased costimulatory marker expression and inflammatory responses. In the case of RNA viruses, important TLRs include TLR3, which recognizes double-stranded RNA (1), and TLR7 and TLR8, which recognize single-stranded RNA (33). In addition, viral glycoproteins have been reported to activate TLR2 or TLR4 in the case of mouse mammary tumor virus, MeV, and RSV (reviewed in reference 8).
TLR ligands have been proposed as new therapeutic tools to promote innate and adaptive immunity, and agonists for TLR-4, -5, -7, and -9 have shown great promise as vaccine adjuvants (reviewed in reference 27). As a TLR5 ligand, bacterial flagellin is a potent stimulus that when instilled into the respiratory tract results in a localized and transient infiltration of neutrophils and the production of immunomodulatory cytokines (23). Flagellin has a number of properties that make it a highly attractive adjuvant: (i) it is effective at very low doses; (ii) it does not promote immunoglobulin E responses; (iii) prior immunity to flagellin does not impair its adjuvant activity; (iv) antigen sequences can be inserted into selected regions of the protein without any loss of TLR5 signaling; (v) when given intranasally or intramuscularly, flagellin does not exhibit any detectable toxicity in adult mice or nonhuman primates; and (vi) the binding of flagellin to TLR5 on DC may enhance the uptake and thus the processing of antigens (reviewed in reference 24).
Given that WT SV5 is a poor inducer of DC maturation (2, 3), we tested the hypothesis that the DC-activating properties of flagellin could be transferred to SV5 vectors. Here we demonstrate that engineered expression of flagellin overcomes the inherent nonstimulatory properties of SV5 infection of human DC in vitro, resulting in increased ability of these infected DC to activate T-cell functions. Our results with SV5-directed expression of flagellin may be applicable to other vectors or pathogenic viruses, for which it has been proposed that overcoming impairment of DC activation could contribute to development of safer and more effective vaccines (3, 18, 32, 40).
MATERIALS AND METHODS
Viruses, cytokines and cells.
An SV5 expressing green fluorescent protein (GFP) was recovered as described previously (44) from a cDNA plasmid kindly provided by Robert Lamb (Northwestern University) and Biao He (Penn State University). P/V-CPI− virus was recovered from cDNA and grown in Vero cells as described previously (44). WT SV5 was engineered to contain the flagellin gene inserted between the HN and L genes using standard molecular biology techniques (details available upon request). Briefly, the previously described DNA encoding flagellin from Salmonella enterica serovar Enteritidis (34) was modified by PCR to contain StuI and SalI sites in the 5′ and 3′ noncoding regions, respectively. The resulting DNA fragment was ligated into the EcoRV and SalI sites located between the HN-L gene end and gene start sequences, and the virus (SV5-flagellin) was recovered as described previously (44). Viruses were concentrated by centrifugation through a glycerol cushion, and titers were determined on CV-1 cells (2). IL-4 and granulocyte-macrophage colony-stimulating factor (GM-CSF) were produced from recombinant baculoviruses (details available on request).
Monolayer cultures of A549 were grown in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum. Human immature DC were prepared from peripheral blood mononuclear cells (PBMC) (2). Briefly, PBMC were isolated from randomly selected healthy donors by standard density gradient centrifugation on Ficoll-Hypaque. CD14+ cells were isolated by positive selection using CD14 microbeads. Typically, the purity of monocytes isolated by this procedure was ≥95%. Enriched CD14+ cells (106 cells/ml in 5 ml) were cultured for 6 days in culture medium containing 10 ng/ml human IL-4 and 10 ng/ml human GM-CSF. On day 3, half of the medium was replaced with fresh medium supplemented with cytokines to the above final concentrations. More than 95% of the resulting cell population was CD11c+ (data not shown). Cells were collected on day 6 and infected as described previously (2). In some experiments, flagellin or lipopolysaccharide (LPS) was added to cultures immediately after resuspending in medium or at 7 h postinfection (p.i.) (3) at final concentrations indicated in the figure legends.
Purification and assays for biologically active flagellin.
Recombinant His-tagged flagellin was produced and purified using metal affinity chromatography and Acrodisc Mustang Q and E membranes (Pall Corporation, Ann Arbor, MI) to remove endotoxins and nucleic acids as described previously (34). Contaminating endotoxin levels were verified to be less than 10 pg LPS per μg of protein by Limulus amebocyte lysate assay (Associates of Cape Cod, Inc., East Falmouth, MA). To determine the biological activity of flagellin from infected cells, immature DC were mock infected or infected at a multiplicity of infection (MOI) of 5 with SV5-flagellin. Medium was collected at 4, 10, and 22 h p.i. and treated with UV light to inactivate virus as described previously (44). TLR5-negative RAW264.7 cells or RAW264.7 cells stably expressing murine TLR5 fused to enhanced yellow fluorescent protein (45) were incubated with medium samples for 4 h, and then levels of released TNF-α were assayed by enzyme-linked immunosorbent assay (ELISA).
Western blotting, immunofluorescence, and flow cytometry.
Six-well dishes of cells were infected with viruses as described in the figure legends. At each time point, cells were washed with phosphate-buffered saline and lysed in 1% sodium dodecyl sulfate (SDS). The protein concentrations of cell lysates were determined by a bicinchoninic acid assay (Pierce Chemicals), and equivalent amounts of protein were analyzed by SDS-polyacrylamide gel electrophoresis and Western blotting using rabbit polyclonal antiserum specific for SV5 P or for flagellin or with a mouse monoclonal antibody specific for actin (Sigma A5316). Extracellular medium was concentrated by trichloroacetic acid precipitation for analysis of released flagellin by SDS-polyacrylamide gel electrophoresis. Samples were analyzed by horseradish peroxidase-conjugated secondary antibodies and enhanced chemiluminescence (Pierce Chemicals).
