Abstract
In recent years, the highly pathogenic avian influenza virus H5N1 has raised serious worldwide concern about an influenza pandemic; however, the biology of H5N1 pathogenesis is largely unknown. To elucidate the mechanism of H5N1 pathogenesis, we prepared primary airway epithelial cells from alveolar tissues from 1-year-old pigs and measured the growth kinetics of three avian H5 influenza viruses (A/Crow/Kyoto/53/2004 [H5N1], A/Duck/Hong Kong/342/78 [H5N2], and A/Duck/Hong Kong/820/80 [H5N3]), the resultant cytopathicity, and possible associated mechanisms. H5N1, but not the other H5 viruses, strongly induced cell death in porcine alveolar epithelial cells (pAEpC), although all three viruses induced similar degrees of cytopathicity in chicken embryonic fibroblasts. Intracellular viral growth and the production of progeny viruses were comparable in pAEpC infected with each H5 virus. In contrast, terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling-positive cells were detected only in H5N1-infected pAEpC, and the activities of caspases 3, 8, and 9 were significantly elevated in pAEpC infected with H5N1, but not with H5N2 and H5N3. These results suggest that only H5N1 induces apoptosis in pAEpC. H5N1 cytopathicity was inhibited by adding the caspase inhibitor z-VAD-FMK; however, there were no significant differences in viral growth or release of progeny viruses. Further investigations using reverse genetics demonstrated that H5N1 hemagglutinin protein plays a critical role in inducing caspase-dependent apoptosis in infected pAEpC. H5N1-specific cytopathicity was also observed in human primary airway epithelial cells. Taken together, these data suggest that avian H5N1 influenza virus leads to substantial cell death in mammalian airway epithelial cells due to the induction of apoptosis.
The first outbreak in humans caused by the highly pathogenic avian influenza virus H5N1 occurred in Hong Kong in 1997. In this outbreak, H5N1 caused respiratory disease in 18 people, 6 of whom died (8). To date, more than 380 human H5N1 infections have been identified, more than 240 of which have been fatal (60), raising serious worldwide concern about a severe influenza pandemic. The high mortality among H5N1-infected patients results from acute respiratory distress syndrome (ARDS) linked with diffuse alveolar damage (DAD) from desquamation of alveolar cells and hemorrhage (10, 11, 32, 50, 54, 55). Though broad-view analyses of pathogenesis have been conducted, such as epidemiological studies and histopathological analyses of human lungs after infection, little attention has been paid to the biological basis of H5N1 pathogenesis in humans.
Recently, Shinya et al. (42) reported that the surface glycoprotein hemagglutinin (HA) of avian influenza viruses preferentially recognizes receptors terminating in sialic acid α-2,3-galactose (SAα2,3Gal) in the respiratory bronchioles and alveoli. The specificity of the HA receptor is thought to be one factor associated with the pathogenesis of H5N1 in humans; however, the pathogenesis after the virus has entered the cells is still unclear. Although avian influenza viruses are all potentially virulent in poultry (1, 19, 23, 35, 41, 43, 46, 47), currently circulating H5N1 is highly virulent in humans, as well as in birds (5, 59, 63), suggesting that it can induce cell damage in mammalian airway organs. To elucidate why H5N1 influenza virus infection leads to DAD in human lungs, it is essential to clarify the difference(s) between recently emerged H5N1 viruses and previously circulating avian influenza viruses with respect to viral replication and processes leading to cell death at the molecular level.
So far, several susceptible cell lines, including MDCK and A549, have been used to evaluate the pathogenesis of influenza virus. These cell lines are useful models for tracing viral entry, replication, and production of progeny viruses; however, they are less suitable for investigating the mechanisms of cell death associated with viral replication, because these cancer-derived cells have been immortalized. For many years, pigs, which possess both the avian influenza SAα2,3Gal receptor and the human influenza SAα2,6Gal receptor (20), have been known to be mixing vessels because of their susceptibility to both avian and human influenza viruses (6, 57, 58). Thus, primary porcine cells, such as airway epithelial cells, are potentially good models for investigating cell death caused by the replication of human and avian influenza viruses. To clarify the mechanisms of H5N1 pathogenesis in the human lung, we focused on the postentry steps and tried to find a difference(s) between the highly pathogenic (high-mortality) avian influenza virus H5N1 and two low-pathogenic (low- or no-mortality) avian influenza viruses, H5N2 and H5N3, in humans. We used porcine alveolar epithelial cells (pAEpC) as a human lung model and investigated the relationship between viral replication kinetics and cytopathicity. Finally, we tried to assess how the H5N1 avian influenza virus leads to cytopathicity in porcine and human alveolar epithelial cells.
MATERIALS AND METHODS
Reagents.
The components used in airway epithelial cell basic medium (AEC basic) and proliferation medium (AEC plus) were based on the methods of You et al. (62) and Rowe et al. (36) and are described in Table S1 in the supplemental material.
Viruses and cells.
Influenza virus strain A/crow/Kyoto/53/2004 (H5N1) was isolated from embryonated eggs inoculated with tracheal homogenates from dead crows; A/Duck/Hong Kong/342/78 (H5N2) and A/Duck/Hong Kong/820/80 (H5N3) were kindly provided by Yoshinobu Okuno, Osaka Prefectural Institute of Public Health. Chicken embryonic fibroblasts (CEF) were prepared from 10-day-old embryonated eggs. MDCK cells were purchased from the Riken BioResource Center Cell Bank (Ibaragi, Japan). Human lung epithelial carcinoma A549 cells were kindly provided by the Cell Resource Center for Biomedical Research (Tohoku University, Sendai, Japan), and human primary small airway epithelial cells (SAEC) were purchased from Lonza Corporation (Walkersville, MD).
Generation of recombinant H5N3 viruses.
Viral RNA was isolated using Trizol reagent (Invitrogen), and cDNA was synthesized using random hexamers. The full-length HA sequence of A/crow/Kyoto/53/2004 (H5N1) was constructed by PCR. The HA sequence of A/Thailand/Kan353/2004 (H5N1) was constructed by PCR using overlapping deoxyoligonucleotides corresponding to the published sequence of the HA open reading frame of the virus. The virulent HA sequence of A/Duck/Hong Kong/820/80 (H5N3) was constructed by changing single basic amino acids to multiple basic amino acids (N′-TR-C′ to N′-RRKKR-C′) at the HA cleavage site. The noncoding regions were identical to those of influenza A/WSN/33 (H1N1) virus. The HA genes were cloned into the pPOLI plasmid (15).
Recombinant viruses were generated by using a previously described reverse-genetics system (3, 15, 52) with slight modifications. Briefly, the pPOLI-HA plasmid derived from A/crow/Kyoto/53/2004 (H5N1), A/Thailand/Kan353/2004 (H5N1), or virulent HA of A/Duck/Hong Kong/820/80 (H5N3) was transfected together with pCAGGS expression plasmids (3) for A/crow/Kyoto/53/2004 (H5N1) PA, PB1, PB2, and NP into 293T cells that had been cocultured with CEF (7:3). At 24 h posttransfection, A/Duck/Hong Kong/820/80 (H5N3) was infected at a multiplicity of infection (MOI) of 1 as a helper virus. At 72 h postinfection (p.i.), recombinant viruses in the supernatant were plaque purified in MDCK cells in the absence of trypsin. The HA gene of each recombinant virus was confirmed by sequencing.
pAEpC isolation and culture conditions.
Isolation and culture of primary pAEpC were performed according to the procedures of You et al. (62), Rowe et al. (36), and Steimer et al. (45) with slight modifications. Briefly, lungs from 1-year-old pigs were obtained from a local abattoir. The fresh organs were transferred into sterile phosphate-buffered saline (PBS) and kept on ice during transport to the laboratory. The time between collecting the tissue and starting the isolation procedure was between 1 and 2 h. Tissue pieces were mechanically excised from diaphragmal and cranial regions of the pulmonary lobe, followed by washes and the removal of visible bronchioles. Tissue cubes of approximately 3-cm edge length were injected with 0.15% (wt/vol) pronase (Calbiochem, San Diego, CA) in F-12 medium (Gibco Co., Carlsbad, CA) at a rate of 2 ml/tissue cube, followed by incubation in polyethylene tubes at 37°C for 1 h to accelerate enzymatic digestion. Subsequent to enzymatic treatment, the reaction samples were mixed with PBS (10 ml/tube), and the resulting tissue was minced with scissors in each tube. The triturated tissues were filtered through stainless steel mesh with 1.5-mm pores, followed by filtration through nylon gauze with 0.5-mm pores and 70-μm cell strainers (BD Biosciences). Airway epithelial cells collected by enzymatic treatment were pooled and isolated by centrifugation at 880 × g for 10 min at 4°C. The cells were resuspended in Ham's F-12 pen-strep containing crude pancreatic DNase I (0.5 mg/ml; Roche Molecular Biochemicals, Indianapolis, IN) and bovine serum albumin (10 mg/ml; Sigma-Aldrich, St. Louis, MO). The cells were then incubated on ice for 5 min, centrifuged at 880 × g for 5 min at 4°C, and resuspended in AEC basic medium (see Table S1 in the supplemental material) with 5% fetal calf serum. The cells were incubated in tissue culture plates (Asahi Techno Glass Co., Ltd., Chiba, Japan) for 2 h at 37°C and aerated with 5% CO2 and 95% air to adhere the fibroblasts (36, 62). Nonadherent cells were collected by centrifugation, resuspended in AEC plus medium (see Table S1 in the supplemental material), and counted. An average of 9.66 (±0.35) g starting tissue mass yielded 2.4 × 106 (±0.36 × 106) cells/g. Variances in this yield may reflect differences in the age, gender, or state of health of the respective donor animals, but this was not investigated further. Cell viability determined by trypan blue exclusion was >95%. The resuspended cells were cultured on tissue culture plates (Asahi Techno Glass) coated with permeable fibronectin (0.06 mg/cm2; BD Biosciences, Bedford, MA) and collagen (0.2 mg/cm2; Nitta Gelatin Inc., Osaka, Japan). The cells were plated at a density of 105 cells/cm2 and grown with AEC plus medium supplemented with d-valine to hinder the growth of fibroblasts (26, 44). The cells were cultivated at 37°C in an atmosphere of 5% CO2 and 95% air, and the cell culture fluid was replaced at least every second day. Subsequent isolation batches are referred to as “pAEpC-n” with n indicating the batch number. Staining pAEpC with a mouse monoclonal antipancytokeratin antibody (Sigma-Aldrich) revealed that >99% of the cells expressed cytokeratin (see Fig. S1 in the supplemental material).
