Abstract
Human metapneumovirus (hMPV) is a recently discovered paramyxovirus that causes upper and lower respiratory tract infections in infants, the elderly, and immunocompromised individuals worldwide. Here, we developed Venezuelan equine encephalitis virus replicon particles (VRPs) encoding hMPV fusion (F) or attachment (G) glycoproteins and evaluated the immunogenicity and protective efficacy of these vaccine candidates in mice and cotton rats. VRPs encoding hMPV F protein, when administered intranasally, induced F-specific virus-neutralizing antibodies in serum and immunoglobulin A (IgA) antibodies in secretions at the respiratory mucosa. Challenge virus replication was reduced significantly in both the upper and lower respiratory tracts following intranasal hMPV challenge in these animals. However, vaccination with hMPV G protein VRPs did not induce neutralizing antibodies or protect animals from hMPV challenge. Close examination of the histopathology of the lungs of VRP-MPV F-vaccinated animals following hMPV challenge revealed no enhancement of inflammation or mucus production. Aberrant cytokine gene expression was not detected in these animals. Together, these results represent an important first step toward the use of VRPs encoding hMPV F proteins as a prophylactic vaccine for hMPV.
Human metapneumovirus (hMPV) is a paramyxovirus recently discovered in young children with respiratory tract disease (40). Subsequent studies showed that hMPV is a causative agent for both upper and lower respiratory tract infections in infants and young children (6, 15, 16, 46, 47). The spectrum of clinical illness ranges from mild upper respiratory tract disease to severe bronchiolitis and pneumonia, similar to that seen in respiratory syncytial virus (RSV) infection. Children and adults with comorbid conditions, such as those with congenital heart or lung diseases, cancer, or immunodeficiency, are at particular risk for severe respiratory disease from hMPV infection (28, 48). Epidemiology studies have suggested that hMPV infection causes lower respiratory tract disease in 5 to 15% of otherwise-healthy infants and young children (6, 16, 22, 27, 28, 47). Recurrent infection with hMPV also has been documented (14, 50). This newly identified virus represents one of the leading causes of acute viral respiratory tract infections in this population and thus represents a valid target for the development of candidate vaccines.
The fusion (F) and attachment (G) proteins are the major surface glycoproteins on hMPV, and they exhibit significant homology with the F and G proteins of RSV. Genetic analysis divides hMPV into two major subgroups (A and B) based on sequence comparison of the F and G genes in various clinical isolates (2, 4). The subgroups can be further divided into sublineages designated A1, A2, B1, and B2. The percent amino acid homology in the F protein among isolates reaches >95% and is highly conserved between the subgroups (7, 34). The G protein, however, shows significant amino acid diversification, with nucleotide sequence homology among field isolates ranging from 34 to 100%, depending on inter- and intrasubgroup comparisons (1, 4). For RSV, F and G proteins are the major antigenic targets for neutralizing antibodies. High titers of serum neutralizing antibodies are sufficient to protect the lower respiratory tract against RSV infection (11). Therefore, F and G proteins have been used singly or in combinations in various experimental RSV vaccines.
A number of experimental vaccines have been described for hMPV. These include subunit F protein vaccine (13), live-attenuated hMPV with gene deletions (5), and a chimeric, live-attenuated parainfluenza virus vaccine that incorporates the hMPV F, G, or SH gene (33, 35, 36). Although proven to be immunogenic in animal models, there are significant hurdles for some of these vaccines to be used in very young infants, the principal target population for hMPV vaccines. The presence of circulating maternal antibodies against hMPV glycoproteins and most of the candidate viral vectors, such as parainfluenza virus, is of concern and could blunt the efficacy of these vaccines in vivo. Furthermore, the ability to induce a mucosal response is desirable for successful immunization against respiratory viruses.
In this study, we developed alphavirus replicon particles (VRPs) based on Venezuelan equine encephalitis virus (VEE) that encode hMPV F or G proteins and tested their immunogenicity in mice and cotton rats. There is no data to date on immunization for hMPV with VRPs, virus-like particles, or related nonreplicating particle vaccine candidates. VEE replicon particles have several significant advantages over other viral vaccine candidates. First, there is limited preexisting immunity to VEE in the target population, making them less likely to be neutralized in vaccine recipients. Second, these replicons are potential vaccine vectors for use in very young infants, since they are encapsidated in a heterologous VEE coat that shields them from maternal hMPV-specific antibodies. Recently, these replicons were found to induce neutralizing antibody responses in young mice, regardless of the maternal immune status (45). In addition, these VEE replicon particles appear to induce novel aspects of mucosal immunity that other approaches do not. In particular, VRPs target lymph nodes, and they have systemic and mucosal adjuvant properties (38). Prior experience with VRPs has proven them to be safe for use in a variety of animals and healthy young adult human subjects (10). Human clinical trials to evaluate safety and immunogenicity have been conducted or are in the process of testing for at least four antigenic targets, including human immunodeficiency virus, cytomegalovirus, influenza virus, and carcinoembryonic antigen. In animals, these particles induce mucosal immune responses after parenteral inoculation and confer protection to the primary mucosal target tissue (38).
Here, we demonstrate that VRPs encoding subgroup A MPV F protein induce both systemic and mucosal humoral responses. High-titer neutralizing antibodies against subgroup A1 and A2 viruses were induced in vaccinated animals; however, these antibodies were not effective in neutralizing subgroup B viruses in vitro. When vaccinated animals were challenged with an hMPV subgroup A2 strain intranasally, virus replication was reduced significantly in both the lungs and nasal turbinates. Histopathology showed no enhanced inflammation or mucus production in vaccinated mice compared to animals that received live hMPV vaccine. In contrast, animals vaccinated with VRPs encoding MPV G did not generate neutralizing antibodies and were not protected against hMPV live virus challenge. These findings provide proof-of-principle that VEE VRPs expressing the MPV F protein can be used in hMPV prophylaxis.
MATERIALS AND METHODS
Animals and cell lines.
Five- to 6-week-old DBA/2 mice and cotton rats were purchased from Harlan (Indianapolis, IN) and Virion Systems (Rockville, MD), respectively. Animals were housed in microisolator cages throughout the study. All experimental procedures performed were approved by the Institutional Animal Care and Use Committee at Vanderbilt University Medical Center.
LLC-MK2 cells were obtained from ATCC (CCL-7) and maintained in OptiMEM I medium (Invitrogen) supplemented with 2% fetal bovine serum, 4 mM l-glutamine, 5 μg/ml amphotericin B, and 50 μg/ml gentamicin sulfate at 37°C with 5% CO2. BHK-21 cells were obtained from ATCC (CCL-10) and maintained in Eagle's minimum essential medium supplemented with 10% fetal bovine serum, 4 mM l-glutamine, 5 μg/ml amphotericin B, and 50 μg/ml gentamicin sulfate at 37°C with 5% CO2.
VEE constructs and generation of VRPs encoding hMPV F or G genes.
The method of construction and packaging of VRPs was described previously (32). Briefly, the hMPV F or G protein-encoding DNA sequences from the subgroup A2 hMPV wild-type strain TN/94-49 were inserted behind the 26S subgenomic promoter in a VEE replicon plasmid, pVR21. pVR21 was derived from mutagenesis of a cDNA clone of the Trinidad donkey strain of VEE.
For generation of VRPs, capped RNA transcripts of the pVR21 plasmid containing hMPV F or G genes were generated in vitro with the mMESSAGE mMACHINE T7 kit (Ambion, Austin, TX). Similarly, helper transcripts that encoded the VEE virus capsid and glycoprotein genes derived from the attenuated recombinant V3014 strain were generated in vitro. BHK-21 cells then were cotransfected by electroporation with the pVR21 and helper RNAs, and culture supernatants were harvested at 30 h after transfection. The generation of VRPs expressing the F protein of the related virus RSV (used in the present studies as a heterologous virus control) was previously described (24).
VRP titration.
