Abstract
Varicella-zoster virus (VZV) is a herpesvirus and is the causative agent of chicken pox (varicella) and shingles (herpes zoster). Active immunization against varicella became possible with the development of live attenuated varicella vaccine. The Oka vaccine strain was isolated in Japan from a child who had typical varicella, and it was then attenuated by serial passages in cell culture. Several manufacturers have obtained this attenuated Oka strain and, following additional passages, have developed their own vaccine strains. Notably, the vaccines Varilrix and Varivax are produced by GlaxoSmithKline Biologicals and Merck & Co., Inc., respectively. Both vaccines have been well studied in terms of safety and immunogenicity. In this study, we report the complete nucleotide sequence of the Varilrix (Oka-VGSK) and Varivax (Oka-VMerck) vaccine strain genomes. Their genomes are composed of 124,821 and 124,815 bp, respectively. Full genome annotations covering the features of Oka-derived vaccine genomes have been established for the first time. Sequence analysis indicates 36 nucleotide differences between the two vaccine strains throughout the entire genome, among which only 14 are involved in unique amino acid substitutions. These results demonstrate that, although Oka-VGSK and Oka-VMerck vaccine strains are not identical, they are very similar, which supports the clinical data showing that both vaccines are well tolerated and elicit strong immune responses against varicella.
Varicella-zoster virus (VZV) is a human alphaherpesvirus that causes chicken pox (varicella) and shingles (herpes zoster) (75). VZV has a linear, double-stranded DNA genome of approximately 125 kb that encodes at least 71 proteins (12). Primary infection with VZV results in varicella, which is a widespread, highly contagious disease. Varicella is commonly regarded as a mild childhood illness, but it may lead to serious complications, such as secondary bacterial infection, pneumonia, encephalitis, congenital infection, and death (76).
Like other herpesviruses, VZV has the capacity to persist in the body after the primary acute infection as a latent infection in sensory nerve ganglia. This lifelong latent infection commonly reactivates to cause herpes zoster, typically in elderly or immunocompromised patients (65).
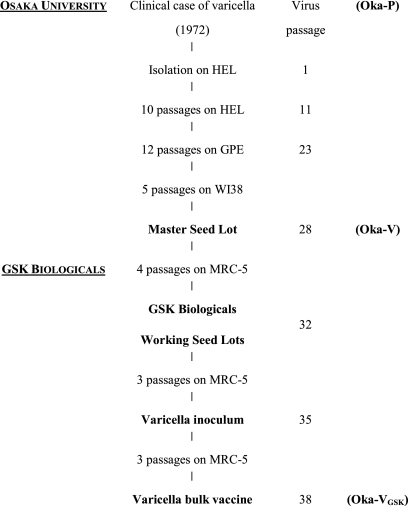
In 1974, Takahashi et al. reported the development of a live-attenuated varicella vaccine through serial passages of wild-type virus in cell culture (67). The parental virus, Oka-P, was isolated in primary human embryo lung cell culture from vesicle fluid from a 3-year-old boy with typical varicella. The virus was attenuated by 10 passages in HEL and 12 passages in guinea pig embryo cells, plaque-purified, and passaged five times further in human diploid cells (WI38) to prepare a strain suitable for use as a vaccine (Oka-V) (Fig. 1) (67).
FIG. 1.
Passage history of the live attenuated Oka varicella vaccine. HEL, human embryonic lung cells; GPE, guinea pig embryo cells; WI38 and MRC-5, human diploid cells.
The Oka-V strain was first supplied in 1976 under license from the Biken Institute in Japan. Several manufacturers (SmithKline RIT, Merck Sharp & Dohme, and Pasteur Mérieux) subsequently used the Oka-V strain in the development of proprietary vaccines. A product license was obtained for Varilrix (frozen formulation) in 1984 by SmithKline RIT for use in groups at high risk for severe varicella and their healthy close contacts. SmithKline RIT—now GlaxoSmithKline (GSK) Biologicals—subsequently developed a refrigerator-stable formulation of this varicella vaccine. Varilrix is indicated in many countries for use in healthy and immunocompromised subjects from 9 months of age. GSK Biologicals' varicella vaccine production is based on the seed lot system (6, 14) using classical cell culture methods (Fig. 1). A manufacturer's working cell bank of human diploid cells, MRC-5, was prepared and tested according to World Health Organization requirements.
The Biken vaccine was licensed in Japan and Korea, in 1986 and 1988, respectively, for use in healthy subjects, and a license for Varivax with the same indication was granted in the United States in 1995 (1). In 1993, the vaccine manufactured by Pasteur Mérieux was licensed in France for use in potentially immunocompromised subjects.
Although the varicella vaccine is licensed in many countries, it is not routinely used because complications associated with varicella disease are often underestimated. Universal mass vaccination against varicella is implemented only in few countries; however, it is under consideration in many others (38, 40, 54, 72). The incidence of varicella disease and the rate of varicella-related hospitalizations in the United States have declined by about 80% since implementation of universal mass vaccination against varicella (using Varivax) in 1996 (8, 16, 81). A similar decrease was observed in Uruguay since the introduction of varicella vaccination (using Varilrix) into the routine childhood immunization program in 1999, with the greatest reduction in children aged 1 to 4 years (51). Most pre- and postlicense studies showed that vaccination with one dose of varicella-containing vaccine provides 70% to 90% protection from chicken pox and over 95% protection against the most severe forms of the disease for a 7- to 10-year period after vaccination (2, 17, 33, 33a, 34, 40, 61). However, vaccine-induced immunity wanes over time (9), leading countries such as the United States to recommend a two-dose schedule for varicella vaccination (40). This strategy aims to overcome primary vaccine failures and to improve long-term protection, thereby reducing the risk of breakthrough varicella (4). Combined vaccine products containing the VZV Oka strain have been developed as well. For instance, GSK Biologicals and Merck & Co., Inc., developed combined tetravalent measles-mumps-rubella-varicella vaccines (Priorix-Tetra and ProQuad, respectively), providing the benefits of measles-mumps-rubella and varicella vaccination in a single injection (19, 30, 35, 48, 71, 72, 79).
Different sets of serological readouts have been used to characterize the adaptive humoral immune response after varicella vaccination or infection (4, 13, 26, 31, 34, 58, 73, 74). Comparative analysis has raised the possibility that differences in the genetic code between the vaccine strains could be responsible for disparity in vaccine-induced humoral responses (36).
Oka-V, and presumably its derivative vaccine strains, was not cloned during the development and the preparation of vaccine (67). Sequencing of the complete genome of the original Oka-V vaccine preparation revealed that it contained multiple variants that could be separated in cell culture (20, 22).
The aim of the present study was to analyze the complete consensus nucleotide sequences of Oka-V strain viruses contained in Varilrix (GSK Biologicals; Oka-VGSK) and Varivax (Merck & Co., Inc.; Oka-VMerck) and to compare them to the published sequences of Oka-V and Oka-P (22). The full-length genomic sequences were also compared to published partial sequencing information on Oka-VGSK and Oka-VMerck (3, 32, 60, 63).
MATERIALS AND METHODS
Nucleic acid extraction.
Total DNA was extracted from a single vial of recent production lots of Varilrix (lot VAV10118, produced in April 2002; GSK Biologicals, Rixensart, Belgium) and Varivax (lot 0895 M, purchased in 2003; Merck & Co., Inc., Whitehouse Station, NJ) vaccines using a High Pure viral nucleic acid kit from Roche (Basel, Switzerland). In brief, 100 μl of sample was lysed in a lysing-binding buffer in the presence of proteinase K. The lysis mixture was then applied to a glass fiber filter, which binds the nucleic acids in the presence of the lysis and binding buffer containing chaotropic salts. Bound nucleic acids were eluted in 50 μl of nuclease-free water by centrifugation and stored at −70°C.
PCR.
Around 540 primers were designed using Primer D software (GSK in-house software) and the nucleotide sequence of the Dumas strain (GenBank accession no. X04370) (12). Overlapping primers were designed approximately 500 bases apart to cover the entire genomic sequence of VZV. Sequences of primers used for amplification and sequencing are available upon request. The reaction mixtures for PCR contained 15 μl of HotStarTaq Plus Master Mix solution (Qiagen, Valencia, CA), 0.3 μM of each primer, and 5 ng of template DNA. A Tetrad thermal cycler (MJ Research, Waltham, MA) was used for all amplifications. An initial hot-start PCR step of 96°C for 15 min was followed by 35 cycles of amplification (95°C for 20 s, 55°C for 30 s, and 72°C for 45 s) and a final elongation step at 72°C for 3 min. All amplified products were then purified using QIAquick PCR purification kit (Qiagen). Direct sequencing of both DNA strands was performed on the generated amplicons.
Sequencing.
Direct sequencing of purified PCR products and plasmid DNA was performed with BigDye Terminator cycle sequencing kit and a 3730XL genetic analyzer (both from Applied Biosystems, Foster City, CA). The viral sequences were compiled and analyzed with Sequencher software (Gene Codes Corp, Ann Arbor, MI). The following GenBank sequences were used for comparison: for the European (The Netherlands) reference strain (Dumas), X04370 (12); for Oka-P, AB097933 (22); and for Oka-V, AB097932 (22), AF206304 (3), AY016450 (15), and the sequencing information provided by Schmidt et al. (60). Unless otherwise stated, all described nucleotide sequence positions in this paper correspond to the genome of Dumas strain, X04370 (12).
Cloning of PCR products.
When direct sequencing did not generate information of sufficient quality or when particular single nucleotide polymorphisms (SNPs) could not be reliably confirmed, additional subcloning was performed, followed by sequencing of numerous generated clones to confirm the consensus sequence of the region. Direct sequencing of the PCR products derived from regions with highly complex secondary structure (flanking regions between internal repeat long and internal repeat short regions, and the R3 repeat region) was complemented by subcloning of amplicons and sequencing of plasmid clones. PCR products containing these regions were individually inserted into a pCR2.1 vector (Invitrogen, Carlsbad, CA) and then transformed into competent Escherichia coli by the TOPO TA cloning method (Invitrogen). The plasmid DNAs were purified from cultured bacteria with a QIAprep spin kit (Qiagen). DNA sequences of the cloned inserts were determined using vector-specific sequencing primers.
Sequencing of ends of the viral genomes.
The direct sequencing data for viral genome ends were complemented by sequencing of overlapping amplicons generated after circularization using T4 DNA ligase (Roche). The PCR mixtures contained 500 μM of each deoxynucleoside triphosphate, 10 pmol of each primer, and 2.5 U high-fidelity Platinum Taq polymerase (Invitrogen). PCR products were inserted into a pCR4-TOPO vector and transformed into competent E. coli TOP10 bacteria by the TOPO TA cloning method (Invitrogen). The plasmid DNAs were purified with a QIAprep spin kit (Qiagen). The consensus sequence of the cloned amplicons was confirmed by sequencing and alignment of multiple E. coli plasmid clones.
Nucleotide sequence accession numbers.
The complete nucleotide consensus sequences of the Oka-VGSK (Varilrix) and Oka-VMerck (Varivax) strains are available in GenBank under the accession numbers DQ008354 and DQ008355, respectively.
RESULTS
Oka-VGSK and Oka-VMerck genome organization.
The full-length consensus sequence of Oka-VGSK and Oka-VMerck vaccine strains was essentially determined by bidirectional sequencing of overlapping PCR-amplified fragments. Occasionally, when the amplified region contained SNPs that could not be conclusively resolved, the amplified fragments were subcloned and a consensus sequence was derived from multiple plasmid clones. The obtained sequences were assembled and the complete genomes of the vaccines were annotated using the VZV sequence of the Dumas strain published by Davison and Scott as a template (12). The full annotations for Oka-VGSK and Oka-VMerck are presented in Tables 1 and 2, respectively.
TABLE 1.
Complete Oka-VGSK genome annotation
| Start | Stop | Featurea | ORF | Function or comment |
|---|---|---|---|---|
| 88 | 89 | Miscellaneous | TRL/UL boundary | |
| 914 | 587 | Gene | 1 | |
| 592 | 587 | Poly(A) signal | ||
| 914 | 588 | CDS | 1 | |
| 1133 | 1861 | Gene | 2 | |
| 1133 | 1849 | CDS | 2 | |
| 1856 | 1861 | Poly(A) signal | ||
| 2446 | 1889 | Gene | 3 | |
| 1894 | 1889 | Poly(A) signal | ||
| 2446 | 1907 | CDS | 3 | |
| 4140 | 2781 | Gene | 4 | |
| 2781 | 2776 | Poly(A) signal | ||
| 4140 | 2782 | CDS | 4 | Transactivator, tegument protein |
| 5273 | 4251 | Gene | 5 | |
| 5273 | 4251 | CDS | 5 | gK |
| 8576 | 5325 | Gene | 6 | |
| 8606 | 9398 | Gene | 7 | |
| 9393 | 9398 | Poly(A) signal | ||
| 8606 | 9385 | CDS | 7 | |
| 10666 | 9425 | Gene | 8 | |
| 9430 | 9425 | Poly(A) signal | ||
| 10666 | 9476 | CDS | 8 | Deoxyuridine triphosphatase |
| 10641 | 10904 | CDS | 9Ab | gN |
| 11008 | 11963 | Gene | 9 | |
| 11958 | 11963 | Poly(A) signal | ||
| 11008 | 11916 | CDS | 9 | Syncytium formation, virion protein |
| 12159 | 13420 | Gene | 10 | |
| 13415 | 13420 | Poly(A) signal | ||
| 12159 | 13391 | CDS | 10 | Transactivator, tegument protein |
| 13589 | 16076 | Gene | 11 | |
| 13936 | 14196 | Repeat region | Reiteration R1 | |
| 16071 | 16076 | Poly(A) signal | ||
| 13589 | 16003 | CDS | 11 | |
| 16168 | 18153 | Gene | 12 | |
| 18695 | 19350 | Gene | 13 | |
| 19345 | 19350 | Poly(A) signal | ||
