Abstract
In order to analyze whether measles virus (MV) is transported via transmigrating leukocytes across endothelial barriers or whether virus spreads via infection of endothelial cells and basolateral release, we investigated the migratory behavior of infected human primary T lymphocytes across polarized cell layers of human brain microvascular endothelial cells. We found that the capacity of lymphocytes to migrate through filter pores was only slightly affected by wild-type MV infection, whereas their capacity to migrate through endothelial barriers was drastically reduced. MV infection stimulated the expression and activation of the leukocyte integrins LFA-1 and VLA-4, mediating a strong adherence to the surface of endothelial cells. Furthermore, the formation of engulfing membrane protrusions by endothelial cells, so-called transmigratory cups, was induced, but transmigration was impaired. As a consequence of this close cell-cell contact, MV infection was transmitted from lymphocytes to the endothelium. MV envelope proteins were expressed on the apical and basolateral surfaces of infected polarized endothelial cells, and virus was released from both sides. Wild-type MV infection did not induce the formation of syncytia, suggesting virus spread from cell to cell via cell processes and contacts. Our data indicate that transendothelial migration of infected T cells is strongly inhibited, whereas virus can cross endothelial barriers by productive infection of the endothelium and subsequent bipolar virus release.
Leukocyte transmigration across vascular endothelium is a highly regulated process and is central to inflammation and the immune response (23, 24). Underlying mechanisms such as interaction between adhesion molecules of both cell types and cytoskeletal rearrangements may be deregulated by viral infections. After transmission to the respiratory tract, measles virus (MV) is transported via dendritic cells or monocytes to draining lymph nodes, where a massive infection in CD150-positive activated dendritic cells, lymphocytes, and macrophages is initiated (10, 13, 22, 38). The virus is then carried via infected leukocytes in the bloodstream to different organs, thereby establishing the systemic infection. To distribute infection into various organs, including the skin and the brain, MV must overcome endothelial cell barriers either within an infected leukocyte transmigrating through the barrier as a “Trojan horse” or by infection of endothelial cells and basolateral (abluminal) virus release or cell-to-cell spread. During the rash, MV-infected microvascular endothelial cells of the skin have been observed (18, 39); however, from these histological analyses it is not clear whether virus disseminates from there to underlying epithelial cell layers, leading to local cellular infiltrations, or whether the virus is first carried to epithelial cell layers via transmigrating leukocytes.
MV is a highly immunosuppressive virus known to impair various functions of leukocytes, but it is not known if the infection affects transendothelial migration. MV-induced immunosuppression is a multifactorial process influenced by virus-induced lymphopenia, inhibition of T-cell proliferation, cytokine imbalance, and suppression of effector functions (for a review, see reference 32). Interestingly, the proliferative inhibition is not necessarily based on the infection of peripheral blood mononuclear cells (PBMC) but can be induced by surface contact with MV envelope proteins (hemagglutinin H and fusion protein F) present on virus particles or on the surface of infected cells (31). The glycoproteins of both vaccine and wild-type MV strains induce the inhibition of cellular Akt kinase and downstream pathways in vitro (3, 4). Within minutes, contact of T cells with intact or UV-inactivated MV impedes their capacity for the cytoskeletal remodeling required for cell spreading, polarization, and CD3 clustering (27). It is not known how long this effect lasts and whether and for how long MV may affect chemokine-dependent motility and migration of T cells. Transendothelial migration beginning with rolling, arrest, adhesion strengthening, and spreading of leukocytes at endothelial surfaces depends on proper signal transduction and the activation of adhesion receptors such as LFA-1 and VLA-4 that are required for full cytoskeletal dynamics of the attracted leukocyte (9, 20, 23, 33, 34), mechanisms that might be impaired by MV.
Hypothetically, MV may be transported via patrolling infected leukocytes or via infection of the endothelium across endothelial cell barriers. We investigated the effects of MV infection in tissue culture on the capacity of primary human T cells to migrate against chemokine gradients and to transmigrate through layers of polarized endothelial cells. We found that MV infection specifically blocked the transmigratory capacity of infected leukocytes and induced an enhanced integrin-mediated adhesion to endothelial cells. The close contact of infected leukocytes to endothelial cells led to the efficient infection of endothelial cells and apical and basolateral virus release, enabling virus spread to the abluminal side of the endothelium.
MATERIALS AND METHODS
Cells and viruses.
Vero (African green monkey) cells and Vero cells expressing human CD150 (Vero-hSLAM; a kind gift of Y. Yanagi, Kyushu University, Fukuoka, Japan) (29) were grown in Eagle's minimal essential medium (Gibco) supplemented with 10% fetal calf serum (Biochrom), 100 U/ml penicillin, and 100 μg/ml streptomycin. Human brain microvascular endothelial cells (HBMEC) and human umbilical vein endothelial cells (HUVEC) were cultured in an endothelial cell basal medium 2 kit (PromoCell). HUVEC were prepared from umbilical cords obtained from the maternity ward of the University Hospital, Würzburg, with permission of the ethics council of the university, as described previously (2, 25). Primary human PBMC obtained from buffy coats of healthy adult volunteers were diluted 1:6 in Versene (GIBCO) and enriched with lymphocyte separation medium LSM 1077 (PAA Laboratories GmbH, Pasching, Austria). Fresh PBMC were washed three times with phosphate-buffered saline (PBS) and suspended in RPMI 1640 (GIBCO) medium containing 10% fetal calf serum. Cells were stimulated with phytohemagglutinin L (PHA) (Roche Diagnostics) at a concentration of 5 μg/ml for 48 h before infection. Activation of leukocytes was controlled by flow cytometry, monitoring the activation markers CD69 and anti-CD150. For calcein labeling, 106 PBMC in 5 ml were incubated with 5 μl of calcein solution (1 mg/ml in DMSO; Molecular Probes) for 15 min at 37°C and then washed three times with medium. The recombinant wild-type MV expressing enhanced green fluorescent protein (GFP) (rMVIC323eGFP) was grown and titrated using Vero-hSLAM cells (a kind gift of Y. Yanagi, Kyushu University, Fukuoka, Japan) (14). For UV inactivation of MV, 1 ml of virus solution in a 35-mm dish was irradiated with UV light (1.5 J/cm2) for 30 min at room temperature.
Antibodies.
