Abstract
Recent research has established that the terminal rectum is the predominant colonization site of enterohemorrhagic Escherichia coli O157:H7 in cattle. The main aim of the present work was to investigate pathological changes and associated immune responses at this site in animals colonized with E. coli O157:H7. Tissue and gastrointestinal samples from a total of 22 weaned Holstein-cross calves challenged with E. coli O157:H7 were analyzed for bacterial colonization and pathology. Five unexposed age-matched calves were used as comparative negative controls. E. coli O157:H7 bacteria induced histopathological alterations of the rectal mucosa with enterocyte remodeling. This was often associated with removal of the colonized epithelial layer. Immunogold labeling and transmission electron microscopy (TEM) showed E. coli O157 bacteria on pedestals, as part of attaching and effacing lesions. These pathological changes induced a local infiltration of neutrophils that was quantified as larger in infected animals. Rectal mucosal immunoglobulin A responses were detected against the E. coli O157:H7 antigen. This work presents evidence that E. coli O157:H7 is not a commensal bacteria in the bovine host and that the mucosal damage produced by E. coli O157:H7 colonization of the terminal rectum induces a quantifiable innate immune response and production of specific mucosal antibodies.
Enterohemorrhagic Escherichia coli (EHEC) infection has emerged in the last 20 years as a cause of diarrhea that can lead to the more serious consequence of hemolytic-uremic syndrome and thrombotic microangiopathy. The majority of EHEC infections are caused by E. coli O157:H7 (24), and this serotype has been isolated frequently from cattle feces. Many human EHEC O157 infections originate, either directly or indirectly, from exposure to cattle feces (17), and a key step in protecting humans from EHEC infection is to understand and control E. coli O157:H7 colonization of cattle.
Experimental challenges have suggested a variety of colonization sites in cattle (4, 5, 12). However, more recently, the terminal rectal mucosa has been identified as the major site of E. coli O157:H7 colonization (25), and this finding has been confirmed in slaughter animals (20). From an understanding of where E. coli O157:H7 colonizes the bovine intestinal tract, there is an opportunity to examine pathological changes at the site and to determine whether these changes correlate with the development of immunological responses. The main aim of the research is to underpin methods to control this pathogen in its main animal reservoir.
A feature of E. coli O157:H7 infection is the formation of attaching and effacing (A/E) lesions, characterized by the elimination of the microvilli and intimate enterocyte attachment (7, 16). In vivo, A/E lesions are present at the terminal rectum of naturally and experimentally infected cattle, and inactivation of the type III secretion apparatus that is essential for this phenotype prevents E. coli O157:H7 colonization of cattle (27). The profound alteration of enterocyte morphology associated with A/E lesions has also been reported to be accompanied by an increase in neutrophils and eosinophils in the lamina propria of the large intestine (37), the colon and cecum (6), the gall bladder (35), and sections of ligated ileal loops (32). This type of inflammatory reaction has been described in the intestinal tract exclusively for experimental infections of gnotobiotic, neonatal, or immunosuppressed calves and in sites other than the terminal rectum. However, to date, the response to colonization at the principal colonization site has not been investigated.
Identification of the terminal rectum as the tissue targeted by E. coli O157:H7 in cattle allows the study of the pathological changes and associated innate and adaptive mucosal responses. Thus, this study had two objectives: first, to determine if E. coli O157:H7 colonization of the bovine terminal rectum induces pathological changes, in terms of both ultrastructural change to the mucosal epithelium and evidence of inflammation, and second, to investigate local immune responses to colonization. Additionally, this work aimed to confirm in a larger number of animals the previously reported findings of E. coli O157:H7 tropism for the terminal rectum.
MATERIALS AND METHODS
Animals.
Fifty-four weaned Holstein-cross calves were reared conventionally on a farm before transfer to Moredun Research Institute for the experimental procedures (authorized by Home Office license 60/3179). The animals were between 8 and 14 weeks of age on arrival and were then penned individually for the study. Calves were fed concentrate twice daily and had access ad libitum to hay and water. Feed, water, and bedding were provided separately for each animal to minimize cross-contamination, and each was haltered to reduce the opportunity for fecal-oral transmission. Five unexposed calves of similar age, breed, and background were used as controls for the histopathological studies, and a separate group of four calves was challenged specifically for ultrastructural investigations of the colonized rectal mucosae.
Bacterial strains.
The challenge strain of E. coli O157:H7 was ZAP 198 isolated from a human patient in Washington state and was used previously in experimental studies (25). ZAP 198 has been naturally cured of the verocytotoxin-carrying bacteriophage, and the strain was selected for spontaneous resistance to nalidixic acid to facilitate recovery from feces and tissues. ZAP 198 possess genes for enterohemolysin, intimin-γ, EspA, and EspB. For the preliminary examination of adaptive responses, whole-cell extracts of E. coli K-12 MG1655 (2) and E. coli O26 ZAP 1082 (28) were used as controls in Western blots.