Virus infection.
Cells on culture plates were washed twice with PBS and then infected with virus at an MOI of 1 or 10 and incubated at 37°C for 1 h. After the removal of virus and one wash, the cells were cultured with minimum essential medium (Sigma-Aldrich) containing 10% fetal calf serum for CEF and A549 cells and were cultured with AEC plus for pAEpC and human SAEC at 37°C in 5% CO2 and 95% air. Aliquots of the supernatants were collected at 8, 16, and 24 h p.i. and titrated on MDCK cells by 50% tissue culture infective dose (TCID50) or antigen-capture enzyme-linked immunosorbent assay (ELISA) as described below. Cell lysates prepared from infected cells after the removal of media were used for detection of viral proteins by Western blotting (53).
TCID50 assays.
Aliquots of supernatants were 10-fold serially diluted with PBS, applied in quadruplicate to 2.5 × 104 MDCK cells/well of a 96-well plate, and incubated at 37°C for 1 h. The inoculum was removed, and the cells were washed with PBS and supplied with Dulbecco's modified Eagle's medium/F-12 containing 0.2% bovine serum albumin and trypsin (5 μg/ml). On the fourth day after infection, the TCID50 was determined on the basis of the Reed- Muench method (34).
Antigen-capture ELISA.
A 96-well plate (Asahi Techno Glass) was coated with a 1:1,000 dilution of a rabbit polyclonal antibody obtained from a rabbit immunized with A/Duck Hong Kong/342/78 (H5N2); this antibody reacts with common sequences of avian influenza virus nucleoprotein (NP) and matrix protein 1 (M1). After the plate was incubated overnight at 4°C, PBS containing 5% nonfat milk was added to the wells to block nonspecific signals, and the plate was incubated for 1 h at 37°C. An authentic standard sample of A/Duck/Hong Kong/820/80 (H5N3) and test samples were inactivated with 0.04% paraformaldehyde, diluted to 0.01% paraformaldehyde in PBS, and added to the wells, and the plate was incubated for 1 h at 37°C. After five washes with PBS-T (PBS containing 0.05% Tween 20), a mouse monoclonal antibody (3C11) diluted 1:2,000 in PBS-T containing 5% nonfat milk was reacted with the immobilized viral antigen in each well. The 3C11 antibody was produced by a hybridoma derived from mice immunized with A/crow/Kyoto/53/2004 (H5N1) and was able to react with the HA sequence common to H5 avian influenza viruses. The plate was incubated for 1 h at 37°C and washed four times with PBS-T. A horseradish peroxidase-conjugated donkey anti-mouse immunoglobulin G (IgG) secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA) diluted 1:2,000 in PBS containing 5% nonfat milk was added to the wells, followed by incubation at 37°C for 1 h. The number of virus particles was determined at 492 nm by a colorimetric method using o-phenylenediamine dihydrochloride as a chromogenic substrate.
Immunofluorescence assay for viral-antigen detection.
An immunofluorescence assay was conducted on 2.5 × 104 cells/well of a 96-well plate (Asahi Techno Glass). At 24 h after infection, the cells were fixed with paraformaldehyde in PBS containing 0.1% Triton X-100 and washed with PBS three times. A monoclonal antibody (3C11) was used as a primary antibody to detect viral antigen (H5N1, H5N2, or H5N3). Antibody binding to viral proteins was detected with an Alexa Fluor 488-conjugated secondary antibody (Molecular Probes, Carlsbad, CA) diluted 1:500 in PBS containing 1% bovine serum albumin. Cell nuclei were counterstained with Hoechst 33342 (Sigma-Aldrich).
Cell proliferation assays.
To quantify cell proliferation after infection of CEF or pAEpC with H5N1, H5N2, or H5N3, we used a mitochondrial tetrazolium dye reduction assay. Following viral infection of 2.5 × 104 cells/well of a 96-well plate (Asahi Techno Glass) at an MOI of 1 or 10, CEF and pAEpC were cultured with minimum essential medium containing 10% fetal calf serum and AEC plus, respectively. At regular intervals after infection (8, 16, 24, and 32 h), 10 μl of MTT reagent (Promega Co., Madison, WI) was added per 100-μl culture volume, and the cells were cultured for 1 h (CEF) or 2 h (pAEpC). After incubation for 1 or 2 h, 25 μl of 2% sodium dodecyl sulfate (SDS) was added to each well, followed by incubation for 20 min at room temperature. The absorbance at 492 nm (A492) of each sample well was measured with an automated plate reader (Multiskan MS-UV; Labsystems, Helsinki, Finland). The results were calculated as the absorbance ratio of infected and uninfected cells and were analyzed statistically with Student's t test, to compare differences between proliferation after H5N1 infection and after H5N2 or H5N3 infection, with statistical significance considered to be a P value of <0.05.
Western blotting.
After the removal of supernatant at each sampling time (8, 16, and 24 h p.i.), the cells were washed three times with ice-cold PBS and harvested with SDS lysis buffer (PBS containing 2% SDS). Lysate containing 10 μg of protein per lane (bicinchoninic acid protein quantification; Pierce Biotechnology, Rockford, IL) was loaded on a 10% SDS-polyacrylamide gel. After electrophoresis, the proteins were blotted onto nitrocellulose, and the membranes were blocked with PBS-T supplemented with a final concentration of 5% nonfat milk overnight at 4°C. The rabbit polyclonal virus antibody (1:2,000 dilution) and a mouse monoclonal antibody against α-tubulin (B-5-1-2; Sigma-Aldrich) were used as primary antibodies. The primary antibody was reacted with the membrane in PBS-T supplemented with a final concentration of 5% nonfat milk. A horseradish peroxidase-conjugated donkey anti-rabbit IgG (Jackson ImmunoResearch Laboratories) was used as a secondary antibody for detection of virus antigen, and a donkey anti-mouse IgG was used as a secondary antibody for detection of α-tubulin as an internal control, with both antibodies diluted 1:1,000 in PBS-T at room temperature. Washing with PBS-T was performed between all steps. All Western blots were visualized using an enhanced chemiluminescence system (Nacalai Pharmaceutical Co., Ltd., Kyoto, Japan) and Fuji XR film. The staining intensity of each band was calculated with Image J software.
TUNEL assay.
Following virus infection of 2.5 × 104 cells/well of a 96-well plate (Asahi Techno Glass) at an MOI of 1 (no caspase inhibitor) or 10 (in the presence of the caspase inhibitor z-VAD-FMK), pAEpC and human SAEC were cultured with AEC plus for 16 h. Parallel samples were cultured with the caspase inducer staurosporine (0.2 μM) as a positive control or with the pancaspase inhibitor z-VAD-FMK (200 μM) after H5N1 infection as a negative control. Apoptotic cells were characterized by positive terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) staining according to the manufacturer's instructions (DeadEnd Fluorometric TUNEL System; Promega). Briefly, 16 h after infection with H5N1, H5N2, or H5N3, cells were fixed with paraformaldehyde in PBS containing 0.1% Triton X-100 and washed with PBS three times. Then, the fixed cells were incubated in the labeling reaction mixture containing terminal deoxynucleotidyl transferase enzyme, fluorescein isothiocyanate-conjugated nucleotide, and labeling buffer. The reaction was quenched in stop buffer, and the cells were washed with PBS several times. To detect viral antigens after TUNEL staining, the cells were incubated at room temperature for 40 min with the aforementioned rabbit polyclonal antibody (1:2,000 dilution) as a primary antibody, and antibody binding to viral proteins was detected with an Alexa Fluor 555-conjugated secondary antibody (Molecular Probes) diluted 1:1,000 in PBS containing 1% bovine serum albumin. Cell nuclei were counterstained with Hoechst 33342 (Sigma-Aldrich).
Assay for caspase activity.
