Abstract
CC chemokine receptor 4 (CCR4) and its two ligands, CCL17 and CCL22, are critically involved in different immune processes. In models of lipopolysaccharide-induced shock, CCR4-deficient (CCR4−/−) mice showed improved survival rates associated with attenuated proinflammatory cytokine release. Using CCR4−/− mice with a C57BL/6 background, this study describes for the first time the role of CCR4 in a murine model of polymicrobial abdominal sepsis, the colon ascendens stent peritonitis (CASP). CASP-induced sepsis led to a massive downregulation of CCR4 in lymphoid and nonlymphoid tissues, whereas the expression of CCL17 and CCL22 was independent of the presence of CCR4. After CASP, CCR4−/− animals showed a strongly enhanced bacterial clearance in several organs but not in the peritoneal lavage fluid and the blood. In addition, significantly reduced levels of proinflammatory cytokines/chemokines were measured in organ supernatants as well as in the sera of CCR4−/− mice. CCR4 deficiency consequently resulted in an attenuated severity of systemic sepsis and a strongly improved survival rate after CASP or CASP with intervention. Thus, our data provide clear evidence that CCR4 plays a strictly detrimental role in the course of polymicrobial sepsis.
Chemokines and chemokine receptors are crucially involved in innate and adaptive immune responses (6). Chemokines attract leukocytes and therefore are important for lymphocyte migration and development but also for recruitment of immune cells to sites of inflammation or infection. Regarding functional aspects, chemokines were recently classified into three subfamilies: inflammatory, homeostatic, and dual-function chemokines (48). CCL17 (thymus- and activation-regulated chemokine) and CCL22 (macrophage-derived chemokine) are assigned to the dual-function subfamily, as they are involved in T-cell development as well as in effector cell recruitment to sites of inflammation. CCL17 and CCL22 are the functional ligands of CC chemokine receptor 4 (CCR4) (31, 32). CCR4 is expressed on T cells with the Th2 phenotype, Tregs, dendritic cells, macrophages, NK cells, basophils, and platelets (11). As Th2 cells express CCR4, the receptor and its ligands have been extensively studied in the context of Th2-triggered immune responses, especially concerning chronic diseases of the respiratory tract such as allergic rhinitis, bronchial asthma, or allergic aspergillosis (27, 36, 42, 43, 51, 67). It could be demonstrated that blocking CCL17 as well as CCL22 by specific antibodies results in prevention of airway hyperresponsiveness and attenuates OVA-induced airway eosinophilia (26, 37).
However, there is emerging evidence that CCR4 is involved in several immune reactions other than allergy and chronic airway disease. In a murine model of cardiac transplantation, CCR4-deficient recipients showed a significantly prolonged allograft survival accompanied by a reduced number of graft-infiltrating CD4+ cells (30). Recent reports on different types of lymphatic neoplasm but also on other kinds of cancer such as lung carcinoma or melanoma revealed an important role for CCR4 in this context and opened ways to new treatment options by using small-molecule antagonists of CCR4 (5, 14, 34, 35, 49, 70).
Surprisingly, in a model of lipopolysaccharide (LPS)-induced systemic shock, CCR4−/− mice showed a significantly reduced mortality rate associated with decreased serum levels of interleukin-1β (IL-1β) and tumor necrosis factor (TNF) (17). Similar results were recently reported by Ness et al. (50) using models of LPS-induced shock and intraperitoneal (i.p.) injection of Escherichia coli. In the absence of CCR4, an augmented activity of innate immune mechanisms could be demonstrated. After LPS challenge, CCR4−/− animals displayed increased expression of Toll-like receptor 2 (TLR-2), TLR-4, TLR-6, and TLR-9 on peritoneal cells as well as elevated secretion of type 2 cytokines by in vitro-stimulated macrophages (50). These results gained from bolus shock models indicate a role for CCR4 in sepsis.
However, immunomodulatory therapy regimens based on results from such kinds of experiments have failed to improve the outcome of abdominal sepsis in human patients (1-3, 21-23, 57, 61). Thus, experiments using animal models of LPS-induced shock or monomicrobial shock, though easy to perform and to reproduce, do not stress the whole complexity of the problem. Colon ascendens stent peritonitis (CASP) is a model of polymicrobial abdominal sepsis that was shown to be highly reproducible and clinically relevant, as it represents a common course of systemic infection of surgical intensive care patients (45, 69). In this study, we show for the first time that CCR4 plays a detrimental role in sepsis caused by polymicrobial abdominal infection.
MATERIALS AND METHODS
Mice.
For all experiments, 8- to 12-week-old C57BL/6 female mice (20 to 25 g) were used. C57BL/6 mice were purchased from Charles River (Sulzfeld, Germany). Prior to surgery, mice were kept for at least 2 weeks in our animal facility to recover after transport. CCR4−/− mice with C57BL/6 genetic background were obtained from Y. Chvatchko (Serono Pharmaceutical Research Institute, Geneva, Switzerland). CCR4−/− animals were bred in our conventional animal facility. The phenotype of CCR4−/− mice is similar to that of wild-type (WT) mice, as they show normal behavior and do not develop diseases. All experimental procedures were approved by the “Landesveterinär- und Lebensmitteluntersuchungsamt Mecklenburg-Vorpommern,” Rostock, Germany (LVL M-V/TSD/7221.3-1.2-003/04 and LALLF M-V/TSD/7221.3-1.1-003/06), and performed according to German animal safety regulations. For all surgical procedures, Avertin (Sigma-Aldrich Chemie, Taufkirchen, Germany) anesthesia was used.
CASP surgery.
The surgical procedure for CASP and CASP with intervention (CASPI) was performed as previously described (69). Under complete anesthesia and after disinfection, the lower abdominal wall was opened through a 10-mm midline incision. The ascending colon was exposed, and a prepared catheter (16-gauge, Venflon; BOC, Ohmeda AB, Sweden) was inserted through the antimesenteric wall into the lumen of the ascending colon and fixed with two sutures (7/0 Ethilon thread; Johnson-Johnson, Brüssel, Belgium). Consecutively, the inner needle of the stent was removed and the stent cut at the prepared site. To ensure proper intraluminal positioning of the stent, stool was milked from the cecum into the stent until a small amount appeared. Fluid resuscitation of animals was performed by flushing 0.5 ml of sterile saline solution into the peritoneal cavity before closure of the abdominal wall (single layer; 4/0 polyester; Catgut, Marktneukirchen, Germany). In animals subjected to CASPI, the stent was removed 5 hours after insertion and the remaining defect in the colonic wall was closed (7/0 Ethilon thread; Johnson-Johnson, Brüssel, Belgium).
Bacteriology.
For detection of the bacterial load in liver, lung, kidney, and spleen, these organs where removed 20 h after surgical treatment and placed in 5 ml ice-cold sterile saline solution (0.9% NaCl). The organs where homogenized for 30 s (2,500 turns/min) by using an Ultra-Turrax (Ultra-Turrax T25 basic; IKA, Staufen, Germany). Homogenates were plated at different dilutions on blood-containing agar (Columbus 5% SB; BD Bioscience, Heidelberg) onto petri dishes. The dishes were incubated for 18 h at 37°C. The numbers of CFU in relation to the whole organ were calculated. Whole blood was collected in EDTA-containing vials (Becton Dickinson, Heidelberg), 10 μl was plated onto agar plates to analyze bacterial loads, and results were normalized to 1 ml of whole blood after an incubation period of 18 h. For detection of the bacterial load in the peritoneal lavage fluid, 6 ml of sterile saline solution was instilled into the abdominal cavity. Four milliliters of this fluid was aspirated, and 10 μl was plated. The bacterial load was subsequently calculated in relation to 1 ml of fluid.
Cytokines/chemokines.