Serial dilutions of VRPs encoding hMPV F (designated VRP-MPV.F) or hMPV G (designated VRP-MPV.G) were used to infect BHK cells in eight-chamber slides (Nunc) for 20 h at 37°C. Infected BHK cells were fixed and immunostained for VEE nonstructural proteins. Infectious units then were calculated from the number of VEE protein-stained cells per dilution and converted to infectious units (IU) per milliliter.
Formalin-inactivated hMPV (FI-hMPV) preparation.
Sucrose gradient-purified hMPV strain A2 (TN 94-49) was prepared as previously described (49). Purified hMPV was inactivated by the addition of 37% formaldehyde solution (one part Formalin per thousand parts hMPV) for 72 h at 37°C. The solution then was centrifuged at 50,000 × g for 1 hour at 4°C. The resulting pellet was then resuspended 1:25 in serum-free OptiMEM and precipitated with aluminum hydroxide (4 mg/ml) for 30 min. The precipitate was collected by centrifugation for 30 min at 1,000 × g, resuspended 1:4 in serum-free OptiMEM, and stored at 4°C (44).
Immunofluorescence staining.
BHK cells were infected at a multiplicity of infection (MOI) of 5 with VRP-MPV.F or VRP-MPV.G in eight-chamber slides (Nunc) for 18 h at 37°C. Infected BHK cells were fixed in 80% methanol for 1 hour at 4°C. The cells then were blocked with phosphate-buffered saline (PBS)-3% bovine serum albumin (BSA) for 2 hours at room temperature. Monoclonal antibody against hMPV F or hMPV polyclonal guinea pig serum (1:1,000 dilution in PBS-1% BSA) was added and allowed to incubate for 1 hour at room temperature. Cells were washed extensively with Tris-buffered saline-0.5% Tween 20 (TBST) after incubation with primary antibodies, and secondary goat anti-mouse or goat anti-guinea pig AlexaFluor C568-conjugated antibodies were added (1:1,000 dilution in TBST-1% BSA) to the cells for an additional hour. The slide then was washed with TBST and mounted with Prolong antifade medium (Invitrogen, Carlsbad, CA). The slide was visualized using an LSM510 inverted laser scanning confocal microscope (Carl Zeiss Microimaging, Thornwood, NY).
Vaccination and challenge of mice or cotton rats.
DBA/2 mice were anesthetized with isoflurane and vaccinated intranasally with various titers of VRP-MPV.F or VRP-MPV.G in a 100-μl inoculum. Control groups were inoculated via the same route with PBS, 105.9 PFU of hMPV subgroup A2 wild-type strain TN/94-49, or 106 infectious units of VRPs encoding the RSV F gene (VRP-RSV.F). Mice that were vaccinated with VRPs were boosted with the same dose 2 weeks later. For histopathology and cytokine gene expression studies, a subgroup of animals was vaccinated once with 50 μl of FI-hMPV in each hind leg intramuscularly. The mice then were observed for clinical signs daily and bled on day 42 to follow immune responses.
Twenty-eight days after the second immunization (day 42), mice from VRP-MPV.F- and VRP-MPV.G-vaccinated groups and mice from the control groups were challenged with 105.9 PFU of the hMPV subgroup A2 strain TN/94-49 or subgroup B1 strain TN/98-242 intranasally. To monitor virus replication in the upper and lower respiratory tracts, nasal turbinates and lungs were harvested on day 4 postchallenge and subsequently assayed for virus titer. Similarly, cotton rats were vaccinated on day 0 and day 14 with 106 IU of VRP-MPV.F or VRP-MPV.G intranasally in groups of four. Control groups were inoculated intranasally with PBS, 105.9 PFU of hMPV TN/94-49, or 106 IU of VRP-RSV.F. They then were bled on day 35 to monitor immune responses, were challenged with 105.9 PFU of hMPV TN/94-49 on day 42, and were sacrificed on day 46. Lung and nasal turbinates were harvested separately and homogenized to determine viral titers.
BAL fluid and nasal wash collection.
A subset of animals was sacrificed on day 42 (28 days after the second immunization) to collect bronchoalveolar lavage (BAL) fluid and nasal wash fluid. BAL fluids were collected by ligation of the trachea with a suture and insertion of a 23-gauge blunt needle into the distal trachea, followed by three in-and-out flushes of the airways with 3 ml of sterile PBS. Nasal washes were obtained by flushing 3 ml PBS through the upper trachea and out the nasal orifice into a sterile receptacle. Both BAL and nasal washes were concentrated 10-fold using 10-kDa molecular weight cutoff Centricon concentrators (Millipore, Bedford, MA).
F protein- and G protein-specific antibody assay.
Sera collected at day 42 from DBA/2 mice were tested for the presence of F or G protein-specific antibodies. Concentrated nasal washes and BAL fluids also were tested. Briefly, 150 ng/well of purified hMPV F protein or hMPV G protein was adsorbed onto Immulon 2B plates overnight in carbonate buffer (pH 9.8) at 4°C. Recombinant F protein was generated as described previously (13), and recombinant G protein was produced by similar methods (A. B. Ryder, A. B. Podsiad, S. J. Tollefson, and J. V. Williams, unpublished data). The plates then were blocked with 3% BSA in PBS for 2 h at room temperature. After thorough washing with TBST-1% BSA, serial dilutions of serum, nasal wash, or BAL fluid samples were added to the plate and allowed to incubate for 1 hour at room temperature. The plates were washed again, and horseradish peroxidase (HRP)-conjugated anti-mouse immunoglobulin A (IgA; 1:500 dilution) or IgG (1:5,000 dilution) antibodies were added (Southern Biotechnology, Birmingham, AL) and allowed to incubate for another hour. Finally, the plates were washed and 100 μl of One-Step Turbo TMB peroxidase substrate (Pierce, Rockford, IL) was added per well to quantify the relative amounts of F-specific or G-specific IgA or IgG in the samples. The reactions then were stopped by adding 50 μl of 1 M HCl and the absorbance of the samples was read at 450 nm. The enzyme-linked immunosorbent assay (ELISA) titers were expressed as the reciprocal titer of serum in which the absorbance was twice the background absorbance. Background absorbance was determined from the average optical density at 450 nm in PBS-incubated control wells.
Virus-neutralizing antibody assay.
Sera collected were used to study the presence of hMPV-neutralizing antibodies as previously described (49). Serum samples were tested for neutralizing activity against subgroup A1 strain TN/96-12, subgroup A2 strain TN/94-49, subgroup B1 strain TN/98-242, and subgroup B2 strain TN/99-419 of hMPV. Briefly, a viral suspension that was standardized to yield 50 plaques per well in a 24-well plate was used. An aliquot of the hMPV suspension was incubated with serial dilutions of the serum samples. After an hour, the suspension was absorbed onto LLC-MK2 cells and then overlaid an hour later with a semisolid methylcellulose overlay containing 5 μg/ml of trypsin. After 4 days, the cell culture monolayers were fixed and stained by immunoperoxidase using hMPV-specific polyclonal guinea pig serum to identify plaques. Plaques were counted, and plaque reduction was calculated by regression analysis to provide a 60% plaque reduction titer.
Viral plaque titer assay.
Serial dilutions of nasal turbinate or lung homogenates were inoculated onto LLC-MK2 cell monolayer cultures, and plaque assays were performed as described above. The viral titer was determined by multiplying the number of plaques by the reciprocal sample dilution, divided by tissue weights, and expressed as PFU per g of tissue.
Lung histopathology studies.
Four days after hMPV challenge, mice were euthanized via CO2 inhalation and lungs were harvested. To preserve the structural integrity of the lungs, 1 ml of 10% neutral buffered formalin was instilled into the lungs via tracheotomy, followed by ligation of the trachea with sutures. The whole lung then was immersed in 10% neutral buffered formalin overnight. After fixation, the lungs were dehydrated by immersing in 70% ethanol for another day. The lungs then were embedded in paraffin, sectioned, and stained with hematoxylin-eosin solution. The severity of airway inflammation was evaluated separately for the alveolar tissue, peribronchial tissue, and perivascular spaces in a group-blind fashion. The degree of inflammation in the alveolar tissue was graded as follows: 0, normal; 1, increased thickness of the interalveolar septa by edema and cell infiltration; 2, luminal cell infiltration; 3, abundant cell infiltration; 4, inflammatory patches evident. The degree of inflammation in the peribronchial and perivascular spaces was graded as follows: 0, no infiltrate; 1, slight cell infiltration; 2, moderate cell infiltration; 3, abundant cell infiltration. In each tissue section, 10 alveolar tissue fields, 10 airways, and 10 blood vessels were analyzed using 200× magnification. Mean scores were calculated for each mouse, and an average score was reported for each animal group.
