Abstract
Osteomyelitis is an inflammatory disease of the bone that is characterized by the presence of necrotic bone tissue and increased osteoclast activity. Staphylococcus aureus is responsible for approximately 80% of all cases of human osteomyelitis. While the disease is especially difficult to treat, the pathogenesis of S. aureus-induced osteomyelitis is poorly understood. Elucidating the molecular mechanisms by which S. aureus induces osteomyelitis could lead to a better understanding of the disease and its progression and development of new treatments. Osteoblasts can produce several soluble factors that serve to modulate the activity or formation of osteoclasts. Receptor activator of NF-κB ligand (RANK-L) and prostaglandin E2 (PGE2) are two such molecules which can promote osteoclastogenesis and stimulate bone resorption. In addition, previous studies in our laboratory have shown that osteoblasts produce inflammatory cytokines, such as interleukin 6, following infection with S. aureus, which could induce COX-2 and in turn PGE2, further modulating osteoclast recruitment and differentiation. Therefore, we hypothesized that following infection with S. aureus, osteoblasts will express increased levels of RANK-L and PGE2. The results presented in this study provide evidence for the first time that RANK-L mRNA and protein and PGE2 expression are upregulated in S. aureus-infected primary osteoblasts. In addition, through the use of the specific COX-2 inhibitor NS 398, we show that when PGE2 production is inhibited, RANK-L production is decreased. These data suggest a mechanism whereby osteoblasts regulate the production of RANK-L during infection.
Osteomyelitis is a severe infection of bone tissue that results in progressive inflammatory destruction of bone (41). The gram-positive organism Staphylococcus aureus is the most common causative agent of osteomyelitis, accounting for approximately 80% of all human cases (27). It is often necessary that infected bone be debrided, tissues reconstructed, and long-term antibiotic therapy utilized (18). The current treatment for osteomyelitis is often traumatic and expensive and often leads to further selection of antibiotic-resistant strains of S. aureus. The growing incidence of antibiotic-resistant S. aureus strains can explain the recurrent attacks of osteomyelitis in patients undergoing therapy. In addition, the pathogenesis of S. aureus-induced osteomyelitis is poorly understood. Elucidating the mechanisms by which S. aureus induces osteomyelitis could therefore lead to a better understanding of the disease, its progression, and development of new treatments.
Bone remodeling involves a continuous, coordinated equilibrium between bone synthesis and bone resorption, and two cell populations are responsible for the continual process (23). Osteoclasts drive the resorption of bone by acidification and release of lysosomal enzymes, while osteoblasts produce components of the bone matrix, principally type I collagen. Osteoblasts also produce factors which serve to modulate the activity or formation of osteoclasts (1). One such key molecule produced by osteoblasts is receptor activator of NF-κB ligand (RANK-L), which on binding to RANK, found on osteoclast precursors, triggers intricate and distinct signaling cascades that control lineage commitment and activation of osteoclasts (53). RANK-L is therefore thought to be the essential and final common signal required both in vitro and in vivo for full osteoclastic differentiation from multipotential hematopoietic precursor cells into mature multinucleated bone-resorptive osteoclasts (22, 24, 25). Thus, osteoblasts can regulate both net bone formation and resorption.
Prostaglandin E2 (PGE2) is produced in bone mainly by osteoblasts and acts as a potent stimulator of bone resorption by inducing osteoclast formation in both mouse bone marrow cultures and calvarial cultures (1, 7, 37, 40, 46, 48). PGE2 synthesis is regulated by three metabolic steps: the release of arachidonic acid from the membranous phospholipids by phospholipase A2, the conversion of arachidonic acid to prostaglandin H2 (PGH2) by cyclooxygenase (COX), and the synthesis of PGE2 catalyzed by PGE synthase (9, 20, 26, 34, 42, 44, 50). Although both constitutive COX (COX-1) and inducible COX (COX-2) are expressed in mouse osteoblasts, the expression of COX-2 is markedly induced by several proinflammatory cytokines, such as interleukin 6 (IL-6). Bost et al. (5, 11) have reported that S. aureus-infected osteoblasts produce high levels of IL-6, which have been shown to modulate the activity of bone-resorptive osteoclasts either directly or indirectly (21, 45). It has also been determined that PGE2 can upregulate RANK-L expression in osteoblasts (16, 17, 54, 57).
Bacterial pathogens can stimulate osteoclastogenesis, which can potentiate bone resorption, leading to bone destruction (31). Okahashi et al. have also demonstrated that normal mouse osteoblasts infected with Streptococcus pyogenes increase expression of RANK-L mRNA and protein (36). In addition, elevated levels of RANK-L have been detected in the bone lesions of mandibular osteomyelitis (33). We therefore propose that following an intracellular challenge with S. aureus, osteoblasts can increase RANK-L expression, which could stimulate osteoclast activity and thereby potentiate the massive bone loss that occurs in S. aureus-infected bone tissue. The current study investigates RANK-L mRNA and protein expression during normal mouse osteoblast infection with S. aureus. Such a proposed upregulation of RANK-L from infected osteoblasts could be mediated either directly or indirectly. Since it has been determined that PGE2 can upregulate RANK-L, we propose in the current study that PGE2 production by S. aureus-infected osteoblasts will be increased and that inhibition of PGE2 by the specific COX-2 inhibitor NS-398 will result in decreased levels of PGE2 and RANK-L by infected osteoblasts.
MATERIALS AND METHODS
Bacterial strains.
Staphylococcus aureus strain UAMS-1 (ATCC 49230) is a human osteomyelitis clinical isolate (15). Staphylococcus carnosus (ATCC 51365) is a nonpathogenic species with a reduced ability to invade osteoblasts (11). Strains N1, N2, N3, N4, and N5 are Staphylococcus aureus nasopharyngeal isolates kindly provided by Vance G. Fowler, Jr. (Duke University Medical Center).
Isolation and culture of mouse osteoblasts.
Osteoblasts were isolated from the calvariae of 2-day-old CD-1 mice by sequential collagenase and protease digestions as described previously (38).
Invasion assay.
