Abstract
Rhesus TRIM5α (rhTRIM5α), but not human TRIM5α (huTRIM5α), potently inhibits human immunodeficiency virus (HIV) infection and is thus a potentially valuable therapeutic tool. Primary human CD4 T cells engineered to express rhTRIM5α were highly resistant to cell-free HIV type 1 (HIV-1) infection. However, when cocultured with unmodified T cells, rhTRIM5α-expressing cells became highly permissive to HIV-1 infection. Physical separation of rhTRIM5α-expressing cells and unmodified cells revealed that rhTRIM5α efficiently restricts cell-free but not cell-associated HIV transmission. Furthermore, we observed that HIV-infected human cells could infect rhesus CD4 T cells by cell-to-cell contact, but the infection was self-limiting. Subsequently, we noted that a spreading infection ensued when HIV-1-infected rhTRIM5α-expressing human cells were cultured with huTRIM5α- but not rhTRIM5α-expressing cells. Our results suggest that cell-associated HIV transmission in humans is blocked only when both donor and recipient cells express rhTRIM5α. These studies further define the role of rhTRIM5α in cell-free and cell-associated HIV transmission and delineate the utility of rhTRIM5α in anti-HIV therapy.
The identification of rhesus TRIM5α (rhTRIM5α) as a potent intracellular human immunodeficiency virus (HIV) restriction factor (37) has fueled intensive investigation into how this factor interferes with HIV type 1 (HIV-1) infection. rhTRIM5α has also attracted attention as a potential therapeutic for HIV disease (28). TRIM5α proteins belong to the tripartite motif family, and they contain RING, B-box 2, and coiled-coil domains, as well as a C-terminal PRY/SPRY (B30.2) domain (15, 18). Observations from multiple groups support the notion that rhTRIM5α restricts HIV at multiple points of the viral replication cycle (3). Acting after viral entry but prior to integration, rhTRIM5α accelerates cytosolic capsid disassembly (9, 38) and prevents accumulation of reverse transcriptase products (37). Proteasome inhibitors restore reverse transcript accumulation while still preserving restriction of HIV-1, leading to the proposal that disruption of preintegration complex trafficking to the nucleus is a key component of rhTRIM5α-mediated restriction of HIV infection (4, 41). More recently, rhTRIM5α overexpression was shown to limit production of infectious virus, but not the infectivity of released virions, in cells previously infected with HIV-1 (32). However, there is also evidence that this may be an in vitro phenomenon observed with the overexpression of either human TRIM5α (huTRIM5α) or rhTRIM5α protein in cell lines (43). The human counterpart of rhTRIM5α, huTRIM5α, can restrict replication of some retroviruses, but it is largely ineffective against HIV-1 (28). But a huTRIM5α derivative containing five rhesus monkey amino acid residues in the human PRY/SPRY (B30.2) domain restricts HIV-1 replication nearly as potently as rhTRIM5α (39). In fact, simply replacing a single arginine residue with proline at position 332 in huTRIM5α confers significant HIV-1 and simian immunodeficiency virus (SIV) restriction (24).
HIV-1 can spread between cells by cell-free (released virions) or cell-associated (direct transfer between cells) mechanisms. While cell-free viral transmission is most commonly studied in vitro, there is growing appreciation that cell-associated spread of HIV-1 is an important, if not the primary, mode of HIV-1 spread in vivo (17). Cell-associated HIV-1 spread is estimated to be several orders of magnitude more efficient than cell-free transmission (13). This difference is due to multiple factors, such as avoidance of the humoral immune response (27), as well as concentration of adhesion molecules, viral receptors, and key cellular signaling components at the site of cell contact (19, 35). Moreover, directional mechanisms such as establishment of filopodial bridges that direct the virus between cells (33) and polarization of the cytoskeleton toward the target cell (18) contribute to the increased efficiency of cell-associated transmission.
The intent of this study was to evaluate the suitability of rhTRIM5α for anti-HIV-1 adoptive T-cell therapy (22, 23). We found that while rhTRIM5α could effectively prevent cell-free HIV-1 infection of primary human CD4 T cells, it was unable to prevent HIV infection mediated by cell-to-cell contact between rhTRIM5α-expressing and HIV-1-infected human CD4 T cells. HIV-1-infected rhTRIM5α-expressing cells produced infectious virions, but they were unable to spread the infection efficiently to other rhTRIM5α-expressing cells, even via cell-to-cell contact. Thus, our studies suggest that rhTRIM5α may be an effective in vivo antiviral agent only when expressed by the majority of cells.
MATERIALS AND METHODS
Lentiviral vector construction and production.
Replication-defective lentiviral vectors were generated and concentrated, and their titers were determined, as previously described (30). TRIM5α-expressing lentiviral vectors were constructed by inserting cDNAs encoding huTRIM5α, rhTRIM5α, or huTRIM5αr323-332 (39) into the lentiviral expression vector pELNS. pELNS was derived from pRRL-SIN-CMV-eGFP-WPRE (14) by replacing the internal cytomegalovirus promoter with the EF-1α promoter. A green fluorescent protein (GFP) cassette and an 18-amino-acid T2A motif cassette including a flexible linker (40) were inserted upstream of the TRIM5α coding sequence, permitting bicistronic expression of GFP and TRIM5α. pCLPS mCD28 167* was constructed by inserting a stop codon into pCLPS mCD28-h28 after residue 167, eliminating the CD28 cytoplasmic tail signaling domains (30). The monomeric red fluorescent protein (RFP) (6)-encoding vector VRX1031, in which RFP expression is driven by the HIV-1NL4-3 viral long terminal repeat, was produced using a protocol designed for clinical-grade lentiviral vector production (25).
Western blot analysis.
Cytoplasmic protein lysates were made as previously described (35), using 1% Nonidet P-40 lysis buffer and a complete cocktail of protease inhibitors (Roche, Indianapolis, IN); 250,000 cell equivalents/lane were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to Immobilon-P polyvinylidene difluoride membranes (Millipore, Bedford, MA). Blots were incubated with either a 1:1,000 dilution of anti-huTRIM5α rabbit polyclonal antibody CT (Imgenex, San Diego, CA) or a 1:1,000 dilution of anti-AKT rabbit polyclonal antibody (Cell Signaling Technology, Danvers, MA), followed by incubation with a 1:1,000 dilution of goat anti-rabbit-horseradish peroxidase (Cell Signaling Technology). Proteins were visualized using ECL (GE Healthcare, Piscataway, NJ) and analyzed on a Gel-Doc densitometer (Bio-Rad, Hercules, CA).
Cell isolation, culture, and transduction.
Peripheral blood mononuclear cells (PBMCs) were obtained by leukapheresis of healthy volunteer donors by the University of Pennsylvania Human Immunology Core. All specimens were collected under a University Institutional Review Board-approved protocol, and informed consent was obtained from each donor. Primary human CD4 T cells were purified by negative selection as previously described (10), and residual red blood cells were removed by ACK lysis (BioWhittaker, Walkersville, MD). Purified human CD4 T cells were transduced by adding 250 to 350 μl of concentrated lentiviral vector (approximately 5 × 107 transducing units) to 250,000 anti-CD3/CD28 bead-activated cells plated at 1 × 106/ml (250 μl) in a 48-well plate. Antibody-coated beads were removed after 5 days of culture. Expression of introduced huTRIM5α, rhTRIM5α, or huTRIM5αr323-332 was monitored using the GFP signal from the bicistronic GFP-2A-TRIM5α transcript.
Rhesus CD4 T cells were purified from PBMCs 4 days after stimulation with concanavalin A (ConA) (1 μg/ml) (Sigma-Aldrich, St. Louis, MO) and interleukin-2 (IL-2) (300 IU/ml). Activated rhesus CD4 T cells were isolated by negative selection using StemSep rhesus monkey CD4+ T-cell enrichment cocktail and human EasySep magnetic nanoparticles (both from StemCell Technologies, Vancouver, Canada). Rhesus and human CD4 T cells were distinguished by staining with species-specific fluorescein isothiocyanate-conjugated anti-CD3 antibodies (human-specific OKT3 [eBioscience] or rhesus-specific FN-18 [Biosource, Camarillo, CA]) combined with allophycocyanin-conjugated pan-reactive anti-CD4 antibody L200 (BD Pharmingen, San Diego, CA). GHOST-CCR5 cells (8) were maintained in selective media as previously described (26).