Cytokine gene expression in the lungs after hMPV challenge.
Lungs from unvaccinated and vaccinated mice were harvested 4 days after hMPV challenge and placed in RNAlater solution (Ambion, Austin, TX) until further analysis. Lungs were homogenized using the Omni-tip PCR kit (Omni International, Marietta, GA), and RNA was extracted using the RNeasy Mini kit (Qiagen, Valencia, CA) according to the manufacturer's protocol. Primers and probes for real-time quantitative PCR were purchased from Applied Biosystems (Foster City, CA) to measure Th1 or Th2 cytokine transcript levels based on GenBank sequences for murine glyceraldehyde-3-phosphate dehydrogenase, gamma interferon (IFN-γ), and interleukin-2 (IL-2), IL-4, IL-5, IL-10, and IL-12. Probes were labeled at the 5′ end with 6-carboxyfluorescein and at the 3′ end with the nonfluorescent quencher Blackhole Quencher 1 (Operon Biotechnologies, Huntsville, AL). Reverse-transcribed real-time PCR was performed using a Quantitect probe RT-PCR kit (Qiagen, Valencia, CA) and a SmartCycler II (Cepheid, Sunnyvale, CA) using 1 μg of extracted mRNA. The parameters used were 1 cycle of 50°C for 2 min, 1 cycle of 95°C for 10 min, and 40 cycles of 95°C for 15 s and 60°C for 1 min. Reactions were performed in triplicate, with a no-template sample used as a negative control. Relative amounts of cytokine gene transcripts expressed were normalized to those of the glyceraldehyde-3-phosphate dehydrogenase housekeeping gene, and uninfected mice were used as baseline controls. Differences in mRNA levels were computed using the ΔΔCt method and compared to values for uninfected mice.
Statistics.
Prism software was used to plot and analyze the data (Graphpad Software Inc., San Diego, CA). All data were expressed as geometric means and their standard deviations. Experimental groups were compared using Mann-Whitney rank sum tests.
RESULTS
Cloning and expression of hMPV antigens using VRPs.
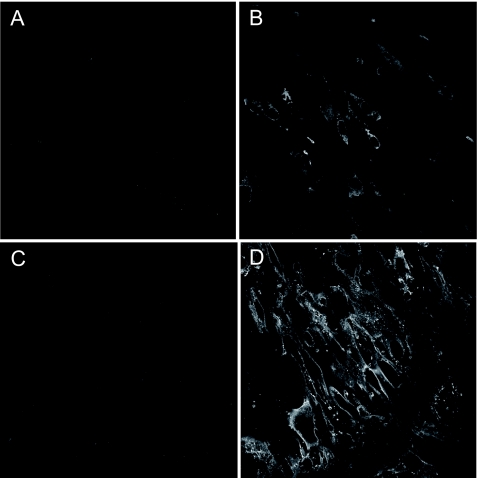
hMPV fusion (MPV.F) and attachment (MPV.G) genes were cloned into the VEE replicon vector as previously described (32). VRPs then were produced in BHK cells by cotransfecting RNA transcribed in vitro from the replicon vector with transcripts of two separate plasmids encoding VEE capsid and envelope proteins in trans. To ensure these replicons expressed the desired antigens, BHK cells were infected at an MOI of 5 with VRPs. Antigen expression then was measured by immunostaining infected cells with guinea pig polyclonal hMPV-specific antibodies. A robust amount of hMPV F or G protein was expressed, as evidenced by the intense staining of infected BHK cells with hMPV-specific antibodies (Fig. 1B and D) compared to uninfected cells (Fig. 1A and C). Examination of infected cells by confocal microscopy showed a Golgi apparatus and membrane-bound expression pattern for hMPV F protein, while staining of cells infected with MPV.G VRPs showed a membrane-bound pattern. Western blot assays also were used to confirm the presence of hMPV F or G protein expression in BHK-infected cell lysates (data not shown).
FIG. 1.
Expression of hMPV proteins from VRP-infected BHK cells. BHK cells were either mock infected (A and C), infected at an MOI of 5 with VRP-MPV.F (B), or infected at an MOI of 5 with VRP-MPV.G (D). Cells then were fixed after 18 h and immunostained for hMPV F (A and B) or hMPV G (C and D) protein expression using guinea pig polyclonal anti-hMPV antibodies.
Systemic IgG and mucosal IgA responses in VRP-vaccinated mice.
To assess if VRPs induced systemic humoral immune responses, we measured the reciprocal endpoint titers of hMPV F- or G-specific IgG antibodies in the serum of vaccinated mice by ELISA. Intranasal inoculation of hMPV F-VRPs induced significantly higher titers of hMPV F-specific IgG in the sera of vaccinated mice (about eightfold higher in both the 106 and 105 IU groups) than in unvaccinated animals. These animals possessed twofold-higher antibody titers compared to mice infected once with hMPV, a difference that did not reach statistical significance (P = 0.22). Similarly, mice that were vaccinated with VRP-MPV.G showed robust levels of hMPV G-specific IgG in serum (298-fold and 20-fold higher in 106 and 105 IU groups, respectively) compared to unvaccinated control animals (Table 1).
TABLE 1.
Serum antibody responses against hMPV F and G proteins in immunized DBA/2 mice
| Immunization | Dose (log10 IU or PFU) | Serum reciprocal endpoint ELISA titer (mean log2 titer ± SD) againsta:
|
|
|---|---|---|---|
| hMPV-F | hMPV-G | ||
| PBS | 9.6 ± 0.5 | 4.4 ± 0.2 | |
| VRP-RSV.F | 6 | 9.8 ± 0.5 | ≤4.3 |
| VRP-MPV.F | 6 | 12.9 ± 1.5** | 4.6 ± 0.6 |
| 5 | 12.8 ± 1.7* | ND | |
| 4 | 10.4 ± 0.8 | ND | |
| VRP-MPV.G | 6 | 9.8 ± 0.4 | 12.3 ± 1.1** |
| 5 | ND | 8.7 ± 1.5** | |
| 4 | ND | 7.3 ± 2.1 | |
| hMPV | 5.9 | 11.8 ± 1.0** | 5.0 ± 1.5 |
Statistical significance of the serum reciprocal endpoint ELISA titer compared to the PBS-vaccinated group. *, P < 0.05; **, P < 0.01. ND, not determined.
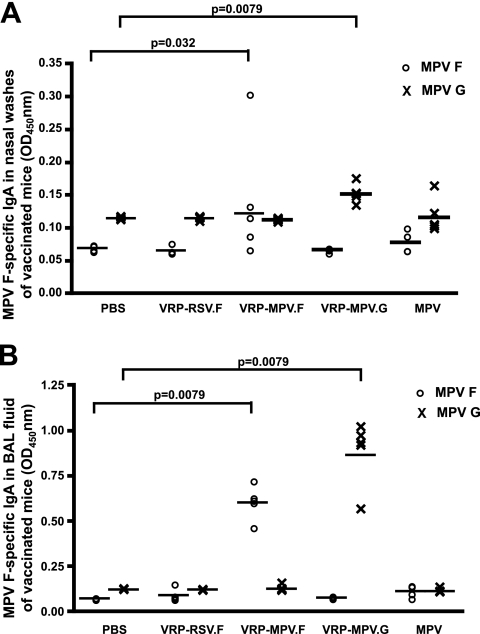
Mucosal hMPV F-specific or G-specific IgA antibodies also were detected in the nasal washes and BAL fluids of VRP-MPV.F- or VRP-MPV.G-vaccinated mice, respectively, which represent immunity in the upper or lower respiratory tracts of vaccinated animals (Fig. 2A and B). Significantly higher titers of hMPV F-specific or hMPV G-specific antibodies were observed in the BAL fluids of VRP-MPV.F- or VRP-MPV.G-vaccinated mice compared to hMPV-infected mice (P = 0.008), possibly due to repeated exposure to antigens during priming and boosting of the VRP-vaccinated animals. Alternatively, the higher anti-F and anti-G BAL antibody titers could be due to presentation of the viral antigens from a different target cell in the case of VRP vaccination.