The invasion assay with live or UV-killed S. aureus strain UAMS-1, live S. carnosus, or live nasopharyngeal isolates was carried out as previously described (11, 12). Briefly, bacteria were grown overnight (16 h) in 5 ml of tryptic soy broth in a shaking water bath at 37°C. The bacteria were harvested by centrifugation for 10 min at 4,300 × g at 4°C and washed twice. The pellets were then resuspended in 5 ml of growth medium lacking antibiotics/antimycotics. Confluent cell layers of osteoblasts were washed three times to remove the growth medium. The cultures were then infected at a multiplicity of infection (MOI) of 25:1 or 75:1 with S. aureus strain UAMS-1 or with S. carnosus, UV-killed S. aureus, or the nasopharyngeal isolates at a MOI of 25:1 in 4 ml of growth medium lacking antibiotics/antimycotics. Following a 45-min infection period, infected cell cultures were washed three times and incubated for 1 h in growth medium containing 25 μg of gentamicin/ml to kill the remaining extracellular bacterial cells. The osteoblast cultures were washed and subsequently lysed at different time points by the addition of 1.2 ml of 0.1% Triton X-100 (Fisher Biotech, Fair Lawn, NJ) with incubation for 5 min at 37°C.
Quantitative real-time PCR.
To quantify RANK-L mRNA, real-time PCR was performed using a Roche LightCycler 2.0 instrument with the Sybr green reagent (Qiagen, Valencia, CA) according to the manufacturer's instructions. Amplicons of RANK-L and glyceraldehyde-3-phosphate dehydrogenase (G3PDH) were used to develop a standard curve. Samples were subjected to 40 cycles of amplification consisting of 95°C for 15 s followed by 53°C for 20 s. Each assay was normalized to G3PDH mRNA. PCR primers were derived from published sequences (IDT, Coralville, IA) (36). Positive- and negative-strand primers were as follows: RANK-L (89 bp), 5′-TACTTTCGAGCGCAGATGGAT-3′ and 5′-ACCTGCGTTTTCATGGAGTCT-3′; G3PDH (63 bp), 5′-AACTACATGGTCTACATGTTCCA-3′ and 5′-CCATTCTCGGCCTTGACTGT-3′.
Protein isolation.
Osteoblasts were isolated and seeded at 106 cells per well in six-well plates and infected once the cells had reached confluence (6 to 8 days). Following a 45-min incubation with or without bacteria and a 1-h treatment with 25 μg/ml gentamicin sulfate, total protein was isolated from the osteoblasts at 0, 6, and 24 h using a tissue protein extraction reagent (Pierce, Rockford, IL) with additional protease inhibitors, aprotinin (2 μg/ml) and leupeptin (2.5 μg/ml) (Sigma Chemical Company, St. Louis, MO). Two hundred μl of the tissue protein extraction reagent was added to each well at the appropriate time point, and cells were removed from the culture plates with a cell scraper (Costar). Samples were collected in 1.5-ml microcentrifuge tubes and then centrifuged at 10,000 × g for 2 min to pellet cell debris. Supernatants were collected, and placed in fresh tubes, and stored at −80°C.
Inhibitor study.
Osteoblasts were isolated and cultured in six-well plates as described previously. Forty-eight hours prior to infection, cells were treated with either 0.025% dimethyl sulfoxide (DMSO) or 20 μM NS-398 (Cayman Chemicals, Ann Arbor, MI), a specific COX-2 inhibitor, dissolved in DMSO at an optimal dose that was previously determined to provide inhibition of COX-2 (6, 58). Following the 48-h treatment, cells were rinsed one time with osteoblast growth medium (OBGM) containing no gentamicin sulfate or amphotericin B. One ml of OBGM, containing no gentamicin sulfate or amphotericin B and containing either DMSO or NS-398, was then added to each appropriate well. The osteoblasts were either uninfected (MOI = 0) or infected with the bacterial strains noted above using the method described previously. Following the 45-min incubation with or without bacteria, each well was rinsed one time with OBGM. One ml of fresh medium, containing either DMSO or NS-398 and 25 μg/ml of gentamicin, was then added to each well. Cells were then incubated for 1 h at 37°C in a 5%-CO2 atmosphere to allow the remaining extracellular bacteria to be killed. Following the 1-h incubation period, protein samples were isolated as described previously (time zero). Samples were also isolated at 6 h and 24 h following time zero. In addition, culture supernatants were collected and stored at −80°C.
Quantification of RANK-L protein and PGE2.
Using protein samples collected as described previously, a mouse TRANCE/TNSF11/RANK ligand enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, MN) was used to detect the levels of RANK-L in osteoblast whole-cell extracts. A PGE2 correlate enzyme immunoassay (EIA) (Assay Designs, Ann Arbor, MI) was performed with the cell culture supernatants following the manufacturer's instructions.
Statistical analysis.
Data were analyzed using a two-way analysis of variance followed by the Tukey-Kramer multiple-comparison analysis. Results are presented as the means ± standard errors. For all tests, P values of <0.05 were considered significant.
RESULTS
Induction of RANK-L mRNA expression in osteoblasts following exposure to bacteria.
Staphylococcal osteomyelitis is associated with increased osteoclast activity, and RANK-L is a key factor required for osteoclastogenesis (24, 25, 46, 57). Therefore, it is important to determine whether osteoblasts infected with S. aureus produce RANK-L and if the levels of RANK-L increase in a time- and dose-dependent manner. Quantitative analysis by real-time reverse transcription-PCR demonstrated that RANK-L expression in osteoblasts infected with S. aureus strain UAMS-1 (MOI = 75:1) was 10-fold greater than RANK-L expression in uninfected cells at 4 h following infection (Fig. 1). RANK-L mRNA expression by osteoblasts also increased in a dose-dependent manner. In addition, we showed that there was a time-dependent increase in RANK-L mRNA (Fig. 1), with the expression of RANK-L mRNA by infected cells being 10-fold greater than the expression in the uninfected controls at 4 h.
FIG. 1.
