Abstract
Alphaviruses such as Ross River virus (RRV) and chikungunya virus are mosquito-transmitted viruses that cause explosive epidemics of debilitating arthritis and myositis affecting millions of humans worldwide. Previous studies using a mouse model of RRV-induced disease demonstrated that viral infection results in a severe inflammatory arthritis and myositis and that complement component 3 (C3) contributes to the destructive phase of the inflammatory disease but not the recruitment of cellular infiltrates to the sites of RRV-induced inflammation. Here, we demonstrate that mice deficient in complement receptor 3 (CR3) (CD11b−/−), a signaling receptor activated by multiple ligands including the C3 cleavage fragment iC3b, develop less-severe disease signs and decreased tissue destruction compared to RRV-infected wild-type mice. CR3 deficiency had no effect on viral replication, nor did it diminish the magnitude, kinetics, and composition of the cellular infiltrates at the sites of inflammation. However, the genetic absence of CR3 diminished the expression of specific proinflammatory and cytotoxic effectors, including S100A9/S100A8 and interleukin-6, within the inflamed tissues, suggesting that CR3-dependent signaling at the sites of inflammation contributes to tissue damage and severe disease.
Arthritis/myositis-associated alphaviruses, such as Ross River virus (RRV), chikungunya virus, o'nyong-nyong virus, mayaro virus, and others, are mosquito-transmitted viruses that cause debilitating inflammatory disease in humans (18, 37, 46). In addition to causing endemic disease, this group of viruses is capable of causing explosive epidemics that can involve millions of infected individuals. Past epidemics include a 1979-to-1980 epidemic of RRV disease in the South Pacific that involved more than 60,000 patients (20) and a 1959-to-1962 epidemic of o'nyong-nyong fever in Africa that involved at least 2 million patients (57). More recently, a reemergence of chikungunya virus has resulted in an unprecedented epidemic in multiple countries, including Indian Ocean islands such as the Comoros, Reunion, Mayotte, Seychelles, and Mauritius as well as India, Sri Lanka, and Indonesia (36, 37). The number of infected individuals involved thus far in this ongoing epidemic is in the millions, with an estimated 1.4 to 6.5 million cases in India alone (29). In addition, from July to September 2007, an outbreak of chikungunya virus occurred in northeastern Italy, resulting in 205 confirmed human cases and detection of the virus within the local mosquito population (4, 40).
Clinical signs and symptoms following infection with arthritis/myositis-associated alphaviruses include fever, rash, muscle pain, and severe joint pain, with the peripheral joints, such as the ankles, knees, wrists, and joints of the hands, most commonly affected (19, 20, 37). Treatment is palliative and based on analgesics and anti-inflammatory drugs. The resolution of disease symptoms generally occurs over several weeks; however, many patients complain of chronic pain lasting 6 months or longer, suggesting that these viruses may be responsible for long-term health effects (20, 39).
The disease caused by this group of viruses is believed to be initiated by viral replication and the induction of host inflammatory responses within the affected tissues. This is based on the detection of RRV RNA and antigen in joint tissues of affected patients as well as the presence of large number of inflammatory cellular infiltrates, consisting primarily of monocytes and macrophages, within synovial effusions (16, 21, 45). Additionally, chikungunya virus antigen, severe myositis with inflammatory macrophages and T lymphocytes, and myofibril pathology have been detected in quadriceps muscle biopsy samples collected from chikungunya virus-infected patients (34).
The complement system, which consists of more than 30 soluble and cell surface proteins, is a major component of innate immunity that functions to recognize and eliminate invading pathogens (9). Activation of the complement system occurs through multiple mechanisms that include three well-described pathways: the classical, lectin, and alternative complement activation pathways. Activation of the complement system results in a cascade of enzymatic proteolysis of various components of the complement system, including complement component 3 (C3), C4, and C5. The proteolytic processing of C3 generates an array of cleavage products that are involved in the amplification of complement activity through the formation of C3 and C5 convertases, the opsonization of pathogens, and the attraction and activation of leukocytes of both the innate and adaptive arms of the immune response.
Regulation of trafficking and effector functions of leukocytes via C3-, C4-, and C5-derived cleavage products occurs through interactions with a number of cell surface receptors. Complement receptor 3 (CR3) (CD11b/CD18, Mac-1, αmβ2) is a member of the β2 integrins, which are heterodimeric cell surface proteins composed of a common β chain (CD18) and one of four α chains (14, 41). The β2 integrins regulate the accumulation and activation of neutrophils, macrophages, and natural killer cells at sites of tissue inflammation and injury. CR3 binds a number of different ligands, including iC3b, ICAM-1 and ICAM-2, and fibrinogen, within the I-domain of the CD11b subunit (3, 11, 44, 58, 59). Additionally, CR3 contains a lectin domain within the C-terminal region of CD11b that interacts with polysaccharides (42, 50, 60).
Recently, our group developed an experimental mouse model to investigate the immunopathogenesis of arthritis/myositis-associated alphaviruses based on RRV infection of C57BL/6 mice (32). Following the inoculation of 3- to 4-week-old mice, RRV rapidly spreads to joint and skeletal muscle tissue via a high-titer serum viremia (32). Viral titers peak in tissues at 24 to 48 h postinfection and decline thereafter. At later times postinfection, RRV-infected mice develop severe inflammation within joint and skeletal muscle tissues that correlates with observable disease signs such as a loss of gripping ability, altered gait, and hind-limb weakness (28, 32). Utilizing this model, we demonstrated that C3 activation products, such as the CR3 ligand iC3b, were detected at the sites of RRV-induced inflammation and that C3 was critical for the tissue destruction phase of RRV-induced inflammatory disease (31). In addition, work from that study indicated that C3 was not required for recruitment of inflammatory leukocytes to the sites of RRV-induced inflammation, suggesting that complement activation may regulate the activation phenotypes of inflammatory leukocytes at the sites of RRV-induced inflammation.
In this study, we assessed the role of CR3 in the pathogenesis of RRV infection. We found that mice deficient in CR3 (CD11b−/−) developed inflammation of joint and skeletal muscle tissue following RRV infection. However, CD11b−/− mice exhibited less-severe disease signs and tissue damage compared to wild-type (WT) mice, suggesting that like C3, CR3 contributes to the tissue destruction phase of the inflammatory disease. These findings are consistent with a model in which CR3-dependent regulation of inflammatory cell function at the sites of inflammation promotes alphavirus-induced disease.
MATERIALS AND METHODS
Viruses and cells.
Viral stocks of the mouse-virulent T48 strain of RRV were generated from the full-length T48 cDNA clone (provided by Richard Kuhn, Purdue University) as previously described (32). The T48 stain of RRV was isolated from Aedes vigilax mosquitoes in Queensland, Australia (13). Prior to cDNA cloning, the virus was passaged 10 times in suckling mice followed by two passages on Vero cells (10). Viral stocks were titrated by plaque assay on BHK-21 cells as described previously (32).
BHK-21 cells were grown in α-minimal essential medium (Gibco) supplemented with 10% donor calf serum, 10% tryptose phosphate broth, and 0.29 mg/ml l-glutamine.
Mouse experiments.