Subpopulations of leukocytes were determined with directly labeled (phycoerythrin conjugated) monoclonal antibodies (MAbs) to CD3, CD19, and CD14 (BD Biosciences). As activation markers we used antibodies to CD69 (BD Biosciences) and CD150 (SLAM; clone 5C6) (11). In addition, we used antibodies against ICAM-1 (Chemicon), VCAM-1 (Chemicon), and zona occludens-1 (ZO-1) (Zytomed Systems). As LFA-1 (CD11a/CD18) antibodies we used anti-activated αL-integrin (CD11a) antibody clone NKI-L16 in the presence of 1 mM Ca2+ (a kind gift of T. Geijtenbeek) (17), anti-CD11a (BD Biosciences), and anti-CD18 (β2-integrin; Chemicon). As VLA-4 (CD49d/CD29) antibodies we used anti-CD29 (β1-integrin; Serotec) and the activated β1-integrin-specific clone HUTS4 (Chemicon). MV hemagglutinin (H) was detected using MAb K83, and MV fusion protein (F) was detected using MAb A504 (our laboratory). For costainings, we used biotinylated primary antibodies against VCAM-1 (Acris) and Alexa-594-conjugated strepavidin (Acris) and CD29-specific primary and Alexa-633-conjugated goat anti-mouse antibodies (Invitrogen). Adhesion-inhibiting MAbs used in the adhesion assay were anti-CD18 (CBL 18; Millipore), anti-CD29 (MAB 1987; Millipore), anti-VCAM-1 (CBL 206; Millipore), and anti-ICAM-1 (MAB 2146Z; Millipore).
Flow cytometry.
Flow cytometry (fluorescence-activated cell sorter [FACS] analysis) was done as described previously (2). Briefly, to evaluate the transmigration of T lymphocytes, an aliquot of 5 × 104 PBMC was stained with phycoerythrin-conjugated antibodies to CD3 and 7-aminoactinomycin (7AAD). Dot plots were gated on CD3-positive T lymphocytes, excluding 7AAD-positive dead cells.
Transmigration assay.
For the transmigration assays, 1 × 106 cells were seeded on a six-well cell culture filter insert (transwell filter) with an 8-μm pore size (Falcon, BD Bioscience) 3 days prior to addition of leukocytes. The confluence of the endothelial cell monolayers was assessed by measuring their permeability to 4-kDa fluorescein isothiocyanate (FITC)-dextran (Sigma) and by staining of tight-junction proteins such as ZO-1 with anti-ZO-1 (Zytomed Systems). Polarization was controlled by demonstrating different patterns of surface proteins on the apical and basolateral membranes of polarized cells (see “Biotinylation of surface proteins on polarized endothelial cells” below). Confluent HBMEC were stimulated with human tumor necrosis factor alpha (TNF-α) (25 ng/ml; Strathmann Biotech) for 6 h to induce ICAM-1. Recombinant human MCP-1 (5 ng/ml; PromoKine) and RANTES (5 ng/ml; PromoKine) were added to the basolateral medium. PBMC (1 × 106 per well) were added to the top chamber and allowed to transmigrate for 16 h at 37°C in a humid atmosphere with 5% CO2. Transmigrated cells in samples from the bottom chamber were counted by microscopy using a hemacytometer. Percentages of leukocyte subpopulations and infection rates (GFP positivity) were determined by flow cytometry (FACSCalibur) and CellQuest analysis software at 48 h postinfection (p.i.).
Adhesion assay.
HBMEC (1 × 104) were seeded in black 96-well flat transparent-bottom plates (Greiner) and cultured overnight until confluence. Six hours before the adhesion assay, HBMEC were stimulated with TNF-α (2 ng/ml). Uninfected and MV-infected (rMVIC323eGFP; multiplicity of infection [MOI] = 1) cells were labeled for 15 min at 37°C with R18 (4 μM in RPMI medium containing 10% fetal calf serum; Molecular Probes). Labeled PBMC were washed three times with RPMI, and incubated with adhesion-inhibiting antibodies at a dilution of 1:30 for 1 h at 37°C. A total of 2 × 105 leukocytes per well were added to the endothelial cells. Adhesion was allowed to occur for 1 h at 37°C. Nonadherent PBMC were removed by washing three times with 100 μl PBS for 2 min on a rotator. Fluorescence was measured at a wavelength of 560 nm (excitation) and 590 nm (emission) using a Safire II fluorometer (Tecan). Background fluorescence was determined after adhesion of unlabeled PBMC under the same conditions and subtracted.
Confocal microscopy.
For confocal microscopy, endothelial cells were grown on transwell filter units, fixed for 10 min with 4% paraformaldehyde, permeabilized with 0.25% Triton X-100 for 10 min, washed three times with PBS, and blocked with 5% bovine serum albumin for 1 h. Cells were incubated for 1 h at 4°C with primary MAbs and 1 h at 4°C with Alexa-594-conjugated goat anti-mouse secondary antibodies (Invitrogen). After final washing steps in PBS and finally water, fluorochrome G (Southern Biotech) was used as mounting medium, and cells were analyzed by confocal laser scanning microscopy (Laser Scan Microscope LSM510 Meta; Zeiss). Vertical Z-stacks were acquired (20 optical planes, 0.5-μm distance), and three-dimensional reconstructions and deconvolutions were performed using the Zeiss software.
Biotinylation of surface proteins on polarized endothelial cells.
Endothelial cells (1 × 106) were seeded on transwell filters (0.4-μm pore size; Falcon), cultured for 3 days to establish a tight monolayer, and selectively labeled from either the apical or the basolateral side with biotin (sulfo-NHS-LC-biotin; Pierce) as described previously (26). Cells were lysed in lysis buffer (1% NP-40, 50 mM HEPES, 0.125 M NaCl, 1 mM EDTA) containing a protease inhibitor tablet (Boehringer) to achieve a concentration of 1 mg/ml protein and centrifuged at 100,000 × g, and supernatants were mixed with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer. Proteins were separated by 12% SDS-PAGE, and the polarization of the cells was controlled by demonstrating the presence of several different biotinylated surface proteins on the apical and basolateral sides by Western blotting using streptavidin-horseradish peroxidase and chemiluminescence (West Pico SuperSignal).
RESULTS
Effect of MV infection on T-cell migration.