Calf colonization.
Fecal samples were taken at least twice from each calf, prior to experimental challenge, and were confirmed negative for E. coli O157:H7 by immunomagnetic separation. The challenge E. coli O157:H7 strain was grown overnight in Luria-Bertani (LB) broth at 37°C with aeration and diluted in sterile phosphate-buffered saline (PBS) to achieve an inoculum of 109 CFU per animal in a total volume of 10 ml. The oral inoculum was administered to the calves via stomach tube and washed down with 500 ml of sterile PBS or by direct administration through a cannula to the rumen in six calves. Initial analysis showed that the ruminal inoculation and the challenge via nasogastric tube did not differ in terms of efficacy, and the former method was halted. Therefore, for analytical purposes the ruminally challenged animals are included within the orally challenged group. Rectal challenge was carried out by loading a large cotton swab with the challenge inoculum, followed by direct application to the mucosal surface of the recto-anal junction (RAJ). In total, 24 calves were challenged via the rectal route and 30 by oral or ruminal administration.
Postmortem examinations were carried out with 22 animals shedding detectable levels of E. coli O157:H7 bacteria beyond day 14 postchallenge. Ten of these animals had been experimentally challenged with E. coli O157:H7 by direct rectal application and 12 by oral administration as described recently (26). Tissue samples were collected from the gastrointestinal tract for bacterial counts and histopathology. Tissue samples were taken from the rumen, jejunum, Peyer's patch, cecum, ileocecocolic valve lymphoid patch, proximal colon, proximal colon lymphoid patch, spiral colon, and distal colon and at the proximal, mid, and distal rectum (at 20, 10, and 5 cm proximal to the RAJ) and from the RAJ itself. Luminal contents were collected from the same sites for bacterial culture, together with bile from the gall bladder.
Rectal biopsy samples were taken from 11 animals, under local epidural anesthesia, at prechallenge and on two further occasions at 6 and 11 days after challenge. Local anesthesia was achieved by epidural administration of 1 ml of lidocaine into the intercoccygeal space between C1 and C2. The biopsies consisted of pieces of rectal mucosa weighing between 50 and 75 mg that were excised from the terminal rectum.
Isolation of E. coli O157:H7 and enumeration.
Feces were caught upon defecation and separated into core and surface components. The concentrations of E. coli O157:H7 bacteria in feces, intestinal contents, and tissues were estimated as described previously (25).
Histopathology.
Tissues taken at postmortem examination for histopathology were immediately fixed in 4% paraformaldehyde. Thirteen animals were selected for histopathological examination. Cell counts were made of total granulocytes, eosinophils, neutrophils (total granulocytes minus eosinophils), mast cells, and γδ T cells from the lamina propria of the rectum and large intestine. Total granulocyte numbers were obtained from sections stained with hematoxylin and eosin, mast cells from sections stained with toluidine blue (0.5% in 0.5 N HCl [pH 0.5]) for 1 h (9), and eosinophils from sections stained with carbol chromotrope solution for 1 h and counterstained for 10 s with hematoxylin (18). IL A29 was used to detect the γδ T-cell marker BoWC1 (15) in immunostained sections.
Paraffin sections (5 μm) of paraformaldehyde-fixed tissues were used to detect E. coli O157:H7 by the horseradish peroxidase (HRP)-streptavidin method. The primary antibody was rabbit anti-O157 polyclonal antibody (1:100 for 60 min at room temperature; Mast-Assure), followed by HRP-streptavidin-conjugated mouse anti-rabbit monoclonal antibodies (Sigma-Aldrich). In some studies, paraformaldehyde-fixed tissues were used to detect E. coli O157 by immunofluorescent antibody labeling. These cells were processed as described above, except that the secondary antibody was a fluorescein isothiocyanate-conjugated goat anti-rabbit antibody (Sigma-Aldrich).
Cell counts.
These were performed for eosinophils, neutrophils, mast cells, and γδ T cells from the lamina propria of the rectum and large intestine. Using a power calculation of 0.8 and 95% significance level, we counted five fields and two different areas for each of the sections. Cell counts were expressed as cells per 0.25 mm2. Bacterial counts were obtained after counting 10 colonies in two different RAJ tissues from each of five animals.
Electron microscopy.
Two different techniques were used to identify E. coli O157 in ultrastructural studies. From the group of thirteen animals selected for histological examination, areas of three tissue sections from two animals where E. coli O157 had been identified by immunofluorescent antibody were circumscribed, and the tissue in the matching areas of the paraffin blocks were precisely excised and reprocessed for transmission electron microscopy (TEM). From the group of four calves challenged specifically for electron microscopy studies, tissues were fixed in 2% formaldehyde and 0.1% glutaraldehyde, and bacteria were labeled with the same primary antibody used for light microscopy, with a secondary goat anti-rabbit immunoglobulin G (IgG) 10-μm gold-labeled antibody (1:500 for 60 min at room temperature; British Biocell). Images were processed with PaintShop Pro (Jasc Software).
