Abstract
Entamoeba histolytica pathogenesis in the colon occurs in a stepwise fashion. It begins with colonization of the mucin layer, which is followed by stimulation of a proinflammatory response that causes nonspecific tissue damage that may facilitate parasite invasion of the underlying colonic mucosa. Unfortunately, the parasite and/or host factors that stimulate a proinflammatory response in the gut are poorly understood. In this study, we found that live E. histolytica or secretory or proteins (SP) and soluble ameba components (SAP) can markedly increase interleukin-8 (IL-8) mRNA expression and protein production in colonic epithelial cells. The IL-8-stimulating molecule produced by live amebae was identified as prostaglandin E2 (PGE2) as trophozoites treated with cyclooxygenase inhibitors inhibited the biosynthesis of PGE2 and eliminated IL-8 production induced by live parasites or ameba components. Moreover, using specific prostaglandin EP2 and EP4 receptor agonists and antagonists, we found that PGE2 binds exclusively through EP4 receptors in colonic epithelial cells to stimulate IL-8 production. Silencing of EP4 receptors with EP4 small interfering RNA completely eliminated SP- and SAP-induced IL-8 production. These studies identified bioactive PGE2 as a one of the major virulence factors produced by E. histolytica that can stimulate the potent neutrophil chemokine and activator IL-8, which can trigger an acute host inflammatory response. Thus, the induction of IL-8 production in response to E. histolytica-derived PGE2 may be a mechanism that explains the initiation and amplification of acute inflammation associated with intestinal amebiasis.
Entamoeba histolytica is an enteric protozoan parasite and the fourth leading cause of death due to a parasite (26). Humans are the only known host for E. histolytica, and about 50 million people are affected worldwide each year. The pathogenesis of amebiasis is believed to be a multistep and multifactorial process. Although a large number of studies have attempted to unravel the factors or molecules responsible for the pathogenesis of amebiasis, the processes involved in pathogenesis are not well understood. In most infected individuals, E. histolytica trophozoites exist as commensals. However, in a small percentage of infections, amebae can elude luminal and epithelial barrier host defense mechanisms and invade the intestinal mucosa, causing ulcers and amebic colitis. Even though host inflammatory responses play an important role in the onset and progression of invasive amebiasis, little is known about the parasite factors that initiate this event. Even less is known about the parasite components that are secreted or released in the gut and can modulate colonic epithelial cell functions.
Some of the important molecules that are involved in the pathogenesis of intestinal amebiasis have been identified. For example, E. histolytica trophozoites bind with high affinity to Gal and GalNAc residues on mucus glycoproteins by using their surface adherence N-acetyl-d-galactosamine-specific lectin (20) in colonization. The N-acetyl-d-galactosamine-specific lectin also mediates binding to target cells, and ameba pore-forming proteins (17) can be inserted into lipid bilayers of target cells, forming ion channels and subsequently causing cell death. We recently showed that cysteine protease 5 secreted by E. histolytica specifically cleaves the C-terminal polymerization domain of mucin polymer and dissolves the protective mucus layer (18). This process allows E. histolytica to come in direct contact with epithelial cells. In addition to the direct cytolysis of host cells by amebae, the parasite also activates host epithelial cell immune responses in contact-dependent and contact-independent manners. Lysed epithelial cells release pre-interleukin-1β (pre-IL-1β), which is processed by ameba cysteine proteinases to its active form (29). Studies using SCID-human mouse models of intestinal amebiasis have shown that there is stimulation of additional inflammatory mediators, including IL-6, growth-related oncogene α, cyclooxygenase 2 (COX-2), and granulocyte-macrophage colony-stimulating factor (GM-CSF), by adjacent intestinal cells through the nuclear factor κB-dependent signaling pathway (10, 22). Collectively, these events result in tissue destruction and subsequent invasion of tissue by amebae in the colon.
Amebiasis is characterized by infiltration of inflammatory and immune cells in the amebic lesions (11). We hypothesized that release of IL-8 by colonic epithelial cells is a major factor that can initiate the onset of inflammation. IL-8 is a potent chemoattractant and activator of neutrophils, which can cause nonspecific tissue damage after activation (10, 28). IL-8 is a member of the CXC family of chemokines, has a molecular mass of 8 to 10 kDa, and is activated after cleavage of 20-amino-acid signal sequences. A variety of cells, including macrophages, T lymphocytes, epithelial cells, and neutrophils, produce IL-8. We have shown previously (9) that E. histolytica synthesizes prostaglandin E2 (PGE2) through a novel COX-like enzyme that is believed to play a major role in maintaining the cell cycle in amebae. However, the mechanism of IL-8 induction by ameba PGE2 during invasive amebiasis is not known, and it is also not clear if ameba components themselves can directly induce production of this chemokine in the gut. Here, we shown that the presence of PGE2 endogenously synthesized by live E. histolytica or the presence of PGE2 in soluble amebic proteins (SAP) or in secretory components or proteins (SP) can induce IL-8 production by a unique pathway involving EP4 receptors on colonic epithelial cells.