FIG. 2.
VRP-MPV.F induced hMPV-F- or hMPV-G-specific antibodies in the mucosal secretions of VRP-vaccinated mice. DBA/2 mice were vaccinated intranasally with 106 infectious units of VRP-MPV.F or VRP-MPV.G on days 0 and 14. Nasal washes (A) or BAL fluids (B) were obtained from vaccinated mice 28 days postvaccination. An MPV-F- or MPV-G-specific enzyme-linked immunosorbent assay was performed on the samples with HRP-conjugated anti-mouse IgA antibodies. The amount of binding was determined from absorbance (optical density [OD]) of HRP-substrate complexes at 450 nm.
Neutralizing activity of antibodies in the sera of VRP-vaccinated animals.
The presence of circulating neutralizing antibodies is an important parameter that has been implicated in protecting the lower respiratory tract against respiratory viral infection, including against hMPV. Therefore, we measured neutralizing activity in the sera from VRP-vaccinated mice or cotton rats against subgroup A or B hMPV strains in a 60% plaque reduction assay. Mice vaccinated with PBS or VRP expressing RSV F protein, used as a heterologous virus control, did not generate any detectable neutralizing titer against either subgroup A or B hMPV strains. Intranasal vaccination with VRP-MPV.F induced at least a 2.3-log2 (5-fold) or 1.8-log2 (3.5-fold) increase in serum neutralizing antibody titer against the A2 or A1 subgroup of hMPV compared to PBS-vaccinated mice (Table 2). Neutralizing activity against subgroup A2 strain MPV was higher than against the subgroup A1 strain in these animals. When these sera were tested against subgroup B hMPV in our 60% plaque reduction assay in vitro, all sera tested had minimal neutralizing activity toward subgroup B hMPV. There was some neutralizing activity at the lowest serum dilution 1:20 which, however, did not reach our 60% plaque reduction criterion in two separate experiments (Table 2). Surprisingly, infection with subgroup A2 hMPV did not induce serum antibodies that could neutralize subgroup B viruses in vitro. Neutralizing titers also were not detected in mice vaccinated with VRP-MPV.G, despite the presence of hMPV G-specific IgG in these animals (Table 1). Mice that were infected with a subgroup A2 strain of hMPV had a neutralizing titer of 7.7 log2 and 6.3 log2 against subgroup A2 and A1 strains of hMPV, respectively, but very little neutralizing activity against subgroup B hMPV.
TABLE 2.
Serum neutralizing antibody responses against various hMPV strains in immunized DBA/2 mice or cotton rats
| Immunization | Dose (log10 IU or PFU) | 60% plaque reduction serum neutralizing titer (mean log2 titer ± SD) against hMPVa in:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| DBA/2 mice
|
Cotton rats
|
||||||||
| A1 | A2 | B1 | B2 | A1 | A2 | B1 | B2 | ||
| PBS | ≤4.3b | ≤4.3 | ≤4.3 | ≤4.3 | ≤4.3 | ≤4.3 | ≤4.3 | ≤4.3 | |
| VRP-RSV.F | 6.0 | ≤4.3 | ≤4.3 | ≤4.3 | ≤4.3 | ≤4.3 | ≤4.3 | ≤4.3 | ≤4.3 |
| VRP-MPV.F | 6.0 | 6.1 ± 1.7 | 6.6 ± 1.9 | ≤4.3 | ≤4.3 | 5.7 ± 1.2 | 6.7 ± 2.3 | ≤4.3 | ≤4.3 |
| VRP-MPV.G | 6.0 | ≤4.3 | ≤4.3 | ≤4.3 | ≤4.3 | ≤4.3 | ≤4.3 | ≤4.3 | ≤4.3 |
| MPV A2 | 5.9 | 6.3 ± 1.2 | 7.7 ± 1.3 | ≤4.3 | ≤4.3 | 6.0 ± 0.6 | 9.6 ± 0.9 | ≤4.3 | ≤4.3 |
The hMPV subgroup A1 strain was TN/96-12, the subgroup A2 strain was TN/94-49, the subgroup B1 strain was TN/98-242, and the subgroup B2 strain was TN/99-419.
The lower limit of detection was 4.3 log2 for the hMPV neutralization titer.
In cotton rats, a similar trend was observed for neutralizing activity against subgroup A hMPV. Intranasal vaccination with 106 IU of VRP-MPV.F induced reciprocal neutralizing titers of 6.7 log2 and 5.7 log2 against subgroup A2 and A1 strains of hMPV, compared to 9.6 log2 and 6.0 log2 from hMPV-infected animals (Table 2). The neutralization responses were higher in cotton rats than mice when immunized with hMPV, likely because the cotton rat is a more permissive model for hMPV infection, as evidenced by the higher viral titers in the nasal turbinates of these animals (Table 3).
TABLE 3.
hMPV titers in lungs or nasal turbinates of immunized DBA/2 mice or cotton rats following wild-type subgroup A2 or B1 hMPV challenge
| Immunization | MPV titer following challenge (mean log10 PFU/g tissue ± SD)
|
|||||
|---|---|---|---|---|---|---|
| DBA/2 (A2)a
|
DBA/2 (B1)a
|
Cotton rats (A2)a
|
||||
| Lungs | Nasal turbinates | Lungs | Nasal turbinates | Lungs | Nasal turbinates | |
| PBS | 3.9 ± 0.4 | 3.5 ± 0.2 | 3.5 ± 0.3 | 3.5 ± 0.3 | 3.4 ± 0.8 | 4.5 ± 0.4 |
| VRP-RSV.F | 3.4 ± 0.2 | 3.4 ± 0.1 | 3.3 ± 0.5 | 3.8 ± 0.2 | 4.2 ± 0.0 | 4.5 ± 0.6 |
| VRP-MPV.F | ≤1.7b | 2.5 ± 0.5d | ≤1.7b | 3.0 ± 0.3 | ≤1.5c | 2.2 ± 0.5f |
| VRP-MPV.G | 3.0 ± 0.7 | 3.0 ± 0.3 | 3.6 ± 0.2 | 3.4 ± 0.4 | 3.5 ± 0.3 | 4.6 ± 0.3 |
| MPV A2 | ≤1.7b | ≤2.0b | ≤1.7b | 2.2 ± 0.3e | ≤1.5c | ≤2.0c |
The designation in parentheses indicates the subgroup of hMPV used for challenge.
The lower limit of detection was 1.7 log10 or 2.0 log10 for the lungs or nasal turbinates of DBA/2 mice, respectively.
The lower limit of detection was 1.5 log10 or 2.0 log10 for the lungs or nasal turbinates of cotton rats, respectively.
Two of five mice had an undetectable hMPV A2 titer in the nasal turbinates.
Two of five mice had an undetectable hMPV B1 titer in the nasal turbinates.
Three of four cotton rats had an undetectable hMPV A2 titer in the nasal turbinates.
Viral titers in lungs and nasal turbinates after challenge in vaccinated animals.
