Abstract
Leptospirosis is a global zoonotic disease. The causative agent, pathogenic Leptospira species, survives in the renal tubules of chronically infected hosts, from where leptospires are shed via urine into the environment. Infection of new hosts can present as an array of acute and chronic disease processes reflecting variations in host-pathogen interactions. The present study was designed to reproduce the carrier phase of infection in Rattus norvegicus, thus facilitating shedding of leptospires in urine. Leptospires shed in urine were collected for proteomic analysis because these organisms reflect a naturally virulent form of Leptospira associated with infection of new hosts. Experimentally infected rats remained clinically asymptomatic but shed leptospires in urine for several months at concentrations of up to 107 leptospires/ml of urine. Proteomic analysis of rat urine-isolated leptospires compared to in vitro-cultivated leptospires confirmed differential protein and antigen expression, as demonstrated by two-dimensional gel electrophoresis and immunoblotting. Furthermore, while serum from chronically infected rats reacted with many antigens of in vitro-cultivated Leptospira, few antigens of rat urine-isolated Leptospira were reactive. Results confirm that differential protein expression by Leptospira during chronic infection facilitates its persistence in the presence of a specific host antibody response.
Leptospirosis is a disease of global significance (3). Chronically infected mammalian hosts harbor pathogenic Leptospira species in renal tubules of the kidney, from which they are shed via urine into the environment and survive under suitable moist conditions. The rat has long been recognized as a carrier host for Leptospira species (8, 31), with recent studies confirming its role in disease outbreaks in urban settings (22, 29, 32). Humans are infected via broken skin and mucosal surfaces during contact with contaminated environments (9). Clinical manifestations of acute human leptospirosis range from mild to severe forms. More recently, a severe pulmonary form of leptospirosis (SPFL) has been recognized that results in rapid disease progression with high mortality among patients (24-26, 30, 34). While there are indications that such severe manifestations of disease are due to an autoimmune response (12) and the emergence of a virulent clonal isolate of Leptospira (20, 21, 27), pathogenic mechanisms of the pulmonary form of leptospirosis remain unclear.
Isolates of Leptospira interrogans were cultured from patients suffering from SPFL in 1998 at the National Leptospirosis Reference Laboratory at the Oswaldo Cruz Institute-FIOCRUZ, Brazil (23). These isolates have been used to develop relevant animal models of disease, including an acute lethal infection with pulmonary hemorrhage in guinea pigs, marmosets, and mice (12, 14, 19). To date, the guinea pig model of SPFL has provided two significant insights into pathogenic mechanisms of severe forms of leptospirosis: first, alveolar septal deposition of immunoglobulin and complement parallels pulmonary hemorrhage in the absence of detectable leptospiral antigen, suggesting that lung hemorrhage is mediated in part by autoimmune mechanisms (12); and second, novel assays employed to extract intact motile leptospires from in vivo sources during acute disease has confirmed that these host-tissue-derived Leptospira isolates differ dramatically in the expression of lipopolysaccharide and protein constituents from the same isolates cultured in vitro (13, 17).
Specific serovars of Leptospira species are associated with clinically asymptomatic chronic infection of specific mammalian host species (28). Rattus norvegicus is a reservoir host of those strains of Leptospira associated with human SPFL. Such host adaptation was first recognized by Babudieri, who observed a “biological equilibrium” between rodent hosts and certain Leptospira serovars (2). Leptospiruria in maintenance hosts is of high intensity, constant, and of long duration compared to that in accidental hosts, where it is of low intensity, intermittent, and of short duration (4). In contrast to previous studies which focused on characterization of the proteome of in vitro-cultivated Leptospira (IVCL) (5, 16), the present study has exploited leptospiruria during a chronic animal disease model to provide, for the first time, a proteomic analysis of leptospires as excreted in urine of chronically infected hosts. Results confirm that leptospires differentially express proteins in the host, which in turn likely facilitates persistence in the presence of a specific host antibody response. A clearer understanding of changes in protein expression by pathogenic species of Leptospira during infection is likely to lead to elucidation of pathogenic mechanisms of leptospirosis.
MATERIALS AND METHODS
Bacteria.
An isolate of Leptospira interrogans serovar Copenhageni, designated RJ16441, was obtained from blood cultures of a patient suffering from SPFL, who was admitted to Antonio Pedro University Hospital, Rio de Janeiro, Brazil (23). Cultures were maintained in Ellinghausen-McCullough-Johnson-Harris (EMJH) liquid (Becton Dickinson, Maryland) or EMJH semisolid medium (EMJH liquid medium containing 0.2% noble agar and 200 μg/ml 5-fluorouracil). Isolates were passaged through guinea pigs and rats to maintain virulence as described previously (12).
Animals.