MATERIALS AND METHODS
Cells, reagents, and ameba components.
The Caco-2 human adenocarcinoma cell line was obtained from the ATCC and grown to obtain confluent monolayers in minimal essential medium containing 5% fetal bovine serum and 5 mg/ml penicillin-streptomycin. EP receptor-specific agonists and antagonists were obtained from Cayman Chemicals unless indicated otherwise. SAP were prepared by using three cycles of freeze-thaw lysis of log-phase E. histolytica virulent strain HM1:IMSS (passaged three times in gerbil livers) and were quantified by the bicinchoninic acid protein assay (Pierce). E. histolytica SP were prepared as described previously (18). For transwell studies, trophozoites were added to Corning transwell inserts with a pore diameter of 0.6 μm, with Caco-2 cells in the bottom well.
Real-time PCR.
Total RNA was extracted with TRIzol reagent (Invitrogen) and quantified. One microgram of RNA was reverse transcribed by using Moloney murine leukemia virus reverse transcriptase (Invitrogen) and oligo(dT) according to the manufacturer's instructions. One-tenth of the cDNA reaction mixture was used for real-time PCR. Amplification was carried out with a Quantitech SYBR green PCR kit (Qiagen) using the following cycling conditions: 94°C for 15 min, followed by 45 cycles of denaturation at 94°C for 20 s, annealing at 60°C for 30 s, and extension at 72°C for 60 s. The primers used were IL-8 forward (5′CGTGGCTCTCTTGGCAGC3′), IL-8 reverse (5′TCTTTAGCACTCCTTGGCAAAAC3′), GAPDH forward (5′GAAGGTGAAGGTCGGAGT3′), and GAPDH reverse (5′GAAGATGGTGATGGGATTTC3′). The specificity of amplification was checked by performing a melting curve analysis. IL-8 mRNA expression was normalized using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA. The change compared with control levels was determined by using the comparative cycle threshold method as described previously (4).
Coculturing of epithelial cells with amebae.
Confluent Caco-2 cells (106 cells) grown in either regular or transwell plates for 7 to 10 days were used for all studies. For small interfering RNA (siRNA) experiments, subconfluent (40 to 50%) Caco-2 cells grown for 2 to 3 days were used. Transwells with 2 × 106 amebae were incubated in culture plates containing 106 epithelial cells for 24 h at 37°C and then removed from coculture and immediately used for subsequent experiments. Epithelial cells were kept under low-serum conditions (5% fetal bovine serum) during coculturing and subsequent treatments and without antibiotics for siRNA studies.
IL-8 assays.
IL-8 production was measured by using a Titerzyme kit (Assay Designs Inc.) and a monoclonal antibody against human IL-8 according to the manufacturer's instructions.
Stimulation of cells with agonists, antagonists, and inhibitors.
Cells were seeded in six-well plates (106 cells/well) and allowed to attach overnight. Prior to the experiments, cells were fasted in serum-free medium overnight and then stimulated with or without PGE2 and with or without other drugs. Cells were pretreated with AH6809 (an EP2 receptor antagonist) or with AH23848 or L161982 (EP4 receptor antagonists) for 30 to 60 min prior to stimulation with PGE2- or EP receptor-specific agonists. Cells were then processed for RNA extraction by the TRIzol method for real-time PCR analysis or used for other studies. Most compounds were used at a final concentration of 1 μM; the only exception was AH6809, which was used at a concentration of 50 μM. The concentrations of PGE2 and several EP receptor-specific agonists and antagonists used in the experiments were optimal for intestinal epithelial cells, as determined in our previous studies and in studies performed by other workers (5, 7, 14, 28).
RNA interference.