Several cytokines/chemokines were detected in the sera and in the supernatants of liver, lung, spleen and kidneys at 20 h after surgical treatment. Serum was separated by centrifugation of blood (10 min, 16,100 × g) (5415R centrifuge; Eppendorf, Germany). For the isolation of organ supernatants, liver, lung, spleen, and kidneys were explanted and 1 ml of a membrane-dissolving solution {1 mM EDTA, 0.25% [wt/vol] 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate [CHAPS] [Merck], 0.25% [vol/vol] Tween 20, 1 mM PEFA-Bloc [Roche], 1 mM phenylmethylsulfonyl fluoride, 1 tablet Complete in 25 ml phosphate-buffered saline [PBS] [Roche]} per 100 mg organ weight was added. Organs were homogenized using an Ultra-Turrax (Ultra-Turrax T25 basic; IKA, Staufen, Germany) and then centrifuged twice (355 × g, 20 min, 4°C). The supernatant was separated. For cytokine detection, a commercially available CBA detection kit (BD cytometric bead array mouse inflammation kit; BD Bioscience, Heidelberg, Germany) was used according to the manufacturer's instructions. Cytokine levels in the organ supernatants were normalized to protein detected in the supernatant by the Bradford reaction.
Detection of apoptotic cells.
Cells of the thymus and the mesenteric lymph nodes were isolated by passing the organs through a 100-μm nylon mesh (BD Falcon cell strainer; BD Bioscience) after explantation. Cells were stored on ice and washed three times with 2% fetal calf serum (Biochrom) in PBS. After the first washing step, a buffer(155 mM NH4Cl, 10 mM KHCO3, 0.1 mM EDTA) was added and left for 10 min to destroy remaining erythrocytes. The volume of the added buffer was 10 times the initial reaction volume. A total of 105 cells were ethanol fixed and incubated with 0.025 M sodium citrate and 0.067 M disodium phosphate (pH 7.8) at room temperature. The pellets were washed with PBS plus 5% fetal calf serum, resuspended in 30 μl RNase (1 mg/ml), and stained with 25 μg/ml propidium iodide (Sigma Aldrich, Steinheim). Apoptosis was measured by flow cytometry (FACScan; Becton Dickinson). The hypodiploid DNA peak in single-parameter DNA histograms typical of apoptotic cells was identified.
RNA isolation.
For RNA isolation, the thymus, spleen, liver, and mesenteric lymph nodes were explanted at 3 h and 20 h after CASP surgery. To produce a standardized RNA master mix, the kidneys of five native female C57BL/6 mice (one kidney each) were collected. The samples were immediately frozen in liquid nitrogen. After homogenization (Ultra-Turrax T25 basic; IKA, Staufen, Germany), total RNA was isolated using the RNeasy Midi kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. RNA concentration and quality were assessed by absorbance measurement using a spectrophotometer (GeneRay UV photometer; Biometra).
Real-time reverse transcriptase PCR (RT-PCR).
Total RNA was reverse transcribed in a 25-μl reaction volume containing 1 μg RNA each using the TaqMan reverse transcription kit (Applied Biosystems, Weiterstadt, Germany) as described in the manufacturer's instructions. The RNA amounts for CCR4, CCL17, and CCL22 were measured by TaqMan real-time PCR with an ABI Prism 7000 sequence detection system (Applied Biosystems). We used commercial available TaqMan assays for CCR4 (Mm99999052_s1; Applied Biosystems), CCL17 (Mm99999167_s1; Applied Biosystems), and CCL22 (Mm00436439_m1; Applied Biosystems) and a TaqMan universal PCR master mix (Applied Biosystems). The cycler protocol (2 min at 50°C, 15 min at 95°C, and 1 min at 60°C) was repeated 40 times. All levels of CCL17, CCL22, and CCR4 measured in thymus, spleen, mesenteric lymph nodes, and liver were normalized to the respective RNA amounts found in kidneys of native mice (see above).
Culture and infection of macrophages.
Bone marrow-derived macrophages were cultivated in serum-free medium. Briefly, tibias and femurs from either C57BL/6 WT or CCR4−/− mice were aseptically removed, and bone marrow cells were flushed with sterile PBS and then centrifuged at 150 × g for 10 min. Cells were resuspended in RPMI 1640 (Biochrom) containing 5% modified Panexin (PAN Biotech, Aidenbach, Germany), 2 ng/ml recombinant murine granulocyte-macrophage colony-stimulating factor (PAN), and 50 μM mercaptoethanol and cultivated for 10 days at 37°C and 5% CO2. Twenty-four hours prior to infection, bone marrow-derived macrophages were seeded in 48-well plates (1.5 × 105 cells per well) and stimulated with 100 U/ml recombinant murine gamma interferon (IFN-γ) (Roche). Bone marrow-derived macrophages were infected with either Burkholderia pseudomallei strain E8 (66) or Salmonella enterica serovar Typhimurium ATCC 14028 with multiplicities of infection (MOI) as indicated for 30 min. The medium was then removed and exchanged for medium containing 100 μg/ml kanamycin (B. pseudomallei experiments) or 50 μg/ml gentamicin (S. enterica serovar Typhimurium experiments) to eliminate extracellular bacteria. At the indicated time points (time zero was 20 min after incubation in antibiotic-containing medium), the number of intracellular CFU was determined as previously described (12).
Statistical methods.
Statistical analysis was performed using GraphPad Prism for Windows (GraphPad Software, Inc., San Diego, CA). Statistical differences in survival were assessed using Kaplan-Meier survival curves and the log rank test. Results for RNA expression, bacterial cultures, cytokine/chemokine levels, and apoptotic cells were analyzed using the two-tailed Mann-Whitney U test for nonparametric probes. A significance level of 0.05 was determined for all calculations.
RESULTS
Regulation of CCR4 in polymicrobial sepsis.
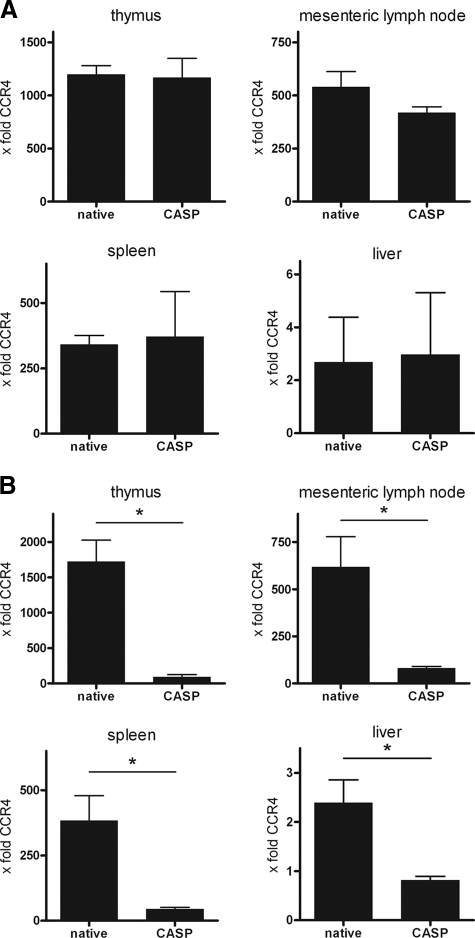
To address the role of CCR4 in experimental peritonitis, RNA levels in the livers, spleens, mesenteric lymph nodes, and thymuses of CCR+/+ mice were analyzed using real-time RT-PCR. All RNA levels were normalized to CCR4 expression in kidneys of untreated mice. In native mice, the strongest expression was observed in the thymus, followed by the mesenteric lymph nodes, the spleen, and the liver, revealing a tissue-specific distribution of CCR4. In the early phase of sepsis (3 h), RNA levels of CCR4 were comparable to those found in the native controls (Fig. 1A). At 20 h after CASP, a massive downregulation of CCR4 could be detected in all organs examined (Fig. 1B). The amount of constitutive expression of CCR4 RNA in organs correlated with its decrease after sepsis induction.
FIG. 1.
Regulation of CCR4. At 3 and 20 h after CASP, RNA was isolated from thymus, spleen, mesenteric lymph nodes, and liver, and real-time RT-PCR was performed. All levels of CCR4 were normalized to those found in kidneys of native WT animals and are given as x-fold the expression in the kidneys (n = 4/group; *, P < 0.05). Error bars indicate standard errors of the means. At 3 h after CASP, no changes in CCR4 RNA levels were detected (A). At 20 h after CASP, a massive downregulation of CCR4 was found in all organs examined (B).
