Abstract
The presence, at the time of challenge, of antiviral effector T cells in the vaginal mucosa of female rhesus macaques immunized with live-attenuated simian-human immunodeficiency virus 89.6 (SHIV89.6) is associated with consistent and reproducible protection from pathogenic simian immunodeficiency virus (SIV) vaginal challenge (18). Here, we definitively demonstrate the protective role of the SIV-specific CD8+ T-cell response in SHIV-immunized monkeys by CD8+ lymphocyte depletion, an intervention that abrogated SHIV-mediated control of challenge virus replication and largely eliminated the SIV-specific T-cell responses in blood, lymph nodes, and genital mucosa. While in the T-cell-intact SHIV-immunized animals, polyfunctional and degranulating SIV-specific CD8+ T cells were present in the genital tract and lymphoid tissues from the day of challenge until day 14 postchallenge, strikingly, expansion of SIV-specific CD8+ T cells in the immunized monkeys was minimal and limited to the vagina. Thus, protection from uncontrolled SIV replication in animals immunized with attenuated SHIV89.6 is primarily mediated by CD8+ T cells that do not undergo dramatic systemic expansion after SIV challenge. These findings demonstrate that despite, and perhaps because of, minimal systemic expansion of T cells at the time of challenge, a stable population of effector-cytotoxic CD8+ T cells can provide significant protection from vaginal SIV challenge.
Sexual transmission remains the predominant mode of human immunodeficiency virus (HIV) infection globally. Thus, identification of a vaccine strategy that can induce immune responses capable of containing the virus in the genital tract before the establishment of a systemic infection would be a significant advance in the effort to stop the HIV pandemic. Uncertainty as to the nature of the immunological response that is needed to control viral replication remains the greatest hurdle in developing an effective HIV vaccine (51). We have repeatedly shown that infection with simian-human immunodeficiency virus 89.6 (SHIV89.6) consistently protects 60% of female rhesus macaques from uncontrolled viral replication after vaginal simian immunodeficiency virus (SIV) challenge (2, 34). Thus, defining protective immune responses in this model may provide an important advance in the effort to develop an HIV vaccine. As the envelope (env) gene in the immunizing SHIV is derived from HIV and only distantly related to SIV env, it is unlikely that neutralizing antibodies have a role in this model of protection (2, 34). We recently reported that SIV Gag-specific CD4+ and CD8+ T cells are present in the vagina of female rhesus macaques 8 months after systemic SHIV89.6 immunization (18). These effector T cells are located at the portal of entry at the time when SHIV immunization is highly effective against vaginal SIV challenge (2, 34). Thus, an indirect association between CD8+ T-cell responses in the vagina and the consistent protection observed in this model has been established (18).
Recent studies suggest that the quality of the T-cell responses may be critical for control of HIV replication (4, 6). Similarly, in live-attenuated SHIV-immunized macaques, polyfunctional T-cell responses are associated with better control of challenge virus replication (17). The ability of HIV/SIV to replicate robustly in the first hours after vaginal transmission is likely the single most important factor in determining if vaginal exposure will result in systemic infection (35). Thus, as noted above (18) and suggested by others (5, 45), the presence of a T-cell response with cytolytic capacity at the portal of entry is likely a desirable feature for an HIV vaccine. Additionally, since survival and apoptotic signals are also induced through the cognate antigen-T-cell receptor interaction (11), the relative resistance or sensitivity of T cells to apoptosis upon antigenic stimulation may be an important factor in an effective anti-HIV T-cell response. Thus, defining the expression of pro- and antiapoptotic molecules in vaccine-induced T cells is necessary to fully understand the nature of the protective CD8+ T-cell response in this model.
While it is generally accepted that virus-specific CD8+ T lymphocytes play a central role in controlling HIV and SIV replication (8, 16, 23, 26, 46, 47), a recently halted efficacy trial of a HIV T-cell vaccine failed to show any evidence of protection from infection, or uncontrolled viral replication, in vaccine recipients (43, 51), raising some doubts as to the ability of vaccine-elicited T-cell responses to control HIV replication (20, 36, 41, 54). To define the role of CD8+ T cells in live, attenuated vaccine-induced protection from vaginal SIV challenge, we determined the frequency, function, and survival capacity of SHIV89.6-induced Gag-specific CD8+ T-cell responses in blood and tissues at early time points after intravaginal SIVmac239 pathogenic challenge. We found that, with the exception of the T-cell responses in the vagina, anti-SIV CD8+ T-cell responses in the tissues and blood of SHIV-immunized animals were consistently present but that they did not dramatically expand after SIV challenge. After SIV challenge, the antiviral CD8+ T-cell responses were most consistently found in the genital lymph nodes (Gen LN) and vagina of the SHIV-immunized animals. In the unimmunized monkeys, the number of functions, cytotoxicity, and survival potential of antiviral CD8+ T cells were clearly restricted relative to the antiviral CD8+ T-cell responses in immunized monkeys. Importantly, after SIV challenge, both T-cell proliferation and T-cell death were blunted in the immunized monkeys compared to the unimmunized monkeys. Finally, CD8+ T-cell depletion on the day of challenge eliminated the SIV-specific CD8+ T-cell response in most tissues through day 14 postchallenge (p.c.) and abrogated the protection conferred by SHIV immunization against vaginal SIV challenge.
MATERIALS AND METHODS
Animals, immunization, and challenge.
Female rhesus macaques (Macaca mulatta) were housed at the California National Primate Research Center in accordance with the American Association for Accreditation of Laboratory Animal Care standards. The experiments were approved by the Institutional Animal Use and Care Committee of the University of California, Davis. All animals were negative for antibodies to HIV type 2, SIV, type D retrovirus, and simian T-cell lymphotropic virus type 1 at the time the study was initiated.
A total of 35 females were intravenously infected with live, virulence-attenuated SHIV89.6 for 6 to 8 months, as previously described (2). After the 6- to 8-month immunization period, at the day zero time point, nine of these animals were necropsied; a detailed study of the SIV-specific T-cell responses has been published (18). The remaining immunized macaques (n = 26), were challenged with pathogenic SIVmac239 by intravaginal inoculation, as described previously (2, 34), and necropsied at 3 (n = 3), 7 (n = 6), or 14 (n = 12) days p.c. Furthermore, five immunized macaques were infused intravenously with a depleting monoclonal antibody (MAb) directed against CD8α (cM-T807; 50 mg/kg of body weight; Centocor, Malvern, PA) on the day of challenge and necropsied at 14 days p.c. In addition, 21 naive female macaques that were vaginally challenged with pathogenic SIVmac239 and necropsied at 3 (n = 3), 7 (n = 9), and 14 (n = 9) days p.c. were used as unimmunized control animals.
The animals were randomly assigned to the immunized or unimmunized group and were distributed as evenly as possible for the necropsy dates according to their major histocompatibility complex (MHC) class I genotype and country of origin (Table 1). Even though the immunized and unimmunized groups did not contain the same number of specific MHC alleles, the frequency of alleles associated with better control of protection was higher in the SIV control macaques than in the immunized macaques, and conversely, among the SHIV-immunized macaques, there was an increased frequency of animals with the Mamu-B*01 allele, associated with poor control of viral replication (9).
TABLE 1.
MHC class I genotypes, country of origin, and plasma vRNA at the time of necropsy for individual rhesus macaques
| Group | Monkey | MHC class I allelea | Country of origin | Highest plasma vRNA (log10) |
|---|---|---|---|---|
| SHIV-immunized 7 days | 26820 | - | India | <2.01 |
| 26833 | B01 | India | 2.12 | |
| 27076 | A01/A02 | India | <2.01 | |
| 28415 | A11/B17/B29 | India | <2.01 | |
| 31631 | B01 | 1/4 China | <2.01 | |
| 27373 | A02 | India | <2.01 | |
| SHIV-immunized 14 days | 31371 | - | India | <2.01 |
| 27130 | A02/B01 | India | <2.01 | |
| 28843 | B01 | India | 5.20 | |
| 31475 | B01 | India | <2.01 | |
| 25988 | A01 | India | <2.01 | |
| 32330 | B01 | 1/8 China | 4.09 | |
| 26960 | A02 | India | 3.01 | |
| 28850 | A01/A08 | India | <2.01 | |
| 30906 | - | India | 2.24 | |
| 30933 | B01 | 1/2 China | <2.01 | |
| 32427 | A01/B17/B29 | India | <2.01 | |
| 32578 | A02 | India | <2.01 | |
| SIV 7 days | 29612 | A01/A02 | Indiab | <2.01 |
| 29683 | A02 | 1/4 China | 4.04 | |
| 30678 | A02/B17/B29 | 1/8 China | <2.01 | |
| 27768 | - | Indiab | <2.01 | |
| 28953 | A01/A02 | Indiab | 2.16 | |
| 28726 | A08 | India | <2.01 | |
| 30322 | ND | 1/4 China | 4.34 | |
| 28752 | A01/ND | India | 5.51 | |
| 26715 | ND | India | 5.33 | |
| SIV 14 days | 30946 | A02/A011 | India | 7.32 |
| 31391 | A01 | India | 6.01 | |
| 32604 | A02 | 1/8 China | 7.29 | |
| 28629 | - | India | 7.08 | |
| 28827 | A02 | Indiab | 6.86 | |
| 28366 | A01 | 1/8 China | 6.87 | |
| 28630 | A01/ND | India | 7.50 | |
| 26984 | ND | India | 6.73 | |
| 29967 | ND | 1/2 China | 5.59 | |
| SHIV-immunized CD8-depleted 14 days | 30863 | B01 | India | 6.98 |
| 30924 | - | 1/2 China | 6.94 | |
| 33175 | - | India | 6.92 | |
| 30921 | - | India | 7.21 | |
| 33982 | B01 | India | 6.69 |
MHC class I loci and alleles were typed by PCR. A dash indicates no known alleles. ND (not determined) indicates that MHC genotyping was performed only for the A01 allele.
Indian origin from 100 to 50%; one parent's pedigree was not verified.
Isolation of lymphocytes from the blood, LN, and cervical and vaginal mucosa.
At necropsy, fresh cervixes and vaginas were collected in complete RPMI 1640 medium containing 10% fetal calf serum, 2 mM l-glutamine, 100 U/ml penicillin, 0.1 mg/ml streptomycin, and 1% amphotericin B (Sigma-Aldrich), and lymphocytes were isolated from genital mucosal tissues as previously described (30, 44). Mononuclear cell suspensions were prepared from LN as previously described (33), and peripheral blood mononuclear cells (PBMC) were isolated from heparinized blood using Lymphocyte Separation Medium (ICN Biomedicals). PBMC samples were frozen in 10% dimethyl sulfoxide (DMSO) (Sigma-Aldrich)/90% fetal bovine serum (Gemini BioProducts) and stored in liquid nitrogen until future analysis in immunological assays (34).
Viral-load measurement.
Plasma was analyzed for viral RNA (vRNA) by a quantitative branched-chain DNA assay with a detection limit of 125 vRNA copies/ml plasma, as previously described (14). The viral loads in plasma samples are reported as the vRNA copy number per ml of plasma.
MHC class I genotyping by PCR.
MHC class I genotyping by PCR was performed by the Rhesus Macaque MHC Typing Core, University of Wisconsin Hospital and Clinics.
Flow cytometric analysis of T-cell populations in blood.
The percentages of CD3+ CD4+ T cells and CD3+ CD8+ T cells within the lymphocyte populations were determined by flow cytometric analysis using a FACSCalibur (Becton Dickinson Immunocytometry Systems) and rhesus macaque-reactive antibodies (Pharmingen/Becton Dickinson; anti-CD3 clone no. SP34, anti-CD4 clone no. M-T477, and anti-CD8 clone no. SK1).