C57BL/6J, C3−/−, and CD11b−/− mice (on a C57BL/6 background), were obtained from Michael Carroll or The Jackson Laboratory (Bar Harbor, ME) and bred in-house. Animal husbandry and experiments were performed in accordance with all UNC-CH Institutional Animal Care and Use Committee guidelines. Although RRV is classified as a biosafety level 2 pathogen, due to its exotic nature all mouse studies were performed in a biosafety level 3 laboratory. Twenty-four-day-old mice were used for all in vivo studies. Mice were inoculated in the left rear footpad with 103 PFU of virus in diluent (phosphate-buffered saline [PBS]-1% bovine calf serum) in a 10-μl volume. Mock-infected animals received diluent alone. Mice were monitored for disease signs and weighed at 24-h intervals. Disease scores were determined by assessing grip strength, hind-limb weakness, and altered gait as previously described (31, 32).
Viral titers.
RRV tissue titers were determined by plaque assay on BHK-21 cells as previously described (32).
Histological analysis.
At the times indicated, mice were sacrificed and extensively perfused with 4% paraformaldehyde, pH 7.3. Tissues were embedded in paraffin and 5-μm sections were prepared. To determine the extent of inflammation and tissue pathology, tissues were stained with hematoxylin and eosin (H&E) (32). Stained sections were blinded and scored for (i) the extent of inflammatory cell infiltration of the tissue and (ii) the extent of tissue damage. Both scoring systems utilized a 10-point scale where a score of 0 indicated no inflammation or damage, a score of 5 indicated moderate inflammation or damage, and a score of 10 indicated total inflammatory involvement or destruction of the tissue.
In situ hybridization.
In situ hybridization was performed as described previously (22). Briefly, a 35S-labeled RRV-specific riboprobe (complementary for RRV nucleotides 7300 to 7775) was generated using SP6 RNA polymerase (Promega) and a NotI-linearized DNA plasmid template. A 35S-labeled riboprobe complementary for the EBER2 gene from Epstein-Barr virus was used as a negative control. Deparaffinized tissue sections were hybridized with 5 × 104 cpm/μl of 35S-labeled riboprobes overnight. Tissues were washed, dehydrated through graded ethanol, and immersed in NTB autoradiography emulsion (Kodak). Following development, sections were counterstained with hematoxylin and silver grain deposition was analyzed by light microscopy.
Flow cytometry.
Mice were inoculated as described above, sacrificed by exsanguination at 7 and 10 days postinfection (dpi), and perfused with 1× PBS. Quadriceps muscles were dissected, minced, and incubated for 2 h with vigorous shaking at 37°C in digestion buffer (RPMI, 10% fetal bovine serum, 15 mM HEPES, 2.5 mg/ml collagenase A [Roche], 1.7 mg/ml DNase I [Sigma]). Digested tissues were passed through a 40-μm cell strainer, pelleted, resuspended in RPMI medium, layered on 5 ml lympholyte-M (Cedarlane), and centrifuged for 30 min at 2,500 rpm. Banded cells were collected and washed in wash buffer (1× Hanks balanced salt solution, 15 mM HEPES), and viable cell totals were determined by trypan blue exclusion. Isolated cells were incubated with anti-mouse FcγRII/III (2.4G2; BD Pharmingen) for 20 min on ice and then stained in fluorescence-activated cell sorter staining buffer (1× Hanks balanced salt solution, 1% fetal bovine serum, 2% normal rabbit serum) with the following antibodies: anti-NK1.1-phycoerythrin (PE) (eBioscience), anti-CD3-fluorescein isothiocyanate (FITC) (eBioscience), anti-F4/80-FITC (eBioscience) or F4/80-PE (Serotec), CD115-FITC (eBioscience), anti-CD11b-allophycocyanin, or GR-1-PE (eBioscience). Biotin conjugates were detected with Streptavidin-PerCP (eBioscience). Cells were fixed overnight in 2% paraformaldehyde and analyzed on a cyan cytometer (Becton Dickinson) using Summit software.
Statistical analyses.
Disease scores were evaluated for statistically significant differences by the Mann-Whitney test with the Bonferroni correction (a P value of <0.008 was considered significant) or the Kruskal-Wallis test (nonparametric analysis of variance [ANOVA]). Viral titers were evaluated for statistically significant differences by ANOVA (a P value of ≤0.05 was considered significant). Histology scores, inflammatory cell numbers, and gene expression data were evaluated for statistically significant differences by the Kruskal-Wallis test or the Mann-Whitney test (a P value of ≤0.05 was considered significant) using GraphPad InStat3 software.
RESULTS
CD11b−/− mice develop less-severe disease following RRV infection.
In previous studies, complement activation products, such as C3a and the CR3 ligand iC3b, were detected at the sites of RRV-induced inflammation in mice and humans (31). Furthermore, despite similar cellular infiltrates at the sites of inflammation, mice deficient in C3 developed less-severe disease signs and tissue destruction following RRV infection (31). Since earlier studies demonstrated a role for macrophages in mediating the destructive phase of RRV-induced disease (27, 28), we tested whether CR3, which regulates the activation of macrophages and NK cells, contributed to RRV-induced disease by use of mice deficient in the CD11b component of CR3 (CD11b−/−). Twenty-four-day-old WT (n = 6) and CD11b−/− (n = 16) mice were inoculated with 1,000 PFU of RRV and scored for the development of disease signs including loss of gripping ability, hind-limb weakness, and altered gait. Consistent with previous studies (31, 32), WT mice developed severe disease signs that peaked at 7 to 10 dpi (Fig. 1A). In contrast, RRV infection of CD11b−/− mice resulted in significantly reduced disease scores from 7 to 14 dpi (Fig. 1A). To further confirm that CD11b−/− mice develop less-severe signs of disease following RRV infection, we plotted disease scores at 7 dpi (Fig. 1B, left) and 10 dpi (Fig. 1B, right) for all other WT (n = 31) and CD11b−/− (n = 30) mice infected during the course of these studies. At both time points, CD11b−/− mice had significantly reduced disease scores compared to WT mice (P < 0.0001). These findings indicate that the genetic absence of CD11b significantly reduced the severity of RRV-induced disease and suggested that CR3 may regulate the host inflammatory response.
FIG. 1.
RRV-induced disease is less severe in CD11b−/− mice. Twenty-four-day-old C57BL/6J WT or CD11b−/− mice were inoculated with 103 PFU of RRV by injection in the left rear footpad. (A) In a representative experiment, RRV-infected WT (n = 6) and CD11b−/− (n = 16) mice were scored for the development of RRV-induced disease signs including loss of gripping ability, hind-limb weakness, and altered gait. Each data point represents the arithmetic mean ± standard error of the mean (SEM). *, P value of <0.008 as determined by the Mann-Whitney test with the Bonferroni correction. (B) Disease scores at 7 dpi (left) and 10 dpi (right) for all other RRV-infected WT and CD11b−/− mice not shown in panel A but infected during the course of these studies. Horizontal bars indicate the means. **, P value of <0.001 as determined by the Kruskal-Wallis test.
Viral loads and tissue tropisms are similar in WT and CD11b−/− mice.