| 18395 | 19300 | CDS | 13 | |
| 21067 | 19296 | Gene | 14 | |
| 19301 | 19296 | Poly(A) signal | ||
| 20526 | 20851 | Repeat region | Reiteration R2 | |
| 21067 | 19385 | CDS | 14 | |
| 22432 | 21198 | Gene | 15 | |
| 21203 | 21198 | Poly(A) signal | ||
| 22432 | 21212 | CDS | 15 | |
| 23748 | 22522 | Gene | 16 | |
| 24103 | 25468 | Gene | 17 | |
| 25463 | 25468 | Poly(A) signal | ||
| 24103 | 25467 | CDS | 17 | |
| 26444 | 25501 | Gene | 18 | |
| 25506 | 25501 | Poly(A) signal | ||
| 26444 | 25524 | CDS | 18 | Ribonucleotide reductase, small subunit |
| 28796 | 26469 | Gene | 19 | Ribonucleotide reductase, big subunit |
| 30426 | 28956 | Gene | 20 | |
| 28961 | 28956 | Poly(A) signal | ||
| 30426 | 28975 | CDS | 20 | |
| 30710 | 33856 | Gene | 21 | |
| 33851 | 33856 | Poly(A) signal | ||
| 30710 | 33826 | CDS | 21 | Nucleocapsid |
| 34034 | 42341 | Gene | 22 | |
| 41405 | 41470 | Repeat region | Reiteration R3 | |
| 42336 | 42341 | Poly(A) signal | ||
| 34034 | 42325 | CDS | 22 | |
| 43090 | 42378 | Gene | 23 | |
| 42383 | 42378 | Poly(A) signal | ||
| 43090 | 42383 | CDS | 23 | |
| 43973 | 43163 | Gene | 24 | |
| 43168 | 43163 | Poly(A) signal | ||
| 43973 | 43164 | CDS | 24 | |
| 44570 | 44083 | Gene | 25 | |
| 44088 | 44083 | Poly(A) signal | ||
| 44570 | 44100 | CDS | 25 | |
| 44458 | 46125 | Gene | 26 | |
| 46079 | 47195 | Gene | 27 | |
| 47190 | 47195 | Poly(A) signal | ||
| 46079 | 47080 | CDS | 27 | |
| 50588 | 46983 | Gene | 28 | |
| 46988 | 46983 | Poly(A) signal | ||
| 50588 | 47004 | CDS | 28 | DNA polymerase |
| 50809 | 54460 | Gene | 29 | |
| 54455 | 54460 | Poly(A) signal | ||
| 50809 | 54408 | CDS | 29 | ssDNA binding protein |
| 54587 | 56899 | Gene | 30 | |
| 56944 | 59584 | Gene | 31 | |
| 59579 | 59584 | Poly(A) signal | ||
| 56944 | 59550 | CDS | 31 | gB, fusogen |
| 59703 | 60150 | Gene | 32 | |
| 60145 | 60150 | Poly(A) signal | ||
| 59703 | 60134 | CDS | 32 | Substrate for ORF 47 kinase |
| 62074 | 60245 | Gene | 33 | |
| 60250 | 60245 | Poly(A) signal | ||
| 62074 | 60257 | CDS | 33 | Protease |
| 63846 | 62107 | Gene | 34 | |
| 64689 | 63913 | CDS | 35 | |
| 64300 | 64306 | promoter | TATA element | |
| 64321 | 64325 | 5′end of dPyKmRNA | ||
| 64743 | 65800 | Gene | 36 | |
| 65795 | 65800 | Poly(A) signal | ||
| 64743 | 65768 | CDS | 36 | Thymidine kinase |
| 65817 | 65821 | 3′end of dPyKmRNA | ||
| 66010 | 68552 | Gene | 37 | |
| 68747 | 68552 | Poly(A) signal | ||
| 66010 | 68535 | CDS | 37 | gH |
| 70229 | 68583 | Gene | 38 | |
| 68588 | 68583 | Poly(A) signal | ||
| 70229 | 68604 | CDS | 38 | |
| 70569 | 71305 | Gene | 39 | |
| 71300 | 71305 | Poly(A) signal | ||
| 70569 | 71291 | CDS | 39 | |
| 71476 | 75699 | Gene | 40 | |
| 75694 | 75699 | Poly(A) signal | ||
| 71476 | 75666 | CDS | 40 | Major nucleocapsid protein |
| 75783 | 76748 | Gene | 41 | |
| 76743 | 76748 | Poly(A) signal | ||
| 75783 | 76733 | CDS | 41 | |
| 77974 | 76791 | Gene | 42 | |
| 76786 | 76791 | Poly(A) signal | ORF 45+ORF 42 | |
| 77974 | 76787 | CDS | 42 | |
| 78105 | 80136 | Gene | 43 | |
| 80131 | 80136 | Poly(A) signal | ||
| 78105 | 80135 | CDS | 43 | |
| 80295 | 81449 | Gene | 44 | |
| 81444 | 81449 | Poly(A) signal | ||
| 80295 | 81386 | CDS | 44 | |
| 82529 | 81474 | CDS | 45 | |
| 82654 | 83253 | CDS | 46 | |
| 83103 | 84635 | CDS | 47 | Protein kinase, tegument protein |
| 84602 | 86257 | CDS | 48 | |
| 86161 | 86429 | Gene | 49 | |
| 86424 | 86429 | Poly(A) signal | ||
| 86161 | 86406 | CDS | 49 | |
| 87807 | 86466 | Gene | 50 | |
| 86471 | 86466 | Poly(A) signal | ||
| 87807 | 86500 | CDS | 50 | |
| 87806 | 90313 | CDS | 51 | Origin binding protein |
| 90418 | 92771 | Gene | 52 | |
| 92766 | 92771 | Poly(A) signal | ||
| 90418 | 92733 | CDS | 52 | |
| 93775 | 92775 | Gene | 53 | |
| 92780 | 92775 | Poly(A) signal | ||
| 93775 | 92780 | CDS | 53 | |
| 95909 | 93600 | CDS | 54 | |
| 95921 | 98566 | CDS | 55 | |
| 98493 | 99280 | Gene | 56 | |
| 99275 | 99280 | Poly(A) signal | ||
| 98493 | 99224 | CDS | 56 | |
| 99548 | 99309 | Gene | 57 | |
| 99314 | 99309 | Poly(A) signal | ||
| 99548 | 99333 | CDS | 57 | Cytoplasmic protein |
| 100194 | 99529 | CDS | 58 | |
| 101141 | 100224 | CDS | 59 | Uracil-DNA glycosylase |
| 101574 | 101092 | CDS | 60 | gL, chaperone for gH |
| 104410 | 102926 | Gene | 61 | |
| 102931 | 102926 | Poly(A) signal | ||
| 104410 | 103007 | CDS | 61 | Transactivator, transrepressor |
| 104849 | 104850 | Miscellaneous | UL/IRL boundary | |
| 104938 | 104939 | Miscellaneous | IRL/IRS boundary | |
| 109061 | 105065 | Gene | 62 | |
| 105071 | 105065 | Poly(A) signal | ||
| 109061 | 105129 | CDS | 62 | Transactivator, tegument protein |
| 109693 | 109718 | Repeat region | Reiteration 4 | |
| 110017 | 110278 | Origin of replication | Origin of replication | |
| 110507 | 111359 | Gene | 63 | |
| 111352 | 111357 | Poly(A) signal | ||
| 110507 | 111343 | CDS | 63 | Tegument protein |
| 111491 | 112072 | Gene | 64 | |
| 112067 | 112072 | Poly(A) signal | ||
| 111491 | 112033 | CDS | 64 | |
| 112571 | 112107 | Gene | 65 | |
| 112112 | 112107 | Poly(A) signal | ||
| 112571 | 112263 | CDS | 65 | Virion protein |
| 112263 | 112264 | Miscellaneous | IRS/US boundary | |
| 112968 | 114172 | Gene | 66 | |
| 114167 | 114172 | Poly(A) signal | ||
| 112968 | 114149 | CDS | 66 | Protein kinase |
| 114427 | 115523 | Gene | 67 | |
| 115518 | 115523 | Poly(A) signal | ||
| 114427 | 115491 | CDS | 67 | gI |
| 115739 | 117652 | Gene | 68 | |
| 117647 | 117652 | Poly(A) signal | ||
| 115739 | 117610 | CDS | 68 | gE |
| 117498 | 117499 | Miscellaneous | US/TRS boundary | |
| 118266 | 117690 | Gene | 69 | |
| 117495 | 117490 | Poly(A) signal | ||
| 118266 | 117724 | CDS | 69 | |
| 119250 | 118400 | Gene | 70 | |
| 118405 | 118400 | Poly(A) signal | ||
| 119250 | 118414 | CDS | 70 | Tegument protein |
| 119479 | 119742 | Origin of replication | Origin of replication | |
| 119921 | 120066 | Repeat region | Reiteration R4 | |
| 120698 | 124694 | Gene | 71 | |
| 124689 | 124694 | Poly(A) signal | ||
| 120698 | 124630 | CDS | 71 | Transactivator, tegument protein |
CDS, coding sequence; dPyKmRNA, deoxypyrimidine kinase mRNA.
ORF was annotated according to the work of Gomi et al. (20)
TABLE 2.
Complete Oka-VMerck genome annotation
| Start | Stop | Featurea | ORF | Function or comment |
|---|---|---|---|---|
| 88 | 89 | Miscellaneous | TRL/UL boundary | |
| 914 | 587 | Gene | 1 | |
| 592 | 587 | Poly(A) signal | ||
| 914 | 588 | CDS | 1 | |
| 1133 | 1861 | Gene | 2 | |
| 1133 | 1849 | CDS | 2 | |
| 1856 | 1861 | Poly(A) signal | ||
| 2446 | 1889 | Gene | 3 | |
| 1894 | 1889 | Poly(A) signal | ||
| 2446 | 1907 | CDS | 3 | |
| 4140 | 2781 | Gene | 4 | |
| 2781 | 2776 | Poly(A) signal | ||
| 4140 | 2782 | CDS | 4 | Transactivator, tegument protein |
| 5273 | 4251 | Gene | 5 | |
| 5273 | 4251 | CDS | 5 | gK |
| 8576 | 5325 | Gene | 6 | |
| 8606 | 9398 | Gene | 7 | |
| 9393 | 9398 | Poly(A) signal | ||
| 8606 | 9385 | CDS | 7 | |
| 10666 | 9425 | Gene | 8 | |
| 9430 | 9425 | Poly(A) signal | ||
| 10666 | 9476 | CDS | 8 | Deoxyuridine triphosphatase |
| 10641 | 10904 | CDS | 9Ab | gN |
| 11008 | 11963 | Gene | 9 | |
| 11958 | 11963 | Poly(A) signal | ||
| 11008 | 11916 | CDS | 9 | Syncytium formation, virion protein |
| 12159 | 13420 | Gene | 10 | |
| 13415 | 13420 | Poly(A) signal | ||
| 12159 | 13391 | CDS | 10 | Transactivator, tegument protein |
| 13589 | 16076 | Gene | 11 | |
| 13936 | 14196 | Repeat region | Reiteration R1 | |
| 16071 | 16076 | Poly(A) signal | ||
| 13589 | 16003 | CDS | 11 | |
| 16168 | 18153 | Gene | 12 | |
| 18695 | 19350 | Gene | 13 | |
| 19345 | 19350 | Poly(A) signal | ||
| 18395 | 19300 | CDS | 13 | |
| 21067 | 19296 | Gene | 14 | |
| 19301 | 19296 | Poly(A) signal | ||
| 20526 | 20851 | Repeat region | Reiteration R2 | |
| 21067 | 19385 | CDS | 14 | |
| 22432 | 21198 | Gene | 15 | |
| 21203 | 21198 | Poly(A) signal | ||
| 22432 | 21212 | CDS | 15 | |
| 23748 | 22522 | Gene | 16 | |
| 24103 | 25468 | Gene | 17 | |
| 25463 | 25468 | Poly(A) signal | ||
| 24103 | 25467 | CDS | 17 | |
| 26444 | 25501 | Gene | 18 | |
| 25506 | 25501 | Poly(A) signal | ||
| 26444 | 25524 | CDS | 18 | Ribonucleotide reductase, small subunit |
| 28796 | 26469 | Gene | 19 | Ribonucleotide reductase, big subunit |
| 30426 | 28956 | Gene | 20 | |
| 28961 | 28956 | Poly(A) signal | ||
| 30426 | 28975 | CDS | 20 | |
| 30710 | 33856 | Gene | 21 | |
| 33851 | 33856 | Poly(A) signal | ||
| 30710 | 33826 | CDS | 21 | Nucleocapsid |
| 34034 | 42341 | Gene | 22 | |
| 41405 | 41470 | Repeat region | Reiteration R3 | |
| 42336 | 42341 | Poly(A) signal | ||
| 34034 | 42325 | CDS | 22 | |
| 43088 | 42376 | Gene | 23 | |
| 42381 | 42376 | Poly(A) signal | ||
| 43088 | 42381 | CDS | 23 | |
| 43971 | 43161 | Gene | 24 | |
| 43166 | 43161 | Poly(A) signal | ||
| 43971 | 43162 | CDS | 24 | |
| 44568 | 44081 | Gene | 25 | |
| 44086 | 44081 | Poly(A) signal | ||
| 44568 | 44098 | CDS | 25 | |
| 44456 | 46123 | Gene | 26 | |
| 46077 | 47193 | Gene | 27 | |
| 47188 | 47193 | Poly(A) signal | ||
| 46077 | 47078 | CDS | 27 | |
| 50586 | 46981 | Gene | 28 | |
| 46986 | 46981 | Poly(A) signal | ||
| 50586 | 47002 | CDS | 28 | DNA polymerase |
| 50807 | 54458 | Gene | 29 | |
| 54453 | 54458 | Poly(A) signal | ||
| 50807 | 54406 | CDS | 29 | Single-stranded-DNA binding protein |
| 54585 | 56897 | Gene | 30 | |
| 56942 | 59582 | Gene | 31 | |
| 59577 | 59582 | Poly(A) signal | ||
| 56942 | 59548 | CDS | 31 | gB, fusogen |
| 59701 | 60148 | Gene | 32 | |
| 60143 | 60148 | Poly(A) signal | ||
| 59701 | 60132 | CDS | 32 | Substrate for ORF 47 kinase |
| 62071 | 60242 | Gene | 33 | |
| 60247 | 60242 | Poly(A) signal | ||
| 62071 | 60254 | CDS | 33 | Protease |
| 63843 | 62104 | Gene | 34 | |
| 64686 | 63910 | CDS | 35 | |
| 64297 | 64303 | Promoter | TATA element | |
| 64321 | 64325 | 5′ end of dPyKmRNA | ||
| 64740 | 65797 | Gene | 36 | |
| 65793 | 65797 | Poly(A) signal | ||
| 64741 | 65765 | CDS | 36 | Thymidine kinase |
| 65814 | 65818 | 3′end of dPyKmRNA | ||
| 66007 | 68549 | Gene | 37 | |
| 68744 | 68549 | Poly(A) signal | ||
| 66007 | 68532 | CDS | 37 | gH |
| 70226 | 68580 | Gene | 38 | |
| 68585 | 68580 | Poly(A) signal | ||
| 70226 | 68601 | CDS | 38 | |
| 70566 | 71302 | Gene | 39 | |
| 71297 | 71302 | Poly(A) signal | ||
| 70566 | 71288 | CDS | 39 | |
| 71473 | 75696 | Gene | 40 | |
| 75691 | 75696 | Poly(A) signal | ||
| 71473 | 75663 | CDS | 40 | Major nucleocapsid protein |
| 75780 | 76745 | Gene | 41 | |
| 76740 | 76745 | Poly(A) signal | ||
| 75780 | 76730 | CDS | 41 | |
| 77971 | 76788 | Gene | 42 | |
| 76783 | 76788 | Poly(A) signal | ORF 45+ORF 42 | |
| 77971 | 76784 | CDS | 42 | |
| 78102 | 80133 | Gene | 43 | |
| 80128 | 80133 | Poly(A) signal | ||
| 78102 | 80132 | CDS | 43 | |
| 80292 | 81446 | Gene | 44 | |
| 81441 | 81446 | Poly(A) signal | ||
| 80292 | 81383 | CDS | 44 | |
| 82526 | 81471 | CDS | 45 | |
| 82651 | 83250 | CDS | 46 | |
| 83100 | 84632 | CDS | 47 | Protein kinase, tegument protein |
| 84599 | 86254 | CDS | 48 | |
| 86158 | 86426 | Gene | 49 | |
| 86421 | 86426 | Poly(A) signal | ||
| 86158 | 86403 | CDS | 49 | |
| 87804 | 86463 | Gene | 50 | |
| 86468 | 86463 | Poly(A) signal | ||
| 87804 | 86497 | CDS | 50 | |
| 87803 | 90310 | CDS | 51 | Origin binding protein |
| 90415 | 92768 | Gene | 52 | |
| 92763 | 92768 | Poly(A) signal | ||
| 90415 | 92730 | CDS | 52 | |
| 93772 | 92772 | Gene | 53 | |
| 92777 | 92772 | Poly(A) signal | ||
| 93772 | 92777 | CDS | 53 | |
| 95906 | 93597 | CDS | 54 | |
| 95918 | 98563 | CDS | 55 | |
| 98490 | 99277 | Gene | 56 | |
| 99272 | 99277 | Poly(A) signal | ||
| 98490 | 99221 | CDS | 56 | |
| 99545 | 99306 | Gene | 57 | |
| 99311 | 99306 | Poly(A) signal | ||
| 99545 | 99330 | CDS | 57 | Cytoplasmic protein |
| 100191 | 99526 | CDS | 58 | |
| 101138 | 100221 | CDS | 59 | Uracil-DNA glycosylase |
| 101571 | 101089 | CDS | 60 | gL, chaperone for gH |
| 104407 | 102923 | Gene | 61 | |
| 102928 | 102923 | Poly(A) signal | ||
| 104407 | 103004 | CDS | 61 | Transactivator, transrepressor |
| 104846 | 104847 | Miscellaneous | UL/IRL boundary | |
| 104935 | 104936 | Miscellaneous | IRL/IRS boundary | |
| 109058 | 105062 | Gene | 62 | |
| 105068 | 105062 | Poly(A) signal | ||
| 109058 | 105126 | CDS | 62 | Transactivator, tegument protein |
| 109690 | 109715 | Repeat region | Reiteration 4 | |
| 110014 | 110277 | Origin of replication | Origin of replication | |
| 110506 | 111356 | Gene | 63 | |
| 111351 | 111356 | Poly(A) signal | ||
| 110506 | 111342 | CDS | 63 | Tegument protein |
| 111490 | 112067 | Gene | 64 | |
| 112062 | 112067 | Poly(A) signal | ||
| 111490 | 112032 | CDS | 64 | |
| 112566 | 112102 | Gene | 65 | |
| 112107 | 112102 | Poly(A) signal | ||
| 112566 | 112258 | CDS | 65 | Virion protein |
| 112258 | 112259 | Miscellaneous | IRS/US boundary | |
| 112963 | 114167 | Gene | 66 | |
| 114162 | 114167 | Poly(A) signal | ||
| 112963 | 114144 | CDS | 66 | Protein kinase |
| 114422 | 115518 | Gene | 67 | |
| 115513 | 115518 | Poly(A) signal | ||
| 114422 | 115486 | CDS | 67 | gI |
| 115734 | 117647 | Gene | 68 | |
| 117642 | 117647 | Poly(A) signal | ||
| 115734 | 117605 | CDS | 68 | gE |
| 117490 | 117491 | Miscellaneous | US/TRS boundary | |
| 118260 | 117682 | Gene | 69 | |
| 117687 | 117682 | Poly(A) signal | ||
| 118260 | 117718 | CDS | 69 | |
| 119244 | 118394 | Gene | 70 | |
| 118399 | 118394 | Poly(A) signal | ||
| 119244 | 118408 | CDS | 70 | Tegument protein |
| 119473 | 119736 | Origin of replication | Origin of replication | |
| 119915 | 120060 | Repeat region | Reiteration R4 | |
| 120692 | 124688 | Gene | 71 | |
| 124683 | 124688 | Poly(A) signal | ||
| 120692 | 124624 | CDS | 71 | Transactivator, tegument protein |
CDS, coding sequence; dPyKmRNA, deoxypyrimidine kinase mRNA.