To assess the protective efficacy of VRP vaccines in vivo, we measured hMPV titers in the lungs and nasal turbinates of mice and cotton rats following intranasal hMPV subgroup A2 challenge. Mice or cotton rats vaccinated with VRP-MPV.F had no detectable challenge hMPV titers in the lungs (at least a 2.2-log10 [158-fold] or 1.9-log10 [79-fold] reduction in mice or cotton rats, respectively). Reduced amounts of hMPV also were observed in the nasal turbinates of VRP-MPV.F-vaccinated animals (1.0-log10- [10-fold] or 2.3-log10 [200-fold] reduction in mice or cotton rats, respectively). Previous infection with hMPV subgroup A2 induced immunity resulting in a reduction of hMPV challenge titers to undetectable levels in both the upper and lower respiratory tracts. In contrast, mice or cotton rats vaccinated with VRP-MPV.G were not protected from hMPV challenge in either the lungs or nasal turbinates (Table 3), which is consistent with the lack of serum neutralizing antibodies we observed. In addition, we challenged our vaccinated mice with a subgroup B1 strain hMPV. In the lungs of VRP-MPV.F-vaccinated mice, viral titers were reduced 1.8 log10 (63-fold) compared with the PBS-vaccinated group. This surprising reduction was possibly due to the presence of a low level of neutralizing antibodies in these animals. In a semipermissive mouse model, a low amount of neutralizing antibodies may be sufficient to reduce hMPV replication in the lower respiratory tract. In animals previously infected with an MPV subgroup A2 strain, we observed a similar magnitude of viral titer reduction in the lungs when animals were challenged with a subgroup B1 strain virus.
Histopathology of lungs after challenge in vaccinated animals.
We evaluated the extent of cellular infiltrates in the perivascular, peribronchial, and alveolar spaces in the lungs of mice vaccinated with VRPs and then challenged with wild-type hMPV. In animals that received mock PBS vaccination, a minimal amount of infiltration was observed 4 days post-hMPV infection. In animals that were previously infected with hMPV, reinfection of mice with hMPV caused a dramatic increase in cellular infiltrates in the perivascular, peribronchial, and alveolar spaces of the lungs. There was also a moderate increase in mononuclear infiltrates in the alveolar, peribronchial, and perivascular spaces of animals that received VRP-MPV.F or VRP-MPV.G when challenged with wild-type hMPV. The histopathology scores were comparable and not statistically different between animals that were vaccinated with VRP-MPV.F and those previously infected with hMPV when both groups were challenged with wild-type hMPV, although mice vaccinated with VRP-MPV.F did show a trend of decreased severity of inflammation in the peribronchial and perivascular tissues upon challenge. In contrast, animals that were vaccinated with a single dose of formalin-inactivated hMPV and challenged with wild-type virus exhibited extensive cell infiltrations in the perivascular, peribronchial, and alveolar spaces, which were evidenced by the increased histopathology scores compared to other vaccination groups (Table 4). This phenomenon is consistent with previous findings (51).
TABLE 4.
Histopathology scores of lung tissues in vaccinated mice 4 days after wild-type MPV challengea
| Immunization | Histopathology score
|
||
|---|---|---|---|
| Alveolar | Peribronchial | Perivascular | |
| PBS | 0.4 ± 0.4 | 0.1 ± 0.1 | 0.2 ± 0.1 |
| MPV | 0.8 ± 0.2 | 0.9 ± 0.2 | 1.2 ± 0.1 |
| VRP-MPV.F | 1.0 ± 0.3 | 0.6 ± 0.2 | 0.7 ± 0.3 |
| VRP-MPV.G | 0.8 ± 0.5 | 0.3 ± 0.1 | 0.4 ± 0.2 |
| VRP-RSV.F | 0.7 ± 0.2 | 0.5 ± 0.4 | 0.4 ± 0.3 |
| FI-MPV | 1.4 ± 0.2 | 1.1 ± 0.2 | 1.8 ± 0.5 |
Lung sections were viewed and scored by a pathologist in a group-blind fashion. Scores ranged from 0 (normal) to 3 or 4 (severe), as described in Materials and Methods.
Cytokine mRNA expression in lungs of vaccinated mice after challenge.
Aberrant cytokine responses and enhanced disease after subsequent natural exposure have been observed in animals or humans vaccinated with certain nonreplicating paramyxovirus vaccines. Recently, formalin-inactivated hMPV has been shown to induce a Th2-biased cytokine response and aggravated disease in experimental animals (51). We measured cytokine mRNA levels in the lungs of VRP-vaccinated mice after hMPV challenge to investigate if VRP vaccines would cause such biased responses. For each of the cytokine mRNAs tested, hMPV-infected mice had increased lung cytokine mRNA levels over uninfected controls. The mRNA expression levels of IFN-γ, IL-4, IL-10, IL-12p40, and IL-13 were not statistically different between groups, with two exceptions. There was a 2.6-fold reduction of IFN-γ gene expression in the lungs of VRP-MPV.F-vaccinated mice compared to PBS controls and a 2.1-fold increase in IL-10 gene expression in the lungs of VRP-MPV.G-vaccinated mice compared to PBS controls. As predicted, in formalin-inactivated hMPV-vaccinated animals, there was a statistically significant decrease in IFN-γ and IL-12p40 mRNA and a statistically significant increase in IL-13 compared to PBS controls (Table 5).
TABLE 5.
Cytokine mRNA expression in lungs of immunized DBA/2 mice following wild-type subgroup A2 hMPV challenge
| Immunization | Mean fold difference in cytokine gene expression vs uninfected controls (range)a
|
||||
|---|---|---|---|---|---|
| IFN-γ | IL-4 | IL-10 | IL-12 p40 | IL-13 | |
| PBS | 8.3 (4.6-17.9) | 2.2 (1.3-5.1) | 3.7 (2.6-7.0) | 9.3 (6.2-14.2) | 15.3 (7.4-46.6) |
| VRP-RSV.F | 5.4 (4.1-8.6) | 1.8 (1.4-3.2) | 3.9 (2.5-5.2) | 9.7 (3.7-16.9) | 11.6 (4.2-24.0) |
| VRP-MPV.F | 3.2 (2.2-4.9)* | 2.2 (1.1-3.7) | 4.4 (2.1-6.7) | 15.5 (10.9-23.9) | 10.8 (6.7-14.5) |
| VRP-MPV.G | 10.8 (6.3-19.5) | 1.9 (1.1-2.9) | 7.8 (4.7-10.0)* | 12.3 (6.3-19.8) | 15.8 (6.3-24.5) |
| MPV | 8.3 (4.0-10.5) | 2.1 (1.4-3.6) | 4.9 (2.3-9.3) | 14.8 (9.0-21.9) | 6.0 (3.3-13.9) |
| FI-MPV | 3.0 (2.1-6.8)* | 4.0 (2.1-8.1) | 2.9 (1.5-7.4) | 4.7 (2.3-7.5)* | 82.7 (27-208)* |
Values in parentheses indicate the range of fold differences between five mice in each group. *, statistically significant group difference compared to PBS-vaccinated group (P < 0.05; Mann-Whitney test).
DISCUSSION
In this study, we have described the molecular construction of a new experimental vaccine and approach to hMPV vaccination and provided a comprehensive preclinical evaluation of this new vaccine candidate. The studies provide a thorough investigation of immunogenicity (binding and neutralizing antibodies and mucosal and systemic responses), wild-type challenge with homologous or separate clade and separate subgroup viruses, cross-neutralization studies of all four clades, histopathology, cytokine profiling of the response, and data in two animal species. We demonstrated that VEE replicon particles encoding hMPV F protein were immunogenic in mice and cotton rats when delivered intranasally. The extent of responses was comparable to those elicited by wild-type hMPV infection.
Robust hMPV protein expression by VRPs was confirmed by immunostaining of infected BHK cells with polyclonal hMPV antisera. When these VRPs were inoculated into mice and cotton rat intranasally, they elicited significant levels of hMPV-specific IgA antibodies in both the upper and lower respiratory tracts. Local virus-specific IgA secretion on the mucosal surfaces has been shown to be associated with protection of individuals from respiratory virus infections (17, 19, 26). Moreover, we detected systemic IgG antibodies against F or G antibodies in vaccinated animals. hMPV F-specific antibodies also possessed neutralizing activity against hMPV. The cross-neutralizing activities of sera from VRP-vaccinated animals against four different strains of the hMPV representing the four major genotypes were variable. Since our hMPV F vaccines were constructed based on the nucleotide sequence obtained from an hMPV subgroup A2 clinical isolate, neutralizing activity toward the homologous subgroup A2 strain was the highest. VRP vaccination induced a significant, but lower, neutralizing antibody titer toward an hMPV subgroup A1 strain. It was surprising that serum from VRP-vaccinated animals neutralized hMPV subgroup B viruses only somewhat at the lowest dilution (1:20), given that the homology of the F gene between the subgroups is >95%. This result is different from some of the published data, in which serum cross-neutralization and cross-protection were found when animals were vaccinated with one hMPV subgroup and challenged with another hMPV subgroup (21, 33, 35, 41). We reasoned that the difference in hMPV F sequences between the subgroups, although small, may contribute to conformational differences in structure that are important for neutralization. This lack of cross-neutralization deserves further study.