Regulation patterns of CCL17 and CCL22.
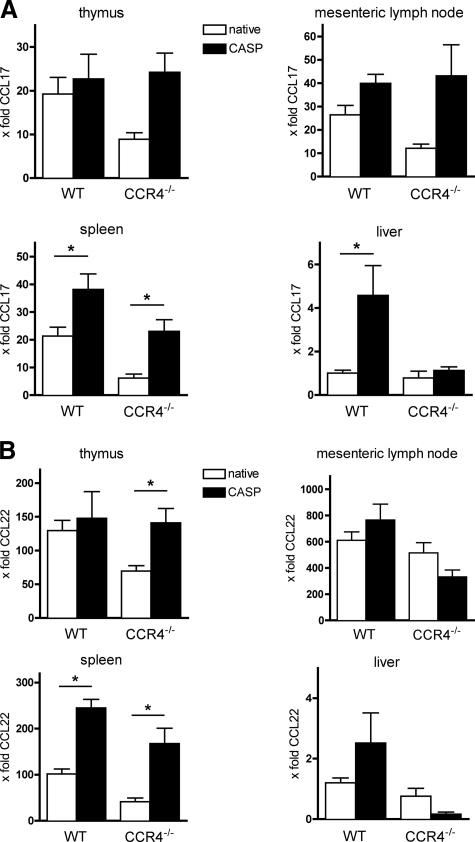
Real-time RT-PCR analysis of CCL17 and CCL22 was performed. In WT animals, CASP led to a significant increase of CCL17 and CCL22 in the spleen (Fig. 2A and B). In the liver, only small amounts of CCL17 and CCL22 could be detected in native mice, whereas a significant increase of CCL17 was found after CASP. In the mesenteric lymph nodes and in the thymus, a strong constitutive expression of CCL17 and CCL22 was observed. After CASP, no significant alteration of CCL17 and CCL22 was detected in either organ.
FIG. 2.
Patterns of regulation of CCL17 and CCL22 in WT and CCR4−/− mice. At 20 h after CASP induced peritonitis, RNA was isolated from thymus, spleen, mesenteric lymph nodes, and liver, and real-time RT-PCR was performed. All levels of CCL17 and CCL22 were normalized to those found in kidneys of native WT animals and are given as x-fold the expression in the kidneys (n = 4/group; *, P < 0.05). Error bars indicate standard errors of the means. The constitutive expression of CCL17 was lower in CCR4−/− mice. After CASP, increased levels of CCL17 were detected in WT and CCR4−/− animals (A). The regulation of CCL22 was predominantly analogous to that of CCL17, yet in the mesenteric lymph nodes and the livers of CCR4−/− mice, a mild decrease in CCL22 RNA was found after CASP (B).
To assess the effect of CCR4 on the expression of its ligands, RNA expression of CCL17 and CCL22 in CCR4−/− mice was determined (Fig. 2A and 2B). In all organs analyzed, the constitutive RNA expression was slightly lower than that in the native WT mice. Under septic conditions, a significant increase of CCL17 and CCL22 was detected in the spleen, similar to the findings for CCR4+/+ mice. No relevant changes could be found in the liver, whereas a remarkable increase of both chemokines was measured in the thymus. In the mesenteric lymph nodes, an increased expression of CCL17 could be observed that was accompanied by a small decrease of CCL22.
Taking the results together, WT and CCR4−/− mice showed comparable patterns of expression of CCL17 and CCL22 in the native and septic status. The ligand regulation in polymicrobial sepsis was therefore independent of the presence of CCR4.
Improved bacterial clearance in CCR4-deficient mice.
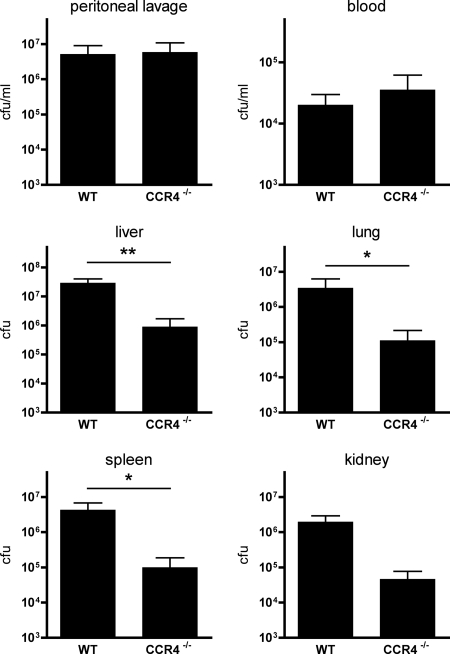
To address the influence of CCR4 deficiency on the local and systemic levels of infection after CASP, bacteriological cultures of liver, lung, spleen, kidney, blood, and peritoneal lavage fluid were performed. Untreated control WT and CCR4−/− mice were completely sterile (data not shown). CASP led to comparably large amounts of bacteria in the blood and in the peritoneal lavage fluid of WT and CCR4−/− animals (Fig. 3). In marked contrast, all examined solid organs of CCR4−/− mice showed a strongly reduced number of CFU compared to that in the septic WT animals. This result indicates that CCR4 is critically involved in local defense against bacteria, as in its absence bacterial clearance is strongly improved.
FIG. 3.
Bacterial cultures of various organs. CASP was performed in WT and CCR4−/− mice, and 20 h afterwards, peritoneal lavage fluid and blood were taken, organs were homogenized, and bacterial cultures of the specimens were performed with appropriate dilutions. Values are given as CFU per ml and CFU per organ as indicated (n ≥ 5/group; *, P < 0.05; **, P < 0.01). Error bars indicate standard errors of the means. CCR4−/− mice showed strongly reduced amounts of bacteria in all solid organs compared to the WT controls. In the peritoneal lavage fluid and in the blood, comparable numbers of bacteria were found in both groups.
CCR4 deficiency does not affect bactericidal activity of macrophages ex vivo.
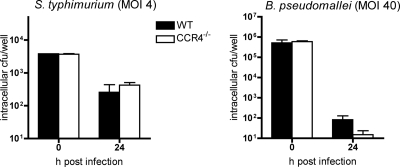
In order to investigate any influence of CCR4 deficiency on the inherent bactericidal function of macrophages against bacterial pathogens, an ex vivo assay was performed using bone marrow-derived macrophages from CCR4−/− and WT mice. IFN-γ-stimulated macrophages were infected with either Salmonella enterica serovar Typhimurium or Burkholderia pseudomallei, bacterial pathogens which reside in a membrane-bound vacuole or escape into the cytosol of the host cell, respectively (13, 53). WT and CCR4−/− mouse-derived macrophages showed no significant difference in killing activity at 24 h after infection. Approximately 90% of intracellular S. enterica serovar Typhimurium organisms and more than 99.99% of B. pseudomallei organisms were eliminated in both WT and CCR4−/− mouse-derived macrophages (Fig. 4). Thus, the observed increased bacterial clearance in solid organs of CCR4−/− mice seems to be independent of an inherent increased bactericidal activity of macrophages from these mice.
FIG. 4.
Bactericidal activity of CCR4−/− macrophages. Bone marrow-derived macrophages isolated from WT and CCR4−/− mice were IFN-γ stimulated and subsequently infected with B. pseudomallei (MOI, 40:1) and S. enterica serovar Typhimurium (MOI, 4:1). Intracellular bacterial numbers were determined 24 h after infection (results are for at least duplicate determinations; values from representative experiments are shown.). Error bars indicate standard errors of the means. Macrophages of both groups showed comparable bacterial uptake and intracellular clearance.
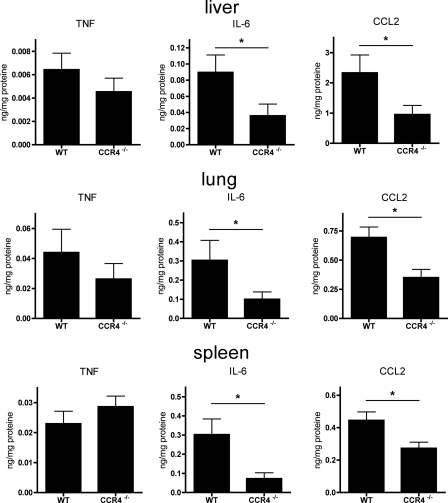
Local cytokine alterations in septic CCR4−/− mice.