A colorimetric assay for caspase 3 activity (CaspACE Assay System; Promega) was used according to the manufacturer's instructions. Caspase 8 and caspase 9 activities were determined by the same method using specific substrates (substrate for caspase 8, Ac-IETD-pNA; for caspase 9, Ac-LEHD-pNA; purchased from Calbiochem). Following virus infection of 1 × 106 cells/well of a six-well plate (Asahi Techno Glass) at an MOI of 1, CEF and A549 cells were cultured with minimum essential medium containing 10% fetal calf serum, and pAEpC and human SAEC were cultured with AEC plus for 16 h. Parallel samples were cultured with the caspase inducer staurosporine (1 μM for CEF, 2 μM for A549 cells, and 0.2 μM for pAEpC) as a positive control or with the pancaspase inhibitor z-VAD-FMK (200 μM) after H5N1 infection as a negative control. At 16 h p.i., the infected cells were harvested by centrifugation at 4°C, washed with ice-cold PBS, and resuspended in cell lysis buffer at a concentration of 108 cells/ml. The cells were lysed by repeated freeze-thaw cycles and incubated on ice for 15 min before centrifugation to collect the supernatant fraction. Caspase activities were determined with 100 μg of whole-cell lysate in a 100-μl volume in 96-well plates. The plates were sealed and incubated at 37°C for 4 h, and the A405 was measured using an automated plate reader. The results were analyzed statistically with Student's t test to compare differences between activities in H5N1-infected cells and those in H5N2- or H5N3-infected cells, with statistical significance considered to be a P value of <0.05.
Nucleotide sequence accession numbers.
The GenBank accession number of the full-length HA sequence of A/crow/Kyoto/53/2004 (H5N1) is AB189053, and that of the HA sequence of A/Thailand/Kan353/2004 (H5N1) is EF541411.
RESULTS
Cytopathicity of avian influenza virus in CEF and pAEpC.
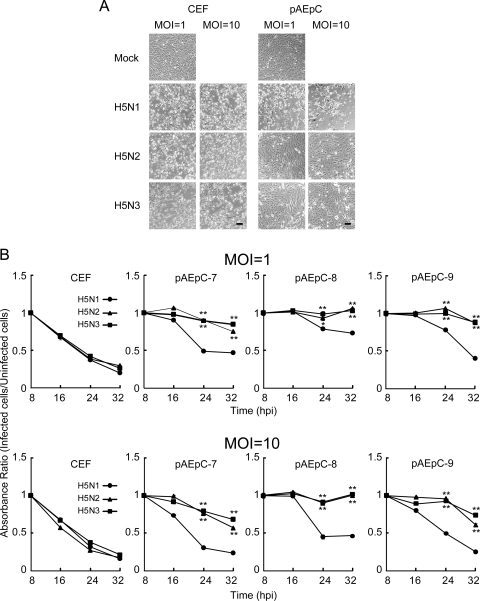
We infected CEF and pAEpC with H5N1, H5N2, and H5N3 at MOIs of 1 and 10 and observed the cytopathic effect (CPE) in infected cells at 24 h p.i.. Each H5 virus produced a similar obvious CPE in infected CEF (Fig. 1A). In contrast, in pAEpC from three pigs, H5N1 produced a severe CPE in infected cells but the other H5 viruses did not (Fig. 1A). In order to analyze the CPE quantitatively, we performed MTT assays in infected CEF and pAEpC with each H5 virus at 8, 16, 24, and 32 h p.i.; uninfected cells were used as controls. The cytopathicity observed in three H5 virus-infected CEF cultures increased over time (Fig. 1B). In pAEpC, H5N1 was significantly more cytopathic (P < 0.01 or P < 0.05) than H5N2 or H5N3 at 24 and 32 h p.i. (Fig. 1B). The absorbance ratio decreased during the observation period for H5N1, but the cytopathicity induced by H5N2 and H5N3 viruses was slight even at 32 h p.i. (Fig. 1B). In order to elucidate whether the lower cytopathicity in pAEpC infected with H5N2 and H5N3 viruses depended on the infectivity of the viruses, we immunostained viral antigens on CEF and pAEpC at 24 h p.i. The immunostaining using an anti-H5 HA monoclonal antibody revealed that all three viruses infected CEF and pAEpC at similar rates, even when different amounts were inoculated (MOIs of 1 and 10) (Fig. 2).
FIG. 1.
Cell damage in CEF and pAEpC infected with avian influenza virus. CEF and three distinct pAEpC cultures isolated from three different pigs (batch numbers 7, 8, and 9) were infected with avian influenza viruses A/crow/Kyoto/53/2004 (H5N1), A/Duck/Hong Kong/342/78 (H5N2), and A/Duck/Hong Kong/820/80 (H5N3) at an MOI of 1 or 10. (A) Morphological changes in CEF and pAEpC (pAEpC-7, -8, and -9) at 24 h p.i. with avian influenza virus. Scale bars, 100 μm. (B) Time-dependent assessment of cell damage in CEF and pAEpC after avian influenza virus infection with an MTT assay at 8, 16, 24, and 32 h p.i. The data are presented as means ± standard errors (n = 3 wells). *, P < 0.05, and **, P < 0.01 versus the same time point in H5N1.
FIG. 2.
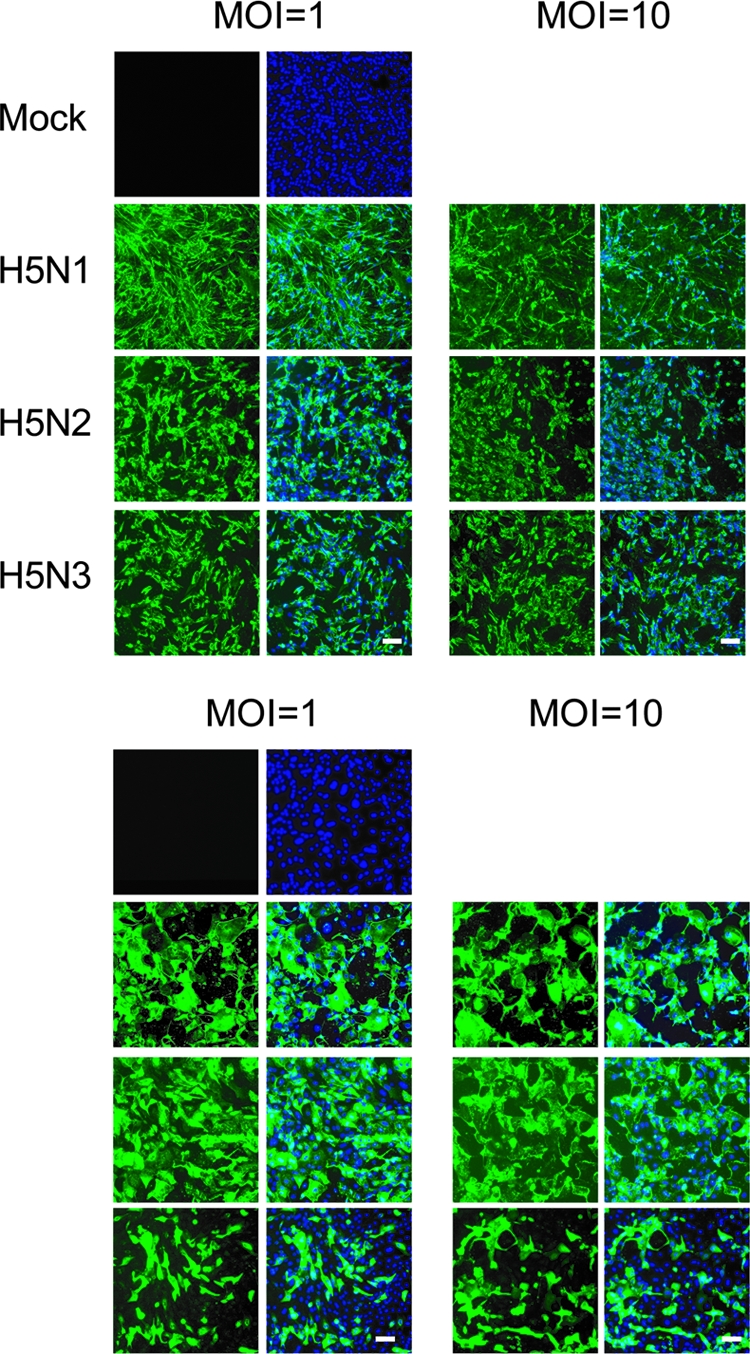
Expression of virus antigen in CEF (top) and pAEpC (bottom) at 24 h p.i. with avian influenza viruses. Virus antigen was detected by immunostaining using a monoclonal antibody (3C11) that recognizes H5 HA (green). Counterstaining with Hoechst 33342 was used for detection of cellular nuclei (blue). Scale bars, 100 μm.
Growth kinetics of avian influenza virus in pAEpC.