HIV-1 infections.
HIV-1SF162 and HIV-1BK132 viral stocks were prepared by harvesting supernatant from infected phytohemagglutinin/IL-2-stimulated human PBMC cultures. Anti-CD3/CD28 bead-stimulated human CD4 T cells were challenged with cell-free HIV-1 on a p24-normalized basis (50 ng p24/0.5 × 106 cells) at 48 h after bead removal, i.e., 7 days poststimulation. Zidovudine (AZT) (NIH AIDS Research and Reference Reagent Program, Germantown, MD) was added where indicated to a final concentration of 10 μg/ml. Transwell assays were performed using 12-well or 24-well 0.4-μm polyester-membrane dishes (Corning Life Sciences, Corning, NY). Cell-free HIV was added to both wells for HIV challenge. Cell-associated HIV-1 infections used untransduced human CD4 T cells infected with cell-free HIV-1SF162 3 days previously as donor cells; target cells were uninfected human or rhesus CD4 T cells.
HIV detection.
HIV-1 infection was monitored by intracellular HIV-1 Gag staining using the Caltag Fix and Perm kit (Invitrogen, Carlsbad, CA) and the KC57 anti-Gag-RD1 antibody (Coulter, Hialeah, FL), following the manufacturers' instructions. Cell populations were acquired using a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA) and analyzed using Flowjo software (Tree Star, Ashland, OR). p24 core antigen concentrations in culture supernatants were measured by p24 enzyme-linked immunosorbent assay (NEN-Perkin Elmer Life Sciences, Boston, MA). Enzyme-linked immunosorbent assays were performed by the University of Pennsylvania Center for AIDS Research (Penn-CFAR) (http://www.uphs.upenn.edu/aids/). The relative infectivity of culture supernatants was measured by infection of GHOST-CCR5 cells. Dilutions of culture supernatant were added to GHOST-CCR5 cells, and 48 h later, cultures were analyzed for GFP and Gag expression by fluorescence-activated cell sorter (FACS) analysis. Copies of HIV gag DNA were measured by real-time PCR and normalized to the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene, using reagents and conditions standardized by Penn-CFAR. Standard curves were constructed using the HIV-1-infected ACH-2 cell line (11). The primers used were gag-6F (5′-CATGTTTTCAGCATTATCAGAAGGA-3′) and gag-84R (5′-TGCTTGATGTCCCCCCACT-3′). The sequence of the probe was (6-carboxyfluorescein)-5′-CCACCCCACAAGATTTAAACACCATGCTAA-3′-(Black Hole Quencher 1).
Sorting and characterization of HIV-1-infected TRIM5α-expressing human CD4 T cells.
Viable human CD4 T cells transduced with rhTRIM5α, huTRIM5αrh323-332, or huTRIM5α were sorted as GFP-positive (GFP+), 4′,6′-diamidino-2-phenylindole-negative (DAPI −) cells from HIV-1SF162-infected cultures using a FACSVantage SE (BD Biosciences, San Jose, CA) by the BSL3 Cell Sorting Facility at The Children's Hospital of Philadelphia (http://stokes.chop.edu/cores/bsl3/index.php). Sorted human CD4 T cells were washed and resuspended in complete RPMI supplemented with 300 U/ml IL-2, counted, plated at 1 × 106 cells/ml, and restimulated with anti-CD3/CD28 antibody-coated beads. On various days after restimulation, supernatants and cell pellets were collected. In some experiments, on day 6 after restimulation, sorted (donor) cells were mixed at a 1:1 ratio with similarly activated TRIM5α-expressing or untransduced (recipient) CD4 T cells. Donor and recipient populations were distinguished by using cells with different HLA A*02 status. HLA A*02 staining was performed using anti-A2 biotin-conjugated primary antibody (One Lambda, Canoga Park, CA) and streptavidin-allophycocyanin as the secondary antibody (BD Pharmingen, San Diego, CA).
RESULTS
Expression of rhTRIM5α restricts HIV-1 infection in primary human CD4 T cells.
To date, most studies examining rhTRIM5α-mediated lentiviral restriction in human cells have used transformed cell lines and pseudotyped HIV vectors (4, 24, 37, 42). To determine whether rhTRIM5α could restrict replication-competent HIV-1 in primary human CD4 T cells, we constructed lentiviral vectors that coexpressed GFP and either huTRIM5α, rhTRIM5α, or a modified huTRIM5α (huTRIM5αr323-332) via a T2A sequence that induces a ribosomal skipping mechanism and results in the production of two proteins from a single transcript (Fig. 1A) (39). The latter construct is a huTRIM5α derivative in which five amino acids in the PRY/SPRY (B30.2) domain were replaced with their corresponding residues from the rhTRIM5α protein (39). We included huTRIM5αr323-332-expressing constructs in our analyses because of its reported potency (39) and the probability that it may be less immunogenic than wild-type rhTRIM5α as a human therapeutic.
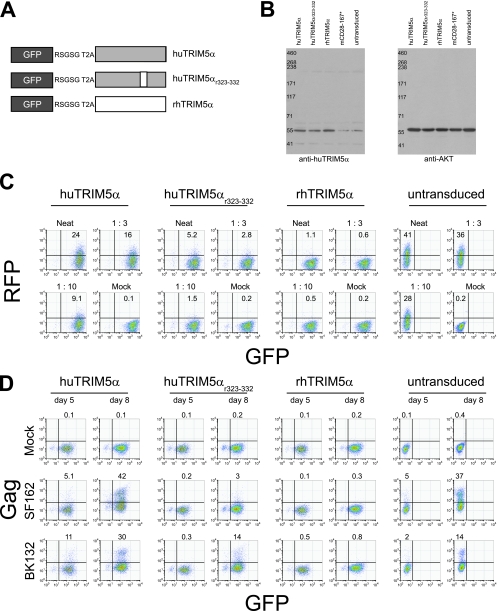
FIG. 1.
Expression of rhTRIM5α restricts HIV-1 infection in primary human CD4 T cells. (A) Schematic diagram of the constructs used in this study. Expression of huTRIM5α (gray), rhTRIM5α (white), or a modified version of human TRIM5α (huTRIM5αr323-332) (mix of gray and white) was linked to GFP expression via a T2A sequence and a flexible RSGSG linker. In this configuration, two additional residues (ProArg) occupy the TRIM5α N terminus. (B) Western blot analysis of TRIM5α expression in primary human CD4 T cells transduced with the indicated lentiviral vectors. TRIM5α expression in cells transduced with a control mCD28 167* construct, as well as in untransduced CD4 T cells, is also shown. Western blot analysis of cellular AKT levels was used as a loading control. All analyses were performed at day 6 posttransduction. (C) Single-round challenge of TRIM5α-transduced human CD4 T cells with the VSV-G-pseudotyped RFP-expressing lentiviral vector VRX1031. Primary human CD4 T cells were activated with anti-CD3/CD28-coated beads and transduced with the indicated TRIM5α-encoding lentiviral vectors. At 5 days posttransduction, the cells were challenged with serial dilutions of VRX1031. Nontransduced CD4 cells were also challenged. At 3 days postchallenge, cells were assayed for RFP expression by FACS. GFP expression is used as a marker for TRIM5α overexpression. The dilutions of added vector supernatant are indicated above the individual FACS plots. (D) Cell-free HIV-1 challenge of homogeneous TRIM5α-transduced human CD4 T cells. Primary human CD4 T cells were activated with anti-CD3/CD28-coated beads and transduced with the indicated TRIM5α lentiviral vectors. Control cell populations were mock transduced. AT 8 days poststimulation (7 days posttransduction), cells were challenged with cell-free HIV-1SF162 (SF162) or HIV-1BK132 (BK132) as described in Materials and Methods. At days 5 and 8 postchallenge, HIV-1 infection was assessed by intracellular HIV-1 Gag staining. GFP expression was used as a marker for TRIM5α overexpression. Percentages of cells staining positive for intracellular HIV-1 Gag are indicated. Results are representative of five independent experiments.