The increased local bacterial clearance in CCR4−/− mice was associated with altered cytokine/chemokine secretion in the liver, lung, and spleen. IL-6, TNF, and CCL2 were measured by cytometric bead array in the respective organ supernatants after CASP. No cytokines could be detected in untreated control WT and CCR4−/− mice (not shown). Small amounts of TNF were measured in liver, lung, and spleen in both groups; however, no difference between WT and CCR4−/− mice could be observed (Fig. 5). Both IL-6 and CCL2 were elevated in septic WT animals in all three organs examined. Interestingly, CCR4 deficiency resulted in a significantly reduced secretion of IL-6 and CCL2 in liver, lung, and spleen (Fig. 5), indicating that the absence of CCR4 leads to an attenuated local proinflammatory response to polymicrobial infection.
FIG. 5.
Cytokines/chemokines in organ supernatants. At 20 h after CASP-induced sepsis, the liver, lung, and spleen were homogenized, and levels of TNF, IL-6, and CCL2 in the organ supernatants were measured by cytometric bead array (n ≥ 10/group; *, P < 0.05). Error bars indicate standard errors of the means. Whereas secretion of TNF was comparable in WT and CCR4−/− animals, significantly reduced amounts of IL-6 and CCL2 were detected in CCR4-deficient animals.
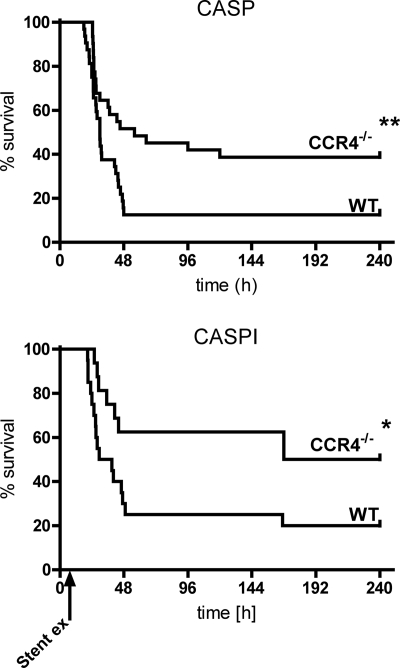
Improved survival rates of CCR4-deficient mice in polymicrobial sepsis.
CASP was performed in WT and CCR4−/− animals, and survival rates were analyzed (Fig. 6). Sham operations revealed a survival rate of 100% in both groups (not shown). According to previous findings in this model of polymicrobial sepsis, WT animals showed a survival rate of 12% after an observation period of 10 days. In marked contrast, CCR4−/− mice revealed a delayed mortality in the first days and a strikingly improved survival rate of 42% in the end of the observation period. In a second experiment, CASPI was performed: the stent in the ascending colon was removed after 5 h (Fig. 6). WT animals showed a survival rate of 20%, whereas CCR4−/− mice again had a noticeably better outcome of 50%. Taking the results together, in both CASP and CASPI a strong survival benefit for CCR4−/− mice could clearly be demonstrated.
FIG. 6.
Survival analysis. Survival rates of WT and CCR4−/− mice were assessed following CASP and CASPI. CCR4−/− animals had a significantly improved outcome after CASP (n ≥ 31/group; **, P < 0.01; data are from four independent experiments with comparable results). The significant survival benefit for CCR4−/− mice could be confirmed in the CASPI model (n ≥ 16/group; *, P < 0.05; data are from three independent experiments with comparable results). Error bars indicate standard errors of the means.
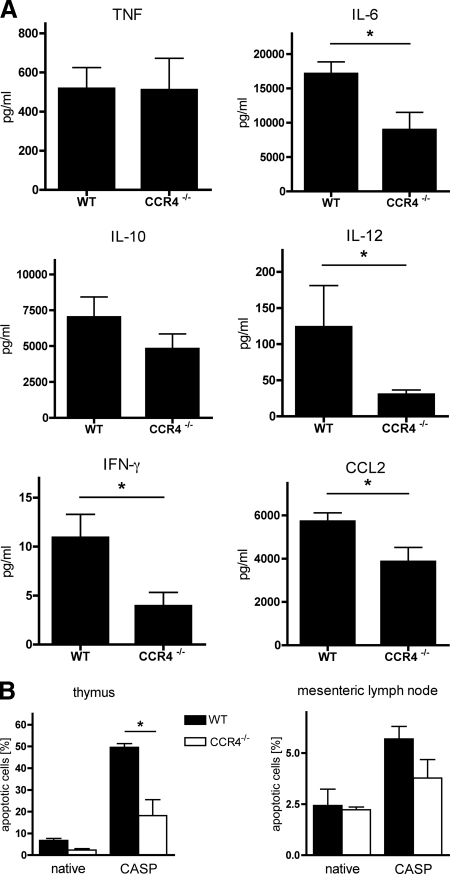
CCR4 deficiency results in attenuated sepsis.
The improved survival rate of CCR4−/− mice was associated with an attenuated systemic cytokine response to CASP-derived sepsis. Serum levels of TNF, IL-6, IL-10, IL-12p70, IFN-γ, and CCL2 were measured 20 h after CASP. No cytokines could be detected in untreated control WT and CCR4−/− mice (not shown). All measured molecules except TNF, which showed comparable amounts in both septic groups, were considerably reduced in CCR4−/− mice (Fig. 7A).
FIG. 7.
Serum cytokines/chemokines and apoptotic cells. (A) At 20 h after CASP, levels of TNF, IL-6, IL-10, IL-12p70, IFN-γ, and CCL2 in the sera of WT and CCR4−/− animals were measured using cytometric bead array (n ≥ 8/group; *, P < 0.05). Whereas secretion of TNF was comparable in WT and CCR4−/− animals, strongly reduced amounts of all other examined cytokines/chemokines were detected in CCR4−/− animals. (B) Apoptotic cells in the thymus and the mesenteric lymph nodes of WT and CCR4−/− animals at 20 h after CASP were detected by fluorescence-activated cell sorter analysis. Values of apoptotic cells are given as percentage of all analyzed cells (n = 3/group; *, P < 0.05). Error bars indicate standard errors of the means. Septic CCR4−/− mice showed reduced levels of apoptotic cells in comparison to the WT controls.
A second experiment to address the systemic extent of sepsis in the respective groups was performed by monitoring the amount of apoptotic cells in the thymus and the mesenteric lymph nodes as described previously (9). At 20 h after CASP-induced sepsis, the fraction of apoptotic cells in the thymus of WT mice reached 50% (Fig. 7B). In marked contrast, septic CCR4−/− animals revealed a significantly lower rate of 18% apoptotic cells. An analogous trend was found in the mesenteric lymph nodes.
DISCUSSION
CCR4 and its two known ligands, CCL17 and CCL22, are crucially involved in Th2-triggered immune reactions such as chronic airway diseases or inflammatory skin reactions (27, 36, 37, 42, 43, 51, 55, 63, 67). However, CCR4−/− mice revealed no phenotype in a model of OVA-induced airway inflammation but, unexpectedly, showed reduced mortality after LPS-induced shock, accompanied by an attenuated release of macrophage-associated proinflammatory cytokines (17). Analogous findings were recently reported by Ness et al. using models of LPS-induced shock and i.p. injection of E. coli (50).
In this study, we examined for the first time the role of CCR4 in the context of polymicrobial abdominal sepsis using CCR4−/− mice in the model of CASP (38, 39, 45, 47, 69). CCR4−/− animals showed reduced organ infection associated with an attenuated proinflammatory cytokine response, resulting in a milder form of systemic sepsis and improved survival rates.