Intracellular staining for cytokine and degranulation markers.
One million freshly isolated mononuclear cells from cervix and vagina were incubated with anti-CD28 and anti-CD49d antibodies (1 μg/ml final concentration; BD-Biosciences) as costimulatory molecules in a total volume of 200 μl RPMI 1640/10% fetal calf serum. In all experiments, the following samples were prepared: SIV Gag p27 peptide pool (20mer peptides overlapping by 10 residues; Anaspec, Inc.) at 5 μg/ml, background controls containing costimulatory molecules and DMSO, and a positive control stimulated with staphylococcal enterotoxin B (0.2 μg/ml; Sigma-Aldrich). One million cells were divided further into two panels, and in the second, a mixture of anti-CD107a- and anti-CD107b-fluorescein isothiocyanate (FITC) MAbs (clones H4A3 and H4B4, respectively; BD-Pharmingen) was added at a pretitrated volume. The cells were incubated for 6 h at 37°C in the presence of brefeldin A (Sigma-Aldrich) and monensin (GolgiStop; BD Biosciences). Following incubation, the cells were washed and surface stained with different combinations of MAbs, depending on the panel: (i) anti-CD3-Pacific Blue, anti-CD4-allophycocyanin (APC), and anti-CD8-APC-Cy7 and (ii) anti-CD3-Pacific Blue, anti-CD4-peridinin chlorophyll protein-Cy5.5, and anti-CD8-APC-Cy7. 7-Aminoactinomycin D (7-AAD) (1/10 diluted; Molecular Probes) was added before permeabilization as a dead-cell marker. Samples were fixed (1% paraformaldehyde) and permeabilized (0.5% saponin) for intracellular staining and then stained intracellularly with B-cell lymphoma/leukemia 2 (Bcl-2)-FITC clone Bcl-2/100 and caspase 3-phycoerythrin (PE) clone C92-605 (both from BD-Pharmingen) for 1 h at 4°C (first panel) or with gamma interferon (IFN-γ)-APC clone B27, tumor necrosis factor alpha (TNF-α)-PE-Cy7 clone MAb11, and interleukin 2 (IL-2)-PE clone MQ1-17H12 for 20 min at room temperature (second panel). All MAbs were from Pharmingen/Becton Dickinson, San Diego, CA, unless otherwise specified. After being washed with permeabilizing buffer, the cells were fixed in phosphate-buffered saline containing 1% paraformaldehyde.
For intracellular staining of PBMC, cryopreserved samples of axillary (Ax), mesenteric (Mes), and Gen LN were thawed and rested overnight at 37°C in a 5% CO2 atmosphere in AIM V medium (Gibco, Invitrogen Inc.) containing 20% fetal calf serum. The next day, the cells were adjusted to 1 × 106/200 μl and stimulated and stained in the same manner as for fresh cells, except an anti-HLA-DR-FITC MAb was substituted for the anti-CD107ab-FITC MAb in the second panel.
Data were acquired using a FACSAria flow cytometer (Becton Dickinson) and analyzed using FlowJo software (Treestar, Inc.) and a Macintosh G5 computer (Apple, Inc.). At least 100,000 events in the forward scatter/side scatter lymphocyte gate were acquired for all tissues except the vagina and cervix. As noted in Results, due to the low frequency of T cells in genital tract tissues, all the detectable lymphocyte events in a sample were collected. Importantly, to maintain consistency between the tissue samples analyzed, samples with fewer than 5,000 CD3+ 7-AAD− T cells and/or fewer than 3,000 CD8+ 7-AAD− events were excluded from the analyses. Further, samples that had a large discrepancy between the number of events in the DMSO control and the Gag peptide-stimulated tubes were eliminated. Thus, of the 253 samples available, 231 were analyzed (and 22 were excluded). For the CD8-depleted animals, an exception in the criteria of exclusion was made, as most of the tissues had only 1 to 3,000 CD8+ 7-AAD− cells and samples with fewer than 1,000 CD8+ 7-AAD− cells were excluded from analysis.
The background level of cytokine staining varied from sample to sample but was typically <0.05% of the unstimulated CD8+ T lymphocytes. Samples considered positive were those in which, after the DMSO control was subtracted, there were at least 5 positive events for a single functional marker and 3 positive events for two or more functional markers and the sum of the different combinations of responses represented at least 10 events. In addition, a sample was not considered positive for a particular combination of functions if the frequency of T cells responding with that particular combination of functions was lower than 0.02%. All data were reported after subtraction of the DMSO control cultures. The software program Simplified Presentation of Incredibly Complex Evaluations (SPICE) (a gift from M. Roederer, Vaccine Research Center, NIAID/NIH) was used to create the pie charts that represent each individual response.
Intracellular staining for Ki-67 and caspase 3 in PBMC and LN.
Cell suspensions were labeled with the following surface-staining cocktail: anti-CD3-Pacific Blue, anti-CD4-APC (clone L200), anti-CD8-Cy7-APC, anti-CD16-Cy7-PE (clone 3G8), and anti-CD20-Cy5-PE (clone 2H7). Samples were permeabilized with 0.5% saponin for intracellular staining with Ki67-FITC (Clone B56) and caspase 3-PE incubated for 1 h at 4°C (all directly conjugated MAbs were from Pharmingen/Becton Dickinson). After being washed with the permeabilizing buffer, the cells were fixed with 1% paraformaldehyde. Data were acquired using a FACSAria flow cytometer (Becton Dickinson) and analyzed using FlowJo software (Treestar, Inc.) and Macintosh G5 computers (Apple, Inc.). At least 100,000 small lymphocyte events were collected from each tube analyzed.
MHC tetramer staining.
Fresh mononuclear cells from the vaginal mucosa or cryopreserved PBMC and LN mononuclear cells (1 × 106 cells) were stained with 5 μl (0.1 mg/ml) of a pretitrated stock of the Mamu-A*01 tetramers Gag181-189 CM9 (CTPYDINQM), Tat28-35SL8 (STPESANL), Gag340-349VT10 (VNPTLEEMLT), and Env620-628TL9 (TVPWPNASL) or Mamu-A*02 Nef221-229 YY9 (YTSGPGIRY), Vif97-104WY8 (WTDVTPNY), Gag71-79GY9 (GSENLKSLY), Env788-795RY8 (RTLLSRVY), all conjugated to APC (a generous gift from D. Watkins). After 1 h of incubation at 37°C, the cells were stained for an additional 45 min at room temperature with surface markers, using a cocktail containing pretitrated amounts of the following MAbs (all from BD Biosciences): CD3-FITC (clone SP34), CD4-PE (clone L200), and CD8-peridinin chlorophyll protein (clone SK1). Samples were washed twice with fluorescence-activated cell sorter wash buffer, red blood cells were lysed, and the cells were fixed and stabilized with paraformaldehyde using a Coulter TQ-prep (Coulter Corporation). PBMC from Mamu-A*01+ uninfected macaques were used as negative controls. Sample data were acquired on a FACSCalibur (BD Biosciences) and analyzed using FlowJO software (BD Immunocytometry Systems). Positive tetramer responses were defined as the percentage of CD3+ CD8+ T cells that were three times the mean percentage observed in samples without the tetramer and were a minimum of 10 positive events after background subtraction.
In situ MHC tetramer detection.
In situ tetramer staining in LN, vagina, and cervix was performed essentially as previously described (49, 50).
Statistical analyses.
Data were reported as the mean and the standard error of the mean for each animal group using Prism 4.0 software (GraphPad Software). Statistical analyses were performed by Student's t test or by a one-way analysis of variance (ANOVA) with Tukey's multiple-comparison test if more than two groups were compared. Statistical analysis of the ratio between Bcl-2 and caspase 3 frequencies and the Ki-67 or caspase 3 frequency used the Kruskal-Wallis nonparametric ANOVA, followed by Dunn's multiple-comparison test. The flow cytometric data analysis program SPICE was used to analyze T-cell responses detected by polychromatic flow cytometry. P values of <0.05 were considered significant.
RESULTS
Experimental design.
The results from 26 female rhesus macaques with regular menstrual cycles and immunized by SHIV89.6 infection are reported here. In previous studies, this attenuated, persistent infection protected about 60% of immunized monkeys from uncontrolled, high-level viral replication after vaginal SIVmac239 challenge (2, 34). To determine the role of CD8+ T lymphocytes in SHIV89.6-mediated protection, we compared Gag-specific CD8+ T-cell responses in unimmunized SIV control and SHIV-immunized macaques 7 and 14 days after vaginal SIVmac239 challenge (Fig. 1A). In addition, a group of SHIV89.6-immunized monkeys were depleted of CD8 alpha-chain-positive T cells and natural killer cells on the day of challenge (Fig. 1A). In rhesus monkeys, specific MHC class I alleles are associated with better control of viral replication (39, 56), and Chinese-origin rhesus monkeys are also inherently better able to control SIV replication than Indian-origin monkeys (27, 31). Thus, we evenly distributed animals among the experimental groups based on the country of lineage origin and MHC genotype (Table 1).
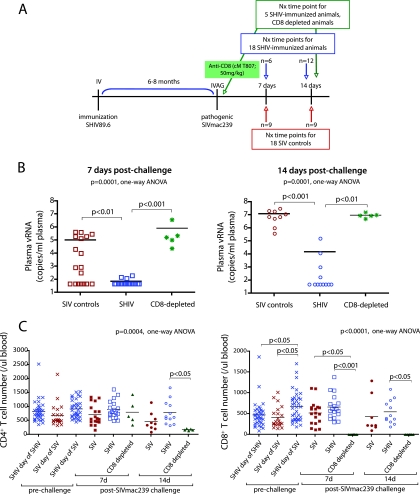
FIG. 1.
Experimental design, SIVmac239 plasma vRNA levels and T-cell counts in SHIV-immunized and unimmunized rhesus macaques. (A) Timing of interventions and necropsy for SHIV89.6-immunized and unimmunized macaques. (B) Plasma vRNA levels at 7 and 14 days after vaginal SIVmac239 challenge. (C) T-cell numbers before and after SIVmac239 challenge. The red symbols represent unimmunized monkeys (SIV controls), the blue symbols represent SHIV89.6-immunized monkeys (SHIV plus SIV) and the green symbols represent SHIV89.6-immunized and CD8+ T-cell-depleted (SHIV plus SIV αCD8) monkeys. Uninfected monkeys are labeled “SHIV day of SHIV” or “SIV day of SIV.” The P values are based on ANOVA analyses and Tukey's multiple-comparison post hoc test.
Outcome of vaginal challenge with SIVmac239.
On day 7 p.c., 11 of 18 unimmunized control monkeys and 3 of 18 immunized monkeys were plasma vRNA positive (Fig. 1B). At 14 days p.c., 9 of 9 controls and 4 of 12 immunized monkeys were plasma vRNA+. At 7 and 14 days p.c., the mean plasma vRNA level of the unimmunized control monkeys was significantly higher than that of the SHIV-immunized monkeys (Fig. 1B and Table 1). Strikingly, CD8+ lymphocyte depletion on the day of vaginal SIVmac239 challenge eliminated the protective effect of the SHIV immunization, and five of five animals were plasma vRNA+ at 7 and 14 days p.c. (Fig. 1B). At 7 and 14 days p.c., CD8-depleted monkeys had the highest plasma vRNA levels of all groups (Fig. 1B and Table 1). Based on the results of quantitative reverse transcriptase PCR for SIV env and HIV env, at 14 days p.c., SIV replication was the source of more than 90% of the vRNA in the plasma of the CD8 lymphocyte-depleted monkeys (unpublished data). Of the four SHIV-immunized animals with detectable vRNA in plasma at 14 days p.c., two monkeys (28843 and 32330) had >104 vRNA copies/ml plasma, a level of viral replication associated with progression toward simian AIDS by 6 months p.c. (2). The other two animals (26960 and 30906) had 102 to 103 vRNA copies/ml plasma, a level that could lead to uncontrolled viral replication or complete containment of the challenge virus (2). Importantly, the frequency of viremic animals within the SHIV-immunized group at 14 days p.c. (4 of 12; 33%) was consistent with data from a previously published SHIV immunization study (2) in which the frequency of SHIV-immunized macaques with detectable vRNA at 14 days p.c. was 5 of 15 animals (33%). Thus, the level of protection conferred by SHIV89.6 immunization against vaginal SIVmac239 challenge in this study is consistent with previous experiments in which 60% of immunized animals were protected through 6 months p.c. (2, 34).