To determine whether CD11b deficiency alters viral loads within tissues, the amounts of infectious virus present in joint and skeletal muscle tissues of RRV-infected WT and CD11b−/− mice at 1, 3, 5, and 7 dpi were quantified by plaque assay. No significant differences in viral loads were detected at any time point examined for joint tissue (Fig. 2A and B) or quadriceps muscle tissue (Fig. 2C and D) harvested from either the injected limb (Fig. 2A and C) or the noninjected limb (Fig. 2B and D). These findings suggest that (i) the ability of RRV to replicate and spread within these tissues is not significantly impacted by the genetic deficiency of CD11b and (ii) the differences in RRV-induced disease signs in CD11b−/− mice (Fig. 1) is not explained by differences in viral loads.
FIG. 2.
RRV titers in joint and skeletal muscle tissue. Twenty-four-day-old C57BL/6J WT (filled circles) or CD11b−/− (open circles) mice were infected with 103 PFU of RRV by injection in the left rear footpad. At 1 (n = 6), 3 (n = 5), 5 (n = 5 or 6), and 7 (n = 5) dpi, mice were sacrificed and extensively perfused with 1× PBS. Ankles and quadriceps were dissected, weighed, and homogenized, and the amount of infectious virus present was quantified by plaque assays. (A) Left ankles. (B) Right ankles. (C) Left quadriceps. (D) Right quadriceps. Horizontal bars indicate the means. No statistically significant differences between WT mice and CD11b−/− mice were detected by ANOVA at any time point.
To assess whether the genetic deficiency of CD11b altered viral tropism for specific cells within tissues or impacted viral replication at later time points that are difficult to accurately assess by plaque assay, the specific sites of RRV replication in WT and CD11b−/− mice were assessed at 2, 7, and 10 dpi by in situ hybridization using a 35S-labeled riboprobe specific for RRV. At 2 dpi, a time point at which previous studies identified high levels of viral replication (31, 32), RRV-specific signal was detected within the perimysium and myofibers of skeletal muscle tissue from both WT and CD11b−/− mice, indicating that the virus targets similar cells in both strains of mice at a time of high viral loads (Fig. 3, top). Similar distribution and intensity of RRV-specific in situ signal were observed at 7 dpi (Fig. 3, middle). At the time of peak disease scores, 10 dpi, only rare, small foci of RRV-specific in situ signal were observed for skeletal muscle tissue from RRV-infected WT and CD11b−/− mice (Fig. 3, bottom). Collectively, these experiments suggest that CD11b−/− mice develop less-severe RRV-induced disease signs despite similar viral loads and viral distributions within the affected tissues.
FIG. 3.
RRV infection in skeletal muscle tissue. Twenty-four-day-old C57BL/6J WT or CD11b−/− mice were infected with 103 PFU of RRV by injection in the left rear footpad. Mice were sacrificed at 2, 7, and 10 dpi and perfused with 4% paraformaldehyde. Five-micrometer-thick paraffin-embedded sections derived from the quadriceps muscles were hybridized with a 35S-labeled riboprobe complementary either to the EBER2 gene from Epstein-Barr virus (control probe) or to RRV. Images (magnification, ×100) are representative of three or four mice per group.
RRV infection induces inflammation but less-severe tissue destruction in CD11b−/− mice.
To investigate whether the reduced disease signs in CD11b−/− mice correlated with differences in tissue inflammation and pathology, histological analyses of hind-limb joint and skeletal muscle tissues were performed by staining fixed sections with H&E. In contrast to what was seen for mock-infected mice, inflammatory infiltrates were readily observed bilaterally in synovial tissues of the metatarsophalangeal joints of both WT and CD11b−/− mice at 7 and 10 dpi, suggesting that CD11b is not strictly required for cellular recruitment to these sites of inflammation (Fig. 4A). Similarly, inflammatory infiltrates were observed in skeletal muscle tissue of RRV-infected WT and CD11b−/− mice at 7 dpi (data not shown) and 10 dpi (Fig. 4B, top). Skeletal muscle tissue sections derived from RRV-infected CD11b−/− mice appeared similar to the corresponding sections derived from RRV-infected C3−/− mice from earlier studies (31). That is, despite extensive tissue inflammation at similar levels, the overall levels of tissue destruction in both C3−/− and CD11b−/− mice were less severe than that seen for WT mice. Therefore, tissue sections were derived from WT, CD11b−/−, and C3−/− mice at 10 dpi, a time point of peak disease scores, inflammation, and tissue pathology, and at 20 dpi, a time point at which inflammation was resolved but tissue destruction was readily observed. These sections were then scored in a blinded manner as described in Materials and Methods for the extent of inflammatory cell infiltration (inflammation score) and the extent of tissue damage. As shown in Fig. 4B and C, no significant differences were detected in the extents of inflammatory cell infiltration of skeletal muscle tissue of all three strains of mice at 10 or 20 dpi. Consistent with previous findings (31), the degree of tissue damage was reduced in C3−/− mice at 10 dpi. Similarly, skeletal muscle tissue from RRV-infected CD11b−/− mice lacked the severe signs of tissue damage observed for RRV-infected WT mice (Fig. 4B), and this was reflected in reduced tissue damage scores, although these differences did not reach statistical significance as determined by the Kruskal-Wallis test (nonparametric ANOVA). At 20 dpi, both CD11b−/− and C3−/− mice had significantly reduced tissue damage compared to RRV-infected WT mice (Fig. 4B and 4C). Taken together, these studies suggest that similar to C3, CD11b promotes tissue damage following RRV infection by a mechanism(s) that is independent of the recruitment of cellular infiltrates to the sites of RRV-induced inflammation.
FIG. 4.
RRV-induced inflammation and tissue damage. Twenty-four-day-old C57BL/6J WT, CD11b−/−, or C3−/− mice were inoculated with PBS or 103 PFU of RRV by injection in the left rear footpad. At 7, 10, and 20 dpi mice were sacrificed and perfused with 4% paraformaldehyde. After decalcification, 5-μm-thick paraffin-embedded sections generated from the ankle/foot (A) or quadriceps muscle (B) of PBS- or RRV-infected mice were stained with H&E. Arrows indicate areas of severe tissue damage. Images are representative of three to eight mice per group. (C) H&E-stained sections of quadriceps muscle tissue from mock-infected mice (PBS) or from RRV-infected WT, RRV-infected C3−/−, or RRV-infected CD11b−/− mice were scored in a blinded manner for the extents of inflammatory cell infiltration (left) and tissue damage (right) as described in Materials and Methods. Each bar represents the arithmetic mean ± SEM. *, P value of <0.05 as determined by the Kruskal-Wallis test (nonparametric ANOVA).