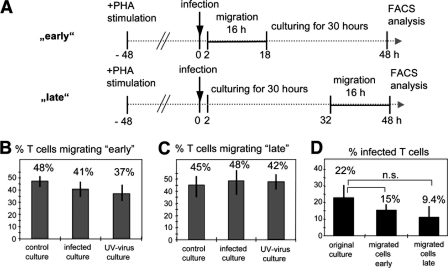
We first analyzed the capacity of T lymphocytes to migrate across transwell filters through 8-μm pores toward the chemokines MCP-1 and RANTES in the absence of endothelial cells. Migration of PHA-activated uninfected PBMC, PBMC infected with recombinant wild-type MV IC323 expressing enhanced GFP (rMVIC323eGFP) at an MOI of 1.0, and PBMC treated with UV-inactivated virus corresponding to the same MOI was assessed during two time periods of 16 h: “early” (2 to 18 h) after infection, when small amounts of viral proteins are present in the infected cells, and “late” (32 to 48 h p.i.), when large amounts of viral proteins have been synthesized and virus is released but most of the infected cells are still viable (Fig. 1A). To determine the infection rate, the percentage of GFP-expressing cells in an original aliquot of infected PBMC (not undergoing the migration assay) was quantified by FACS analysis at 48 h p.i. In order to analyze exclusively living T lymphocytes, FACS analyses were done by gating on CD3-positive T lymphocytes and excluding 7AAD-positive cells. There were no significant differences in the migratory capacities of uninfected, infected, and UV-inactivated virus-treated cultures, and 37 to 48% of input T cells migrated within the 16-h “early” and “late” test periods (Fig. 1B and C). The percentage of infected T cells was 22% in the original culture not undergoing the migration assay and was 15% and 9.4% in the populations of “early” and “late” migrated cells, respectively (Fig. 1D). Although fewer infected cells were detected in samples of migrated cells, these differences were not statistically significant (P = 0.15). Therefore, the data indicate that virus contact and infection have no major effect on T-cell migration per se and that there is only a statistically nonsignificant tendency for infected T cells to migrate less efficiently toward chemokine gradients.
FIG. 1.
Effect of wild-type MV on the migration of T cells through porous filter membranes. (A) Experimental setup. After 48 h of prestimulation with PHA, human PBMC were infected with recombinant wild-type MV (rMVIC323eGFP) at an MOI of 1.0 or incubated with a corresponding amount of UV-inactivated virus. Cells were seeded in the upper compartments of transwell filters. The migration through porous filter membranes was analyzed “early” (migration between 2 and 18 h p.i.) and “late” (migration between 32 and 48 h p.i.) after infection for T cells by flow cytometry. To determine the percentages of infected T cells by FACS, an aliquot of the original culture, and the cells which migrated to the lower compartment were cultured until 48 h p.i. (B) Percentages of T cells that migrated “early” through 8-μm filter pores. (C) Percentages of T cells that migrated “late” through filter pores. (D) Percentages of infected T cells in the original culture and present in the “early” and “late” migrated cells (n = 3, with PBMC from three different donors; experiments were performed in triplicate). Error bars indicate standard deviations.
Block of transendothelial migration by MV infection.
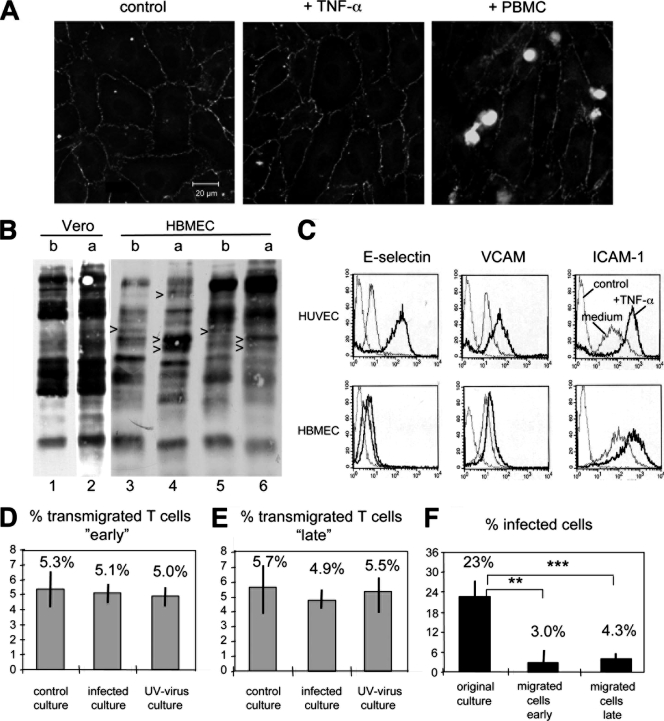
To investigate the capacity of infected T cells to transmigrate through endothelial barriers, we used transwell filter cultures of polarized HBMEC. When these confluent monolayers were controlled for their impermeability (density) using FITC-conjugated dextran, no significant differences were detected in the absence and presence of infection with wild-type MV. The polarization of the endothelial cells was controlled by visualization of the tight-junction molecule ZO-1 (Fig. 2A) and detection of differentially expressed proteins at the basolateral and apical sides (Fig. 2B). Treatment of the endothelial cells for 6 h with TNF-α, as used in the transmigration assay to stimulate the expression of the adhesion molecules E-selectin, VCAM, and ICAM-1 (Fig. 2C), or addition of lymphocytes did not impair the morphology of polarized endothelial cells (Fig. 2A).
FIG. 2.