Wild-type Caco-2 cells were transfected with EP4 siRNA (Smartpool M-005714-00) or control siRNA (D-001210-02 or D-001140-01) obtained from Dharmacon, Inc. (Lafayette, CO) using the manufacturer's protocol. Briefly, subconfluent (50 to 60%) cells were transfected using the X-Treme Gene siRNA transfection reagent (Roche) for 36 h at an siRNA concentration of 40 nM, and cells were immediately used for experiments.
Statistical analysis.
Data are expressed below as means ± standard errors of the means and were analyzed using Student's t test for unpaired data using Graphpad Prism software. P values of ≤0.05 were considered significant.
RESULTS
Induction of IL-8 by E. histolytica.
To determine the kinetics by which E. histolytica induces IL-8 production in colonic epithelial cells, we treated 106 confluent Caco-2 cells with different concentrations of SP or SAP or with live amebae. Treatment or coculture with live amebae was carried out in transwell plates in a contact-independent manner.
As shown in Fig. 1A and B, the induction of IL-8 mRNA expression and protein production was dose dependent. Maximum stimulation occurred with 40 μg/ml SP, which significantly (P > 0.001) induced both IL-8 mRNA expression (fivefold) and protein production (ninefold) compared with untreated controls. With SAP, the peak stimulatory responses occurred with 100 μg/ml, which induced IL-8 mRNA expression and protein production 7- and 11-fold compared with the control, respectively. Higher doses of SP and SAP did not induce IL-8 production significantly more than 40 μg/ml of SP or 100 μg/ml of SAP. Using these protein concentrations, we then determined the time dependence of IL-8 mRNA expression and protein production. As shown in Fig. 2A and B, 40 μg/ml of SP and 100 μg/ml of SAP induced both IL-8 mRNA expression and protein production significantly (P > 0.001) compared with controls in a time-dependent manner; peak mRNA expression occurred after 4 h, and peak IL-8 protein production occurred after 12 h. Interestingly, live E. histolytica trophozoites in transwells separated from colonic cells by membranes also significantly stimulated IL-8 mRNA expression and IL-8 production at 6, 8, and 12 h compared to untreated controls. At these time points in cocultures, about 90% of the amebae were alive as determined by a trypan blue exclusion assay (data not shown).
FIG. 1.

E. histolytica components induce IL-8 mRNA expression and protein production in colonic epithelial cells. (A) Confluent Caco-2 cells (106 cells) were treated with different concentrations of SP or SAP or with 2 × 106 live E. histolytica cells for 4 h. Live E. histolytica trophozoites were incubated in transwells with colonic cell monolayers. Total cellular RNA was extracted by the TRIzol method, and real-time PCR was performed as described in Materials and Methods. The data indicate the changes in mRNA expression compared with controls. (B) IL-8 production was quantified using enzyme-linked immunosorbent assay kits following treatment of Caco-2 cells with SP or SAP or with live E. histolytica for 12 h. The bars indicate the means and the error bars indicate the standard errors of the means for three different experiments. The asterisks indicate the results of comparisons with the controls (*, P < 0.1; **, P < 0.01; ***, P < 0.001). Eh, E. histolytica.
FIG. 2.

Induction of IL-8 mRNA expression and protein production. Confluent Caco-2 cells (106 cells) were treated with 40 μg/ml of SP, 100 μg/ml of SAP, or 2 × 106 live E. histolytica cells for different times as described in the legend to Fig. 1. (A) Increases in IL-8 mRNA expression compared with controls were determined by real-time PCR as previously described. (B) IL-8 production was quantified using an enzyme-linked immunosorbent assay kit. The bars indicate the means and the error bars indicate the standard errors of the means for three different experiments. The asterisks indicate the results of comparisons with the controls (*, P < 0.1; **, P < 0.01; ***, P < 0.001). Eh, E. histolytica.
E. histolytica induces IL-8 production through a lipid molecule.
To determine the biochemical characteristics of the ameba-derived component(s) that stimulated IL-8 production, we boiled SP, SAP, and PGE2 for 30 min or delipidized the ameba components with chloroform-methanol (2:1, vol/vol) (21) prior to testing. As shown in Fig. 3, SP, SAP, PGE2, and live E. histolytica stimulated robust IL-8 production 27-, 44-, 57-, and 37-fold, respectively, after 4 h compared to untreated controls. However, following boiling or delipidization of the ameba components, IL-8 production was completely eliminated (P > 0.001). In parallel, we determine that proteinase K treatment of ameba components had no effect on inhibition of IL-8 production (data not shown). These data clearly suggest that lipid or glycolipid molecules may be responsible for stimulating IL-8 production in colonic cells.