For immunofluorescence, A549 cells were mock infected or infected at an MOI of 10, and at 20 h p.i., cells were fixed in 4% paraformaldehyde and permeabilized with 0.01% saponin in phosphate-buffered saline for 10 min at room temperature. Cells were stained with chicken antiflagellin (1:200 dilution) followed by Alexafluor 568-conjugated goat anti-chicken antibody (1:500 dilution) raised against purified flagellin. Alternatively, DC were stained with a monoclonal antibody specific for the WT sequence of the shared N-terminal region of the SV5 P and V proteins (V5 antibody; Invitrogen) followed by phycoerythrin (PE)-conjugated anti-mouse antibody. Microscopy was carried out as described previously (3) using a Nikon Eclipse fluorescence microscope and a 20× lens. Images were captured using a QImaging digital camera and processed using QCapture software. Exposure times were manually set to be constant between samples.
For detection of surface markers, DC were stained with the following conjugated monoclonal antibodies according to the manufacturer's recommendations: CD11c (B-ly6), CD14 (M5E2), CD80 (L307.4), and CD86 (FUN-1), all from BD Pharmingen (San Diego, CA). Samples were analyzed on a FACScan instrument using Cell Quest software (Becton Dickinson, San Diego, CA). Apoptotic markers were measured by intracellular staining for active caspase-3 using PE-conjugated polyclonal anti-caspase-3 antibody (Pharmingen, San Diego, CA) along with CD11c-allophycocyanin or by measuring cell surface staining with PE-conjugated annexin V. Samples were analyzed by flow cytometry.
ELISAs.
ELISAs were performed using OptEIA sets for human IL-6 and IL-12 (p40) or for mouse TNF-α (BD Pharmingen) per the manufacturer's instructions. For experiments with A549 cells, a parallel sample of uninfected cells was counted at 24 h p.i. for use in normalizing secretion levels to the value obtained for 106 cells.
T-cell-activation assay.
Immature DC were mock infected or infected at an MOI of 5 with SV5 viruses. In some samples, LPS (200 ng/ml; Invivogen, San Diego, CA) or flagellin (5 ng/ml) was added at 7 h p.i. as described previously (3). At 22 h p.i., samples were irradiated (3,000 rads), and 2.5 × 104 DC were cultured with 2.5 × 105 allogeneic responder T cells. Mixed cultures were incubated for 3 days in 96-well round-bottom plates in a total volume of 200 μl/well of complete culture medium at 37°C in 5% CO2. Cells were harvested and stimulated with or without 5 × 105 DC in the presence of Golgi-plug (BD Biosciences) for 5 h. Cells were then stained with CD8 and CD4 antibodies, followed by fixation, permeabilization, and intracellular staining with anti-IFN-γ antibodies. Flow cytometry was used to measure intracellular IFN-γ levels in CD8+ and CD4+ T cells. Student's t test was used to determine significance between samples. No differences between results obtained with and without DC restimulation were seen.
RESULTS
Intracellular expression of flagellin from WT SV5.
We tested the hypothesis that expression of a TLR agonist in the context of SV5-infected cells promotes more extensive cytokine production and costimulatory molecule expression than those seen with WT SV5. Bacterial flagellin was chosen as the agonist for engineered expression, since it is a polypeptide and its stimulatory effect in vitro is well characterized (21, 24, 35). As shown schematically in Fig. 1A, a recombinant virus (SV5-flagellin) was generated such that the GFP gene between HN and L in the WT SV5-GFP genome was replaced with the open reading frame for flagellin from S. enterica serovar Enteritidis. The P/V-CPI− virus was used as a positive control, since we showed previously that this mutant SV5 induces small increases in DC maturation markers and cytokines and that infected DC are capable of stimulating T cells in vitro (2).
FIG. 1.
Recombinant SV5 expressing flagellin. (A) The SV5 genome structure is shown schematically as negative-sense RNA, with an additional gene (GFP or flagellin) inserted between HN and L. The shaded box shows the shared N-terminal region of P and V protein, which contains the six CPI− amino acid substitutions. le, leader; tr, trailer. (B) Flagellin expression. A549 cells were mock infected or infected at an MOI of 5 with SV5-GFP or SV5-flagellin. At 20 h p.i., cell lysates were analyzed by Western blotting for P protein, flagellin, or cellular actin. (C) Intracellular expression of flagellin. A549 cells that had been mock infected or infected with SV5-flagellin were permeabilized at 20 h p.i. and stained with chicken antiflagellin antiserum as described in Materials and Methods. DAPI (4′,6′-diamidino-2-phenylindole) staining was used to visualize the nucleus.
As shown in the Western blot in Fig. 1B, high levels of flagellin were expressed in A549 cells infected with SV5-flagellin. Flagellin accumulated in the cytoplasm of A549 cells infected with SV5-flagellin (Fig. 1C), consistent with the SV5-expressed flagellin lacking a signal sequence for secretion. The expression of flagellin from the SV5 genome had no appreciable effect on virus multistep or single-step growth kinetics (Fig. 2A and B), and virus yields from infected A549 cells were similar to the yield seen with WT SV5-GFP and the P/V-CPI− virus.
FIG. 2.
Growth and cytopathic effect of SV5-flagellin infection. A549 cells were infected at an MOI of 0.05 (A) or 5 (B) with the indicated viruses, and virus titers were determined at the indicated times p.i.. Results are means for triplicate samples, and error bars represent standard deviations from the means. (C and D) A549 cells were mock infected or infected at an MOI of 10 with the indicated viruses. Levels of cleaved caspase-3 and cell surface annexin V staining were determined at 24 and 48 h p.i. as described in Materials and Methods.
WT SV5-GFP is largely noncytopathic in human epithelial cells (12, 44), while the P/V-CPI− induces high levels of apoptotic markers (19, 44). At late times after infection with SV5-flagellin, A549 cells showed levels of cleaved caspase-3 (Fig. 2C) and cell surface annexin V staining (Fig. 2D) that were only slightly higher than those seen with WT SV5-GFP and well below those seen with P/V-CPI−. Thus, intracellular expression of bacterial flagellin has little effect on SV5 growth or on the noncytopathic nature of SV5 infections of epithelial cells.