Also surprising was the finding that the presence of elevated titers of hMPV G-specific antibodies in vaccinated animals did not neutralize hMPV. Unlike RSV, the G protein did not seem to be a neutralizing antigen for hMPV in these studies and did not contribute to protection against challenge. This finding is not unique to VRP vaccination. The lack of neutralizing antibody induction by hMPV G was demonstrated recently by our group using purified hMPV G protein as an immunogen in cotton rats (Ryder et al., unpublished) and by another group using a recombinant parainfluenza virus vector to deliver hMPV G protein in hamsters (33). The role of hMPV G protein in viral pathogenesis is still not defined, although it is presumed to be an attachment protein, possibly with immuno-modulation properties based on its homology with the RSV G protein (8, 30, 39).
When mice or cotton rats vaccinated with VRPs encoding the hMPV F gene were challenged with wild-type hMPV A2, the challenge virus replication was reduced to lower than detectable levels in the lungs. The reduction correlated well with the level of hMPV serum neutralizing antibody in the animals. This finding is similar to that seen in RSV; a RSV serum neutralizing titer of >1:380 protected cotton rats from RSV infection (31). The challenge hMPV titer in the nasal turbinates, however, was not completely reduced in some animals. VRP-MPV.F-vaccinated animals did have significantly reduced titers in the nasal turbinates, possibly due to the presence of mucosal hMPV-specific IgA antibodies. The incomplete protection of the nose could be due to several factors. One is that the hMPV-specific IgA level induced in the nasal turbinates appeared to be at a lower level than in the lungs. In the lungs, both hMPV-specific IgA in the BAL fluids and serum Ig antibodies contribute to protection, while in the nasal turbinates, hMPV-specific IgA was solely responsible for protection. This has been demonstrated with Sendai virus in mice (25). Second, cellular immune responses may be important in reducing viral replication in the nasal turbinates. In the RSV animal model, both RSV-specific CD4+ and CD8+ cells were found to be important in conferring protection to animals against RSV challenge (9, 12, 29). Therefore, cellular immunity also may contribute partly to protection in the upper respiratory tract. However, in our experience, significant levels of activated T cells specific for the hMPV F protein are not detected in DBA/2 animals (data not shown). Several groups also have found limited cytotoxic T-cell responses against the hMPV F protein in mice. T-cell epitopes were found to be restricted exclusively to the M2-1 protein (23) and M2-2 protein in H-2d major histocompatibility complex class I (MHC-I) alleles and N protein in H-2b MHC-I alleles (18). It is, however, likely that a cellular response against hMPV F epitopes would be found in humans, because of the diverse MHC alleles. Another surprising finding is that VRP-MPV.F-vaccinated mice did not exhibit a significant neutralizing antibody titer against subgroup B hMPV, yet they were still protected in the lungs when challenged with a subgroup B1 hMPV. These mice may have produced low levels of neutralizing antibodies that could not be detected. In a semipermissive model, such an immune response may be sufficient to restrict hMPV B1 replication in vivo; however, this level of immunogenicity is unlikely to be protective in humans. Based on amino acid homology, we expected that the antigen of one clade in the A subgroup (A2) would induce cross-reacting antibodies and protection against an A subgroup virus in the other clade (A1), and this was found to be the case. It was surprising that our VEE replicon vaccine encoding a subgroup A virus F protein was not able to induce complete protection in mice against a subgroup B hMPV challenge, given the F gene sequence similarities between viruses of these subgroups. Possibly, immunization with F proteins from both subgroups is needed to generate a broadly effective vaccine for hMPV. In addition, when mice were immunized with subgroup A2 hMPV and challenged with subgroup B1 virus, we observed a similar pattern of protection against B1 hMPV. This intrinsic lack of cross-neutralization of serum antibodies for subgroup B clinical isolates in animals infected with subgroup A antigens in experimental animals deserves further study. The extent of cross-protection induced by infection with hMPV A or hMPV B is still under debate. van der Hoogen et al. described the two major genetic lineages of hMPV as separate serotypes. These investigators found that when ferrets or nonhuman primates were infected with hMPV, homologous neutralizing titers were, in general, greater than heterologous titers, especially at early time points (41, 42). In addition, a heterologous reinfection associated with severe disease within 1 month of primary infection in an otherwise-healthy infant has been documented (14). On the other side of the argument, others have demonstrated cross-protection between the two subgroups in animals that were immunized with recombinant hMPV infection or inoculation with MPV F protein (3, 34). The extent of homologous and heterologous cross-protection remains unresolved. In this study, the data resemble those from the van der Hoogen group, as we observed homologous neutralizing titers higher than heterologous titers. This finding could be attributed in part to the differences between the various clinical hMPV isolates used among the studies.
One concern for paramyxovirus vaccines is that they could enhance pulmonary disease by inducing biased Th2-dominant responses that are exacerbated when the immunized individual is exposed to natural infection. This is the case for formalin-inactivated RSV vaccine in infants and more recently FI-hMPV vaccine in cotton rats (51). Vaccination with VRPs had been shown to skew toward Th1-type responses (20, 37). We therefore evaluated lung histopathology and cytokine gene expression in VRP-vaccinated animals after wild-type hMPV challenge. Upon hMPV challenge, we found that lungs from mice that were vaccinated with VRP had similar inflammation scores as mice that were immunized with hMPV. Lungs from all hMPV-challenged animals exhibited slight alveolar, peribronchiolar, and perivascular infiltrates and no significant airway mucus production. As expected, mice vaccinated with formalin-inactivated hMPV showed significant cellular inflammation in the lungs after hMPV challenge and a corresponding increase in Th2 cytokine mRNAs. These data establish that the formalin inactivation effect of altering fusion protein antigenicity applies not only to RSV but also to MPV.
Overall, cytokine gene expression was increased in all hMPV-infected animals compared to uninfected controls. However, the increase in IFN-γ gene expression was lower when comparing animals vaccinated with VRP-MPV.F to other groups. This finding may be due to the absence of T-cell reactivity in DBA/2 mice toward peptides comprising the hMPV F protein. In the case of RSV, pulmonary disease is aggravated by aberrant T-cell responses in animal models (9, 43). This finding suggests that the humoral response against hMPV did not predispose animals to imbalanced immune responses in vaccinated animals following hMPV exposure.
In summary, we have demonstrated that VEE replicon particles encoding hMPV F protein induce strong systemic and mucosal antigen-specific humoral responses and protect animals against intranasal hMPV challenge. This study provides strong preliminary evidence that suggests that further development of this vaccine candidate for hMPV is warranted.
Acknowledgments
We thank Nancy L. Davis and Martha Collier for their technical assistance with VRP replicon cloning and packaging, the Vanderbilt Immunohistochemistry Core for assistance with specimen processing, and the VUMC Cell Imaging Shared Resources for support of confocal imaging experiments.
This work was supported by a grant from the National Institute of Allergy and Infectious Diseases, National Institutes of Health (R01 AI-59597 [J.E.C.]) and a Burroughs Wellcome Fund Clinical Scientist Award in Translation Research to J.E.C. VUMC Cell Imaging Shared Resources is supported by NIH grants CA68485, DK20593, DK58404, and HD15052.
Footnotes
Published ahead of print on 10 September 2008.