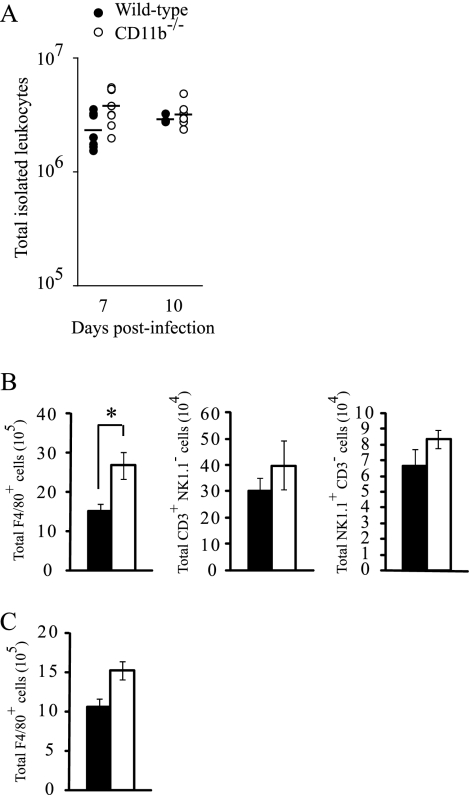
To further confirm that inflamed tissues from RRV-infected WT and CD11b−/− mice contain similar numbers and compositions of inflammatory infiltrates, which are composed primarily of macrophages, NK cells, and T lymphocytes (31, 32), we isolated and quantified infiltrating leukocytes from skeletal muscle tissue at 7 and 10 dpi. Interestingly, at 7 dpi we isolated more inflammatory cells from skeletal muscle tissue of RRV-infected CD11b−/− mice than from WT mice (Fig. 5A). However, similar total numbers of inflammatory infiltrates were isolated from tissues at 10 dpi (Fig. 5A) suggesting that the increase observed at 7 dpi was a transient effect. Previous work from other researchers demonstrated that treatment of mice with macrophage-depleting agents, such as clodronate-containing liposomes, dramatically reduced the severity of RRV-induced disease, implicating an important role for macrophages in RRV pathogenesis (27, 28). Therefore, flow cytometry was utilized to quantify the number of macrophages present in inflamed skeletal muscle tissue of WT and CD11b−/− mice at times of peak disease scores and tissue damage (7 and 10 dpi). Reflecting the higher total number of cellular infiltrates isolated from RRV-infected CD11b−/− mice at 7 dpi, significantly more F4/80+ cells were detected in tissue from CD11b−/− mice at this time point (Fig. 5B). Similar numbers of F4/80+ cells were detected in tissue from WT and CD11b−/− mice at 10 dpi (Fig. 5C). These cell populations were CD11b+ and CD11b− in WT mice and CD11b-deficient mice, respectively, confirming (i) their macrophage phenotype and (ii) the lack of CD11b protein expression in CD11b−/− mice (data not shown). In addition to macrophages, the total numbers of CD3+/NK1.1− cells (T lymphocytes) and CD3−/NK1.1+ cells (NK cells) isolated trended toward being higher in CD11b−/− mice than in WT mice at 7 dpi (Fig. 5B). These results confirm that the reduced disease signs observed following RRV infection of CD11b−/− mice is not due to a diminished recruitment of inflammatory macrophages or other inflammatory cell populations to the sites of RRV-induced inflammation and are consistent with the notion that CR3 plays a more important role in the activation of leukocyte effector functions than in their recruitment and migration (24).
FIG. 5.
Quantification of inflammatory infiltrates in skeletal muscle from RRV-infected WT and CD11b−/− mice. Twenty-four-day-old C57BL/6J WT or CD11b−/− mice were infected with 103 PFU of RRV by injection in the left rear footpad. (A) At 7 dpi (n = 8) and 10 dpi (n = 3 to 7), infiltrating leukocytes were isolated from whole quadriceps muscles of WT and CD11b−/− mice as described in Materials and Methods and the total cell number was determined. Horizontal bars indicate the means. No statistically significant differences were detected between WT mice and CD11b−/− mice at each time point by the Kruskal-Wallis test. (B) Total numbers of F4/80+, CD3+/NK1.1−, and NK1.1+/CD3− cells isolated from RRV-infected WT (filled bars) and CD11b−/− (open bars) mice at 7 dpi. Each bar represents the arithmetic mean ± SEM of eight mice per group. *, P value of <0.05 as determined by the Mann-Whitney test. (C) Total numbers of F4/80+ cells isolated from skeletal muscle from RRV-infected WT (filled bars) and CD11b−/− (open bars) mice at 10 dpi. Each bar represents the arithmetic mean ± SEM of three mice per group. Data are representative of two independent experiments.
CD11b regulates the expression of specific proinflammatory and cytotoxic effectors.
The data outlined above indicated that CR3 deficiency significantly reduced the severity of RRV-induced disease but did not diminish the recruitment of effector cells that have been implicated in RRV pathogenesis, such as macrophages, to the sites of inflammation. Therefore, we evaluated the RNA transcript levels of genes that are associated with inflammatory diseases, cellular cytotoxicity, and macrophage activation phenotypes within skeletal muscle tissue of mock- or RRV-infected WT and CD11b−/− mice at 7 dpi by quantitative real-time PCR. To further evaluate the role of the complement system in the regulation of gene expression within the inflamed tissues, we included mock- and RRV-infected C3−/− mice in these analyses. As shown in Fig. 6, three major patterns of gene expression profiles were observed. First, we identified genes, such as those for tumor necrosis factor alpha, interleukin-1β (IL-1β), and IL-10, that were induced to similar levels in RRV-infected WT, C3−/−, and CD11b−/− mice compared to mock-infected mice (Fig. 6, top). These findings suggest that the induction of these genes following RRV infection is regulated by C3- and CD11b-independent mechanisms and further confirm that many aspects of the RRV-induced inflammatory response are similar in WT, C3−/−, and CD11b−/− mice. RRV-induced expression of a second set of transcripts, including the proinflammatory genes for S100A9, S100A8, and IL-6, was significantly reduced in RRV-infected C3−/− and CD11b−/− mice compared to WT mice (Fig. 6, middle). These findings suggest that these genes may be regulated in both a C3- and a CD11b-dependent manner following RRV infection, which implicates an interaction of CR3 with its C3-derived ligand iC3b, as the regulatory mechanism. Intriguingly, S100A9 and S100A8 form a heterodimeric complex that is secreted from activated leukocytes such as macrophages and neutrophils (33, 62). Levels of this complex are elevated in inflamed tissues of rheumatoid arthritis patients and in patients with inflammatory muscle diseases, and the expression of these proteins by inflammatory macrophages has been associated with the degeneration of muscle fibers (33, 43, 47). Finally, we detected significantly reduced induction of RNA transcripts encoding arginase I in RRV-infected C3−/− mice compared to what was seen for WT and CD11b−/− mice (Fig. 6, bottom). Arginase I is an enzyme involved in l-arginine metabolism that is associated with a distinct macrophage activation phenotype, termed alternatively activated, which is implicated in tissue repair, fibrosis, and in some cases exacerbated pathology (7, 17, 49). These findings suggest that the induction of this gene following RRV infection occurs by a C3-dependent, but CD11b-independent, pathway. Taken together, these analyses suggest that the activation of the complement system following RRV infection regulates the expression of specific effector genes at the sites of inflammation. Furthermore, the findings suggest that this complement-mediated regulation occurs by both CD11b-dependent and CD11b-independent mechanisms.
FIG. 6.
CR3 regulates RRV-induced gene expression at the inflammatory site. Twenty-four-day-old C57BL/6J WT, C3−/−, or CD11b−/− mice were mock infected or infected with 103 PFU of RRV by injection in the left rear footpad. Total RNA from the right quadriceps muscle was isolated at 7 dpi and analyzed for mRNA expression by quantitative real-time PCR. Data are normalized to 18S rRNA levels and are expressed as the relative expression (n-fold increase) over RNA isolated from quadriceps muscles of mock-infected controls. Each bar represents the arithmetic mean ± SEM of six mice per group. *, Statistically significant differences compared to WT values as determined by the Kruskal-Wallis test (P < 0.05).