Effect of MV infection on the transendothelial migration of T cells. (A) HBMEC were cultured on transwell filters until a confluent monolayer was formed (left panel), treated with TNF-α for 6 h (middle panel), or treated with TNF-α and overlaid with calcein-labeled PBMC (right panel). Cells were fixed and stained with a MAb to ZO-1 and FITC-conjugated secondary antibodies. (B) Vero cells, as a control for nonpolarized cells, and HBMEC were cultured on transwell filters until a confluent monolayer was formed, followed by basolateral and apical surface biotinylation of separate samples. Proteins were separated by SDS-PAGE, blotted on nitrocellulose filters, and visualized using strepavidin-peroxidase and ECL. Polarization of HBMEC is confirmed by the presence of several differentially expressed proteins (arrowheads) on the basolateral (b) and apical (a) sides in the absence (lanes 3 and 4) and presence (lanes 5 and 6) of TNF-α. (C) Expression of the adhesion molecules E-selectin, VCAM-1, and ICAM-1 determined by FACS analysis on HUVEC and HBMEC in medium and after treatment with TNF-α. (D and E) Transmigration assay of T cells migrating “early” (D) and “late” (E) through polarized HBMEC on transwell filters according to the protocol given in Fig. 1A. PBMC were either uninfected (control culture), infected with rMVIC323eGFP at an MOI of 1.0 (infected culture), or treated with UV-inactivated virus (UV-virus culture), and the percentage of transmigrated T cells was determined by counting cell numbers in the basolateral compartment and flow cytometry. (F) The percentage of infected T cells was determined by FACS in an aliquot of the original culture and in cells which migrated to the lower chamber “early” and “late” (n = 3, with PBMC from three different donors; experiments were performed in triplicate). Error bars indicate standard deviations.
PHA-activated PBMC were infected with rMVIC323eGFP at an MOI of 1.0, or treated with UV-inactivated MV, and overlaid on the endothelial cells at 2 or 32 h as indicated in Fig. 1A. After 16 h, the numbers of transmigrated cells were determined by counting cells in the basolateral compartment, and the percentages of uninfected and infected T lymphocytes were determined by flow cytometry. Approximately 5 to 6% of input T cells migrated through the monolayer of polarized endothelial cells, independently of whether the cultures were uninfected, infected (containing 23% infected T cells), or treated with UV-inactivated virus (Fig. 2D and E). Interestingly, the percentage of infected T cells in samples containing transmigrated T cells (in the basolateral compartments) was considerably reduced in comparison to the original culture. In contrast to 23% infected T cells in the original culture, only 3.0% and 4.3% of T cells were infected in cultures of “early” and “late” migrated cells (Fig. 2F). This corresponded to approximately 0.9% (early) and 1.8% (late) of input T cells that were able to transmigrate through the endothelial cell barrier. These data indicate that MV infection drastically impaired the capacity of T cells to migrate through endothelial cell barriers.
Viral effects on adhesion of leukocytes to endothelial cells.
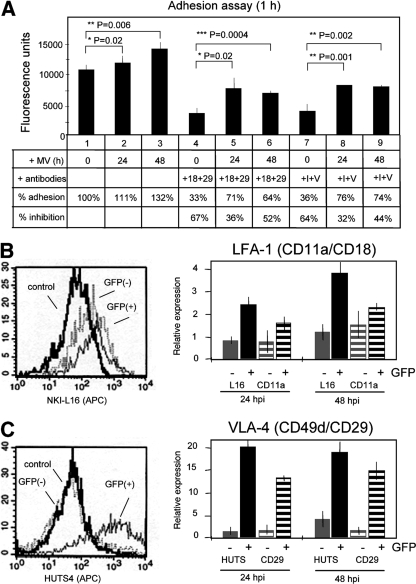
After finding that T-cell migration through filter pores was only slightly reduced by MV infection, whereas transmigration through endothelial cell layers was drastically impaired after infection, we wondered which step of the transendothelial migration might be specifically affected. The experiment presented in Fig. 2 gave a first hint that the adhesion of PBMC to endothelial cells may be increased following infection. When PBMC were incubated in this experiment for 16 h with either “early” or “late” endothelial cells, the majority of leukocytes adhered and could not be washed off from the endothelial cell surface. We detected an infection-mediated increase of adherent cells from 80 to 93% (“early”) and 82 to 85% (“late”), whereas UV-inactivated virus had no significant influence on adhesion. To quantify the adhesion of leukocytes in more detail and to identify the adhesion molecules involved, we performed a specific adhesion assay in which leukocytes are allowed to adhere for 1 hour to polarized endothelial cells. This test confirmed that after infection more leukoctyes adhered (at 24 h p.i. 111% and at 48 h p.i. 132%) than in the absence of infection (100%) (Fig. 3A, bars 1 to 3). Prior to and during transmigration, the adhesion between leukocytes and endothelial cells is mediated predominantly by the integrins LFA-1 (CD11a/CD18) and VLA-4 (CD49d/CD29) on the leukocytes and ICAM-1 and VCAM-1 on the endothelium (20, 33). We therefore added antibodies that inhibit these molecules to assess the specificity of the observed adhesion. Whereas antibodies to CD18 and CD29 inhibited the adhesion of uninfected leukocytes by 67%, the adhesion of infected leukocytes was inhibited only by 36% and 52% at 24 and 48 h p.i., respectively (Fig. 3A, bars 4 to 6). Antibodies to ICAM-1 (expressed on leukocytes and endothelial cells) and VCAM-1 inhibited the adhesion of uninfected leukocytes by 64% and at 24 and 48 h p.i. by 32% and 44%, respectively (Fig. 3A, bars 7 to 9), confirming the results obtained with antibodies to LFA-1 and VLA-4.
FIG. 3.
Adhesion of MV-infected leukocytes to endothelial cells and effect of infection on adhesion receptors. (A) Uninfected or 24- and 48-h-infected R18-labeled PBMC were bound to endothelial cell monolayers (HBMEC) and washed under standard conditions, and the fluorescence was quantified using a fluorescence reader. Adhesion-inhibiting antibodies to CD18 and CD29 (+18+29) or ICAM-1 and VCAM-1 (+I+V) were added as indicated. The percent adhesion is given with respect to the adhesion of uninfected PBMC (lane 1, 100%). The percent inhibition is given with respect to the values obtained for 0-, 24-, and 48-h-infected PBMC (n = 3, with PBMC from 3 different donors). (B and C) LFA-1 (B) and VLA-4 (C) expression on infected T cells was determined using MAbs NKI-L16 and HUTS4 to activation epitopes of the integrins and anti-CD11a and anti-CD29 to activation-independent epitopes on infected and uninfected leukocytes. Left panels, examples of FACS diagrams obtained at 48 h p.i. using MAbs NKI-L16 (B) and HUTS4 (C). Right panels, expression of activation epitopes, NKI-L16 and HUTS (gray bars, noninfected GFP-negative cells; black bars, = infected GFP-positive cells), in comparison to the expression of activation-independent epitopes, CD11a and CD29 (gray striped bars, noninfected GFP-negative cells; black striped bars, infected GFP-positive cells), at 24 and 48 h p.i. Error bars indicate standard deviations.