FIG. 3.

Involvement of lipid mediators (PGE2) in IL-8 production. Confluent Caco-2 cells (106 cells) were treated with 40 μg/ml of SP, 100 μg/ml of SAP, 1 μM PGE2, or 2 × 106 live E. histolytica cells for 12 h, and IL-8 production was measured. All components were boiled for 30 min or delipidized before treatment, and IL-8 production was assayed. Ctl, control (no treatment). The bars indicate the means and the error bars indicate the standard errors of the means for three different experiments. ***, P < 0.001. Eh, E. histolytica.
Effect of COX-1/2 inhibitors on IL-8 production.
Based on the results shown in Fig. 3, we thought that PGE2 produced by amebae was a likely candidate for the molecule that was responsible for stimulating IL-8 production. We have previously shown (9) that E. histolytica synthesizes PGE2 by using a novel COX-like enzyme that was inhibited by high concentrations (1 mM) of the nonspecific COX inhibitor aspirin but not by other COX-1/2-specific inhibitors. Moreover, live E. histolytica amebae incubated with 100 μM arachidonic acid, the precursor of PGE2, produced high levels of bioactive PGE2 in a time-dependent manner (9). Thus, to determine the biological function of ameba-derived PGE2 in stimulation of IL-8 production in colonic cells, live amebae were treated with a COX-1/2-specific inhibitor (indomethcin), a COX-2-specific inhibitor (nimesulide), and a nonspecific COX-1/2 inhibitor (aspirin) for 16 h. As shown in Fig. 4, only treatment with aspirin significantly inhibited (eightfold; P > 0.001) IL-8 production in Caco-2 cells following exposure to live amebae in transwells compared to the results obtained with untreated amebae or amebae treated with a COX-1/2-specific inhibitor. Furthermore, SAP and SP derived from aspirin-treated live amebae also significantly inhibited IL-8 production after 12 h (sevenfold for both; P > 0.001) compared to the results obtained with untreated controls or cells treated with a COX-1/2-specific inhibitor. These data suggest that PGE2 synthesized by the COX-like enzyme in amebae was responsible for IL-8 induction in colonic epithelial cells.
FIG. 4.

Role of COX inhibitors in inhibiting E. histolytica-induced IL-8 production. Confluent Caco-2 cells were treated with 40 μg/ml of SP, 100 μg/ml of SAP, or 2 × 106 live E. histolytica cells for 12 h, and IL-8 production was quantified. For inhibition studies, live amebae were pretreated with the COX-1/2 inhibitor indomethcin (50 μM), the COX-2-specific inhibitor nimesulide (40 μM), and the nonspecific COX-1/2 inhibitor aspirin (1 mM) for 16 h, and SP and SAP were prepared from drug-treated amebae as previously described. Caco-2 cells were treated with SP and SAP components, and IL-8 production was quantified by an enzyme-linked immunosorbent assay. Ctl, control (no treatment). The bars indicate the means and the error bars indicate the standard errors of the means for three different experiments. ***, P < 0.001. Eh, E. histolytica; Indo, indomethcin; NS, nimesulide; ASA, aspirin.
Effect of EP receptor-specific agonist and antagonist on IL-8 production.
PGE2 exerts its biological effects by coupling and signaling through EP2 or EP4 receptors to induce intracellular cyclic AMP production. Cyclic AMP responsive element (bp 828 to 835) regulatory sequences have been identified in the 5′ untranslated region of the IL-8 gene (15). Therefore, to unequivocally demonstrate that PGE2 derived from amebae was responsible for the induction of IL-8 production in colonic cells, we treated cells with PGE2, SP, SAP, live E. histolytica, and several EP2 or EP4 receptor-specific agonists or antagonists and quantified IL-8 production. As shown in Fig. 5, PGE2, the EP4 receptor-specific agonist ONO-AE1-329, SP, SAP, and live E. histolytica induced IL-8 production 29-, 26-, 9-, 15-, and 13-fold compared with the results obtained for untreated control Caco-2 cells. The EP2 receptor-specific agonist butaprost and the antagonist AH6809 did not induce IL-8 production. Remarkably, pretreatment of cells with the EP4 receptor-specific antagonist AH23848 or L161982 completely eliminated PGE2-, SP-, or SAP-induced IL-8 production (P > 0.001). These data clearly indicate that PGE2 endogenously synthesized by amebae efficiently binds EP4 receptors to induce IL-8 production in colonic epithelial cells.
FIG. 5.