DISCUSSION
Alphaviruses are a significant cause of infectious arthritis and myositis in humans, and we and others have used mouse models of RRV to define the mechanisms underlying these virus-induced inflammatory diseases. Chikungunya virus- and RRV-induced arthritides in humans are characterized by inflammatory cell infiltration into afflicted joint and muscle tissue, which strongly suggests that immune-mediated pathology contributes to virus-induced disease. This idea is supported by work with the RRV mouse model, in which the ablation of macrophages or the blockade of proinflammatory cytokines significantly reduced RRV-induced disease and tissue damage (27, 28). Additional studies demonstrated that the host complement system plays a major role in the pathogenesis of RRV-induced inflammatory disease, since mice deficient in the C3 component of complement exhibited significantly reduced RRV-induced disease signs and tissue destruction (31). The complement system plays a prominent role in a number of inflammatory diseases, including several murine models of immune-mediated arthritis (2, 23, 25). Interestingly, the role of the complement system in the RRV model of virus-induced arthritis/myositis appeared to differ from other arthritis models in that C3 was required for inflammatory tissue destruction but not the recruitment of inflammatory cells into the affected tissues (31). This suggested that complement contributes to tissue destruction through one or more downstream effector pathways. Given the prominent role of macrophages in the destructive disease process (27, 28) and the fact that CR3 signaling regulates macrophage effector functions, including cytotoxic activity (14, 41), we assessed whether CR3 contributed to the pathogenesis of RRV-induced inflammatory disease. As shown in this report, mice lacking CR3 show significantly reduced disease signs and reduced tissue destruction following RRV infection. The decreased disease signs were not accompanied by any significant differences in RRV replication or diminished recruitment of inflammatory leukocytes, including macrophages, into the inflamed muscle or joint tissue. These results, which are similar to previous findings with C3-deficient mice, suggest that CR3 may act downstream of inflammatory cell invasion and complement activation to promote tissue destruction during RRV infection.
CR3 is expressed to high levels on monocytes/macrophages, NK cells, and neutrophils, plays a major role in regulating several aspects of the inflammatory response, including leukocyte adhesion/migration, phagocytosis, and inflammatory cell activation/degranulation, and binds a wide range of ligands, including iC3b and ICAM-1 and -2, and fibrinogen. CR3 regulates cellular adhesion via interactions with ICAM-1, ICAM-2, and the extracellular matrix (14, 41). Therefore, there was a strong possibility that CR3-deficient mice would exhibit a defect in inflammatory cell recruitment or invasion into the sites of inflammation. However, our studies indicated that CR3-deficient mice exhibited no measurable defect in the recruitment or invasion of cellular infiltrates into joint and skeletal muscle tissue (Fig. 4 and 5). In fact, higher numbers of cellular infiltrates were detected in skeletal muscle tissue of RRV-infected CD11b−/− mice at 7 dpi (Fig. 5). These finding are consistent with other studies where the absence of CR3 enhanced or did not affect leukocyte recruitment into the inflammatory sites (24, 56). These results also suggest that the RRV inflammatory disease model differs from antibody-induced arthritis, where CR3 deficiency did not impact disease severity (56).
CR3 has been shown to enhance the infection of monocytic cells and immature dendritic cells by complement-opsonized human immunodeficiency virus (1, 5, 6, 38) and flaviviruses (8), and CR3-dependent signaling has been shown to enhance human immunodeficiency virus replication (48). These findings raised the possibility that CR3 deficiency may affect RRV-induced disease by alteration of viral replication or spread. However, we did not detect any significant differences in viral loads or viral distributions within the affected joint and skeletal muscle tissues of RRV-infected CD11b−/− mice compared to WT mice at multiple early and late time points within the disease process (Fig. 2 and 3). These results suggest that the effects of CR3 on RRV-induced disease are not due to effects on viral replication; however, we cannot rule out that CR3 may promote nonproductive infection of specific cell types that could lead to alterations in cellular functions.
CR3 signals in a Syk- and phosphatidylinositol 3 kinase-dependent manner to induce cytotoxic activation of macrophages, NK cells, and neutrophils (26, 30). The ligation of CR3 on peritoneal macrophages leads to activation, including enhanced production of reactive oxygen intermediates (12), while the activation of CR3 by iC3b and beta glucans significantly enhances macrophage and NK cell cytotoxic activity against tumor cells (55, 61). CR3 has also been shown to enhance the Toll-like receptor 4-dependent activation of murine macrophages (35) and has been shown to act in concert with Fas to promote smooth muscle cell killing by activated macrophages (54). Therefore, it is likely that the effects of CR3 in the RRV inflammatory disease model are at least partly due to its ability to regulate macrophage and/or NK cell activation and subsequent cytotoxicity. This is supported by the findings in this study that CR3 was required for the upregulation of at least a subset of genes within the inflamed tissue (Fig. 6). The genetic absence of CR3 significantly diminished the RRV-induced expression of S100A9, S100A8, and IL-6 genes within inflamed skeletal muscle tissue. In addition, the induction of these genes was also decreased in RRV-infected C3−/− mice, suggesting that the induction was regulated, at least in part, by CR3 interaction with its C3-derived ligand iC3b. However, the interaction of CR3 with other ligands may also contribute to the effects. The diminished induction of IL-6 was similar to what was found in previous studies utilizing a mouse model of collagen-induced arthritis that found CR3 interaction with fibrin was required for the induction of IL-6 and that this effect correlated with a reduced severity of arthritis (15). However, in contrast to what was found in that study, CR3 deficiency had no effect on RRV-induced expression of tumor necrosis factor alpha or IL-1β. Interestingly, IL-6 induces skeletal muscle breakdown and the presence of IL-6 in inflamed muscle tissue contributes to skeletal muscle degeneration (53). In addition, IL-6 transgenic mice suffer skeletal muscle atrophy which can be reversed by treatment with IL-6 receptor antibody (51, 52), suggesting that high levels of IL-6 within the diseased tissues may contribute to RRV pathogenesis. Our findings that C3 and CR3 were both required for full induction of S100A9 and S100A8 at the sites of RRV-induced inflammation potentially identifies a novel regulatory mechanism for these secreted proinflammatory and cytotoxic factors. As mentioned above, the S100A9/S100A8 complex is elevated in inflamed tissues of rheumatoid arthritis patients and patients with inflammatory muscle diseases (33, 43, 47). Furthermore, the expression of these proteins by inflammatory macrophages has been associated with the degeneration of muscle fibers (43), and these proteins have been shown to have direct cytotoxic effects, including plasma membrane damage, on cultured muscle cells and endothelial cells (43, 54). Since CR3 deficiency had no effect on viral replication or clearance, the results support the idea that complement-dependent inflammatory cell activation represents an immunopathologic response that contributes to the tissue damage incurred during infection with arthritic alphaviruses. These findings suggest that interfering with CR3-dependent signaling may represent a potential therapeutic approach to treating alphavirus-induced inflammatory disease, which is significant given that there are no approved vaccines or targeted therapeutics for any of these pathogens.