ORF was annotated according to the work of Gomi et al. (20).
The complete genomes of Oka-VGSK and Oka-VMerck strains are comprised of 124,821 and 124,815 bp, respectively. Like the wild-type Dumas strain and the parental Japanese Oka-V strain, the Oka-VGSK and Oka-VMerck genomes consist of a unique long region flanked by terminal repeat long and internal repeat long inverted repeat regions, as well as a unique short region flanked by internal repeat short (IRS) and terminal repeat short (TRS) inverted repeat regions. An origin of replication was found in both the IRS and TRS regions. Four unique reiteration regions (R1 to R4) were found along the genome, with R4 duplicated in the IRS and TRS regions.
All the open reading frames (ORFs) described for the Dumas VZV strain (12) and the Oka vaccine parental strain (22) were found in the two Oka-derived vaccine strains (Tables 1 and 2). The 72 ORFs predicted to encode proteins were evenly distributed on both DNA strands. Three genes were located within the repeat sequences and were therefore duplicated within the VZV genome, so that ORFs 69 to 71 in the IRS region correspond to ORFs 62 to 64 in the TRS region.
Comparison of Oka strain genomes to the Dumas strain genome.
The obtained sequences of Oka-VGSK and Oka-VMerck were aligned with the full-length VZV genomes of Oka-P, Oka-V, and Dumas strains. All sequence differences between the four Oka strains and the Dumas strain are given in Table 3. A total of 326 nucleotide positions displaying differences relative to the genome of Dumas strain (X04370 [12]) were identified. Among these, 228 were common to the four Oka strains, and the remaining 98 were specific to one, two, or three of the Oka strains. Several deletions or insertions were found, but most mutations were substitutions of one nucleotide, i.e., SNPs. Frequently, the original nucleotide was nonetheless preserved, resulting in a mixture of two nucleotides present at the same position (Table 3). Because, to our knowledge, the vaccine strains were never cloned, this is consistent with the existence of multiple viral species that evolved during the attenuation process. Multiple SNPs were found to still contain the original Oka-P-specific nucleotide. This supports the cooperative effect of the overall pattern of nucleotide substitutions in the expression of the attenuation phenotype and, to a lesser extent, the contribution of individual SNPs.
TABLE 3.
Comparison of complete genomic sequences of Dumas and Oka strains of VZVa
| Feature relative to WT (Dumas) | Position (WT) | Feature in:
|
Position in:
|
||||
|---|---|---|---|---|---|---|---|
| Oka-P | Oka-V | Oka-VGSK | Oka-VMerck | Oka-VGSK | Oka-VMerck | ||
| A→G | 1 | X | X | X | X | 1 | 1 |
| G→C | 3 | X | X | X | X | 3 | 3 |
| Deletion of C (from WT) | 109 | X | X | X | X | 109 | 109 |
| G→C | 178 | - | - | X | X | 177 | 177 |
| A→G | 236 | X | X | X | X | 235 | 235 |
| C→T | 262 | X | X | X | X | 261 | 261 |
| T→C | 560 | - | X | X | X | 559 | 559 |
| G→A (ORF 1), N, silent | 685 | X | X | X | X | 684 | 684 |
| T→T/C (ORF 1), Q, silent | 703 | - | X | C | C | 702 | 702 |
| T→T/C (ORF 1), P, silent | 763 | - | X | C | C | 762 | 762 |
| T→C (ORF 1), T→A | 789 | X | X | X | X | 788 | 788 |
| T→C (ORF 1), Q→R | 790 | X | X | X | X | 789 | 789 |
| T→C (ORF 1), Q→R | 791 | X | X | X | X | 790 | 790 |
| C→G (ORF 2), G, silent | 1838 | - | X | - | - | 1837 | 1837 |
| T→T/C | 2515 | - | X | C | - | 2514 | 2514 |
| A→G (ORF 4), T, silent | 3764 | X | X | X | X | 3763 | 3763 |
| C→T (ORF 5), K, silent | 4258 | X | X | X | X | 4257 | 4257 |
| A→G (ORF 6), S→P | 5745 | - | X | X | A/G | 5744 | 5744 |
| G→T (ORF 6), H→Q | 6853 | X | X | X | X | 6852 | 6852 |
| C→A (ORF 6), G→V | 7091 | X | X | X | X | 7090 | 7090 |
| C→T (ORF 6), P, silent | 7753 | X | X | X | X | 7752 | 7752 |
| T→C | 9460 | X | X | X | X | 9459 | 9459 |
| G→A (ORF 8), P→S | 10079 | X | X | X | X | 10078 | 10078 |
| T→C/T (ORF 9A), W→R | 10900 | - | X | X | - | 10899 | 10899 |
| T→G (ORF 9), S, silent | 11890 | X | X | X | X | 11889 | 11889 |
| A→G (ORF 9), T→A | 11906 | X | X | X | X | 11905 | 11905 |
| C→A (ORF 10), P→H | 12188 | X | X | X | X | 12187 | 12187 |
| T→C (ORF 10), F→S | 12284 | X | X | X | X | 12283 | 12283 |
| T→C (ORF 10), F→S | 12285 | X | X | X | X | 12284 | 12284 |
| C→C/T (ORF 10), A→V | 12779 | - | X | X | - | 12778 | 12778 |
| T→G (ORF 10), G, silent | 13173 | X | X | X | X | 13172 | 13172 |
| G→A | 13407 | X | X | X | X | 13406 | 13406 |
| Deletion (ORF 11, R1), ATTGACGACGAGGGAGAGGCGGAGGAGGGAGAGGCGGAGGAGGGAGAGGCGGAGGAGGGAGAG, IDDEGEAEEGEAEEGEAEEGE | 14088 | X | X | X | X | 14086 | 14086 |
| Deletion (ORF 11, R1), GCGGAGGAGGACGCG, AEEDA | 14199-213 | X | X | X | X | 14134-48 | 14134-48 |
| Insertion (ORF 11, R1), CGCGATCGACGACGAGGGAGAGGCGGAGGAGGA | 14242 | X | - | X | X | 14164-96 | 14164-96 |
| T→C | 14390 | X | X | X | X | 14344 | 14344 |
| C→T (ORF 12), V, silent | 17404 | X | X | X | X | 17358 | 17358 |
| C→T (ORF 12), L, silent | 17834 | X | X | X | X | 17788 | 17788 |
| C→T (ORF 12), T, silent | 18082 | X | X | X | X | 18036 | 18036 |
| G→A (ORF 13), K, silent | 18467 | X | X | X | X | 18421 | 18421 |
| T→T/C (ORF 14), stop | 19431 | - | X | - | - | 19385 | 19385 |
| A→G (ORF 14), I, silent | 19719 | X | X | X | X | 19673 | 19673 |
| T→A (ORF 14), Y→F | 20656 | X | X | X | X | 20610 | 20610 |
| T→C (ORF 14), T→A | 20684 | X | X | X | X | 20638 | 20638 |
| C→C/T (ORF 14), K, silent | 20703 | - | - | X | - | 20657 | 20657 |
| A→T (ORF 14), E→V | 20711 | X | X | - | - | 20665 | 20665 |
| C→A (ORF 14), C→A | 20745 | X | X | - | - | 20699 | 20699 |
| T→A (ORF 14), T→S | 20753 | X | X | X | X | 20707 | 20707 |
| C→A (ORF 14), K→N | 20787 | X | X | C/A | - | 20741 | 20741 |
| C→A (ORF 14), K→N | 20829 | X | X | C/A | C/A | 20783 | 20783 |
| T→A/T (ORF 14), T→S | 20837 | - | - | X | X | 20791 | 20791 |
| C→A (ORF 14), K→N | 20871 | - | - | C/A | C/A | 20825 | 20825 |
| A→T (ORF 14), S→T | 20879 | - | - | A/T | 20833 | 20833 | |
| C→A (ORF 14), K→N | 20913 | - | - | A/C | A/C | 20867 | 20867 |
| T→A (ORF 14), T→S | 21005 | X | X | X | X | 20959 | 20959 |
| G→A (ORF 15), L, silent | 21371 | X | X | X | X | 21325 | 21325 |
| G→T (ORF 15), R, silent | 21734 | X | X | X | X | 21688 | 21688 |
| G→A (ORF 15), S, silent | 22311 | X | X | X | X | 22265 | 22265 |
| A→G | 22504 | X | X | X | X | 22458 | 22458 |
| A→G (ORF 16), M→T | 22794 | X | X | X | X | 22748 | 22748 |
| A→G (ORF 16), F, silent | 23294 | X | X | X | X | 23248 | 23248 |
| Deletion (ORF 17), dCAT (delS) | 24516 | X | X | X | X | 24469 | 24469 |
| A→G (ORF 17), T→A | 24578 | X | X | X | X | 24529 | 24529 |
| C→T (ORF 17), T→M | 24654 | X | X | X | X | 24605 | 24605 |
| G→A (ORF 17), V→I | 25067 | X | X | X | X | 25018 | 25018 |
| A→G (ORF 18), N, silent | 26125 | - | X | X | A/G | 26076 | 26076 |
| A→G (ORF 19), H, silent | 27523 | X | X | X | X | 27474 | 27474 |
| T→G (ORF 20), G, silent | 29201 | X | X | X | X | 29152 | 29152 |
| C→T/C (ORF 21), T→I | 31732 | - | X | - | - | 31683 | 31683 |
| A→G (ORF 21), T→A | 32274 | X | X | X | X | 32225 | 32225 |
| T→C (ORF 21), H, silent | 33722 | X | X | X | X | 33673 | 33673 |
| T→C (ORF 21), D, silent | 33725 | X | X | X | X | 33676 | 33676 |
| T→C (ORF 21), N, silent | 33728 | X | X | X | X | 33679 | 33679 |
| T→C (ORF 22), V, silent | 35543 | X | X | X | X | 35494 | 35494 |
| A→G (ORF 22), L, silent | 37649 | X | X | X | X | 37600 | 37600 |
| A→G (ORF 22), I→V | 37902 | X | X | X | X | 37853 | 37853 |
| T→C (ORF 22), T, silent | 38036 | - | - | C/T | C/T | 37987 | 37987 |
| T→C (ORF 22), Y→H | 38055 | X | X | X | X | 38006 | 38006 |
| A→C (ORF 22), P, silent | 38081 | X | X | X | X | 38032 | 38032 |
| G→A (ORF 22), E, silent | 38177 | X | X | X | X | 38128 | 38128 |
| G→T (ORF 22), T, silent | 38714 | X | X | X | X | 38665 | 38665 |
| C→T (ORF 22), A, silent | 38717 | X | X | X | X | 38668 | 38668 |
| A→G (ORF 22), R, silent | 39023 | X | X | X | X | 38974 | 38974 |
| T→T/G (ORF 22), P, silent | 39227 | - | X | X | - | 39178 | 39178 |
| G→A (ORF 22), Q, silent | 39263 | X | X | X | X | 39214 | 39214 |
| G→A (ORF 22), R→H | 39394 | X | X | X | X | 39345 | 39345 |
| A→G (ORF 22), V, silent | 39530 | X | X | X | X | 39481 | 39481 |
| A→G (ORF 22), Q, silent | 40388 | X | X | X | X | 40339 | 40339 |
| T→C (ORF 22), P, silent | 41057 | X | X | X | X | 41008 | 41008 |
| G→A | 41452 | X | X | X | X | 41403 | 41403 |
| C→T (R3 repeat), A→V | 41458 | X | X | X | X | 41409 | 41409 |
| G→C (R3 repeat), A→V | 41459 | X | X | X | X | 41410 | 41410 |
| C→T (R3 repeat), A→V | 41476 | X | - | X | X | 41427 | 41427 |
| Deletion, GCGCAGCCC | 41475-83 | - | X | - | - | 41426-34 | 41426-34 |
| G→C (R3 repeat), A→V | 41476 | X | - | X | X | 41427 | 41427 |
| Deletion, GCGCAGCCCGCGCAGACCGTCCAGCCCGCGCAGCCC, AQPAQTVQPAQP | 41484-519 | X | X | - | - | 41435-70 | 41435-70 |
| C→T (R3 repeat), A→V | 41485 | - | - | X | - | 41436 | 41436 |
| C→C/T (R3 repeat), A→V | 41494 | - | - | X | - | 41445 | 41445 |
| A→C (R3 repeat), T→P | 41499 | - | - | X | X | 41450 | 41450 |
| C→T (ORF 22), T, silent | 41618 | X | X | X | X | 41569 | 41569 |
| G→A (ORF 22), S→N | 41764 | X | X | X | X | 41715 | 41715 |
| C→G (ORF 22), Q→E | 42069 | X | X | X | X | 42020 | 42020 |
| C→T (ORF 22), R, silent | 42176 | X | X | X | X | 42127 | 42127 |
| A→C (ORF 22), A, silent | 42242 | X | X | X | X | 42193 | 42193 |
| 42403 | Del AAA | Del AA | Ins A | Del A | 42355 | 42353 | |
| T→G (ORF 23), S, silent | 42476 | X | X | X | X | 42428 | 42426 |
| T→C (ORF 24), I→V | 43262 | X | X | X | X | 43214 | 43212 |
| C→T (ORF 26), C, silent | 44835 | X | X | X | X | 44787 | 44785 |
| A→G (ORF 28), C→R | 47162 | X | X | X | X | 47114 | 47112 |
| C→T (ORF 28), L, silent | 47940 | X | X | X | X | 47892 | 47890 |
| T→C (ORF 28), S→G | 48050 | X | X | X | X | 48002 | 48000 |
| G→A (ORF 28), T, silent | 48825 | X | X | X | X | 48777 | 48775 |
| G→A (ORF 28), L, silent | 49535 | X | X | X | X | 49487 | 49485 |
| C→A (ORF 28), G→C | 50081 | X | X | X | X | 50033 | 50031 |
| C→T (ORF 29), S, silent | 51168 | X | X | X | X | 51120 | 51118 |
| A→G (ORF 29), Q, silent | 52917 | X | X | X | X | 52869 | 52867 |
| A→C (ORF 29), I→L | 53482 | X | X | X | X | 53434 | 53432 |
| G→A (ORF 29), A→T | 53938 | X | X | X | X | 53890 | 53888 |
| Deletion (ORF 29), ACATTTCAGGGTCAA, NISGS | 54359-73 | X | X | X | X | 54310 | 54308 |
| Deletion, T | 54562 | X | X | X | X | 54498 | 54496 |
| T→C | 54564 | X | X | X | X | 54500 | 54498 |
| A→G (ORF 30), P, silent | 55820 | X | X | X | X | 55756 | 55754 |
| A→C (ORF 31), T→P | 57224 | X | X | X | X | 57160 | 57158 |
| A→C (ORF 31), A, silent | 57301 | X | X | X | X | 57237 | 57235 |
| G→T (ORF 31), A, silent | 57397 | X | X | X | X | 57333 | 57331 |
| A→G (ORF 31), I→V | 58595 | - | A/G | A/G | X | 58531 | 58529 |
| A→A/G (ORF 31), P, silent | 59287 | - | X | X | X | 59223 | 59221 |
| Insertion, G | 59760 | X | X | X | X | 59697 | 59695 |
| Deletion | 60278 | Del5A | Del5A | Del A | Del AA | 60214 | 60211 |
| A→C | 60279 | X | X | X | X | 60215 | 60212 |
| C→A (ORF 33), A, silent | 60405 | X | X | X | X | 60341 | 60338 |
| T→G (ORF 33), Y→S | 60781 | X | X | X | X | 60717 | 60714 |
| G→A (ORF 33), P→L | 61018 | X | X | X | X | 60954 | 60951 |
| G→A (ORF 33), P→L | 61019 | X | X | X | X | 60955 | 60952 |
| T→C (ORF 33), N→G | 61201 | X | X | X | X | 61137 | 61134 |
| T→C (ORF 33), N→G | 61202 | X | X | X | X | 61138 | 61135 |
| A→G (ORF 35), A, silent | 64067 | - | X | X | A/G | 64003 | 64000 |
| A→G (ORF 35), C, silent | 64136 | X | X | X | X | 64072 | 64069 |
| T→C (ORF 35), P, silent | 64259 | X | X | X | X | 64195 | 64192 |
| T→C (ORF 35), M→V | 64375 | X | X | X | X | 64311 | 64308 |
| C→T (ORF 36), A, silent | 64989 | X | X | X | X | 64925 | 64922 |
| C→T (ORF 36), S→L | 65669 | X | X | X | X | 65605 | 65602 |
| G→T (ORF 37), L, silent | 66646 | X | X | X | X | 66582 | 66579 |
| C→T (ORF 37), P→L | 66879 | X | X | X | X | 66815 | 66812 |
| G→A (ORF 37), R→K | 68172 | X | X | X | X | 68108 | 68105 |
| A→G (ORF 38), T, silent | 69349 | X | X | X | X | 69285 | 69282 |