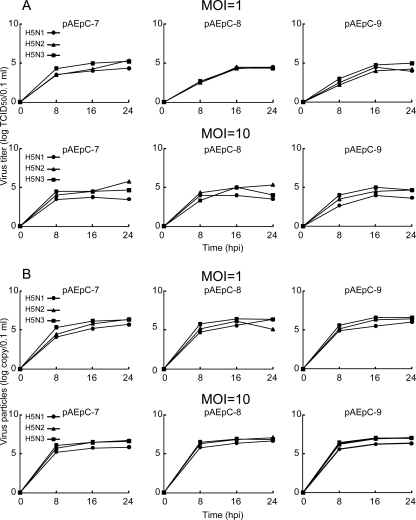
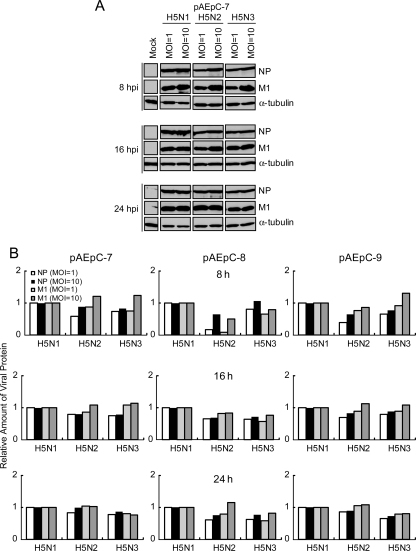
In order to assess the relationship between cytopathicity and viral replication in pAEpC, we focused on the growth kinetics of the H5 viruses. The culture supernatants from the infected cells at different time points were harvested for measurement of progeny virus production. The infectious virus titers (TCID50) (Fig. 3A) and the concentrations of progeny virions (Fig. 3B) measured by quantitative antigen capture ELISA were similar among the three H5 viruses, although H5N1 produced slightly fewer progeny virus than the other two viruses. We then analyzed the intracellular expression level of viral proteins in infected cells at 8, 16, and 24 h p.i. in Western blots using antibodies against the viral proteins NP and M1. Although the expression level of H5N1 protein at 8 h p.i. in pAEpC-8 was slightly higher than those of the other two viruses, the intracellular expression levels of viral proteins were comparable among H5N1, H5N2, and H5N3 (Fig. 4). In addition, the expression level of viral protein at an MOI of 10 was slightly higher than that at an MOI of 1 in the early phase of infection (8 h p.i.); however, these differences disappeared at 16 and 24 h p.i. (Fig. 4). The ratios of infectious virions to all progeny virions were also comparable among the three H5 viruses. These results suggest that intracellular, as well as extracellular, viral growth rates were comparable among H5N1, H5N2, and H5N3 viruses in pAEpC (Fig. 3 and 4) in spite of the differences in the HA cleavage sites between the H5N1 virus (N′-RRKKR-C′) and the H5N2 and H5N3 viruses (N′-TR-C′).
FIG. 3.
Avian influenza virus production from pAEpC. pAEpC-7, -8, and -9 were infected with H5N1, H5N2, or H5N3 virus at MOIs of 1 and 10. The numbers of progeny viruses at 8, 16, and 24 h p.i. were measured as TCID50 (A) and by antigen-capture ELISA (B).
FIG. 4.
Western blot analysis of the growth kinetics of viral proteins in pAEpC at 8, 16, and 24 h p.i. with avian influenza virus. pAEpC-7, -8, and -9 were infected with H5N1, H5N2, or H5N3 virus at MOIs of 1 and 10. (A) Viral NP and M1 were detected by anti-NP and anti-M polyclonal antibodies, respectively. α-Tubulin was used as a control. (B) Relative band intensities of the viral proteins (NP and M1) in panel A. The intensities of H5N1 viral proteins at each time point were set to 1.
H5N1-induced apoptosis in pAEpC.
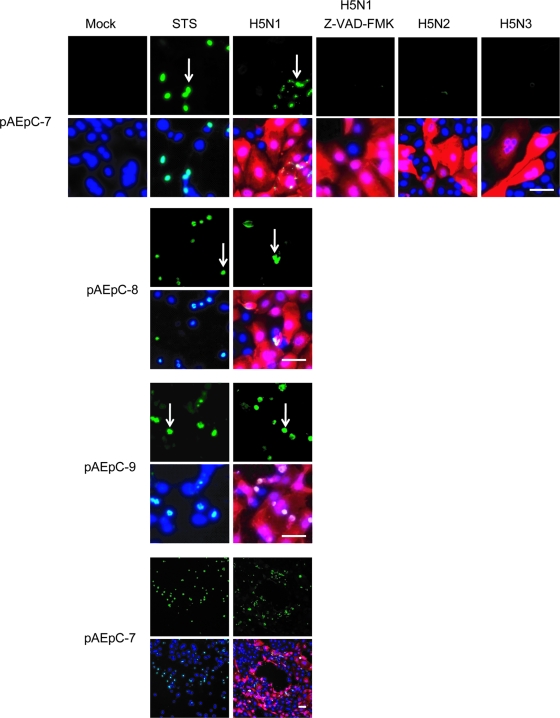
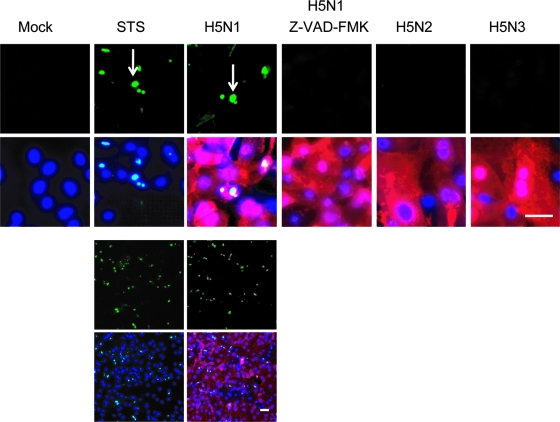
In order to evaluate the mechanism of H5N1-specific cell death in pAEpC, we examined apoptosis using TUNEL staining. TUNEL-positive cells were abundantly detected in H5N1-infected cells (Fig. 5), but few TUNEL-positive cells were detected in H5N2- or H5N3-infected cells. Generally, apoptosis is mediated by the activation of a family of cysteine-containing aspartate-directed proteases called caspases (12, 38, 39). TUNEL-positive cells were not detected in the presence of the pancaspase inhibitor z-VAD-FMK (Fig. 5), suggesting that H5N1 kills cells through caspase-dependent apoptosis.
FIG. 5.
TUNEL assay for detection of apoptosis in pAEpC at 16 h p.i. with avian influenza virus. pAEpC-7, -8, and -9 were infected with H5N1, H5N2, or H5N3 virus at an MOI of 1 (in the absence of caspase inhibitor) or 10 (in the presence of the caspase inhibitor z-VAD-FMK). Parallel samples were cultured with the caspase inducer staurosporine (STS) as a control or cultured with the pancaspase inhibitor z-VAD-FMK in H5N1-infected cells. The arrows indicate apoptotic cells (green). Viral antigen was detected simultaneously with a polyclonal antibody against the H5 virus (red). Cellular nuclei were counterstained with Hoechst 33342 (blue). Scale bars, 80 μm. The results are shown as low-magnification (bottom) and high-magnification (top) micrographs.
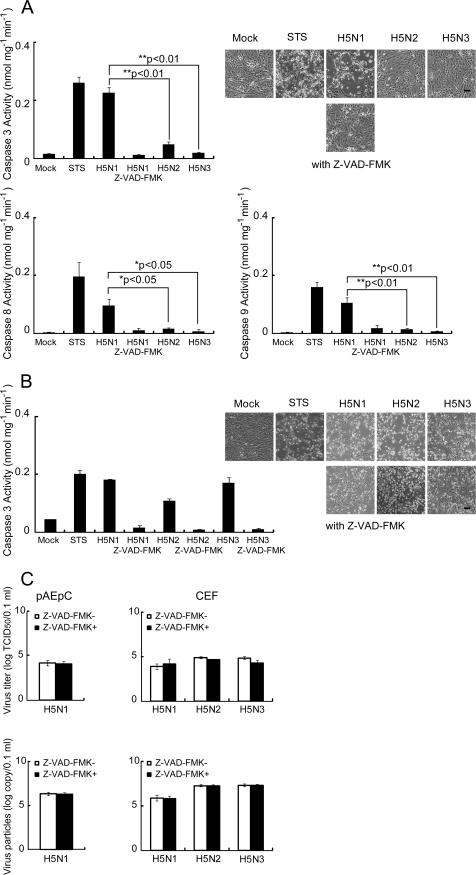
There are two main pathways for induction of apoptosis, the extrinsic pathway and the intrinsic pathway (13). In the extrinsic pathway, apoptosis is triggered by death ligands, such as TRAIL, tumor necrosis factor alpha, or Fas ligand, with these ligands binding their respective membrane receptors and activating the initiation of caspase 8, which directly activates other caspases, including caspase 3, or activates the mitochondrion-dependent pathway described below (2). In contrast to death receptor-mediated apoptosis, the key event of the intrinsic pathway is represented by mitochondrial damage, release of cytochrome c from mitochondria to the cytosol, and subsequent formation of the so-called apoptosome, composed of cytochrome c, caspase 9, and Apaf-1 (37). The apoptosome also activates caspase 3 and results in apoptosis represented by typical characteristics, such as intranucleosomal DNA fragmentation and morphological changes. Therefore, we performed quantitative analyses of caspase 3, 8, and 9 activities in H5-infected pAEpC. The activity of caspase 3 was significantly higher (P < 0.01) in H5N1-infected cells than in H5N2- or H5N3-infected cells (Fig. 6A, top). In addition, caspases 8 and 9 were more highly activated (P < 0.05 for caspase 8; P < 0.01 for caspase 9) in H5N1-infected cells than in H5N2- or H5N3-infected cells (Fig. 6A, bottom). These results indicate that H5N1 induces both the extrinsic and intrinsic apoptotic pathways. In CEF, the H5N1, H5N2, and H5N3 viruses elevated caspase 3 activity to similar levels (Fig. 6B). These caspase activities induced by the H5 viruses were completely inhibited by z-VAD-FMK in pAEpC and CEF (Fig. 6A and B), but progeny viruses were produced similarly from infected cells in the presence or absence of z-VAD-FMK in pAEpC, as well as CEF (Fig. 6C).