Purified human CD4 T cells were stimulated with anti-CD3/CD28-coated beads, transduced with TRIM5α-expressing lentiviral vectors, and expanded using conditions that permit CCR5 reexpression (7, 12). TRIM5α expression was examined at 6 days posttransduction by Western blotting and densitometry (Fig. 1B), using a polyclonal antiserum that recognizes both huTRIM5α and rhTRIM5α. We observed at least threefold overexpression of TRIM5α in transduced cells compared to untransduced cells or cells transduced with the control vector mCD28 167*. Before challenging human CD4 T cells with replication-competent HIV-1, we first determined whether introduced TRIM5α could protect primary human CD4 T cells from challenge with a replication-defective lentiviral vector. We observed efficient restriction of challenge with cell-free preparations of the RFP-encoding lentiviral vector VRX1031 in both rhTRIM5α- and huTRIM5αr323-332-expressing human CD4 T cells, with rhTRIM5α expression conferring the more potent resistance. In contrast, only modest restriction of the incoming RFP vector was seen in huTRIM5α-overexpressing human CD4 T cells (Fig. 1C). These data indicate that rhTRIM5α and huTRIM5αr323-332 can protect primary human CD4 T cells in a single-round challenge assay and are consistent with previously published observations (39).
We next performed challenge experiments with either cell-free HIV-1SF162 (an R5 strain) or the primary, highly cytopathic X4 isolate HIV-1BK132. Expression of rhTRIM5α in primary human CD4 T cells efficiently restricted both HIV-1SF162 and HIV-1BK132 cell-free viral challenge (Fig. 1D). Resistance to infection was robust: ∼40% of untransduced CD4 T cells became HIV-1SF162 infected, whereas HIV Gag expression remained at background levels in rhTRIM5α-expressing cells. CD4 T cells expressing huTRIM5αr323-332 were less susceptible to HIV-1SF162 infection, but they were not completely protected. huTRIM5αr323-332-expressing cells displayed only slight resistance to HIV-1BK132 infection, indicating that the huTRIM5αr323-332 antiviral effect is less potent than that of rhTRIM5α. In contrast, overexpression of huTRIM5α had little effect against either virus compared to untransduced cells. These data indicate that rhTRIM5α is a potent HIV-1 restriction factor when expressed in primary human CD4 T cells; furthermore, because huTRIM5αr323-332 was unable to completely recapitulate the effects of rhTRIM5α, our results imply that additional residues and regions within the rhTRIM5α PRY/SPRY (B30.2) domain contribute to the potency of HIV-1 restriction.
Human rhTRIM5α-expressing CD4 T cells are not protected from HIV-1 infection when cocultured with unmodified, HIV-1-infected human CD4 T cells.
To model an adoptive T-cell therapy scenario in which ex vivo expanded, modified CD4 T cells are infused into an environment of infected, unmodified CD4 T cells, we mixed equal numbers of TRIM5α-expressing cells and untransduced CD4 T cells and then performed cell-free HIV challenge to determine if rhTRIM5α-expressing cells were preferentially protected from HIV-1 infection. Surprisingly, we did not observe preferential protection of rhTRIM5α- or huTRIM5αr323-332-expressing CD4 T cells, as cocultured TRIM5α-expressing and untransduced cells contained similar levels of intracellular HIV-1 Gag (Fig. 2A). Moreover, we predicted that rhTRIM5α-expressing cells would be preferentially expanded in HIV-1-infected cultures, since they would be less sensitive to HIV-1 cytopathic effects. However, we observed minimal if any preferential expansion, as the ratio of rhTRIM5α-expressing cells to untransduced cells remained essentially unchanged throughout the experiment. To rule out that these findings were unique to HIV-1SF162 and HIV-1BK132, we performed identical experiments challenging the mixed T-cell cultures with HIV-1Ba-L and HIV-1US-1 and obtained similar results (data not shown).
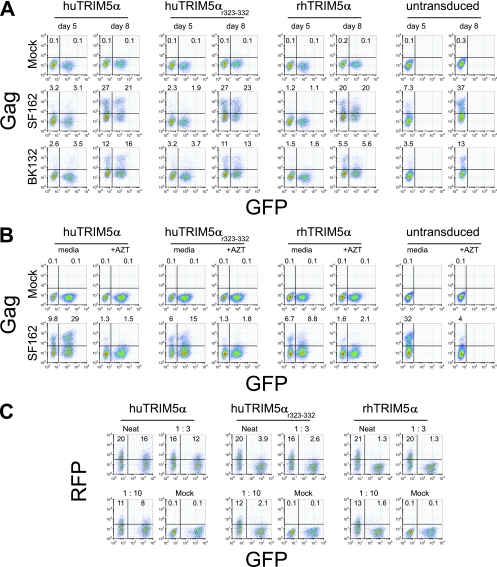
FIG. 2.
Human CD4 T cells expressing rhTRIM5α are not preferentially protected from HIV-1 infection when cocultured with untransduced cells. (A) Cell-free HIV-1 challenge of mixed cocultures of TRIM5α-overexpressing and untransduced human CD4 T cells. Primary human CD4 T cells were activated with anti-CD3/CD28-coated beads and transduced with the indicated vector. Portions of each culture were left as untransduced T cells. AT 7 days posttransduction, TRIM5α-expressing and untransduced cells were mixed at a 1:1 ratio and challenged with 100 ng p24/106 cells of cell-free HIV-1SF162 (SF162) or HIV-1BK132 (BK132). At days 5 and 8 postchallenge, HIV-1 infection was quantified by intracellular HIV-1 Gag staining. On the x axis, GFP was used as a marker for TRIM5α overexpression. The percentage of cells staining positive for intracellular HIV-1 Gag is indicated above each FACS plot and reflects the extent of HIV-1 infection in untransduced (GFP−) and TRIM5α-expressing (GFP+) CD4 T cells. (B) Effect of AZT on cell-associated HIV-1 challenge of TRIM5α-overexpressing human CD4 T cells. Primary human CD4 T cells were activated with anti-CD3/CD28-coated beads, and one half of the culture was transduced with the indicated lentiviral vector; the remainder was left as an untransduced culture. Untransduced cells were preinfected with 100 ng p24/106 cells of HIV-1SF162 (SF162) or HIV-1BaL (BaL) 3 days prior to mixing with uninfected TRIM5α-overexpressing CD4 T cells. TRIM5α-overexpressing cells were either pretreated with 10 μM AZT for 15 min or left untreated. After 3 days of coculture in the presence or absence of AZT, cell-associated HIV transmission was measured by HIV-1 Gag intracellular staining and FACS. (C) Single-round VSV-G-pseudotyped lentiviral vector challenge of mixed cocultures of TRIM5α-transduced and untransduced human CD4 T cells. Primary human CD4 T cells were activated with anti-CD3/CD28-coated beads and either transduced with the indicated TRIM5α-encoding lentiviral vector, or left as an untransduced population. At 5 days after transduction with TRIM5α-encoding lentiviral vectors, TRIM5α-overexpressing and untransduced CD4 T cells were mixed at a 1:1 ratio and then challenged with the indicated dilutions of cell-free RFP-encoding replication-defective lentiviral vector VRX1031. At 3 days postchallenge, cells were assayed for RFP expression by FACS. GFP expression is used as a marker for TRIM5α overexpression. Results are representative of five independent experiments.