In order to investigate the effect of infection on the expression and activity of LFA-1 and VLA-4, we used antibodies recognizing specifically either the activated conformation of these integrins or activation-independent epitopes. To determine the LFA-1 and VLA-4 expression levels, we used anti-CD11a and anti-CD29 antibodies. As conformation-specific antibodies, we used clones NKI-L16 and HUTS4 for activated LFA-1 and VLA-4, respectively. FACS analysis revealed that the activated extended form of the α chain of LFA-1 (NKI-L16 epitope) was less expressed by uninfected control cultures and on uninfected GFP-negative T cells in infected cultures than by 48-h-infected GFP-positive T cells (Fig. 3B, left panel). Further quantification of the activated LFA-1 specific NKI-L16 epitope (mean fluorescence intensity) revealed that it was stimulated by the infection by factors of approximately 2.5 and 3.8 at 24 and 48 h p.i., respectively, whereas the non-activation-specific epitope was stimulated by factors of approximately 1.6 and 2.2, respectively (Fig. 3B, right panel). Thus, the infection-mediated increase of the activated form of LFA-1 was slightly stronger than the increase of the protein level. An even more pronounced difference between uninfected and infected leukocytes was detected for VLA-4 (Fig. 3C, left panel). The activated conformation of VLA-4 (HUTS4-positive cells) was strongly induced by the MV infection, and the mean fluorescence intensity increased by factors of approximately 20 and 18 at 24 and 48 h p.i., respectively (Fig. 3C, right panel), whereas the signal for the activation-independent epitope increased by factors of approximately 13 and 14, respectively. These differences between activation-dependent and -independent VLA-4 epitopes were statistically significant at 24 h p.i. and nonsignificant at 48 h p.i. Taken together, these data indicated that MV infection stimulates the expression and activity of the adhesion molecules LFA-1 and VLA-4 on T cells and that these molecules mediate the enhanced adhesion of infected T cells to endothelial cells.
Formation of transmigratory cups and infection of endothelial cells by adherent infected PBMC.
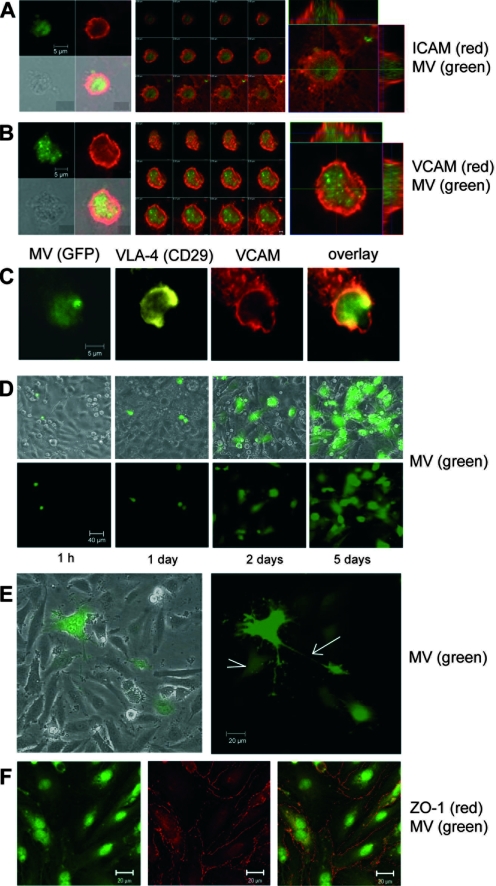
Next, we investigated whether adhesion of infected leukocytes is followed by proper formation of so-called docking structures or transmigratory cups. Transmigratory cups are formed when endothelial cells engulf adherent leukocytes with cup-like membrane protrusions as the first step of transcellular or intercellular migration (6, 7). Infected PBMC were overlaid on confluent endothelial cell layers, and interactions between single infected GFP-positive leukocytes and endothelial cells were visualized by confocal microscopy. Within the first 24 h of contact, the presence of transmigratory cups was detected with antibodies to ICAM-1 and VCAM-1, the binding partners of LFA-1 and VLA-4 on leukocytes (Fig. 4A and B). Costainings of LFA-1 and ICAM-1 (not shown) and VLA-4 (CD29) and VCAM-1 (Fig. 4C) confirmed the presence of these molecules on and around infected GFP-positive leukocytes.
FIG. 4.
Formation of transmigratory cups and infection of endothelial cells. (A) Transmigratory cups visualized with antibodies to ICAM-1 and Alexa-594-conjugated secondary antibodies (red) formed around adhering rMVIC323eGFP-infected leukocytes (green). Left, single fluorescence, showing phase-contrast and composite pictures. Middle, single sections from top to bottom. Note that at the bottom section, the ICAM-1-positive endothelial cell membrane is seen. Right, enlargement with Z-stacks. (B) A transmigratory cup as in panel A visualized using antibodies to VCAM. (C) Costaining of VLA-4 (CD29; yellow) and VCAM (red) on a GFP-positive MV-infected leukocyte in a transmigratory cup. Primary antibodies were mouse MAb anti-CD29 and MAb anti-VCAM-biotin, and secondary reagents were Alexa-633-conjugated goat anti-mouse antibodies and streptavidin-Alexa-594. (D) Phase-contrast plus fluorescence composite pictures and fluorescence alone of leukocytes infected with rMVIC323eGFP for 24 h, which were overlaid on a confluent HBMEC monolayer and incubated for 1 h and 1, 2, and 5 days as indicated. (E) rMVIC323eGFP-infected leukocytes cocultivated with HBMEC for 7 days. A typical GFP-positive cell process connects the small syncytium with a single infected cell (arrow). A weakly GFP-positive cell is seen in contact with processes from the syncytium (arrowhead). (F) The tight-junction molecule ZO-1 was stained with primary MAb and Alexa-594-conjugated secondary antibodies (red) in formaldehyde-fixed and permeabilized rMVIC323eGFP-infected HUVEC. ZO-1 expression in uninfected (Fig. 2A) and wild-type MV-infected endothelial cells is unchanged.