FIG. 6.
Caspase activities in pAEpC and CEF at 16 h p.i. with avian influenza virus. pAEpC-7, -8, and -9 and CEF were infected with H5N1, H5N2, or H5N3 virus at an MOI of 1. Parallel samples were cultured with the caspase inducer staurosporine (STS) as a control or with the pancaspase inhibitor z-VAD-FMK in the H5N1-infected cells. Caspase activity was determined with substrates appropriate for each caspase (caspase 3, Ac-DEVD-pNA; caspase 8, Ac-IETD-pNA; caspase 9, Ac-LEHD-pNA). (A) Caspase 3, 8, and 9 activities in pAEpC at 16 h p.i. with avian influenza virus. Each bar is an average of three pAEpC. The cell morphology of infected cells is shown. (B) Caspase 3 activity in CEF at 16 h p.i. with avian influenza virus. The cell morphology of infected cells is shown. (C) Virus production from CEF and pAEpC in the presence (+) or absence (−) of z-VAD-FMK. The viral titer was determined by the TCID50 (top) or by ELISA (bottom). The results (except those in panel B) were obtained individually from three different pAEpC and are presented as means ± standard errors. *, P < 0.05, and **, P < 0.01 versus H5N1. Scale bars, 100 μm.
A critical role for the H5N1 HA gene in apoptosis induction.
In order to evaluate whether the HA cleavage sequence is critical for apoptosis induction, we generated a recombinant form of H5N3 (rH5N3HAvir) possessing multiple basic amino acids (N′-RRKKR-C′) at the HA cleavage site (Fig. 7B). When pAEpC were infected with rH5N3HAvir at an MOI of 1, CPE and induction of caspase 3 were nearly the same as those of pAEpC infected with wild-type H5N3 possessing a single basic amino acid at its HA cleavage site. These results suggest that the HA cleavage sequence is not critical for caspase 3 activation and CPE. In contrast, when pAEpC were infected with recombinant H5N3 (rH5N3KYHA) containing the HA gene of H5N1 (A/crow/Kyoto/53/2004) under the same conditions described above, significant activation of caspase 3 (P < 0.01) and severe CPE were observed in the infected cells (Fig. 7A). Taken together, these results suggest that H5N1 HA protein is one of the main factors involved in the induction of caspase-dependent apoptosis, although the contributions of other viral proteins to apoptosis will have to be evaluated. In order to confirm that this was a feature of other H5N1 viruses as well, we replaced the HA gene of recombinant H5N3 (rH5N3ThaiHA) with that of human-isolated H5N1 (A/Thailand/Kan353/2004). Upon infection of pAEpC with rH5N3ThaiHA at an MOI of 1, caspase 3 activity and CPE similar to those induced by rH5N3KYHA were detected (Fig. 7A). The caspase 3 activities induced by recombinant viruses with H5N1-HA were completely inhibited by z-VAD-FMK (Fig. 7A).
FIG. 7.
Caspase 3 activity in pAEpC at 16 h p.i. with recombinant H5N3 viruses. pAEpC-7, -8, and -9 were infected with recombinant H5N3 viruses (rH5N3HAvir, rH5N3KYHA, and rH5N3ThaiHA) at an MOI of 1. Parallel samples were cultured with the caspase inducer staurosporine (STS) as a control or with the pancaspase inhibitor z-VAD-FMK in the cases of rH5N3KYHA- and rH5N3ThaiHA-infected cells. Caspase 3 activity was determined by using the appropriate substrate, Ac-DEVD-pNA. (A) Caspase 3 activity in pAEpC at 16 h p.i. with recombinant H5N3 viruses. The cell morphology of the infected cells is shown. (B) Sequence identities between H5N1 HAs and H5N3 HAs. The results were obtained individually from three different pAEpC and are presented as means plus standard errors. **, P < 0.01 versus H5N3HAvir. Scale bar, 100 μm.
CPE and growth kinetics of avian influenza virus in human airway epithelial cells.
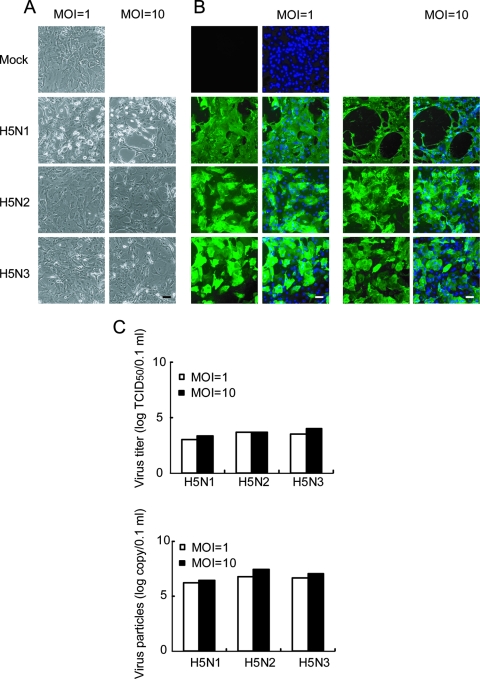
Finally, we evaluated whether the above-mentioned results obtained from pAEpC can be applied to human airway epithelial cells. When human SAEC, which are derived from human bronchiolar epithelium, were infected with H5N1, H5N2, and H5N3 at MOIs of 1 and 10, only H5N1-infected cells showed an obvious CPE (Fig. 8A). Corresponding to the results obtained from pAEpC, H5N1, H5N2, and H5N3 viruses replicated similarly in human SAEC at 24 h p.i. (Fig. 8B), and the levels of production of progeny viruses from infected cells were also comparable among the three H5 viruses, as measured by TCID50 (Fig. 8C, top), as well as quantitative ELISA (Fig. 8C, bottom). Furthermore, TUNEL-positive cells were detected only in H5N1-infected cells (Fig. 9). These results indicate that the CPE and growth kinetics of avian H5 influenza viruses in human airway epithelial cells are comparable to those in pAEpC.
FIG. 8.
Cytopathicity and virus propagation in human SAEC at 24 h p.i. with avian influenza virus. Human SAEC were infected with H5N1, H5N2, or H5N3 at an MOI of 1 or 10. (A and B) Morphological changes (A) and virus antigen expression (B) in SAEC at 24 h p.i. Virus antigen was detected by immunostaining, as for Fig. 2. (C) Progeny virus production from infected SAEC at 24 h p.i. The viral titer was determined by TCID50 (top) or ELISA (bottom). Scale bars, 100 μm.
FIG. 9.
TUNEL assay for detection of apoptosis in human SAEC at 16 h p.i. with avian influenza virus. Human SAEC were infected with H5N1, H5N2, or H5N3 at an MOI of 1 (in the absence of caspase inhibitor) or 10 (in the presence of the caspase inhibitor z-VAD-FMK). Parallel samples were cultured with the caspase inducer staurosporine (STS) as a positive control or with the pancaspase inhibitor z-VAD-FMK in H5N1-infected cells. The arrows indicate apoptotic cells (green). Virus antigen was detected simultaneously with a polyclonal antibody that recognizes all avian influenza viruses (red). Cellular nuclei were counterstained with Hoechst 33342 (blue). Scale bars, 80 μm. The results are shown as low-magnification (bottom) and high-magnification (top) micrographs.
DISCUSSION
In the present study, we obtained five main findings from our investigation of the cytopathicity induced by the H5N1 avian influenza virus in pAEpC and its possible mechanisms. (i) H5N1 and the previously isolated, less virulent H5N2 and H5N3 viruses were highly and equally cytopathic in CEF; in sharp contrast, only H5N1 was cytopathic in pAEpC. (ii) The H5N1, H5N2, and H5N3 viruses showed similar growth kinetics and efficient production of progeny virions in both pAEpC and avian CEF. (iii) H5N1 specifically induced caspase-dependent apoptosis in pAEpC. (iv) Recombinant H5N3 viruses (rH5N3KYHA and rH5N3ThaiHA) carrying the H5N1 HA gene induced caspase-dependent apoptosis, while rH5N3HAvir, possessing multiple basic amino acids within its HA, did not. (v) H5N1-specific cytotoxicity and apoptosis were also observed in human airway epithelial cells.
In this study, highly pathogenic H5N1 and the less pathogenic H5N2 and H5N3 were highly and equally cytopathic in CEF. Avian influenza H5 viruses, including H5N2 and H5N3, can cause severe outbreaks of disease in poultry (35), and the virulence of avian influenza viruses toward poultry in vivo does not depend on the HA0 cleavage site, although the extent of mortality does (1, 19, 23, 35, 41, 43, 46, 47). Our present results in primary cultured CEF might correspond to the pathogenesis of avian influenza virus in vivo. Supporting this relationship between cytopathicity in vitro and biological phenotype in vivo, a 2-amino-acid change in the hepatitis B virus X protein induces cytopathicity in primary hepatocytes of the tree shrew, which is susceptible to hepatitis B virus, and this mutation is directly linked to the pathological disease, fulminant hepatitis (4). The present results in CEF and a report by Baumer et al. (4) suggest that primary cultured cells can reflect pathogenesis in vivo.