SHIV89.6 immunization increases total CD8+ T-cell counts in blood but blunts proliferative and apoptotic signals in T cells.
SHIV infection resulted in a significant increase in the absolute number of CD8+ T cells in blood compared to preinfection levels (Fig. 1C). After SIV challenge, there were no significant changes in the lymphocyte populations in the unimmunized control animals or the SHIV-immunized animals, although at 14 days p.c. there was a trend toward a decrease in the CD4+ T-cell counts in the unimmunized control group (Fig. 1C). T-cell counts in the anti-CD8 MAb-treated SHIV-immunized monkeys not only confirmed the pharmacologically induced and sustained depletion of CD8+ T cells in the blood at 7 and 14 days p.c., but also revealed massive CD4+ T-cell depletion at 14 days p.c. compared to the T-cell-intact SHIV-immunized macaques (P < 0.05) (Fig. 1C). There were significant correlations between the loss of CD4+ CD95+ T cells from day 7 to 14 p.c. and the plasma viral load at day 14 p.c. for both CD8-depleted immunized and unimmunized animal groups (r [Pearson correlation coefficient] = −0.94, P = 0.018 for the CD8-depleted group and r = −0.69, P = 0.038 for the unimmunized controls). Additionally, in both CD8+ and CD4+ T cells, the magnitude of the T-cell loss from day 0 to 14 days p.c. correlated with plasma vRNA levels at 14 days p.c. in the SIV control group only (r = 0.72 and P = 0.03) for both T-cell subsets.
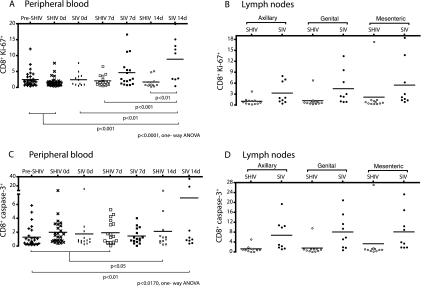
At 7 and 14 days p.c., the frequency of CD8+ T lymphocytes expressing Ki-67, a nuclear protein that is expressed in dividing T cells from S phase to mitosis of the cell cycle and on activated T cells blocked at G0 (13, 42), was much higher in SIV controls than in the immunized macaques (Fig. 2). SHIV-immunized macaques, before and after challenge, maintained a constant frequency of Ki-67+ CD8+ T cells in blood (between 1.7 and 2.5%, which is in the same range found in naïve monkeys). In contrast, SIV controls showed an increase in Ki-67+ CD8+ T cells at 7 days p.c. (4.7%) and at 14 days p.c. (8.9%; P < 0.01 compared to SHIV-immunized macaques) (Fig. 2A). A trend toward an increase in Ki-67+ CD8+ T cells was apparent in LN of the SIV control animals at 14 days p.c. compared to the low and stable frequencies detected in LN of SHIV-immunized macaques (particularly in the Gen LN: 4.5% versus 1.2%) (Fig. 2B). Of note, the immunized macaque with the highest plasma vRNA at 14 days p.c., monkey 28843, had consistently high levels of Ki-67+ CD8+ T cells in LN (Fig. 2B). Thus, the value from this one outlier monkey increased the mean number of Ki-67+ CD8+ T cells in the SHIV-immunized group, which eliminated the statistically significant difference between the groups.
FIG. 2.
Increased activation and cell death in peripheral CD8+ T cells of unimmunized macaques but not in T cells of SHIV-immunized macaques after SIV challenge. Shown are the frequencies on days 0, 7, and 14 p.c. of CD8+ T cells expressing the activation/proliferation marker Ki-67 in PBMC (A) and LN (B) and the frequency of CD8+ T cells in LN expressing the proapoptotic marker caspase 3 (C). “Pre-SHIV” is the time point prior to SHIV infection. The P values are based on ANOVA analyses and Tukey's multiple-comparison post hoc test.
Ki-67+ CD4+ T-cell frequencies in the SIV controls transiently increased to 4.6% at 7 days p.c. and then decreased by 14 days p.c. (data not shown). In contrast, SHIV-immunized animals maintained stable frequencies of ∼2% Ki-67+ CD4+ T cells in blood. No increase was observed in the Ki-67+ CD4+ T-cell frequency in the LN of SHIV-immunized or SIV control macaques (data not shown). Like the unimmunized SIV-challenged controls, CD8+ T-cell-depleted macaques had an increase in Ki-67+ CD4+ T cells at 7 days p.c. in PBMC compared to SHIV-immunized monkeys (4.7%; P = 0.008). Additionally, an increase in Ki-67+ CD4+ T-cell frequency in Mes LN of the CD8+ T-cell-depleted macaques was detected (2.5%), which was significantly higher than SIV controls (0.61%) and SHIV-immunized macaques (0.79%) (P = 0.037 and P = 0.034, respectively) (data not shown).
The frequencies of CD8+ T cells expressing caspase 3, a mediator of apoptotic cell death, in the PBMC of SHIV-immunized macaques were 1 to 2% before and after vaginal SIV challenge, but this was not significantly different from the SIV controls (Fig. 2C). In the Ax, Gen, and Mes LN, there was a trend toward an increase in the frequency of CD8+ caspase 3+ T cells at 14 days p.c. in the SIV controls compared to the SHIV-immunized macaques (6.8% versus 1.3%, 8.1% versus 1.6%, and 8.1% versus 3.3%, respectively) (Fig. 2D). We found consistent low frequencies of <1% caspase 3+ CD4+ T cells in the blood of all the animal groups at all time points, except for the CD8-depleted group, which by 14 days p.c. had an average frequency of 1.4% caspase 3+ CD4+ T cells, and this was significantly higher than the SHIV-immunized macaques at 14 days p.c. (P = 0.041). Also in the CD8-depleted group, frequencies of ∼1% CD4+ caspase 3+ T cells were detected in LN, which were significantly higher than the ∼0.2% caspase 3+ CD4+ T cells in the SHIV-immunized macaques (P values of <0.01 for all LN). In the Mes LN, the caspase 3+ CD4+ T-cell frequency in CD8+ T-cell-depleted animals was also significantly higher than in the SIV controls (1.26% versus 0.37%; P = 0.03). Thus, the increased immune activation present in the SIV control macaques and CD8-depleted immunized macaques was accompanied by increased apoptosis (presumably activation induced cell death), while SHIV-immunized macaques maintained a steady low frequency of proliferating T cells.
SHIV-immunized macaques maintain polyfunctional anti-SIV CD8+ T-cell responses in blood p.c.
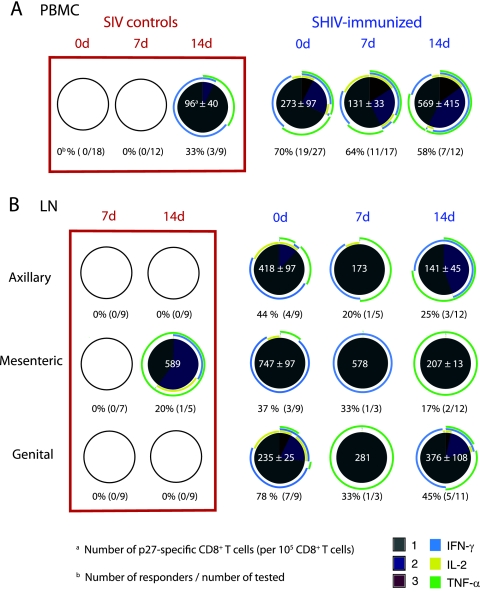
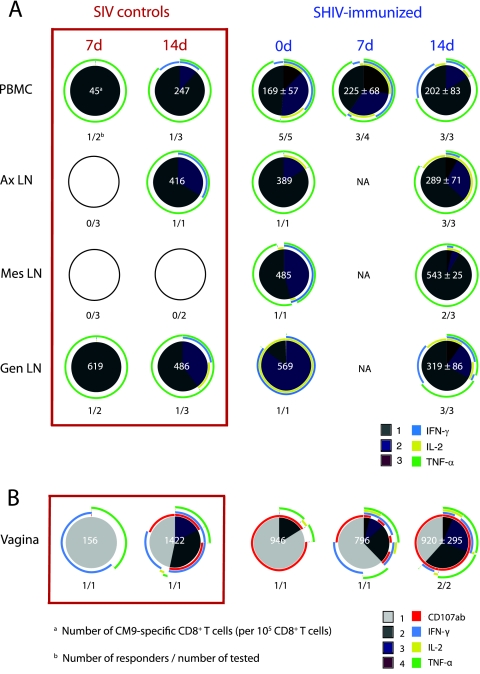
In PBMC, we detected Gag-specific CD8+ T-cell responses in three of the nine SIV control animals at 14 days p.c., but no responses were detected at day 7 days p.c. These responses were characterized by monofunctional T cells secreting IFN-γ or TNF-α (Fig. 3A). In contrast, at days 0, 7, and 14 p.c., 58 to 70% of the SHIV-immunized monkeys had SIV-specific CD8+ T-cell responses in PBMC (Fig. 3A). Among the 19 immunized animals with Gag-specific CD8+ T cells in blood on the day of challenge, half (9 of 19) had T cells that secreted IFN-γ only while the other monkeys had cells that also secreted IL-2 and/or TNF-α (Fig. 3A). After challenge, a similar proportion of SHIV-immunized animals had T-cell responses at 7 days and 14 days p.c. in PBMC (64 and 58%, respectively) (Fig. 3A). The number of SIV-specific CD8+ T cells and the number of functions, or polyfunctionality, exhibited by each SIV-specific T cell were unchanged after challenge (Fig. 3A). However, at 14 days p.c., the CD8+ T-cell responses of SHIV-immunized monkeys were highly variable. The difference in polyfunctionality of the T-cell responses in SIV control monkeys compared to the immunized monkeys was statistically significant at 14 days p.c. only for IL-2 secretion (P = 0.045). In the CD8-depleted group, the SIV-specific CD8+ T-cell response in PBMC could not be detected due to the effective elimination of the circulating CD8+ T cells.
FIG. 3.
Polyfunctional SIV-specific CD8+ T cells in blood and LN of SHIV-immunized macaques, but not of unimmunized macaques, after vaginal challenge with SIVmac239. The frequency and functional capacity of SIV-specific CD8+ T cells after stimulation with a p27-SIV peptide pool as described in Materials and Methods are shown. The samples included PBMC and LN mononuclear cells from unimmunized, SIV control, or SHIV89.6-immunized macaques at days 0, 7, and 14 p.c. Each pie chart represents the average of the positive responses of the animals in a group. The empty circles indicate that there were no positive responses in those samples. Below each pie chart is shown the number of monkeys with a positive response over the number of monkeys tested. For positive responses, the frequency of SIV-specific CD8+ T cells was normalized to 105 CD8+ T cells, and the mean frequency is shown, along with the standard error, in white at the center of the pie chart. Each colored portion of a pie chart indicates the percentage of SIV-specific CD8+ T cells that responded with one, two, or three functions, and the colored arcs around the pie show the function or combination of functions to which the specific response corresponds.