When infected PBMC were overlaid on confluent endothelial cell layers for increasing time periods, we observed the spread of infection from leukocytes to endothelial cells. Small, round GFP-positive lymphocytes were seen on top of the endothelial cell monolayer after 1 and 24 h (Fig. 4D). With increasing time (at 2 days and later), larger GFP-positive endothelial cells in the underlying monolayer appeared (Fig. 4D and E). While the number of infected endothelial cells increased, syncytia were only rarely observed. Infected endothelial cells also possess long cellular processes contacting neighboring cells (Fig. 4E). Tight junctions between endothelial cells, as visualized by the expression of markers such as ZO-1, remained conserved in the confluent monolayers even after infection with rMVIC323eGFP (Fig. 4F; compare to Fig. 2D), indicating that the monolayer was not destroyed by the infection.
Release of MV from polarized endothelial cells.
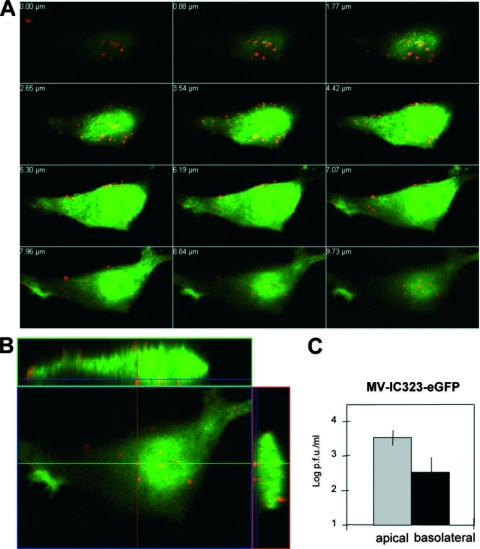
To investigate virus release, endothelial cells were cultivated for 3 days on transwell filters to achieve confluent monolayers. Confluence of the monolayer and polarization of the cells were confirmed as described for Fig. 2. Endothelial cells were infected with rMVIC323eGFP, and the expression of GFP and the distribution of the viral envelope glycoprotein H (MV-H) were analyzed by confocal microscopy. Subsequent pictures of cell layers from top to cell bottom, as well as the composite picture with Z-stacks, revealed the expression of MV-H on both the apical and basolateral sides of the polarized endothelial cells (Fig. 5A and B). Analysis of virus release from rMVIC323eGFP-infected endothelial cells by titration of supernatants from the upper and lower filter chambers revealed that virus was released from the apical and the basolateral sides (Fig. 5C).
FIG. 5.
Surface distribution of wild-type MV-H glycoproteins and virus release from polarized HBMEC. (A) Sectioning through an rMVIC323eGFP-infected polarized HBMEC from top (upper left) to bottom (lower right). (B) Representation of one section with Z-stacks at 48 h p.i. Apical and basolateral cell surfaces were labeled with anti-MV-H MAb K83 and secondary Alexa-594-conjugated goat anti-mouse antibodies (red). (C) Virus release into the apical and basolateral medium was quantified by plaque titration. The apical (gray bar) and basolateral (black bar) virus titers at 48 h p.i. are presented. Error bars indicate standard deviations.
DISCUSSION
We demonstrated here in tissue culture that wild-type MV-infected human T lymphocytes are drastically impaired in their capacity to migrate through endothelial cell barriers. MV infection enhanced the adhesion of T cells to endothelial cells, and the subsequent close cell contact frequently mediated infection of the endothelial cells. The enhanced adhesion of leukocytes obviously allowed the first steps of transendothelial migration, including the formation of transmigratory cups, to take place; however, it may also be the reason why completion of the process is inhibited. Interestingly, the interaction of UV-inactivated MV with leukocytes did not inhibit transendothelial migration. This indicates that the impairment of cytoskeletal rearrangement, polarization, CD3 clustering, and cell spreading of T cells, as described earlier for a time scale of 2 h after virus-cell interaction (27), is only transient. In addition, MV infection did not strongly affect diapedesis per se. However, the capacity of the leukocytes to transmigrate through the endothelial cell layer was strongly affected, suggesting a specific effect on mechanisms required for the interaction with endothelial cells. VLA-4 and LFA-1 activation is necessary for transmigration, where VLA-4 acts as a “rolling receptor” and contributes to the activation of LFA-1 and, vice versa, LFA-1 can stimulate the activity of VLA-4 (8, 15, 30). Active LFA-1 causes T-cell attachment and lamellipodial movement induced by myosin light-chain kinase at the leading edge of a T cell, whereas RhoA and ROCK cause T-cell detachment at the trailing edge. In our hands, both the expression and activation of VLA-4 and LFA-1 were strongly stimulated in infected T cells and mediated efficient adhesion. However, this strong adhesion may also cause a failure of detachment and thus inhibit the motility of the cells. This fits well with our finding that T-cell adhesion and the formation of transmigratory cups did occur, whereas actively transmigrating infected T cells were barely found. Interestingly, it has also been demonstrated that MV interacts and interferes with signal transduction from T-cell lipid rafts (5), a microdomain in which integrins and other signaling molecules are found and which is involved in the regulation of integrin activity (21). This may contribute to an aberrant infection-mediated activation of the integrins.
Increased LFA-1 expression, adhesion to endothelial cells, and virus transmission was observed earlier using the monocytic cell line U937 infected with various MV strains (16, 35). However, since U937 cells are CD150 negative (11) and the infection rates with the viruses used in that study varied considerably, predominantly only the CD46-using attenuated MV strains infected U937 cells and stimulated the expression of LFA-1. We demonstrated here that wild-type MV strongly stimulates the expression and activity of LFA-1, and even more so those of VLA-1, on primary human T cells.
A consequence of integrin activation in vivo may be the adhesion-mediated depletion of leukocytes from peripheral blood, as observed during MV-induced leukopenia (28). It is likely that this mechanism also contributes to the massive MV infection of lymphoid organs in humans and as recently impressively demonstrated in various animal models using recombinant GFP-expressing morbilliviruses (10, 37). A further consequence of the integrin activation in vivo may be endothelial cell infection and virus release from the abluminal side of the endothelium. It is not known how MV is transported into the brain or other organs; however, infection of endothelial cells may indeed play a role, since some infected endothelial cells are found during the rash, where virus disseminates in underlying epithelial cell layers and leads to local inflammatory cell infiltrations (13, 18, 39), and in lethal cases of acute measles encephalitis and brains of patients with subacute sclerosing panencephalitis (1, 12, 19). Although our findings suggest that it is unlikely that MV is transported across endothelial cell barriers via infected leukocytes, we cannot exclude the possibility that infected leukocytes can invade the brain when the barrier is already disrupted at certain sites and many cells infiltrate via migratory paths paved by uninfected cells, like “doors” in the basement membrane (23).