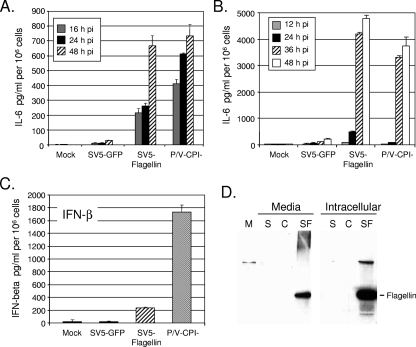
To determine if the flagellin-expressing virus induced proinflammatory cytokine secretion, A549 cells were infected at a high MOI, and IL-6 released into the media at various times p.i. was quantitated. As shown in Fig. 3A, infection with SV5-GFP induced very little IL-6 secretion, whereas the P/V-CPI− virus induced high levels of IL-6, as shown previously (46). Cells infected with SV5-flagellin responded similarly to those infected with P/V-CPI−, and high levels of IL-6 secretion were detected as early as 16 h p.i.. After low-MOI infection, the flagellin-expressing virus induced a level of IL-6 secretion similar to that seen with the P/V-CPI− virus and much lower than that seen with parental SV5-GFP (Fig. 3B). Final levels of IL-6 secretion after high- and low-MOI infections with SV5-flagellin differed ∼6-fold, which is within the variability typically seen in our experiments with SV5-infected A549 cells (16, 46). It is unlikely that this reflects differences in cytopathic effect or virus growth, since these parameters were similar for SV5-GFP and the flagellin-expressing viruses. However, this could reflect differences in cell number, cell confluence, or time in culture. The activation of beta interferon (IFN-β) secretion by SV5-flagellin differed from activation of IL-6. This is evident in Fig. 3C, which shows that A549 cells infected with SV5-flagellin secreted low levels of IFN-β compared to cells infected with P/V-CPI− (44). Thus, SV5-directed expression of flagellin in human epithelial cells induces high levels of IL-6 secretion similar to P/V-CPI−, but IFN-β secretion is low, similar to that seen in cells infected with WT SV5-GFP.
FIG. 3.
Release of flagellin and IL-6 from epithelial cells infected with SV5-flagellin. (A and B) IL-6 secretion. A549 cells were mock infected or infected at an MOI of 5 (A) or 0.05 (B) with the indicated viruses. Levels of IL-6 released into the media at the indicated times p.i. were determined by ELISA and were normalized to the value obtained for 106 cells. (C) A549 cells were infected at an MOI of 10 with the indicated viruses, and levels of extracellular IFN-β were determined at 24 h p.i.. Results are means for triplicate samples, and error bars represent standard deviations from the means. (D) Flagellin release. A549 cells were mock infected (lane M) or infected at an MOI of 10 with SV5-GFP (lanes S), P/V-CPI− (lanes C), or SV5-flagellin (lanes SF). At 24 h p.i., media and cell lysates were isolated and analyzed by Western blotting with antiflagellin antiserum as described in Materials and Methods. “Media,” total flagellin released from infected cells; “Intracellular,” one-eighth of total cell lysate.
Since flagellin promotes inflammatory responses through binding to cell surface TLR5 (21, 45), the finding that SV5-flagellin stimulated IL-6 secretion suggested that flagellin was released from infected cells. To directly test this, A549 cells were mock infected or infected at a high MOI with SV5-GFP, P/V-CPI−, or SV5-flagellin. At 24 h p.i., media and cell lysates were isolated and separately analyzed for levels of flagellin by Western blotting. As shown in Fig. 3D, high levels of flagellin were detected in lysates from cells infected with SV5-flagellin (lanes SF) but not in control samples. Much lower levels of flagellin were released from cells infected with SV5-flagellin, consistent with the increased secretion of IL-6 induced by these viruses. Quantitative Western blotting indicated that the level of released flagellin at 24 h p.i. represented ∼0.1% of the accumulated intracellular levels (data not shown). The above data obtained with A549 cells indicate that SV5-flagellin expresses an intracellular form of flagellin that is released at relatively low levels from infected cells. The most dramatic effect of flagellin expression was seen in the case of IL-6 secretion, which was much higher than that induced by parental SV5-GFP.
Biologically active flagellin is released from immature DC infected with SV5-flagellin.
To test the effect of SV5-mediated flagellin expression on DC activation, PBMC-derived DC were generated after in vitro culturing of CD14+ cells with IL-4 and GM-CSF. These cells were operationally defined as immature DC, using the widely accepted criteria of being CD11c+, expressing relatively low levels of the surface costimulatory markers CD80 and CD86, and producing low levels of proinflammatory cytokines (2, 3). As shown in Fig. 4A, the proportions of DC infected with SV5-GFP and SV5-flagellin at an MOI of 5 were similar. Likewise, the kinetics and levels of viral protein expression did not differ appreciably between DC infected with SV5-GFP and SV5-flagellin (Fig. 4B).
FIG. 4.
Biologically active flagellin is released from human DC infected with SV5-flagellin. (A) Infectivity of SV5-flagellin. DC that were mock infected or infected at an MOI of 5 with the indicated viruses were processed for immunofluorescence staining using an antibody specific for the SV5 P and V proteins. (B) Viral gene expression. Lysates collected at 8 and 22 h p.i. from DC infected with SV5-GFP (lanes S) or SV5-flagellin (lanes SF) were assayed by Western blotting for levels of NP, P, M, and actin. (C and D) DC were mock infected (lanes M) or infected at an MOI of 5 with SV5-flagellin (lanes SF). Equal amounts of cell lysate were analyzed by Western blotting for levels of flagellin, viral P protein, and actin (C). Media were analyzed for their ability to stimulate release of TNF-α from RAW 264.7 control cells or RAW cells engineered to express human TLR5, as described in Materials and Methods.
