Abstract
The rectal mucosa is a major site for human immunodeficiency virus entry and CD4 T-cell depletion. The early and near-total loss of these cells from the rectal mucosa severely compromises the ability of the mucosal immune system to control various opportunistic infections. Protecting these cells from infection and destruction can delay disease progression, leading to a better long-term outcome. Here we show that effective suppression of viral infection in memory CD4 T cells from the rectal mucosa and peripheral blood to a very low level with antiretroviral therapy (ART) initiated prior to the peak of infection is associated with opposite outcomes in these tissues. A near-total loss of CD4 T cells in the rectal mucosa contrasted with preservation of most memory CD4 T cells in peripheral blood during the course of treatment. Interestingly, ART significantly reduced viral infection in memory CD4 T cells from both rectal mucosa and peripheral blood. Although early ART was of limited value in protecting the CD4 T cells in the rectal mucosa, the significant preservation of peripheral CD4 T cells could contribute to maintaining immune competence, leading to a better long-term outcome.
Memory CD4 T cells in the periphery and mucosa are the primary targets of human immunodeficiency virus (HIV) infection. These cells are rapidly infected and destroyed within the first few weeks after infection (14, 17, 28). Protecting these cells from infection and subsequent destruction during the acute phase of infection can have a significant potential long-term benefit. Little is known, however, about the ability of antiretroviral therapy (ART) to contain viral infection in memory CD4 T cells and to preserve them during the early acute phase of infection. More importantly, little is known about whether ART can have a significant benefit in mucosal tissues. Studies to date using long-term highly active antiretroviral therapy (HAART) have shown that viral replication continues, and CD4 T cells fail to repopulate the mucosa even after 10 years of continuous HAART (1, 3, 8, 12, 13, 18, 22, 25, 32).
Previous studies (14, 17) showed that viral infection of CD4 T cells in peripheral and mucosal tissues peaked around 10 days after simian immunodeficiency virus (SIV) challenge. We hypothesized that initiating ART at day 7 would contain viral replication, thereby preventing the destruction of CD4 T cells. We tested this hypothesis using rhesus macaques that were challenged with highly pathogenic SIVmac251 and treated with ART that was initiated at day 7 postinfection (p.i.). Peripheral blood and rectal biopsies were collected longitudinally at various time points prior to and after challenge (days −28, 0, 7, 10, 14, 21, 35, and 63 for blood and days −28, 10, and 63 for rectal biopsies). We evaluated the kinetics of viral loads in plasma and memory CD4 T cells and CD4 T-cell dynamics in animals that received ART and compared them to untreated animals.
Plasma viral loads were determined by real-time PCR (ABI Prism 7700 sequence detection system; Applied Biosystems), using reverse-transcribed viral RNA as the template, by previously described methods (11). CD4 T-cell-associated viral DNA was measured by a quantitative PCR assay for SIV gag, using a Perkin-Elmer ABI 7700 instrument as previously described (10, 17) with the SIV gag primers and probe described by Lifson et al. (16). The assay was calibrated using a cell line that carried a single copy of proviral SIV DNA as described previously (17).
Eight (four untreated and four treated [for 8 weeks with 20 mg/kg of body weight of tenofovir and emtricitabine starting at day 7 p.i.; both drugs were obtained from Gilead Sciences, Inc., Foster City, CA]) colony-bred healthy Mamu*A01− rhesus macaques (Macaca mulatta) of Indian origin, housed at Advanced Bioscience Laboratories Inc., MD, were used in this study. Animals were housed in accordance with American Association for Accreditation of Laboratory Animal Care guidelines and were seronegative for SIV, simian retrovirus, and simian T-cell leukemia virus type 1. All of the animals were infected intravenously with 100 animal infectious doses of uncloned pathogenic SIVmac251.
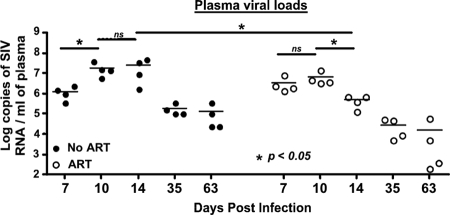
Early therapy significantly suppressed plasma viremia in treated animals.