Determination of mucosal antibody responses.
Rectal mucosa (weighing approximately 2 g) samples were excised at postmortem examination from nine animals and stored at −70°C. After being thawed, 50 to 75 mg of the biopsy sample or rectal mucosal tissue was mixed with 1 ml of ice-cold PBS (pH 7.2) in RiboLyzer tubes (Hybaid, United Kingdom) and then processed in a Hybaid RiboLyzer for 10 s at 5.5 ms−1. The supernatant of resultant homogenate was removed and centrifuged to remove the tissue. The protein content of the supernatant was then determined with the bicinchoninic acid protein assay kit (Pierce) according to the manufacturer's instructions and standardized to a final concentration of 5 mg gram−1. Whole-cell samples of E. coli O157:H7, E. coli O26, and E. coli K-12 were prepared from overnight LB broth cultures, and heated for 10 min at 70°C in loading buffer, 5 μl of sodium dodecyl sulfate, and 2 μl of reducing agent (Invitrogen), and each lane was loaded with 10 μg of bacterial protein and separated using 4% to 12% NuPAGE Novex bis-Tris gels (Novex, Invitrogen) with NuPAGE morpholineethanesulfonic acid-sodium dodecyl sulfate running buffer. Lysates of E. coli O157:H7 were made by trypsinization of whole-cell samples for 45 min at 70°C with sequencing-grade-modified trypsin (Promega). Gels were transferred onto nitrocellulose membranes, using a semidry transfer apparatus at room temperature. After being transferred, the membranes were incubated in a PBS-T80-NaCl buffer consisting of PBS, 0.5% Tween 80, and 0.5 M NaCl for 60 min at room temperature to block nonspecific protein binding. The transferred proteins were incubated with the supernatant from the mucosal homogenates (0.03 mg gram−1 of mucosal protein). Following three washes, the membranes were incubated sequentially with mouse anti-bovine IgA (1/500 in PBS-T80-NaCl; Dako), biotinylated goat anti-mouse Ig (1/2500 in PBS-T80-NaCl; Dako), and streptavidin-HRP (streptavidin-HRP, 1/4,000 in PBS-T80-NaCl; Dako). The incubation steps were performed for 60 min at room temperature with three washes between each step. Peroxidase activity was revealed by chemiluminescence using ECL (Amersham Life Sciences, Bucks, United Kingdom) reagent. Control Western blots consisted of incubations of bovine rectal homogenate at the same concentration from unexposed animals and Western blots in which no primary antibody was added.
Statistical analyses.
E. coli O157:H7 cell counts within fecal and tissue samples were calculated by determining the mean plate count at the most relevant dilution for each sample and by multiplying the dilution factor to convert to CFU g−1 (feces or gastrointestinal tract contents) or to CFU cm−2 (mucosal samples). The concentration (CFU g−1 plus 1) was log10 transformed. Student's t test was used to compare means of samples, with a paired t test used where a natural pair existed. The chi-square test was used to analyze proportions. Where the number of observations was low in some categories, Fisher's exact test was used. The kappa test was used to qualify the level of agreement, where the null hypothesis is that there is no more agreement than might occur by random chance. Data were processed using Excel (Microsoft) and Minitab (Minitab Inc.) software.
RESULTS
Site of colonization.
Out of a total of 54 animals, 46 became colonized for longer than 5 days, and a postmortem examination was carried out on 22 animals still shedding detectable levels of E. coli O157:H7 bacteria beyond day 14 postchallenge. Ten of these animals had been challenged by direct rectal application and 12 by oral administration. E. coli O157:H7 cell counts from tissue washings of the terminal rectum were significantly higher than from tissues of the large intestine (P < 0.001), irrespective of the challenge route. Significantly higher counts (P < 0.01) were detected in the other rectal sites (5, 10, and 20 cm proximal to the RAJ) compared to counts from tissue washings of large-intestinal tissues. For the animals challenged by the direct rectal administration method, E. coli O157:H7 bacteria were not recovered from bile or samples of digesta from nonrectal sites that included the rumen and the small or large intestine. For the orally challenged group, E. coli O157:H7 bacteria were not recovered from bile (data not shown). Terminal rectal tissue collected from colonized animals allowed us to study pathological changes.
Pathological changes at the terminal rectum.