Flagellin released from the infected DC was biologically active (Fig. 4C and D). DC were mock infected or infected at a high MOI with SV5-flagellin. At 4, 10, and 22 h p.i., cell lysates were harvested for Western blot analysis, and extracellular media were harvested to determine the biological activity of released flagellin. As shown in Fig. 4C, DC infected with SV5-flagellin accumulated intracellular flagellin with kinetics matching that of the viral P protein. Medium from infected cells was UV treated to inactivate virus and then tested for the ability to induce cytokine secretion from noninfected reporter cells. TLR5-negative RAW 264.7 cells or RAW 264.7 cells engineered to express murine TLR5 (45) were incubated with medium samples for 4 h, and released TNF-α was quantitated by ELISA. Media collected from DC infected with SV5-flagellin showed an increased ability to induce TNF-α secretion in cells expressing TLR-5 but not in matched control cells lacking TLR-5 (Fig. 4D). These data indicate that biologically active flagellin is released from DC infected with SV5-flagellin.
Cytokine secretion and costimulatory marker expression following infection of DC with SV5-flagellin.
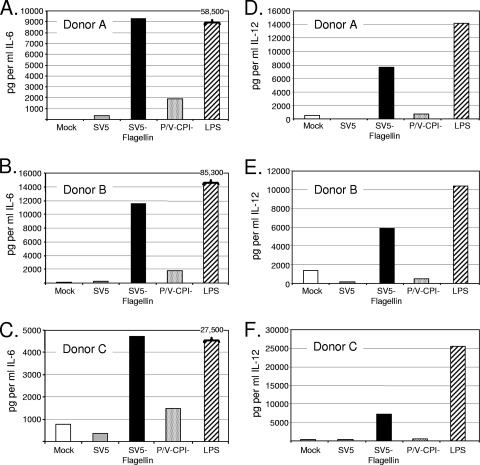
To test the ability of SV5-flagellin to activate DC, cells from three individual donors were mock infected or infected at a high MOI with the viruses indicated in Fig. 5. As a positive control, noninfected cells were treated with LPS. Levels of secreted IL-6 (Fig. 5A to C) and IL-12 p40 (Fig. 5D to F) at 22 h p.i. were determined by ELISA. In confirmation of prior results, SV5-GFP was a poor inducer of both IL-6 and IL-12, whereas P/V-CPI− induced only slightly higher levels of these two cytokines (2). Importantly, secretion of IL-6 and IL-12 was 5- to 20-fold higher in the case of DC infected with SV5-flagellin. Cytokine secretion was dependent on virus replication, since UV-inactivated SV5-flagellin did not induce cytokine secretion (data not shown). The flagellin-expressing virus also induced higher levels of IL-8 and TNF-α secretion than SV5-GFP and P/V-CPI− (data not shown).
FIG. 5.
SV5 expression of flagellin enhances cytokine secretion from infected DC. DC from three individual donors were mock infected or infected at an MOI of 5 with the indicated viruses. Alternatively, cells were treated with LPS as a positive control. At 22 h p.i., levels of secreted IL-6 and IL-12 p40 were determined by ELISA. In panels A through C, the levels of IL-6 induced by LPS treatment were much higher that those seen with infected cells and are presented above the bars.
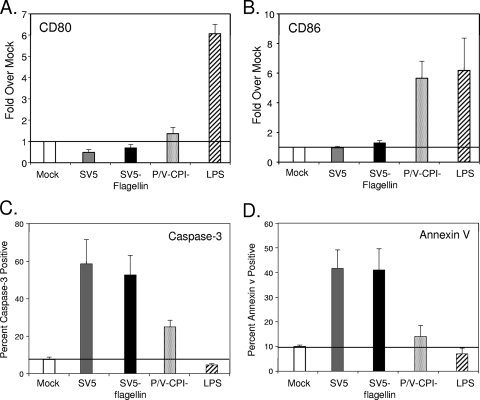
In contrast to the effect on cytokine induction, levels of cell surface CD80 and CD86 were not substantially increased in the case of DC infected with SV5-flagellin compared to parental SV5-GFP (Fig. 6). The changes in cell surface CD80 (Fig. 6A) and CD86 (Fig. 6B) in response to SV5-flagellin were lower than that seen with DC infected with P/V-CPI−. We showed previously that WT SV5 is highly cytopathic for human DC in vitro (2, 3). As shown in Fig. 6C and D, expression of flagellin from the SV5 genome did not substantially change the levels of apoptotic markers such as cleaved caspase-3 (Fig. 6C) or cell surface annexin V staining (Fig. 6D) compared to infection with parental SV5-GFP. Taken together, these data indicate that expression of flagellin by WT SV5 can lead to increases in secretion of immunomodulatory cytokines such as IL-6 and IL-12, but there was relatively little effect on cell surface maturation markers compared to that seen with parental SV5-GFP.
FIG. 6.
SV5 expression of flagellin has little effect on maturation or apoptotic markers on infected DC. DC were mock infected (white bars) or infected at an MOI of 5 with the indicated viruses. Alternatively, cells were treated with LPS as a positive control (hatched bars). Levels of cell surface CD80 (A), cell surface CD86 (B), intracellular cleaved caspase-3 (C), and cell surface annexin V (D) were determined by flow cytometry. Maturation marker expression from three donors (with standard error bars) is expressed as the change relative to mock-infected, mock-treated samples (horizontal line). Levels of apoptotic markers from three donors (with standard error bars) are expressed as percentages of cells positive for caspase-3 or annexin V staining.
DC infected with flagellin-expressing virus are effective stimulators of T-cell function.
Mixed lymphocyte assays were carried out to test the ability of DC infected with SV5-flagellin to activate T cells. DC were mock infected or infected at a high MOI with WT SV5-GFP, SV5-flagellin, or P/V-CPI−. As controls, DC were also mock infected or infected with parental SV5-GFP and then treated with or without exogenous LPS or flagellin. At 22 h p.i., DC were cultured for 3 days with allogeneic responder T cells, and levels of intracellular IFN-γ were assayed by flow cytometry.