In a first series of experiments, we addressed the distribution of CCR4 expression in regard to lymphoid and nonlymphoid organs and the consequences of a septic challenge for its regulation. CCR4 was found predominantly in the thymus, followed by the mesenteric lymph nodes, the spleen, and the liver. This fits with the result that CCR4 is expressed mainly on immune cells such as Th2 cells, CD4+ Foxp3+ Tregs, dendritic cells, NK cells, and monocytes but also on platelets (11, 16, 18, 33, 40, 54, 58, 64, 65). Interestingly, CASP-induced sepsis led to a massive downregulation of CCR4 RNA in all organs examined. The generalized downregulation in crucial organ systems must be seen as a direct consequence of the severe infection resulting from abdominal sepsis. In an additional experiment, splenocytes isolated from native WT mice revealed strongly reduced CCR4 RNA levels after 24 h of stimulation with LPS in vitro (data not shown). This finding indicates that the observed diminution of CCR4 message in several organs following CASP-induced sepsis results more from downregulation on a cellular level than from an efflux of CCR4-bearing cells. Apparently, the presence of CCR4 in peripheral organs during the course of sepsis seems to have adverse effects as the organism tries to diminish its transcription. CCR4 RNA levels did not drop until 3 h after CASP. In previous experiments, the point of no return for the sepsis cascade in the CASP model was located at between 3 and 5 h after the onset of sepsis (69). Thus, the massive drop of CCR4 levels in septic WT mice comes too late as to have a protective effect for these animals.
A simultaneous upregulation of CCL17 and CCL22 found predominantly in the spleen seems to be contrary to the universal downregulation of their receptor. However, the upregulation of CCL17 and CCL22 was independent from the presence of CCR4, as CCR4−/− mice showed similar patterns of regulation of CCL17 and CCL22. The sources of CCL17 and CCL22 are macrophages, dendritic cells, T cells, and B cells but also nonimmune cells such as epithelial cells, fibroblasts, and smooth muscle cells (4, 7, 8, 20, 24, 25, 31, 32, 41, 68). A severe septic challenge such as CASP results in a general activation of these cells and leads to secretion of many cytokines and chemokines, including CCL17 and CCL22. As the patterns of regulation of CCL17 and CCL22 are independent from the presence of CCR4, there seems to be no feedback mechanism between the receptor and its ligands. Moreover, it is possible that there are unknown biological functions of CCL17 and CCL22 apart from those associated with CCR4.
Bacterial cultures showed that infection of the peritoneal cavity resulted in a severe bacteremia independent of the presence of CCR4. Interestingly, local bacterial clearance in several organs seemed to be markedly improved in the absence of CCR4. To evaluate the direct impact of CCR4 on antibacterial functions of macrophages, we infected IFN-γ-stimulated bone marrow-derived macrophages of CCR4−/− mice and WT mice with either B. pseudomallei or S. enterica serovar Typhimurium, both of which are pathogens known to be highly virulent for mice (28, 60). Our results suggest that CCR4−/− deficiency does not augment the inherent bactericidal activity of macrophages per se. However, we cannot exclude that CCR4−/− deficiency might lead to an enhanced bactericidal activity of macrophages in our in vivo model via unknown mechanisms and thereby contribute to the enhanced bacterial clearance in CCR4−/− mice.
We reported recently that depletion of CD4+ cells in mice subjected to CASP results in a strongly increased invasion of granulocytes and monocytes into the peritoneal cavity, leading to reduced bacterial numbers in peritoneal lavage fluid, blood, and all examined organs. This dampening effect of CD4+ cells on the innate immune response could be shown to be detrimental for the survival of severe sepsis (15). As the migration of CD4+ Foxp3+ Tregs into nonlymphoid organs, e.g., liver and lung, is associated mainly with the expression of CCR4 (58), it may be concluded that absence of CCR4 leads to a reduced extravasation of Tregs into nonlymphoid organs, resulting in a diminished repression of the local innate immune defense. In this context, the massive downregulation of CCR4 in septic WT animals could be a kind of self-protection to reduce the recruitment of immune-dampening CD4+ cells to the local sites of acute and severe infection.
The augmented bacterial clearance in CCR4−/− mice was associated with locally as well as systemically reduced secretion of IL-6 and CCL2, whereas levels of TNF were comparable to those in WT controls. Previous studies examining CCR4−/− mice in the context of septic hyperinflammation used models of LPS-induced shock (17, 50). Chvatchko et al. attributed the unexpected survival benefit of CCR4−/− mice to the reduced serum levels of TNF, which were interpreted as an impaired hyperinflammatory response (17). It is known from several studies that TNF is a crucial molecule for survival in LPS-induced shock; furthermore, TNF application alone can cause septic shock in a dose-dependent manner (10, 52, 56, 62). However, in contrast to models of LPS-induced shock, in which blocking of TNF signals is protective, TNF receptor p55 deficiency had no effect in the CASP model (52, 56, 69). Therefore, the comparably large amounts of TNF after CASP in both the CCR4−/− and the WT controls were not surprising, as they merely indicate a status of local and systemic sepsis caused by polymicrobial infection.
In contrast, levels of IL-6 and CCL2 were significantly reduced in CCR4-deficient animals after CASP. CCL2 has been determined to be a proinflammatory chemokine; it is produced by macrophages, monocytes, fibroblasts, and keratinocytes and especially attracts macrophages, monocytes, NK cells, and T cells via its receptor CCR2 (46, 59). IL-6 is a proinflammatory cytokine secreted by macrophages, endothelial cells, and T cells that is critically involved in the acute-phase reaction following infectious stimuli (29). Both IL-6 and CCL2 are local as well as systemic players in the course of sepsis. Their reduced levels in the liver, lung, and spleen of CCR4−/− mice may be seen as a consequence of the decreased number of bacteria found in these organs. The lower release of IL-6 and CCL2 represents an attenuated inflammatory reaction as a sign of enhanced local infection control in the absence of CCR4.
Apoptotic cells in the thymus and the mesenteric lymph nodes after CASP were clearly reduced in CCR4−/− mice. In a recent study, we could demonstrate a rapid loss of T cells in the thymus due to a massive apoptotic activity after CASP-induced polymicrobial sepsis (15). As this phenomenon is a strict consequence of the systemic infection caused by CASP, the diminished number of apoptotic cells in the thymus and the mesenteric lymph nodes of CCR4-deficient mice must be taken as a clear marker for significantly alleviated severity of sepsis. The observed reduction of serum IL-6 and CCL2 levels after CASP additionally underlines the attenuated systemic response in CCR4−/− mice.
The improved survival rates of CCR4−/− mice following CASP as well as CASPI may be seen mainly as a consequence of the reduced level of organ infection due to augmented bacterial clearance. In previous studies using the CASP/CASPI model, it could be demonstrated that reduced bacterial loads were predominantly associated with improved survival rates (15, 38, 39, 44, 69). Furthermore, a shift to a systemic anti-inflammatory cytokine response was shown to be protective (19, 38, 39). In CASPI, the bacterial leakage from the gut into the peritoneal cavity is interrupted by removing the stent 5 h after implantation. Surgical sanitation of the septic focus is commonly performed in clinics as most important therapeutic approach in terms of abdominal sepsis (45, 69). In this study we used CASPI as an additional model to stress the relevance of the improved outcome of CCR4−/− mice. In these animals, sanitation of the bacterial focus led to a strongly decreased mortality rate, underlining that survival in this context depends more on increased bacterial clearance than on an attenuated cytokine response, which seems to be a secondary effect.
In a recent study, CCR4−/− mice revealed a tendency to have reduced bacterial numbers in the peritoneal cavity and the serum after single i.p. injection of E. coli. This finding was associated with reduced serum levels of CCL2, increased expression of TLR-2, TLR-4, TLR-6, and TLR-9 on peritoneal cells, and an increased number of peritoneal leukocytes in CCR4−/− mice (50). According to these data, CCR4 deficiency resulted in an augmented activity of the innate immune system in the monomicrobial model of i.p. E. coli injection. Our results, gained from a more complex animal model of polymicrobial abdominal sepsis, confirm these findings and adjust the pivotal role of CCR4 to generalized polymicrobial infection. For the first time, our data define the role of CCR4 in the context of polymicrobial systemic infection as an exceedingly detrimental one.