The functional capacity of CD8+ T cells was also assessed by stimulation with the superantigen staphylococcal enterotoxin B (SEB). There were clear differences in the cytokine profiles of SEB-stimulated T-cells from immunized and unimmunized macaques. After SEB stimulation of PBMC collected on day zero, immunized monkeys had more IFN-γ-secreting CD8+ T cells than the unimmunized macaques (n = 26 and n = 15, respectively; P < 0.01) (data not shown). At 7 days p.c., IL-2-secreting CD8+ T cells were significantly more frequent in immunized macaques than in controls (n = 18 and n = 15, respectively; P < 0.001). Additionally, the fraction of 7-day-p.c. PBMC secreting all three cytokines simultaneously in response to SEB was significantly higher in the SHIV-immunized monkeys than in control monkeys (P < 0.0001).
Polyfunctional anti-SIV CD8+ T-cell responses in lymphoid tissues of SHIV-immunized animals after SIV challenge.
Detectable SIV Gag-specific CD8+ T-cell responses in the LN of unimmunized monkeys were rare at 7 and 14 days p.c. However, prior to SIV challenge, live-attenuated SHIV89.6 immunization alone induced anti-SIV CD8+ T-cell responses in the Gen LN of most animals (18). Although genital tract effector T-cell responses were common, less than half of the SHIV-immunized animals had a positive SIV Gag-specific T-cell response in Ax and Mes LN at day zero (18). After challenge, there was minimal expansion of the anti-SIV Gag-specific CD8+ T-cell response in LN of immunized macaques, and most of the specific CD8+ T cells were monofunctional for IFN-γ or TNF-α (Fig. 3B). Among the LN tested, the most consistent T-cell responses were in the Gen LN. At 14 days p.c., in the Gen LN of five of the six SHIV-immunized responders, ∼ 25% of the Gag-specific cells were double positive for IFN-γ and TNF-α, and in two Mamu-A*01-positive animals, 5 to 10% of anti-SIV CD8+ T cells in Gen LN were triple positive (IFN-g+ TNF-a+ IL-2+).
Finally, in the CD8-depleted group, only one of five animals had a Gag-specific CD8+ T-cell response in the Mes LN, while none of the CD8-depleted macaques had a detectable response in the Ax or Gen LN (Fig. 4A), indicating that the depletion successfully removed antiviral T cells from tissues through day 14 p.c.
FIG. 4.
SIV-specific CD8+ T cells in tissues of SHIV-immunized CD8-depleted animals after challenge. The frequency and functional capacity of SIV-specific CD8+ T cells after stimulation with a p27-SIV peptide pool as described in Materials and Methods are shown. The samples included LN mononuclear cells (A) and vagina and cervix (B) from SHIV89.6-immunized macaques depleted of CD8 alpha-chain-positive T cells at 14 days p.c. Each pie chart represents the average of the positive responses of the animals in a group. The empty circles indicate that there were no positive responses in those samples. Below each pie is shown the number of monkeys with a positive response over the number of monkeys tested. For positive responses, the frequency of SIV-specific CD8+ T cells was normalized to 105 CD8+ T cells, and the mean frequency is shown, along with the standard error, in white at the center of the pie chart. Each colored portion of a pie chart indicates the percentage of SIV-specific CD8+ T cells that responded with different numbers of functions (note that the legends are different for LN and genital mucosa), and the colored arcs around the pie show the function or combination of functions to which the specific response corresponds.
Although the CD8-depleted animals were effectively depleted of their circulating CD8+ T cells (<2% of PBMC), the effect of anti-CD8 MAb treatment on resident T-cell populations in LN was variable by the time of necropsy at 14 days p.c. CD8 alpha chain+ lymphocyte depletion was most effective in the Gen LN and Ax LN and less evident in the Mes LN (data not shown). Additionally, CD8 antibody staining of tissue sections from SHIV-immunized CD8-depleted macaques confirmed extensive CD8+ T-cell depletion, as no staining of CD8+ T cells in LN or spleen was detected (data not shown). In the cervix or vagina at day 14 p.c., although most regions had no cells labeled by the anti-CD8 antibody, a few clusters of CD8+ T cells were found in the epithelium or in the submucosa near the epithelium (data not shown). Thus, CD8+ lymphocytes were very efficiently depleted from peripheral blood, but in the LN (especially the Mes LN) and mucosal tissues there were a few remaining CD8+ T cells detectable at 14 days p.c. Given the increased frequency of CD8+ Ki-67+ in LN of CD8+ T-cell-depleted animals compared to control and immunized macaques, the CD8+ T-cells in tissues at day 14 p.c. could represent newly generated CD8+ T cells and not necessarily incomplete CD8+ T-cell depletion.
CD8+ T-cell responses in the genital tract of SHIV-immunized macaques are more polyfunctional and cytotoxic than in control monkeys.
SIV Gag-specific CD4+ and CD8+ T cells are present in the genital tract of rhesus macaques 6 to 8 months after inoculation of SHIV89.6 (18). Here, we assessed Gag-specific CD8+ T-cell responses in the vagina and cervix at 7 and 14 days post-SIVmac239 vaginal challenge, using freshly isolated lymphocytes. We measured CD107a and CD107b mobilization, cell surface markers of degranulation or cytotoxic potential, in addition to the expression of IFN-γ, TNF-α, and IL-2. For 5 × 105 fresh cells, the average number of events in the lymphocyte gate was approximately 80,000 ± 55,000 from the vagina and 45,000 ± 35,000 from the cervix. Among the vaginal cells, ∼30% of the CD3+ 7-AAD− viable T cells were CD4+ and >50% were CD8+. In the cervical cells, approximately 20 to 30% of the CD3+ 7-AAD− T cells were CD4+ and ∼60% were CD8+.
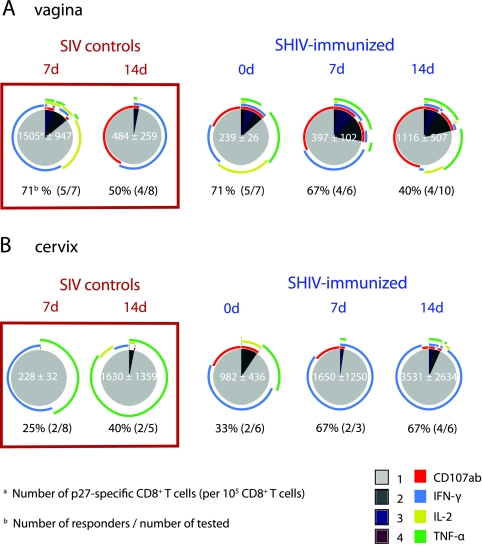
Unimmunized SIV control animals had SIV-specific T-cell responses in the genital tract as early as 7 days p.c. These responses were associated with high levels of viral replication, as four of the five SIV control animals with a T-cell response in the vagina at 7 days p.c. also had detectable vRNA in plasma by 7 days p.c. A similar proportion of unimmunized and SHIV-immunized macaques had SIV Gag-specific CD8+ T-cell responses in the vagina at 7 days (∼70%) and 14 days (∼45%) p.c. (Fig. 5A). Further, at 7 days p.c., the numbers of SIV-specific T cells in the vagina of the control monkeys were higher than in the SHIV-immunized monkeys (Fig. 5A); however, the responses in the control animals declined in strength by day 14 p.c., while the responses significantly increased in SHIV-immunized animals (P = 0.045; one-tailed unpaired t test). After SIV challenge, the proportion of SHIV-immunized macaques with SIV-specific T-cell responses in the cervix increased (Fig. 5B). Thus, an increase in genital tract SIV-specific T cells occurred in SHIV-immunized monkeys after SIV challenge; however, expansion of SIV-specific T cells was not observed in other tissues.
FIG. 5.
Polyfunctional and cytotoxic CD8+ T cells in the genital tracts of SHIV-immunized macaques after challenge. The frequency and functional capacity of SIV Gag-specific CD8+ T cells after stimulation with a p27-SIV peptide pool as described in Materials and Methods are shown. The samples included vagina (A) and cervix (B) of unimmunized SIV control monkeys or of SHIV89.6-immunized macaques at days 0, 7, and 14 p.c. Below each pie chart, the percentage of responders is shown with the fraction of the positive samples in parentheses. The empty circles indicate that there were no positive responses in those samples. See the Fig. 3 legend for an explanation of the pie charts.
Notably, at all time points examined, the polyfunctionality of SIV-specific CD8+ T cells in the cervix and vagina of the SHIV-immunized animals was markedly higher than in control animals (Fig. 5). While all four functions assessed were represented in the SIV-specific CD8+ T cells in the vaginal mucosa of the SHIV-immunized macaques, monofunctional CD107+ SIV-specific CD8+ T cells were very common, particularly after SIV challenge (Fig. 5). In the SIV control monkeys, IFN-γ-secreting cells predominated, and degranulation was not evident in the SIV-specific T-cell population in the vagina until 14 days p.c. In fact, at 7 days p.c., SHIV-immunized macaques had significantly higher numbers of CD107+ cells than SIV control monkeys (P = 0.0084). In the cervix, the SIV-specific CD8+ T-cell responses of immunized macaques consisted of a few degranulating cells among a large population of IFN-γ+ T cells. In contrast, among the SIV-specific CD8+ T cells of the SIV control macaques, TNF-α was the predominant function in the cervix (Fig. 5).
Finally, among the five CD8-depleted animals, only two had a SIV Gag-specific CD8+ T-cell response in the vagina at day 14 p.c., and no SIV-specific CD8+ T-cell responses were detected in the cervix of the CD8-depleted animals (Fig. 4B).
Additionally, at 3 days p.c., three of three SHIV-immunized macaques had SIV-specific CD8+ T-cell responses in the genital tract, but SIV-specific CD8+ T cells were undetectable in the genital tissues of three SIV control monkeys at day 3 p.c. (data not shown). The main function of the T-cell response in the vaginas of the immunized animals at day 3 p.c. was cytotoxicity (CD107+). In the cervixes of the SHIV-immunized macaques at 3 days p.c., IFN-γ was the principal function of SIV-specific CD8+ T cells, but degranulation was also detected, and >25% of SIV-specific CD8+ T cells were both IFN-γ+ and CD107+. Furthermore, SEB stimulation demonstrated very high cytotoxic potential in the T cells in the vaginas of the SHIV-immunized macaques at 3 days p.c. compared to the SIV control macaques (P = 0.003) (data not shown).
CD8+ T-cell responses to immunodominant Gag epitopes are highly polyfunctional in SHIV-immunized, Mamu-A*01+ macaques.
Six months after SHIV89.6 infection, there were strong CM9-specific CD8+ T-cell responses in all tissues of a Mamu-A*01+ SHIV-immunized macaque (18). CM9 (CTPYDINQM; Gag 181 to 189) is the immunodominant Gag epitope restricted by this allele (3). On the day of challenge, five of five SHIV-immunized Mamu-A*01+ macaques had highly polyfunctional T-cell responses to the CM9 peptide in blood (Fig. 6A). With the exception of PBMC samples, the number of functions displayed by the CM9-specific CD8+ T cells of these Mamu-A*01+ SHIV-immunized macaques increased in all tissues after SIV challenge. The increase in the number of functions in the vaginal T cells was particularly striking, as these CM9+ CD8+ T cells expressed more functions than any other SIV Gag-specific response measured in any sample collected in this study (Fig. 6B). However, IL-2 secretion was rare in vaginal antiviral T cells responding to CM9. There was also a trend toward an increase in the frequency of polyfunctional CM9-specific CD8+ T cells in the Gen and Ax LN of immunized macaques after SIV challenge (Fig. 6A). Notably, at 14 days p.c., these LN responses had the highest proportion of IL-2-secreting T cells of any SIV-specific T-cell response measured (Fig. 3 and 6A).