When bacterial concentrations exceeded 105 CFU per cm2, E. coli O157:H7 bacteria could be readily detected in association with the epithelium by immunostaining and microscopy. In these positive tissues, at 15 to 21 days after challenge, the immunopositive bacteria were usually but not exclusively colonizing focal areas of the absorptive epithelium or the scarcer follicle-associated epithelium. Bacterial microcolonies ranged from those containing less than 30 bacteria to those with several hundred. The distribution of the colonies appeared random, with some microcolonies close together and others separated by large areas of noncolonized rectal tissue. In all cases, affected epithelial cells had effaced microvilli, and bacteria were intimately associated with their apical membranes. Occasionally, immunostained bacteria were present without producing major morphological alterations of the rectal epithelium. Generally, the mucosal border in foci with attached bacteria was low columnar to cuboidal (Fig. 1A and B). There was frequent exfoliation of the mucosal epithelium from the basal membrane, and bacteria were often seen in cavities of evacuated enterocytes (Fig. 1C and D). Groups of loose bacteria were also present in the mucus 40 to 100 μm from the intestinal surface and were not always associated with adherent microcolonies. On rare occasions, E. coli O157:H7 bacteria were also attached to areas of the squamous epithelium of the perianal region and crypts of the rectal mucosa. Rarely, an immunopositive bacterium was detectable along lymphatic lacteals of the lamina propria in areas lacking an epithelial surface. E. coli O157:H7 microcolonies were also detected by immunostaining at day 6, from rectal biopsy samples from three animals that were shedding >105 CFU g−1 of feces of E. coli O157:H7 bacteria. The distribution and intimate bacterial attachment to enterocytes were similar to those observed for the cases examined at postmortem at days 15 and 21 postchallenge.
FIG. 1.
Histopathological changes induced by E. coli O157:H7 colonization at the terminal rectum of cattle. (A) Apical surface of the enterocyte partly eroded by an E. coli O157 microcolony (arrow). (B) A larger E. coli O157 microcolony causing further erosion of the cytoplasm. An E. coli O157 colony is illustrated (arrow). (C) Enterocytes (arrows) heavily colonized with E. coli O157 are shown sloughing off from the basal membrane. Bacterial aggregates surround the enterocyte. (D) A larger E. coli O157 microcolony is shown on the lamina propria, following the shedding of the epithelial layer. Histopathology was carried out as described in Materials and Methods.
The pathological changes were further examined by scanning electron microscopy, which revealed multifocal clusters of rod-shaped bacteria of up to 2 μm in length, distributed randomly over the surface of the absorptive epithelium of the rectum. TEM studies and gold particle immunolabeling allowed us to identify E. coli O157:H7 bacteria on pedestals as part of A/E lesions (Fig. 2A and B). Pedestal heights varied but in some cases were up to 10 μm long. Some microcolonies appeared to consist of bacteria in layers, forming a stack, and individual bacteria were observed in the process of dividing while attached to the host cells. Bacterial microcolonies were associated with different degrees of enterocyte erosion (Fig. 2C). On occasion, granulocytes were present, interspersed among enterocytes, and exuded leukocytes formed aggregations in the gut lumen (microabscesses) (Fig. 2D). Lateral and basal membrane detachment and enterocyte exfoliation were often evident but present only when the bacteria had caused enough damage to approach the cell basal nucleus.
FIG. 2.
Ultrastructure analysis of E. coli O157 interaction with the bovine terminal rectum. (A) Immunogold staining of E. coli O157 organisms. The image shows a single bacterium intimately attached through a pedestal to the host enterocyte, as part of an A/E lesion. Immunolabeling and TEM were carried out as described in Materials and Methods. (B) Cross-section of an E. coli O157 microcolony at the bovine terminal rectum. The bacteria are all intimately attached to the damaged epithelium (black arrows), inducing effacement of the microvilli. Unaffected brush border is visible on the neighboring cell (white arrow). (C) Aggregates of bacteria eroding the apical surface of colonized enterocytes (black arrows). Bacteria are intimately attached to the apical surface of the enterocyte (white arrows). (D) Extravasated polymorphic mononuclear leukocyte (N) adjacent to a bacterial cluster (black arrow).
Cellular infiltration and inflammation.
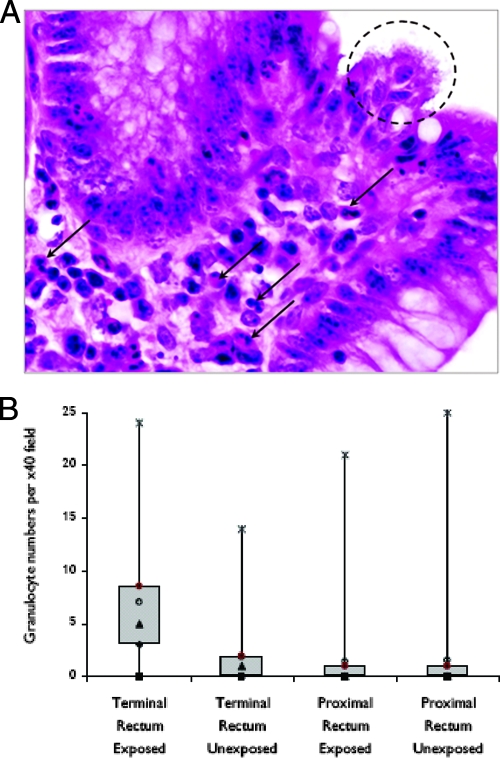
When E. coli O157:H7 was isolated from mucosal washings of tissues at levels higher than 105 CFU cm−2 or from biopsy samples taken from colonized animals that had similar numbers of E. coli O157:H7 bacteria in feces, there was a diffuse, low to mild granulocytic focal infiltration of the lamina propria of the rectum (Fig. 3A). In the terminal rectum, a significant (P < 0.001) leukocytic infiltrate was present in E. coli O157:H7-colonized animals (mean, 6.6 ± 1.4 per 0.25 mm2) compared with controls (mean, 1.9 ± 0.9 per 0.25 mm2). However, no differences were detected between the numbers of eosinophils, mast cells, and γδ T cells (P > 0.65, P > 0.69, and P > 0.68, respectively) in colonized animals compared with those in control animals. For colonized animals, significantly more granulocytes (P < 0.001) were found in the terminal rectum than in the proximal rectum (+20 cm). However, in the area 20 cm proximal from the terminal rectum, no significant differences (P > 0.42) were detected between the neutrophil count of infected cases and that of noninfected cases (controls/mean of 1.5 ± 1.1 per 0.25 mm2; cases/mean of 1.7 ± 1.0 per 0.25 mm2) (Fig. 3).