Early ART significantly suppressed viral replication compared to that in the untreated animals. A significant increase in plasma viremia was observed in untreated animals between days 7 and 10. In contrast, the plasma viral loads increased marginally in treated animals. By day 14 p.i., there was a significant decline in plasma viral loads in treated animals compared to those at day 10 p.i. (Fig. 1), whereas they continued to increase and peaked at day 14 p.i. in untreated animals. In line with our findings, Verhoeven et al. (31), using a similar ART regimen (30 mg/kg of body weight) that was initiated at day 7 p.i., reported a significant decline in plasma viral loads at 2 weeks p.i. in treated animals compared to those in untreated animals. However, unlike our results, Verhoeven et al. (31) found no major change in plasma viral loads between days 7 and 14 p.i. in the animals that received ART.
FIG. 1.
Kinetics of plasma viral loads. Plasma viral loads peaked at day 14 in untreated animals, whereas they peaked at day 10 in animals treated with ART starting at day 7 p.i. There was a significant suppression of viral replication between days 10 and 14 p.i. in animals that received ART. Lines indicate mean values. The limit of detection is <30 copies/ml of plasma. *, significant; ns, not significant.
It is difficult to determine at this point why plasma viral loads did not decline more rapidly, as often observed when ART is initiated during chronic infection. It is likely that during the ramp-up phase of infection, when viral replication is increasing dramatically, the rate at which virus is produced is far greater than the rate at which plasma virus decays. On the other hand, during chronic infection, viral production is much lower and at a steady state, and initiation of ART is rapidly followed by the decay of existing plasma virus. Brandin et al. (5) showed that the mean half-life of plasma virus in chronically infected animals treated with ART was ∼0.5 day, with the decay rate of plasma SIV ranging from 1 to 4 days after treatment. Others (19) have shown that the decay rates in SIV-infected animals range from 0.7 to 1.4 days. Our results suggest that during the early ramp-up phase of active viral replication, effective suppression with ART may take much longer than with initiation of ART during the chronic steady phase of viral replication.
Interestingly, two of the four animals treated with ART had ∼104 RNA copies at the end of 8 weeks of therapy, suggesting that these animals likely failed therapy. This failure could be due to the virus escaping the drugs during the course of early therapy, as some previous studies (27) have shown. This raises a concern that initiating ART during the ramp-up phase of viral replication may increase the potential for viral escape.
Early therapy protected memory CD4 T cells in the periphery but not in the mucosa.
To determine if initiating ART at day 7 prevented the depletion of mucosal CD4 T cells, we evaluated CD4 T-cell dynamics in the mucosa and peripheral blood by using a combination of the following antibodies: CD3-Cy7-allophycocyanin, CD8-Alexa 700, CD4-phycoerythrin, CD4-Qdot605, CD45RA-TRPE, CD95-allophycocyanin, CD28-Cy5-phycoerythrin, and CD20-fluorescein isothiocyanate. All antibodies except for CD4-Qdot605 (courtesy of Mario Roederer) were obtained from BD Biosciences (San Diego, CA). Flow cytometric data were analyzed using FlowJo, version 8.1 (Tree Star, Inc., Ashland, OR). Student's t test was used for statistical analysis with Prism software (GraphPad Software, Inc., La Jolla, CA).
As expected (14, 17, 28), in untreated animals there was a massive loss of the memory CD4 T cells in peripheral blood (Fig. 2a) by day 21 following infection. In contrast to the untreated animals, animals that received ART at day 7 preserved a significant majority of their memory CD4 T cells in the periphery (Fig. 2a and c). The marginal decline in memory CD4 T cells in the peripheral blood of ART-treated animals compared to a significant loss in untreated animals likely reflects the loss of cells that were infected prior to initiation of ART. Surprisingly, early ART failed to protect the CD4 T cells in the rectal mucosa (Fig. 2b). A near-total destruction of CD4 T cells occurred in the rectal mucosae of treated animals and did not differ from that in untreated animals.
FIG. 2.