FIG. 6.
Highly polyfunctional responses to an immunodominant Gag epitope. The frequency and functional capacity of SIV Gag CM9+ CD8+ T cells after stimulation with the CM9 SIV Gag peptide as described in Materials and Methods are shown. Samples from Mamu A01+ SIV control monkeys and SHIV89.6-immunized monkeys at days 0, 7, and 14 p.c. in PBMC and LN mononuclear cells (A) and vagina and cervix (B) are included. For an explanation of the pie charts, see the Fig. 4 legend.
By day 14 p.c., Mamu-A*01+ SIV control monkeys also had strong and consistent CM9-specific CD8+ T-cell responses in most tissues (Fig. 6). Thus, a CM9-specific CD8+ T-cell response could be detected in the Ax LN, but not in the Mes LN. The magnitude of the CM9-specific response, the proportion of Mamu-A*01+ responding animals, the extent of anatomic distribution, and the number of functions expressed by responding cells were clearly lower in the SIV control Mamu-A*01+ macaques than in the SHIV-immunized Mamu-A*01+ macaques.
Frequencies of CD8+ T cells binding immunodominant SIV MHC class I tetramers in tissues and blood after SIV challenge.
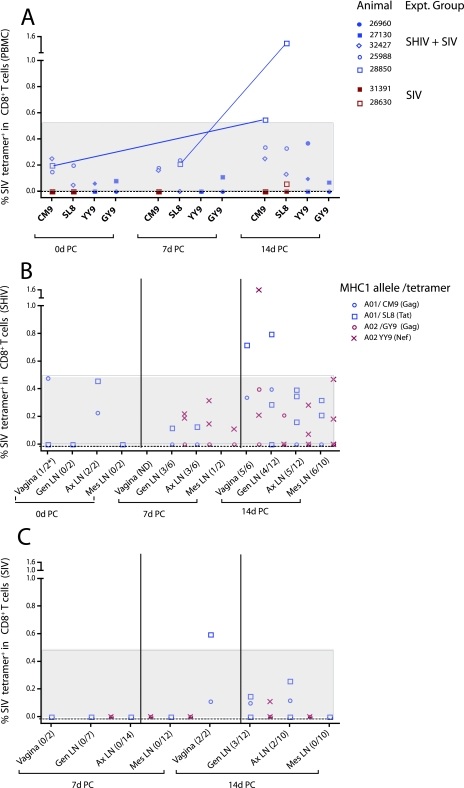
In addition to the Gag CM9 tetramer, we determined the frequency of T cells binding three previously defined Mamu-A*01+-restricted epitopes (Gag VT10, Tat SL8, and Env TL9) (44) and four Mamu-A*02+-restricted epitopes (Gag GY9, Vif WY8, Nef YY9, and Env RY8) (29). Three of four Mamu-A*01+ immunized macaques had 0.15% ± 0.06% CM9+ T cells and 0.15% ± 0.05% SL8 tetramer+ CD8+ T cells in blood on the day of challenge (data not shown), while the one Mamu-A*02+ immunized animal (out of four total) with detectable tetramer-positive cells at day zero had 0.06% YY9+ CD8+ T cells and 0.08% GY9 tetramer+ CD8+ T cells in the PBMC (data not shown). After challenge of SHIV-immunized monkeys, the frequencies of the individual epitope tetramer-binding CD8+ T cells in PBMC were similar for the most common epitopes (CM9, SL8, YY9, and GY9), and these frequencies were all much higher than in control animals (data not shown). Only 1 (28850) of the six SHIV-immunized Mamu-A*01+ or Mamu-A*02+ monkeys had an unequivocal expansion of SIV-specific CD8+ T cells in PBMC after SIV challenge (Fig. 7A). In fact, this animal had increased frequencies of both CM9 and SL8 tetramer-binding CD8+ T cells circulating at day 14 compared to day 0 p.c. Remarkably, at day 14 p.c., the other five SHIV-immunized Mamu-A*01+ or Mamu-A*02+ monkeys had tetramer-specific CD8+ T-cell frequencies within the range found among the SHIV-immunized Mamu-A*01+ or Mamu-A*02+ monkeys on the day of challenge (Fig. 7A).
FIG. 7.
Frequencies of CD8+ T cells binding SIV peptide/MHC class I tetramers in blood and LN of the SHIV-immunized and SIV control macaques before and after SIVmac239 vaginal challenge. (A) Percentages of the most frequent tetramer-binding cells at 0, 7, and 14 days p.c. in blood of Mamu A*01+ and/or Mamu A*02+ SHIV-immunized (n = 6) and unimmunized (n = 2) macaques necropsied at 14 days p.c. The lines connect the data points for the only SHIV-immunized monkey (28850) with an unequivocal expansion of tetramer-binding CD8+ T cells in PBMC after SIV challenge. (B and C) The most frequent epitopes binding Mamu A*01 and Mamu A*02 alelles are shown in the vagina and Gen, Ax, and Mes LN at 0, 7, and 14 days p.c. of the immunized macaques (B) and at 7 and 14 days p.c. of the unimmunized macaques (C). The red symbols represent the YY9 and GY9 SIV epitopes binding Mamu A*02, and the blue symbols represent the CM9 and SL8 SIV epitopes binding Mamu A*01. The shaded areas of the graphs denote the frequency range of tetramer-binding CD8+ T cells in blood or tissues of a single SHIV-immunized monkey on the day of challenge. In panel A, the red symbols represent unimmunized monkeys (SIV controls) and the blue symbols represent SHIV89.6-immunized monkeys. For the tissues, the number of epitopes for which specific tetramer-binding cells were detected divided by the number of epitopes tested at each time point is shown in parentheses below the x axis.
Tetramer-binding CD8+ T cells were frequently detected in the LN of the immunized macaques after SIV challenge, and the most common tetramer-binding cells were SL8+ and YY9+ (Fig. 7B). Note that after SIV challenge, expansion of the SL8 tetramer+ CD8+ T-cell population was observed in the vagina of one Mamu-A*01+ animal (28850) and in the Gen LN of another Mamu-A*01+ monkey (32427) (Fig. 7B). Additionally, YY9 tetramer-binding T cells were remarkably increased in the vagina of a Mamu-A*02+ monkey (26960) that, interestingly, had detectable viremia in plasma (Fig. 7B). In contrast, only two SIV control monkeys at 14 days p.c. had tetramer+ CD8+ T cells in LN tissues: a Mamu-A*01+ monkey that had CM9 and SL8 tetramer+ CD8+ T cells in the vagina, Gen LN, and Ax LN and a Mamu-A*02+ monkey with YY9 tetramer+ CD8+ T cells in Gen LN (Fig. 7C).
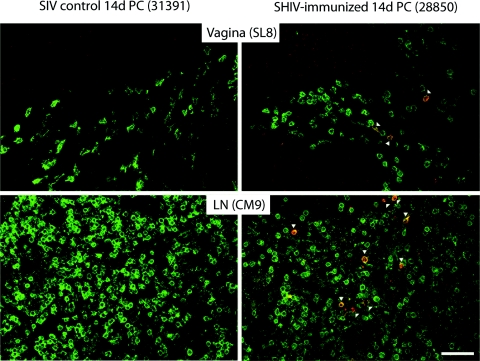
Thus, in agreement with the functional analyses of SIV-specific T-cell responses by flow cytometry, expansion of tetramer-specific CD8+ T cells was limited to the vagina and Gen LN. Furthermore, although five of six animals had detectable tetramer-binding CD8+ T cells in the Ax LN at 14 days p.c., there was no evidence that these populations had recently expanded to a higher frequency than that found prior to challenge (Fig. 7B). These findings were confirmed by in situ tetramer staining, as CM9 tetramer+ CD8+ T cells and SL8 tetramer+ CD8+ T cells were abundantly localized in the LN and genital mucosa of the immunized macaques, but not in the SIV controls (Fig. 8).
FIG. 8.
SL8 and CM9 tetramer+ CD8+ T cells are abundant in the tissues of SHIV-immunized, but not of SIV control, animals. Tetramer+ CD8+ T cells were detected in situ on vagina and LN tissue samples. The representative images are from animals necropsied at 14 days post-SIVmac239 challenge: unimmunized SIV control monkey 31391 and SHIV-immunized monkey 28850. Shown are SL8+ tetramer+ CD8+ T cells in the vagina (upper panels) and CM9+ tetramer+ CD8+ T cells in Ax LN for 28850 and genital LN for 31391 (lower panels). In each image, anti-CD8 staining is green and Mamu A*01/SL8 (upper panels) and Mamu A*01/CM9 (lower panels) tetramer staining is red, indicated by arrowheads. The images are 400× confocal z series projections spanning 10 to 15 μm into the sections. Scale bar, 50 μm.
Attenuated SHIV89.6 infection induces SIV Gag-specific CD8+ T-cell responses with reduced apoptotic susceptibility.
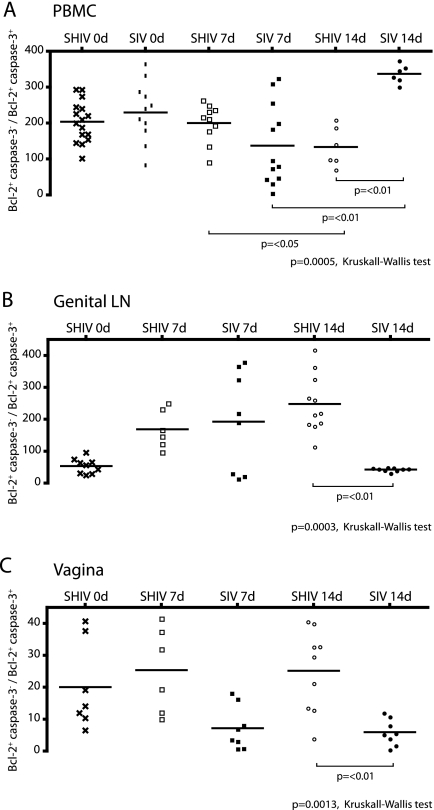
We have previously shown that increased expression of survival signals in SIV-specific, polyfunctional CD8+ T cells is associated with better control of SIVmac239 replication in SHIV-immunized monkeys (17). To determine the balance between proapoptotic and survival signals in the T cells of animals in this study, the frequency of CD8+ T cells expressing the prosurvival molecule, Bcl-2, and the mediator of apoptosis, caspase 3, was analyzed after stimulation with SIV Gag peptide. In PBMC, SHIV-immunized and SIV control macaques had similar ratios of CD8+ T cells expressing survival (Bcl-2) and proapoptotic (caspase 3) molecules on the day of challenge (data not shown). Within the Bcl-2+ fraction of CD8+ T cells, we also determined the ratio of CD8+ T cells that were negative or positive for caspase 3 (Fig. 9). In PBMC, the SIV control animals showed a trend toward a lower Bcl-2+ caspase 3−/Bcl-2+ caspase 3+ CD8+ T-cell ratio at 7 days p.c. compared to the ratio on the day of challenge. However, at 14 days p.c., there was a significant increase in this ratio in PBMC of the SIV controls compared to the ratio at 7 days p.c. (Fig. 9A) (P < 0.01). In PBMC at days 0 and 7 p.c., SHIV-immunized macaques had a Bcl-2+ caspase 3−/Bcl-2+ caspase 3+ CD8+ T-cell ratio similar to the day zero ratio in the SIV control monkeys. However, at 14 days p.c., this ratio decreased significantly in the SHIV-immunized monkeys compared to 7 days p.c. and to SIV controls at 14 days p.c. (P < 0.05 and P < 0.01, respectively) (Fig. 9A).