FIG. 3.
Histological granulocyte quantification. (A) Hematoxylin- and-eosin-stained bovine rectal mucosa colonized with E. coli O157:H7. The dashed outline highlights colonized epithelium. Arrows indicate infiltration of granulocytes. (B) Box plots show the granulocyte counts in the terminal rectums of exposed and unexposed animals. The boxes contain 50% of the data, and the median count is illustrated by the black triangle. ⧫, 1st quartile; ▪, minimum; ▴, median; ○, mean; ×, maximum; •, 3rd quartile. Samples were prepared and granulocytes identified as described in Materials and Methods.
Adaptive responses to E. coli O157:H7 colonization in the rectal mucosa.
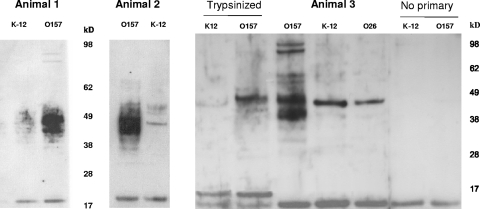
Mucosal antibodies were extracted from rectal mucosal homogenates to determine whether animals colonized by E. coli O157:H7 at the terminal rectum were generating specific mucosal antibody responses to the E. coli O157:H7 antigen. Samples were taken from three animals that shed E. coli O157:H7 at levels consistently higher than 104 CFU gram−1 for at least 2 weeks. In these samples, IgA antibodies were detected that bound to antigens of whole E. coli O157:H7 cells. Between 4 and 11 protein bands, with molecular masses ranging from 38 to 98 kDa, were recognized. The same homogenate samples showed no immune response to whole-cell extracts of the E. coli O26 or E. coli K-12 strains. Trypsinization of the E. coli K-12 and O157:H7 samples removed most of the immunoreactive material and resulted in the detection of a 14-kDa band for both strains (Fig. 4). No specific reactive bands were observed for four noninfected controls or from Western blots where the rectal mucosal homogenate was omitted (data not shown).
FIG. 4.
Detection of mucosal antibody responses to protein antigens of E. coli O157. Mucosal antibody was obtained from homogenates of rectal mucosa sampled from three animals that had histopathological lesions detectable by microscopy. The homogenates were used to blot whole-cell preparations of E. coli strains O157 and K-12 and, in one case, O26. Multiple E. coli O157 immunoreactive bands were detected in comparison to either E. coli K-12 or E. coli O26. Trypsin digestion of the bacterial preparations removed most of the immunoreactive material. This is demonstrated with a blot using the homogenate from animal 3 (lanes labeled “Trypsinized”). Lanes marked “no primary” are controls containing E. coli O157 and K-12 preparations incubated with all the reagents, except for the rectal homogenate. Mucosal homogenates and Western blots were prepared as described in Materials and Methods.
DISCUSSION
Work carried out by our group has demonstrated that E. coli O157:H7 has a tropism for the terminal rectum of cattle (25). The present study has confirmed this finding by examination of over 50 animals colonized by different challenge routes. The postmortem examination of these colonized animals also allowed the identification of minor sites of E. coli O157:H7 carriage. These sites included the rumen, small intestine, and most frequently, the proximal colon and, in particular, the lymphoid-rich tissue immediately distal to the ileocecal valve. In 2 animals out of the 54 studied, E. coli O157:H7 bacteria were distributed throughout the large intestine, given the even distribution of the bacteria throughout the fecal part. This finding is consistent with previous reports (25) and suggests that there is a different mechanism of colonization for a small number of animals, maybe due to the existence of multiple E. coli O157:H7 genetic types with different colonization strategies within one animal (11).