To exclude the possibility that TRIM5α-expressing cells were simply taking up soluble HIV-1 Gag as a result of coculture with HIV-1-infected cells and thus staining positive for intracellular p24 without actually being infected, we pretreated TRIM5α-expressing cells with 10 μM AZT for 15 min prior to the addition of washed, robustly HIV-1SF162-infected (day 3 postinfection), untransduced CD4 T cells from the same donor (Fig. 2B). Cultures were stained for intracellular HIV-1 Gag after 3 days of coculture in the presence or absence of AZT. The presence of AZT reduced the total number of HIV-1 Gag-positive cells and severely limited the number of cells that expressed high levels of Gag, indicating that AZT treatment blocked viral replication and inhibited spread of the infection. The absence of HIV-1 Gag from transduced cells cultured in the presence of AZT, even when cocultured with productively infected cells, suggested that the ability to observe Gag staining (Fig. 2A) resulted from infection of TRIM5α-expressing cells rather than from uptake of released virions. These data indicate that rhTRIM5α antiviral activity is compromised in the presence of untransduced, HIV-1-infected CD4 T cells. In contrast, in single-round lentiviral challenge assays of mixed cocultures using a high-titer replication-defective vesicular stomatitis virus G protein (VSV-G)-pseudotyped RFP-encoding lentiviral vector, potent restriction was observed in rhTRIM5α- and huTRIM5αr323-332-expresssing cells (Fig. 2C); modest restriction was observed in huTRIM5α-overexpressing CD4 T cells despite coculture with untransduced CD4 T cells, comparable to that seen in uniform cultures (Fig. 1C). Clearly, in contrast to the results obtained with replication-competent HIV-1, coculture with untransduced human CD4 T cells did not compromise the ability of rhTRIM5α-expressing CD4 T cells to restrict incoming lentiviral vectors in single-round assays.
Cell-to-cell contact with HIV-infected, untransduced CD4 T cells is required to circumvent rhTRIM5α-mediated restriction of HIV-1 in human CD4 T cells.
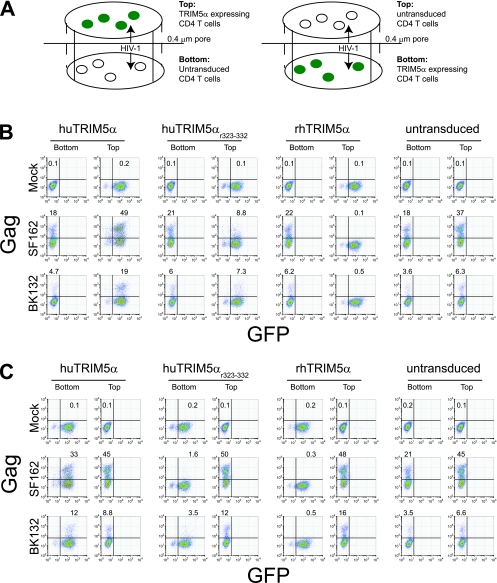
We considered two possibilities to explain how rhTRIM5α robustly protected pure populations of rhTRIM5α-expressing cells from HIV-1 infection yet failed to protect populations of rhTRIM5α-expressing cells when they were combined with untransduced CD4 T cells: (i) the untransduced, HIV-1-infected T cells continually produced cell-free HIV-1, which saturated the rhTRIM5α antiviral effect (37), or (ii) cell-associated HIV-1 transmission circumvents rhTRIM5α-mediated restriction. To distinguish between these two possibilities, we physically separated TRIM5α-expressing cells and untransduced CD4 T cells using a transwell apparatus, permitting HIV-1, but not cells, to pass from one chamber to the other. Both orientations of TRIM5α-expressing and untransduced CD4 cells within the transwell apparatus were examined (Fig. 3A). Cell-free HIV-1 was added to both chambers, and HIV-1 infection was monitored by intracellular HIV-1 Gag staining as before. Expression of rhTRIM5α in human CD4 T cells completely restricted cell-free HIV infection, despite the presence of robustly infected untransduced cells in the opposite transwell chamber, which resulted in continual cell-free viral challenge as well as exposure to soluble p24 (Fig. 3B). Expression of huTRIM5αr323-332 provided modest protection from HIV-1SF162 infection but little or no protection from HIV-1BK132 infection. Overexpression of huTRIM5α did not provide protection from either strain of HIV-1. In fact, slightly higher levels of HIV-1 Gag staining were seen in untransduced cells cocultured with huTRIM5α-expressing cells than in untransduced CD4 T cells cultured alone. Similar results were obtained when we switched the orientation of TRIM5α-expressing and untransduced cells (Fig. 3C), indicating that minor density-dependent effects on the spread of HIV-1 infection observed with cells cultured in the smaller upper chamber did not affect our findings. Together, these data suggest that cell-to-cell contact with HIV-1-infected untransduced CD4 T cells overcomes the rhTRIM5α antiviral effect, which potently inhibits infection with cell-free HIV-1.
FIG. 3.
Cell-to-cell contact with HIV-1-infected untransduced CD4 cells is required to escape rhTRIM5α-mediated restriction of HIV-1. (A) Schematic drawing of transwell experiment. TRIM5α-overexpressing cells express GFP. (B) Cell-free HIV-1 challenge of mixed cocultures of TRIM5α-overexpressing and untransduced CD4 T cells separated by a transwell membrane. Primary human CD4 T cells were activated with anti-CD3/CD28-coated beads and transduced with the indicated vector or left as an untransduced population. At 7 days posttransduction, huTRIM5α-, huTRIM5αr323-332-, or rhTRIM5α-expressing cells were placed in the top chamber and untransduced cells were placed in the bottom chamber of the 0.4-μm transwell apparatus. (C) The layout is reversed. HIV-1 challenge was performed by adding cell-free HIV-1SF162 (SF162) or HIV-1BK132 (BK132) to both wells, and the extent of HIV-1 infection was monitored by intracellular HIV Gag staining at days 5 and 8 postchallenge; results for day 8 are shown. Data are representative of five independent experiments.
Rhesus macaque CD4 T cells can be infected with HIV-1 via cell-to-cell contact with preinfected human CD4 T cells.
Rhesus macaque CD4 T cells are highly resistant to cell-free HIV-1 infection (5, 34). We also were unable to infect primary rhesus CD4 T cells with cell-free HIV-1SF162 under conditions that produced a spreading infection in primary human CD4 T cells (Fig. 4A). Similarly, primary rhesus CD4 T cells were also not infected with HIV-1 despite continual cell-free HIV-1SF162 challenge via coculture across a 0.4-μm transwell from preinfected human CD4 T cells, whereas initially uninfected human CD4 T cells became infected under these conditions (Fig. 4B).
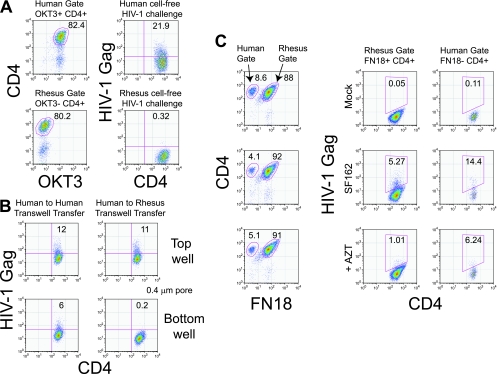
FIG. 4.
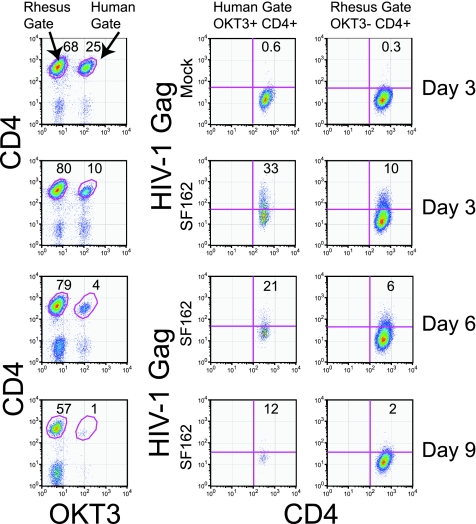
Rhesus CD4 T cells can be infected with HIV-1 via cell-to-cell contact with HIV-1-infected human CD4 T cells. (A) Cell-free HIV-1 challenge of cultures of primary human CD4 T cells or primary rhesus CD4 T cells. The right panels show HIV-1 Gag expression on day 7 postinfection with 100 ng p24/106 cells of cell-free HIV-1SF162. The left panels show the composition of the cultures on day 7 postinfection. (B) Cell-free HIV-1 challenge of human and rhesus CD4 T cells via coculture in opposite wells of a transwell apparatus with HIV-1SF162-preinfected human CD4 T cells. HIV-1SF162-infected human CD4 T cells were placed in the top chamber, while uninfected human and rhesus CD4 T cells were placed in the bottom chamber. HIV-1 transmission was assessed by intracellular HIV-1 Gag staining 3 days later. (C) HIV-1 challenge of rhesus CD4 T cells by direct coculture with HIV-1-preinfected human CD4 T cells. HIV-1SF162-infected human CD4 T cells were mixed at a 1:3 ratio with ConA/IL-2-stimulated rhesus CD4 T cells, and intracellular HIV-1 Gag staining was performed 3 days later. The gating strategy employed is depicted in the left panels. Measurement of intracellular Gag concentrations is shown in the right panels for rhesus and human CD4+ T-cell populations. The top panels depict rhesus cells cocultured with mock-infected human CD4 T cells, the middle panels depict cocultures between rhesus CD4 T cells and HIV-1SF162-infected human CD4 T cells, and the bottom panels represent cocultures between rhesus CD4 T cells and HIV-1SF162-infected human CD4 T cells conducted in the presence of 10 μM AZT.