Recently, it has been described that an epithelial cell receptor-blind recombinant MV maintaining CD150-dependent cell entry successfully infected rhesus monkeys and caused viremia accompanied by the usual symptoms such as rash (22). These findings nicely support the view that CD150 is the functional receptor required for initiation of host infection and viremia. However, this virus was not released by the respiratory epithelium of the infected host, because the epithelial cell receptor interaction is required for the basolateral infection of epithelial cells of the respiratory tract. Thus, for transmission from host to host, MV may require the capacity to interact with CD150 and the epithelial cell receptor (36). In contrast to the epithelial cell infection, the infection of endothelial cells may be an early step during virus spread to various organs, and for this virus spread, infection via the apical (luminal) side and release from the basolateral side of the endothelial cells is required. Therefore, the unknown MV receptor on endothelial cells, which has been postulated earlier (2), is required on the apical side, or its accessibility may be induced after the formation of transmigratory cups and the close contact of infected leukocytes with endothelial cells.
Acknowledgments
We thank Yusuke Yanagi for the recombinant wild-type measles virus and Theo Geijtenbeek for providing the NKI-L16 antibody.
This work was supported by the Deutsche Forschungsgemeinschaft (SPP 1130).
Footnotes
Published ahead of print on 3 September 2008.
REFERENCES
- 1.Allen, I. V., S. McQuaid, J. McMahon, J. Kirk, and R. McConnell. 1996. The significance of measles virus antigen and genome distribution in the CNS in SSPE for mechanisms of viral spread and demyelination. J. Neuropathol. Exp. Neurol. 55471-480. [DOI] [PubMed] [Google Scholar]
- 2.Andres, O., K. Obojes, K. S. Kim, V. ter Meulen, and J. Schneider-Schaulies. 2003. CD46- and CD150-independent endothelial cell infection with wild-type measles viruses. J. Gen. Virol. 841189-1197. [DOI] [PubMed] [Google Scholar]
- 3.Avota, E., A. Avots, S. Niewiesk, L. P. Kane, U. Bommhardt, V. ter Meulen, and S. Schneider-Schaulies. 2001. Disruption of Akt kinase activation is important for immunosuppression induced by measles virus. Nat. Med. 7725-731. [DOI] [PubMed] [Google Scholar]
- 4.Avota, E., H. Harms, and S. Schneider-Schaulies. 2006. Measles virus induces expression of SIP110, a constitutively membrane clustered lipid phosphatase, which inhibits T cell proliferation. Cell. Microbiol. 81826-1839. [DOI] [PubMed] [Google Scholar]
- 5.Avota, E., N. Müller, M. Klett, and S. Schneider-Schaulies. 2004. Measles virus interacts with and alters signal transduction in T-cell lipid rafts. J. Virol. 789552-9559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barreiro, O., M. Yanez-Mo, J. M. Serrador, M. C. Montoya, M. Vicente-Manzanares, R. Tejedor, H. Furthmayr, and F. Sanchez-Madrid. 2002. Dynamic interaction of VCAM-1 and ICAM-1 with moesin and ezrin in a novel endothelial docking structure for adherent leukocytes. J. Cell Biol. 1571233-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carman, C. V., and T. A. Springer. 2004. A transmigratory cup in leukocyte diapedesis both through individual vascular endothelial cells and between them. J. Cell Biol. 167377-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan, J. R., S. J. Hyduk, and M. I. Cybulsky. 2000. α4β1 integrin/VCAM-1 interaction activates αLβ2 integrin-mediated adhesion to ICAM-1 in human T cells. J. Immunol. 164746-753. [DOI] [PubMed] [Google Scholar]
- 9.Constantin, G., M. Majeed, C. Giagulli, L. Piccio, J. Y. Kim, E. C. Butcher, and C. Laudanna. 2000. Chemokines trigger immediate β2 integrin affinity and mobility changes: differential regulation and roles in lymphocyte arrest under flow. Immunity 13759-769. [DOI] [PubMed] [Google Scholar]
- 10.de Swart, R. L., M. Ludlow, L. de Witte, Y. Yanagi, G. van Amerongen, S. McQuaid, S. Yuksel, T. B. Geijtenbeek, W. P. Duprex, and A. D. Osterhaus. 2007. Predominant infection of CD150(+) lymphocytes and dendritic cells during measles virus infection of macaques. PLoS Pathog. 3e178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erlenhoefer, C., W. J. Wurzer, S. Löffler, S. Schneider-Schaulies, V. ter Meulen, and J. Schneider-Schaulies. 2001. CD150 (SLAM) is a receptor for measles virus but is not involved in viral contact-mediated proliferation inhibition. J. Virol. 754499-4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esolen, L. M., K. Takahashi, R. T. Johnson, A. Vaisberg, T. R. Moench, S. L. Wesselingh, and D. E. Griffin. 1995. Brain endothelial cell infection in children with acute fatal measles. J. Clin. Investig. 962478-2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Griffin, D. E., and W. Bellini. 1996. Measles virus, p. p. 1267-1312. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology. Lippincott-Raven Publishers, Philadelphia, PA.