Effects of EP2 and EP4 receptor agonists and antagonists on IL-8 production. Confluent Caco-2 cells were treated with 1 μM PGE2, ONO-AE1-329 (an EP4 receptor agonist), or butaprost (an EP2 receptor agonist) for 12 h. Cells were pretreated with either 1 μM AH23848 or L161982 (EP4 receptor antagonists) or 50 μM AH6809 (an EP2 receptor antagonist) for 60 min prior to treatment with PGE2, SP, or SAP for 12 h, and IL-8 production was measured by an enzyme-linked immunosorbent assay. The bars indicate the means and the error bars indicate the standard errors of the means for three different experiments. ***, P < 0.001. Ctl, control; Eh, E. histolytica.
Silencing of EP4 receptors inhibits IL-8 production.
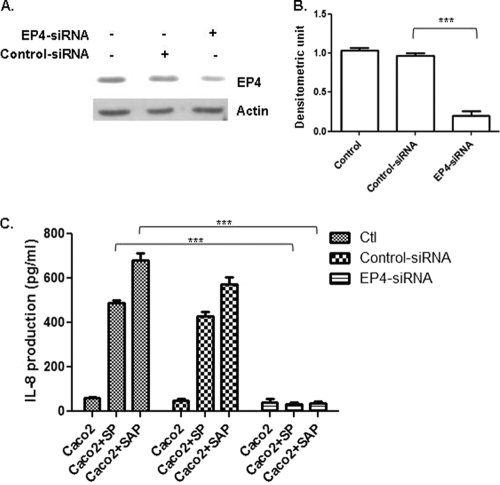
To determine the specificity for EP4 receptors in PGE2-induced IL-8 production in epithelial cells, we silenced EP4 receptors using EP4 siRNA and determined IL-8 production following stimulation with SP and SAP. As shown in Fig. 6A and B, treatment with EP4 siRNA almost completely inhibited EP4 receptor expression (86% inhibition compared with a control) in wild-type Caco-2 cells. More importantly, EP4 siRNA treatment inhibited SP- and SAP-induced IL-8 production 15- and 18-fold compared to homologous controls (P > 0.001) (Fig. 6C). Interestingly, silencing of EP2 receptor expression by EP2 siRNA did not inhibit PGE2-induced IL-8 production, suggesting that the EP2 receptor plays a minimal role in this process (data not shown). Using green fluorescent protein as a control, the transfection efficiency was routinely found to be between 65 and 75% (data not shown). During these silencing experiments with EP2 siRNA and EP4 siRNA, more than 90% of transfected Caco-2 cells were alive as determined by a trypan blue exclusion assay. Taken together, these data clearly demonstrate that EP4 receptors have an important role in PGE2-induced IL-8 production in colonic epithelial cells.
FIG. 6.
Treatment with EP4 siRNA inhibits IL-8 production. Caco-2 cells were transfected with or without EP4 receptor siRNA and control siRNAs as described in Materials and Methods. (A) Expression of EP4 receptor as determined by immunoblot analysis. (B) Densitometric analysis of EP4 receptor expression normalized using β-actin. (C) Colonic cells were treated with SP and SAP for 16 h, and IL-8 production was measured by an enzyme-linked immunosorbent assay. The bars indicate the means and the error bars indicate the standard errors of the means for three different experiments. ***, P < 0.001 compared to untransfected control cells.
DISCUSSION
E. histolytica invades the intestinal mucosa and causes amebic colitis and severe ulceration. Analysis of the inflammatory response during intestinal amebiasis in human and animal models of the disease has revealed an important regulatory role for chemokines and cytokines. Recruitment and activation of inflammatory cells can also be modulated by secreted amebic factors, such as amebapores and monocyte locomotion inhibitory factor. Several Th1/2 cytokines, such as IL-6 and IL-4, and regulatory cytokines, like IL-10 and transforming growth factor β, have been shown to be associated with the development of amebiasis (13).