REFERENCES
- 1.Bastien, N., L. Liu, D. Ward, T. Taylor, and Y. Li. 2004. Genetic variability of the G glycoprotein gene of human metapneumovirus. J. Clin. Microbiol. 423532-3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bastien, N., S. Normand, T. Taylor, D. Ward, T. C. Peret, G. Boivin, L. J. Anderson, and Y. Li. 2003. Sequence analysis of the N, P, M and F genes of Canadian human metapneumovirus strains. Virus Res. 9351-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biacchesi, S., Q. N. Pham, M. H. Skiadopoulos, B. R. Murphy, P. L. Collins, and U. J. Buchholz. 2005. Infection of nonhuman primates with recombinant human metapneumovirus lacking the SH, G, or M2-2 protein categorizes each as a nonessential accessory protein and identifies vaccine candidates. J. Virol. 7912608-12613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biacchesi, S., M. H. Skiadopoulos, G. Boivin, C. T. Hanson, B. R. Murphy, P. L. Collins, and U. J. Buchholz. 2003. Genetic diversity between human metapneumovirus subgroups. Virology 3151-9. [DOI] [PubMed] [Google Scholar]
- 5.Biacchesi, S., M. H. Skiadopoulos, L. Yang, E. W. Lamirande, K. C. Tran, B. R. Murphy, P. L. Collins, and U. J. Buchholz. 2004. Recombinant human metapneumovirus lacking the small hydrophobic SH and/or attachment G glycoprotein: deletion of G yields a promising vaccine candidate. J. Virol. 7812877-12887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boivin, G., Y. Abed, G. Pelletier, L. Ruel, D. Moisan, S. Cote, T. C. Peret, D. D. Erdman, and L. J. Anderson. 2002. Virological features and clinical manifestations associated with human metapneumovirus: a new paramyxovirus responsible for acute respiratory-tract infections in all age groups. J. Infect. Dis. 1861330-1334. [DOI] [PubMed] [Google Scholar]
- 7.Boivin, G., I. Mackay, T. P. Sloots, S. Madhi, F. Freymuth, D. Wolf, Y. Shemer-Avni, H. Ludewick, G. C. Gray, and E. LeBlanc. 2004. Global genetic diversity of human metapneumovirus fusion gene. Emerg. Infect. Dis. 101154-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bukreyev, A., M. E. Serra, F. R. Laham, G. A. Melendi, S. R. Kleeberger, P. L. Collins, and F. P. Polack. 2006. The cysteine-rich region and secreted form of the attachment G glycoprotein of respiratory syncytial virus enhance the cytotoxic T-lymphocyte response despite lacking major histocompatibility complex class I-restricted epitopes. J. Virol. 805854-5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cannon, M. J., P. J. Openshaw, and B. A. Askonas. 1988. Cytotoxic T cells clear virus but augment lung pathology in mice infected with respiratory syncytial virus. J. Exp. Med. 1681163-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chulay, J., D. Burke, S. Karim, N. Russel, M. Wecker, M. Allen, G. Ferarri, and P. Gilbert. 2006. Safety and immunogenicity of an alphavirus replicon HIV Gag vaccine (AVX101) in healthy HIV-uninfected adults. Antivir. Ther. 11P1-P109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Connors, M., P. L. Collins, C. Y. Firestone, and B. R. Murphy. 1991. Respiratory syncytial virus (RSV) F, G, M2 (22K), and N proteins each induce resistance to RSV challenge, but resistance induced by M2 and N proteins is relatively short-lived. J. Virol. 651634-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crowe, J. E., Jr., C. Y. Firestone, and B. R. Murphy. 2001. Passively acquired antibodies suppress humoral but not cell-mediated immunity in mice immunized with live attenuated respiratory syncytial virus vaccines. J. Immunol. 1673910-3918. [DOI] [PubMed] [Google Scholar]
- 13.Cseke, G., D. W. Wright, S. J. Tollefson, J. E. Johnson, J. E. Crowe, Jr., and J. V. Williams. 2007. Human metapneumovirus fusion protein vaccines that are immunogenic and protective in cotton rats. J. Virol. 81698-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ebihara, T., R. Endo, N. Ishiguro, T. Nakayama, H. Sawada, and H. Kikuta. 2004. Early reinfection with human metapneumovirus in an infant. J. Clin. Microbiol. 425944-5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Esper, F., R. A. Martinello, D. Boucher, C. Weibel, D. Ferguson, M. L. Landry, and J. S. Kahn. 2004. A 1-year experience with human metapneumovirus in children aged <5 years. J. Infect. Dis. 1891388-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Falsey, A. R., D. Erdman, L. J. Anderson, and E. E. Walsh. 2003. Human metapneumovirus infections in young and elderly adults. J. Infect. Dis. 187785-790. [DOI] [PubMed] [Google Scholar]
- 17.Fischer, D., D. Rood, R. W. Barrette, A. Zuwallack, E. Kramer, F. Brown, and L. K. Silbart. 2003. Intranasal immunization of guinea pigs with an immunodominant foot-and-mouth disease virus peptide conjugate induces mucosal and humoral antibodies and protection against challenge. J. Virol. 777486-7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herd, K. A., S. Mahalingam, I. M. Mackay, M. Nissen, T. P. Sloots, and R. W. Tindle. 2006. Cytotoxic T-lymphocyte epitope vaccination protects against human metapneumovirus infection and disease in mice. J. Virol. 802034-2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lazar, A., N. Okabe, and P. F. Wright. 1980. Humoral and cellular immune responses of seronegative children vaccinated with a cold-adapted influenza A/HK/123/77 (H1N1) recombinant virus. Infect. Immun. 27862-866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ljungberg, K., A. C. Whitmore, M. E. Fluet, T. P. Moran, R. S. Shabman, M. L. Collier, A. A. Kraus, J. M. Thompson, D. C. Montefiori, C. Beard, and R. E. Johnston. 2007. Increased immunogenicity of a DNA-launched Venezuelan equine encephalitis virus-based replicon DNA vaccine. J. Virol. 8113412-13423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MacPhail, M., J. H. Schickli, R. S. Tang, J. Kaur, C. Robinson, R. A. Fouchier, A. D. Osterhaus, R. R. Spaete, and A. A. Haller. 2004. Identification of small-animal and primate models for evaluation of vaccine candidates for human metapneumovirus (hMPV) and implications for hMPV vaccine design. J. Gen. Virol. 851655-1663. [DOI] [PubMed] [Google Scholar]
- 22.McAdam, A. J., M. E. Hasenbein, H. A. Feldman, S. E. Cole, J. T. Offermann, A. M. Riley, and T. A. Lieu. 2004. Human metapneumovirus in children tested at a tertiary-care hospital. J. Infect. Dis. 19020-26. [DOI] [PubMed] [Google Scholar]
- 23.Melendi, G. A., F. Zavala, U. J. Bucholz, G. Boivin, P. L. Collins, S. Kleeberger, and F. P. Polack. 2007. Mapping and characterization of the primary and anamnestic H-2d-restricted cytotoxic T-lymphocyte response in mice against human metapneumovirus. J. Virol. 8111461-11467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mok, H., S. Lee, T. J. Utley, B. E. Shepherd, V. V. Polosukhin, M. L. Collier, N. L. Davis, R. E. Johnston, and J. E. Crowe, Jr. 2007. Venezuelan equine encephalitis replicon particles encoding respiratory syncytial virus surface glycoproteins induce protective mucosal responses in mice and cotton rats. J. Virol. 8113710-13722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nedrud, J. G., X. P. Liang, N. Hague, and M. E. Lamm. 1987. Combined oral/nasal immunization protects mice from Sendai virus infection. J. Immunol. 1393484-3492. [PubMed] [Google Scholar]
- 26.Oien, N. L., R. J. Brideau, E. E. Walsh, and M. W. Wathen. 1994. Induction of local and systemic immunity against human respiratory syncytial virus using a chimeric FG glycoprotein and cholera toxin B subunit. Vaccine 12731-735. [DOI] [PubMed] [Google Scholar]
- 27.Osterhaus, A., and R. Fouchier. 2003. Human metapneumovirus in the community. Lancet 361890-891. [DOI] [PubMed] [Google Scholar]