In summary, this study clearly demonstrates a harmful role for CCR4 during the course of severe sepsis caused by polymicrobial abdominal infection. The massive downregulation of CCR4 in WT animals after septic challenge might reflect an attempt to circumvent this deleterious effect. Our data show that the absence of CCR4 results in augmented bacterial clearance and a significantly reduced mortality rate. These findings suggest that the presence of CCR4-positive cells is responsible for maintaining infection in several organ systems.
Acknowledgments
This work was supported by the Bundesministerium für Forschung und Technologie (BMBF/NBL3 grant DM5-KINC-03) and by the Deutsche Forschungsgemeinschaft (GRK-840).
We are grateful to C. Powers and Y. Chvatchko (Serono Pharmaceutical Research Institute, Geneva, Switzerland) for the kind gift of CCR4−/− mice. We thank K. Mülling, A. Müller, and T. Rossmann-Bloeck for expert technical assistance. We thank Barbara M. Broeker for helpful discussions during the process of this work.
We declare that we have no financial conflicts of interest.
Editor: F. C. Fang
Footnotes
Published ahead of print on 2 September 2008.
REFERENCES
- 1.Abraham, E. 1999. Why immunomodulatory therapies have not worked in sepsis. Intensive Care Med. 25556-566. [DOI] [PubMed] [Google Scholar]
- 2.Abraham, E., A. Anzueto, G. Gutierrez, S. Tessler, G. San Pedro, R. Wunderink, A. Dal Nogare, S. Nasraway, S. Berman, R. Cooney, H. Levy, R. Baughman, M. Rumbak, R. B. Light, L. Poole, R. Allred, J. Constant, J. Pennington, S. Porter, et al. 1998. Double-blind randomised controlled trial of monoclonal antibody to human tumour necrosis factor in treatment of septic shock. Lancet 351929-933. [PubMed] [Google Scholar]
- 3.Abraham, E., M. P. Glauser, T. Butler, J. Garbino, D. Gelmont, P. F. Laterre, K. Kudsk, H. A. Bruining, C. Otto, E. Tobin, C. Zwingelstein, W. Lesslauer, A. Leighton, et al. 1997. p55 Tumor necrosis factor receptor fusion protein in the treatment of patients with severe sepsis and septic shock. A randomized controlled multicenter trial. JAMA 2771531-1538. [PubMed] [Google Scholar]
- 4.Andrew, D. P., M. S. Chang, J. McNinch, S. T. Wathen, M. Rihanek, J. Tseng, J. P. Spellberg, and C. G. Elias III. 1998. STCP-1 (MDC) CC chemokine acts specifically on chronically activated Th2 lymphocytes and is produced by monocytes on stimulation with Th2 cytokines IL-4 and IL-13. J. Immunol. 1615027-5038. [PubMed] [Google Scholar]
- 5.Baatar, D., P. Olkhanud, D. Newton, K. Sumitomo, and A. Biragyn. 2007. CCR4-expressing T cell tumors can be specifically controlled via delivery of toxins to chemokine receptors. J. Immunol. 1791996-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bachmann, M. F., M. Kopf, and B. J. Marsland. 2006. Chemokines: more than just road signs. Nat. Rev. Immunol. 6159-164. [DOI] [PubMed] [Google Scholar]
- 7.Berin, M. C., M. B. Dwinell, L. Eckmann, and M. F. Kagnoff. 2001. Production of MDC/CCL22 by human intestinal epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 280G1217-G1226. [DOI] [PubMed] [Google Scholar]
- 8.Berin, M. C., L. Eckmann, D. H. Broide, and M. F. Kagnoff. 2001. Regulated production of the T helper 2-type T-cell chemoattractant TARC by human bronchial epithelial cells in vitro and in human lung xenografts. Am. J. Respir. Cell Mol. Biol. 24382-389. [DOI] [PubMed] [Google Scholar]
- 9.Bernstorff, W. V., J. N. Glickman, R. D. Odze, F. A. Farraye, H. G. Joo, P. S. Goedegebuure, and T. J. Eberlein. 2002. Fas (CD95/APO-1) and Fas ligand expression in normal pancreas and pancreatic tumors. Implications for immune privilege and immune escape. Cancer 942552-2560. [DOI] [PubMed] [Google Scholar]
- 10.Beutler, B., I. W. Milsark, and A. C. Cerami. 1985. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science 229869-871. [DOI] [PubMed] [Google Scholar]
- 11.Bonecchi, R., G. Bianchi, P. P. Bordignon, D. D'Ambrosio, R. Lang, A. Borsatti, S. Sozzani, P. Allavena, P. A. Gray, A. Mantovani, and F. Sinigaglia. 1998. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J. Exp. Med. 187129-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Breitbach, K., S. Klocke, T. Tschernig, N. van Rooijen, U. Baumann, and I. Steinmetz. 2006. Role of inducible nitric oxide synthase and NADPH oxidase in early control of Burkholderia pseudomallei infection in mice. Infect. Immun. 746300-6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brumell, J. H., and S. Grinstein. 2004. Salmonella redirects phagosomal maturation. Curr. Opin. Microbiol. 778-84. [DOI] [PubMed] [Google Scholar]
- 14.Burdi, D. F., S. Chi, K. Mattia, C. Harrington, Z. Shi, S. Chen, S. Jacutin-Porte, R. Bennett, K. Carson, W. Yin, V. Kansra, J. A. Gonzalo, A. Coyle, B. Jaffee, T. Ocain, M. Hodge, G. LaRosa, and G. Harriman. 2007. Small molecule antagonists of the CC chemokine receptor 4 (CCR4). Bioorg. Med. Chem. Lett. 173141-3145. [DOI] [PubMed] [Google Scholar]
- 15.Busse, M., T. Traeger, C. Potschke, A. Billing, A. Dummer, E. Friebe, C. Kiank, U. Grunwald, R. S. Jack, C. Schutt, C. D. Heidecke, S. Maier, and B. M. Broker. 2008. Detrimental role for CD4+ T lymphocytes in murine diffuse peritonitis due to inhibition of local bacterial elimination. Gut. 57188-195. [DOI] [PubMed] [Google Scholar]
- 16.Chantry, D., A. J. DeMaggio, H. Brammer, C. J. Raport, C. L. Wood, V. L. Schweickart, A. Epp, A. Smith, J. T. Stine, K. Walton, L. Tjoelker, R. Godiska, and P. W. Gray. 1998. Profile of human macrophage transcripts: insights into macrophage biology and identification of novel chemokines. J. Leukoc Biol. 6449-54. [DOI] [PubMed] [Google Scholar]
- 17.Chvatchko, Y., A. J. Hoogewerf, A. Meyer, S. Alouani, P. Juillard, R. Buser, F. Conquet, A. E. Proudfoot, T. N. Wells, and C. A. Power. 2000. A key role for CC chemokine receptor 4 in lipopolysaccharide-induced endotoxic shock. J. Exp. Med. 1911755-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clemetson, K. J., J. M. Clemetson, A. E. Proudfoot, C. A. Power, M. Baggiolini, and T. N. Wells. 2000. Functional expression of CCR1, CCR3, CCR4, and CXCR4 chemokine receptors on human platelets. Blood 964046-4054. [PubMed] [Google Scholar]
- 19.Emmanuilidis, K., H. Weighardt, S. Maier, K. Gerauer, T. Fleischmann, X. X. Zheng, W. W. Hancock, B. Holzmann, and C. D. Heidecke. 2001. Critical role of Kupffer cell-derived IL-10 for host defense in septic peritonitis. J. Immunol. 1673919-3927. [DOI] [PubMed] [Google Scholar]