Expression of RANK-L mRNA by osteoblasts. Primary mouse osteoblasts either were not infected (MOI = 0) or were infected with S. aureus strain UAMS-1 (MOI = 25:1 or 75:1). Following a 45-min incubation with or without bacteria (time zero hours), total RNA was extracted at various time points and subjected to quantitative real-time reverse transcription-PCR analysis for RANK-L mRNA. All values were normalized to G3PDH and are expressed as n-fold change over baseline levels (0 h; MOI = 0). Data are expressed as the means ± standard errors for three separate osteoblast cultures. *, P < 0.05 versus results for MOI of 75:1 at 0 min; #, P < 0.05 versus results for MOI of 0 at time zero min; $, P < 0.05 versus results for MOI of 25:1 at 4 h.
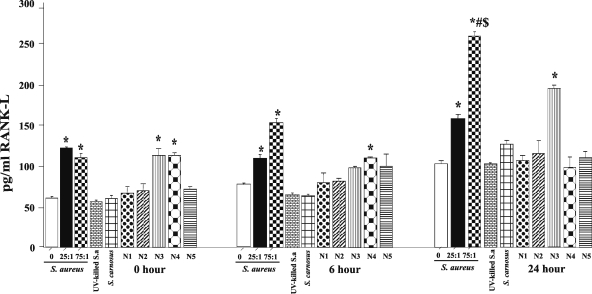
To confirm that the changes in RANK-L mRNA result in corresponding protein expression, cell-associated RANK-L was measured by specific-capture ELISA. As shown in Fig. 2, there was a time- and dose-dependent response to S. aureus strain UAMS-1. At 24 h and at an MOI of 75:1, there was a 2.5-fold increase in the RANK-L protein compared to results with the control. Although the documented effects of RANK-L have been shown to be mediated by the membrane-bound form, we still measured the levels of soluble RANK-L as well and found its levels to be undetectable (data not shown). Osteoblasts infected with S. carnosus and UV-killed S. aureus strain UAMS-1 demonstrated the same levels of RANK-L protein expression as uninfected osteoblasts (Fig. 2). Additionally, osteoblasts infected with S. aureus nasopharyngeal isolates N3 and N4 induced RANK-L production at levels similar to those for strain UAMS-1 at 0 h. Strains N4 and N3 sustained this increased RANK-L expression following 6 and 24 h of incubation, respectively.
FIG. 2.
Expression of RANK-L protein by osteoblasts. Primary mouse osteoblasts were either uninfected (MOI 0) or infected with either S. aureus strain UAMS-1 (MOI = 25:1 or 75:1), UV-killed strain UAMS-1 (MOI = 25:1), S. carnosus (MOI = 25:1), or S. aureus nasopharyngeal isolate N1, N2, N3, N4, or N5 (MOI = 25:1). Following a 45-min incubation with or without bacteria and a 1-h treatment with 25 μg/ml gentamicin sulfate, whole-cell extracts were taken at time zero hours, 6 h, and 24 h. RANK-L concentrations were determined using an ELISA, and the results are displayed in pg/ml. Data are expressed as the means ± standard errors for three separate osteoblast cultures. *, P < 0.05 versus results with MOI of 0 at all time points; #, P < 0.05 versus results with MOI of 75:1 0 h and 6 h; $, P < 0.05 versus results with MOI of 25:1 at 6 h or 24 h.
Induction of PGE2 expression by osteoblasts following exposure to S. aureus.
Choi et al. demonstrated that PGE2 is a main mediator in RANK-L-dependent osteoclastogenesis induced by several periodontal pathogens (8). Therefore, it is possible that RANK-L production in osteoblasts infected with S. aureus involves a PGE2-dependent mechanism. The current study examined levels of PGE2 following infection of osteoblasts by S. aureus. Figure 3 demonstrates that PGE2 is upregulated by S. aureus strain UAMS-1-infected osteoblasts at all time points examined. Although there is no significant difference between infection at an MOI of 25:1 and that at 75:1, there is a significant difference in PGE2 expression between S. aureus strain UAMS-1-infected and uninfected osteoblasts. Figure 3 also shows that the increase in levels of PGE2 occurs early following S. aureus infection and the levels remain elevated throughout the 24-h duration of the experiment. PGE2 expression in response to UV-killed S. aureus strain UAMS-1- or S. carnosus-infected osteoblasts is not significantly different from that observed for uninfected osteoblasts (Fig. 3). Although S. aureus nasopharyngeal isolates N1 to N5 significantly increase PGE2 expression at time zero, PGE2 levels following 6 and 24 h of infection are comparable to those for uninfected osteoblasts.
FIG. 3.
Expression of PGE2 by osteoblasts. Primary mouse osteoblasts were either uninfected (MOI = 0) or infected with either S. aureus strain UAMS-1 (MOI = 25:1 or 75:1), UV-killed strain UAMS-1 (MOI = 25:1), S. carnosus (MOI = 25:1,) or S. aureus nasopharyngeal isolate N1, N2, N3, N4, or N5 (MOI = 25:1). Following a 45-min incubation with or without bacteria and a 1-h treatment with 25 μg/ml gentamicin sulfate, supernatants were collected at time zero, 6 h, and 24 h. PGE2 concentrations were determined by an EIA and are expressed in pg/ml. Data are expressed as the means ± standard errors for three separate osteoblast cultures. *, P < 0.05 versus results with an MOI of 0 at 0, 6 h, or 24 h.
PGE2 production mediates S. aureus-induced increases in RANK-L production by osteoblasts.
One of the steps in the PGE2 synthesis pathway involves the conversion of arachidonic acid to PGH2, which is then metabolized to PGE2, by COX. The addition of NS-398, a selective inhibitor of COX-2, inhibits IL-1-induced PGE2 synthesis (7, 48). Therefore, in the current study, it is hypothesized that inhibition of COX-2 by NS-398 will result in decreased levels of PGE2 production by S. aureus-infected osteoblasts. Figure 4 demonstrates that NS-398 significantly attenuates PGE2 production in both uninfected and S. aureus-infected osteoblasts. Infected control cells treated with DMSO show the same dose response to S. aureus strain UAMS-1 seen in Fig. 3.
FIG. 4.
