Abstract
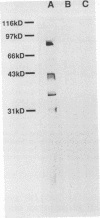
The immunological basis for protection against Brazilian purpuric fever (BPF), a fulminant infection of young children associated with bacteremia with Haemophilus influenzae biogroup aegyptius, is unknown. Candidate antigens to which protective antibodies may be directed include cell surface proteins and lipooligosaccharide (LOS). We studied the activity of antisera to LOS purified from a BPF H. influenzae biogroup aegyptius isolate. Anti-LOS antisera contained anti-LOS antibody by enzyme immunoassay and immunoblot and no detectable anti-outer membrane protein antibodies by immunoblot. Anti-LOS antisera had minimal bactericidal activity and were not protective against the homologous strain in an infant rat model of bacteremia. Antiserum to whole bacterial cells had a titer of anti-LOS antibody similar to that of anti-LOS antisera and was bactericidal and protective. Removal of anti-LOS antibodies from anti-whole cell antiserum by affinity chromatography did not result in a loss of bactericidal activity. Serum from a normal adult contained anti-LOS antibodies and had bactericidal activity. However, anti-LOS antibodies purified from this serum did not have detectable bactericidal activity. These studies suggest that anti-LOS antibodies produced in rats are not bactericidal and do not contribute to protection against experimental bacteremia with BPF strains of H. influenzae biogroup aegyptius.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carlone G. M., Gorelkin L., Gheesling L. L., Erwin A. L., Hoiseth S. K., Mulks M. H., O'Connor S. P., Weyant R. S., Myrick J., Rubin L. Potential virulence-associated factors in Brazilian purpuric fever. Brazilian Purpuric Fever Study Group. J Clin Microbiol. 1989 Apr;27(4):609–614. doi: 10.1128/jcm.27.4.609-614.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erwin A. L., Munford R. S. Comparison of lipopolysaccharides from Brazilian purpuric fever isolates and conjunctivitis isolates of Haemophilus influenzae biogroup aegyptius. Brazilian Purpuric Fever Study Group. J Clin Microbiol. 1989 Apr;27(4):762–767. doi: 10.1128/jcm.27.4.762-767.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulig P. A., Hansen E. J. Coprecipitation of lipopolysaccharide and the 39,000-molecular-weight major outer membrane protein of Haemophilus influenzae type b by lipopolysaccharide-directed monoclonal antibody. Infect Immun. 1985 Sep;49(3):819–827. doi: 10.1128/iai.49.3.819-827.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison L. H., da Silva G. A., Pittman M., Fleming D. W., Vranjac A., Broome C. V. Epidemiology and clinical spectrum of Brazilian purpuric fever. Brazilian Purpuric Fever Study Group. J Clin Microbiol. 1989 Apr;27(4):599–604. doi: 10.1128/jcm.27.4.599-604.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks M. I., Ziegler E. J., Douglas H., Corbeil L. B., Braude A. I. Induction of immunity against lethal Haemophilus influenzae type b infection by Escherichia coli core lipopolysaccharide. J Clin Invest. 1982 Apr;69(4):742–749. doi: 10.1172/JCI110512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin L. G., Gloster E. S., Carlone G. M. An infant rat model of bacteremia with Brazilian purpuric fever isolates of Hemophilus influenzae biogroup aegyptius. Brazilian Purpuric Fever Study Group. J Infect Dis. 1989 Sep;160(3):476–482. doi: 10.1093/infdis/160.3.476. [DOI] [PubMed] [Google Scholar]
- Shenep J. L., Munson R. S., Jr, Barenkamp S. J., Granoff D. M. Further studies of the role of noncapsular antibody in protection against experimental Haemophilus influenzae type b bacteremia. Infect Immun. 1983 Oct;42(1):257–263. doi: 10.1128/iai.42.1.257-263.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenep J. L., Munson R. S., Jr, Granoff D. M. Human antibody responses to lipopolysaccharide after meningitis due to Haemophilus influenzae type b. J Infect Dis. 1982 Feb;145(2):181–190. doi: 10.1093/infdis/145.2.181. [DOI] [PubMed] [Google Scholar]
- Weiser J. N., Lindberg A. A., Manning E. J., Hansen E. J., Moxon E. R. Identification of a chromosomal locus for expression of lipopolysaccharide epitopes in Haemophilus influenzae. Infect Immun. 1989 Oct;57(10):3045–3052. doi: 10.1128/iai.57.10.3045-3052.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]