FIG. 9.
Increased survival potential of SIV Gag-stimulated CD8+ T cells in immunized macaques. The ratios of CD8+ T cells expressing only survival signals (Bcl-2+ caspase 3−) to cells with proapoptotic signals (Bcl-2+ caspase 3+) are shown in the immunized and unimmunized macaques on the day of challenge and 7 and 14 days post-SIVmac challenge in peripheral blood (A), Gen LN (B), and vagina (C). PBMC or LN mononuclear cells were stimulated for 6 h with a Gag p27 peptide pool. The P values were determined by Kruskal-Wallis and Dunn's multiple-comparison post hoc tests.
In the Gen LN, the ratio of Bcl-2+ caspase 3− to Bcl-2+ caspase 3+ CD8+ T cells was significantly lower in the SIV control animals than in SHIV-immunized animals at 14 days p.c. (P < 0.01) (Fig. 9B). Moreover, at 7 and 14 days p.c., SIV controls had significantly lower ratios than the SHIV-immunized monkeys in CD8+ T cells from the vagina (Fig. 9C) and cervix (data not shown). Thus, during the acute p.c. period, the balance of survival to proapoptotic signaling in CD8+ T cells in the genital tract was significantly biased toward survival in the SHIV-immunized monkeys, but this antiapoptotic bias was not seen in the blood of the immunized monkeys.
DISCUSSION
Understanding the mechanisms that provide viral control and containment in a rigorous animal model of heterosexual HIV transmission would help to identify the immune responses that HIV candidate vaccines should emulate. Here, we show that in rhesus macaques immunized with an attenuated lentivirus, the protection from vaginal SIV challenge is primarily mediated by CD8+ T cells. The SIV-specific CD8+ T-cell response in mucosal tissues, at the time of and after SIV challenge, consisted largely of effector T cells with cytolytic potential. Strikingly, there was little evidence for widespread expansion or proliferation of SIV-specific T cells in the blood or tissues of immunized animals after vaginal SIV challenge. In fact, of the six tissues examined, significant expansion of SIV-specific T cells occurred only in the vagina. Thus, the presence of SIV-specific CD8+ T cells in the vagina on the day of vaginal SIV challenge and a modest expansion of effector T cells were sufficient to stop viral dissemination and uncontrolled SIV replication p.c.
We recently reported that immunization with SHIV89.6 induced and maintained SIV-specific CD4+ and CD8+ T cells in the genital mucosa of female rhesus macaques (18) and that the frequency of immunized monkeys with these responses was correlated with the consistent protection of 60% of immunized monkeys observed in this model (2, 34). Here, we found that immunized animals maintained anti-SIV CD8+ T-cell responses after pathogenic SIV challenge, and the most consistent responses were found in the Gen LN and vagina. In the SIV controls, detection of early responses was restricted to the genital compartment, yet the number of functions, cytotoxicity, and survival potential of the antiviral CD8+ T cells were clearly restricted in these unimmunized animals. Additionally, after SHIV89.6 infection, we found a significant increase in the absolute number of CD8+ T cells in blood compared to preinfection levels, suggesting that immunization with SHIV89.6 induced a new pool of circulating T cells that was constantly renewed by sustained, low levels of attenuated viral replication. Confirmation of a critical role for CD8+ T cells in SHIV-induced protection comes from the fact that immunized monkeys depleted of CD8α+ lymphocytes on the day of challenge were unable to control SIV replication after challenge. Critically, since natural killer cells also express CD8α, we cannot rule out the possibility that depletion of these innate immune effector cells might contribute to uncontrolled viral replication. However, a recent study showed that depletion of CD16+ lymphocytes, which contain CD8+ and CD8− natural killer cells, has no effect on SIV replication during acute infection (12). Preliminary analyses of the frequency and the phenotype of natural killer cells in the present study did not suggest their participation in SHIV-mediated protection (data not shown); however, the role of natural killer cells controlling SIV/HIV replication in acute infection is still unclear (7) and should be assessed further.
Importantly, the presence and strength of a CD8+ T-cell response in the different tissues did not correlate with control of viral replication. However, the quality of the CD8+ T-cell response, including the cytokine profile and survival capacity of CD8+ T cells, appears to be an important component of an effective immune response against SIV. Significantly, protection was associated with the presence of CD8+ T cells with lytic function (CD107+) in the genital tract. Although SIV-specific CD8+ T cells were detected in the genital tracts of both groups at day 14 p.c., polyfunctional and degranulating CD8+ T cells were limited to the immunized monkeys. In unimmunized SIV controls, there was significantly less degranulation capacity in the vaginal CD8+ T cells than in the immunized macaques, particularly at early time points. These findings suggest that SHIV89.6-induced SIV Gag-specific CD8+ T cells with lytic function at the portal of entry are critical to SHIV89.6-mediated protection from vaginal SIV challenge. Although cell surface markers that distinguish memory CD8+ T-cell subsets were not measured here, the existence of IL-2-secreting Gag-specific CD8+ T cells in LN suggests that there are central-memory CD8+ T cells in the LN of SHIV-immunized monkeys and that these cells are capable of continuously providing effector cytotoxic T lymphocytes to the genital mucosa. Importantly, in SHIV-immunized, Mamu-A*01+ macaques, CM9+ CD8+ T cells from the vaginal mucosa were CD107+ before (18) and after SIV challenge. This cytolytic effector T-cell response in the mucosa was accompanied by polyfunctional IL-2-secreting CM9+ CD8+ T cells in LN, suggesting that a balance exists between a memory CD8+ T-cell pool in LN and effector-cytolytic CD8+ T-cell populations in the vagina. In contrast, in unimmunized Mamu-A*01+ monkeys, there was a delay in the detection of degranulating CD8+ T cells in the vagina, in agreement with a previous report (45). The presence of CM9+ CD8+ T cells in tissues was confirmed by in situ tetramer staining. Of note, the frequency of SL8 tetramer+ CD8+ T cells in tissues of SHIV89.6-immunized macaques was much higher than in the SIV control monkeys. CD8+ T cells that recognize the SL8 epitope in the regulatory protein Tat have been reported to be more effective than CD8+ T cells recognizing CM9 at suppressing SIV replication in vitro and in vivo during the acute phase of infection (28). Thus, at the time of challenge, the SHIV-immunized macaques had highly functional and lytic T-cell responses to immunodominant epitopes, and these responses became more polyfunctional after challenge. In contrast, even at 14 days p.c., the Mamu-A*01+ SIV control animals had T-cell responses that were relatively monofunctional with little evidence of cytolytic function.
Contrary to what is observed in most conventional HIV vaccine strategies, we did not detect a strong anamnestic CD8+ T-cell response in the immunized macaques after SIVmac239 challenge. Thus, the frequencies of specific CD8+ T cells in most tissues were similar at 0, 7, and 14 days p.c. in the SHIV-immunized macaques. In peripheral blood, the only tissue that allowed a longitudinal sampling of the CD8+ T-cell response after SIV challenge, there was a decrease in the number of Gag-specific CD8+ T cells at 7 days p.c. in the animals that had a positive response at day zero. Additionally, the SIV-specific CD8+ T-cell responses detected in blood in three of the SIV controls at 14 days p.c. were of the same order of magnitude as the responses in the SHIV-immunized responders. Only in the vagina was there a significant increase in the number of SIV-Gag-specific CD8+ T cells from days 0 to 14 p.c. in the SHIV-immunized animals. The expansion of SIV-specific T-cell responses in the vagina occurred without bystander T-cell proliferation in the LN. Accordingly, SHIV-infected animals had constant levels of Ki-67 expression throughout the p.c. period, while expression of this marker increased in the CD8+ T cells of SIV controls consistent with expansion or recruitment of specific CD8+ T cells to the initial site of viral dissemination after vaginal SIV inoculation (33). The lack of T-cell proliferation in the immunized monkeys supports the conclusion that an anamnestic T-cell response after vaginal SIV challenge was restricted in these monkeys.
Importantly, immune activation enhances SIV replication in mucosal sites. Topical application of the toll-like receptor 7/8 agonist imiquimod to the vagina produces immune activation in the vaginal mucosa, and this enhances vaginal SIV transmission/replication (55). Administration of the proinflammatory cytokine IL-15 in acute SIV infection increases plasma vRNA levels at set point, despite the fact that the treatment induces stronger SIV-specific T-cell responses (37). The most direct evidence that immune activation drives viral replication comes from recently completed studies in SIV-infected rhesus macaques using a cytotoxic-T-lymphocyte antigen 4 (CTLA-4) antagonist (10). CTLA-4 is a negative regulatory molecule expressed on activated T cells and a subset of regulatory T cells, and administration of a blocking anti-CTLA-4 MAb in acute SIV infection leads to T-cell activation and enhanced viral replication (10). Finally, it has been reported that CD8+ lymphocyte depletion of SIV-infected rhesus macaques induces nonspecific activation of the CD4+ T cells and transient increases in viral target cells (52; L. J. Picker, personal communication). Systemic immune activation may contribute to SIV replication in CD8 lymphocyte-depleted monkeys; however, it seems unlikely that immune activation alone can account for the almost 8-log10-unit difference in plasma vRNA levels in the CD8-depleted and CD8-intact immunized macaques.
Traditionally, vaccines have been used to antigenically prime the immune system so that during a subsequent encounter, a rapid anamnestic immune response eliminates the pathogen. Prime-boost SIV vaccine regimens with plasmid DNA and viral vectors induce robust anamnestic T-cell responses following pathogenic SIVmac challenge, including cytotoxic T lymphocytes, although control of viral replication is inconsistent (15, 22, 32, 53). However, monkeys vaccinated with live, attenuated SIV do not produce a strong anamnestic T-cell response following pathogenic-virus challenge (1, 25, 40, 48). There are three nonexclusive mechanisms that could account for the difference in the vaccine-induced T-cell responses in monkeys vaccinated with live, attenuated SIV or SHIV and those vaccinated with prime-boost strategies. (i) The “danger signal” threshold for immune activation is raised by chronic SHIV89.6 infection, and lack of T-cell activation is part of broad immune modulation. (ii) Rapid control of challenge virus replication by CD8+ effector T cells in the genital tract may limit the antigenic stimulation of memory T cells in the LN below a threshold required to drive T-cell proliferation. (iii) The SIV-specific CD8+ T-cell pool maintained by low-level SHIV89.6 replication has more effector than proliferative potential, as shown by Genesca et al. (18), and thus cannot respond with a proliferative burst upon SIV challenge. The concept that a persistent, attenuated viral vaccine that maintains a pool of CD8+ effector T cells capable of controlling but not eliminating the pathogen is necessary for efficacy against persistent pathogens, like HIV or SIV, was proposed by Zinkernagel (57). Primary antiviral CD8+ T cells undergo a poorly understood process of proliferation and differentiation en route to becoming short-lived terminal effector cells or long-lived memory T cells. The level and duration of antigenic stimulation and the inflammatory milieu are critical factors, in addition to CD4+ T-cell helper activity, in dermining the nature of the CD8+ T-cell response. Recently, it was observed that prolonged inflammation or stimulation by the T-cell transcription factor T-bet results in a predominantly short-lived effector CD8+ T-cell population rather than a long-lived memory population with proliferative potential (19, 21, 24). Thus, the CD8+ T-cell response that is observed in SHIV89.6-vaccinated monkeys that are protected from pathogenic SIVmac239 challenge is consistent with a continuously antigen-driven effector T-cell population rather than a strong memory T-cell pool associated with anamnestic proliferation. The presence of central-memory T cells in the draining LN and a balance between these cells and effector CD8+ T cells in the mucosa may be required to maintain an effective, yet homeostatic, anti-SIV T-cell response.