Expression of PGE2 and the effects of NS-398. Primary mouse osteoblasts were cultured in the presence of 0.025% DMSO or 20 μM NS-398 in DMSO 48 h prior to infection. Osteoblasts were either uninfected (MOI = 0) or infected with S. aureus strain UAMS-1 (MOI = 25:1 or 75:1). Following a 45-min incubation with or without bacteria and a 1-h treatment with 25 μg/ml gentamicin sulfate, supernatants were collected at time zero, 6 h, and 24 h. PGE2 concentrations were determined by an EIA and are expressed in ng/ml. Data are expressed as the means ± standard errors for three separate osteoblast cultures. *, P < 0.05 versus results with MOI of 0 and treatment with DMSO; #, P < 0.05 versus results for all groups treated with NS-398 at all time points.
Since it is known that PGE2 upregulates RANK-L, we also examined the effects of NS-398 on RANK-L expression by S. aureus-infected osteoblasts. When infected osteoblasts are treated with NS-398, RANK-L levels are attenuated, indicating S. aureus-induced PGE2 plays a role in expression of RANK-L by infected cells (Fig. 5).
FIG. 5.
Expression of RANK-L and the effects of NS-398. Primary mouse osteoblasts were cultured in the presence of 0.025% DMSO or 20 μM NS-398 in DMSO 48 h prior to infection. Osteoblasts were either uninfected (MOI = 0) or infected with S. aureus strain UAMS-1 (MOI = 25:1 or 75:1). Following a 45-min incubation with or without bacteria and a 1-h treatment with 25 μg/ml gentamicin sulfate, whole-cell extracts were collected at time zero, 6 h, and 24 h. RANK-L concentrations were determined by an ELISA and are expressed in pg/ml. Data are expressed as the means ± standard errors for three separate osteoblast cultures. *, P < 0.05 versus results with MOI of 0 and treatment with DMSO at 0 h, 6 h, or 24 h; #, P < 0.05 versus results for all groups treated with NS-398 at all time points; $, P < 0.05 versus MOI of 25:1 at 24 h.
DISCUSSION
Osteomyelitis is a disease associated with abnormal bone remodeling and massive bone resorption. The mechanisms responsible for such bone loss are largely unclear. The generation of proinflammatory cytokines and chemokines helps recruit immune cells, such as leukocytes, which could alter the balance between bone resorption and formation. Recent studies in our laboratory have demonstrated S. aureus-infected osteoblasts as a hitherto unrecognized source of soluble immune modulators both in vivo and in vitro (3-5, 14, 29, 30). Osteoblasts can also produce other soluble factors that can modulate the activity or formation of osteoclasts. RANK-L is one such key molecule that stimulates differentiation in osteoclasts and is an inducing agent involved in bone destruction (24, 25, 46, 49, 57). Following the binding of RANK-L to its receptor, RANK, on osteoclast precursors, the signal transduction can diverge into diverse pathways that can regulate distinct aspects of osteoclast functions (10, 18, 35, 39, 56).
Bacterial pathogens can stimulate osteoclastogenesis, either directly or indirectly, which can potentiate bone resorption, leading to bone destruction (31, 36). Ishida et al. have recently shown elevated RANK-L expression in S. aureus-infected NRG cells, a fibroblast cell line that has osteoblast-like features (19). Based on these findings, we undertook this study to evaluate the production of RANK-L from S. aureus-infected primary osteoblasts. Results outlined in Fig. 1 and 2 demonstrate that the viable S. aureus strain UAMS-1 is a potent inducer of membrane-bound, and not secreted, RANK-L mRNA and protein production by osteoblasts. In addition, UV-killed S. aureus strain UAMS-1 did not induce RANK-L expression, thus indicating that active bacterial gene expression may be required for maximal RANK-L production. Similarly, S. carnosus, an invasion-deficient species, did not stimulate increased RANK-L expression. Although osteoblasts infected with S. aureus nasopharyngeal isolates N3 and N4 induce RANK-L expression comparable to expression from osteoblasts infected with the osteomyelitis clinical isolate UAMS-1 at time zero and the strains maintain induction at 6 h in one case and at 24 h in the other, it is possible that strains N3 and N4 are capable of inducing osteomyelitis if they gain access to the bloodstream and subsequently to bone. The majority of the nasopharyngeal isolates are not capable of inducing RANK-L production. Taken together, these findings substantiate that live S. aureus is required for maximal RANK-L production from infected osteoblasts. An increase in RANK-L production by infected osteoblasts can further osteoclast differentiation and activation, which can potentiate the exacerbated bone destruction observed during osteomyelitis.
While RANK-L potentiates osteoclastogenesis, Osteoprotegerin (OPG), produced and secreted by osteoblasts, is a negative regulator of osteoclast formation. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) (43) is another key molecule produced by osteoblasts which can bind to OPG. Alexander et al. have demonstrated that when osteoblasts are infected with S. aureus, TRAIL expression is increased (2). Since OPG is known to bind both TRAIL and RANK-L, competition may limit the amount of OPG available to block the interaction between RANK-L and RANK during infection of osteoblasts by S. aureus. Taken together, it is highly likely that the increased levels of RANK-L contribute to the massive bone loss and aberrant bone remodeling associated with the pathology of staphylococcal osteomyelitis.
We next attempted to delineate the mechanism involved in upregulation of RANK-L expression in osteoblasts following exposure to S. aureus. PGE2 is produced in bone mainly by osteoblasts and acts as a potent stimulator of bone resorption (37, 40, 46). It has been demonstrated that PGE2 can mediate RANK-L-dependent osteoclastogenesis in periodontal diseases (8). Furthermore, the production of PGE2 by osteoblasts is regulated by several cytokines and is also reported to induce osteoclast formation in mouse bone marrow cultures and stimulate bone resorption in mouse calvarial cultures (1, 7, 48). We therefore hypothesized that increased RANK-L expression in infected osteoblasts occurs as a result of PGE2 activation upstream in the RANK-L pathway. The current study demonstrates that live S. aureus strain UAMS-1 induces significant PGE2 production in infected osteoblasts (Fig. 3). However, UV-killed S. aureus strain UAMS-1 and S. carnosus do not induce PGE2 production by osteoblasts. Osteoblasts infected with S. aureus nasopharyngeal isolates N1 to N5 demonstrate increased PGE2 expression initially and decreased expression subsequently comparable to uninfected osteoblasts. Increased and sustained levels of PGE2 production are induced only by live osteomyelitis clinical isolate UAMS-1, indicating the strain is capable of potentiating the bone damage observed in osteomyelitis. These results, along with the RANK-L data, are consistent with our previous findings that live S. aureus strain UAMS-1 is more effective at eliciting immune modulator production by cultured osteoblasts than UV-killed bacteria or nonpathogenic species (3-5, 11).