| T→C (ORF 38), S→G | 69756 | X | X | X | X | 69692 | 69689 |
| T→C (ORF 39), M→T | 71252 | - | X | X | C/T | 71188 | 71185 |
| C→T (ORF 40), V, silent | 72997 | X | X | X | X | 72933 | 72930 |
| T→C (ORF 40), T, silent | 73993 | X | X | X | X | 73929 | 73926 |
| C→T (ORF 41), V, silent | 76530 | X | X | X | X | 76466 | 76463 |
| Deletion, T | 78144 | X | X | X | X | 78079 | 78076 |
| G→T | 80244 | X | X | X | X | 80179 | 80176 |
| A→G (ORF 44), N→D | 80840 | X | X | X | X | 80775 | 80772 |
| C→T (ORF 44), A, silent | 81187 | X | X | X | X | 81122 | 81119 |
| A→A/G (ORF 45), P, silent | 82225 | - | X | - | - | 82160 | 82157 |
| G→A/G (ORF 47), E, silent | 84091 | - | X | - | - | 84026 | 84023 |
| A→G (ORF 47), T, silent | 84616 | X | X | X | X | 84551 | 84548 |
| G→A (ORF 48), R→H | 84983 | X | X | X | X | 84918 | 84915 |
| C→T (ORF 48), D, silent | 85563 | X | X | X | X | 85498 | 85495 |
| A→A/G (ORF 48), T→A | 85594 | - | - | X | X | 85529 | 85526 |
| C→A (ORF 48), Q→K | 86170 | X | X | X | X | 86105 | 86102 |
| Deletion, CCTGATAAAC | 86484-93 | X | X | X | X | 86418 | 86415 |
| T→G | 86556 | X | X | X | X | 86481 | 86478 |
| A→A/G (ORF 50), C, silent | 87280 | - | X | - | - | 87205 | 87202 |
| T→C/T (ORF 50), S→G | 87306 | - | X | - | - | 87231 | 87228 |
| C→T (ORF 50), S, silent | 87841 | X | X | X | X | 87766 | 87763 |
| G→T (ORF 51), S, silent | 88477 | X | X | X | X | 88402 | 88399 |
| A→G (ORF 51), T, silent | 89734 | - | X | X | - | 89659 | 89656 |
| T→C (ORF 51), T, silent | 89905 | X | X | X | X | 89830 | 89827 |
| G→T (ORF 51), Q→H | 90202 | X | X | X | X | 90127 | 90124 |
| T→C (ORF 51), S, silent | 90217 | X | X | X | X | 90142 | 90139 |
| G→A | 90392 | X | X | X | X | 90317 | 90314 |
| A→A/G (ORF 52), I→V | 90535 | - | X | X | - | 90460 | 90457 |
| C→T (ORF 52), G, silent | 91191 | X | X | X | X | 91116 | 91113 |
| A→G (ORF 52), T→A | 92026 | X | X | X | X | 91951 | 91948 |
| A→G (ORF 52), T→A | 92092 | X | X | X | X | 92017 | 92014 |
| A→G (ORF 52), H→R | 92375 | X | X | X | X | 92300 | 92297 |
| T→C (ORF 53), V, silent | 92999 | X | X | X | X | 92924 | 92921 |
| T→C (ORF 54), L, silent | 94167 | - | X | X | T/C | 94092 | 94089 |
| A→G (ORF 54), V, silent | 94632 | X | X | X | X | 94557 | 94554 |
| A→T (ORF 54), T, silent | 94641 | X | X | X | X | 94566 | 94563 |
| T→C (ORF 54), G, silent | 95241 | X | X | X | X | 95166 | 95163 |
| G→A (ORF 54), L, silent | 95546 | X | X | X | X | 95471 | 95468 |
| T→G (ORF 54), E→D | 95601 | X | X | X | X | 95526 | 95523 |
| T→C (ORF 55), L, silent | 97141 | X | X | X | X | 97066 | 97063 |
| T→T/C (ORF 55), V→A | 97479 | - | - | - | X | 97404 | 97401 |
| C→T (ORF 55), I, silent | 97591 | X | X | X | X | 97516 | 97513 |
| G→A/G (ORF 55), A→T | 97748 | - | X | X | X | 97673 | 97670 |
| T→C/T (ORF 55), C→R | 97796 | - | X | X | - | 97721 | 97718 |
| T→C (ORF 55), G, silent | 98437 | X | X | X | X | 98362 | 98359 |
| T→C (ORF 56), V, silent | 98765 | X | X | X | X | 98690 | 98687 |
| A→C (ORF 56), T, silent | 98807 | X | X | X | X | 98732 | 98729 |
| Deletion (ORF 56), TTC, S | 99227-29 | X | X | X | X | 99148 | 99145 |
| T→G (ORF 57), H→P | 99421 | X | X | X | X | 99343 | 99340 |
| A→G (ORF 58), Y, silent | 99709 | X | X | X | X | 99631 | 99628 |
| C→T (ORF 58), V→I | 99981 | X | X | X | X | 99903 | 99900 |
| T→A (ORF 58), K→N | 100114 | X | X | X | X | 100036 | 100033 |
| T→G (ORF 58), N→T | 100151 | X | X | X | X | 100073 | 100070 |
| A→G | 100283 | X | X | X | X | 100205 | 100202 |
| A→A/G (ORF 59), L→P | 101089 | X | X | X | X | 101011 | 101008 |
| C→T (ORF 60), A→T | 101331 | X | X | X | X | 101253 | 101250 |
| Insertion (ORF 60), ATC | 101623 | X | X | X | X | 101543-101545 | 101540-101542 |
| T→C | 101886 | X | X | X | X | 101811 | 101808 |
| C→T | 101991 | X | X | X | X | 101916 | 101913 |
| G→A | 102192 | X | X | X | X | 102117 | 102114 |
| A→G | 102203 | X | X | X | X | 102128 | 102125 |
| Insertion, TCAAGCTTTAAAAACGTACCCCAAACTTAAAACGCTCAAATTGCCTTTTGGAGGCCTGCCCAACGGCCATTATCCCTTGGATCTAAGATTGATTTGCGGTAACGTTTGCCAA | 102219 | X | X | - | - | 102144 | 102141 |
| C→A | 102309 | X | X | X | X | 102234 | 102231 |
| A→C | 102351 | X | X | X | X | 102276 | 102273 |
| A→G | 102458 | X | X | X | X | 102383 | 102380 |
| T→G | 102601 | X | X | X | X | 102526 | 102523 |
| T→C | 103043 | X | X | X | X | 102968 | 102965 |
| A→G | 104898 | X | X | X | X | 104823 | 104820 |
| C→G | 105010 | X | X | X | X | 104935 | 104932 |
| T→C | 105012 | X | X | X | X | 104937 | 104934 |
| T→C | 105015 | X | X | X | X | 104940 | 104937 |
| T→C | 105017 | X | X | X | X | 104942 | 104939 |
| Insertion, C | 105020 | X | - | X | X | 104946 | 104943 |
| Deletion, G | 105054 | X | X | X | X | 104979 | 104976 |
| Deletion, G | 105071 | X | X | X | X | 104995 | 104992 |
| Insertion, ACAA | 105145 | X | X | X | X | 105075 | 105072 |
| A→A/G | 105169 | - | X | X | X | 105097 | 105094 |
| A→A/G (ORF 62), L→S | 105310 | - | X | X | X | 105238 | 105235 |
| A→G (ORF 62), G, silent | 105312 | X | X | X | X | 105240 | 105237 |
| T→C (ORF 62), I→V | 105356 | - | X | X | T/C | 105284 | 105281 |
| A→G (ORF 62), L→P | 105451 | X | X | X | X | 105379 | 105376 |
| A→C (ORF 62), S→A | 105512 | X | X | X | X | 105440 | 105437 |
| A→G (ORF 62), V→A | 105544 | - | X | X | X | 105472 | 105469 |
| T→C (ORF 62), A, silent | 105705 | - | X | X | X | 105633 | 105630 |
| T→C (ORF 62), R→G | 106262 | - | X | X | X | 106190 | 106187 |
| T→C (ORF 62), A, silent | 107136 | - | X | X | T/C | 107064 | 107061 |
| C→T (ORF 62), A→T | 107165 | X | X | X | X | 107093 | 107090 |
| T→C (ORF 62), S→G | 107252 | - | X | X | X | 107180 | 107177 |
| T→C (ORF 62), R, silent | 107307 | X | X | X | X | 107235 | 107232 |
| A→A/G (ORF 62), V→A | 107599 | - | X | X | - | 107527 | 107524 |
| C→A (ORF 62), T, silent | 107607 | X | X | X | X | 107535 | 107532 |
| T→C (ORF 62), A, silent | 107715 | X | X | X | X | 107643 | 107640 |
| T→C (ORF 62), P, silent | 108111 | - | X | X | X | 108039 | 108036 |
| A→G (ORF 62), L, silent | 108747 | X | X | X | X | 108675 | 108672 |
| A→A/G (ORF 62), M→T | 108838 | - | X | X | X | 108766 | 108763 |
| G→A (ORF 62), H, silent | 108951 | X | X | X | X | 108879 | 108876 |
| C→G (ORF 62), A, silent | 109044 | X | X | X | X | 108972 | 108969 |
| A→A/G | 109137 | - | X | X | X | 109065 | 109062 |
| A→A/G | 109200 | - | X | X | - | 109128 | 109125 |
| T→C/T | 109546 | - | - | X | - | 109474 | 109471 |
| G→T | 109654 | X | X | X | X | 109582 | 109579 |
| Insertion, CAT | 109696 | X | X | X | X | 109625-109627 | 109622-109624 |
| Insertion, GGGAGGGGGCGCGGTACCCCGCCGATGGGGAGGGGGCGCGGTACCCCGCCGATGGGGAGGGGGCGCGGTACCCCGCCGATGGGGAGGGGGCGCGGTACCCCGCCGATG | 109907 | X | X | - | - | 109838 | 109835 |
| Insertion, GGGAGGGGGCGCGGTACCCCGCCGATG | 109907 | X | - | - | - | 109838 | 109835 |
| G→A | 110003 | X | X | X | X | 109934 | 109931 |
| Deletion, G | 110058 | X | X | X | X | 109988 | 109985 |
| G→A (Ori), -, silent | 110112 | X | X | X | X | 109934 | 109931 |
| Deletion, AT | 110212 | - | X | X | - | 110142 | 110140-110141 |
| T→G | 110214 | - | - | - | X | 110141 | |
| Insertion, ATATAG | 110214 | X | - | - | - | 110142 | 110141 |
| T→G (Ori) | 110216 | X | X | X | X | 110144 | 110143 |
| T→G (Ori) | 110218 | X | X | X | X | 110146 | 110145 |
| T→G (Ori) | 110220 | X | X | X | X | 110148 | 110147 |
| T→G (Ori) | 110222 | X | X | X | X | 110150 | 110149 |
| T→G (Ori) | 110224 | X | X | X | X | 110152 | 110151 |
| T→G (Ori) | 110226 | X | X | X | X | 110154 | 110153 |
| A→G (Ori) | 110232 | X | X | X | X | 110160 | 110159 |
| A→G (Ori) | 110235 | X | X | X | X | 110163 | 110162 |
| Deletion, GC | 110378-110379 | X | X | X | X | 110305 | 110304 |
| A→G (ORF 63), T, silent | 111312 | X | X | X | X | 110238 | 110237 |
| A→G (ORF 64), Q→R | 111650 | - | X | A/G | A/G | 111576 | 111575 |
| T→C (ORF 64), Y→H | 112093 | X | X | X | X | 112019 | 112018 |
| Deletion/insertion | 112128 | Del A | Del A | Ins 5a | Ins A | 112064-112068 | 112063 |
| G→A | 112198 | X | X | X | X | 112129 | 112124 |
| A→G (ORF 66), S, silent | 114140 | X | X | X | X | 114071 | 114066 |
| G→A (ORF 67), P, silent | 115041 | X | X | X | X | 114072 | 114967 |
| C→T (ORF 68), T→I | 115926 | X | X | X | X | 115857 | 115852 |
| C→T | 117699 | X | X | X | X | 117630 | 117625 |
| Deletion/insertion | 117769 | Del T | Del T | Ins 5T | Ins T | 117701-117705 | 117696 |
| A→G (ORF 69), Y→H | 117804 | X | X | X | X | 117740 | 117731 |
| T→C (ORF 69), Q→R | 118247 | - | X | T/C | T/C | 118183 | 118174 |
| T→C (ORF 70), T, silent | 118585 | X | X | X | X | 118521 | 118512 |
| Deletion, GC | 119518-119519 | X | X | X | X | 119453 | 119444 |
| Insertion, CTCTCT | 119654 | X | X | - | - | 119588 | 119579 |
| T→C (Ori) | 119656 | X | X | - | - | 119590 | 119581 |
| T→C (Ori) | 119665 | X | X | X | X | 119599 | 119590 |
| A→C (Ori) | 119671 | X | X | X | X | 119605 | 119596 |
| A→C (Ori) | 119673 | X | X | X | X | 119607 | 119598 |
| A→C (Ori) | 119675 | X | X | X | X | 119609 | 119600 |
| A→C (Ori) | 119677 | X | - | X | X | 119611 | 119602 |
| Deletion, ATATATAT | 119677-119684 | - | X | - | 119611-119618 | 119602-119609 | |
| A→C (Ori) | 119679 | X | - | X | X | 119613 | 119604 |
| A→C (Ori) | 119681 | X | - | X | X | 119615 | 119606 |
| A→C (Ori) | 119683 | X | - | X | A/C | 119617 | 119608 |
| C→T (Ori) | 119785 | X | X | X | X | 119719 | 119710 |
| Deletion, C | 119847 | X | X | X | X | 119780 | 119771 |
| C→T, | 119894 | X | X | X | X | 119827 | 119818 |
| Insertion, TACCGCGCCCCCTCCCCATCGGCGGGGTACCGCGCCCCCTCCCCATCGGCGGGGTACCGCGCCCCCTCCCCATCGGCGGGGTACCGCGCCCCCTCCCCATCGGCGGGGTACCGCGCCCCCTCCCCATCGGCGGGG | 120135 | X | X | - | - | 120068 | 120059 |
| Insertion, TACCGCGCCCCCTCCCCATCGGCGGGG | 120135 | X | - | - | 120068 | 120060 | |
| Insertion, GAT | 120202 | X | X | X | X | 120136-120138 | 120127-120129 |
| C→A | 120243 | X | X | X | X | 120179 | 120170 |
| A→A/G | 120351 | - | - | X | - | 120287 | 120278 |
| T→T/C | 120697 | - | X | X | - | 120633 | 120624 |
| T→T/C | 120760 | - | X | X | X | 120696 | 120687 |
| G→C (ORF 71), A, silent | 120853 | X | X | X | X | 120789 | 120780 |
| C→T (ORF 71), H, silent | 120946 | X | X | X | X | 120882 | 120873 |
| T→C/T (ORF 71), M→T | 121059 | - | X | X | X | 120995 | 120986 |
| T→C (ORF 71), L, silent | 121150 | X | X | X | X | 121086 | 121077 |
| A→G (ORF 71), P, silent | 121786 | - | X | X | X | 121722 | 121713 |
| A→G (ORF 71), A, silent | 122182 | X | X | X | X | 122118 | 122109 |
| G→T (ORF 71), T, silent | 122290 | X | X | X | X | 122226 | 122217 |
| T→C/T (ORF 71), V→A | 122298 | - | X | X | - | 122234 | 122225 |
| A→G (ORF 71), R, silent | 122590 | X | X | X | X | 122526 | 122517 |
| A→G (ORF 71), S→G | 122645 | - | X | X | X | 122581 | 122572 |
| G→A (ORF 71), A→T | 122732 | X | X | X | X | 122668 | 122659 |
| A→G (ORF 71), A, silent | 122761 | - | X | X | A/G | 122697 | 122688 |
| A→G (ORF 71), R→G | 123635 | - | X | X | X | 123571 | 123563 |
| A→G (ORF 71), A, silent | 124192 | - | X | X | X | 124128 | 124119 |
| T→C (ORF 71), V→A | 124353 | - | X | X | X | 124289 | 124280 |
| T→G (ORF 71), S→A | 124385 | X | X | X | X | 124321 | 124312 |
| T→C (ORF 71), L→P | 124446 | X | X | X | X | 124382 | 124373 |
| A→G (ORF 71), I→V | 124541 | - | X | X | A/G | 124477 | 124468 |
| T→C (ORF 71), G, silent | 124585 | X | X | X | X | 124521 | 124512 |
| T→C (ORF 71), L→S | 124587 | - | T/C | T/C | T/C | 124523 | 124514 |
| T→T/C | 124728 | - | X | X | X | 124664 | 124655 |
| Insertion, TGTT | 124750 | X | X | X | X | 124687-124690 | 124678-124681 |
| Deletion, C | 124834 | X | X | X | X | 124773 | 124764 |
| Deletion, C | 124851 | X | X | X | X | 124789 | 124780 |
| A→G | 124880 | NA | NA | X | X | 124818 | 124809 |
| A→G | 124882 | NA | NA | X | X | 124820 | 124811 |
A partial analysis of 20 of these nucleotide differences was published previously (70). Nucleotide positions within ORFs are indicated, as well as the encoded amino acids. Ori, origin of replication; WT, wild type. X, difference relative to Dumas strain; -, identical nucleotide relative to Dumas strain; NA, not applicable; Del, deletion; Ins, insertion. Where applicable, the resulting codon switch is specified.