Two SHIV-immunized monkeys had viremia on days 7 and 14 post-SIV challenge, compatible with failure to control pathogenic SIV replication. Compared to the other SHIV-immunized animals in this study, these two monkeys had no detectable SIV-specific response in vaginal CD8+ T cells, lack of IL-2 secretion at 7 days p.c. by CD8+ T cells in blood, and a lower ratio of Bcl-2+ caspase 3−/Bcl-2+ caspase 3+ CD8+ T cells. Thus, unprotected SHIV-immunized macaques might have an impaired capacity to maintain an effective CD8+ T-cell response, leading to antigen-driven exhaustion of the specific immune response. Antigen-driven defects in survival and maturation of SIV-specific CD8+ T cells have been shown to appear during the first week of infection and are comparable to similar defects in HIV-specific CD8+ T cells (38).
In summary, this study demonstrates that CD8+ T cells are necessary mediators of the protection induced by previous SHIV89.6 immunization in rhesus macaques challenged intravaginally with SIVmac239 (2, 18, 34). CD8α lymphocyte depletion on the day of challenge in SHIV-immunized macaques consistently resulted in the highest levels of viral replication, detected as early as 7 days p.c. This indicates that, in the absence of antiviral CD8+ effector T cells, the presence of vaccine-induced, SIV-specific CD4+ T cells in the genital tract (18) might be counterproductive, providing more viral substrate and increasing burst size. Effective anti-SIV CD8+ T-cell responses were characterized by cells with multiple functions, but especially by the presence of T cells in the vagina that had cytolytic potential. Further, the CD8+ T cells in an effective response have increased survival capacity and do not dramatically proliferate upon antigenic stimulation. Thus, T-cell-based vaccine strategies that can elicit mucosal effector CD8+ T-cell responses with minimal T-cell proliferation upon exposure to HIV have the greatest potential for mimicking the success of live, attenuated lentiviral vaccines.
Acknowledgments
This work was supported by Public Health Service grants U51RR00169 from the National Center for Research Resources and P01 AI066314 and R01 AI44480 from the National Institute of Allergy and Infectious Diseases and by a gift from the James B. Pendleton Charitable Trust.
We thank M. Busch, A. Haase, N. Miller, and K. Reimann for helpful discussions and the Primate Services Unit at the CNPRC, Lili Guo, and Roxana Colon for excellent technical assistance. We also thank Mario Roederer, National Institutes of Health, for providing the SPICE and PESTLE software and David Watkins, University of Wisconsin, for providing MHC-peptide tetramers.
Footnotes
Published ahead of print on 10 September 2008.
REFERENCES
- 1.Abdel-Motal, U. M., J. Gillis, K. Manson, M. Wyand, D. Montefiori, K. Stefano-Cole, R. C. Montelaro, J. D. Altman, and R. P. Johnson. 2005. Kinetics of expansion of SIV Gag-specific CD8+ T lymphocytes following challenge of vaccinated macaques. Virology 333226-238. [DOI] [PubMed] [Google Scholar]
- 2.Abel, K., L. Compton, T. Rourke, D. Montefiori, D. Lu, K. Rothaeusler, L. Fritts, K. Bost, and C. J. Miller. 2003. Simian-human immunodeficiency virus SHIV89.6-induced protection against intravaginal challenge with pathogenic SIVmac239 is independent of the route of immunization and is associated with a combination of cytotoxic T-lymphocyte and alpha interferon responses. J. Virol. 773099-3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen, T. M., J. Sidney, M. F. del Guercio, R. L. Glickman, G. L. Lensmeyer, D. A. Wiebe, R. DeMars, C. D. Pauza, R. P. Johnson, A. Sette, and D. I. Watkins. 1998. Characterization of the peptide binding motif of a rhesus MHC class I molecule (Mamu-A*01) that binds an immunodominant CTL epitope from simian immunodeficiency virus. J. Immunol. 1606062-6071. [PubMed] [Google Scholar]
- 4.Almeida, J. R., D. A. Price, L. Papagno, Z. A. Arkoub, D. Sauce, E. Bornstein, T. E. Asher, A. Samri, A. Schnuriger, I. Theodorou, D. Costagliola, C. Rouzioux, H. Agut, A. G. Marcelin, D. Douek, B. Autran, and V. Appay. 2007. Superior control of HIV-1 replication by CD8+ T cells is reflected by their avidity, polyfunctionality, and clonal turnover. J. Exp. Med. 2042473-2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Appay, V., D. F. Nixon, S. M. Donahoe, G. M. Gillespie, T. Dong, A. King, G. S. Ogg, H. M. Spiegel, C. Conlon, C. A. Spina, D. V. Havlir, D. D. Richman, A. Waters, P. Easterbrook, A. J. McMichael, and S. L. Rowland-Jones. 2000. HIV-specific CD8+ T cells produce antiviral cytokines but are impaired in cytolytic function. J. Exp. Med. 19263-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Betts, M. R., M. C. Nason, S. M. West, S. C. De Rosa, S. A. Migueles, J. Abraham, M. M. Lederman, J. M. Benito, P. A. Goepfert, M. Connors, M. Roederer, and R. A. Koup. 2006. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 1074781-4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bimber, B., and D. H. O'Connor. 2008. KIRigami: the case for studying NK cell receptors in SIV+ macaques. Immunol. Res. 40235-243. [DOI] [PubMed] [Google Scholar]
- 8.Borrow, P., H. Lewicki, B. H. Hahn, G. M. Shaw, and M. B. Oldstone. 1994. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 686103-6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyer, J. D., S. Kumar, T. Robinson, R. Parkinson, L. Wu, M. Lewis, and D. B. Weiner. 2006. Initiation of antiretroviral therapy during chronic SIV infection leads to rapid reduction in viral loads and the level of T-cell immune response. J. Med. Primatol. 35202-209. [DOI] [PubMed] [Google Scholar]
- 10.Cecchinato, V., E. Tryniszewska, Z. M. Ma, M. Vaccari, A. Boasso, W. P. Tsai, C. Petrovas, D. Fuchs, J. M. Heraud, D. Venzon, G. M. Shearer, R. A. Koup, I. Lowy, C. J. Miller, and G. Franchini. 2008. Immune activation driven by CTLA-4 blockade augments viral replication at mucosal sites in simian immunodeficiency virus infection. J. Immunol. 1805439-5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheroutre, H., and L. Madakamutil. 2005. Mucosal effector memory T cells: the other side of the coin. Cell Mol. Life Sci. 622853-2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi, E. I., K. A. Reimann, and N. L. Letvin. 2008. In vivo natural killer cell depletion during primary simian immunodeficiency virus infection in rhesus monkeys. J. Virol. 826758-6761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Combadere, B., C. Blanc, T. Li, G. Carcelain, C. Delaugerre, V. Calvez, R. Tubiana, P. Debre, C. Katlama, and B. Autran. 2000. CD4+Ki67+ lymphocytes in HIV-infected patients are effector T cells accumulated in the G1 phase of the cell cycle. Eur. J. Immunol. 303598-3603. [DOI] [PubMed] [Google Scholar]
- 14.Dailey, P. J., M. Zamroud, R. Kelso, J. Kolberg, and M. Urdea. 1995. Quantitation of simian immunodeficiency virus (SIV) RNA in plasma of acute and chronically infected rhesus macaques using a branched DNA (bDNA) signal amplification assay. J. Med. Primatol. 24209. [Google Scholar]
- 15.Egan, M. A., W. A. Charini, M. J. Kuroda, J. E. Schmitz, P. Racz, K. Tenner-Racz, K. Manson, M. Wyand, M. A. Lifton, C. E. Nickerson, T. Fu, J. W. Shiver, and N. L. Letvin. 2000. Simian immunodeficiency virus (SIV) gag DNA-vaccinated rhesus monkeys develop secondary cytotoxic T-lymphocyte responses and control viral replication after pathogenic SIV infection. J. Virol. 747485-7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egan, M. A., M. J. Kuroda, G. Voss, J. E. Schmitz, W. A. Charini, C. I. Lord, M. A. Forman, and N. L. Letvin. 1999. Use of major histocompatibility complex class I/peptide/β2M tetramers to quantitate CD8+ cytotoxic T lymphocytes specific for dominant and nondominant viral epitopes in simian-human immunodeficiency virus-infected rhesus monkeys. J. Virol. 735466-5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Genesca, M., T. Rourke, J. Li, K. Bost, B. Chohan, M. B. McChesney, and C. J. Miller. 2007. Live attenuated lentivirus infection elicits polyfunctional simian immunodeficiency virus Gag-specific CD8+ T cells with reduced apoptotic susceptibility in rhesus macaques that control virus replication after challenge with pathogenic SIVmac239. J. Immunol. 1794732-4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Genesca, M., P. J. Skinner, K. Bost, D. Lu, Y. Wang, T. L. Rourke, A. T. Haase, M. B. McChesney, and C. J. Miller. 2008. Protective attenuated lentivirus immunization induces SIV-specific T cells in the genital tract of rhesus monkeys. Mucosal Immunol. 1219-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harty, J. T., and V. P. Badovinac. 2008. Shaping and reshaping CD8+ T-cell memory. Nat. Rev. Immunol. 8107-119. [DOI] [PubMed] [Google Scholar]
- 20.Haynes, B. F., and R. J. Shattock. 2008. Critical issues in mucosal immunity for HIV-1 vaccine development. J. Allergy Clin. Immunol. 1223-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hikono, H., J. E. Kohlmeier, S. Takamura, S. T. Wittmer, A. D. Roberts, and D. L. Woodland. 2007. Activation phenotype, rather than central- or effector-memory phenotype, predicts the recall efficacy of memory CD8+ T cells. J. Exp. Med. 2041625-1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horton, H., T. U. Vogel, D. K. Carter, K. Vielhuber, D. H. Fuller, T. Shipley, J. T. Fuller, K. J. Kunstman, G. Sutter, D. C. Montefiori, V. Erfle, R. C. Desrosiers, N. Wilson, L. J. Picker, S. M. Wolinsky, C. Wang, D. B. Allison, and D. I. Watkins. 2002. Immunization of rhesus macaques with a DNA prime/modified vaccinia virus Ankara boost regimen induces broad simian immunodeficiency virus (SIV)-specific T-cell responses and reduces initial viral replication but does not prevent disease progression following challenge with pathogenic SIVmac239. J. Virol. 767187-7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin, X., D. E. Bauer, S. E. Tuttleton, S. Lewin, A. Gettie, J. Blanchard, C. E. Irwin, J. T. Safrit, J. Mittler, L. Weinberger, L. G. Kostrikis, L. Zhang, A. S. Perelson, and D. D. Ho. 1999. Dramatic rise in plasma viremia after CD8+ T cell depletion in simian immunodeficiency virus-infected macaques. J. Exp. Med. 189991-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joshi, N. S., W. Cui, A. Chandele, H. K. Lee, D. R. Urso, J. Hagman, L. Gapin, and S. M. Kaech. 2007. Inflammation directs memory precursor and short-lived effector CD8+ T cell fates via the graded expression of T-bet transcription factor. Immunity 27281-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khatissian, E., V. Monceaux, M. C. Cumont, M. P. Kieny, A. M. Aubertin, and B. Hurtrel. 2001. Persistence of pathogenic challenge virus in macaques protected by simian immunodeficiency virus SIVmacDeltanef. J. Virol. 751507-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 684650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ling, B., R. S. Veazey, A. Luckay, C. Penedo, K. Xu, J. D. Lifson, and P. A. Marx. 2002. SIVmac pathogenesis in rhesus macaques of Chinese and Indian origin compared with primary HIV infections in humans. AIDS 161489-1496. [DOI] [PubMed] [Google Scholar]