The functions of PGE2 in target cells are mediated by four different G-protein-coupled receptor subtypes, EP1, EP2, EP3, and EP4 (28, 47). Among these PGE2 receptor subtypes, EP4 is the main receptor expressed on osteoblasts and is responsible for mediating PGE2-induced RANK-L expression by osteoblasts (13, 47, 52). In addition, PGE2 synergistically promotes the differentiation of bone marrow macrophages into osteoclasts induced by RANK-L and macrophage colony-stimulating factor (51, 55). Thus, PGE2 stimulates osteoclastic bone resorption through the following two different pathways: the induction of RANK-L expression in osteoblasts and the direct enhancement of RANK-L-induced differentiation of osteoclast precursor cells into osteoclasts. The mechanism of the synergistic effect of PGE2 on the RANK-L-induced osteoclastic differentiation of precursor cells has not yet been explained (23).
PGE2 synthesis is regulated by three metabolic steps: the release of arachidonic acid from the membranous phospholipids by phospholipase A2, the conversion of arachidonic acid to PGH2 by COX, and the synthesis of PGE2 by PGE synthase (26, 34, 44). Both constitutive COX (COX-1) and inducible COX (COX-2) are expressed in mouse osteoblasts, with COX-2 being markedly induced by several bone-resorbing, inflammatory factors, including IL-1 and IL-6, in osteoblasts (32, 47). To establish the role of COX-2 with respect to production of PGE2 during infection of osteoblasts with S. aureus, we used NS-398 to inhibit COX-2 and examined its effect on PGE2 expression. When osteoblasts were treated with NS-398, PGE2 levels were significantly attenuated in both uninfected and S. aureus-infected osteoblasts (Fig. 4). These results indicate that COX-2 is important in the regulation of PGE2 production during S. aureus infection of osteoblasts.
We next examined the effects of NS-398 on the production of RANK-L. Although NS-398 significantly reduced the amount of RANK-L produced by S. aureus-infected osteoblasts, levels of RANK-L still remained detectable and were still responsive to S. aureus infection (Fig. 5). These data suggest that COX-2 and PGE2 play a significant role in potentiating RANK-L production by S. aureus-infected osteoblasts.
The increasing incidence of bacterial infections caused by S. aureus and the emergence of antibiotic-resistant strains of this organism have made it imperative that we understand the mechanisms of initiation and maintenance of inflammation, as well as those of bone loss observed in sites of infection, in order to develop safe and effective therapies. Data presented here clearly outline a mechanism by which RANK-L, an essential factor for osteoclastogenesis, is upregulated by S. aureus strain UAMS-1-infected osteoblasts, further substantiating the role that osteoblasts may have in the aberrant bone remodeling observed in staphylococcal osteomyelitis. In addition, this upregulation of RANK-L appears to involve a COX-2-mediated PGE2-dependent pathway. Potentially, agents that inhibit COX-2 or block RANK-L production could ameliorate bone loss. Further studies are warranted to investigate the in vivo significance of RANK-L in S. aureus-induced osteomyelitis.
Acknowledgments
This work was supported by grant AR049263 from the National Institutes of Health and by a faculty research grant from UNC Charlotte (to M.C.H.).
Editor: S. R. Blanke
Footnotes
Published ahead of print on 2 September 2008.
REFERENCES
- 1.Akatsu, T., N. Takahashi, N. Udagawa, K. Imamura, A. Yamaguchi, K. Sato, N. Nagata, and T. Suda. 1991. Role of prostaglandins in interleukin-1-induced bone resorption in mice in vitro. J. Bone Miner. Res. 6183-189. [DOI] [PubMed] [Google Scholar]
- 2.Alexander, E. H., J. L. Bento, F. M. Hughes, Jr., I. Marriott, M. C. Hudson, and K. L. Bost. 2001. Staphylococcus aureus and Salmonella enterica serovar Dublin induce tumor necrosis factor-related apoptosis-inducing ligand expression by normal mouse and human osteoblasts. Infect. Immun. 691581-1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bost, K. L., J. L. Bento, J. K. Ellington, I. Marriott, and M. C. Hudson. 2000. Induction of colony-stimulating factor expression following Staphylococcus or Salmonella interaction with mouse or human osteoblasts. Infect. Immun. 685075-5083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bost, K. L., J. L. Bento, C. C. Petty, L. W. Schrum, M. C. Hudson, and I. Marriott. 2001. Monocyte chemoattractant protein-1 expression by osteoblasts following infection with Staphylococcus aureus or Salmonella. J. Interferon Cytokine Res. 21297-304. [DOI] [PubMed] [Google Scholar]
- 5.Bost, K. L., W. K. Ramp, N. C. Nicholson, J. L. Bento, I. Marriott, and M. C. Hudson. 1999. Staphylococcus aureus infection of mouse or human osteoblasts induces high levels of interleukin-6 and interleukin-12 production. J. Infect. Dis. 1801912-1920. [DOI] [PubMed] [Google Scholar]