Wistar male rats (University College Dublin Biomedical Facility), 80 to 110 g in weight, ∼5 weeks of age, were injected intraperitoneally with either 105 or 106 low-passage RJ16441 leptospires in a final volume of 500 μl EMJH liquid medium. Negative-control animals were injected with EMJH liquid medium alone. Animals were weighed weekly and monitored daily for signs of illness. Animals were housed in metabolism cages and urine collected for enumeration of leptospires by dark-field microscopy as described previously (10). For proteomic analysis, urine samples were collected and placed in 10 mM Tris-1 mM EDTA buffer containing 20% methanol. Urine samples were first centrifuged at 4°C, 1,000 × g, for 5 min to remove debris before a further centrifugation step at 4°C, 12,000 × g, for 10 min. Pellets were stored at −20°C until analysis. Serum was collected from rats at the time of euthanasia at day 160 postinfection. All study protocols were approved by the University College Dublin Animal Research Ethics Committee and conducted under license from the Department of Health and Children.
Histopathology and immunohistochemistry.
After sacrifice, rat kidneys were immediately fixed in neutral buffered 10% formalin, processed routinely, embedded in paraffin, cut into 4-μm sections, and stained with hematoxylin and eosin. Immunohistochemistry was performed on paraffin-embedded tissue sections using antiserum generated against outer membrane vesicles (OMV) of Leptospira species as described previously with slight modifications (12). Paraffin-embedded tissue sections were incubated at 60°C for 20 min. Two xylene washes were applied for 5 min, followed by 100% ethanol for 1 min (two times), 95% ethanol for 1 min (two times), 70% ethanol for 1 min (two times), H2O for 5 min (once), and incubation in peroxidase blocking reagent (DakoCytomation) for 15 min. After three 5-min washes in phosphate-buffered saline (PBS), sections were incubated in a trypsin solution (1 mg/ml) (Sigma) for 5 min at 37°C. Sections were again washed three times in PBS for 5 min before being processed using the Vectastain Elite ABC kit (Vector Laboratories). Sections were first blocked with normal goat serum for 20 min and then incubated with anti-OMV (1:400) diluted in PBS for 30 min. Anti-OMV was detected with anti-rabbit immunoglobulin G (IgG) (1:200; Vector Laboratories). Finally, sections were washed three times with PBS for 5 min, incubated with Vectastain Elite ABC reagent for 30 min, and again washed three times with PBS for 5 min. Staining was visualized with a Vector NovaRED substrate for 5 min and the reaction stopped by rinsing sections with H2O. Sections were counterstained in weak hematoxylin and mounted with Vectashield mounting medium.
Gel electrophoresis and immunoblotting.
IVCL and rat urine-isolated Leptospira (RUIL) samples were processed for one- and two-dimensional (1-D and 2-D) gel electrophoresis as described previously (13, 16). Protein concentrations were determined using the RC/DC protein assay kit (Bio-Rad). Separated proteins were visualized using the protocol from the PlusOne silver staining kit (GE Healthcare). For immunoblotting, proteins were transferred to an Immobilon-P transfer membrane (Millipore) and blocked with 5% (wt/vol) nonfat dry milk (Marvel) in PBS-0.1% Tween 20. Membranes were incubated for 1 h with antiserum raised against OMV (1:2,000) (13), followed by a 1-h incubation with horseradish peroxidase-donkey anti-rabbit IgG conjugate (1:2,500; Sigma) or with chronic rat serum (CRS) (1:1,000), followed by incubation with horseradish peroxidise-goat anti-rat IgG conjugate (1:2,500; Sigma). Antisera specific for Qlp42, LipL41, LipL32, LipL21, and Loa22 were all used at 1:2,000. Bound conjugates were detected using the SuperSignal WestPico substrate (Pierce) and images acquired using a UVP Biospectrum-AC w/Bio Chemi camera (Cambridge, United Kingdom).
RESULTS
Chronic infection of rats with L. interrogans serovar Copenhageni.
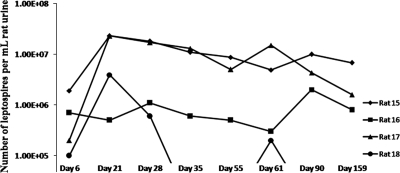
Experimental infection of male rats with L. interrogans serovar Copenhageni RJ16441 elicits a chronic infection with no obvious clinical signs. All infected rats displayed similar weight increases compared to noninfected controls (data not shown), but infected rats shed leptospires in their urine from day 6 postinfection until day 159, in numbers ranging from 105 to 107 per ml urine (Fig. 1). Microscopic analysis of kidney sections from infected animals taken at day 160 postinfection displayed discrete areas of multifocal, mild to severe granulomatous interstitial nephritis (Fig. 2A and B ) compared to noninfected controls (Fig. 2D and E). The inflammatory infiltrate was lymphocyte rich with a discrete aggregation of macrophages and primarily located in the interstitium in the region of proximal tubules. The presence of leptospires in renal tubules was confirmed by immunohistochemistry using antiserum specific for OMV of Leptospira species (Fig. 2C, G, and H), compared to a negative control (Fig. 2F). In some regions, tubules which were positive for Leptospira were surrounded by inflammatory infiltrate (Fig. 2H) while other positive tubules were devoid of any obvious inflammation (Fig. 2G).