Recent studies have provided evidence that chemokines, such as IL-8, are crucial mediators in inflammation and in tissue injury in intestinal inflammation. IL-8 is a small, 8- to 11-kDa secreted protein and may participate in immune and inflammatory responses through chemoattraction and activation of neutrophils or leukocytes (1). The precise nature of the IL-8 signaling pathway related to epithelial cell signaling has not been defined yet. However, the initial signaling events during inflammation ultimately lead to activation and translocation of various transcription factors that control the transcription of genes encoding the various chemokines and cytokines secreted by epithelial cells (24). It has been found that both the C-X-C and C-C members of the chemokine family of proteins, as well as the proinflammatory cytokines IL-1β, IL-6, and tumor necrosis factor alpha and the cell growth factor GM-CSF, are released by epithelial cells after bacterium-enterocyte interactions (27). Recently, we showed that in colonic epithelial cells, monocyte chemoattractant protein 1 is secreted in response to soluble ameba components via the phosphatidylinositol 3-kinase/P65 pathway (16). The release of this cytokine in vivo by the epithelium would be an effective means of initiating a mucosal inflammatory response. Cytokines, particularly IL-8, growth-related oncogene α, and monocyte chemoattractant protein 1, are potent chemoattractants for neutrophils and monocytes, while GM-CSF prolongs the survival of these cells and increases their response to other proinflammatory agonists (3).
In this study, we established that endogenously synthesized PGE2 present in soluble and secretory ameba components could induce robust IL-8 production in colonic epithelial cells via activation of the EP4 receptor. During amebic invasion, the epithelium responds by mounting a protective inflammatory response. This may cause release of epithelium cytokines and chemokines, such as CXCL1, CXCL8, CCL2, CCL3, CCL5, IL-6, GM-CSF, gamma interferon, and tumor necrosis factor alpha. Altogether, histological analyses of human biopsies have shown that there is mild infiltration of neutrophils, accompanied by hyperplasic lymphoid aggregates with macrophages and dendritic cells, in the submucosa at the beginning of amebic ulceration along with neutrophils, macrophages, and T cells as the infection progresses (11). A member of the transmembrane kinase family, phagosome-associated TMK96, is required for amebic infection (6). Since neutrophils predominantly recruit to the submucosa during amebic infection, in vivo experiments have shown that neutrophils are not capable of killing the parasite, probably because parasite superoxide dismutases and oxidoreductases are produced, which may inhibit the neutrophil respiratory burst (8). Furthermore, neutrophil depletion in murine models of infection resulted in more severe ulceration of susceptible strains than of resistant strains (2). Moreover, ameba trophozoites interact with β2 integrins on the surface of neutrophils, induce their apoptosis through the phosphatidylinositol 3-kinase-mediated pathway (23), and activate intracellular signaling (19). Interestingly, the monocyte locomotion inhibitory factor is an anti-inflammatory oligopeptide produced by E. histolytica that inhibits locomotion of human monocytes (19).
In previous studies, it was shown that neutrophils play a major protective role in resolving hepatic E. histolytica infection in mice (25). Thus, IL-8 may play an important role in chemoattraction and activation of neutrophils during the onset of amebic colitis, which may help control ameba infection. Our current findings are of considerable interest, as it is not known how E. histolytica triggers a host inflammatory response in the gut in the absence of cellular contact. We have shown previously that the SP and SAP components are able to induce IL-8 production in colonic epithelial cells (28); however, the identity of the ameba component responsible for the induction of IL-8 production was not determined. Here we found that ameba-derived PGE2 is responsible for stimulating IL-8 production through EP4 receptors in colonic epithelial cells. Thus, it is not surprising that silencing EP4 receptor expression completely eliminated SP- and SAP-induced IL-8 production. All attempts to date to knock down the in vitro expression of the COX-like gene by using an antisense strategy or by silencing the gene using siRNA treatments have failed. As amebae died slowly in culture following these treatments, it appears that the COX-like enzyme responsible for PGE2 biosynthesis may play a critical role in the ameba cell cycle. These findings showed that PGE2 produced in the gut by amebae is a major player in the initiation of inflammation because it induces IL-8 production in the pathogenesis of intestinal amebiasis.
The local induction of production of chemokines, such as IL-8, by epithelial cells could explain the histopathological findings for E. histolytica infection and thus may provide a mechanism for initiation and exacerbation of the inflammation seen during intestinal amebiasis. Moreover, modulation of host chemokines like IL-8 could perhaps be used as a virulence marker for E. histolytica. In summary, we show here that PGE2 produced by E. histolytica stimulates IL-8 mRNA expression and protein production in human colonic epithelial cells through an EP4 receptor signaling mechanism in a contact-independent manner. This observation is very important for understanding ameba pathogenic mechanisms as EP4 antagonists targeted against EP4 receptors have the potential to become therapeutically important in the treatment of amebiasis. Perhaps directly targeting the production of IL-8 or blocking EP4 receptors might alter the course of invasive amebiasis, but this is only speculation at this time.