- 20.Faffe, D. S., T. Whitehead, P. E. Moore, S. Baraldo, L. Flynt, K. Bourgeois, R. A. Panettieri, and S. A. Shore. 2003. IL-13 and IL-4 promote TARC release in human airway smooth muscle cells: role of IL-4 receptor genotype. Am. J. Physiol. Lung Cell Mol. Physiol. 285L907-914. [DOI] [PubMed] [Google Scholar]
- 21.Fisher, C. J., Jr., J. M. Agosti, S. M. Opal, S. F. Lowry, R. A. Balk, J. C. Sadoff, E. Abraham, R. M. Schein, E. Benjamin, et al. 1996. Treatment of septic shock with the tumor necrosis factor receptor:Fc fusion protein. N. Engl. J. Med. 3341697-1702. [DOI] [PubMed] [Google Scholar]
- 22.Fisher, C. J., Jr., J. F. Dhainaut, S. M. Opal, J. P. Pribble, R. A. Balk, G. J. Slotman, T. J. Iberti, E. C. Rackow, M. J. Shapiro, R. L. Greenman, et al. 1994. Recombinant human interleukin 1 receptor antagonist in the treatment of patients with sepsis syndrome. Results from a randomized, double-blind, placebo-controlled trial. JAMA 2711836-1843. [PubMed] [Google Scholar]
- 23.Fisher, C. J., Jr., G. J. Slotman, S. M. Opal, J. P. Pribble, R. C. Bone, G. Emmanuel, D. Ng, D. C. Bloedow, M. A. Catalano, et al. 1994. Initial evaluation of human recombinant interleukin-1 receptor antagonist in the treatment of sepsis syndrome: a randomized, open-label, placebo-controlled multicenter trial. Crit. Care Med. 2212-21. [DOI] [PubMed] [Google Scholar]
- 24.Fukuda, K., Y. Fujitsu, K. Seki, N. Kumagai, and T. Nishida. 2003. Differential expression of thymus- and activation-regulated chemokine (CCL17) and macrophage-derived chemokine (CCL22) by human fibroblasts from cornea, skin, and lung. J. Allergy Clin. Immunol. 111520-526. [DOI] [PubMed] [Google Scholar]
- 25.Godiska, R., D. Chantry, C. J. Raport, S. Sozzani, P. Allavena, D. Leviten, A. Mantovani, and P. W. Gray. 1997. Human macrophage-derived chemokine (MDC), a novel chemoattractant for monocytes, monocyte-derived dendritic cells, and natural killer cells. J. Exp. Med. 1851595-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gonzalo, J. A., Y. Pan, C. M. Lloyd, G. Q. Jia, G. Yu, B. Dussault, C. A. Powers, A. E. Proudfoot, A. J. Coyle, D. Gearing, and J. C. Gutierrez-Ramos. 1999. Mouse monocyte-derived chemokine is involved in airway hyperreactivity and lung inflammation. J. Immunol. 163403-411. [PubMed] [Google Scholar]
- 27.Hartl, D., K. F. Buckland, and C. M. Hogaboam. 2006. Chemokines in allergic aspergillosis—from animal models to human lung diseases. Inflamm. Allergy Drug Targets 5219-228. [DOI] [PubMed] [Google Scholar]
- 28.Hoppe, I., B. Brenneke, M. Rohde, A. Kreft, S. Haussler, A. Reganzerowski, and I. Steinmetz. 1999. Characterization of a murine model of melioidosis: comparison of different strains of mice. Infect. Immun. 672891-2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horn, F., C. Henze, and K. Heidrich. 2000. Interleukin-6 signal transduction and lymphocyte function. Immunobiology 202151-167. [DOI] [PubMed] [Google Scholar]
- 30.Huser, N., C. Tertilt, K. Gerauer, S. Maier, T. Traeger, V. Assfalg, R. Reiter, C. D. Heidecke, and K. Pfeffer. 2005. CCR4-deficient mice show prolonged graft survival in a chronic cardiac transplant rejection model. Eur. J. Immunol. 35128-138. [DOI] [PubMed] [Google Scholar]
- 31.Imai, T., M. Baba, M. Nishimura, M. Kakizaki, S. Takagi, and O. Yoshie. 1997. The T cell-directed CC chemokine TARC is a highly specific biological ligand for CC chemokine receptor 4. J. Biol. Chem. 27215036-15042. [DOI] [PubMed] [Google Scholar]
- 32.Imai, T., D. Chantry, C. J. Raport, C. L. Wood, M. Nishimura, R. Godiska, O. Yoshie, and P. W. Gray. 1998. Macrophage-derived chemokine is a functional ligand for the CC chemokine receptor 4. J. Biol. Chem. 2731764-1768. [DOI] [PubMed] [Google Scholar]
- 33.Inngjerdingen, M., B. Damaj, and A. A. Maghazachi. 2000. Human NK cells express CC chemokine receptors 4 and 8 and respond to thymus and activation-regulated chemokine, macrophage-derived chemokine, and I-309. J. Immunol. 1644048-4054. [DOI] [PubMed] [Google Scholar]
- 34.Ishida, T., S. Iida, Y. Akatsuka, T. Ishii, M. Miyazaki, H. Komatsu, H. Inagaki, N. Okada, T. Fujita, K. Shitara, S. Akinaga, T. Takahashi, A. Utsunomiya, and R. Ueda. 2004. The CC chemokine receptor 4 as a novel specific molecular target for immunotherapy in adult T-Cell leukemia/lymphoma. Clin. Cancer Res. 107529-7539. [DOI] [PubMed] [Google Scholar]
- 35.Ishida, T., T. Ishii, A. Inagaki, H. Yano, S. Kusumoto, M. Ri, H. Komatsu, S. Iida, H. Inagaki, and R. Ueda. 2006. The CCR4 as a novel-specific molecular target for immunotherapy in Hodgkin lymphoma. Leukemia 202162-2168. [DOI] [PubMed] [Google Scholar]
- 36.Kaplan, A. P. 2001. Chemokines, chemokine receptors and allergy. Int. Arch. Allergy Immunol. 124423-431. [DOI] [PubMed] [Google Scholar]
- 37.Kawasaki, S., H. Takizawa, H. Yoneyama, T. Nakayama, R. Fujisawa, M. Izumizaki, T. Imai, O. Yoshie, I. Homma, K. Yamamoto, and K. Matsushima. 2001. Intervention of thymus and activation-regulated chemokine attenuates the development of allergic airway inflammation and hyperresponsiveness in mice. J. Immunol. 1662055-2062. [DOI] [PubMed] [Google Scholar]
- 38.Kessler, W., T. Traeger, A. Westerholt, F. Neher, M. Mikulcak, A. Muller, S. Maier, and C. D. Heidecke. 2006. The vagal nerve as a link between the nervous and immune system in the instance of polymicrobial sepsis. Langenbecks Arch. Surg. 39183-87. [DOI] [PubMed] [Google Scholar]
- 39.Kiank, C., P. Koerner, W. Kessler, T. Traeger, S. Maier, C. D. Heidecke, and C. Schuett. 2007. Seasonal variations in inflammatory responses to sepsis and stress in mice. Crit. Care Med. 352352-2358. [DOI] [PubMed] [Google Scholar]
- 40.Kunkel, E. J., J. Boisvert, K. Murphy, M. A. Vierra, M. C. Genovese, A. J. Wardlaw, H. B. Greenberg, M. R. Hodge, L. Wu, E. C. Butcher, and J. J. Campbell. 2002. Expression of the chemokine receptors CCR4, CCR5, and CXCR3 by human tissue-infiltrating lymphocytes. Am. J. Pathol. 160347-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kunkel, E. J., and E. C. Butcher. 2002. Chemokines and the tissue-specific migration of lymphocytes. Immunity 161-4. [DOI] [PubMed] [Google Scholar]
- 42.Lloyd, C. M., and S. M. Rankin. 2003. Chemokines in allergic airway disease. Curr. Opin. Pharmacol. 3443-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lukacs, N. W. 2001. Role of chemokines in the pathogenesis of asthma. Nat. Rev. Immunol. 1108-116. [DOI] [PubMed] [Google Scholar]
- 44.Maier, S., K. Emmanuilidis, M. Entleutner, N. Zantl, M. Werner, K. Pfeffer, and C. D. Heidecke. 2000. Massive chemokine transcription in acute renal failure due to polymicrobial sepsis. Shock 14187-192. [DOI] [PubMed] [Google Scholar]