Despite the similar susceptibilities of pAEpC to H5N1, H5N2, and H5N3 infection, only H5N1 was intensely cytopathic, consistent with the fact that among avian H5 influenza viruses, only H5N1 is virulent to humans in vivo (5, 59, 63). Therefore, considering that results in primary cultured cell systems can be closely correlated with pathogenesis in vivo, the H5N1 avian virus may be as virulent as human-derived influenza virus toward mammalian airway epithelial cells in vitro.
When pAEpC were independently infected with H5N1, H5N2, and H5N3 and TUNEL staining was performed, the key feature of apoptosis, intranucleosomal DNA fragmentation (25), was observed only in H5N1-infected cells (Fig. 5). Thus, the intense cytopathicity observed in pAEpC infected with H5N1 is due to apoptosis. These results led us to examine whether the cytopathicity in H5 virus-infected pAEpC is quantitatively or qualitatively regulated by a viral factor(s). The growth kinetics of H5N1, H5N2, and H5N3 were comparable (Fig. 2 to 4), although in one batch of pAEpC (pAEpC-8) at 8 h p.i., intracellular viral-protein synthesis was slightly higher in H5N1-infected pAEpC than in H5N2- or H5N3-infected pAEpC (Fig. 4). Thus, the intense cytopathicity and apoptosis observed in H5N1-infected pAEpC could depend on one or more H5N1-specific viral factors.
Several viral factors, including M1, NS1, and NA proteins, have been reported to be related to apoptosis induction (24, 30, 40, 66). Recently, PB1-F2, an 87-amino-acid protein synthesized from an alternate reading frame of the influenza A PB1 gene, was shown to induce cell death (9). Zamarin et al. (64) have also found that the PB1-F2 protein sensitizes cells to apoptotic stimuli, as demonstrated by increased cleavage of caspase 3 substrates in PB1-F2-expressing cells. In this study, we demonstrated that rH5N3KYHA and rH5N3ThaiHA, but not rH5N3HAvir, induced significant levels of caspase-dependent apoptosis (Fig. 7), suggesting that apoptotic cell death was caused by the H5N1 HA protein and not by differences in HA0 cleavage. Therefore, H5N1 HA could be one of the major factors involved in the induction of apoptosis. The importance of HA in apoptotic cell death in vitro is further supported by recent findings showing that the HA of the 1918 pandemic influenza virus enhances mortality in mice (21, 31, 51). Further investigations will be required to determine the minimal HA domain(s) required for apoptosis induction and if there is a correlation between high pathogenicity and the ability of viruses to induce apoptosis in vivo.
Wurzer et al. recently reported that caspase 3 enhances virus production from MDCK cells infected with avian H7N7 influenza virus and that the pancaspase inhibitor z-VAD-FMK inhibits virus production from infected cells (61). However, in our present study, the production levels of progeny virus from pAEpC and CEF infected with avian influenza virus were comparable in the presence and absence of z-VAD-FMK (Fig. 6). This incongruity in the regulation of virus production by caspase 3 could be due to differences in intracellular signal transduction between different cell types. Apoptosis induced by influenza viruses has been shown in a variety of cell lines (17, 24, 30, 33, 40, 48, 64, 66); however, in the last few years, a viral protein, NS1, has been shown to block apoptosis (14, 65). These opposite effects make the precise mechanism of virus-induced apoptosis unclear. The discrepancy might be based on the different cell lines and virus strains used in each experiment. In this study, H5N1-infected MDCK and A549 cells, derived from human lung carcinoma, showed little cytopathicity at 24 h p.i., although large amounts of viral proteins were made in the two cell lines (data not shown). In addition, infection of A549 with H5N1 did not change the activity of caspase 3, despite the large amounts of viral proteins synthesized (see Fig. S2 in the supplemental material). This lack of H5N1-induced cytopathicity might suggest that immortalized cell lines are not suitable as in vitro models for analyzing virus-induced CPE. We therefore investigated whether primary cells derived from mammalian airway epithelium could be a better system to evaluate the pathogenesis of influenza virus in humans.
The use of primary human airway epithelial cells is the most suitable for analyzing the pathogenesis of influenza virus in humans. Although primary human cells have been used for this purpose (7, 18, 22, 27, 28, 49, 56), infection experiments with these cells are restricted in practice because of the limited number of donors, the limited number of cells per person, and/or ethical issues. As shown in this study, the similar H5 virus growth and cytopathicity in pAEpC and human SAEC suggest that pAEpC may be a good experimental system to model the human airway epithelium. pAEpC can be prepared on a large scale, and large numbers of cells allow us to perform many kinds of in vitro infection experiments, such as evaluation of viral growth kinetics, virus-induced cytopathicity, and expression profiles of host genes, including those in apoptotic pathways. Furthermore, this system has the advantage of being able to compare viral growth and cytopathicity in different types of airway epithelial cells, from trachea to alveoli, in a single animal. We did not use ciliated cells in this study; however, the pAEpC were monolayered and were confirmed to be epithelial cells by staining them with an antibody against pancytokeratin, an epithelial cell marker (see Fig. S1 in the supplemental material). Further investigations using well-differentiated primary epithelial cells could generate a lot of useful information, and comparative experiments using ciliated and nonciliated primary cells might be necessary in future studies.
Generally, the major cause of death in patients infected with H5N1 has been shown to be ARDS concomitant with DAD, including the loss of alveolar epithelial cells and widespread hemorrhage in the lung (10, 11, 32, 50, 54, 55). Uiprasertkul et al. (54) reported that TUNEL-positive cells, together with a loss of alveolar epithelial cells and widespread hemorrhage, were found in autopsy lung alveolar sections from H5N1-infected humans. This report indicates that the pathogenesis of H5N1, including DAD, is possibly caused by apoptosis. In addition, several reports suggested that apoptosis is a prerequisite for DAD followed by ARDS (16, 29). Our established in vitro system to evaluate apoptotic cell death in mammalian airway epithelial cells caused by H5N1 virus could be useful for revealing the molecular mechanisms by which the recently emerged H5N1 virus induces severe ARDS in humans.
For the past several years, the pathogenesis of H5N1 has been of major concern worldwide. While knowledge of the mechanisms governing viral entry, propagation, and budding has advanced considerably, we still know relatively little about other aspects of H5N1 virulence, such as cytopathicity, in part because of a lack of suitable in vitro infection systems that properly mimic the human airway epithelium. In this study, we used primary airway epithelial cells prepared from porcine lung and presented evidence that the pathogenicity of H5N1 in humans is based on virus-induced apoptosis, which could lead to DAD. Given that H5N1-induced cytopathicity is due to caspase-dependent apoptosis, decreasing the activity of caspase 3, 8, or 9 by pharmaceutical, neutraceutical, or other techniques may provide patient relief from the DAD induced by H5N1. Further studies are also required to determine the host factors that interact with H5N1 HA protein in the apoptotic pathway that elicits apoptosis in mammalian alveolar epithelium.
Supplementary Material
Acknowledgments
We thank Ritsuko Koketsu, Masanobu Yamate, Yohei Watanabe, Takuhiro Matsumura, Yo Sugawara, Yohei Hayashi, and Yuki Takegahara (RIMD, Osaka University); Hiroshi Yokota, Hidetomo Iwano, and Katsuro Hagiwara (Rakuno Gakuen University); and Masahiko Nakamura (Kyoto Gakuen University) for helpful discussions. We also thank Yoshinobu Okuno, Osaka Prefectural Institute of Public Health, for providing viruses and helpful discussions.
This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports, Culture, and Technology (MEXT) to T.N.; a Grant-in-Aid for Young Scientists from the Japan Society for the Promotion of Science (JSPS) to T.D.; the Sasakawa Scientific Research Grant from the Japan Science Society to T.D.; a grant from Kato Memorial Bioscience Foundation to T.D.; and a project for the International Research Center for Infectious Diseases, RIMD, Osaka University from the MEXT to T.D. and T.N. M.U. is a research fellow of JSPS Research Fellowships for Young Scientists.