The observation that cell-to-cell contact facilitated escape from rhTRIM5α-mediated restriction in human CD4 T cells raised the question of whether rhesus CD4 T cells could become infected by cell-to-cell contact with HIV-infected human CD4 T cells. To address this possibility, we mixed HIV-1SF162-infected human CD4 T cells with ConA- and IL-2-stimulated rhesus CD4 T cells. HIV-1 infection was then monitored every 3 days by intracellular HIV-1 Gag staining, with rhesus and human CD4 T-cell populations distinguished based on staining with rhesus-specific anti-CD3 antibody (FN-18), combined with the pan-reactive anti-CD4 monoclonal antibody L200. To our surprise, rhesus CD4 T cells were infected with HIV-1 after 3 days of coculture with HIV-1-infected human CD4 T cells (Fig. 4C). The disparity in outcomes between this coculture experiment and the Transwell experiment (Fig. 4B) indicates that cell-to-cell contact with preinfected human CD4 T cells and, presumably therefore, cell-associated HIV-1 transmission are sufficient to infect rhesus CD4 T cells with HIV-1. We also examined the ability of AZT to block cell-associated HIV-1 transmission to cocultured rhesus CD4 T cells (Fig. 4C). HIV-1SF162-preinfected human CD4 T cells were washed and then mixed with primary rhesus CD4 T cells that had been pretreated with 10 μM AZT for 15 min. The cells were cocultured in the presence of AZT for an additional 3 days. The ability to detect HIV-infected rhesus CD4 T cells was greatly reduced in the presence of AZT, indicating that AZT was able to block the further spread of HIV-1 infection from the preinfected human CD4 T cells and suggesting that the Gag positivity observed in the absence of AZT treatment represented actual HIV-1 infection of rhesus CD4 T cells.
HIV-infected human CD4 T cells cannot establish a sustained infection in cocultured rhesus CD4 T cells.
There has been considerable interest in using rhesus macaques as a model of HIV pathogenesis (21). However, because rhesus macaques are resistant to HIV-1 infection, SIV or SHIV strains have been employed almost exclusively in these studies, limiting the evaluation of agents that specifically target HIV-1 pathogenesis. Since rhesus CD4 T cells are susceptible to cell-to-cell transmission of HIV-1, we wanted to determine whether a sustainable, spreading infection of rhesus CD4 T cells ensued when a small number of HIV-1-infected human cells were mixed with rhesus CD4 T cells and allowed to expand for longer periods of time (Fig. 5). As the cultures expanded, the human CD4 T cells were diluted out, presumably due to the cytopathic effects of HIV-1. Notably, we were unable to observe a spreading HIV-1 infection among the rhesus CD4 T cells, suggesting that adoptive transfer of HIV-1-infected human CD4 T cells to rhesus monkeys would not lead to a productive infection. Thus, HIV-1 infection could be established via cell-to-cell transfer in cultures containing human and rhesus CD4 T cells but could not be propagated further among the rhesus CD4 T-cell population.
FIG. 5.
HIV-infected human CD4 T cells cannot establish a sustained infection in cocultured rhesus CD4 cells. HIV-1SF162-infected human CD4 T cells were combined with ConA/IL-2-stimulated rhesus CD4 T cells in a 1:3 ratio. The cultures were monitored by intracellular HIV Gag staining at 3-day intervals. The left panels show the gating strategy: human and rhesus CD4 cells were distinguished by staining with human anti-CD3 (OKT3) and the pan-CD4 antibody L200. The right panels depict intracellular HIV-1 Gag levels in human and rhesus CD4+ cell populations. Data shown are representative of three independent experiments.
HIV-1-infected rhTRIM5α-expressing cells produce infectious HIV.
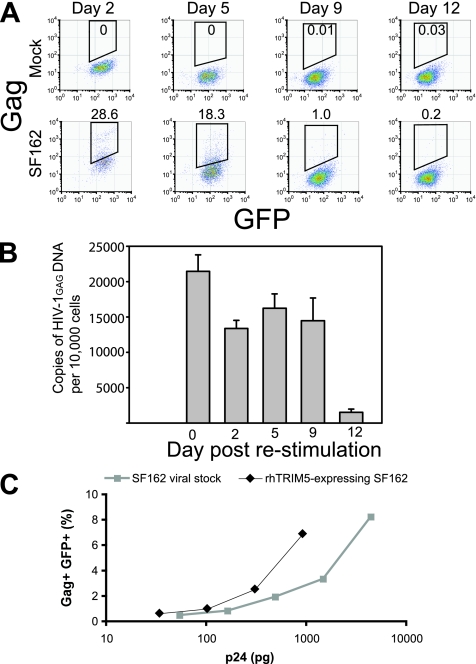
Our inability to establish a spreading HIV-1 infection within rhesus CD4 T cells suggested that cell-to-cell transmission of HIV-1 between infected and uninfected rhesus CD4 T cells was blocked. In addition to restricting infection by HIV-1, it has been reported that rhTRIM5α, when expressed in an HIV-1-infected cell, blocks the production but not the infectivity of released virions (32), but this is controversial (43). Therefore, we measured the ability of HIV-infected, rhTRIM5α-expressing human CD4 T cells to produce infectious virions (Fig. 6). rhTRIM5α-expressing human CD4 T cells were cocultured with untransduced human CD4 T cells and challenged with cell-free HIV-1SF162. Twelve days later, rhTRIM5α-expressing cells were collected by viable cell sorting. The sorted cells were restimulated with anti-CD3/CD28-coated beads and cultured for an additional 12 days. At early time points, the restimulated cultures contained many HIV Gag-positive cells, but by day 9, intracellular Gag was virtually undetectable (Fig. 6A). This is consistent with the observed inability of HIV-1 to establish a spreading infection in primary rhesus CD4 T cells (Fig. 5). At various time points after restimulation, cell pellets were analyzed for HIV-1 gag DNA by PCR (Fig. 6B). The cultures were strongly gag positive until day 9, but the gag copy number declined by approximately 10-fold by day 12, reinforcing the notion that HIV-1 is unable to establish a spreading infection in rhTRIM5α-expressing cells. Interestingly, we did observe that after 9 days of culture, intracellular p24 staining was reduced whereas HIV-1 gag DNA levels remained constant. This likely reflects the stability of HIV-1 gag DNA in this culture system. We also tried to culture huTRIM5α-expressing cells side by side with the rhTRIM5α-expressing cells. Unfortunately, these cultures died after 5 days due to cytopathic effects of the virus (data not shown). Next, we measured the ability of the HIV-1-infected, rhTRIM5α-expressing human CD4 T cells to produce infectious virions. Using culture supernatants harvested at 2 days after restimulation, we compared the infectivity of virions produced by HIV-infected rhTRIM5α-expressing CD4 T cells with the infectivity of a conventional HIV-1SF162 viral stock using CCR5-expressing GHOST cells (Fig. 6C). When normalized for p24 input, virus produced by rhTRIM5α-expressing human CD4 T cells was at least as infectious as a conventional viral stock. These data indicate that (i) rhTRIM5α-expressing CD4 T cells can be infected with HIV-1 and (ii) the infected cells produce infectious virions.