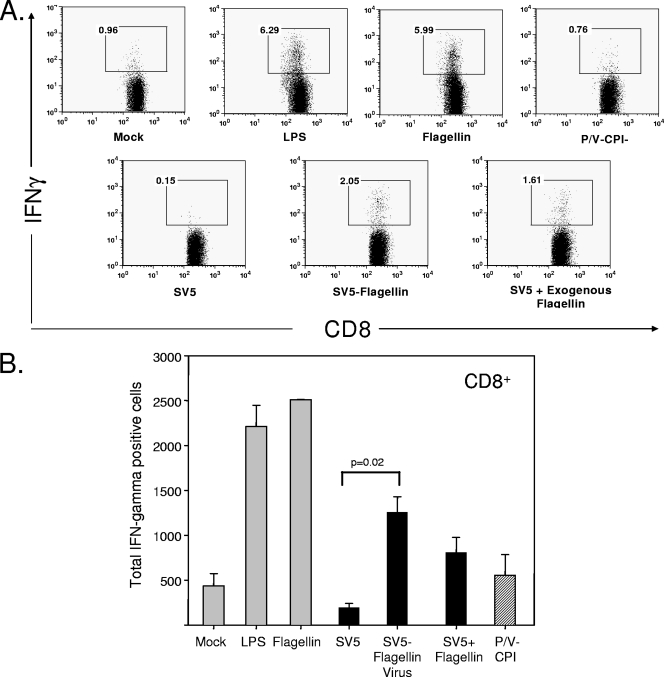
Figure 7A shows results from a representative experiment measuring activation of CD8+ T cells. DC treated with either exogenous flagellin or LPS induced an ∼6-fold increase in the number of CD8+ T cells expressing IFN-γ (0.96 to ∼6%). As expected, infection of DC with WT SV5 did not induce T-cell activation (2). Most importantly, DC infected with SV5-flagellin showed an enhanced ability to stimulate IFN-γ production in CD8+ T cells relative to DC infected with parental SV5-GFP. Furthermore, the stimulatory effect was equivalent to or greater than that seen by adding exogenous flagellin to SV5-infected DC. Infection with the flagellin-expressing viruses also increased the ability of DC to stimulate TNF-α production from responding T cells (not shown). As shown in Fig. 7B, DC infected with SV5-flagellin induced a reproducible, statistically significant increase in total number of IFN-γ-positive CD8+ T cells compared to DC infected with parental SV5-GFP.
FIG. 7.
DC infected with SV5-flagellin are more effective than parental viruses at activating CD8+ T-cell function. DC were mock infected or infected at an MOI of 5 with SV5-GFP, SV5-flagellin, or P/V-CPI−. In addition, mock-infected DC or DC infected with SV5 were treated with LPS (200 μg/ml) or flagellin (5 ng/ml) at 7 h p.i. as controls. At 22 h p.i., DC were cultured for 3 days with allogeneic responder T cells. Cells were stained with antibodies for CD8 and for IFN-γ before analysis by flow cytometry as described in Materials and Methods. (A) Levels of CD8 and IFN-γ from a representative experiment. For each sample, the internal box shows the position of IFN-γ-positive cells. Numbers are percentages of the population that stained positive for IFN-γ. (B) Average total number of IFN-γ-positive CD8+ cells in each culture; error bars indicate standard deviations. Results are averages from three experiments.
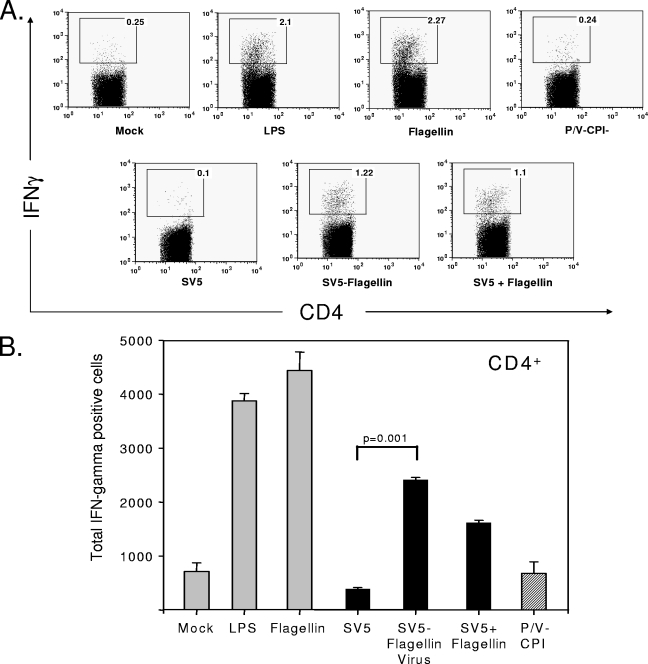
Similar results were seen when activation of CD4+ T cells was assayed in mixed lymphocyte reactions (Fig. 8). SV5-flagellin was more effective than WT SV5 at activating DC to stimulate CD4+ T cells (Fig. 8B), with more IFN-γ-positive T cells being activated than with SV5-infected DC. Taken together, our data indicate that the flagellin-expressing SV5 viruses are more effective than their corresponding parental viruses at inducing DC cytokine secretion but induce relatively little change in cell surface levels of CD80 and CD86. Cell surface levels of these costimulatory markers are nevertheless sufficiently high to allow these infected DC to stimulate T cells in vitro.
FIG. 8.
Activation of CD4+ T-cell proliferation and function by DC infected with SV5-flagellin. DC were treated and cultured with allogeneic responder T cells as described in the legend to Fig. 7, except that T cells were stained with anti-CD4 antibodies. (A) Levels of CD4 and IFN-γ from a representative experiment; (B) average total number of IFN-γ positive CD4+ cells in each culture. Results are averages from three experiments, and error bars indicate standard deviations.