Footnotes
Published ahead of print on 11 September 2008.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Alexander, D. J. 2007. An overview of the epidemiology of avian influenza. Vaccine 255637-5644. [DOI] [PubMed] [Google Scholar]
- 2.Ashkenazi, A. 2002. Targeting death and decoy receptors of the tumour-necrosis factor superfamily. Nat. Rev. Cancer 2420-430. [DOI] [PubMed] [Google Scholar]
- 3.Basler, C. F., A. H. Reid, J. K. Dybing, T. A. Janczewski, T. G. Fanning, H. Zheng, M. Salvatore, M. L. Perdue, D. E. Swayne, A. Garcia-Sastre, P. Palese, and J. K. Taubenberger. 2001. Sequence of the 1918 pandemic influenza virus nonstructural gene (NS) segment and characterization of recombinant viruses bearing the 1918 NS genes. Proc. Natl. Acad. Sci. USA 982746-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baumert, T. F., C. Yang, P. Schurmann, J. Kock, C. Ziegler, C. Grullich, M. Nassal, T. J. Liang, H. E. Blum, and F. von Weizsacker. 2005. Hepatitis B virus mutations associated with fulminant hepatitis induce apoptosis in primary Tupaia hepatocytes. Hepatology 41247-256. [DOI] [PubMed] [Google Scholar]
- 5.Beigel, J. H., J. Farrar, A. M. Han, F. G. Hayden, R. Hyer, M. D. de Jong, S. Lochindarat, T. K. Nguyen, T. H. Nguyen, T. H. Tran, A. Nicoll, S. Touch, and K. Y. Yuen. 2005. Avian influenza A (H5N1) infection in humans. N. Engl. J. Med. 3531374-1385. [DOI] [PubMed] [Google Scholar]
- 6.Castrucci, M. R., L. Campitelli, A. Ruggieri, G. Barigazzi, L. Sidoli, R. Daniels, J. S. Oxford, and I. Donatelli. 1994. Antigenic and sequence analysis of H3 influenza virus haemagglutinins from pigs in Italy. J. Gen. Virol. 75371-379. [DOI] [PubMed] [Google Scholar]
- 7.Chan, M. C., C. Y. Cheung, W. H. Chui, S. W. Tsao, J. M. Nicholls, Y. O. Chan, R. W. Chan, H. T. Long, L. L. Poon, Y. Guan, and J. S. Peiris. 2005. Proinflammatory cytokine responses induced by influenza A (H5N1) viruses in primary human alveolar and bronchial epithelial cells. Respir. Res. 6135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan, P. K. 2002. Outbreak of avian influenza A(H5N1) virus infection in Hong Kong in 1997. Clin. Infect. Dis. 34(Suppl. 2)S58-S64. [DOI] [PubMed] [Google Scholar]
- 9.Chen, W., P. A. Calvo, D. Malide, J. Gibbs, U. Schubert, I. Bacik, S. Basta, R. O'Neill, J. Schickli, P. Palese, P. Henklein, J. R. Bennink, and J. W. Yewdell. 2001. A novel influenza A virus mitochondrial protein that induces cell death. Nat. Med. 71306-1312. [DOI] [PubMed] [Google Scholar]
- 10.Chokephaibulkit, K., M. Uiprasertkul, P. Puthavathana, P. Chearskul, P. Auewarakul, S. F. Dowell, and N. Vanprapar. 2005. A child with avian influenza A (H5N1) infection. Pediatr. Infect. Dis. J. 24162-166. [DOI] [PubMed] [Google Scholar]
- 11.Chotpitayasunondh, T., K. Ungchusak, W. Hanshaoworakul, S. Chunsuthiwat, P. Sawanpanyalert, R. Kijphati, S. Lochindarat, P. Srisan, P. Suwan, Y. Osotthanakorn, T. Anantasetagoon, S. Kanjanawasri, S. Tanupattarachai, J. Weerakul, R. Chaiwirattana, M. Maneerattanaporn, R. Poolsavathitikool, K. Chokephaibulkit, A. Apisarnthanarak, and S. F. Dowell. 2005. Human disease from influenza A (H5N1), Thailand, 2004. Emerg. Infect. Dis. 11201-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cummings, B. S., J. McHowat, and R. G. Schnellmann. 2000. Phospholipase A(2)s in cell injury and death. J. Pharmacol. Exp. Ther. 294793-799. [PubMed] [Google Scholar]
- 13.Degterev, A., M. Boyce, and J. Yuan. 2003. A decade of caspases. Oncogene 228543-8567. [DOI] [PubMed] [Google Scholar]
- 14.Ehrhardt, C., T. Wolff, S. Pleschka, O. Planz, W. Beermann, J. G. Bode, M. Schmolke, and S. Ludwig. 2007. Influenza A virus NS1 protein activates the PI3K/Akt pathway to mediate antiapoptotic signaling responses. J. Virol. 813058-3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fodor, E., L. Devenish, O. G. Engelhardt, P. Palese, G. G. Brownlee, and A. Garcia-Sastre. 1999. Rescue of influenza A virus from recombinant DNA. J. Virol. 739679-9682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guinee, D., Jr., E. Brambilla, M. Fleming, T. Hayashi, M. Rahn, M. Koss, V. Ferrans, and W. Travis. 1997. The potential role of BAX and BCL-2 expression in diffuse alveolar damage. Am. J. Pathol. 151999-1007. [PMC free article] [PubMed] [Google Scholar]
- 17.Hinshaw, V. S., C. W. Olsen, N. Dybdahl-Sissoko, and D. Evans. 1994. Apoptosis: a mechanism of cell killing by influenza A and B viruses. J. Virol. 683667-3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ibricevic, A., A. Pekosz, M. J. Walter, C. Newby, J. T. Battaile, E. G. Brown, M. J. Holtzman, and S. L. Brody. 2006. Influenza virus receptor specificity and cell tropism in mouse and human airway epithelial cells. J. Virol. 807469-7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Isoda, N., Y. Sakoda, N. Kishida, G. R. Bai, K. Matsuda, T. Umemura, and H. Kida. 2006. Pathogenicity of a highly pathogenic avian influenza virus, A/chicken/Yamaguchi/7/04 (H5N1) in different species of birds and mammals. Arch. Virol. 1511267-1279. [DOI] [PubMed] [Google Scholar]
- 20.Ito, T., J. N. Couceiro, S. Kelm, L. G. Baum, S. Krauss, M. R. Castrucci, I. Donatelli, H. Kida, J. C. Paulson, R. G. Webster, and Y. Kawaoka. 1998. Molecular basis for the generation in pigs of influenza A viruses with pandemic potential. J. Virol. 727367-7373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobasa, D., A. Takada, K. Shinya, M. Hatta, P. Halfmann, S. Theriault, H. Suzuki, H. Nishimura, K. Mitamura, N. Sugaya, T. Usui, T. Murata, Y. Maeda, S. Watanabe, M. Suresh, T. Suzuki, Y. Suzuki, H. Feldmann, and Y. Kawaoka. 2004. Enhanced virulence of influenza A viruses with the haemagglutinin of the 1918 pandemic virus. Nature 431703-707. [DOI] [PubMed] [Google Scholar]
- 22.Kogure, T., T. Suzuki, T. Takahashi, D. Miyamoto, K. I. Hidari, C. T. Guo, T. Ito, Y. Kawaoka, and Y. Suzuki. 2006. Human trachea primary epithelial cells express both sialyl(alpha2-3)Gal receptor for human parainfluenza virus type 1 and avian influenza viruses, and sialyl(alpha2-6)Gal receptor for human influenza viruses. Glycoconj. J. 23101-106. [DOI] [PubMed] [Google Scholar]
- 23.Kwon, Y. K., H. W. Sung, S. J. Joh, Y. J. Lee, M. C. Kim, J. G. Choi, E. K. Lee, S. H. Wee, and J. H. Kim. 2005. An outbreak of highly pathogenic avian influenza subtype H5N1 in broiler breeders, Korea. J. Vet. Med. Sci. 671193-1196. [DOI] [PubMed] [Google Scholar]
- 24.Lam, W. Y., J. W. Tang, A. C. Yeung, L. C. Chiu, J. J. Sung, and P. K. Chan. 2008. Avian influenza virus A/HK/483/97(H5N1) NS1 protein induces apoptosis in human airway epithelial cells. J. Virol. 822741-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lazebnik, Y. A., S. Cole, C. A. Cooke, W. G. Nelson, and W. C. Earnshaw. 1993. Nuclear events of apoptosis in vitro in cell-free mitotic extracts: a model system for analysis of the active phase of apoptosis. J. Cell Biol. 1237-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lazzaro, V. A., R. J. Walker, G. G. Duggin, A. Phippard, J. S. Horvath, and D. J. Tiller. 1992. Inhibition of fibroblast proliferation in l-valine reduced selective media. Res. Commun. Chem. Pathol. Pharmacol. 7539-48. [PubMed] [Google Scholar]
- 27.Matrosovich, M. N., T. Y. Matrosovich, T. Gray, N. A. Roberts, and H. D. Klenk. 2004. Human and avian influenza viruses target different cell types in cultures of human airway epithelium. Proc. Natl. Acad. Sci. USA 1014620-4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matrosovich, M. N., T. Y. Matrosovich, T. Gray, N. A. Roberts, and H. D. Klenk. 2004. Neuraminidase is important for the initiation of influenza virus infection in human airway epithelium. J. Virol. 7812665-12667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matute-Bello, G., W. C. Liles, K. P. Steinberg, P. A. Kiener, S. Mongovin, E. Y. Chi, M. Jonas, and T. R. Martin. 1999. Soluble Fas ligand induces epithelial cell apoptosis in humans with acute lung injury (ARDS). J. Immunol. 1632217-2225. [PubMed] [Google Scholar]
- 30.Morris, S. J., G. E. Price, J. M. Barnett, S. A. Hiscox, H. Smith, and C. Sweet. 1999. Role of neuraminidase in influenza virus-induced apoptosis. J. Gen. Virol. 80137-146. [DOI] [PubMed] [Google Scholar]