The postmortem and rectal biopsy materials collected from the colonized animals enabled a detailed study of the histological and ultrastructural changes associated with rectal colonization. A/E lesions were detected in animals with bacterial counts of more than 105 CFU g−1 in rectal tissues several weeks after experimental inoculation, and this is consistent with the previous finding that bacterial type III secretion system and A/E lesion formation are essential for the colonization and persistence of the organism in cattle (26). The long-term persistence is of a duration similar to the natural carriage observed for animals in field studies (1). The “shotgun” distribution of the microcolonies on the rectal mucosa may be caused by the dispersion of cells from the microcolonies into the surrounding environment, in the same manner proposed for E. coli spreading from biofilms (36) formed in response to shear forces and turbulent flow (8). In addition to A/E lesions, the major histopathological changes consisted of a reduction in enterocyte cellular width, a degeneration of cytoplasm in heavily colonized cells, and a frequent sloughing of enterocytes. These alterations were associated with a quantifiable neutrophilic response. The microscopic examination was made in animals shedding bacterial numbers similar to those animals considered super-shedders in field studies (10, 22, 34). Similar lesions have been reported in weaned calves 4 days postchallenge (7). Given the severe nature of the enterocyte changes observed, it is possible that most of the mucosal damage observed is due to enterocyte desquamation. In vitro studies have consistently reported decreased transepithelial resistance and opening of the tight junctions following E. coli O157:H7 colonization of monolayers (29), and similar findings have been reported in vivo in a mouse model of enteropathogenic E. coli/EHEC infection (13). The destructive alterations induced by E. coli O157:H7 infection in the bovine epithelial barrier induced a quantifiable neutrophilic response. This type of inflammatory reaction has been described previously in the intestinal tract for experimental infections of gnotobiotic or neonatal calves (0 to 1 day old) that are not immunocompetent. A quantified increase in neutrophils and eosinophils in the lamina propria has been described on one occasion (37), while other studies report similar increases in leukocytes in the colon and cecum (6) and sections of ligated ileal loops (32). In weaned calves, an eosinophilic infiltrate was reported for the perianal skin that bounds the rectal epithelium and in areas where A/E lesions were present (30). However, the quantified response in this study is the first for the predominant colonization site in experimentally challenged cattle.
The work also determined that E. coli O157:H7 infection induces a mucosal humoral immune response in cattle. Western blot analysis indicated 4 to 11 IgA-reactive bands of between 38 and 98 kDa. This pattern of bands was almost entirely removed by protein lysis of the bacterial cell sample. These responses may be the result of the high-level experimental challenge with E. coli O157:H7. However, this seems unlikely given that other experimental work has shown no immunological response to nonpathogenic strains of E. coli (14) and that challenge by E. coli O157:H7 leads to serological responses to proteins encoded by the locus of enterocyte effacement (3). This study has, therefore, provided evidence that cattle develop specific mucosal antibodies following colonization. Further studies and the application of more sensitive mucosal antibody detection methods (23) are required to determine whether these mucosal antibodies are involved in a protective immune response.
The terminal rectum is an area rich in lymphoid follicles (21), and it has been suggested that adherence to these sites may explain the tropism of E. coli O157:H7 bacteria for the bovine terminal rectum (25). In this study, extensive histological examination of terminal rectal tissues did not reveal a prominent association between E. coli O157:H7 microcolonies and follicle-associated epithelium. Thus, the reason for the terminal rectum tropism of E. coli O157:H7 is still obscure (19). Two of the main features of this area are a potentially reduced width of the mucous barrier, based on measurements taken with mice over Peyer's patches (33) and the fact that the recto-anal junction is adjacent to the anal sphincter. The combined effect of a reduced protective mucous barrier coupled with raised intrarectal pressure during defecation may facilitate colonization by the promotion of cell-to-cell contact that is one of the key mechanisms considered to induce type III secretion (31).
For many years, E. coli O157:H7 has been regarded as causing no clinical signs of infection in cattle. This study identifies pathological change and production of a local immune response in the terminal rectum in animals shedding high numbers of E. coli O157:H7 bacteria and infiltration of granulocytes and production of local IgA antibodies. This is the first report of local innate immune responses to E. coli O157:H7 rectal colonization in weaned calves, and so E. coli O157:H7 should not be regarded as a commensal organism in this host species. The findings may be of value in the development of methods for the control of E. coli O157:H7 carriage by cattle.
Acknowledgments
This research was supported by a research grant from DEFRA (CSA6372/OZ0712) to J.C.L. and D.L.G. The Scottish government provides financial support to SAC, Moredun Research Institute, and BioSS.
Editor: B. A. McCormick
Footnotes
Published ahead of print on 2 September 2008.