Acknowledgments
This work was supported by grants from the Canadian Institute for Health Research and by a Canadian Association of Gastroenterology-Axcan Pharma-CIHR research and fellowship award.
We thank Mark Giembycz of the University of Calgary for providing the EP4 agonist ONO-AE1-329 and EP4 antagonist ONO-AE3-208 (Ono Pharmaceutical Co. Ltd., Japan).
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 18 August 2008.
REFERENCES
- 1.Ajuebor, M. N., and M. G. Swain. 2002. Role of chemokines and chemokine receptors in the gastrointestinal tract. Immunology 105137-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asgharpour, A., C. Gilchrist, D. Baba, S. Hamano, and E. Houpt. 2005. Resistance to intestinal Entamoeba histolytica infection is conferred by innate immunity and Gr-1+ cells. Infect. Immun. 734522-4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baggiolini, M., B. Dewbald, and B. Moser. 1994. Interleukin-8 and related chemotactic cytokines: CXC and CC chemokines. Adv. Immunol. 5597-197. [PubMed] [Google Scholar]
- 4.Bas, A., G. Forsberg, S. Hammarström, and M. L. Hammarström. 2004. Utility of the housekeeping genes 18S rRNA, β-actin and glyceraldehyde-3-phosphate-dehydrogenase for normalization in real-time quantitative reverse transcriptase-polymerase chain reaction analysis of gene expression in human T lymphocytes. Scand. J. Immunol. 59566-573. [DOI] [PubMed] [Google Scholar]
- 5.Belley, A., and K. Chadee. 1999. Prostaglandin E2 stimulates rat and human colonic mucin exocytosis via the EP4 receptor. Gastroenterology 1171352-1362. [DOI] [PubMed] [Google Scholar]
- 6.Boettner, D. R., C. D. Huston, A. S. Linford, S. N. Buss, E. Houpt, N. E. Sherman, and W. A. Petri, Jr. 2008. Entamoeba histolytica phagocytosis of human erythrocytes involves PATMK, a member of the transmembrane kinase family that participates in erythrophagocytosis and is uniquely required for intestinal infection. PLoS Pathog. 4e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boie, Y., R. Stocco, N. Sawyer, D. M. Slipetz, M. D. Ungrin, F. Neuschafer-Rube, G. P. Puschel, K. M. Metters, and M. Abramovitz. 1997. Molecular cloning and characterization of the four rat prostaglandin E2 prostanoid receptor subtypes. Eur. J. Pharmacol. 340227-241. [DOI] [PubMed] [Google Scholar]
- 8.Bruchhaus, I., and E. Tannich. 1994. Induction of the iron-containing superoxide dismutase in Entamoeba histolytica by a superoxide anion-generating system or by iron chelation. Mol. Biochem. Parasitol. 67281-288. [DOI] [PubMed] [Google Scholar]
- 9.Dey, I., K. Keller, A. Belley, and K. Chadee. 2003. Identification and characterization of a cyclooxygenase-like enzyme from Entamoeba histolytica. Proc. Natl. Acad. Sci. USA 10013561-13566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eckmann, L., S. L. Reed, J. R. Smith, and M. F. Kagnoff. 1995. Entamoeba histolytica trophozoites induce an inflammatory cytokine response by cultured human cells through the paracrine action of cytolytically released interleukin-1α. J. Clin. Investig. 961269-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Espinosa-Cantellano, M., and A. Martinez-Palomo. 2000. Pathogenesis of intestinal amebiasis: from molecules to disease. Clin. Microbiol. Rev. 13318-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reference deleted.