DISCUSSION
The development of robust adaptive immunity to viral infections depends on appropriate innate responses at the time of infection. Key among these responses is the activation of DC (5, 38). Many viruses have evolved mechanisms to prevent DC activation or to interfere with DC functions (28). Our previous cell culture experiments showed that WT SV5 is a poor activator of human DC in vitro and that these infected cells are not effective at inducing T-cell functions (2, 3). Likewise, gene expression for the P/V-CPI− mutant is highly restricted in immature DC, and infected DC show only low-level increases in cell surface maturation markers (2). Recent work has highlighted the potential for nonsegmented RNA viruses to serve as vaccine vectors (reviewed in reference 10), including SV5 (37, 42) and our P/V-CPI− mutant (11). Here we tested the hypothesis that the DC-stimulating capacity of SV5 would be enhanced by providing a strong DC-activating signal in the form of the TLR5 agonist flagellin. Our results clearly demonstrate that DC infected with flagellin-expressing virus are induced to become more effective activators of T-cell function than DC infected with the parental SV5.
A main determinant for selection of flagellin as the DC-activating TLR ligand was the need to express a polypeptide agonist from the viral genome, which would be impractical with nonprotein TLRs such as CpG DNA or lipid conjugates. Expression of flagellin from the viral genome would have the advantage in vaccination protocols that the TLR agonist is provided “at the right place at the right time” to stimulate DC maturation. In addition, flagellin has strong immunostimulatory properties, and previous work has shown that flagellin can act as a potent adjuvant to promote adaptive responses to selected antigens from human pathogens (6, 23, 24, 25, 31). We engineered SV5 to express an intracellular form of flagellin, since attempts to express flagellin containing a conventional signal sequence for secretion were unsuccessful (data not shown). Intracellular expression was predicted to result in release of flagellin by cells undergoing low levels of apoptosis. We showed previously that only ∼2% of WT SV5-infected A549 cells show signs of apoptosis (44), and as expected, our results here show that relatively low levels of flagellin were released by human lung epithelial cells infected with SV5-flagellin. Release may also involve nonapoptotic pathways, such as necrosis or autophagy. While there are cellular pathways by which intracellular flagellin can be detected (39), the findings that flagellin released from infected cells is biologically active and that flagellin binds to cell surface TLR5 with high affinity (34) support the proposal that flagellin released from infected DC is responsible for activating DC.
We have operationally defined our cultured DC as immature on the basis that they express relatively low levels of surface costimulatory markers and secreted proinflammatory cytokines (2, 3). It is important to note that although DC cultured in vitro with IL-4 and GM-CSF express a baseline level of cell surface CD80, CD86, and CD40, it is widely accepted that an elevation in the expression of these markers is a hallmark of DC maturation. SV5-flagellin induced DC to secrete large amounts of IL-6 and IL-12, which is not seen with DC infected with the P/V-CPI− virus or WT SV5. By contrast, the level of cell surface maturation markers on infected DC was not substantially increased by infection with SV5-flagellin. Nevertheless, this surface level of costimulatory proteins was at a sufficient level to allow SV5-flagellin-infected DC to markedly enhance activation of T cells in vitro compared to DC infected with the parental SV5. Consistent with a previous proposal (41), it may be that the ability of our SV5 vectors to induce cytokine secretion from DC is a more important parameter in determining the effectiveness of DC in stimulating T cells than is the relative change in maturation markers. This would suggest a model whereby activation of T-cell function by DC infected with SV5-flagellin is driven by low levels of costimulatory markers but high levels of cytokines such as IL-12.
Our results with the expression of a TLR5 ligand provide proof-of-principle for engineering viruses to be more effective inducers of DC activation and function. Flagellin was a highly effective ligand for testing our hypothesis that viral expression of a TLR agonist could overcome the inherent nonstimulatory properties of a WT SV5 infection. Other TLR ligands may function similarly to flagellin in the context of an SV5 infection, including the viral glycoproteins from MeV and CMV, which activate TLR2 (7, 13), or the RSV F protein, which activates TLR4 (29). Our previous results showed that treatment of SV5-infected immature DC with exogenous TLR2 and TLR4 agonists can increase maturation marker expression but can also override cell death signals normally seen in the case of WT-SV5-infected DC (3). While expression of foreign ligands for TLR2 or TLR4 from the SV5 genome may result in less apoptosis of activated DC, it remains to be determined whether these changes in DC survival have an effect on their ability to activate T-cell function.
In engineering flagellin-expressing viruses, the timing of TLR agonist expression may be a critical factor for balancing activation of infected DC and the induction of an antiviral response that could prevent viral infection. This was evident in our previous work where infected DC responded well to exogenous TLR agonists that were added after 7 h of infection when STAT1 was degraded, but earlier stimulation resulted in a restricted infection (3). In this regard, our strategy of expressing an intracellular form of flagellin may have advantages over incorporating a TLR agonist into the SV5 envelope. The latter approach may result in a virus that induces an abortive infection of DC, since antiviral responses can be induced as a consequence of interactions of the incoming virion particle with cell surface receptors.
Nonsegmented negative-strand viruses have been engineered to increase their immunogenicity through expression of genes encoding selected cytokines, such as IFN-γ, IL-2, and GM-CSF (reviewed in reference 10). In many cases, these cytokine-expressing viruses elicit altered immune responses in vivo. With the flagellin-expressing SV5, we present an alternative approach that elicits a broad cytokine response in infected DC through expression of a potent inducer of TLR signaling pathways. Our results with SV5 may be applicable to other pathogenic viruses, for which it has been proposed that overcoming virus-induced impairment of DC activation could contribute to development of safer and more effective vaccines (3, 18, 32, 40).
Acknowledgments
We thank Doug Lyles and members of the Parks lab for comments on the manuscript, James Phipps and Ellen Young for excellent technical help, and Robert Lamb (Northwestern University) and Biao He (Penn State University) for the initial gift of the SV5 infectious clone.
This work was supported by NIH grant AI-060642 (S.B.M.).
Footnotes
Published ahead of print on 10 September 2008.