REFERENCES
- 1.Besser, T. E., D. D. Hancock, L. C. Pritchett, E. M. McRae, D. H. Rice, and P. I. Tarr. 1997. Duration of detection of fecal excretion of Escherichia coli O157:H7 in cattle. J. Infect. Dis. 175726-729. [DOI] [PubMed] [Google Scholar]
- 2.Blattner, F. R., G. Plunkett III, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 2771453-1474. [DOI] [PubMed] [Google Scholar]
- 3.Bretschneider, G., E. M. Berberov, and R. A. Moxley. 2007. Isotype-specific antibody responses against Escherichia coli O157:H7 locus of enterocyte effacement proteins in adult beef cattle following experimental infection. Vet. Immunol. Immunopathol. 118229-238. [DOI] [PubMed] [Google Scholar]
- 4.Brown, C. A., B. G. Harmon, T. Zhao, and M. P. Doyle. 1997. Experimental Escherichia coli O157:H7 carriage in calves. Appl. Environ. Microbiol. 6327-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cray, W. C., Jr., and H. W. Moon. 1995. Experimental infection of calves and adult cattle with Escherichia coli O157:H7. Appl. Environ. Microbiol. 611586-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dean-Nystrom, E. A., B. T. Bosworth, and H. W. Moon. 1997. Pathogenesis of O157:H7 Escherichia coli infection in neonatal calves. Adv. Exp. Med. Biol. 41247-51. [DOI] [PubMed] [Google Scholar]
- 7.Dean-Nystrom, E. A., B. T. Bosworth, and H. W. Moon. 1999. Pathogenesis of Escherichia coli O157:H7 in weaned calves. Adv. Exp. Med. Biol. 473173-177. [DOI] [PubMed] [Google Scholar]
- 8.Donlan, R. M. 2002. Biofilms: microbial life on surfaces. Emerg. Infect. Dis. 8881-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Enerback, L. 1966. Mast cells in rat gastrointestinal mucosa. I. Effects of fixation. Acta Pathol. Microbiol. Scand. 66289-302. [DOI] [PubMed] [Google Scholar]
- 10.Fegan, N., P. Vanderlinde, G. Higgs, and P. Desmarchelier. 2004. The prevalence and concentration of Escherichia coli O157 in faeces of cattle from different production systems at slaughter. J. Appl. Microbiol. 97362-370. [DOI] [PubMed] [Google Scholar]
- 11.Fox, J. T., X. Shi, and T. G. Nagaraja. 2008. Escherichia coli O157 in the rectoanal mucosal region of cattle. Foodborne Pathog. Dis. 569-77. [DOI] [PubMed] [Google Scholar]
- 12.Grauke, L. J., I. T. Kudva, J. W. Yoon, C. W. Hunt, C. J. Williams, and C. J. Hovde. 2002. Gastrointestinal tract location of Escherichia coli O157:H7 in ruminants. Appl. Environ. Microbiol. 682269-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guttman, J. A., Y. Li, M. E. Wickham, W. Deng, A. W. Vogl, and B. B. Finlay. 2006. Attaching and effacing pathogen-induced tight junction disruption in vivo. Cell. Microbiol. 8634-645. [DOI] [PubMed] [Google Scholar]
- 14.Hoffman, M. A., C. Menge, T. A. Casey, W. Laegreid, B. T. Bosworth, and E. A. Dean-Nystrom. 2006. Bovine immune response to Shiga-toxigenic Escherichia coli O157:H7. Clin. Vaccine Immunol. 131322-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howard, C. J., W. I. Morrison, A. Bensaid, W. Davis, L. Eskra, J. Gerdes, M. Hadam, D. Hurley, W. Leibold, J. J. Letesson, N. Machugh, J. Naessens, K. O'Reilly, K. R. Parsons, D. Scholte, P. Sopp, G. Splitter, and R. Wilson. 1991. Summary of workshop findings for leukocyte antigens of cattle. Vet. Immunol. Immunopathol. 2721-27. [DOI] [PubMed] [Google Scholar]
- 16.Ismaili, A., E. McWhirter, M. Y. Handelsman, J. L. Brunton, and P. M. Sherman. 1998. Divergent signal transduction responses to infection with attaching and effacing Escherichia coli. Infect. Immun. 661688-1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karmali, M. A. 2004. Infection by Shiga toxin-producing Escherichia coli: an overview. Mol. Biotechnol. 26117-122. [DOI] [PubMed] [Google Scholar]
- 18.Lendrum, A. C. 1944. The staining of eosinophil polymorphs and entrochromaffin cells in histological sections. J. Pathol. Bacteriol. 56441. [Google Scholar]
- 19.Lim, J. Y., J. Li, H. Sheng, T. E. Besser, K. Potter, and C. J. Hovde. 2007. Escherichia coli O157:H7 colonization at the rectoanal junction of long-duration culture-positive cattle. Appl. Environ. Microbiol. 731380-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Low, J. C., I. J. McKendrick, C. McKechnie, D. Fenlon, S. W. Naylor, C. Currie, D. G. Smith, L. Allison, and D. L. Gally. 2005. Rectal carriage of enterohemorrhagic Escherichia coli O157 in slaughtered cattle. Appl. Environ. Microbiol. 7193-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahajan, A., S. Naylor, A. D. Mills, J. C. Low, A. Mackellar, D. E. Hoey, C. G. Currie, D. L. Gally, J. Huntley, and D. G. Smith. 2005. Phenotypic and functional characterisation of follicle-associated epithelium of rectal lymphoid tissue. Cell Tissue Res. 321365-374. [DOI] [PubMed] [Google Scholar]