Dynamics of memory CD4 T cells in the rectal mucosa and peripheral blood. ART was initiated at day 7 p.i., and data are shown for peripheral blood and rectal mucosa from animals that were treated with ART (n = 4) and animals that were not treated with ART (n = 4). Naïve and memory CD4 T cells were discriminated on the basis of CD28 and CD95 expression, with all memory CD4 T cells expressing CD95. Acute infection was associated with a significant decrease in the frequency (a) and absolute count (c) of memory CD4 T cells in peripheral blood of untreated animals, whereas there was no significant difference in the animals that received ART. (b) Unlike in peripheral blood, there was a significant loss of CD4 T cells in the mucosae of both untreated and treated animals by day 63 p.i. compared to day 10 p.i. and preinfection values. Lines represent mean values. P, preinfection samples collected at −day 28; *, significant; ns, not significant.
The difference in protection of memory cells in blood versus mucosa of treated animals might have been due to the inability of the drugs to adequately suppress viral replication in mucosal tissues. In this regard, numerous studies (8, 12, 22, 25, 31) have shown that low levels of HIV replication continue in the mucosal tissues of HIV-infected patients and SIV-infected macaques undergoing therapy.
ART significantly suppressed the level of infection in peripheral and mucosal memory CD4 T cells.
To determine if the tissue-specific bioavailability of the drugs was the cause for the selective loss of mucosal CD4 T cells, we evaluated the levels of viral DNA in subsets of CD4 T cells from the mucosa and compared them to those from peripheral blood. Naïve and memory CD4 T cells (discriminated based on the expression of CD28 and CD95 [21]) were sorted and used in a highly quantitative PCR assay for SIV gag DNA as a measure of the efficacy of the drugs to stop viral transcription. We previously demonstrated that this assay was highly sensitive for measuring viral infection (17) in CD4 T cells.
Our results showed that the initiation of ART dramatically reduced the frequency of SIV gag DNA in both mucosal and peripheral blood CD4 T cells of treated animals compared to that in untreated controls (Fig. 3a and b), suggesting that ART effectively limited infection in both tissues. At day 10 p.i., the average frequency of SIV gag copies was ∼8 × 103 copies/105 memory CD4 T cells in the mucosa and ∼16 × 103 copies/105 memory CD4 T cells in peripheral blood of ART-treated animals. We previously showed that there were ∼2 copies of SIV gag/infected memory CD4 T cell at day 10 p.i. (17). This suggests that at the peak of viral infection (day 10 p.i.), <5% of mucosal CD4 T cells and <10% of memory CD4 T cells in peripheral blood were infected in treated animals, whereas most of the memory CD4 T cells in both tissues were infected in untreated animals.
FIG. 3.
Kinetics of cell-associated viral loads during early ART. Far fewer cells were infected in animals that received ART at day 7 p.i. The cell-associated SIV gag DNA levels in sorted memory CD4 T cells in peripheral blood (a) and rectal mucosa (b) were determined for untreated animals (n = 4) and compared to animals that received ART (n = 4). ART significantly suppressed viral transcription in memory CD4 T cells from both peripheral blood and rectal mucosa within days of initiating therapy. Error bars represent standard errors. *, significant; ns, not significant.
It is interesting that at day 7 p.i. there was a significant amount of virus in the plasma but few cells were infected. One reason for this discordance may be that initial infection targets highly activated memory CD4 T cells, which are more efficient at replicating the virus. Thus, even though very few of these cells are infected, they likely contribute to a significant level of early plasma viremia. After the initial phase, infection disseminates to encompass the resting memory CD4 T cells, which are not as efficient as the activated memory CD4 T cells at viral replication (14). Additionally, it is possible that other cells, such as macrophages and dendritic cells, likely contribute to early plasma viremia. Numerous studies (7, 20) have shown that both of these cell types can be productively infected.
Our findings suggest that the higher level of memory CD4 T-cell preservation seen in peripheral blood of treated animals than in untreated animals was primarily due to the loss of fewer memory cells by direct viral infection. In contrast, the massive destruction of CD4 T cells in the rectal mucosa even when infection was controlled to very low levels indicates that most (>90%) of the mucosal CD4 T cells in treated animals were destroyed by mechanisms upstream of viral reverse transcription, such as SIV gp120 binding to CD4 T cells or viral entry.