The 98 differences between the Oka-VGSK (124,821 bp), Oka-VMerck (124,815 bp), Oka-P (125,125 bp), and Oka-V (125,078 bp) genomes were found in 25 ORFs (ORFs 1, 2, 6, 9A, 10, 11, 14, 18, 21, 22, 31, 35, 39, 45, 47, 48, 50, 51, 52, 54, 55, 62, 64, and 71), the R1 and R3 repeat regions (in ORFs 11 and 22, respectively), and one origin of replication (Table 3).
The total number of differences between the four Oka strains was determined (Table 4). Of the 98 differences identified, 69 were found between Oka-P and Oka-V, 51 between Oka-V and Oka-VGSK, and 68 between Oka-V and Oka-VMerck. Consequently, Oka-VMerck contains 17 more differences that discriminate it from Oka-V compared with Oka-VGSK.
TABLE 4.
Numbers of genomic sequence differences between the four Oka strains
Although the highest convergence was found for Oka-VGSK and Oka-VMerck, they still had 36 nucleotide differences (Table 4). For 12 of these positions, Oka-VMerck had nucleotides matching the Oka-P strain, whereas the Oka-VGSK strain had only a single position (119683) where the sequence was Oka-P-like. Overall, for the positions in which Oka-VGSK differed from Oka-VMerck, the Oka-VGSK sequence was closer to Oka-V, whereas the Oka-VMerck sequence was closer to Oka-P.
Sixty-nine nucleotide changes between the Oka-V and the Oka-P strains were identified (Table 5). Among these 69 differences, 56 positions in Oka-P were identical to the reference Dumas strain, whereas only 11 positions in Oka-V were identical to the Dumas strain. Identical nucleotides for many of these positions were also present in Oka-VGSK and Oka-VMerck.
TABLE 5.
Comparison of complete genomic sequences of Oka-P and Oka-V strains of VZVa
| Feature relative to WT (Dumas) | Position (WT) | Feature in:
|
Position in:
|
||||
|---|---|---|---|---|---|---|---|
| Oka-P | Oka-V | Oka-VGSK | Oka-VMerck | Oka-VGSK | Oka-VMerck | ||
| T→C | 560 | - | X | X | X | 559 | 559 |
| T→T/C (ORF 1), Q, silent | 703 | - | X | C | C | 702 | 702 |
| T→T/C (ORF 1), P, silent | 763 | - | X | C | C | 762 | 762 |
| C→G (ORF 2), G, silent | 1838 | - | X | - | - | 1837 | 1837 |
| T→T/C | 2515 | - | X | C | - | 2514 | 2514 |
| A→G (ORF 6), S→P | 5745 | - | X | X | A/G | 5744 | 5744 |
| T→C/T (ORF 9A), W→R | 10900 | - | X | X | - | 10899 | 10899 |
| C→C/T (ORF 10), A→V | 12779 | - | X | X | - | 12778 | 12778 |
| Insertion (ORF 11, R1), CGCGATCGACGACGAGGGAGAGGCGGAGGAGGA | 14242 | X | X | X | 14164-14196 | 14164-14196 | |
| T→T/C (ORF 14), stop | 19431 | - | X | - | - | 19385 | 19385 |
| A→G (ORF 18), N, silent | 26125 | - | X | X | A/G | 26076 | 26076 |
| C→T/C (ORF 21), T→I | 31732 | - | X | - | - | 31683 | 31683 |
| T→T/G (ORF 22), P, silent | 39227 | - | X | X | - | 39178 | 39178 |
| C→T (R3 repeat), A→V | 41476 | X | X | X | 41427 | 41427 | |
| Deletion, GCGCAGCCC | 41475-83 | - | X | - | - | 41426-41434 | 41426-41434 |
| G→C (R3 repeat), A→V | 41476 | X | X | X | 41427 | 41427 | |
| Deletion/insertion | 42403 | Del AAA | Del AA | Ins A | Del A | 42355 | 42353 |
| A→G (ORF 31), I→V | 58595 | - | A/G | A/G | X | 58531 | 58529 |
| A→A/G (ORF 31), P, silent | 59287 | - | X | X | X | 59223 | 59221 |
| A→G (ORF 35), A, silent | 64067 | - | X | X | A/G | 64003 | 64000 |
| T→C (ORF 39), M→T | 71252 | - | X | X | C/T | 71188 | 71185 |
| A→A/G (ORF 45), P, silent | 82225 | - | X | - | - | 82160 | 82157 |
| G→A/G (ORF 47), E, silent | 84091 | - | X | - | - | 84026 | 84023 |
| A→A/G (ORF 50), C, silent | 87280 | - | X | - | - | 87205 | 87202 |
| T→C/T (ORF 50), S→G | 87306 | - | X | - | - | 87231 | 87228 |
| A→G (ORF 51), T, silent | 89734 | - | X | X | - | 89659 | 89656 |
| A→A/G (ORF 52), I→V | 90535 | - | X | X | - | 90460 | 90457 |
| T→C (ORF 54), L, silent | 94167 | - | X | X | T/C | 94092 | 94089 |
| G→A/G (ORF 55), A→T | 97748 | - | X | X | X | 97673 | 97670 |
| T→C/T (ORF 55), C→R | 97796 | - | X | X | - | 97721 | 97718 |
| Insertion, C | 105020 | X | X | X | 104946 | 104943 | |
| A→A/G | 105169 | - | X | X | X | 105097 | 105094 |
| A→A/G (ORF 62), L→S | 105310 | - | X | X | X | 105238 | 105235 |
| T→C (ORF 62), I→V | 105356 | - | X | X | T/C | 105284 | 105281 |
| A→G (ORF 62), V→A | 105544 | - | X | X | X | 105472 | 105469 |
| T→C (ORF 62), A, silent | 105705 | - | X | X | X | 105633 | 105630 |
| T→C (ORF 62), R→G | 106262 | - | X | X | X | 106190 | 106187 |
| T→C (ORF 62), A, silent | 107136 | - | X | X | T/C | 107064 | 107061 |
| T→C (ORF 62), S→G | 107252 | - | X | X | X | 107180 | 107177 |
| A→A/G (ORF 62), V→A | 107599 | - | X | X | - | 107527 | 107524 |
| T→C (ORF 62), P, silent | 108111 | - | X | X | X | 108039 | 108036 |
| A→A/G (ORF 62), M→T | 108838 | - | X | X | X | 108766 | 108763 |
| A→A/G | 109137 | - | X | X | X | 109065 | 109062 |
| A→A/G | 109200 | - | X | X | 109128 | 109125 | |
| Insertion, GGGAGGGGGCGCGGTACCCCGCCGATG | 109907 | X | - | - | - | 109838 | 109835 |
| Deletion, AT | 110212 | - | X | X | - | 110142 | 110140-110141 |
| Insertion, ATATAG | 110214 | X | - | - | - | 110142 | 110141 |
| A→G (ORF 64), Q→R | 111650 | - | X | A/G | A/G | 111576 | 111575 |
| T→C (ORF 69), Q→R | 118247 | - | X | T/C | T/C | 118183 | 118174 |
| A→C (Ori) | 119677 | X | - | X | X | 119611 | 119602 |
| Deletion, ATATATAT | 119677-119684 | - | X | - | - | 119611-119618 | 119602-119609 |
| A→C (Ori) | 119679 | X | - | X | X | 119613 | 119604 |
| A→C (Ori) | 119681 | X | - | X | X | 119615 | 119606 |
| A→C (Ori) | 119683 | X | - | X | A/C | 119617 | 119608 |
| Insertion, TACCGCGCCCCCTCCCCATCGGCGGGG | 120135 | X | - | - | - | 120068 | 120060 |
| T→T/C | 120697 | - | X | X | 120633 | 120624 | |
| T→T/C | 120760 | - | X | X | X | 120696 | 120687 |
| T→C/T (ORF 71), M→T | 121059 | - | X | X | X | 120995 | 120986 |
| A→G (ORF 71), P, silent | 121786 | - | X | X | X | 121722 | 121713 |
| T→C/T (ORF 71), V→A | 122298 | - | X | X | 122234 | 122225 | |
| A→G (ORF 71), S→G | 122645 | - | X | X | X | 122581 | 122572 |
| A→G (ORF 71), A, silent | 122761 | - | X | X | A/G | 122697 | 122688 |
| A→G (ORF 71), R→G | 123635 | - | X | X | X | 123571 | 123563 |
| A→G (ORF 71), A, silent | 124192 | - | X | X | X | 124128 | 124119 |
| T→C (ORF 71), V→A | 124353 | - | X | X | X | 124289 | 124280 |
| A→G (ORF 71), I→V | 124541 | - | X | X | A/G | 124477 | 124468 |
| T→C (ORF 71), L→S | 124587 | - | T/C | T/C | T/C | 124523 | 124514 |
| T→T/C | 124728 | - | X | X | X | 124664 | 124655 |
Ori, origin of replication; WT, wild type. X, difference relative to Dumas strain; -, identical nucleotide relative to Dumas strain; Del, deletion; Ins, insertion. Where applicable, the resulting codon switch is specified. Boldface highlights homologies between genomic sequences of Oka-V and genomic sequences of Oka-VGSK and/or Oka-VMerck.
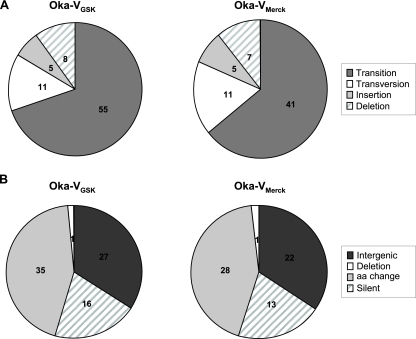
To better characterize the observed differences, the substitution spectra were analyzed (Fig. 2). The large majority of mutations were SNPs and only partial, with two different nucleotides at the same position. Compared to Oka-P, transitions (i.e., mutations resulting in substitution of a purine for a purine [A↔ G] or a pyrimidine for a pyrimidine [C↔ T]) were more frequently (64% to 69%) observed for the Oka-VGSK and Oka-VMerck strains than transversions (i.e., mutations resulting in substitution of a purine for a pyrimidine and vice versa; 13% to 17%). Transversions were more common than insertions or deletions (≤10%). The majority of the identified mutations were silent mutations, either because they were located in intergenic regions or because of the degenerated genetic code. A significant proportion of mutations in intragenic regions (∼45%) caused single amino acid substitutions in both the Oka-VGSK and Oka-VMerck strains (Fig. 2). No stop or frameshift mutations were identified. All deletions and insertions either were located in intergenic regions or, when located within coding regions, were multiples of three bases.
FIG. 2.
Type (A) and function (B) of the mutations between Oka-P and the Oka-VGSK and Oka-VMerck vaccine strains of VZV. The numbers indicate the number of events identified for each category of mutations. aa, amino acid.
Comparison of Oka-VGSK and Oka-VMerck genomes.
Sequence differences observed between the Oka-VGSK and Oka-VMerck strains are described in Table 6. Only 36 differences were found throughout the complete genomes (i.e., ∼125 kb), three of which were repeated in ORF 62 and its duplicate, ORF 71. These 33 nucleotide unique position changes resulted in 14 amino acid changes, 1 each in ORFs 6, 9A, 10, 31, 39, and 52 and 2 each in ORFs 14, 55, and 62/71 and the R3 repeat region.
TABLE 6.
Comparison of complete genomic sequences of Oka-VGSK and Oka-VMerck vaccine strains of VZVa
| Feature relative to WT (Dumas) | Position (WT) | Feature in:
|
Position in:
|
||||
|---|---|---|---|---|---|---|---|
| Oka-P | Oka-V | Oka-VGSK | Oka-VMerck | Oka-VGSK | Oka-VMerck | ||
| T→T/C | 2515 | - | X | C | - | 2514 | 2514 |
| A→G (ORF 6), S→P | 5745 | - | X | X | A/G | 5744 | 5744 |
| T→C/T (ORF 9A), W→R | 10900 | - | X | X | - | 10899 | 10899 |
| C→C/T (ORF 10), A→V | 12779 | - | X | X | - | 12778 | 12778 |
| C→C/T (ORF 14), K, silent | 20703 | - | - | X | - | 20657 | 20657 |
| C→A (ORF 14), K→N | 20787 | X | X | C/A | - | 20741 | 20741 |
| A→T (ORF 14), S→T | 20879 | - | - | A/T | - | 20833 | 20833 |
| A→G (ORF 18), N, silent | 26125 | - | X | X | A/G | 26076 | 26076 |
| T→T/G (ORF 22), P, silent | 39227 | - | X | X | - | 39178 | 39178 |
| C→T (R3 repeat), A→V | 41485 | - | - | X | - | 41436 | 41436 |
| C→C/T (R3 repeat), A→V | 41494 | - | - | X | - | 41445 | 41445 |
| Deletion/insertion | 42403 | Del AAA | Del AA | Ins A | Del A | 42355 | 42353 |
| A→G (ORF 31), I→V | 58595 | - | A/G | A/G | X | 58531 | 58529 |
| Deletion | 60278 | Del 5A | Del 5A | Del A | Del AA | 60214 | 60211 |
| A→G (ORF 35), A, silent | 64067 | - | X | X | A/G | 64003 | 64000 |
| T→C (ORF 39), M→T | 71252 | - | X | X | C/T | 71188 | 71185 |
| A→G (ORF 51), T, silent | 89734 | - | X | X | - | 89659 | 89656 |
| A→A/G (ORF 52), I→V | 90535 | - | X | X | - | 90460 | 90457 |
| T→C (ORF 54), L, silent | 94167 | - | X | X | T/C | 94092 | 94089 |
| T→T/C (ORF 55), V→A | 97479 | - | - | - | X | 97404 | 97401 |
| T→C/T (ORF 55), C→R | 97796 | - | X | X | - | 97721 | 97718 |
| T→C (ORF 62), I→V | 105356 | - | X | X | T/C | 105284 | 105281 |
| T→C (ORF 62), A, silent | 107136 | - | X | X | T/C | 107064 | 107061 |
| A→A/G (ORF 62), V→A | 107599 | - | X | X | - | 107527 | 107524 |
| A→A/G | 109200 | - | X | X | - | 109128 | 109125 |
| T→C/T | 109546 | - | - | X | - | 109474 | 109471 |
| Deletion, AT | 110212 | - | X | X | - | 110142 | 110140-110141 |
| T→G | 110214 | - | - | - | X | - | 110141 |
| Deletion/insertion | 112128 | Del A | Del A | Ins 5a | Ins A | 112064-112068 | 112063 |
| Deletion/insertion | 117769 | Del T | Del T | Ins 5T | Ins T | 117701-117705 | 117696 |
| A→C (Ori) | 119683 | X | - | X | A/C | 119617 | 119608 |
| A→A/G | 120351 | - | - | X | - | 120287 | 120278 |
| T→T/C | 120697 | - | X | X | - | 120633 | 120624 |
| T→C/T (ORF 71), V→A | 122298 | - | X | X | - | 122234 | 122225 |
| A→G (ORF 71), A, silent | 122761 | - | X | X | A/G | 122697 | 122688 |
| A→G (ORF 71), I→V | 124541 | - | X | X | A/G | 124477 | 124468 |
WT, wild type. X, difference relative to Dumas strain; -, identical nucleotide relative to Dumas strain; Ori, origin of replication; Del, deletion; Ins, insertion. When applicable, the resulting codon switch is specified. Boldface highlights homologies between genomic sequences of Oka-V and genomic sequences of Oka-VGSK and/or Oka-VMerck.
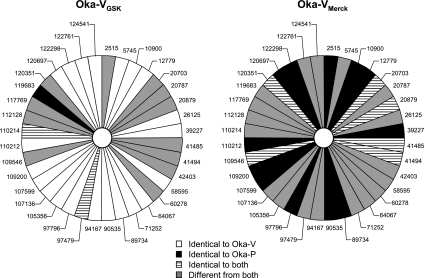
Among these 36 position differences between Oka-VGSK and Oka-VMerck, Oka-VGSK had 23 nucleotide sequences identical to Oka-V but only 3 identical to Oka-P. In contrast, Oka-VMerck had 18 positions identical to Oka-P but only 6 identical to Oka-V (Table 6 and Fig. 3).