- 22.Matthews, L., I. J. McKendrick, H. Ternent, G. J. Gunn, B. Synge, and M. E. Woolhouse. 2006. Super-shedding cattle and the transmission dynamics of Escherichia coli O157. Epidemiol. Infect. 134131-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McNeilly, T. A., S. W. Naylor, M. C. Mitchell, S. McAteer, A. Mahajan, D. G. E. Smith, D. L. Gally, J. C. Low, and J. F. Huntley. Simple methods for measurement of bovine mucosal antibody responses in vivo. Vet. Immunol. Immunopathol. 118160-167. [DOI] [PubMed]
- 24.Mead, P. S., and P. M. Griffin. 1998. Escherichia coli O157:H7. Lancet 3521207-1212. [DOI] [PubMed] [Google Scholar]
- 25.Naylor, S. W., J. C. Low, T. E. Besser, A. Mahajan, G. J. Gunn, M. C. Pearce, I. J. McKendrick, D. G. Smith, and D. L. Gally. 2003. Lymphoid follicle-dense mucosa at the terminal rectum is the principal site of colonization of enterohemorrhagic Escherichia coli O157:H7 in the bovine host. Infect. Immun. 711505-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naylor, S. W., P. Nart, J. Sales, A. Flockhart, D. L. Gally, and J. C. Low. 2007. Impact of the direct application of therapeutic agents to the terminal recta of experimentally colonized calves on Escherichia coli O157:H7 shedding. Appl. Environ. Microbiol. 731493-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naylor, S. W., A. J. Roe, P. Nart, K. Spears, D. G. Smith, J. C. Low, and D. L. Gally. 2005. Escherichia coli O157: H7 forms attaching and effacing lesions at the terminal rectum of cattle and colonization requires the LEE4 operon. Microbiology 1512773-2781. [DOI] [PubMed] [Google Scholar]
- 28.Pearce, M. C., J. Evans, I. J. McKendrick, A. W. Smith, H. I. Knight, D. J. Mellor, M. E. Woolhouse, G. J. Gunn, and J. C. Low. 2006. Prevalence and virulence factors of Escherichia coli serogroups O26, O103, O111, and O145 shed by cattle in Scotland. Appl. Environ. Microbiol. 72653-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Philpott, D. J., D. M. McKay, W. Mak, M. H. Perdue, and P. M. Sherman. 1998. Signal transduction pathways involved in enterohemorrhagic Escherichia coli-induced alterations in T84 epithelial permeability. Infect. Immun. 661680-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pohlenz, J. F., and E. A. Dean-Nystrom. 2004. Colonisation of Escherichia coli O157:H7 on squamous epithelial cells at the rectal-anal junction. Vet. Rec. 155248. [PubMed] [Google Scholar]
- 31.Roe, A. J., D. E. Hoey, and D. L. Gally. 2003. Regulation, secretion and activity of type III-secreted proteins of enterohaemorrhagic Escherichia coli O157. Biochem. Soc. Trans. 3198-103. [DOI] [PubMed] [Google Scholar]
- 32.Sandhu, K. S., and C. L. Gyles. 2002. Pathogenic Shiga toxin-producing Escherichia coli in the intestine of calves. Can. J. Vet. Res. 6665-72. [PMC free article] [PubMed] [Google Scholar]
- 33.Sansonetti, P. J. 2004. War and peace at mucosal surfaces. Nat. Rev. Immunol. 4953-964. [DOI] [PubMed] [Google Scholar]
- 34.Stanford, K., S. J. Bach, T. H. Marx, S. Jones, J. R. Hansen, G. L. Wallins, H. Zahiroddini, and T. A. McAllister. 2005. Monitoring Escherichia coli O157:H7 in inoculated and naturally colonized feedlot cattle and their environment. J. Food Prot. 6826-33. [DOI] [PubMed] [Google Scholar]
- 35.Stoffregen, W. C., J. F. Pohlenz, and E. A. Dean-Nystrom. 2004. Escherichia coli O157:H7 in the gallbladders of experimentally infected calves. J. Vet. Diagn. Investig. 1679-83. [DOI] [PubMed] [Google Scholar]
- 36.Van, H. R., and C. W. Michiels. 2005. Role of bacterial cell surface structures in Escherichia coli biofilm formation. Res. Microbiol. 156626-633. [DOI] [PubMed] [Google Scholar]
- 37.Woodward, M. J., D. Gavier-Widen, I. M. McLaren, C. Wray, M. Sozmen, and G. R. Pearson. 1999. Infection of gnotobiotic calves with Escherichia coli o157:h7 strain A84. Vet. Rec. 144466-470. [DOI] [PubMed] [Google Scholar]