Li et al. (14) demonstrated that a majority of mucosal CD4 T cells were not productively infected and were likely killed at a high rate due to SIV gp120-mediated apoptosis. Additionally, Boirivant et al. (4) showed that binding of HIV-1 gp120 accelerated the Fas-mediated apoptosis of human lamina propria T cells. Previous studies (6, 23, 29, 30) have shown that mucosal CD4 T cells express high levels of CCR5, making them highly susceptible to infection. Additionally, the recent demonstration (2) that HIV can use the activated form of α4β7 receptor for high-affinity binding and entry into cells lends support to this hypothesis, as most mucosal CD4 T cells express high levels of α4β7 receptor on their surfaces (26). Cummins et al. (9) showed that high levels of α4β7 integrin expression on CD4 T cells exposed to an HIV-1 X4 strain were associated with bystander death in these cells. Vajdy et al. (26) demonstrated that acute SIV infection was associated with a profound loss of CD4 T cells expressing α4β7 in the rectal mucosa.
We cannot rule out that viral RNA was present in the mucosal CD4 T cells due to viral entry, since our assay measures only viral DNA; however, our results suggest that either gp120 binding, viral entry, or bystander mechanisms, rather than productive viral infection, are sufficient to kill most of the mucosal CD4 T cells. It is highly unlikely that SIV-specific immune responses played a major role in the depletion of these mucosal CD4 T cells, as previous studies (24) have shown that mucosal CD4 T cells are destroyed much earlier than the emergence of SIV-specific immune responses. Additionally, it is important to indicate that we did not sample rectal mucosa between days 10 and 63 p.i., and it is likely that the continuous viral replication in these tissues contributed to the ongoing attrition of CD4 T cells in the rectal mucosa, leading to their loss. Numerous studies have shown that low levels of viral replication continue in the mucosa even during continuous HAART (8, 12, 22, 25, 32).
The plasma viral loads at day 7 p.i. were essentially similar in both the ART-treated and untreated animals (Fig. 1), suggesting that by the time ART was initiated there were enough virions to interact with CD4 T cells resident in the mucosa. This likely explains why mucosal CD4 T cells in both groups displayed similar kinetics of depletion even though <5% of these cells were infected at day 10 p.i. in treated animals.
It is unlikely that the rapid and widespread dissemination of the virus across the mucosal tissue compartment immediately after intravenous challenge could have more readily exposed the mucosal CD4 T cells to the virus. A similar intravenous challenge was associated with the protection of mucosal CD4 T cells when ART was initiated within 24 h after challenge (15). The protection observed by Lifson et al. (15) was probably due to the rapid containment of early viral replication, thereby preventing the dissemination of SIV across the entire mucosal compartment. On the other hand, by day 7 p.i., viral replication was already in the ramp-up phase, with the virus disseminating rapidly across the mucosa.
Though mechanisms other than productive viral infection may explain the near-total loss of mucosal CD4 T cells during ART, a higher level of preservation of memory CD4 T cells (Fig. 2a) in peripheral blood of these animals than in the mucosa (Fig. 2b) suggests that mucosal CD4 T cells are much more susceptible to loss than cells in the periphery. Thus, although the plasma viral loads at day 10 p.i. were essentially similar between treated and untreated animals, fewer peripheral blood memory CD4 T cells were infected and lost in treated animals.
In conclusion, our data show that initiating ART prior to peak viremia has a selective effect on CD4 T cells in peripheral tissues relative to the rectal mucosa. Mucosal CD4 T cells are more easily lost following infection, and minimal viral interaction is probably sufficient for the loss of these cells. Most likely, the highly activated microenvironment in which the cells reside contributes to this process. On the other hand, the ability of peripheral blood CD4 memory T cells to survive even in the presence of high viremia suggests that peripheral memory CD4 T cells have a much higher threshold for loss than their mucosal counterparts. These findings may explain why HAART is associated with immediate and lasting repopulation in the periphery compared to the lack of or transient repopulation in mucosal tissues (1, 3, 8, 13, 18); the continuous low level of viral replication in the mucosa (8, 12, 22, 25, 32) is likely sufficient to destroy the repopulating cells.