FIG. 6.
HIV-1-infected rhTRIM5α-expressing human CD4 T cells productive infectious HIV-1 upon restimulation. rhTRIM5α-expressing human CD4 T cells were cocultured with HIV-1SF162-infected, untransduced human CD4 T cells. Twelve days later, the cells were viably sorted and rhTRIM5α-positive cells (GFP+ DAPI− singlets) were collected. In parallel, rhTRIM5α-positive cells were purified from cocultures with mock-infected, untransduced human CD4 T cells. Sorted cells were washed extensively and restimulated with anti-CD3/CD28-coated beads. (A) Restimulated purified cell populations were analyzed for intracellular HIV-1 Gag expression at the indicated days after restimulation. Results are shown for cells purified from HIV-1-infected and mock-infected cocultures. (B) At the indicated days after restimulation, cells from the cultures in panel A were pelleted and assayed for gag DNA by real-time PCR. Cell copy numbers were determined by GAPDH gene PCR, and gag copy numbers were determined using an ACH-2 reference standard. Error bars indicate standard deviations.(C) GHOST-CCR5 cells were infected with supernatants from sort-purified HIV-1-infected rhTRIM5α-expressing CD4 cells at 48 h after restimulation. In parallel, GHOST-CCR5 cells were infected with a standard HIV-1SF162 viral stock preparation. Forty-eight hours later, the GHOST-CCR5 cells were analyzed for GFP and HIV-1 Gag expression by flow cytometry. Viral input (expressed as pg of p24 added) is displayed on the x axis. The percentage of GFP+/Gag+ GHOST-CCR5 cells is shown on the y axis.
The ability of HIV-1-infected rhTRIM5α-expressing human CD4 T cells to induce a spreading infection depends on the TRIM5α status of the target cell.
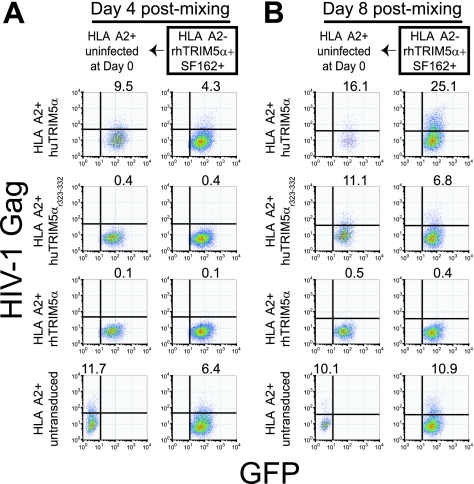
Our inability to establish a spreading HIV-1 infection within rhesus CD4 T cells suggested that cell-to-cell transmission of HIV-1 between infected and uninfected rhesus CD4 T cells was blocked. Our previous data (Fig. 4) indicating that rhTRIM5α can restrict cell-free but not cell-associated HIV-1 transmission were obtained using untransduced HIV-infected donor CD4 T cells. We next asked whether rhTRIM5α expression could prevent cell-associated HIV-1 spread if expressed in both donor and recipient cells (Fig. 7). Because rhesus CD4 T cells contain other restriction factors such as APOBEC3G (16), we used primary human CD4 T cells transduced with rhTRIM5α to examine the restrictive function of rhTRIM5α in isolation. We first purified HLA-A*02-negative, HIV-infected, rhTRIM5α-expressing human CD4 T cells, as described for Fig. 6. These cells were cultured with uninfected TRIM5α-expressing or untransduced CD4 T cells from an HLA-A*02 positive donor. Cocultures were then evaluated for the presence of a spreading HIV-1 infection, using cell surface HLA-A*02 expression to gate donor (HLA-A*02-negative) and recipient (HLA-A*02-positive) populations. At day 4 postmixing, we observed that sorted, HIV-1-infected rhTRIM5α-expressing human CD4 T cells could productively infect untransduced and huTRIM5α-overexpressing human CD4 T cells, and a spreading infection ensued in both recipient and donor populations (Fig. 7A). By day 8 postmixing, it was apparent that expression of huTRIM5αr323-332 in the recipient cell delayed but did not eliminate HIV-1 infection induced by coculture with sorted rhTRIM5α-expressing donor cells, as well as the spread of the infection between both donor and recipient populations. In contrast, there was little evidence of HIV-1 infection of fresh recipient rhTRIM5α-expressing cells cocultured with sorted rhTRIM5α-expressing donor cells at days 4 and 8 postmixing, and a spreading infection did not ensue in either HLA-A*02-positive recipient or HLA-A*02-negative donor populations; HIV-1 infection appeared to have been effectively contained (Fig. 7B). Together, these data suggest that rhTRIM5α expression in both donor and recipient human CD4 T cells can effectively block cell-associated transmission of HIV-1 and prevent the spread of HIV-1 infection.
FIG. 7.
HIV-1-infected rhTRIM5α-expressing human CD4 T cells are not able to efficiently transmit HIV-1 to uninfected rhTRIM5α-expressing human CD4 T cells. Human rhTRIM5α-expressing CD4 T cells from an HLA-A*02-negative individual were viably sorted (GFP+ DAPI− singlets) from an HIV-1SF162-infected mixed coculture with untransduced human CD4 T cells. Sorted rhTRIM5α-expressing human CD4 T cells were subsequently restimulated and mixed 1:1 with anti-CD3/CD28-stimulated and untransduced or TRIM5α-overexpressing human CD4 T cells from an HLA-A*02-positive individual; uninfected HLA-A*02-negative rhTRIM5α-expressing cells were manipulated similarly and used as a control in subsequent mixing experiments. HIV-1 infection in both HLA-A*02-positive target and HLA-A*02-negative donor CD4 T-cell populations was monitored by intracellular HIV-1 Gag staining at day 4 and at day 8 postmixing. HIV-1 Gag intracellular staining is shown on the y axis, and GFP expression is shown on the x axis. For each postmixing time point, two panels of FACS plots are shown. The left panel for each time point shows the HLA-A*02-positive target population; the right panel for each time point shows the HIV-1-infected HLA-A*02-negative rhTRIM5α-expressing donor population. Similar results were observed in other experiments (n = 3).
DISCUSSION
rhTRIM5α is a potent HIV-1 restriction factor, and as such, it has generated considerable attention as a potential anti-HIV therapeutic. The studies presented here reveal that while rhTRIM5α efficiently blocks infection by cell-free HIV-1 (Fig. 1), it is considerably less effective at preventing cell-associated HIV-1 transfer. As shown in Fig. 2 and 3, when rhTRIM5α is expressed only in target cells, it is unable to block infection by cell-associated HIV-1. Furthermore, primary rhesus CD4 cells can also be infected by coculture with HIV-1-infected human CD4 cells (Fig. 4). However, rhTRIM5α is able to block HIV transmission by either cell-free or cell-associated mechanisms when it is expressed in both donor and recipient cells (Fig. 5, 6, and 7), explaining why spreading infections do not ensue when a small amount of infected human cells is mixed with rhesus CD4 cells. Thus, our studies indicate that rhTRIM5α is the first cell-intrinsic antiviral mechanism of which we are aware that can block one form of cell entry (cell free) but not another (cell associated).
While HIV-1 can be transmitted via cell-free or cell-associated mechanisms, robust HIV-1 infection requires cell-to-cell spread, at least in vitro (36). Cell-free HIV-1 can be restricted very efficiently by rhTRIM5α (37), although it is saturable. It is conceivable that the inability of rhTRIM5α to restrict cell-associated HIV-1 transmission is solely a consequence of saturating rhTRIM5α's functional capacity, perhaps due to locally high virion concentration at the site of cell contact. However, other factors could contribute to the differential ability of rhTRIM5α to restrict cell-free versus cell-associated infection. Since the virological synapse shares many features with the immunological synapse (31), it is likely that significant sections of the membrane of the donor cell are transferred with HIV-1 to the recipient cell during cell-to-cell transfer (33). This membrane component may form a shield that protects the incoming virus from rhTRIM5α restriction and thus allows infection of rhTRIM5α-expressing cells.