The results presented in this study clearly demonstrate a role for CR3 in the pathogenesis of RRV-induced arthritis/myositis; however, RRV-infected CD11b−/− mice trended to exhibit more-severe disease signs and tissue pathology compared to infected C3−/− mice. Therefore, it remains a possibility that other complement effector pathways contribute to RRV-induced disease, and this requires further evaluation. For example, other complement-dependent signaling pathways, such as the C3a receptor, which also regulates leukocyte effector functions, may be required for full activation and subsequent tissue destruction by the inflammatory cells. This is supported by our findings that some genes are regulated by both C3 and CR3, but other genes that are affected by C3 deficiency are not affected in tissues from CR3-deficient mice (Fig. 6). While this may reflect other inflammatory changes within the tissue rather than direct signaling through CR3 or other complement receptors, the possibility that multiple complement receptors are activated and drive the inflammatory destructive process needs to be addressed further.
In summary, the results presented in this study define a role for CR3 in mediating alphavirus-induced inflammatory disease, which further establishes that the host complement cascade plays a major role in driving virus-induced pathology within the mouse model. Not only do these results extend our understanding of the processes underlying alphavirus-induced arthritis/myositis, they also suggest that interfering with the complement cascade and complement receptor signaling may represent a useful route for therapeutic intervention.
Acknowledgments
This research was supported by NIH research grant R01 AR47190. T.E.M. was supported by NIH postdoctoral fellowship F32 AR052600-01.
We thank members of the Carolina Vaccine Institute and the Johnston laboratory for helpful scientific discussions. We also thank Janice Weaver at the LCCC/DLAM UNC histopathology core facility.
Footnotes
Published ahead of print on 10 September 2008.
REFERENCES
- 1.Bajtay, Z., C. Speth, A. Erdei, and M. P. Dierich. 2004. Cutting edge: productive HIV-1 infection of dendritic cells via complement receptor type 3 (CR3, CD11b/CD18). J. Immunol. 1734775-4778. [DOI] [PubMed] [Google Scholar]
- 2.Banda, N. K., J. M. Thurman, D. Kraus, A. Wood, M. C. Carroll, W. P. Arend, and V. M. Holers. 2006. Alternative complement pathway activation is essential for inflammation and joint destruction in the passive transfer model of collagen-induced arthritis. J. Immunol. 1771904-1912. [DOI] [PubMed] [Google Scholar]
- 3.Beller, D. I., T. A. Springer, and R. D. Schreiber. 1982. Anti-Mac-1 selectively inhibits the mouse and human type three complement receptor. J. Exp. Med. 1561000-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonilauri, P., R. Bellini, M. Calzolari, R. Angelini, L. Venturi, F. Fallacara, P. Cordioli, P. Angelini, C. Venturelli, G. Merialdi, and M. Dottori. 2008. Chikungunya virus in Aedes albopictus, Italy. Emerg. Infect. Dis. 14852-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouhlal, H., N. Chomont, M. Requena, N. Nasreddine, H. Saidi, J. Legoff, M. D. Kazatchkine, L. Belec, and H. Hocini. 2007. Opsonization of HIV with complement enhances infection of dendritic cells and viral transfer to CD4 T cells in a CR3 and DC-SIGN-dependent manner. J. Immunol. 1781086-1095. [DOI] [PubMed] [Google Scholar]
- 6.Bouhlal, H., J. Galon, M. D. Kazatchkine, W. H. Fridman, C. Sautes-Fridman, and N. Haeffner Cavaillon. 2001. Soluble CD16 inhibits CR3 (CD11b/CD18)-mediated infection of monocytes/macrophages by opsonized primary R5 HIV-1. J. Immunol. 1663377-3383. [DOI] [PubMed] [Google Scholar]
- 7.Bronte, V., and P. Zanovello. 2005. Regulation of immune responses by l-arginine metabolism. Nat. Rev. Immunol. 5641-654. [DOI] [PubMed] [Google Scholar]
- 8.Cardosa, M. J., J. S. Porterfield, and S. Gordon. 1983. Complement receptor mediates enhanced flavivirus replication in macrophages. J. Exp. Med. 158258-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carroll, M. C. 2004. The complement system in regulation of adaptive immunity. Nat. Immunol. 5981-986. [DOI] [PubMed] [Google Scholar]
- 10.Dalgarno, L., C. M. Rice, and J. H. Strauss. 1983. Ross River virus 26 s RNA: complete nucleotide sequence and deduced sequence of the encoded structural proteins. Virology 129170-187. [DOI] [PubMed] [Google Scholar]
- 11.Diamond, M. S., D. E. Staunton, A. R. de Fougerolles, S. A. Stacker, J. Garcia-Aguilar, M. L. Hibbs, and T. A. Springer. 1990. ICAM-1 (CD54): a counter-receptor for Mac-1 (CD11b/CD18). J. Cell Biol. 1113129-3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ding, A., S. D. Wright, and C. Nathan. 1987. Activation of mouse peritoneal macrophages by monoclonal antibodies to Mac-1 (complement receptor type 3). J. Exp. Med. 165733-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doherty, R. L., R. H. Whitehead, B. M. Gorman, and A. K. O'Gower. 1963. The isolation of a third group A arbovirus in Australia, with preliminary observations on its relationship to epidemic polyarthritis. Aust. J. Sci. 26183-184. [Google Scholar]
- 14.Ehlers, M. R. 2000. CR3: a general purpose adhesion-recognition receptor essential for innate immunity. Microbes Infect. 2289-294. [DOI] [PubMed] [Google Scholar]
- 15.Flick, M. J., C. M. LaJeunesse, K. E. Talmage, D. P. Witte, J. S. Palumbo, M. D. Pinkerton, S. Thornton, and J. L. Degen. 2007. Fibrin(ogen) exacerbates inflammatory joint disease through a mechanism linked to the integrin alphaMbeta2 binding motif. J. Clin. Investig. 1173224-3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fraser, J. R., A. L. Cunningham, B. J. Clarris, J. G. Aaskov, and R. Leach. 1981. Cytology of synovial effusions in epidemic polyarthritis. Aust. N. Z. J. Med. 11168-173. [DOI] [PubMed] [Google Scholar]
- 17.Gordon, S. 2003. Alternative activation of macrophages. Nat. Rev. Immunol. 323-35. [DOI] [PubMed] [Google Scholar]
- 18.Griffin, D. E. 2001. Alphaviruses, p. 917-962. In D. M. Knipe and P. M. Howley (ed.), Fields virology. Lippincott, Williams, & Wilkins, Philadelphia, PA.