FIG. 3.
Sequence comparisons of Oka-VGSK and Oka-VMerck with Oka-P and Oka-V strains of VZV. The 36 nucleotide positions that are different in Oka-VGSK and Oka-VMerck vaccines were compared to the sequence of the original vaccine strain Oka-V and its parental virus, Oka-P.
DISCUSSION
In this study, we compared the complete genomes of the varicella vaccine strains Oka-VGSK and Oka-VMerck, both derived from the original attenuated Oka-V strain (67). Phylogenetic analyses of these sequences along with 16 other complete VZV genomes were recently reported (50, 69), providing new insight into strain variability (69) and evidence of recombination between major circulating VZV clades (50).
Although VZV is a monotypic virus with a very low rate of interstrain sequence variations (0.061%) compared to other members of the Herpesviridae family of viruses (between 0.32% and 3.0% [47]), the sequence analysis of the Oka vaccine strains is not straightforward due to the presence of heterogeneous genomes with distinct sequences (21). Therefore, consensus sequencing provides only an indication of the most prevalent bases for each position. In the present study, we determined the full-length sequences of both Oka-VGSK and Oka-VMerck largely by bidirectional sequencing of overlapping PCR fragments, but when direct sequencing did not generate results of sufficient quality, fragments were subcloned and the consensus sequence was derived from numerous plasmid clones. All sequences obtained were confirmed on both DNA strands. This approach gave a high-quality assessment of the whole genomes of Oka-VGSK and Oka-VMerck, and this is, to our knowledge, the first published comparative analysis of the complete genomes of these two strains.
Comparison with partial sequencing information published on these strains and the other Oka strains, Oka-P and Oka-V, is shown in Table 7 (3, 22, 32, 59, 60, 63, 69). Argaw et al. sequenced approximately 34 kb from the 3′ ends of Oka-V, Oka-P, and Oka-VMerck strains, and Schmidt et al. sequenced approximately 26 kb of the Oka-VGSK strain (3, 60). Two sequence differences were found for Oka-VMerck in ORF 59 (position 101089; A versus A/G) and ORF 62 (position 105310; G versus A/G) (3). Six differences were observed between the present results and those previously published for Oka-VGSK, and 13 differences were observed for Oka-VMerck (60). Finally, comparison between the present study and a previous one (32) revealed quantitative (number of sequence differences between Oka-VGSK and Oka-VMerck strains) and qualitative (ORFs involved) discrepancies.
TABLE 7.
Comparison of Oka-VGSK and Oka-VMerck genomic sequences with previously published Oka genomic sequencesas
| Reference | Position | ORF | Previously reported feature
|
Feature in present study
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| Dumas | Oka-P | Oka-V | Oka-VGSK | Oka-VMerck | Oka-VGSK | Oka-VMerck | |||
| 3 | 84983 | 48 | G | A | A | A | A | A | |
| 85563 | 48 | C | T | T | T | T | T | ||
| 86484 | cctgataaac | - | cctgataaac | cctgataaac | |||||
| 86556 | T | G | G | G | |||||
| 87841 | 50 | C | T | T | T | ||||
| 88477 | 51 | G | T | T | T | ||||
| 89734 | 51 | A | G | G | A | ||||
| 89905 | 51 | T | C | C | C | ||||
| 90202 | 51 | G | T | T | T | ||||
| 90217 | 51 | T | C | C | C | ||||
| 90392 | G | A | A | A | |||||
| 91191 | 52 | C | T | T | T | ||||
| 92092 | 52 | A | G | G | G | ||||
| 92375 | 52 | A | G | G | G | ||||
| 92999 | 52 | T | C | C | C | ||||
| 94167 | 54 | T | C | C | T/C | ||||
| 94632 | 54 | T | G | G | G | ||||
| 94641 | 54 | A | T | T | T | ||||
| 95241 | 54 | T | C | C | C | C | C | ||
| 95546 | 54 | G | A | A | A | ||||
| 97141 | 55 | T | C | C | C | ||||
| 97470b | 55 | G | C | G | G | ||||
| 97591 | 55 | C | T | T | T | ||||
| 97748 | 55 | G | A | A/G | A/G | ||||
| 97834c | 55 | C | T | C | C | ||||
| 98437 | 55 | T | C | C | C | ||||
| 98765 | 56 | T | C | C | C | ||||
| 98807 | 56 | A | C | C | C | ||||
| 99227 | 56 | TTC | - | - | TTC | TTC | |||
| 99709 | 58 | A | G | G | G | G | G | ||
| 99981 | 58 | C | T | T | T | T | T | ||
| 100114 | 58 | T | A | A | A | A | A | ||
| 100151 | 58 | T | G | G | G | G | G | ||
| 100283 | A | G | G | G | |||||
| 101089 | 59 | A | A | G | A | A/G | A/G | ||
| 101331 | 60 | C | T | T | T | T | T | ||
| 101623 | 60 | - | +ATC | +ATC | +ATC | +ATC | |||
| 101886 | T | C | C | C | |||||
| 101991 | C | T | T | T | |||||
| 102192 | G | A | A | A | |||||
| 102203 | A | G | G | G | |||||
| 102219 | +112 bp | ||||||||
| 102309 | C | A | A | A | |||||
| 102351 | A | C | C | C | |||||
| 102458 | A | G | G | G | |||||
| 102601 | T | G | G | G | |||||
| 103043 | T | C | C | C | |||||
| 104898 | A | G | G | G | |||||
| 105010 | cctcctct | cctcctct | gcccttacccc | cctcctct | cctcctct | cctcctct | |||
| 105054 | G | Del G | Del G | Del G | |||||
| 105063d | G | Del G | Del G | Del G | |||||
| 105145 | +AACA | +AACA | +AACA | +ACAA | |||||
| 105310 | 62 | A | A | G | G | A/G | A/G | ||
| 105312 | 62 | A | G | G | G | G | G | ||
| 105356 | 62 | T | T | C | C | C | C | ||
| 105451 | 62 | A | G | G | G | ||||
| 105512 | 62 | A | C | C | C | ||||
| 105544 | 62 | A | G | G | G | ||||
| 105705 | 62 | T | C | C | C | ||||
| 106262 | 62 | T | T | C | C | C | C | ||
| 107136 | 62 | T | C | C | C | ||||
| 107165 | 62 | C | T | T | T | ||||
| 107252 | 62 | T | C | C | C | ||||
| 107307 | 62 | T | C | C | C | ||||
| 107607 | 62 | C | A | A | A | ||||
| 107715 | 62 | T | C | C | C | ||||
| 108111 | 62 | T | C | C | C | ||||
| 108747 | 62 | A | G | G | G | ||||
| 108951 | 62 | G | A | A | A | ||||
| 109044 | 62 | C | G | G | G | ||||
| 109694e | +ATC | +CAT | +CAT | ||||||
| 109762 | +27 bp | C | C | ||||||
| 110196 | Del TA | No del | No del | ||||||
| 110216 | (ga)9gg | (ta)6(gc)2aaga | (ta)16gag(ga)4 | (ta)10(ga)9aaa(ga)4 | |||||
| 110378 | Del GC | Del GC | Del GC | ||||||
| 111312 | 63 | A | G | G | G | ||||
| 111650 | 64 | A | A | G | A/G | A/G | A/G | ||
| 112093 | 64 | T | C | C | C | C | C | ||
| 112130f | - | - | +A8 | +A8 | +A12 | +A9 | |||
| 112198 | G | A | A | A | A | A | |||
| 114140 | 66 | A | G | G | G | ||||
| 115041 | 67 | G | A | A | A | ||||
| 115926 | 68 | C | T | T | T | ||||
| 60 | 1 | A | G | G | G | A | G | G | |
| 3 | G | C | C | C | G | C | C | ||
| 178 | G | G | G | C | C | C | C | ||
| 560 | T | T | C | C | C | C | C | ||
| 703g | 1 | T | T | T | C | C | C | C | |
| 82225h | 45 | A | A | A | G | A | A | A | |
| 86363 | 49 | A | A | A | T | A | A | A | |
| 87677 | 50 | A | A | A | G | A | A | A | |
| 89734 | 51 | A | A | G | A | A | G | A | |
| 90115i | 51 | A | A | stop | A | A | A | A | |
| 105054 | G | - | - | - | G | Del G | Del G | ||
| 105071 | G | G | - | - | G | Del G | Del G | ||
| 105145 | Poly(A) | - | AACA | AACA | AACA | - | ACAA | ACAA | |
| 105169 | A | A | A/G | A/G | A | A/G | A/G | ||
| 105310 | 62 | A | A | A/G | G | A | A/G | A/G | |
| 105356 | 62 | T | T | C | C | T | C | T/C | |
| 105544 | 62 | A | A | G | G | G | G | G | |
| 124353 | 71 | T | T | C | C | C | C | C | |
| 124541 | 71 | A | A | G | G | G | G | A/G | |
| 124587 | 71 | T | T | C/T | C | T | C | C/T | |
| 124728 | T | T | C/T | C/T | T | C/T | C/T | ||
| 124750 | pA-71 | - | TGTT | TGTT | TGTT | TGTT | TGTT | TGTT | |
| 124834 | C | - | - | - | C | Del C | Del C | ||
| 124851 | C | - | - | - | C | Del C | Del C | ||
| 22 | 560 | T | C | C | C | ||||
| 703 | 1 | T | T/C | C | C | ||||
| 763 | 1 | T | T/C | C | C | ||||
| 2515 | T | T/C | C | T | |||||
| 5745 | 6 | A | G | G | A/G | ||||
| 10900 | 9A | T | T/C | T/C | T | ||||
| 12779j | 10 | T | T/C | T/C | C | ||||
| 19431 | 14 | T | T/C | T | T | ||||
| 26125 | 18 | A | G | G | A/G | ||||
| 31732 | 21 | C | T/C | C | C | ||||
| 38036k | 22 | T | T/C | T/C | T/C | ||||
| 39227 | 22 | T | T/G | T/G | T | ||||
| 58595 | 31 | A | A/G | A/G | G | ||||
| 59287 | 31 | A | A/G | A/G | A/G | ||||
| 64067l | 35 | A | A/G | G | A/G | ||||
| 71252m | 39 | T | T/C | C | T/C | ||||
| 82225 | 45 | A | A/G | A | A | ||||
| 84091 | 47 | G | A/G | G | G | ||||
| 87280 | 50 | A | A/G | A | A | ||||
| 87306 | 50 | T | T/C | T | T | ||||
| 89734n | 51 | A | A/G | G | A | ||||
| 90535 | 52 | A | A/G | A/G | A | ||||
| 94167 | 54 | T | C | C | T/C | ||||
| 97748 | 55 | G | A/G | A/G | A/G | ||||
| 97796 | 55 | T | T/C | T/C | T | ||||
| 101089o | 59 | A | A/G | A/G | A/G | ||||
| 105169 | A | A/G | A/G | A/G | |||||
| 105310 | 62 | A | A/G | A/G | A/G | ||||
| 105356 | 62 | T | C | C | T/C | ||||
| 105544 | 62 | A | G | G | G | ||||
| 105705 | 62 | T | C | C | C | ||||
| 106262 | 62 | T | C | C | C | ||||
| 106710p | 62 | A | A/G | A | A | ||||
| 107136 | 62 | T | C | C | T/C | ||||
| 107252 | 62 | T | C | C | C | ||||
| 107599 | 62 | A | A/G | A/G | A | ||||
| 107797q | 62 | A | A/G | A | A | ||||
| 108111 | 62 | T | C | C | C | ||||
| 108838 | 62 | A | A/G | A/G | A/G | ||||
| 109137 | A | A/G | A/G | A/G | |||||
| 109200 | A | A/G | A/G | A | |||||
| 111650r | 64 | A | A/G | A/G | A/G | ||||
Boldface highlights differences (3, 60) and homologies (22) between results from published studies and results from the present study. Lowercase indicates insertion; -, missing nucleotide position.
Indicated as G in the Oka-V GenBank submission AB097932.
Indicated as C in the Oka-V GenBank submission AB097932.
In the present alignment, this position is 105071.
In the present alignment, this position is 109696.
In the present alignment, this position is 112128.
Indicated as Y in the Oka-V GenBank submission AB097932.
Indicated as R in the Oka-V GenBank submission AB097932.
Indicated as A in the Oka-V GenBank submission AB097932.
Indicated as C in the Oka-P GenBank submission AB097933.
Indicated as T in the Oka-V GenBank submission AB097932.
Indicated as G in the Oka-V GenBank submission AB097932.
Indicated as C in the Oka-V GenBank submission AB097932.
Indicated as G in the Oka-V GenBank submission AB097932.
Indicated as R in the Oka-P GenBank submission AB097933.
Indicated as A in the Oka-V GenBank submission AB097932.
Indicated as A in the Oka-V GenBank submission AB097932.
Indicated as A in the Oka-V GenBank submission AB097932.
Our analysis of Oka-VGSK and Oka-VMerck sequences revealed that they have very few nucleotide differences. When we compared their complete genomic sequences (i.e., ∼125 kb), we found that only 36 positions were different between Oka-VGSK and Oka-VMerck. These differences lead to 14 unique amino acid substitutions, which suggests that although these two vaccine strains are not identical, they are very similar. The differences resulting in amino acid substitutions were found in 10 different ORFs and in the R3 repeat region, while silent nucleotide substitutions were found in 8 different ORFs, and 1 noncoding substitution was found in the origin of replication.
Transactivation.
ORF 62 encodes immediate early protein 62 (IE62), also known as a transcription regulator, which is the major component of the virion tegument and an important transactivating protein for all classes of VZV promoters (28, 49, 56). It is located in the short repeat sequences and has therefore a duplicate gene, ORF 71. These two duplicated genes cover 7% of the whole VZV genome. Recent studies suggested that ORF 62 could play a central role in the attenuated phenotype of the Oka vaccine strains (3, 20-22). Defined amino acid substitutions in ORF 62 that are associated with individual virus variants purified from the vaccine mixture have been linked to enhanced virus growth and spread in monolayer cell culture (22).
The present analysis of vaccine strains confirmed that a high number of mutations could be detected within ORF 62 (20-22). As previously discussed, these SNPs in ORF 62/71 may be important for attenuation of VZV (50, 69). The current analysis identified a nucleotide transition (position 105356 in ORF 62, corresponding to 124541 in ORF 71) that altered an Ile of the IE62 protein to a Val only partially in Oka-VMerck and completely in Oka-VGSK and Oka-V. Because Oka-P encodes only Ile at this position, it is likely that the Oka-VMerck passaging history has selected for minor Oka-P-related species that might be present in the Oka-V vaccine. The second substitution (position 107599 in ORF 62 and 122298 in ORF 71) partially changed a Val to Ala in Oka-VGSK and Oka-V; for Oka-VMerck, this position is identical to the one found in Oka-P and encodes Val only. In both cases, the amino acids involved are small hydrophobic residues. Gomi et al. demonstrated that five amino acid substitutions, including the 105356 mutation, in the carboxyl terminus of IE62 directly reduced transactivational activity (22). Experiments with recombinant VZV will be required to determine how these mutations in ORF 62 modulate VZV gene expression and which amino acid substitutions are responsible for the differences in viral spreading.
The product of ORF 10, the virion-associated transactivator, is a tegument protein that regulates the IE62 promoter (28, 46). A nucleotide substitution (C→C/T) at position 12779 results in a conversion of an Ala in the Dumas, Oka-P, and Oka-VMerck strains to a mixture of Ala and Val in the Oka-V and Oka-VGSK strains. Similarly, minor subspecies from Oka-V that were originally present in Oka-P may have been selected in Oka-VMerck. This position, which corresponds to a location in the middle of the protein, in Oka-V and Oka-VGSK encodes two small hydrophobic amino acids (Val and Ala) that could have similar functions. Indeed, no statistically significant differences in transactivational activity of the ORF 10 gene product could be detected between the wild type and the mutant form, suggesting that this alternative form of ORF 10 has a minimal effect on viral attenuation through modulation of the expression level of IE62 (22). Furthermore, in vitro studies have shown that ORF 10 product was dispensable for VZV replication in vitro (10).
The helicase-primase complex.
The helicase-primase complex consists of three proteins encoded by ORF 6 (primase) and ORFs 52 and 55 (helicase). Interestingly, we found four amino acid substitutions in these proteins, three of which were described previously (22).
The first amino acid substitution was located near the C terminus of ORF 6 (position 5745, A→G), which was a Ser in Dumas and Oka-P, a Pro in Oka-V and Oka-VGSK, and a mixture of both in Oka-VMerck. Pro is a rigid residue that could induce substantial changes in the protein conformation. This nucleotide substitution was also comprised in an AluI restriction site. Interestingly, Quinlivan et al. found no differences between Oka-VGSK and Oka-VMerck by AluI restriction analysis: both Oka-VGSK and Oka-VMerck were A/G (±AluI), whereas Oka-V was G (+AluI) (52).