- 13.García-Zepeda, E. A., A. Rojas-López, M. Esquivel-Velázquez, and P. Ostoa-Saloma. 2007. Regulation of the inflammatory immune response by the cytokine/chemokine network in amoebiasis. Parasite Immunol. 12679-684. [DOI] [PubMed] [Google Scholar]
- 14.Gardiner, P. J. 1986. Characterization of prostanoid relaxant/inhibitory receptors (psi) using a highly selective agonist, TR4979. Br. J. Pharmacol. 8745-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iourgenko, V., W. Zhang, C. Mickanin, I. Daly, C. Jiang, J. M. Hexham, A. P. Orth, L. Miraglia, J. Meltzer, D. Garza, G. W. Chirn, E. McWhinnie, D. Cohen, J. Skelton, R. Terry, Y. Yu, D. Bodian, F. P. Buxton, J. Zhu, C. Song, and M. A. Labow. 2003. Identification of a family of cAMP response element-binding protein coactivators by genome-scale functional analysis in mammalian cells. Proc. Natl. Acad. Sci. USA 10012147-12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kammanadiminti, S. J., I. Dey, and K. Chadee. 2007. Induction of monocyte chemotactic protein 1 in colonic epithelial cells by Entamoeba histolytica is mediated via the phosphatidylinositol 3-kinase/p65 pathway. Infect. Immun. 751765-1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leippe, M., S. Ebel, O. L. Schoenberger, R. D. Horstmann, and H. J. Müller-Eberhard. 1991. Pore-forming peptide of pathogenic Entamoeba histolytica. Proc. Natl. Acad. Sci. USA 887659-7663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lidell, M. E., D. M. Moncada, K. Chadee, and G. C. Hansson. 2006. Entamoeba histolytica cysteine proteases cleave the MUC2 mucin in its C-terminal domain and dissolve the protective colonic mucus gel. Proc. Natl. Acad. Sci. USA 1039298-9303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morales-Martínez, M. E., R. Silva-García, C. Soriano-Correa, J. A. Giménez-Scherer, S. Rojas-Dotor, F. Blanco-Favela, and G. Rico-Rosillo. 2008. The Cys-Asn-Ser carboxyl-terminal end group is the pharmacophore of the amebic anti-inflammatory monocyte locomotion inhibitory factor (MLIF). Mol. Biochem. Parasitol. 15846-51. [DOI] [PubMed] [Google Scholar]
- 20.Petri, W. A., R. D. Smith, P. H. Schlesinger, C. F. Murphy, and J. I. Ravdin. 1987. Isolation of the galactose-binding lectin that mediates the in vitro adherence of Entamoeba histolytica. J. Clin. Investig. 801238-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rothblat, G. H., L. Y. Arbogast, L. Ouellette, and B. V. Howard. 1976. Preparation of delipidized serum protein for use in cell culture systems. In Vitro 8554-557. [DOI] [PubMed] [Google Scholar]
- 22.Seydel, K. B., E. Li, Z. Zhang, and S. L. Stanley, Jr. 1998. Epithelial cell-initiated inflammation plays a crucial role in early tissue damage in amebic infection of human intestine. Gastroenterology 1151446-1453. [DOI] [PubMed] [Google Scholar]
- 23.Sim, S., S. J. Park, T. S. Yong, K. I. Im, and M. H. Shin. 2007. Involvement of beta2-integrin in ROS-mediated neutrophil apoptosis induced by Entamoeba histolytica. Microbes Infect. 111368-1375. [DOI] [PubMed] [Google Scholar]
- 24.Svanborg, C., M. Hedlund, H. Connel, W. Agace, R. Daun, A. Nilsson, and B. Wullt. 1996. Bacterial adherence and mucosal cytokine responses. Ann. N. Y. Acad. Sci. 797177-190. [DOI] [PubMed] [Google Scholar]
- 25.Velázquez, C., M. Shibayama-Salas, J. Aguirre-García, V. Tsutsumi, and J. Calderón. 1998. Role of neutrophils in innate resistance to Entamoeba histolytica liver infection in mice. Parasite Immunol. 20255-262. [DOI] [PubMed] [Google Scholar]
- 26.World Health Organization. 1997. WHO/PAHO/UNESCO report. A consultation with experts on amebiasis. Mexico City, Mexico 28-29 January. Epidemiol. Bull. 813-14. [PubMed] [Google Scholar]
- 27.Yang, S., L. Eckmann, A. Panja, and M. Kagnoff. 1997. Differential and regulated expression of C-X-C, C-C and C-chemokines by human colon epithelial cells. Gastroenterology 1131214-1223. [DOI] [PubMed] [Google Scholar]
- 28.Yu, Y., and K. Chadee. 1998. Prostaglandin E2 stimulates IL-8 gene expression in human colonic epithelial cells by a posttranscriptional mechanism. J. Immunol. 1613746-3752. [PubMed] [Google Scholar]
- 29.Zhang, Z., L. Wang, K. B. Seydel, E. Li, S. Ankri, D. Mirelman, and S. L. Stanley, Jr. 2000. Entamoeba histolytica cysteine proteinases with interleukin-1β converting enzyme (ICE) activity cause intestinal inflammation and tissue damage in amoebiasis. Mol. Microbiol. 37542-548. [DOI] [PubMed] [Google Scholar]