Acknowledgments
We thank Nancy Miller at the SVEU of NIAID for help with the animals; Michael Miller at Gilead Sciences, Inc., for providing PMPA and FTC; Karen Wolcott and Kateryna Lund at the Biomedical Instrumentation Core facility at USUHS for help with flow cytometry; and Deborah Weiss and Jim Treece at ABL, Inc., Rockville, MD, for expert assistance with the animals.
The described project was supported by grant K22AI07812 from the National Institute of Allergy and Infectious Diseases (NIAID), by grant R21DE018339 from the National Institute of Dental and Craniofacial Research (NIDCR) to J.J.M., and in part by federal funds from the National Cancer Institute, National Institutes of Health, under contract NO1-CO-124000.
The content is solely the responsibility of the authors and does not necessarily represent the official views of NIAID, NIDCR, or the National Institutes of Health.
Footnotes
Published ahead of print on 3 September 2008.
REFERENCES
- 1.Anthony, K. B., C. Yoder, J. A. Metcalf, R. DerSimonian, J. M. Orenstein, R. A. Stevens, J. Falloon, M. A. Polis, H. C. Lane, and I. Sereti. 2003. Incomplete CD4 T cell recovery in HIV-1 infection after 12 months of highly active antiretroviral therapy is associated with ongoing increased CD4 T cell activation and turnover. J. Acquir. Immune Defic. Syndr. 33125-133. [DOI] [PubMed] [Google Scholar]
- 2.Arthos, J., C. Cicala, E. Martinelli, K. Macleod, D. Van Ryk, D. Wei, Z. Xiao, T. D. Veenstra, T. P. Conrad, R. A. Lempicki, S. McLaughlin, M. Pascuccio, R. Gopaul, J. McNally, C. C. Cruz, N. Censoplano, E. Chung, K. N. Reitano, S. Kottilil, D. J. Goode, and A. S. Fauci. 2008. HIV-1 envelope protein binds to and signals through integrin alpha4beta7, the gut mucosal homing receptor for peripheral T cells. Nat. Immunol. 9301-309. [DOI] [PubMed] [Google Scholar]
- 3.Belmonte, L., M. Olmos, A. Fanin, C. Parodi, P. Bare, H. Concetti, H. Perez, M. M. de Bracco, and P. Cahn. 2007. The intestinal mucosa as a reservoir of HIV-1 infection after successful HAART. AIDS 212106-2108. [DOI] [PubMed] [Google Scholar]
- 4.Boirivant, M., M. Viora, L. Giordani, A. L. Luzzati, A. M. Pronio, C. Montesani, and O. Pugliese. 1998. HIV-1 gp120 accelerates Fas-mediated activation-induced human lamina propria T cell apoptosis. J. Clin. Immunol. 1839-47. [DOI] [PubMed] [Google Scholar]
- 5.Brandin, E., R. Thorstensson, S. Bonhoeffer, and J. Albert. 2006. Rapid viral decay in simian immunodeficiency virus-infected macaques receiving quadruple antiretroviral therapy. J. Virol. 809861-9864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brenchley, J. M., T. W. Schacker, L. E. Ruff, D. A. Price, J. H. Taylor, G. J. Beilman, P. L. Nguyen, A. Khoruts, M. Larson, A. T. Haase, and D. C. Douek. 2004. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 200749-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cassol, E., M. Alfano, P. Biswas, and G. Poli. 2006. Monocyte-derived macrophages and myeloid cell lines as targets of HIV-1 replication and persistence. J. Leukoc. Biol. 801018-1030. [DOI] [PubMed] [Google Scholar]
- 8.Chun, T. W., D. C. Nickle, J. S. Justement, J. H. Meyers, G. Roby, C. W. Hallahan, S. Kottilil, S. Moir, J. M. Mican, J. I. Mullins, D. J. Ward, J. A. Kovacs, P. J. Mannon, and A. S. Fauci. 2008. Persistence of HIV in gut-associated lymphoid tissue despite long-term antiretroviral therapy. J. Infect. Dis. 197714-720. [DOI] [PubMed] [Google Scholar]
- 9.Cummins, J. E., Jr., W. J. Bunn, S. D. Hall, H. H. Donze, J. Mestecky, and S. Jackson. 2001. In vitro exposure to highly cytopathic HIV-1 X4 strains increases expression of mucosa-associated integrins on CD4(+) T cells. Virology 280262-272. [DOI] [PubMed] [Google Scholar]