- 14.Hashimoto, K., N. Ono, H. Tatsuo, H. Minagawa, M. Takeda, K. Takeuchi, and Y. Yanagi. 2002. SLAM (CD150)-independent measles virus entry as revealed by recombinant virus expressing green fluorescent protein. J. Virol. 766743-6749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hogg, N., M. Laschinger, K. Giles, and A. McDowall. 2003. T-cell integrins: more than just sticking points. J. Cell Sci. 1164695-4705. [DOI] [PubMed] [Google Scholar]
- 16.Hummel, K., W. J. Bellini, and M. K. Offerman. 1998. Strain-specific differences in LFA-1 induction on measles virus-infected monocytes and adhesion and viral transmission to endothelial cells. J. Virol. 728403-8407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keizer, G. D., W. Visser, M. Vliem, and C. G. Figdor. 1988. A monoclonal antibody (NKI-L16) directed against a unique epitope on the alpha-chain of human leukocyte function-associated antigen 1 induces homotypic cell-cell interactions. J. Immunol. 1401393-1400. [PubMed] [Google Scholar]
- 18.Kimura, A., K. Tosaka, and T. Nakao. 1975. Measles rash. I. Light and electron microscopicstudy of skin eruptions. Arch. Virol. 47295-307. [DOI] [PubMed] [Google Scholar]
- 19.Kirk, J., A. L. Zhou, S. McQuaid, S. L. Cosby, and I. V. Allen. 1991. Cerebral endothelial cell infection by measles virus in subacute sclerosing panencephalitis: ultrastructural and in situ hybridization evidence. Neuropathol. Appl. Neurobiol. 17289-297. [DOI] [PubMed] [Google Scholar]
- 20.Laudanna, C., J. Y. Kim, G. Constantin, and E. Butcher. 2002. Rapid leukocyte integrin activation by chemokines. Immunol. Rev. 18637-46. [DOI] [PubMed] [Google Scholar]
- 21.Leitinger, B., and N. Hogg. 2002. The involvement of lipid rafts in the regulation of integrin function. J. Cell Sci. 115963-972. [DOI] [PubMed] [Google Scholar]
- 22.Leonard, V. H., P. L. Sinn, G. Hodge, T. Miest, P. Devaux, N. Oezguen, W. Braun, P. B. McCray, M. B. McChesney, and R. Cattaneo. 2008. Measles virus blind to its epithelial cell receptor remains virulent in rhesus monkeys but cannot cross the airway epithelium and is not shed. J. Clin. Investig. 1182448-2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ley, K., C. Laudanna, M. I. Cybulsky, and S. Nourshargh. 2007. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 7678-689. [DOI] [PubMed] [Google Scholar]
- 24.Liu, C., S. K. Shaw, S. Ma, L. Yang, F. W. Luscinskas, and C. A. Parkos. 2004. Regulation of leukocyte transmigration: cell surface interactions and signaling events. J. Immunol. 1727-13. [DOI] [PubMed] [Google Scholar]
- 25.Marin, V., G. Kaplanski, S. Grès, C. Farnarier, and P. Bongrand. 2001. Endothelial cell culture: protocol to obtain and cultivate human umbilical endothelial cells. J. Immunol. Methods 254183-190. [DOI] [PubMed] [Google Scholar]
- 26.Moll, M., H.-D. Klenk, G. Herrlerr, and A. Maisner. 2001. A single amino acid change in the cytoplasmic domains of measles virus glycoproteins H and F alters targeting, endocytosis, and cell fusion in polarized Madin-Darby canine kidney cells. J. Biol. Chem. 27617887-17894. [DOI] [PubMed] [Google Scholar]
- 27.Müller, N., E. Avota, J. Schneider-Schaulies, H. Harms, G. Krohne, and S. Schneider-Schaulies. 2006. Measles virus contact with T cells impedes cytoskeletal remodeling associated with spreading, polarization, and CD3 clustering. Traffic 7849-858. [DOI] [PubMed] [Google Scholar]
- 28.Nanan, R., B. Chittka, M. Hadam, and H. W. Kreth. 1999. Measles virus infection causes transient depletion of activated T cells from peripheral circulation. J. Clin. Virol. 12201-210. [DOI] [PubMed] [Google Scholar]
- 29.Ono, N., H. Tatsuo, Y. Hidaka, T. Aoki, H. Minagawa, and Y. Yanagi. 2001. Measles virus on throat swabs from measles patients use signalling lymphocytic activation molecule (CDw150) but not CD46 as a cellular receptor. J. Virol. 754399-4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Porter, J. C., and N. Hogg. 1997. Integrin cross talk: activation of lymphocyte function-associated antigen-1 on human T cells alters α4β1- and α5β1-mediated function. J. Cell Biol. 1381437-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schlender, J., J. J. Schnorr, P. Spielhoffer, T. Cathomen, R. Cattaneo, M. A. Billeter, V. ter Meulen, and S. Schneider-Schaulies. 1996. Interaction of measles virus glycoproteins with the surface of uninfected peripheral blood lymphocytes induces immunosuppression in vitro. Proc. Natl. Acad. Sci. USA 9313194-13199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schneider-Schaulies, S., S. Niewiesk, J. Schneider-Schaulies, and V. ter Meulen. 2001. Measles virus induced immunosuppression: targets and effector mechanisms. Curr. Mol. Med. 1163-181. [DOI] [PubMed] [Google Scholar]
- 33.Shamri, R., V. Grabovsky, J. M. Gauguet, S. Feigelson, E. Manevich, W. Kolanus, M. K. Robinson, D. E. Staunton, U. H. von Andrian, and R. Alon. 2005. Lymphocyte arrest requires instantaneous induction of an extended LFA-1 conformation mediated by endothelium-bound chemokines. Nat. Immunol. 6497-506. [DOI] [PubMed] [Google Scholar]
- 34.Shimaoka, M., J. Takagi, and T. A. Springer. 2002. Conformational regulation of integrin structure and function. Annu. Rev. Biophys. Biomol. Struct. 31485-516. [DOI] [PubMed] [Google Scholar]
- 35.Soilu-Hanninen, M., A. Hanninen, J. Ilonen, A. Salmi, and R. Salonen. 1996. Measles virus hemagglutinin mediates monocyte aggregation and increased adherence to measles-infected endothelial cells. Med. Microbiol. Immunol. 18573-80. [DOI] [PubMed] [Google Scholar]
- 36.Takeda, M. 2008. Measles virus breaks through epithelial cell barriers to achieve transmission. J. Clin. Investig. 1182386-2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.von Messling, V., D. Milosevic, and R. Cattaneo. 2004. Tropism illuminated: lymphocyte-based pathways blazed by lethal morbillivirus through the host immune system. Proc. Natl. Acad. Sci. USA 10114216-14221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yanagi, Y., M. Takeda, and S. Ohno. 2006. Measles virus: cellular receptors, tropism and pathogenesis. J. Gen. Virol. 872767-2779. [DOI] [PubMed] [Google Scholar]
- 39.Yanagihara, M., T. Fujii, T. Mochizuki, H. Ishizaki, and T. Sata. 1998. Measles virus was present in the inner cell of the acrosyringium in the skin rash. Pediatr. Dermatol. 15456-458. [DOI] [PubMed] [Google Scholar]