The second substitution occurred in ORF 52 (position 90535, A→A/G). In this case, the amino acid residue in Oka-P and Oka-VMerck was Ile, which was partially changed to Val in Oka-V and Oka-VGSK.
The last two substitutions were located in ORF 55. Val at position 97479 was partially replaced by Ala in Oka-VMerck, and Cys at position 97796 was partially replaced by Arg in Oka-V and Oka-VGSK.
Gomi et al. demonstrated that pathogenicity and spreading of VZV were affected by mutations in ORFs 6, 10, and 62, whereas ORFs 52 and 55 did not seem to be important for efficient VZV spreading (22). Because these substitutions were only partial, they could result in the coexistence of different helicase-primase activities resulting from different isomeric complexes, as shown by restriction fragment length polymorphism analysis (22). For most of these positions Oka-VGSK was similar to Oka-V, whereas Oka-VMerck was similar to Oka-P.
Envelope glycoproteins.
VZV produces at least seven glycoproteins, gK, gC, gB, gH, gL, gI, and gE, which are the products of ORFs 5, 14, 31, 37, 60, 67, and 68, respectively (11). Two putative additional glycoproteins were recently described, gN (ORF 9A) and gM (ORF 50) (55, 77). It is known that VZV glycoproteins induce a strong humoral immune response following either natural infection or vaccination with the Oka strain. The SNPs in the nine VZV glycoproteins were reviewed in a recent comparative analysis (64). Some of them are specific to the vaccine strains and thus could be involved in VZV attenuation.
The product of ORF 68, gE, is the most abundant glycoprotein expressed during infection. A single amino acid substitution in this protein was shown to induce the accelerated replication phenotype of the VZV-MSP mutant strain (57). Recently, Grose at al. reported the sequences of two VZV isolates harboring a D150N mutation within ORF 68 (25). That study identified only one mutation, common to all four Oka strains (position 115926, C→T), which induced the replacement of a Thr by Ile.
The product of ORF 31, gB, is the second most abundant and immunogenic envelope glycoprotein of VZV after gE. Along with gH and gC, it seems to play a role in the attachment and penetration of viral particles. It was also shown to have important fusogenic properties in the presence of gE and to be associated with cell-to-cell infection (39, 45). An A→G transition at position 58595 induced a conversion of Ile to Val in Oka-VMerck and a mixture of Ile and Val in both Oka-VGSK and Oka-V. Both amino acids are small and hydrophobic, suggesting that this substitution would probably not affect the properties of this glycoprotein.
ORF 14 exhibited one silent replacement and two amino acid substitutions in its product, gC. At position 20787, a coexistence of C and A induced the partial replacement of a Lys by Asn in Oka-VGSK, whereas the original C residue was completely replaced by A in Oka-VMerck, entirely replacing Lys with Asn. Both residues are hydrophilic; however, Lys is basic and Asn is polar, with an uncharged side chain. Because Asn was not detected in either Oka-P or Oka-V, it is likely that this amino acid substitution evolved as a result of additional vaccine passages and might reflect additional cell culture adaptation. The second modification (position 20879, A→T) was found only in the Oka-VGSK strain. It partially changed a Ser to Thr. Both residues are hydrophilic and polar with an uncharged side chain. Grinfeld et al. showed that products of ORF 14 and ORF 67 were dispensable for the establishment of latency in a rat model (24). However, experiments with SCID-hu mice showed that gC is important for viral tropism in skin cells and that a decrease in gC plays a critical role in attenuation (44). It was also previously shown that expression of gC is dependent on the strain of VZV, with the gC level of Oka-V being much lower than that of wild-type viruses (29, 37).
A nucleotide substitution at position 71252 induced the replacement of a Met by a Thr in the product of ORF 39, one of the two multiply inserted membrane proteins of VZV. This change was complete in Oka-VGSK and Oka-V but only partial in Oka-VMerck. Although both amino acids are neutral, Thr is polar and Met is hydrophobic. This difference may have some effect on the properties of the protein, although these are unclear at present (23).
We also found a mutation in the gN envelope glycoprotein. This glycoprotein is the product of ORF 9A, a newly identified gene positioned closely upstream of the ORF 9 initiation codon (55). Glycoprotein gN is an 87-amino-acid protein whose amino-terminal extremity overlaps with the first nine amino acids of the ORF 8 product, coded on the complementary strand. The observed T-to-T/C shift at position 10900 involves a Trp-to-Arg switch in the very last amino acid of the protein in Oka-V and Oka-VGSK. This change leads to replacement of a hydrophobic amino acid with a large aromatic side chain at the carboxyl terminus of the protein by a hydrophilic basic amino acid, which could alter the membrane topology of the protein or its stability.
R3 repeat region.
The R3 reiteration region is located in ORF 22, the longest ORF in VZV. The product of ORF 22 is homologous to the UL36 virion tegument phosphoprotein of herpes simplex virus type 1 (41, 42). R3 is a highly variable region consisting of repeated elements that can vary in number and combination (12, 22), but the impact of this region on the function of ORF 22 phosphoprotein remains mostly unknown because the function of the phosphoprotein itself is poorly understood.
Despite the high variability of the R3 region, no frameshift mutations were detected in ORF 22, because the repeated elements are present as multiples of 3 bp. Nevertheless, two mutations (positions 41485 and 41494, C→T) in the Oka-VGSK strain were identified, both of which convert Ala into Val, another amino acid with similar properties that probably does not affect the function of the ORF 22 protein.
Attenuation and reactivation.
Comparison of the four Oka strain sequences of VZV indicated that Oka-VGSK is genetically closer to Oka-V than Oka-VMerck is but that Oka-VMerck is closer to Oka-P than Oka-VGSK is (reference 50 and the present study). In agreement with these findings, Sauerbrei et al. demonstrated that Oka-VMerck is genetically closer to Oka-P than Oka-VGSK is (59). Nevertheless, it is well established that both vaccine strains are attenuated but remain strongly immunogenic (43, 73). Recently, Quinlivan et al. suggested an association of particular SNPs in the VZV genome with frequency of vaccine-induced rash (53). Four SNP positions were suggested to contain nucleotides specific to Oka-P in most of the viruses isolated from vaccine rashes. The first one is a silent nucleotide change within ORF 51 (A→G at position 89734). The nucleotide at this position is A in Oka-VMerck and Oka-P but G in Oka-VGSK and Oka-V. The second SNP, at position 105169, contains mixed A/G nucleotides for Oka-VMerck, Oka-VGSK, and Oka-V, whereas the parental Oka-P contains only A. The third SNP, at position 105356, is located within ORF 62. The change from T to C is responsible for an amino acid switch (Ile→Val) for both Oka-VGSK and Oka-V. At the same position, the parental Oka-P contains T (Ile), and Oka-VMerck contains a mixture of T and C (Ile/Val). The last position (nucleotide 107797) was not identified as a SNP in our sequencing data, which agrees with a previous study (22).
Although the genetic basis of Oka-V attenuation has not been determined, Oka-V and Oka-P genomes have nucleotide differences predicted to change amino acids in every class of viral proteins (3, 21). VZV attenuation is a multifactorial phenomenon whose mechanism remains unclear (80), but it is conceivable that mutations of the vaccine genome, in particular, mutations resulting in amino acid modifications, could affect virulence or latency of the vaccine strain. Recently, Peters and coworkers sequenced 11 VZV genomes from different clades, bringing the current number of available full-length VZV sequences to 18 (50, 69). To assess variations that can occur during serial passage in cell culture, these studies included the four Oka strains (Oka-P and the three Oka vaccine strains) and a VZV strain sequenced at passages 5, 22, and 72. As discussed by Tyler et al. (69), the SNPs in ORF 62/71 found in the three Oka vaccine strains and in the VZV strain at high passage level (S628G, R958G, and I1260V in IE62) could be involved in the attenuation of VZV. In addition, it was suggested that other mutations could play a role, particularly those in regions containing ORFs 30 to 55 (69, 78, 80). However, we found that numerous SNPs contain the original Oka-P nucleotide, supporting the idea that the attenuated phenotype is the result of a cooperative effect between several SNPs rather than the result of selected mutations. Therefore, our analysis of SNP importance is aligned with the conclusions of Tyler et al. regarding VZV attenuation (69).
VZV remains latent in sensory-nerve ganglia and can reactivate later, causing herpes zoster. It was suggested that herpes zoster is less common after vaccination, because initial access of Oka-V to neural cells is reduced by limited skin replication or because Oka-V reactivation and secondary viral infection of skin are less efficient, rather than because of an intrinsic attenuation of Oka-V neurotropism (5). Indeed, even though the Oka-V strain of VZV can cause herpes zoster, it seems to reactivate less often than the wild-type VZV even in immunocompromised children (18, 27, 68), and increased incidences of reactivation after vaccination have not been demonstrated in either clinical studies or postmarketing surveillance (33a, 43, 62, 66, 70, 73). In addition, the Oka vaccines have been shown to elicit a strong and protective immune response against varicella (7, 43, 73).
Conclusion.
Overall, this study shows that, throughout the entire VZV genome, only 36 nucleotide positions differ between the Oka-VGSK and Oka-VMerck vaccine strains. Analysis of the complete genome of VZV also shows that, genetically, Oka-VGSK is closer to Oka-V and that Oka-VMerck is closer to Oka-P. Although Oka-VGSK and Oka-VMerck exhibit differences, there is a high degree of conservation between these strains at both the nucleotide and amino acid levels. This result supports the clinical data showing that both vaccines are well tolerated and elicit strong immune responses against varicella.
Acknowledgments
We are grateful to Catherine Arnaudeau-Bégard, Julie Harriague, and Anne Hepburn for their constructive discussions and editorial assistance in the preparation of the manuscript. Technical support from various sequencing staff members is acknowledged.
Footnotes
Published ahead of print on 10 September 2008.
REFERENCES
- 1.American Academy of Pediatrics Committee on Infectious Diseases. 1995. Recommendations for the use of live attenuated varicella vaccine. Pediatrics 95791-796. [PubMed] [Google Scholar]
- 2.Arbeter, A. M., S. E. Starr, and S. A. Plotkin. 1986. Varicella vaccine studies in healthy children and adults. Pediatrics 78748-756. [PubMed] [Google Scholar]
- 3.Argaw, T., J. I. Cohen, M. Klutch, K. Lekstrom, T. Yoshikawa, Y. Asano, and P. R. Krause. 2000. Nucleotide sequences that distinguish Oka vaccine from parental Oka and other varicella-zoster virus isolates. J. Infect. Dis. 1811153-1157. [DOI] [PubMed] [Google Scholar]
- 4.Arvin, A., and A. Gershon. 2006. Control of varicella: why is a two-dose schedule necessary? Pediatr. Infect. Dis. J. 25475-476. [DOI] [PubMed] [Google Scholar]
- 5.Arvin, A. M. 2001. Varicella vaccine: genesis, efficacy, and attenuation. Virology 284153-158. [DOI] [PubMed] [Google Scholar]
- 6.Arvin, A. M. 2002. Varilrix (GlaxoSmithKline). Curr. Opin. Investig. Drugs 3996-999. [PubMed] [Google Scholar]
- 7.Asano, Y. 1996. Varicella vaccine: the Japanese experience. J. Infect. Dis. 174(Suppl. 3)S310-S313. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. 1996. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recommend. Rep. 451-36. [PubMed] [Google Scholar]
- 9.Chaves, S. S., P. Gargiullo, J. X. Zhang, R. Civen, D. Guris, L. Mascola, and J. F. Seward. 2007. Loss of vaccine-induced immunity to varicella over time. N. Engl. J. Med. 3561121-1129. [DOI] [PubMed] [Google Scholar]
- 10.Cohen, J. I., and K. Seidel. 1994. Varicella-zoster virus (VZV) open reading frame 10 protein, the homolog of the essential herpes simplex virus protein VP16, is dispensable for VZV replication in vitro. J. Virol. 687850-7858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cole, N. L., and C. Grose. 2003. Membrane fusion mediated by herpesvirus glycoproteins: the paradigm of varicella-zoster virus. Rev. Med. Virol. 13207-222. [DOI] [PubMed] [Google Scholar]
- 12.Davison, A. J., and J. E. Scott. 1986. The complete DNA sequence of varicella-zoster virus. J. Gen. Virol. 671759-1816. [DOI] [PubMed] [Google Scholar]
- 13.de Ory, F., J. M. Echevarría, G. Kafatos, C. Anastassopoulou, N. Andrews, J. Backhouse, G. Berbers, B. Bruckova, D. I. Cohen, H. de Melker, I. Davidkin, G. Gabutti, L. M. Hesketh, K. Johansen, S. Jokinen, L. Jones, A. Linde, E. Miller, J. Mossong, A. Nardone, M. C. Rota, A. Sauerbrei, F. Schneider, Z. Smetana, A. Tischer, A. Tsakris, and R. Vranckx. 2006. European seroepidemiology network 2: standardisation of assays for seroepidemiology of varicella zoster virus. J. Clin. Virol. 36111-118. [DOI] [PubMed] [Google Scholar]
- 14.D'Hondt, E., E. Berge, G. Colinet, M. Duchene, and J. Peetermans. 1985. Production and quality control of the Oka-strain live varicella vaccine. Postgrad Med. J. 6153-56. [PubMed] [Google Scholar]
- 15.Faga, B., W. Maury, D. A. Bruckner, and C. Grose. 2001. Identification and mapping of single nucleotide polymorphisms in the varicella-zoster virus genome. Virology 2801-6. [DOI] [PubMed] [Google Scholar]
- 16.Galil, K., B. Lee, T. Strine, C. Carraher, A. L. Baughman, M. Eaton, J. Montero, and J. Seward. 2002. Outbreak of varicella at a day-care center despite vaccination. N. Engl. J. Med. 3471909-1915. [DOI] [PubMed] [Google Scholar]
- 17.Gershon, A. A. 2001. The current status of live attenuated varicella vaccine. Arch. Virol. Suppl. 20011-6. [DOI] [PubMed] [Google Scholar]
- 18.Gershon, A. A., P. LaRussa, I. Hardy, S. Steinberg, and S. Silverstein. 1992. Varicella vaccine: the American experience. J. Infect. Dis. 166(Suppl. 1)S63-S68. [DOI] [PubMed] [Google Scholar]
- 19.Goh, P., F. S. Lim, H. H. Han, and P. Willems. 2007. Safety and immunogenicity of early vaccination with two doses of tetravalent measles-mumps-rubella-varicella (MMRV) vaccine in healthy children from 9 months of age. Infection 35326-333. [DOI] [PubMed] [Google Scholar]
- 20.Gomi, Y., T. Imagawa, M. Takahashi, and K. Yamanishi. 2001. Comparison of DNA sequence and transactivation activity of open reading frame 62 of Oka varicella vaccine and its parental viruses. Arch. Virol. Suppl. 200149-56. [DOI] [PubMed] [Google Scholar]
- 21.Gomi, Y., T. Imagawa, M. Takahashi, and K. Yamanishi. 2000. Oka varicella vaccine is distinguishable from its parental virus in DNA sequence of open reading frame 62 and its transactivation activity. J. Med. Virol. 61497-503. [DOI] [PubMed] [Google Scholar]
- 22.Gomi, Y., H. Sunamachi, Y. Mori, K. Nagaike, M. Takahashi, and K. Yamanishi. 2002. Comparison of the complete DNA sequences of the Oka varicella vaccine and its parental virus. J. Virol. 7611447-11459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Govero, J., S. Hall, and T. C. Heineman. 2007. Intracellular localization of varicella-zoster virus ORF39 protein and its functional relationship to glycoprotein K. Virology 358291-302. [DOI] [PubMed] [Google Scholar]
- 24.Grinfeld, E., C. Sadzot-Delvaux, and P. G. Kennedy. 2004. Varicella-zoster virus proteins encoded by open reading frames 14 and 67 are both dispensable for the establishment of latency in a rat model. Virology 32385-90. [DOI] [PubMed] [Google Scholar]
- 25.Grose, C., S. Tyler, G. Peters, J. Hiebert, G. M. Stephens, W. T. Ruyechan, W. Jackson, J. Storlie, and G. A. Tipples. 2004. Complete DNA sequence analyses of the first two varicella-zoster virus glycoprotein E (D150N) mutant viruses found in North America: evolution of genotypes with an accelerated cell spread phenotype. J. Virol. 786799-6807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hammond, O., Y. Wang, T. Green, J. Antonello, R. Kuhn, C. Motley, P. Stump, B. Rich, N. Chirmule, and R. D. Marchese. 2006. The optimization and validation of the glycoprotein ELISA assay for quantitative varicella-zoster virus (VZV) antibody detection. J. Med. Virol. 781679-1687. [DOI] [PubMed] [Google Scholar]
- 27.Hardy, I., A. A. Gershon, S. P. Steinberg, P. LaRussa, et al. 1991. The incidence of zoster after immunization with live attenuated varicella vaccine. A study in children with leukemia. N. Engl. J. Med. 3251545-1550. [DOI] [PubMed] [Google Scholar]
- 28.Kinchington, P., J. Hougland, A. Arvin, W. Ruyechan, and J. Hay. 1992. The varicella-zoster virus immediate-early protein IE62 is a major component of virus particles. J. Virol. 66359-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kinchington, P. R., P. Ling, M. Pensiero, A. Gershon, J. Hay, and W. T. Ruyechan. 1990. A possible role for glycoprotein gpV in the pathogenesis of varicella-zoster virus. Adv. Exp. Med. Biol. 27883-91. [DOI] [PubMed] [Google Scholar]
- 30.Knuf, M., P. Habermehl, F. Zepp, W. Mannhardt, M. Kuttnig, P. Muttonen, A. Prieler, H. Maurer, H. Bisanz, N. Tornieporth, D. Descamps, and P. Willems. 2006. Immunogenicity and safety of two doses of tetravalent measles-mumps-rubella-varicella vaccine in healthy children. Pediatr. Infect. Dis. J. 2512-18. [DOI] [PubMed] [Google Scholar]
- 31.Krah, D. L., I. Cho, T. Schofield, and R. W. Ellis. 1997. Comparison of gpELISA and neutralizing antibody responses to Oka/Merck live varicella vaccine (Varivax) in children and adults. Vaccine 1561-64. [DOI] [PubMed] [Google Scholar]
- 32.Kraiouchkine, N., A. R. Shaw, P. M. Keller, and D. J. Distefano. 2003. Comparison of the complete genome sequences of Varicella Zoster Virus Oka/Merck (Varivax) and Oka/GSK (Varilrix), abstr. G-1652, p. 295. Abstr. 43rd Annu. Intersci. Conf. Antimicrob. Agents Chemother. Chicago, IL, 14 to 17 September 2003.