- 10.Douek, D. C., J. M. Brenchley, M. R. Betts, D. R. Ambrozak, B. J. Hill, Y. Okamoto, J. P. Casazza, J. Kuruppu, K. Kunstman, S. Wolinsky, Z. Grossman, M. Dybul, A. Oxenius, D. A. Price, M. Connors, and R. A. Koup. 2002. HIV preferentially infects HIV-specific CD4+ T cells. Nature 41795-98. [DOI] [PubMed] [Google Scholar]
- 11.Endo, Y., T. Igarashi, Y. Nishimura, C. Buckler, A. Buckler-White, R. Plishka, D. S. Dimitrov, and M. A. Martin. 2000. Short- and long-term clinical outcomes in rhesus monkeys inoculated with a highly pathogenic chimeric simian/human immunodeficiency virus. J. Virol. 746935-6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finzi, D., M. Hermankova, T. Pierson, L. M. Carruth, C. Buck, R. E. Chaisson, T. C. Quinn, K. Chadwick, J. Margolick, R. Brookmeyer, J. Gallant, M. Markowitz, D. D. Ho, D. D. Richman, and R. F. Siliciano. 1997. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 2781295-1300. [DOI] [PubMed] [Google Scholar]
- 13.Guadalupe, M., E. Reay, S. Sankaran, T. Prindiville, J. Flamm, A. McNeil, and S. Dandekar. 2003. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J. Virol. 7711708-11717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li, Q., L. Duan, J. D. Estes, Z. M. Ma, T. Rourke, Y. Wang, C. Reilly, J. Carlis, C. J. Miller, and A. T. Haase. 2005. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature 4341148-1152. [DOI] [PubMed] [Google Scholar]
- 15.Lifson, J. D., M. Piatak, Jr., A. N. Cline, J. L. Rossio, J. Purcell, I. Pandrea, N. Bischofberger, J. Blanchard, and R. S. Veazey. 2003. Transient early post-inoculation anti-retroviral treatment facilitates controlled infection with sparing of CD4+ T cells in gut-associated lymphoid tissues in SIVmac239-infected rhesus macaques, but not resistance to rechallenge. J. Med. Primatol. 32201-210. [DOI] [PubMed] [Google Scholar]
- 16.Lifson, J. D., J. L. Rossio, M. Piatak, Jr., T. Parks, L. Li, R. Kiser, V. Coalter, B. Fisher, B. M. Flynn, S. Czajak, V. M. Hirsch, K. A. Reimann, J. E. Schmitz, J. Ghrayeb, N. Bischofberger, M. A. Nowak, R. C. Desrosiers, and D. Wodarz. 2001. Role of CD8(+) lymphocytes in control of simian immunodeficiency virus infection and resistance to rechallenge after transient early antiretroviral treatment. J. Virol. 7510187-10199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mattapallil, J. J., D. C. Douek, B. Hill, Y. Nishimura, M. Martin, and M. Roederer. 2005. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 4341093-1097. [DOI] [PubMed] [Google Scholar]
- 18.Mehandru, S., M. A. Poles, K. Tenner-Racz, P. Jean-Pierre, V. Manuelli, P. Lopez, A. Shet, A. Low, H. Mohri, D. Boden, P. Racz, and M. Markowitz. 2006. Lack of mucosal immune reconstitution during prolonged treatment of acute and early HIV-1 infection. PLoS Med. 3e484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nowak, M. A., A. L. Lloyd, G. M. Vasquez, T. A. Wiltrout, L. M. Wahl, N. Bischofberger, J. Williams, A. Kinter, A. S. Fauci, V. M. Hirsch, and J. D. Lifson. 1997. Viral dynamics of primary viremia and antiretroviral therapy in simian immunodeficiency virus infection. J. Virol. 717518-7525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piguet, V., and R. M. Steinman. 2007. The interaction of HIV with dendritic cells: outcomes and pathways. Trends Immunol. 28503-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pitcher, C. J., S. I. Hagen, J. M. Walker, R. Lum, B. L. Mitchell, V. C. Maino, M. K. Axthelm, and L. J. Picker. 2002. Development and homeostasis of T cell memory in rhesus macaque. J. Immunol. 16829-43. [DOI] [PubMed] [Google Scholar]