- 28.Pelletier, G., P. Dery, Y. Abed, and G. Boivin. 2002. Respiratory tract reinfections by the new human Metapneumovirus in an immunocompromised child. Emerg. Infect. Dis. 8976-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Plotnicky-Gilquin, H., D. Cyblat-Chanal, J. P. Aubry, T. Champion, A. Beck, T. Nguyen, J. Y. Bonnefoy, and N. Corvaia. 2002. Gamma interferon-dependent protection of the mouse upper respiratory tract following parenteral immunization with a respiratory syncytial virus G protein fragment. J. Virol. 7610203-10210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Polack, F. P., P. M. Irusta, S. J. Hoffman, M. P. Schiatti, G. A. Melendi, M. F. Delgado, F. R. Laham, B. Thumar, R. M. Hendry, J. A. Melero, R. A. Karron, P. L. Collins, and S. R. Kleeberger. 2005. The cysteine-rich region of respiratory syncytial virus attachment protein inhibits innate immunity elicited by the virus and endotoxin. Proc. Natl. Acad. Sci. USA 1028996-9001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prince, G. A., R. L. Horswood, and R. M. Chanock. 1985. Quantitative aspects of passive immunity to respiratory syncytial virus infection in infant cotton rats. J. Virol. 55517-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pushko, P., M. Parker, G. V. Ludwig, N. L. Davis, R. E. Johnston, and J. F. Smith. 1997. Replicon-helper systems from attenuated Venezuelan equine encephalitis virus: expression of heterologous genes in vitro and immunization against heterologous pathogens in vivo. Virology 239389-401. [DOI] [PubMed] [Google Scholar]
- 33.Skiadopoulos, M. H., S. Biacchesi, U. J. Buchholz, E. Amaro-Carambot, S. R. Surman, P. L. Collins, and B. R. Murphy. 2006. Individual contributions of the human metapneumovirus F, G, and SH surface glycoproteins to the induction of neutralizing antibodies and protective immunity. Virology 345492-501. [DOI] [PubMed] [Google Scholar]
- 34.Skiadopoulos, M. H., S. Biacchesi, U. J. Buchholz, J. M. Riggs, S. R. Surman, E. Amaro-Carambot, J. M. McAuliffe, W. R. Elkins, M. St. Claire, P. L. Collins, and B. R. Murphy. 2004. The two major human metapneumovirus genetic lineages are highly related antigenically, and the fusion (F) protein is a major contributor to this antigenic relatedness. J. Virol. 786927-6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang, R. S., K. Mahmood, M. Macphail, J. M. Guzzetta, A. A. Haller, H. Liu, J. Kaur, H. A. Lawlor, E. A. Stillman, J. H. Schickli, R. A. Fouchier, A. D. Osterhaus, and R. R. Spaete. 2005. A host-range restricted parainfluenza virus type 3 (PIV3) expressing the human metapneumovirus (hMPV) fusion protein elicits protective immunity in African green monkeys. Vaccine 231657-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang, R. S., J. H. Schickli, M. MacPhail, F. Fernandes, L. Bicha, J. Spaete, R. A. Fouchier, A. D. Osterhaus, R. Spaete, and A. A. Haller. 2003. Effects of human metapneumovirus and respiratory syncytial virus antigen insertion in two 3′ proximal genome positions of bovine/human parainfluenza virus type 3 on virus replication and immunogenicity. J. Virol. 7710819-10828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomas, C. E., W. Zhu, C. N. Van Dam, N. L. Davis, R. E. Johnston, and P. F. Sparling. 2006. Vaccination of mice with gonococcal TbpB expressed in vivo from Venezuelan equine encephalitis viral replicon particles. Infect. Immun. 741612-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thompson, J. M., A. C. Whitmore, J. L. Konopka, M. L. Collier, E. M. Richmond, N. L. Davis, H. F. Staats, and R. E. Johnston. 2006. Mucosal and systemic adjuvant activity of alphavirus replicon particles. Proc. Natl. Acad. Sci. USA 1033722-3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tripp, R. A., L. P. Jones, L. M. Haynes, H. Zheng, P. M. Murphy, and L. J. Anderson. 2001. CX3C chemokine mimicry by respiratory syncytial virus G glycoprotein. Nat. Immunol. 2732-738. [DOI] [PubMed] [Google Scholar]
- 40.van den Hoogen, B. G., J. C. de Jong, J. Groen, T. Kuiken, R. de Groot, R. A. Fouchier, and A. D. Osterhaus. 2001. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat. Med. 7719-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van den Hoogen, B. G., S. Herfst, M. de Graaf, L. Sprong, R. van Lavieren, G. van Amerongen, S. Yuksel, R. A. Fouchier, A. D. Osterhaus, and R. L. de Swart. 2007. Experimental infection of macaques with human metapneumovirus induces transient protective immunity. J. Gen. Virol. 881251-1259. [DOI] [PubMed] [Google Scholar]
- 42.van den Hoogen, B. G., S. Herfst, L. Sprong, P. A. Cane, E. Forleo-Neto, R. L. de Swart, A. D. Osterhaus, and R. A. Fouchier. 2004. Antigenic and genetic variability of human metapneumoviruses. Emerg. Infect. Dis. 10658-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Varga, S. M., X. Wang, R. M. Welsh, and T. J. Braciale. 2001. Immunopathology in RSV infection is mediated by a discrete oligoclonal subset of antigen-specific CD4+ T cells. Immunity 15637-646. [DOI] [PubMed] [Google Scholar]
- 44.Waris, M. E., C. Tsou, D. D. Erdman, S. R. Zaki, and L. J. Anderson. 1996. Respiratory syncytial virus infection in BALB/c mice previously immunized with formalin-inactivated virus induces enhanced pulmonary inflammatory response with a predominant Th2-like cytokine pattern. J. Virol. 702852-2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.White, L. J., M. M. Parsons, A. C. Whitmore, B. M. Williams, A. de Silva, and R. E. Johnston. 2007. An immunogenic and protective alphavirus replicon particle-based dengue vaccine overcomes maternal antibody interference in weanling mice. J. Virol. 8110329-10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Williams, J. V., J. E. Crowe, Jr., R. Enriquez, P. Minton, R. S. Peebles, Jr., R. G. Hamilton, S. Higgins, M. Griffin, and T. V. Hartert. 2005. Human metapneumovirus infection plays an etiologic role in acute asthma exacerbations requiring hospitalization in adults. J. Infect. Dis. 1921149-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Williams, J. V., P. A. Harris, S. J. Tollefson, L. L. Halburnt-Rush, J. M. Pingsterhaus, K. M. Edwards, P. F. Wright, and J. E. Crowe, Jr. 2004. Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N. Engl. J. Med. 350443-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williams, J. V., R. Martino, N. Rabella, M. Otegui, R. Parody, J. M. Heck, and J. E. Crowe, Jr. 2005. A prospective study comparing human metapneumovirus with other respiratory viruses in adults with hematologic malignancies and respiratory tract infections. J. Infect. Dis. 1921061-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Williams, J. V., S. J. Tollefson, J. E. Johnson, and J. E. Crowe, Jr. 2005. The cotton rat (Sigmodon hispidus) is a permissive small animal model of human metapneumovirus infection, pathogenesis, and protective immunity. J. Virol. 7910944-10951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Williams, J. V., C. K. Wang, C. F. Yang, S. J. Tollefson, F. S. House, J. M. Heck, M. Chu, J. B. Brown, L. D. Lintao, J. D. Quinto, D. Chu, R. R. Spaete, K. M. Edwards, P. F. Wright, and J. E. Crowe, Jr. 2006. The role of human metapneumovirus in upper respiratory tract infections in children: a 20-year experience. J. Infect. Dis. 193387-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yim, K. C., R. P. Cragin, M. S. Boukhvalova, J. C. Blanco, M. E. Hamlin, G. Boivin, D. D. Porter, and G. A. Prince. 2007. Human metapneumovirus: enhanced pulmonary disease in cotton rats immunized with formalin-inactivated virus vaccine and challenged. Vaccine 255034-5040. [DOI] [PMC free article] [PubMed] [Google Scholar]