Furthermore, we also established that TRIM5α-mediated restriction of cell-to-cell HIV-1 transmission requires rhTRIM5α expression in both donor and recipient cells. Sakuma et al. observed that rhTRIM5α overexpression reduces virion production by infected cells (32), and we observed less p24 HIV-1GAG expression in HIV-1-infected rhTRIM5α-expressing cells (data not shown). However, we also observed that virions produced by HIV-1-infected rhTRIM5α-expressing cells, on a p24-normalized basis, were at least as infectious as viral stocks prepared in human PBMCs (Fig. 6). Therefore, the presence of rhTRIM5α in the donor cell would presumably reduce the number of virions available for cell-to-cell transmission, perhaps below the threshold required to saturate the rhTRIM5α present in the recipient cell. This is one scenario that could explain how transmission of HIV-1 from an infected rhTRIM5α donor cell to an rhTRIM5α-expressing recipient cell is blocked. Another possible explanation is that the presence of huTRIM5α or rhTRIM5α in the producer cell influences or augments the activity of TRIM5α restriction in the recipient cell. It is known that rhTRIM5α becomes incorporated into virions (32), and this amount of virion-associated rhTRIM5α may be sufficient to block cell-to-cell infection of rhTRIM5α-expressing but not huTRIM5α-expressing cells. In other words, the additional virion-associated rhTRIM5α transferred may tip the balance so that rhTRIM5α-expressing cells can block cell-to-cell transmission of HIV-1 infection. Our data, when combined with these previous observations, suggest but do not prove that factors other than virion concentration may contribute to the differential ability of rhTRIM5α to restrict cell-free and cell-associated infections. In fact, these postulated mechanisms are not mutually exclusive, and indeed they may work in unison to negate the effects of rhTRIM5α during cell-to-cell transmission.
The data presented here provide some insight into the circumstances in which rhTRIM5α may be a useful in vivo anti-HIV therapeutic. Given the fact that only a small fraction of the total T-cell compartment can be reconstituted using current adoptive T-cell therapy guidelines (20, 23), we would predict that rhTRIM5α-expressing cells would not be preferentially protected from HIV-1 infection, since cell-associated transmission plays a pivotal role in transmitting HIV in vivo (17). There is even less optimism for this approach when one considers that cells expressing rhTRIM5α may be immunogenic and thus one would be forced to use a less effective version such as huTRIM5αr323-332. Our studies show that while huTRIM5αr323-332 expression has antiviral effects, it is clearly not as potent as rhTRIM5α. This finding was not unexpected, as other studies have shown that variable regions within the PRY/SPRY (B30.2) domain beside V1 were required for full antiviral activity (24, 29). However, there is still room for optimism regarding the use of rhTRIM5α expression in immune reconstitution. Stem cell therapy (1, 2) or future T-cell adoptive therapy approaches that could repopulate the vast majority of T cells with rhTRIM5α activity would likely be very effective at controlling and possibly eliminating HIV-1 infection, since these cells would be protected from both cell-free and cell-associated HIV-1 infection.
Acknowledgments
This work was supported by NIH/NIAID grant U19AI066290. M.S. and J.S. were funded by a grant (AI63987) from the National Institutes of Health. M.S. was supported by a National Defense Science and Engineering Fellowship and was a Fellow of the Ryan Foundation.
We thank Tatiana Golovina, Tatiana Mikheeva, and Chanelle Case from the Penn-CFAR Immunology Core for providing primary human T cells and Farida Shaheen from Penn-CFAR Virology Core for p24 analysis; Paul Hallberg from the BSL3 Cell Sorting Facility for sorting the HIV-1-infected T cells; James Blanchard and Andrew Lackner from the Tulane National Primate Center for reagents and expertise pertaining to the use of rhesus T cells; and Angel Varela-Rohena, Carl June, Gwen Binder, Bob Doms, and Jim Hoxie for helpful suggestions and discussions.
Footnotes
Published ahead of print on 3 September 2008.
REFERENCES
- 1.Anderson, J., and R. Akkina. 2005. TRIM5alpharh expression restricts HIV-1 infection in lentiviral vector-transduced CD34+-cell-derived macrophages. Mol. Ther. 12687-696. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, J., and R. Akkina. 2008. Human immunodeficiency virus type 1 restriction by human-rhesus chimeric tripartite motif 5alpha (TRIM 5alpha) in CD34(+) cell-derived macrophages in vitro and in T cells in vivo in severe combined immunodeficient (SCID-hu) mice transplanted with human fetal tissue. Hum. Gene Ther. 19217-228. [DOI] [PubMed] [Google Scholar]
- 3.Anderson, J. L., E. M. Campbell, A. Figueiredo, and T. J. Hope. 2008. Heat shock perturbs TRIM5α restriction of human immunodeficiency virus type 1. J. Virol. 822575-2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson, J. L., E. M. Campbell, X. Wu, N. Vandegraaff, A. Engelman, and T. J. Hope. 2006. Proteasome inhibition reveals that a functional preintegration complex intermediate can be generated during restriction by diverse TRIM5 proteins. J. Virol. 809754-9760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Besnier, C., Y. Takeuchi, and G. Towers. 2002. Restriction of lentivirus in monkeys. Proc. Natl. Acad. Sci. USA 9911920-11925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell, R. E., O. Tour, A. E. Palmer, P. A. Steinbach, G. S. Baird, D. A. Zacharias, and R. Y. Tsien. 2002. A monomeric red fluorescent protein. Proc. Natl. Acad. Sci. USA 997877-7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carroll, R. G., J. L. Riley, B. L. Levine, Y. Feng, S. Kaushal, D. W. Ritchey, W. Bernstein, O. S. Weislow, C. R. Brown, E. A. Berger, C. H. June, and L. St. 1997. Differential regulation of HIV-1 fusion cofactor expression by CD28 costimulation of CD4+ T cells. Science 276273-276. [DOI] [PubMed] [Google Scholar]
- 8.Cecilia, D., V. N. KewalRamani, J. O'Leary, B. Volsky, P. Nyambi, S. Burda, S. Xu, D. R. Littman, and S. Zolla-Pazner. 1998. Neutralization profiles of primary human immunodeficiency virus type 1 isolates in the context of coreceptor usage. J. Virol. 726988-6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chatterji, U., M. D. Bobardt, P. Gaskill, D. Sheeter, H. Fox, and P. A. Gallay. 2006. Trim5alpha accelerates degradation of cytosolic capsid associated with productive HIV-1 entry. J. Biol. Chem. 28137025-37033. [DOI] [PubMed] [Google Scholar]
- 10.Chemnitz, J. M., A. R. Lanfranco, I. Braunstein, and J. L. Riley. 2006. B and T lymphocyte attenuator-mediated signal transduction provides a potent inhibitory signal to primary human CD4 T cells that can be initiated by multiple phosphotyrosine motifs. J. Immunol. 1766603-6614. [DOI] [PubMed] [Google Scholar]
- 11.Clouse, K. A., D. Powell, I. Washington, G. Poli, K. Strebel, W. Farrar, P. Barstad, J. Kovacs, A. S. Fauci, and T. M. Folks. 1989. Monokine regulation of human immunodeficiency virus-1 expression in a chronically infected human T cell clone. J. Immunol. 142431-438. [PubMed] [Google Scholar]