REFERENCES
- 1.Alexopoulou, L. A., C. Holt, R. Medzhitov, and R. A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 413:732-738. [DOI] [PubMed] [Google Scholar]
- 2.Arimilli, S., M. A. Alexander-Miller, and G. D. Parks. 2006. A simian virus 5 (SV5) P/V mutant is less cytopathic than wild-type SV5 in human dendritic cells and is a more effective activator of dendritic cell maturation and function. J. Virol. 803416-3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arimilli, S., J. B. Johnson, M. A. Alexander-Miller, and G. D. Parks. 2007. TLR-4 and -6 agonists reverse apoptosis and promote maturation of simian virus 5 infected human dendritic cells through NF-κB-dependent pathways. Virology 365144-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baize, S., J. Kaplon, C. Faure, D. Pannetier, M. C. Georges-Courbot, and V. Deubel. 2004. Lassa virus infection of human dendritic cells and macrophages is productive but fails to activate cells. J. Immunol. 172:2861-2869. [DOI] [PubMed] [Google Scholar]
- 5.Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y. J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18767. [DOI] [PubMed] [Google Scholar]
- 6.Bates, J. T., A. N. Honko, A. H. Graff, N. D. Kock, and S. B. Mizel. 2008. Mucosal adjuvant activity of flagellin in aged mice. Mech. Ageing Dev. 129271-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bieback, K., E. Lien, I. M. Klagge, E. Avota, J. Schneider-Schaulies, W. P. Duprex, H. Wagner, C. J. Kirschning, V. Ter Meulen, and S. Schneider-Schaulies. 2002. Hemagglutinin protein of wild-type measles virus activates TLR2 signaling. J. Virol. 768729-8736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boehme, K. W., and T. Compton. 2004. Innate sensing of viruses by Toll-like receptors. J. Virol. 78:7867-7873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bosio, C. M., M. J. Aman, C. Grogan, R. Hogan, G. Ruthel, D. Negley, M. Mohamadzadeh, S. Bavari, and A. Schmaljohn. 2003. Ebola and Marburg viruses replicate in monocyte-derived dendritic cells without inducing the production of cytokines and full maturation. J. Infect. Dis. 1881630-1638. [DOI] [PubMed] [Google Scholar]
- 10.Bukreyev, A., M. H. Skiadopoulos, B. R. Murphy, and P. L. Collins. 2006. Nonsegmented negative-sense viruses as vaccine vectors. J. Virol. 8010293-10306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Capraro, G. A., J. J. Johnson, N. D. Kock, and G. D. Parks. 2008. Growth and antibody responses to respiratory tract infection of ferrets and mice with WT and P/V mutants of the paramyxovirus simian virus 5. Virology 376416-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choppin, P. W. 1964. Multiplication of myxovirus (SV5) with minimal cytopathic effects and without interference. Virology 23224-233. [DOI] [PubMed] [Google Scholar]
- 13.Compton, T., E. A. Kurt-Jones, K. W. Boehme, J. Bleko, E. Latz, D. T. Golenbock, and R. W. Finberg. 2003. Human cytomegalovirus activates inflammatory cytokine responses via CD14 and TLR2. J. Virol. 774588-4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cousens, L. P., R. Peterson, S. Hsu, A. Dorner, J. D. Altman, R. Ahmed, and C. A. Biron. 1999. Two roads diverged: interferon alpha/beta- and IL-12-mediated pathways in promoting T cell interferon gamma responses during viral infection. J. Exp. Med. 1891315-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Didcock, L., D. F. Young, S. Goodbourn, and R. E. Randall. 1999. The V protein of SV5 inhibits interferon signaling by targeting STAT1 for proteasome-mediated degradation. J. Virol. 739928-9933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dillon, P. J., and G. D. Parks. 2007. Role for the phosphoprotein P subunit of the paramyxovirus polymerase in limiting induction of host cell antiviral responses. J. Virol. 8111116-11127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engelmayer, J., M. Larsson, M. Subklewe, A. Chahorudi, W. I. Cox, R. M. Steinman, and N. Bhardwaj. 1999. Vaccinia virus inhibits the maturation of human dendritic cells: a novel mechanism of immune evasion. J. Immunol. 1636762-6768. [PubMed] [Google Scholar]
- 18.Fernandez-Sesma, A., S. Marukian, B. J. Ebersole, D. Kaminski, M.-S. Park, T. Yuen, S. C. Sealfon, A. Garcia-Sastre, and T. M. Moran. 2006. Influenza virus evades innate and adaptive immunity via the NS1 protein. J. Virol. 806295-6304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gainey, M. D., P. J. Dillon, K. M. Clark, M. J. Manuse, and G. D. Parks. 2008. Paramyxovirus induced shut off of translation: role of P and V proteins in limiting activation of PKR. J. Virol. 82828-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grosjean, I., C. Caux, C. Bella, I. Berger, F. Wild, J. Banchereau, and D. Kaiserlian. 1997. Measles virus infects human dendritic cells and blocks their allostimulatory properties for CD4+ T cells. J. Exp. Med. 186801-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayashi, F., K. D. Smith, A. Ozinsky, T. R. Hawn, E. C. Yi, D. R. Goodlett, J. K. Eng, S. Akira, D. M. Underhill, and A. Aderem. 2001. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 4101099-1103. [DOI] [PubMed] [Google Scholar]
- 22.He, B., R. G. Paterson, N. Stock, J. E. Durbin, R. K. Durbin, S. Goodbourn, R. E. Randall, and R. A. Lamb. 2002. Recovery of paramyxovirus simian virus 5 with a V protein lacking the conserved cysteine-rich domain: the multifunctional V protein blocks both interferon-beta induction and interferon signaling. Virology 30315-32. [DOI] [PubMed] [Google Scholar]