- 33.Krause, P. R., and D. M. Klinman. 1995. Efficacy, immunogenicity, safety, and use of live attenuated chickenpox vaccine. J. Pediatr. 127518-525. [DOI] [PubMed] [Google Scholar]
- 33a.Kreth, H. W., B. W. Lee, P. Kosuwon, J. Salazar, N. Gloriani-Barzaga, H. L. Bock, F. Meurice. 16 years of global experience with the first refrigerator-stable varicella vaccine (Varilrix™). BioDrugs, in press. [DOI] [PubMed]
- 34.Kuter, B., H. Matthews, H. Shinefield, S. Black, P. Dennehy, B. Watson, K. Reisinger, L. L. Kim, L. Lupinacci, J. Hartzel, and I. Chan. 2004. Ten year follow-up of healthy children who received one or two injections of varicella vaccine. Pediatr. Infect. Dis. J. 23132-137. [DOI] [PubMed] [Google Scholar]
- 35.Kuter, B. J., M. L. Brown, J. Hartzel, W. R. Williams, K. A. Eves, S. Black, H. Shinefield, K. S. Reisinger, C. D. Marchant, B. J. Sullivan, M. Thear, S. Klopfer, J. Xu, J. O. Gress, and F. Schodel. 2006. Safety and immunogenicity of a combination measles, mumps, rubella and varicella vaccine (ProQuad). Hum. Vaccines 2205-214. [DOI] [PubMed] [Google Scholar]
- 36.Lau, Y. L., S. J. Vessey, I. S. Chan, T. L. Lee, L. M. Huang, C. Y. Lee, T. Y. Lin, B. W. Lee, K. Kwan, S. M. Kasim, C. Y. Chan, K. M. Kaplan, D. J. Distefano, A. L. Harmon, A. Golie, J. Hartzel, J. Xu, S. Li, H. Matthews, J. C. Sadoff, and A. Shaw. 2002. A comparison of safety, tolerability and immunogenicity of Oka/Merck varicella vaccine and VARILRIX in healthy children. Vaccine 202942-2949. [DOI] [PubMed] [Google Scholar]
- 37.Ling, P., P. R. Kinchington, W. T. Ruyechan, and J. Hay. 1991. A detailed analysis of transcripts mapping to varicella zoster virus gene 14 (glycoprotein V). Virology 184625-635. [DOI] [PubMed] [Google Scholar]
- 38.Macartney, K. K., P. Beutels, P. McIntyre, and M. A. Burgess. 2005. Varicella vaccination in Australia. J. Paediatr. Child Health 41544-552. [DOI] [PubMed] [Google Scholar]
- 39.Maresova, L., T. J. Pasieka, and C. Grose. 2001. Varicella-zoster Virus gB and gE coexpression, but not gB or gE alone, leads to abundant fusion and syncytium formation equivalent to those from gH and gL coexpression. J. Virol. 759483-9492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marin, M., D. Guris, S. S. Chaves, S. Schmid, and J. F. Seward. 2007. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recommend. Rep. 561-40. [PubMed] [Google Scholar]
- 41.McNabb, D. S., and R. J. Courtney. 1992. Analysis of the UL36 open reading frame encoding the large tegument protein (ICP1/2) of herpes simplex virus type 1. J. Virol. 667581-7584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McNabb, D. S., and R. J. Courtney. 1992. Characterization of the large tegument protein (ICP1/2) of herpes simplex virus type 1. Virology 190221-232. [DOI] [PubMed] [Google Scholar]
- 43.Meurice, F., J. L. De Bouver, D. Vandevoorde, S. Woods, and H. Bogaerts. 1996. Immunogenicity and safety of a live attenuated varicella vaccine (Oka/SB Bio) in healthy children. J. Infect. Dis. 174(Suppl. 3)S324-S329. [DOI] [PubMed] [Google Scholar]
- 44.Moffat, J. F., L. Zerboni, P. R. Kinchington, C. Grose, H. Kaneshima, and A. M. Arvin. 1998. Attenuation of the vaccine Oka strain of varicella-zoster virus and role of glycoprotein C in alphaherpesvirus virulence demonstrated in the SCID-hu mouse. J. Virol. 72965-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Montalvo, E. A., and C. Grose. 1987. Assembly and processing of the disulfide-linked varicella-zoster virus glycoprotein gpII(140). J. Virol. 612877-2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moriuchi, H., M. Moriuchi, and J. I. Cohen. 1995. Proteins and cis-acting elements associated with transactivation of the varicella-zoster virus (VZV) immediate-early gene 62 promoter by VZV open reading frame 10 protein. J. Virol. 694693-4701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muir, W., K. Hawrami, J. Clarke, and J. Breuer. 2001. Investigation of varicella-zoster virus variation by heteroduplex mobility assay, p. 17-25. In C. C. Gershon and A. A. Arvin (ed.), Immunity to and prevention of herpes zoster. Springer-Verlag, Vienna, Austria. [DOI] [PubMed]
- 48.Nolan, T., P. McIntyre, D. Roberton, and D. Descamps. 2002. Reactogenicity and immunogenicity of a live attenuated tetravalent measles-mumps-rubella-varicella (MMRV) vaccine. Vaccine 21281-289. [DOI] [PubMed] [Google Scholar]
- 49.Perera, L. P., J. D. Mosca, W. T. Ruyechan, G. S. Hayward, S. E. Straus, and J. Hay. 1993. A major transactivator of varicella-zoster virus, the immediate-early protein IE62, contains a potent N-terminal activation domain. J. Virol. 674474-4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peters, G. A., S. D. Tyler, C. Grose, A. Severini, M. J. Gray, C. Upton, and G. A. Tipples. 2006. A full-genome phylogenetic analysis of varicella-zoster virus reveals a novel origin of replication-based genotyping scheme and evidence of recombination between major circulating clades. J. Virol. 809850-9860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Quian, J., R. Rüttimann, C. Romero, P. Dall'Orso, A. Cerisola, T. Breuer, M. Greenberg, and T. Verstraeten. 2008. Impact of universal varicella vaccination on 1-year-olds in Uruguay: 1997-2005. Arch. Dis. Child. 93845-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Quinlivan, M., A. A. Gershon, S. P. Steinberg, and J. Breuer. 2005. An evaluation of single nucleotide polymorphisms used to differentiate vaccine and wild type strains of varicella-zoster virus. J. Med. Virol. 75174-180. [DOI] [PubMed] [Google Scholar]
- 53.Quinlivan, M. L., A. A. Gershon, M. M. Al Bassam, S. P. Steinberg, P. LaRussa, R. A. Nichols, and J. Breuer. 2007. Natural selection for rash-forming genotypes of the varicella-zoster vaccine virus detected within immunized human hosts. Proc. Natl. Acad. Sci. USA 104208-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Robert Koch Institut. 2001. Empfehlungen der Ständigen Impfkommission (STIKO). Epidemiol. Bull. 29219-223. [Google Scholar]
- 55.Ross, J., M. Williams, and J. I. Cohen. 1997. Disruption of the varicella-zoster virus dUTPase and the adjacent ORF9A gene results in impaired growth and reduced syncytia formation in vitro. Virology 234186-195. [DOI] [PubMed] [Google Scholar]
- 56.Sadzot-Delvaux, C., and B. Rentier. 2001. The role of varicella zoster virus immediate-early proteins in latency and their potential use as components of vaccines. Arch. Virol. Suppl. 1781-89. [DOI] [PubMed] [Google Scholar]
- 57.Santos, R. A., C. C. Hatfield, N. L. Cole, J. A. Padilla, J. F. Moffat, A. M. Arvin, W. T. Ruyechan, J. Hay, and C. Grose. 2000. Varicella-zoster virus gE escape mutant VZV-MSP exhibits an accelerated cell-to-cell spread phenotype in both infected cell cultures and SCID-hu mice. Virology 275306-317. [DOI] [PubMed] [Google Scholar]
- 58.Sauerbrei, A., I. Farber, A. Brandstadt, M. Schacke, and P. Wutzler. 2004. Immunofluorescence test for sensitive detection of varicella-zoster virus-specific IgG: an alternative to fluorescent antibody to membrane antigen test. J. Virol. Methods 11925-30. [DOI] [PubMed] [Google Scholar]
- 59.Sauerbrei, A., E. Rubtcova, P. Wutzler, D. S. Schmid, and V. N. Loparev. 2004. Genetic profile of an Oka varicella vaccine virus variant isolated from an infant with zoster. J. Clin. Microbiol. 425604-5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schmidt, M., M. Kress, S. Heinemann, and H. Fickenscher. 2003. Varicella-zoster virus isolates, but not the vaccine strain OKA, induce sensitivity to alpha-1 and beta-1 adrenergic stimulation of sensory neurons in culture. J. Med. Virol. 70S82-S89. [DOI] [PubMed] [Google Scholar]
- 61.Seward, J. F., B. M. Watson, C. L. Peterson, L. Mascola, J. W. Pelosi, J. X. Zhang, T. J. Maupin, G. S. Goldman, L. J. Tabony, K. G. Brodovicz, A. O. Jumaan, and M. Wharton. 2002. Varicella disease after introduction of varicella vaccine in the United States, 1995-2000. JAMA 287606-611. [DOI] [PubMed] [Google Scholar]
- 62.Sharrar, R. G., P. LaRussa, S. A. Galea, S. P. Steinberg, A. R. Sweet, R. M. Keatley, M. E. Wells, W. P. Stephenson, and A. A. Gershon. 2000. The postmarketing safety profile of varicella vaccine. Vaccine 19916-923. [DOI] [PubMed] [Google Scholar]
- 63.Shaw, A. R., N. Kraiouchkine, J. Condra, D. Graham, X. Liu, and D. DiStefano. 2001. Genetic differences between OKA varicella vaccines, abstr. 69. 19th Annu. Meet. Eur. Soc. Paediatr. Infect. Dis.
- 64.Storlie, J., L. Maresova, W. Jackson, and C. Grose. 2008. Comparative analyses of the 9 glycoprotein genes found in wild-type and vaccine strains of varicella-zoster virus. J. Infect. Dis. 197(Suppl. 2)S49-S53. [DOI] [PubMed] [Google Scholar]
- 65.Straus, S. E., W. Reinhold, H. A. Smith, W. T. Ruyechan, D. K. Henderson, R. M. Blaese, and J. Hay. 1984. Endonuclease analysis of viral DNA from varicella and subsequent zoster infections in the same patient. N. Engl. J. Med. 3111362-1364. [DOI] [PubMed] [Google Scholar]
- 66.Takahashi, M. 2001. 25 years' experience with the Biken Oka strain varicella vaccine: a clinical overview. Paediatr. Drugs 3285-292. [DOI] [PubMed] [Google Scholar]
- 67.Takahashi, M., T. Otsuka, Y. Okuno, Y. Asano, and T. Yazaki. 1974. Live vaccine used to prevent the spread of varicella in children in hospital. Lancet ii1288-1290. [DOI] [PubMed] [Google Scholar]
- 68.Takayama, N., M. Takayama, and J. Takita. 2000. Herpes zoster in healthy children immunized with varicella vaccine. Pediatr. Infect. Dis. J. 19169-170. [DOI] [PubMed] [Google Scholar]
- 69.Tyler, S. D., G. A. Peters, C. Grose, A. Severini, M. J. Gray, C. Upton, and G. A. Tipples. 2007. Genomic cartography of varicella-zoster virus: a complete genome-based analysis of strain variability with implications for attenuation and phenotypic differences. Virology 359447-458. [DOI] [PubMed] [Google Scholar]
- 70.Vassilev, V. 2005. Stable and consistent genetic profile of Oka varicella vaccine virus is not linked with appearance of infrequent breakthrough cases postvaccination. J. Clin. Microbiol. 435415-5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vesikari, T., M. Baer, and P. Willems. 2007. Immunogenicity and safety of a second dose of measles-mumps-rubella-varicella vaccine in healthy children aged 5 to 6 years. Pediatr. Infect. Dis. J. 26153-158. [DOI] [PubMed] [Google Scholar]
- 72.Vesikari, T., C. Sadzot-Delvaux, B. Rentier, and A. Gershon. 2007. Increasing coverage and efficiency of measles, mumps, and rubella vaccine and introducing universal varicella vaccination in Europe: a role for the combined vaccine. Pediatr. Infect. Dis. J. 26632-638. [DOI] [PubMed] [Google Scholar]
- 73.White, C. J., B. J. Kuter, C. S. Hildebrand, K. L. Isganitis, H. Matthews, W. J. Miller, P. J. Provost, R. W. Ellis, R. J. Gerety, and G. B. Calandra. 1991. Varicella vaccine (VARIVAX) in healthy children and adolescents: results from clinical trials, 1987 to 1989. Pediatrics 87604-610. [PubMed] [Google Scholar]
- 74.Williams, V., A. Gershon, and P. A. Brunell. 1974. Serologic response to varicella-zoster membrane antigens measured by direct immunofluorescence. J. Infect. Dis. 130669-672. [DOI] [PubMed] [Google Scholar]
- 75.Wood, M. J. 2000. History of varicella zoster virus. Herpes 760-65. [PubMed] [Google Scholar]
- 76.World Health Organization. 1998. Varicella vaccines. WHO position paper. Wkly. Epidemiol. Rec. 73241-248. [PubMed] [Google Scholar]
- 77.Yamagishi, Y., T. Sadaoka, H. Yoshii, P. Somboonthum, T. Imazawa, K. Nagaike, K. Ozono, K. Yamanishi, and Y. Mori. 2008. Varicella-zoster virus glycoprotein M homolog is glycosylated, is expressed on the viral envelope, and functions in virus cell-to-cell spread. J. Virol. 82795-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yamanishi, K. 2008. Molecular analysis of the Oka vaccine strain of varicella-zoster virus. J. Infect. Dis. 197(Suppl. 2)S45-S48. [DOI] [PubMed] [Google Scholar]
- 79.Zepp, F., U. Behre, K. Kindler, K. H. Laakmann, H. Pankow-Culot, W. Mannhardt-Laakmann, F. Beckers, D. Descamps, and P. Willems. 2007. Immunogenicity and safety of a tetravalent measles-mumps-rubella-varicella vaccine co-administered with a booster dose of a combined diphtheria-tetanus-acellular pertussis-hepatitis B-inactivated poliovirus-Haemophilus influenzae type b conjugate vaccine in healthy children aged 12-23 months. Eur. J. Pediatr. 166857-864. [DOI] [PubMed] [Google Scholar]
- 80.Zerboni, L., S. Hinchliffe, M. H. Sommer, H. Ito, J. Besser, S. Stamatis, J. Cheng, D. Distefano, N. Kraiouchkine, A. Shaw, and A. M. Arvin. 2005. Analysis of varicella zoster virus attenuation by evaluation of chimeric parent Oka/vaccine Oka recombinant viruses in skin xenografts in the SCIDhu mouse model. Virology 332337-346. [DOI] [PubMed] [Google Scholar]
- 81.Zhou, F., R. Harpaz, A. O. Jumaan, C. A. Winston, and A. Shefer. 2005. Impact of varicella vaccination on health care utilization. JAMA 294797-802. [DOI] [PubMed] [Google Scholar]