- 6.Chang, Y. C., P. C. Li, B. C. Chen, M. S. Chang, J. L. Wang, W. T. Chiu, and C. H. Lin. 2006. Lipoteichoic acid-induced nitric oxide synthase expression in RAW 264.7 macrophages is mediated by cyclooxygenase-2, prostaglandin E2, protein kinase A, p38 MAPK, and nuclear factor-kappaB pathways. Cell Signal. 181235-1243. [DOI] [PubMed] [Google Scholar]
- 7.Chen, Q. R., C. Miyaura, S. Higashi, M. Murakami, I. Kudo, S. Saito, T. Hiraide, Y. Shibasaki, and T. Suda. 1997. Activation of cytosolic phospholipase A2 by platelet-derived growth factor is essential for cyclooxygenase-2-dependent prostaglandin E2 synthesis in mouse osteoblasts cultured with interleukin-1. J. Biol. Chem. 2725952-5958. [DOI] [PubMed] [Google Scholar]
- 8.Choi, B. K., S. Y. Moon, J. H. Cha, K. W. Kim, and Y. J. Yoo. 2005. Prostaglandin E(2) is a main mediator in receptor activator of nuclear factor-kappaB ligand-dependent osteoclastogenesis induced by Porphyromonas gingivalis, Treponema denticola, and Treponema socranskii. J. Periodontol. 76813-820. [DOI] [PubMed] [Google Scholar]
- 9.Clark, J. D., L. L. Lin, R. W. Kriz, C. S. Ramesha, L. A. Sultzman, A. Y. Lin, N. Milona, and J. L. Knopf. 1991. A novel arachidonic acid-selective cytosolic PLA2 contains a Ca(2+)-dependent translocation domain with homology to PKC and GAP. Cell 651043-1051. [DOI] [PubMed] [Google Scholar]
- 10.Darnay, B. G., J. Ni, P. A. Moore, and B. B. Aggarwal. 1999. Activation of NF-kappaB by RANK requires tumor necrosis factor receptor-associated factor (TRAF) 6 and NF-kappaB-inducing kinase. Identification of a novel TRAF6 interaction motif. J. Biol. Chem. 2747724-7731. [DOI] [PubMed] [Google Scholar]
- 11.Ellington, J. K., A. Elhofy, K. L. Bost, and M. C. Hudson. 2001. Involvement of mitogen-activated protein kinase pathways in Staphylococcus aureus invasion of normal osteoblasts. Infect. Immun. 695235-5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellington, J. K., S. S. Reilly, W. K. Ramp, M. S. Smeltzer, J. F. Kellam, and M. C. Hudson. 1999. Mechanisms of Staphylococcus aureus invasion of cultured osteoblasts. Microb. Pathog. 26317-323. [DOI] [PubMed] [Google Scholar]
- 13.Fujita, D., N. Yamashita, S. Iita, H. Amano, S. Yamada, and K. Sakamoto. 2003. Prostaglandin E2 induced the differentiation of osteoclasts in mouse osteoblast-depleted bone marrow cells. Prostaglandins Leukot. Essent. Fatty Acids 68351-358. [DOI] [PubMed] [Google Scholar]
- 14.Gasper, N. A., C. C. Petty, L. W. Schrum, I. Marriott, and K. L. Bost. 2002. Bacterium-induced CXCL10 secretion by osteoblasts can be mediated in part through toll-like receptor 4. Infect. Immun. 704075-4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gillaspy, A. F., S. G. Hickmon, R. A. Skinner, J. R. Thomas, C. L. Nelson, and M. S. Smeltzer. 1995. Role of the accessory gene regulator (agr) in pathogenesis of staphylococcal osteomyelitis. Infect. Immun. 633373-3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hofbauer, L. C., S. Khosla, C. R. Dunstan, D. L. Lacey, W. J. Boyle, and B. L. Riggs. 2000. The roles of osteoprotegerin and osteoprotegerin ligand in the paracrine regulation of bone resorption. J. Bone Miner. Res. 152-12. [DOI] [PubMed] [Google Scholar]
- 17.Hofbauer, L. C., D. L. Lacey, C. R. Dunstan, T. C. Spelsberg, B. L. Riggs, and S. Khosla. 1999. Interleukin-1beta and tumor necrosis factor-alpha, but not interleukin-6, stimulate osteoprotegerin ligand gene expression in human osteoblastic cells. Bone 25255-259. [DOI] [PubMed] [Google Scholar]
- 18.Hsu, H., D. L. Lacey, C. R. Dunstan, I. Solovyev, A. Colombero, E. Timms, H. L. Tan, G. Elliott, M. J. Kelley, I. Sarosi, L. Wang, X. Z. Xia, R. Elliott, L. Chiu, T. Black, S. Scully, C. Capparelli, S. Morony, G. Shimamoto, M. B. Bass, and W. J. Boyle. 1999. Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. Proc. Natl. Acad. Sci. USA 963540-3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishida, I., C. Kohda, Y. Yanagawa, H. Miyaoka, and T. Shimamura. 2007. Epigallocatechin gallate suppresses expression of receptor activator of NF-kappaB ligand (RANKL) in Staphylococcus aureus infection in osteoblast-like NRG cells. J. Med. Microbiol. 561042-1046. [DOI] [PubMed] [Google Scholar]
- 20.Jakobsson, P. J., S. Thoren, R. Morgenstern, and B. Samuelsson. 1999. Identification of human prostaglandin E synthase: a microsomal, glutathione-dependent, inducible enzyme, constituting a potential novel drug target. Proc. Natl. Acad. Sci. USA 967220-7225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaneda, T., T. Nojima, M. Nakagawa, A. Ogasawara, H. Kaneko, T. Sato, H. Mano, M. Kumegawa, and Y. Hakeda. 2000. Endogenous production of TGF-beta is essential for osteoclastogenesis induced by a combination of receptor activator of NF-kappa B ligand and macrophage-colony-stimulating factor. J. Immunol. 1654254-4263. [DOI] [PubMed] [Google Scholar]