- 23.Honko, A. N., and S. B. Mizel. 2004. Mucosal administration of flagellin induces innate immunity in the mouse lung. Infect. Immun. 726676-6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Honko, A. N., and S. B. Mizel. 2005. Effects of flagellin on innate and adaptive immunity. Immunol. Res. 3383-102. [DOI] [PubMed] [Google Scholar]
- 25.Huleatt, J. W., V. Nakaar, P. Desai, Y. Huang, D. Hewitt, A. Jacobs, J. Tang, W. McDonald, L. Song, R. K. Evans, S. Umlauf, L. Tussey, and T. J. Powell. 2008. Potent immunogenicity and efficacy of a universal influenza vaccine candidate comprising a recombinant fusion protein linking influenza m2e to the TLR5 ligand flagellin. Vaccine 26201-214. [DOI] [PubMed] [Google Scholar]
- 26.Iwasaki, A., and R. Medzhitov. 2004. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 5987-995. [DOI] [PubMed] [Google Scholar]
- 27.Kanzler, H., F. J. Barrat, E. M. Hessel, and R. L. Coffman. 2007. Therapeutic targeting of innate immunity with toll-like receptor agonists and antagonists. Nat. Med. 13552-559. [DOI] [PubMed] [Google Scholar]
- 28.Klagge, I. M., and S. Schneider-Schaulies. 1999. Virus infections with dendritic cells. J. Gen. Virol. 80823-833. [DOI] [PubMed] [Google Scholar]
- 29.Kurt-Jones, E. A., L. Popova, L. Kwinn, L. M. Haynes, L. P. Jones, R. A. Tripp, E. E. Walsh, M. W. Freeman, D. T. Golenbock, L. J. Anderson, and R. W. Finberg. 2000. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat. Immunol. 1398-401. [DOI] [PubMed] [Google Scholar]
- 30.Lamb, R. A., and G. Parks. 2007. Paramyxoviridae: the viruses and their replication, p. 1449-1496. In B. Fields, D. Knipe, and P. Howley (ed.), Fields virology, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 31.Levi, R., and R. Arnon. 1996. Synthetic recombinant influenza vaccine induces efficient long-term immunity and cross-strain protection. 1485-92. [DOI] [PubMed] [Google Scholar]
- 32.Lopez, C. B., J. S. Yount, and T. M. Moran. 2006. Toll-like receptor-independent triggering of dendritic cell maturation by viruses. J. Virol. 803128-3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lund, J., M. L. Alexopolou, A. Sato, M. Karow, N. C. Adams, M. W. Gale, A. Iwasaki, and R. A. Flavell. 2004. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc. Natl. Acad. Sci. USA 1015598-5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McDermott, P. F., F. Ciacci-Woolwine, J. A. Snipes, and S. B. Mizel. 2000. High-affinity interaction between gram-negative flagellin and a cell surface polypeptide results in human monocyte activation. Infect. Immun. 685525-5529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Means, T. K., F. Hayashi, K. D. Smith, A. Aderem, and A. D. Luster. 2003. The Toll-like receptor 5 stimulus bacterial flagellin induces maturation and chemokine production in human dendritic cells. J. Immunol. 1705165-5175. [DOI] [PubMed] [Google Scholar]
- 36.Moutaftsi, M., A. M. Mehl, L. K. Borysiewicz, and Z. Tabi. 2002. Human cytomegalovirus inhibits maturation and impairs function of monocyte-derived dendritic cells. Blood 992913-2921. [DOI] [PubMed] [Google Scholar]
- 37.Parks, G. D., and M. A. Alexander-Miller. 2002. High avidity CTL to a foreign antigen are efficiently activated following immunization with a recombinant paramyxovirus, simian virus 5. J. Gen. Virol. 831167-1172. [DOI] [PubMed] [Google Scholar]
- 38.Reis e Sousa, C. 2001. Dendritic cells as sensors of infection. Immunity 14495. [DOI] [PubMed] [Google Scholar]
- 39.Roy, C. R., and D. S. Zamboni. 2006. Cytosolic detection of flagellin: a deadly twist. Nat. Immunol. 7:549-551. [DOI] [PubMed] [Google Scholar]
- 40.Schlender, J., V. Hornung, S. Finke, M. Gunthner-Biller, S. Marozin, K. Brzozka, S. Moghim, S. Endres. G. Hartmann, and K.-K. Conzelmann. 2005. Inhibition of Toll-like receptor 7- and 9-mediated alpha/beta interferon production in human plasmacytoid dendritic cells by respiratory syncytial virus and measles virus. J. Virol. 795507-5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sporri, R., and C. Reis e Sousa. 2005. Inflammatory mediators are insufficient for full dendritic cell activation and promote expansion of CD4+ T cell populations lacking helper function. Nat. Immunol. 6:163-170. [DOI] [PubMed] [Google Scholar]
- 42.Tompkins, S. M., Y. Lin, G. P. Leser, K. A. Kramer, D. L. Haas, E. W. Howerth, J. Xu, M. J. Kennett, R. K. Durbin, J. E. Durbin, R. Tripp, R. A. Lamb, and B. He. 2007. Recombinant parainfluenza virus 5 expressing influenza virus hemagglutinin provides immunity in mice to influenza A virus challenge. Virology 362139-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wahid, R., M. J. Cannon, and M. Chow. 2005. Dendritic cells and macrophages are productively infected by poliovirus. J. Virol. 79401-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wansley, E. K., and G. D. Parks. 2002. Naturally occurring substitutions in the P/V gene convert the noncytopathic paramyxovirus simian virus 5 into a virus that induces alpha/beta interferon synthesis and cell death. J. Virol. 7610109-10121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.West, A. P., B. A. Dancho, and S. B. Mizel. 2005. Gangliosides inhibit flagellin signaling in the absence of an effect on flagellin binding to Toll-like receptor 5. J. Biol. Chem. 2809482-9488. [DOI] [PubMed] [Google Scholar]
- 46.Young, V. A., and G. D. Parks. 2003. Simian virus 5 is a poor inducer of chemokine secretion from human lung epithelial cells: identification of viral mutants that activate IL-8 secretion by distinct mechanisms. J. Virol. 777124-7130. [DOI] [PMC free article] [PubMed] [Google Scholar]