- 19.Harley, D., D. Bossingham, D. M. Purdie, N. Pandeya, and A. C. Sleigh. 2002. Ross River virus disease in tropical Queensland: evolution of rheumatic manifestations in an inception cohort followed for six months. Med. J. Aust. 177352-355. [DOI] [PubMed] [Google Scholar]
- 20.Harley, D., A. Sleigh, and S. Ritchie. 2001. Ross River virus transmission, infection, and disease: a cross-disciplinary review. Clin. Microbiol. Rev. 14909-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hazelton, R. A., C. Hughes, and J. G. Aaskov. 1985. The inflammatory response in the synovium of a patient with Ross River arbovirus infection. Aust. N. Z. J. Med. 15336-339. [DOI] [PubMed] [Google Scholar]
- 22.Heise, M. T., D. A. Simpson, and R. E. Johnston. 2000. Sindbis-group alphavirus replication in periosteum and endosteum of long bones in adult mice. J. Virol. 749294-9299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hietala, M. A., I. M. Jonsson, A. Tarkowski, S. Kleinau, and M. Pekna. 2002. Complement deficiency ameliorates collagen-induced arthritis in mice. J. Immunol. 169454-459. [DOI] [PubMed] [Google Scholar]
- 24.Hirahashi, J., D. Mekala, J. Van Ziffle, L. Xiao, S. Saffaripour, D. D. Wagner, S. D. Shapiro, C. Lowell, and T. N. Mayadas. 2006. Mac-1 signaling via Src-family and Syk kinases results in elastase-dependent thrombohemorrhagic vasculopathy. Immunity 25271-283. [DOI] [PubMed] [Google Scholar]
- 25.Ji, H., K. Ohmura, U. Mahmood, D. M. Lee, F. M. Hofhuis, S. A. Boackle, K. Takahashi, V. M. Holers, M. Walport, C. Gerard, A. Ezekowitz, M. C. Carroll, M. Brenner, R. Weissleder, J. S. Verbeek, V. Duchatelle, C. Degott, C. Benoist, and D. Mathis. 2002. Arthritis critically dependent on innate immune system players. Immunity 16157-168. [DOI] [PubMed] [Google Scholar]
- 26.Li, B., D. J. Allendorf, R. Hansen, J. Marroquin, C. Ding, D. E. Cramer, and J. Yan. 2006. Yeast beta-glucan amplifies phagocyte killing of iC3b-opsonized tumor cells via complement receptor 3-Syk-phosphatidylinositol 3-kinase pathway. J. Immunol. 1771661-1669. [DOI] [PubMed] [Google Scholar]
- 27.Lidbury, B. A., N. E. Rulli, A. Suhrbier, P. N. Smith, S. R. McColl, A. L. Cunningham, A. Tarkowski, N. van Rooijen, R. J. Fraser, and S. Mahalingam. 2008. Macrophage-derived proinflammatory factors contribute to the development of arthritis and myositis after infection with an arthrogenic alphavirus. J. Infect. Dis. 1971585-1593. [DOI] [PubMed] [Google Scholar]
- 28.Lidbury, B. A., C. Simeonovic, G. E. Maxwell, I. D. Marshall, and A. J. Hapel. 2000. Macrophage-induced muscle pathology results in morbidity and mortality for Ross River virus-infected mice. J. Infect. Dis. 18127-34. [DOI] [PubMed] [Google Scholar]
- 29.Mavalankar, D., P. Shastri, and P. Raman. 2007. Chikungunya epidemic in India: a major public-health disaster. Lancet Infect. Dis. 7306-307. [DOI] [PubMed] [Google Scholar]
- 30.Mocsai, A., M. Zhou, F. Meng, V. L. Tybulewicz, and C. A. Lowell. 2002. Syk is required for integrin signaling in neutrophils. Immunity 16547-558. [DOI] [PubMed] [Google Scholar]
- 31.Morrison, T. E., R. J. Fraser, P. N. Smith, S. Mahalingam, and M. T. Heise. 2007. Complement contributes to inflammatory tissue destruction in a mouse model of Ross River virus-induced disease. J. Virol. 815132-5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morrison, T. E., A. C. Whitmore, R. S. Shabman, B. A. Lidbury, S. Mahalingam, and M. T. Heise. 2006. Characterization of Ross River virus tropism and virus-induced inflammation in a mouse model of viral arthritis and myositis. J. Virol. 80737-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Odink, K., N. Cerletti, J. Bruggen, R. G. Clerc, L. Tarcsay, G. Zwadlo, G. Gerhards, R. Schlegel, and C. Sorg. 1987. Two calcium-binding proteins in infiltrate macrophages of rheumatoid arthritis. Nature 33080-82. [DOI] [PubMed] [Google Scholar]
- 34.Ozden, S., M. Huerre, J. P. Riviere, L. L. Coffey, P. V. Afonso, V. Mouly, J. de Monredon, J. C. Roger, M. El Amrani, J. L. Yvin, M. C. Jaffar, M. P. Frenkiel, M. Sourisseau, O. Schwartz, G. Butler-Browne, P. Despres, A. Gessain, and P. E. Ceccaldi. 2007. Human muscle satellite cells as targets of Chikungunya virus infection. PLoS ONE 2e527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perera, P. Y., T. N. Mayadas, O. Takeuchi, S. Akira, M. Zaks-Zilberman, S. M. Goyert, and S. N. Vogel. 2001. CD11b/CD18 acts in concert with CD14 and Toll-like receptor (TLR) 4 to elicit full lipopolysaccharide and taxol-inducible gene expression. J. Immunol. 166574-581. [DOI] [PubMed] [Google Scholar]
- 36.Pialoux, G., B. A. Gauzere, S. Jaureguiberry, and M. Strobel. 2007. Chikungunya, an epidemic arbovirosis. Lancet Infect. Dis. 7319-327. [DOI] [PubMed] [Google Scholar]
- 37.Powers, A. M., and C. H. Logue. 2007. Changing patterns of chikungunya virus: re-emergence of a zoonotic arbovirus. J. Gen. Virol. 882363-2377. [DOI] [PubMed] [Google Scholar]
- 38.Pruenster, M., D. Wilflingseder, Z. Banki, C. G. Ammann, B. Muellauer, M. Meyer, C. Speth, M. P. Dierich, and H. Stoiber. 2005. C-type lectin-independent interaction of complement opsonized HIV with monocyte-derived dendritic cells. Eur. J. Immunol. 352691-2698. [DOI] [PubMed] [Google Scholar]
- 39.Queyriaux, B., F. Simon, M. Grandadam, R. Michel, H. Tolou, and J. P. Boutin. 2008. Clinical burden of chikungunya virus infection. Lancet Infect. Dis. 82-3. [DOI] [PubMed] [Google Scholar]
- 40.Rezza, G., L. Nicoletti, R. Angelini, R. Romi, A. C. Finarelli, M. Panning, P. Cordioli, C. Fortuna, S. Boros, F. Magurano, G. Silvi, P. Angelini, M. Dottori, M. G. Ciufolini, G. C. Majori, and A. Cassone. 2007. Infection with chikungunya virus in Italy: an outbreak in a temperate region. Lancet 3701840-1846. [DOI] [PubMed] [Google Scholar]
- 41.Ross, G. D. 2000. Regulation of the adhesion versus cytotoxic functions of the Mac-1/CR3/alphaMbeta2-integrin glycoprotein. Crit. Rev. Immunol. 20197-222. [PubMed] [Google Scholar]
- 42.Ross, G. D., J. A. Cain, and P. J. Lachmann. 1985. Membrane complement receptor type three (CR3) has lectin-like properties analogous to bovine conglutinin as functions as a receptor for zymosan and rabbit erythrocytes as well as a receptor for iC3b. J. Immunol. 1343307-3315. [PubMed] [Google Scholar]