- 22.Kikuchi, T., T. Matsuguchi, N. Tsuboi, A. Mitani, S. Tanaka, M. Matsuoka, G. Yamamoto, T. Hishikawa, T. Noguchi, and Y. Yoshikai. 2001. Gene expression of osteoclast differentiation factor is induced by lipopolysaccharide in mouse osteoblasts via Toll-like receptors. J. Immunol. 1663574-3579. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi, Y., T. Mizoguchi, I. Take, S. Kurihara, N. Udagawa, and N. Takahashi. 2005. Prostaglandin E2 enhances osteoclastic differentiation of precursor cells through protein kinase A-dependent phosphorylation of TAK1. J. Biol. Chem. 28011395-11403. [DOI] [PubMed] [Google Scholar]
- 24.Kong, Y. Y., U. Feige, I. Sarosi, B. Bolon, A. Tafuri, S. Morony, C. Capparelli, J. Li, R. Elliott, S. McCabe, T. Wong, G. Campagnuolo, E. Moran, E. R. Bogoch, G. Van, L. T. Nguyen, P. S. Ohashi, D. L. Lacey, E. Fish, W. J. Boyle, and J. M. Penninger. 1999. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature 402304-309. [DOI] [PubMed] [Google Scholar]
- 25.Kong, Y. Y., H. Yoshida, I. Sarosi, H. L. Tan, E. Timms, C. Capparelli, S. Morony, A. J. Oliveira-dos-Santos, G. Van, A. Itie, W. Khoo, A. Wakeham, C. R. Dunstan, D. L. Lacey, T. W. Mak, W. J. Boyle, and J. M. Penninger. 1999. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 397315-323. [DOI] [PubMed] [Google Scholar]
- 26.Kudo, I., M. Murakami, S. Hara, and K. Inoue. 1993. Mammalian non-pancreatic phospholipases A2. Biochim. Biophys. Acta 1170217-231. [DOI] [PubMed] [Google Scholar]
- 27.Lew, D. P., and F. A. Waldvogel. 2004. Osteomyelitis. Lancet 364369-379. [DOI] [PubMed] [Google Scholar]
- 28.Li, X., Y. Okada, C. C. Pilbeam, J. A. Lorenzo, C. R. Kennedy, R. M. Breyer, and L. G. Raisz. 2000. Knockout of the murine prostaglandin EP2 receptor impairs osteoclastogenesis in vitro. Endocrinology 1412054-2061. [DOI] [PubMed] [Google Scholar]
- 29.Marriott, I., D. L. Gray, D. M. Rati, V. G. Fowler, Jr., M. E. Stryjewski, L. S. Levin, M. C. Hudson, and K. L. Bost. 2005. Osteoblasts produce monocyte chemoattractant protein-1 in a murine model of Staphylococcus aureus osteomyelitis and infected human bone tissue. Bone 37504-512. [DOI] [PubMed] [Google Scholar]
- 30.Marriott, I., D. L. Gray, S. L. Tranguch, V. G. Fowler, Jr., M. Stryjewski, L. Scott Levin, M. C. Hudson, and K. L. Bost. 2004. Osteoblasts express the inflammatory cytokine interleukin-6 in a murine model of Staphylococcus aureus osteomyelitis and infected human bone tissue. Am. J. Pathol. 1641399-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meghji, S., S. J. Crean, P. A. Hill, M. Sheikh, S. P. Nair, K. Heron, B. Henderson, E. B. Mawer, and M. Harris. 1998. Surface-associated protein from Staphylococcus aureus stimulates osteoclastogenesis: possible role in S. aureus-induced bone pathology. Br. J. Rheumatol. 371095-1101. [DOI] [PubMed] [Google Scholar]
- 32.Miyaura, C., M. Inada, C. Matsumoto, T. Ohshiba, N. Uozumi, T. Shimizu, and A. Ito. 2003. An essential role of cytosolic phospholipase A2α in prostaglandin E2-mediated bone resorption associated with inflammation. J. Exp. Med. 1971303-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montonen, M., T. F. Li, P. L. Lukinmaa, E. Sakai, M. Hukkanen, A. Sukura, and Y. T. Konttinen. 2006. RANKL and cathepsin K in diffuse sclerosing osteomyelitis of the mandible. J. Oral Pathol. Med. 35620-625. [DOI] [PubMed] [Google Scholar]
- 34.Murakami, M., H. Naraba, T. Tanioka, N. Semmyo, Y. Nakatani, F. Kojima, T. Ikeda, M. Fueki, A. Ueno, S. Oh, and I. Kudo. 2000. Regulation of prostaglandin E2 biosynthesis by inducible membrane-associated prostaglandin E2 synthase that acts in concert with cyclooxygenase-2. J. Biol. Chem. 27532783-32792. [DOI] [PubMed] [Google Scholar]
- 35.Nakagawa, N., M. Kinosaki, K. Yamaguchi, N. Shima, H. Yasuda, K. Yano, T. Morinaga, and K. Higashio. 1998. RANK is the essential signaling receptor for osteoclast differentiation factor in osteoclastogenesis. Biochem. Biophys. Res. Commun. 253395-400. [DOI] [PubMed] [Google Scholar]
- 36.Okahashi, N., A. Sakurai, I. Nakagawa, T. Fujiwara, S. Kawabata, A. Amano, and S. Hamada. 2003. Infection by Streptococcus pyogenes induces the receptor activator of NF-κB ligand expression in mouse osteoblastic cells. Infect. Immun. 71948-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raisz, L. G., J. Y. Vanderhoek, H. A. Simmons, B. E. Kream, and K. C. Nicolaou. 1979. Prostaglandin synthesis by fetal rat bone in vitro: evidence for a role of prostacyclin. Prostaglandins 17905-914. [DOI] [PubMed] [Google Scholar]
- 38.Ramp, W. K., L. G. Lenz, and K. K. Kaysinger. 1994. Medium pH modulates matrix, mineral, and energy metabolism in cultured chick bones and osteoblast-like cells. Bone Miner. 2459-73. [DOI] [PubMed] [Google Scholar]
- 39.Ross, F. P. 2000. RANKing the importance of measles virus in Paget's disease. J. Clin. Investig. 105555-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sato, K., Y. Fujii, K. Kasono, M. Saji, T. Tsushima, and K. Shizume. 1986. Stimulation of prostaglandin E2 and bone resorption by recombinant human interleukin 1 alpha in fetal mouse bones. Biochem. Biophys. Res. Commun. 138618-624. [DOI] [PubMed] [Google Scholar]