- 22.Poles, M. A., W. J. Boscardin, J. Elliott, P. Taing, M. M. Fuerst, I. McGowan, S. Brown, and P. A. Anton. 2006. Lack of decay of HIV-1 in gut-associated lymphoid tissue reservoirs in maximally suppressed individuals. J. Acquir. Immune Defic. Syndr. 4365-68. [DOI] [PubMed] [Google Scholar]
- 23.Poles, M. A., J. Elliott, P. Taing, P. A. Anton, and I. S. Chen. 2001. A preponderance of CCR5(+) CXCR4(+) mononuclear cells enhances gastrointestinal mucosal susceptibility to human immunodeficiency virus type 1 infection. J. Virol. 758390-8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reynolds, M. R., E. Rakasz, P. J. Skinner, C. White, K. Abel, Z. M. Ma, L. Compton, G. Napoe, N. Wilson, C. J. Miller, A. Haase, and D. I. Watkins. 2005. CD8+ T-lymphocyte response to major immunodominant epitopes after vaginal exposure to simian immunodeficiency virus: too late and too little. J. Virol. 799228-9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharkey, M. E., I. Teo, T. Greenough, N. Sharova, K. Luzuriaga, J. L. Sullivan, R. P. Bucy, L. G. Kostrikis, A. Haase, C. Veryard, R. E. Davaro, S. H. Cheeseman, J. S. Daly, C. Bova, R. T. Ellison III, B. Mady, K. K. Lai, G. Moyle, M. Nelson, B. Gazzard, S. Shaunak, and M. Stevenson. 2000. Persistence of episomal HIV-1 infection intermediates in patients on highly active anti-retroviral therapy. Nat. Med. 676-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vajdy, M., R. S. Veazey, H. K. Knight, A. A. Lackner, and M. R. Neutra. 2000. Differential effects of simian immunodeficiency virus infection on immune inductive and effector sites in the rectal mucosa of rhesus macaques. Am. J. Pathol. 157485-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Rompay, K. K., J. A. Johnson, E. J. Blackwood, R. P. Singh, J. Lipscomb, T. B. Matthews, M. L. Marthas, N. C. Pedersen, N. Bischofberger, W. Heneine, and T. W. North. 2007. Sequential emergence and clinical implications of viral mutants with K70E and K65R mutation in reverse transcriptase during prolonged tenofovir monotherapy in rhesus macaques with chronic RT-SHIV infection. Retrovirology 425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Veazey, R. S., M. DeMaria, L. V. Chalifoux, D. E. Shvetz, D. R. Pauley, H. L. Knight, M. Rosenzweig, R. P. Johnson, R. C. Desrosiers, and A. A. Lackner. 1998. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280427-431. [DOI] [PubMed] [Google Scholar]
- 29.Veazey, R. S., K. G. Mansfield, I. C. Tham, A. C. Carville, D. E. Shvetz, A. E. Forand, and A. A. Lackner. 2000. Dynamics of CCR5 expression by CD4(+) T cells in lymphoid tissues during simian immunodeficiency virus infection. J. Virol. 7411001-11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Veazey, R. S., P. A. Marx, and A. A. Lackner. 2003. Vaginal CD4+ T cells express high levels of CCR5 and are rapidly depleted in simian immunodeficiency virus infection. J. Infect. Dis. 187769-776. [DOI] [PubMed] [Google Scholar]
- 31.Verhoeven, D., S. Sankaran, M. Silvey, and S. Dandekar. 2008. Antiviral therapy during primary simian immunodeficiency virus infection fails to prevent acute loss of CD4+ T cells in gut mucosa but enhances their rapid restoration through central memory T cells. J. Virol. 824016-4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong, J. K., M. Hezareh, H. F. Gunthard, D. V. Havlir, C. C. Ignacio, C. A. Spina, and D. D. Richman. 1997. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science 2781291-1295. [DOI] [PubMed] [Google Scholar]