- 45.Maier, S., T. Traeger, M. Entleutner, A. Westerholt, B. Kleist, N. Huser, B. Holzmann, A. Stier, K. Pfeffer, and C. D. Heidecke. 2004. Cecal ligation and puncture versus colon ascendens stent peritonitis: two distinct animal models for polymicrobial sepsis. Shock 21505-511. [DOI] [PubMed] [Google Scholar]
- 46.Matsukawa, A., C. M. Hogaboam, N. W. Lukacs, and S. L. Kunkel. 2000. Chemokines and innate immunity. Rev. Immunogenet. 2339-358. [PubMed] [Google Scholar]
- 47.Meissner, K., W. Kessler, H. E. Meyer Zu Schwabedissen, K. Schuster, K. Saalfeld, M. Grube, A. Buck, G. Jedlitschky, S. Maier, T. Traeger, J. Mostertz, G. Homuth, C. D. Heidecke, C. Lehmann, and H. K. Kroemer. 2007. Sepsis affects cardiac expression of multidrug resistance protein 5 (Mrp5, Abcc5), an Abc-type CGMP export pump. Shock 28564-569. [DOI] [PubMed] [Google Scholar]
- 48.Moser, B., M. Wolf, A. Walz, and P. Loetscher. 2004. Chemokines: multiple levels of leukocyte migration control. Trends Immunol. 2575-84. [DOI] [PubMed] [Google Scholar]
- 49.Nakanishi, T., K. Imaizumi, Y. Hasegawa, T. Kawabe, N. Hashimoto, M. Okamoto, and K. Shimokata. 2006. Expression of macrophage-derived chemokine (MDC)/CCL22 in human lung cancer. Cancer Immunol. Immunother. 551320-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ness, T. L., J. L. Ewing, C. M. Hogaboam, and S. L. Kunkel. 2006. CCR4 is a key modulator of innate immune responses. J. Immunol. 1777531-7539. [DOI] [PubMed] [Google Scholar]
- 51.Owen, C. 2001. Chemokine receptors in airway disease: which receptors to target? Pulm. Pharmacol. Ther. 14193-202. [DOI] [PubMed] [Google Scholar]
- 52.Pfeffer, K., T. Matsuyama, T. M. Kundig, A. Wakeham, K. Kishihara, A. Shahinian, K. Wiegmann, P. S. Ohashi, M. Kronke, and T. W. Mak. 1993. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell 73457-467. [DOI] [PubMed] [Google Scholar]
- 53.Pilatz, S., K. Breitbach, N. Hein, B. Fehlhaber, J. Schulze, B. Brenneke, L. Eberl, and I. Steinmetz. 2006. Identification of Burkholderia pseudomallei genes required for the intracellular life cycle and in vivo virulence. Infect. Immun. 743576-3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Power, C. A., A. Meyer, K. Nemeth, K. B. Bacon, A. J. Hoogewerf, A. E. Proudfoot, and T. N. Wells. 1995. Molecular cloning and functional expression of a novel CC chemokine receptor cDNA from a human basophilic cell line. J. Biol. Chem. 27019495-19500. [DOI] [PubMed] [Google Scholar]
- 55.Ritter, M., R. Goggel, N. Chaudhary, A. Wiedenmann, B. Jung, A. Weith, and P. Seither. 2005. Elevated expression of TARC (CCL17) and MDC (CCL22) in models of cigarette smoke-induced pulmonary inflammation. Biochem. Biophys. Res. Commun. 334254-262. [DOI] [PubMed] [Google Scholar]
- 56.Rothe, J., W. Lesslauer, H. Lotscher, Y. Lang, P. Koebel, F. Kontgen, A. Althage, R. Zinkernagel, M. Steinmetz, and H. Bluethmann. 1993. Mice lacking the tumour necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature 364798-802. [DOI] [PubMed] [Google Scholar]
- 57.Russell, D. A., and R. C. Thompson. 1993. Targets for sepsis therapies: tumor necrosis factor versus interleukin-1. Curr. Opin. Biotechnol. 4714-721. [DOI] [PubMed] [Google Scholar]
- 58.Sather, B. D., P. Treuting, N. Perdue, M. Miazgowicz, J. D. Fontenot, A. Y. Rudensky, and D. J. Campbell. 2007. Altering the distribution of Foxp3(+) regulatory T cells results in tissue-specific inflammatory disease. J. Exp. Med. 2041335-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scapini, P., J. A. Lapinet-Vera, S. Gasperini, F. Calzetti, F. Bazzoni, and M. A. Cassatella. 2000. The neutrophil as a cellular source of chemokines. Immunol. Rev. 177195-203. [DOI] [PubMed] [Google Scholar]
- 60.Sha, J., A. A. Fadl, G. R. Klimpel, D. W. Niesel, V. L. Popov, and A. K. Chopra. 2004. The two murein lipoproteins of Salmonella enterica serovar Typhimurium contribute to the virulence of the organism. Infect. Immun. 723987-4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tracey, K. J., and E. Abraham. 1999. From mouse to man: or what have we learned about cytokine-based anti-inflammatory therapies? Shock 11224-225. [PubMed] [Google Scholar]
- 62.Tracey, K. J., Y. Fong, D. G. Hesse, K. R. Manogue, A. T. Lee, G. C. Kuo, S. F. Lowry, and A. Cerami. 1987. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature 330662-664. [DOI] [PubMed] [Google Scholar]
- 63.Tsunemi, Y., H. Saeki, K. Nakamura, D. Nagakubo, T. Nakayama, O. Yoshie, S. Kagami, K. Shimazu, T. Kadono, M. Sugaya, M. Komine, K. Matsushima, and K. Tamaki. 2006. CCL17 transgenic mice show an enhanced Th2-type response to both allergic and non-allergic stimuli. Eur. J. Immunol. 362116-2127. [DOI] [PubMed] [Google Scholar]
- 64.Vulcano, M., C. Albanesi, A. Stoppacciaro, R. Bagnati, G. D'Amico, S. Struyf, P. Transidico, R. Bonecchi, A. Del Prete, P. Allavena, L. P. Ruco, C. Chiabrando, G. Girolomoni, A. Mantovani, and S. Sozzani. 2001. Dendritic cells as a major source of macrophage-derived chemokine/CCL22 in vitro and in vivo. Eur. J. Immunol. 31812-822. [DOI] [PubMed] [Google Scholar]
- 65.Wu, M., H. Fang, and S. T. Hwang. 2001. Cutting edge: CCR4 mediates antigen-primed T cell binding to activated dendritic cells. J. Immunol. 1674791-4795. [DOI] [PubMed] [Google Scholar]
- 66.Wuthiekanun, V., M. D. Smith, D. A. Dance, A. L. Walsh, T. L. Pitt, and N. J. White. 1996. Biochemical characteristics of clinical and environmental isolates of Burkholderia pseudomallei. J. Med. Microbiol. 45408-412. [DOI] [PubMed] [Google Scholar]
- 67.Yanai, M., K. Sato, N. Aoki, Y. Takiyama, K. Oikawa, H. Kobayashi, S. Kimura, Y. Harabuchi, and M. Tateno. 2007. The role of CCL22/macrophage-derived chemokine in allergic rhinitis. Clin. Immunol. 125291-298. [DOI] [PubMed] [Google Scholar]
- 68.Yoshie, O., T. Imai, and H. Nomiyama. 1997. Novel lymphocyte-specific CC chemokines and their receptors. J. Leukoc. Biol. 62634-644. [DOI] [PubMed] [Google Scholar]
- 69.Zantl, N., A. Uebe, B. Neumann, H. Wagner, J. R. Siewert, B. Holzmann, C. D. Heidecke, and K. Pfeffer. 1998. Essential role of gamma interferon in survival of colon ascendens stent peritonitis, a novel murine model of abdominal sepsis. Infect. Immun. 662300-2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang, T., R. Somasundaram, K. Berencsi, L. Caputo, P. Gimotty, P. Rani, D. Guerry, R. Swoboda, and D. Herlyn. 2006. Migration of cytotoxic T lymphocytes toward melanoma cells in three-dimensional organotypic culture is dependent on CCL2 and CCR4. Eur. J. Immunol. 36457-467. [DOI] [PubMed] [Google Scholar]