- 43.Seeliger, S., T. Vogl, I. H. Engels, J. M. Schroder, C. Sorg, C. Sunderkotter, and J. Roth. 2003. Expression of calcium-binding proteins MRP8 and MRP14 in inflammatory muscle diseases. Am. J. Pathol. 163947-956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith, C. W., S. D. Marlin, R. Rothlein, C. Toman, and D. C. Anderson. 1989. Cooperative interactions of LFA-1 and Mac-1 with intercellular adhesion molecule-1 in facilitating adherence and transendothelial migration of human neutrophils in vitro. J. Clin. Investig. 832008-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Soden, M., H. Vasudevan, B. Roberts, R. Coelen, G. Hamlin, S. Vasudevan, and J. La Brooy. 2000. Detection of viral ribonucleic acid and histologic analysis of inflamed synovium in Ross River virus infection. Arthritis Rheum. 43365-369. [DOI] [PubMed] [Google Scholar]
- 46.Suhrbier, A., and M. La Linn. 2004. Clinical and pathologic aspects of arthritis due to Ross River virus and other alphaviruses. Curr. Opin. Rheumatol. 16374-379. [DOI] [PubMed] [Google Scholar]
- 47.Sunahori, K., M. Yamamura, J. Yamana, K. Takasugi, M. Kawashima, H. Yamamoto, W. J. Chazin, Y. Nakatani, S. Yui, and H. Makino. 2006. The S100A8/A9 heterodimer amplifies proinflammatory cytokine production by macrophages via activation of nuclear factor kappa B and p38 mitogen-activated protein kinase in rheumatoid arthritis. Arthritis Res. Ther. 8R69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thieblemont, N., N. Haeffner-Cavaillon, A. Haeffner, B. Cholley, L. Weiss, and M. D. Kazatchkine. 1995. Triggering of complement receptors CR1 (CD35) and CR3 (CD11b/CD18) induces nuclear translocation of NF-kappa B (p50/p65) in human monocytes and enhances viral replication in HIV-infected monocytic cells. J. Immunol. 1554861-4867. [PubMed] [Google Scholar]
- 49.Thompson, R. W., J. T. Pesce, T. Ramalingam, M. S. Wilson, S. White, A. W. Cheever, S. M. Ricklefs, S. F. Porcella, L. Li, L. G. Ellies, and T. A. Wynn. 2008. Cationic amino acid transporter-2 regulates immunity by modulating arginase activity. PLoS Pathogens 4e1000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thornton, B. P., V. Vetvicka, M. Pitman, R. C. Goldman, and G. D. Ross. 1996. Analysis of the sugar specificity and molecular location of the beta-glucan-binding lectin site of complement receptor type 3 (CD11b/CD18). J. Immunol. 1561235-1246. [PubMed] [Google Scholar]
- 51.Tsujinaka, T., C. Ebisui, J. Fujita, M. Kishibuchi, T. Morimoto, A. Ogawa, A. Katsume, Y. Ohsugi, E. Kominami, and M. Monden. 1995. Muscle undergoes atrophy in association with increase of lysosomal cathepsin activity in interleukin-6 transgenic mouse. Biochem. Biophys. Res. Commun. 207168-174. [DOI] [PubMed] [Google Scholar]
- 52.Tsujinaka, T., J. Fujita, C. Ebisui, M. Yano, E. Kominami, K. Suzuki, K. Tanaka, A. Katsume, Y. Ohsugi, H. Shiozaki, and M. Monden. 1996. Interleukin 6 receptor antibody inhibits muscle atrophy and modulates proteolytic systems in interleukin 6 transgenic mice. J. Clin. Investig. 97244-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsujinaka, T., M. Kishibuchi, M. Yano, T. Morimoto, C. Ebisui, J. Fujita, A. Ogawa, H. Shiozaki, E. Kominami, and M. Monden. 1997. Involvement of interleukin-6 in activation of lysosomal cathepsin and atrophy of muscle fibers induced by intramuscular injection of turpentine oil in mice. J. Biochem. 122595-600. [DOI] [PubMed] [Google Scholar]
- 54.Vasudevan, S. S., N. H. Lopes, P. N. Seshiah, T. Wang, C. B. Marsh, D. J. Kereiakes, C. Dong, and P. J. Goldschmidt-Clermont. 2003. Mac-1 and Fas activities are concurrently required for execution of smooth muscle cell death by M-CSF-stimulated macrophages. Cardiovasc. Res. 59723-733. [DOI] [PubMed] [Google Scholar]
- 55.Vetvicka, V., B. P. Thornton, and G. D. Ross. 1996. Soluble beta-glucan polysaccharide binding to the lectin site of neutrophil or natural killer cell complement receptor type 3 (CD11b/CD18) generates a primed state of the receptor capable of mediating cytotoxicity of iC3b-opsonized target cells. J. Clin. Investig. 9850-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Watts, G. M., F. J. Beurskens, I. Martin-Padura, C. M. Ballantyne, L. B. Klickstein, M. B. Brenner, and D. M. Lee. 2005. Manifestations of inflammatory arthritis are critically dependent on LFA-1. J. Immunol. 1743668-3675. [DOI] [PubMed] [Google Scholar]
- 57.Williams, M. C., J. P. Woodall, and J. D. Gillett. 1965. O'nyong-nyong fever: an epidemic virus disease in East Africa. VII. Virus isolations from man and serological studies up to July 1961. Trans. R. Soc. Trop. Med. Hyg. 59186-197. [DOI] [PubMed] [Google Scholar]
- 58.Wright, S. D., P. E. Rao, W. C. Van Voorhis, L. S. Craigmyle, K. Iida, M. A. Talle, E. F. Westberg, G. Goldstein, and S. C. Silverstein. 1983. Identification of the C3bi receptor of human monocytes and macrophages by using monoclonal antibodies. Proc. Natl. Acad. Sci. USA 805699-5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wright, S. D., J. I. Weitz, A. J. Huang, S. M. Levin, S. C. Silverstein, and J. D. Loike. 1988. Complement receptor type three (CD11b/CD18) of human polymorphonuclear leukocytes recognizes fibrinogen. Proc. Natl. Acad. Sci. USA 857734-7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xia, Y., and G. D. Ross. 1999. Generation of recombinant fragments of CD11b expressing the functional beta-glucan-binding lectin site of CR3 (CD11b/CD18). J. Immunol. 1627285-7293. [PubMed] [Google Scholar]
- 61.Xia, Y., V. Vetvicka, J. Yan, M. Hanikyrova, T. Mayadas, and G. D. Ross. 1999. The beta-glucan-binding lectin site of mouse CR3 (CD11b/CD18) and its function in generating a primed state of the receptor that mediates cytotoxic activation in response to iC3b-opsonized target cells. J. Immunol. 1622281-2290. [PubMed] [Google Scholar]
- 62.Zwadlo, G., J. Bruggen, G. Gerhards, R. Schlegel, and C. Sorg. 1988. Two calcium-binding proteins associated with specific stages of myeloid cell differentiation are expressed by subsets of macrophages in inflammatory tissues. Clin. Exp. Immunol. 72510-515. [PMC free article] [PubMed] [Google Scholar]