- 41.Sax, H., and D. Lew. 1999. Osteomyelitis. Curr. Infect. Dis. Rep. 1261-266. [DOI] [PubMed] [Google Scholar]
- 42.Sharp, J. D., D. L. White, X. G. Chiou, T. Goodson, G. C. Gamboa, D. McClure, S. Burgett, J. Hoskins, P. L. Skatrud, J. R. Sportsman, et al. 1991. Molecular cloning and expression of human Ca(2+)-sensitive cytosolic phospholipase A2. J. Biol. Chem. 26614850-14853. [PubMed] [Google Scholar]
- 43.Simonet, W. S., D. L. Lacey, C. R. Dunstan, M. Kelley, M. S. Chang, R. Luthy, H. Q. Nguyen, S. Wooden, L. Bennett, T. Boone, G. Shimamoto, M. DeRose, R. Elliott, A. Colombero, H. L. Tan, G. Trail, J. Sullivan, E. Davy, N. Bucay, L. Renshaw-Gegg, T. M. Hughes, D. Hill, W. Pattison, P. Campbell, S. Sander, G. Van, J. Tarpley, P. Derby, R. Lee, and W. J. Boyle. 1997. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 89309-319. [DOI] [PubMed] [Google Scholar]
- 44.Smith, W. L., R. M. Garavito, and D. L. DeWitt. 1996. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. J. Biol. Chem. 27133157-33160. [DOI] [PubMed] [Google Scholar]
- 45.Suda, K., N. Udagawa, N. Sato, M. Takami, K. Itoh, J. T. Woo, N. Takahashi, and K. Nagai. 2004. Suppression of osteoprotegerin expression by prostaglandin E2 is crucially involved in lipopolysaccharide-induced osteoclast formation. J. Immunol. 1722504-2510. [DOI] [PubMed] [Google Scholar]
- 46.Suda, T., N. Takahashi, N. Udagawa, E. Jimi, M. T. Gillespie, and T. J. Martin. 1999. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr. Rev. 20345-357. [DOI] [PubMed] [Google Scholar]
- 47.Suzawa, T., C. Miyaura, M. Inada, T. Maruyama, Y. Sugimoto, F. Ushikubi, A. Ichikawa, S. Narumiya, and T. Suda. 2000. The role of prostaglandin E receptor subtypes (EP1, EP2, EP3, and EP4) in bone resorption: an analysis using specific agonists for the respective EPs. Endocrinology 1411554-1559. [DOI] [PubMed] [Google Scholar]
- 48.Tai, H., C. Miyaura, C. C. Pilbeam, T. Tamura, Y. Ohsugi, Y. Koishihara, N. Kubodera, H. Kawaguchi, L. G. Raisz, and T. Suda. 1997. Transcriptional induction of cyclooxygenase-2 in osteoblasts is involved in interleukin-6-induced osteoclast formation. Endocrinology 1382372-2379. [DOI] [PubMed] [Google Scholar]
- 49.Takayanagi, H., K. Ogasawara, S. Hida, T. Chiba, S. Murata, K. Sato, A. Takaoka, T. Yokochi, H. Oda, K. Tanaka, K. Nakamura, and T. Taniguchi. 2000. T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-gamma. Nature 408600-605. [DOI] [PubMed] [Google Scholar]
- 50.Tanioka, T., Y. Nakatani, N. Semmyo, M. Murakami, and I. Kudo. 2000. Molecular identification of cytosolic prostaglandin E2 synthase that is functionally coupled with cyclooxygenase-1 in immediate prostaglandin E2 biosynthesis. J. Biol. Chem. 27532775-32782. [DOI] [PubMed] [Google Scholar]
- 51.Tintut, Y., F. Parhami, A. Tsingotjidou, S. Tetradis, M. Territo, and L. L. Demer. 2002. 8-Isoprostaglandin E2 enhances receptor-activated NFkappa B ligand (RANKL)-dependent osteoclastic potential of marrow hematopoietic precursors via the cAMP pathway. J. Biol. Chem. 27714221-14226. [DOI] [PubMed] [Google Scholar]
- 52.Tomita, M., X. Li, Y. Okada, F. N. Woodiel, R. N. Young, C. C. Pilbeam, and L. G. Raisz. 2002. Effects of selective prostaglandin EP4 receptor antagonist on osteoclast formation and bone resorption in vitro. Bone 30159-163. [DOI] [PubMed] [Google Scholar]
- 53.Wada, T., T. Nakashima, N. Hiroshi, and J. M. Penninger. 2006. RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol. Med. 1217-25. [DOI] [PubMed] [Google Scholar]
- 54.Walsh, M. C., and Y. Choi. 2003. Biology of the TRANCE axis. Cytokine Growth Factor Rev. 14251-263. [DOI] [PubMed] [Google Scholar]
- 55.Wani, M. R., K. Fuller, N. S. Kim, Y. Choi, and T. Chambers. 1999. Prostaglandin E2 cooperates with TRANCE in osteoclast induction from hemopoietic precursors: synergistic activation of differentiation, cell spreading, and fusion. Endocrinology 1401927-1935. [DOI] [PubMed] [Google Scholar]
- 56.Wong, B. R., R. Josien, S. Y. Lee, M. Vologodskaia, R. M. Steinman, and Y. Choi. 1998. The TRAF family of signal transducers mediates NF-kappaB activation by the TRANCE receptor. J. Biol. Chem. 27328355-28359. [DOI] [PubMed] [Google Scholar]
- 57.Yasuda, H., N. Shima, N. Nakagawa, K. Yamaguchi, M. Kinosaki, S. Mochizuki, A. Tomoyasu, K. Yano, M. Goto, A. Murakami, E. Tsuda, T. Morinaga, K. Higashio, N. Udagawa, N. Takahashi, and T. Suda. 1998. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc. Natl. Acad. Sci. USA 953597-3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yongchaitrakul, T., K. Lertsirirangson, and P. Pavasant. 2006. Human periodontal ligament cells secrete macrophage colony-stimulating factor in response to tumor necrosis factor-alpha in vitro. J. Periodontol. 77955-962. [DOI] [PubMed] [Google Scholar]