- 28.Loffredo, J. T., E. G. Rakasz, J. P. Giraldo, S. P. Spencer, K. K. Grafton, S. R. Martin, G. Napoe, L. J. Yant, N. A. Wilson, and D. I. Watkins. 2005. Tat28-35SL8-specific CD8+ T lymphocytes are more effective than Gag181-189 CM9-specific CD8+ T lymphocytes at suppressing simian immunodeficiency virus replication in a functional in vitro assay. J. Virol. 7914986-14991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loffredo, J. T., J. Sidney, C. Wojewoda, E. Dodds, M. R. Reynolds, G. Napoe, B. R. Mothe, D. H. O'Connor, N. A. Wilson, D. I. Watkins, and A. Sette. 2004. Identification of seventeen new simian immunodeficiency virus-derived CD8+ T cell epitopes restricted by the high frequency molecule, Mamu-A*02, and potential escape from CTL recognition. J. Immunol. 1735064-5076. [DOI] [PubMed] [Google Scholar]
- 30.Lu, F. X., K. Abel, Z. Ma, T. Rourke, D. Lu, J. Torten, M. McChesney, and C. J. Miller. 2002. The strength of B cell immunity in female rhesus macaques is controlled by CD8+ T cells under the influence of ovarian steroid hormones. Clin. Exp. Immunol. 12810-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marthas, M. L., D. Lu, M. C. Penedo, A. G. Hendrickx, and C. J. Miller. 2001. Titration of an SIVmac251 stock by vaginal inoculation of Indian and Chinese origin rhesus macaques: transmission efficiency, viral loads, and antibody responses. AIDS Res. Hum. Retrovir. 171455-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mattapallil, J. J., D. C. Douek, A. Buckler-White, D. Montefiori, N. L. Letvin, G. J. Nabel, and M. Roederer. 2006. Vaccination preserves CD4 memory T cells during acute simian immunodeficiency virus challenge. J. Exp. Med. 2031533-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller, C. J., Q. Li, K. Abel, E. Y. Kim, Z. M. Ma, S. Wietgrefe, L. La Franco-Scheuch, L. Compton, L. Duan, M. D. Shore, M. Zupancic, M. Busch, J. Carlis, S. Wolinsky, and A. T. Haase. 2005. Propagation and dissemination of infection after vaginal transmission of simian immunodeficiency virus. J. Virol. 799217-9227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller, C. J., M. B. McChesney, X. Lu, P. J. Dailey, C. Chutkowski, D. Lu, P. Brosio, B. Roberts, and Y. Lu. 1997. Rhesus macaques previously infected with simian/human immunodeficiency virus are protected from vaginal challenge with pathogenic SIVmac239. J. Virol. 711911-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller, C. J., and R. J. Shattock. 2003. Target cells in vaginal HIV transmission. Microbes Infect. 559-67. [DOI] [PubMed] [Google Scholar]
- 36.Moore, J. P., P. J. Klasse, M. J. Dolan, and S. K. Ahuja. 2008. AIDS/HIV. A STEP into darkness or light? Science 320753-755. [DOI] [PubMed] [Google Scholar]
- 37.Mueller, Y. M., D. H. Do, S. R. Altork, C. M. Artlett, E. J. Gracely, C. D. Katsetos, A. Legido, F. Villinger, J. D. Altman, C. R. Brown, M. G. Lewis, and P. D. Katsikis. 2008. IL-15 treatment during acute simian immunodeficiency virus (SIV) infection increases viral set point and accelerates disease progression despite the induction of stronger SIV-specific CD8+ T cell responses. J. Immunol. 180350-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mueller, Y. M., C. Petrovas, D. H. Do, S. R. Altork, T. Fischer-Smith, J. Rappaport, J. D. Altman, M. G. Lewis, and P. D. Katsikis. 2007. Early establishment and antigen dependence of simian immunodeficiency virus-specific CD8+ T-cell defects. J. Virol. 8110861-10868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muhl, T., M. Krawczak, P. Ten Haaft, G. Hunsmann, and U. Sauermann. 2002. MHC class I alleles influence set-point viral load and survival time in simian immunodeficiency virus-infected rhesus monkeys. J. Immunol. 1693438-3446. [DOI] [PubMed] [Google Scholar]
- 40.Nixon, D. F., S. M. Donahoe, W. M. Kakimoto, R. V. Samuel, K. J. Metzner, A. Gettie, T. Hanke, P. A. Marx, and R. I. Connor. 2000. Simian immunodeficiency virus-specific cytotoxic T lymphocytes and protection against challenge in rhesus macaques immunized with a live attenuated simian immunodeficiency virus vaccine. Virology 266203-210. [DOI] [PubMed] [Google Scholar]
- 41.Pantaleo, G. 2008. HIV-1 T-cell vaccines: evaluating the next step. Lancet Infect. Dis. 882-83. [DOI] [PubMed] [Google Scholar]
- 42.Picker, L. J., S. I. Hagen, R. Lum, E. F. Reed-Inderbitzin, L. M. Daly, A. W. Sylwester, J. M. Walker, D. C. Siess, M. Piatak, Jr., C. Wang, D. B. Allison, V. C. Maino, J. D. Lifson, T. Kodama, and M. K. Axthelm. 2004. Insufficient production and tissue delivery of CD4+ memory T cells in rapidly progressive simian immunodeficiency virus infection. J. Exp. Med. 2001299-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Priddy, F. H., D. Brown, J. Kublin, K. Monahan, D. P. Wright, J. Lalezari, S. Santiago, M. Marmor, M. Lally, R. M. Novak, S. J. Brown, P. Kulkarni, S. A. Dubey, L. S. Kierstead, D. R. Casimiro, R. Mogg, M. J. DiNubile, J. W. Shiver, R. Y. Leavitt, M. N. Robertson, D. V. Mehrotra, and E. Quirk. 2008. Safety and immunogenicity of a replication-incompetent adenovirus type 5 HIV-1 clade B gag/pol/nef vaccine in healthy adults. Clin. Infect. Dis. 461769-1781. [DOI] [PubMed] [Google Scholar]
- 44.Reynolds, M. R., E. Rakasz, P. J. Skinner, C. White, K. Abel, Z. M. Ma, L. Compton, G. Napoe, N. Wilson, C. J. Miller, A. Haase, and D. I. Watkins. 2005. CD8+ T-lymphocyte response to major immunodominant epitopes after vaginal exposure to simian immunodeficiency virus: too late and too little. J. Virol. 799228-9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rollman, E., M. Z. Smith, A. G. Brooks, D. F. Purcell, B. Zuber, I. A. Ramshaw, and S. J. Kent. 2007. Killing kinetics of simian immunodeficiency virus-specific CD8+ T cells: implications for HIV vaccine strategies. J. Immunol. 1794571-4579. [DOI] [PubMed] [Google Scholar]
- 46.Schmitz, J. E., R. P. Johnson, H. M. McClure, K. H. Manson, M. S. Wyand, M. J. Kuroda, M. A. Lifton, R. S. Khunkhun, K. J. McEvers, J. Gillis, M. Piatak, J. D. Lifson, G. Grosschupff, P. Racz, K. Tenner-Racz, E. P. Rieber, K. Kuus-Reichel, R. S. Gelman, N. L. Letvin, D. C. Montefiori, R. M. Ruprecht, R. C. Desrosiers, and K. A. Reimann. 2005. Effect of CD8+ lymphocyte depletion on virus containment after simian immunodeficiency virus SIVmac251 challenge of live attenuated SIVmac239δ3-vaccinated rhesus macaques. J. Virol. 798131-8141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283857-860. [DOI] [PubMed] [Google Scholar]
- 48.Sharpe, S. A., A. Cope, S. Dowall, N. Berry, C. Ham, J. L. Heeney, D. Hopkins, L. Easterbrook, M. Dennis, N. Almond, and M. Cranage. 2004. Macaques infected long-term with attenuated simian immunodeficiency virus (SIVmac) remain resistant to wild-type challenge, despite declining cytotoxic T lymphocyte responses to an immunodominant epitope. J. Gen. Virol. 852591-2602. [DOI] [PubMed] [Google Scholar]
- 49.Skinner, P. J., M. A. Daniels, C. S. Schmidt, S. C. Jameson, and A. T. Haase. 2000. Cutting edge: in situ tetramer staining of antigen-specific T cells in tissues. J. Immunol. 165613-617. [DOI] [PubMed] [Google Scholar]
- 50.Skinner, P. J., and A. T. Haase. 2002. In situ tetramer staining. J. Immunol. Methods 26829-34. [DOI] [PubMed] [Google Scholar]
- 51.Steinbrook, R. 2007. One step forward, two steps back—will there ever be an AIDS vaccine? N. Engl. J. Med. 3572653-2655. [DOI] [PubMed] [Google Scholar]
- 52.Veazey, R. S., P. M. Acierno, K. J. McEvers, S. H. Baumeister, G. J. Foster, M. D. Rett, M. H. Newberg, M. J. Kuroda, K. Williams, E. Y. Kim, S. M. Wolinsky, E. P. Rieber, M. Piatak, Jr., J. D. Lifson, D. C. Montefiori, C. R. Brown, V. M. Hirsch, and J. E. Schmitz. 2008. Increased loss of CCR5+ CD45RA− CD4+ T cells in CD8+ lymphocyte-depleted simian immunodeficiency virus-infected rhesus monkeys. J. Virol. 825618-5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vogel, T. U., M. R. Reynolds, D. H. Fuller, K. Vielhuber, T. Shipley, J. T. Fuller, K. J. Kunstman, G. Sutter, M. L. Marthas, V. Erfle, S. M. Wolinsky, C. Wang, D. B. Allison, E. W. Rud, N. Wilson, D. Montefiori, J. D. Altman, and D. I. Watkins. 2003. Multispecific vaccine-induced mucosal cytotoxic T lymphocytes reduce acute-phase viral replication but fail in long-term control of simian immunodeficiency virus SIVmac239. J. Virol. 7713348-13360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walker, B. D., and D. R. Burton. 2008. Toward an AIDS vaccine. Science 320760-764. [DOI] [PubMed] [Google Scholar]
- 55.Wang, Y., K. Abel, K. Lantz, A. M. Krieg, M. B. McChesney, and C. J. Miller. 2005. The Toll-like receptor 7 (TLR7) agonist, imiquimod, and the TLR9 agonist, CpG ODN, induce antiviral cytokines and chemokines but do not prevent vaginal transmission of simian immunodeficiency virus when applied intravaginally to rhesus macaques. J. Virol. 7914355-14370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yant, L. J., T. C. Friedrich, R. C. Johnson, G. E. May, N. J. Maness, A. M. Enz, J. D. Lifson, D. H. O'Connor, M. Carrington, and D. I. Watkins. 2006. The high-frequency major histocompatibility complex class I allele Mamu-B*17 is associated with control of simian immunodeficiency virus SIVmac239 replication. J. Virol. 805074-5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zinkernagel, R. M. 2003. On natural and artificial vaccinations. Annu. Rev. Immunol. 21515-546. [DOI] [PubMed] [Google Scholar]