- 31.Pappas, C., P. V. Aguilar, C. F. Basler, A. Solorzano, H. Zeng, L. A. Perrone, P. Palese, A. Garcia-Sastre, J. M. Katz, and T. M. Tumpey. 2008. Single gene reassortants identify a critical role for PB1, HA, and NA in the high virulence of the 1918 pandemic influenza virus. Proc. Natl. Acad. Sci. USA 1053064-3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peiris, J. S., W. C. Yu, C. W. Leung, C. Y. Cheung, W. F. Ng, J. M. Nicholls, T. K. Ng, K. H. Chan, S. T. Lai, W. L. Lim, K. Y. Yuen, and Y. Guan. 2004. Re-emergence of fatal human influenza A subtype H5N1 disease. Lancet 363617-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Price, G. E., H. Smith, and C. Sweet. 1997. Differential induction of cytotoxicity and apoptosis by influenza virus strains of differing virulence. J. Gen. Virol. 782821-2829. [DOI] [PubMed] [Google Scholar]
- 34.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 27493-497. [Google Scholar]
- 35.Rohm, C., T. Horimoto, Y. Kawaoka, J. Suss, and R. G. Webster. 1995. Do hemagglutinin genes of highly pathogenic avian influenza viruses constitute unique phylogenetic lineages? Virology 209664-670. [DOI] [PubMed] [Google Scholar]
- 36.Rowe, R. K., S. L. Brody, and A. Pekosz. 2004. Differentiated cultures of primary hamster tracheal airway epithelial cells. In Vitro Cell Dev. Biol. Anim. 40303-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saelens, X., N. Festjens, L. Vande Walle, M. van Gurp, G. van Loo, and P. Vandenabeele. 2004. Toxic proteins released from mitochondria in cell death. Oncogene 232861-2874. [DOI] [PubMed] [Google Scholar]
- 38.Salvesen, G. S., and V. M. Dixit. 1997. Caspases: intracellular signaling by proteolysis. Cell 91443-446. [DOI] [PubMed] [Google Scholar]
- 39.Saraste, A. 1999. Morphologic criteria and detection of apoptosis. Herz 24189-195. [DOI] [PubMed] [Google Scholar]
- 40.Schultz-Cherry, S., N. Dybdahl-Sissoko, G. Neumann, Y. Kawaoka, and V. S. Hinshaw. 2001. Influenza virus ns1 protein induces apoptosis in cultured cells. J. Virol. 757875-7881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shalaby, A. A., R. D. Slemons, and D. E. Swayne. 1994. Pathological studies of A/chicken/Alabama/7395/75 (H4N8) influenza virus in specific-pathogen-free laying hens. Avian Dis. 3822-32. [PubMed] [Google Scholar]
- 42.Shinya, K., M. Ebina, S. Yamada, M. Ono, N. Kasai, and Y. Kawaoka. 2006. Avian flu: influenza virus receptors in the human airway. Nature 440435-436. [DOI] [PubMed] [Google Scholar]
- 43.Slemons, R. D., and D. E. Swayne. 1992. Nephrotropic properties demonstrated by A/chicken/Alabama/75 (H4N8) following intravenous challenge of chickens. Avian Dis. 36926-931. [PubMed] [Google Scholar]
- 44.Smith, M. A., J. Swann, and D. Acosta. 1988. Isolation and primary culture of rat renal cortical epithelial cells. J. Tissue Cult. Methods 11207-210. [Google Scholar]
- 45.Steimer, A., M. Laue, H. Franke, E. Haltner-Ukomado, and C. M. Lehr. 2006. Porcine alveolar epithelial cells in primary culture: morphological, bioelectrical and immunocytochemical characterization. Pharm. Res. 232078-2093. [DOI] [PubMed] [Google Scholar]
- 46.Swayne, D. E. 1997. Pathobiology of H5N2 Mexican avian influenza virus infections of chickens. Vet. Pathol. 34557-567. [DOI] [PubMed] [Google Scholar]
- 47.Swayne, D. E., M. J. Radin, T. M. Hoepf, and R. D. Slemons. 1994. Acute renal failure as the cause of death in chickens following intravenous inoculation with avian influenza virus A/chicken/Alabama/7395/75 (H4N8). Avian Dis. 38151-157. [PubMed] [Google Scholar]
- 48.Takizawa, T., S. Matsukawa, Y. Higuchi, S. Nakamura, Y. Nakanishi, and R. Fukuda. 1993. Induction of programmed cell death (apoptosis) by influenza virus infection in tissue culture cells. J. Gen. Virol. 742347-2355. [DOI] [PubMed] [Google Scholar]
- 49.Thompson, C. I., W. S. Barclay, M. C. Zambon, and R. J. Pickles. 2006. Infection of human airway epithelium by human and avian strains of influenza A virus. J. Virol. 808060-8068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.To, K. F., P. K. Chan, K. F. Chan, W. K. Lee, W. Y. Lam, K. F. Wong, N. L. Tang, D. N. Tsang, R. Y. Sung, T. A. Buckley, J. S. Tam, and A. F. Cheng. 2001. Pathology of fatal human infection associated with avian influenza A H5N1 virus. J. Med. Virol. 63242-246. [DOI] [PubMed] [Google Scholar]
- 51.Tumpey, T. M., C. F. Basler, P. V. Aguilar, H. Zeng, A. Solorzano, D. E. Swayne, N. J. Cox, J. M. Katz, J. K. Taubenberger, P. Palese, and A. Garcia-Sastre. 2005. Characterization of the reconstructed 1918 Spanish influenza pandemic virus. Science 31077-80. [DOI] [PubMed] [Google Scholar]
- 52.Tumpey, T. M., A. Garcia-Sastre, A. Mikulasova, J. K. Taubenberger, D. E. Swayne, P. Palese, and C. F. Basler. 2002. Existing antivirals are effective against influenza viruses with genes from the 1918 pandemic virus. Proc. Natl. Acad. Sci. USA 9913849-13854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ueda, M., M. Yamate, A. Du, T. Daidoji, Y. Okuno, K. Ikuta, and T. Nakaya. 2008. Maturation efficiency of viral glycoproteins in the ER impacts the production of influenza A virus. Virus Res. 13691-97. [DOI] [PubMed] [Google Scholar]
- 54.Uiprasertkul, M., R. Kitphati, P. Puthavathana, R. Kriwong, A. Kongchanagul, K. Ungchusak, S. Angkasekwinai, K. Chokephaibulkit, K. Srisook, N. Vanprapar, and P. Auewarakul. 2007. Apoptosis and pathogenesis of avian influenza A (H5N1) virus in humans. Emerg. Infect. Dis. 13708-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Uiprasertkul, M., P. Puthavathana, K. Sangsiriwut, P. Pooruk, K. Srisook, M. Peiris, J. M. Nicholls, K. Chokephaibulkit, N. Vanprapar, and P. Auewarakul. 2005. Influenza A H5N1 replication sites in humans. Emerg. Infect. Dis. 111036-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wan, H., and D. R. Perez. 2007. Amino acid 226 in the hemagglutinin of H9N2 influenza viruses determines cell tropism and replication in human airway epithelial cells. J. Virol. 815181-5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Webster, R. G., G. B. Sharp, and E. C. Claas. 1995. Interspecies transmission of influenza viruses. Am. J. Respir. Crit. Care Med. 152S25-S30. [DOI] [PubMed] [Google Scholar]
- 58.Webster, R. G., S. M. Wright, M. R. Castrucci, W. J. Bean, and Y. Kawaoka. 1993. Influenza—a model of an emerging virus disease. Intervirology 3516-25. [DOI] [PubMed] [Google Scholar]
- 59.Wong, S. S., and K. Y. Yuen. 2006. Avian influenza virus infections in humans. Chest 129156-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.World Health Organization. 2008. Cumulative number of confirmed human cases of avian influenza A/(H5N1) reported to WHO. World Health Organization, Geneva, Switzerland. http://www.who.int/csr/disease/avian_influenza/country/cases_table_2008_04_17/en/index.html. Accessed 20 April 2008.
- 61.Wurzer, W. J., O. Planz, C. Ehrhardt, M. Giner, T. Silberzahn, S. Pleschka, and S. Ludwig. 2003. Caspase 3 activation is essential for efficient influenza virus propagation. EMBO J. 222717-2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.You, Y., E. J. Richer, T. Huang, and S. L. Brody. 2002. Growth and differentiation of mouse tracheal epithelial cells: selection of a proliferative population. Am. J. Physiol. Lung Cell Mol. Physiol. 283L1315-L1321. [DOI] [PubMed] [Google Scholar]
- 63.Yu, H., Y. Shu, S. Hu, H. Zhang, Z. Gao, H. Chen, J. Dong, C. Xu, Y. Zhang, N. Xiang, M. Wang, Y. Guo, N. Cox, W. Lim, D. Li, Y. Wang, and W. Yang. 2006. The first confirmed human case of avian influenza A (H5N1) in Mainland China. Lancet 36784. [DOI] [PubMed] [Google Scholar]
- 64.Zamarin, D., A. Garcia-Sastre, X. Xiao, R. Wang, and P. Palese. 2005. Influenza virus PB1-F2 protein induces cell death through mitochondrial ANT3 and VDAC1. PLoS Pathog. 1e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhirnov, O. P., T. E. Konakova, T. Wolff, and H. D. Klenk. 2002. NS1 protein of influenza A virus down-regulates apoptosis. J. Virol. 761617-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhirnov, O. P., A. L. Ksenofontov, S. G. Kuzmina, and H. D. Klenk. 2002. Interaction of influenza A virus M1 matrix protein with caspases. Biochemistry 67534-539. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.