- 12.Creson, J. R., A. A. Lin, Q. Li, D. F. Broad, M. R. Roberts, and S. J. Anderson. 1999. The mode and duration of anti-CD28 costimulation determine resistance to infection by macrophage-tropic strains of human immunodeficiency virus type 1 in vitro. J. Virol. 739337-9347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dimitrov, D. S., R. L. Willey, H. Sato, L. J. Chang, R. Blumenthal, and M. A. Martin. 1993. Quantitation of human immunodeficiency virus type 1 infection kinetics. J. Virol. 672182-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dull, T., R. Zufferey, M. Kelly, R. J. Mandel, M. Nguyen, D. Trono, and L. Naldini. 1998. A third-generation lentivirus vector with a conditional packaging system. J. Virol. 728463-8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goff, S. P. 2004. Retrovirus restriction factors. Mol. Cell 16849-859. [DOI] [PubMed] [Google Scholar]
- 16.Goff, S. P. 2004. Genetic control of retrovirus susceptibility in mammalian cells. Annu. Rev. Genet. 3861-85. [DOI] [PubMed] [Google Scholar]
- 17.Haase, A. T. 1999. Population biology of HIV-1 infection: viral and CD4+ T cell demographics and dynamics in lymphatic tissues. Annu. Rev. Immunol. 17625-656. [DOI] [PubMed] [Google Scholar]
- 18.James, L. C., A. H. Keeble, Z. Khan, D. A. Rhodes, and J. Trowsdale. 2007. Structural basis for PRYSPRY-mediated tripartite motif (TRIM) protein function. Proc. Natl. Acad. Sci. USA 1046200-6205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jolly, C., K. Kashefi, M. Hollinshead, and Q. J. Sattentau. 2004. HIV-1 cell to cell transfer across an Env-induced, actin-dependent synapse. J. Exp. Med. 199283-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.June, C. H. 2007. Principles of adoptive T cell cancer therapy. J. Clin. Investig. 1171204-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LaBonte, J. A., G. J. Babcock, T. Patel, and J. Sodroski. 2002. Blockade of HIV-1 infection of New World monkey cells occurs primarily at the stage of virus entry. J. Exp. Med. 196431-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levine, B. L., W. B. Bernstein, N. E. Aronson, K. Schlienger, J. Cotte, S. Perfetto, M. J. Humphries, S. Ratto-Kim, D. L. Birx, C. Steffens, A. Landay, R. G. Carroll, and C. H. June. 2002. Adoptive transfer of costimulated CD4+ T cells induces expansion of peripheral T cells and decreased CCR5 expression in HIV infection. Nat. Med. 847-53. [DOI] [PubMed] [Google Scholar]
- 23.Levine, B. L., L. M. Humeau, J. Boyer, R. R. MacGregor, T. Rebello, X. Lu, G. K. Binder, V. Slepushkin, F. Lemiale, J. R. Mascola, F. D. Bushman, B. Dropulic, and C. H. June. 2006. Gene transfer in humans using a conditionally replicating lentiviral vector. Proc. Natl. Acad. Sci. USA 10317372-17377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li, Y., X. Li, M. Stremlau, M. Lee, and J. Sodroski. 2006. Removal of arginine 332 allows human TRIM5α to bind human immunodeficiency virus capsids and to restrict infection. J. Virol. 806738-6744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu, X., L. Humeau, V. Slepushkin, G. Binder, Q. Yu, T. Slepushkina, Z. Chen, R. Merling, B. Davis, Y. N. Chang, and B. Dropulic. 2004. Safe two-plasmid production for the first clinical lentivirus vector that achieves >99% transduction in primary cells using a one-step protocol. J. Gene Med. 6963-973. [DOI] [PubMed] [Google Scholar]
- 26.Michael, N. L., J. A. Nelson, V. N. KewalRamani, G. Chang, S. J. O'Brien, J. R. Mascola, B. Volsky, M. Louder, G. C. White, D. R. Littman, R. Swanstrom, and T. R. O'Brien. 1998. Exclusive and persistent use of the entry coreceptor CXCR4 by human immunodeficiency virus type 1 from a subject homozygous for CCR5 Δ32. J. Virol. 726040-6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morita, E., and W. I. Sundquist. 2004. Retrovirus budding. Annu. Rev. Cell Dev. Biol. 20395-425. [DOI] [PubMed] [Google Scholar]
- 28.Nisole, S., J. P. Stoye, and A. Saib. 2005. TRIM family proteins: retroviral restriction and antiviral defence. Nat. Rev. Microbiol. 3799-808. [DOI] [PubMed] [Google Scholar]
- 29.Ohkura, S., M. W. Yap, T. Sheldon, and J. P. Stoye. 2006. All three variable regions of the TRIM5α B30.2 domain can contribute to the specificity of retrovirus restriction. J. Virol. 808554-8565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parry, R. V., C. A. Rumbley, L. H. Vandenberghe, C. H. June, and J. L. Riley. 2003. CD28 and inducible costimulatory protein Src homology 2 binding domains show distinct regulation of phosphatidylinositol 3-kinase, Bcl-xL, and IL-2 expression in primary human CD4 T lymphocytes. J. Immunol. 171166-174. [DOI] [PubMed] [Google Scholar]
- 31.Piguet, V., and Q. Sattentau. 2004. Dangerous liaisons at the virological synapse. J. Clin. Investig. 114605-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sakuma, R., J. A. Noser, S. Ohmine, and Y. Ikeda. 2007. Rhesus monkey TRIM5alpha restricts HIV-1 production through rapid degradation of viral Gag polyproteins. Nat. Med. 13631-635. [DOI] [PubMed] [Google Scholar]
- 33.Sherer, N. M., M. J. Lehmann, L. F. Jimenez-Soto, C. Horensavitz, M. Pypaert, and W. Mothes. 2007. Retroviruses can establish filopodial bridges for efficient cell-to-cell transmission. Nat. Cell Biol. 9310-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shibata, R., M. Kawamura, H. Sakai, M. Hayami, A. Ishimoto, and A. Adachi. 1991. Generation of a chimeric human and simian immunodeficiency virus infectious to monkey peripheral blood mononuclear cells. J. Virol. 653514-3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sol-Foulon, N., M. Sourisseau, F. Porrot, M. I. Thoulouze, C. Trouillet, C. Nobile, F. Blanchet, V. di Bartolo, N. Noraz, N. Taylor, A. Alcover, C. Hivroz, and O. Schwartz. 2007. ZAP-70 kinase regulates HIV cell-to-cell spread and virological synapse formation. EMBO J. 26516-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sourisseau, M., N. Sol-Foulon, F. Porrot, F. Blanchet, and O. Schwartz. 2007. Inefficient human immunodeficiency virus replication in mobile lymphocytes. J. Virol. 811000-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stremlau, M., C. M. Owens, M. J. Perron, M. Kiessling, P. Autissier, and J. Sodroski. 2004. The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature 427848-853. [DOI] [PubMed] [Google Scholar]
- 38.Stremlau, M., M. Perron, M. Lee, Y. Li, B. Song, H. Javanbakht, F. az-Griffero, D. J. Anderson, W. I. Sundquist, and J. Sodroski. 2006. Specific recognition and accelerated uncoating of retroviral capsids by the TRIM5alpha restriction factor. Proc. Natl. Acad. Sci. USA 1035514-5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stremlau, M., M. Perron, S. Welikala, and J. Sodroski. 2005. Species-specific variation in the B30.2(SPRY) domain of TRIM5α determines the potency of human immunodeficiency virus restriction. J. Virol. 793139-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Szymczak, A. L., C. J. Workman, Y. Wang, K. M. Vignali, S. Dilioglou, E. F. Vanin, and D. A. Vignali. 2004. Correction of multi-gene deficiency in vivo using a single ‘self-cleaving’ 2A peptide-based retroviral vector. Nat. Biotechnol. 22589-594. [DOI] [PubMed] [Google Scholar]
- 41.Wu, X., J. L. Anderson, E. M. Campbell, A. M. Joseph, and T. J. Hope. 2006. Proteasome inhibitors uncouple rhesus TRIM5alpha restriction of HIV-1 reverse transcription and infection. Proc. Natl. Acad. Sci. USA 1037465-7470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yap, M. W., S. Nisole, and J. P. Stoye. 2005. A single amino acid change in the SPRY domain of human Trim5alpha leads to HIV-1 restriction. Curr. Biol. 1573-78. [DOI] [PubMed] [Google Scholar]
- 43.Zhang, F., D. Perez-Caballero, T. Hatziioannou, and P. D. Bieniasz. 2008. No effect of endogenous TRIM5alpha on HIV-1 production. Nat. Med. 14235-236. [DOI] [PubMed] [Google Scholar]