FIG. 1.
Chronic infection of rats with L. interrogans serovar Copenhageni results in the shedding of significant numbers of leptospires in urine. Counting by dark-field microscopy reveals numbers of 105 to 107 leptospires per ml urine.
FIG. 2.
Light microscopy studies. (A, B and C) Kidney tissue from experimentally infected rat, 160 days postinfection. Hematoxylin-and-eosin (H&E) studies reveal multifocal, mild-to-severe granulomatous interstitial nephritis. Magnification, ×40 (A) or ×200 (B). Immunohistochemistry confirmed the presence of leptospires in renal tubules (C) (magnification, ×100). (D, E, and F) Kidney tissue from negative-control rat. The architecture of the tissue appears normal after H&E staining (magnification, ×40 [D] or×200 [E]) or immunohistochemistry (F) (magnification, ×100). (G and H) Immunohistochemistry confirms positively stained tubules associated with surrounding inflammatory infiltrate (H) (magnification, ×200), while other tubules are positive for Leptospira and independent of surrounding inflammation (G) (magnification, ×200).
Antigens expressed by IVCL and RUIL.
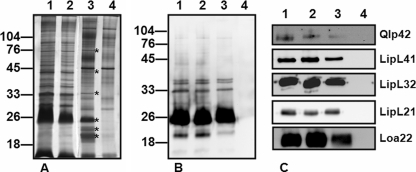
Urine from chronically infected rats was collected into a 20% methanol Tris-EDTA buffer in order to stabilize leptospiral proteins as soon as possible after excretion (1). Total protein silver staining of samples separated by 1-D sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) confirmed that several proteins appeared to be unique to infected rat urine in a comparison with negative control urine (Fig. 3A). Immunoblotting of samples with serum specific for OMV of Leptospira species confirmed that urine derived from infected rats contained antigens of Leptospira as opposed to negative control urine (Fig. 3B). The molecular mass of reactive antigens, in combination with results of previous studies, would suggest that these antigens include the flagellar doublet, LipL32 and lipopolysaccharide (13, 15, 16). In addition, results confirmed that RUIL organisms express antigens similar to those of IVCL. Expression of the previously characterized membrane proteins Qlp42, LipL41, LipL32, LipL21, and Loa22 in both RUIL and IVCL was confirmed by immunoblotting with monospecific antiserum (Fig. 3C).
FIG. 3.
Infected rat urine contains leptospiral antigens. (A) 1-D SDS-PAGE silver staining of IVCL (lane 1), IVCL treated with 20% MeOH-10 mM Tris-1 mM EDTA buffer (IVCL MeOH) (lane 2), L. interrogans-infected rat urine collected into 20% MeOH-10 mM Tris-1 mM EDTA buffer (lane 3), or control rat urine (lane 4). Lanes 1 to 4 contain 4 μg total protein. Asterisks indicate bands present in infected urine as opposed to noninfected urine. (B and C) Immunoblot analysis of IVCL (lane 1), IVCL MeOH (lane 2), RUIL (lane 3), or negative urine (lane 4) probed with antiserum against Leptospira species OMV (B) or antiserum specific for Qlp42, LipL41, LipL32, LipL21, or Loa22 (C). Approximately 107 leptospires are loaded per lane. Molecular mass markers are indicated (in kilodaltons).
Leptospires excreted in rat urine downregulate expression of antigens.
Experimentally infected rats generated antibody specific for IVCL by day 7 postinfection (data not shown). Immunoblotting of IVCL with serum from chronically infected rats at 160 days postinfection confirmed reactivity with many antigens (Fig. 4). In contrast, CRS was reactive with few antigens expressed by RUIL (Fig. 4). Since results shown in Fig. 3 confirm that RUIL organisms do express a significant number of antigens, similar to results with IVCL, results shown in Fig. 4 confirm that leptospires downregulate the expression of many antigens reactive with CRS during chronic disease. This in turn suggests that antigen expression is influenced by the host antibody response.
FIG. 4.
Immunoblot analysis of IVCL or RUIL with chronic rat serum from infected rats numbered 15, 16, 17, and 18. Serum from noninfected rats 13 and 14 acted as negative controls. An asterisk indicates rat IgG detected in urine. Approximately 107 leptospires are loaded per lane. Molecular mass markers are indicated (in kilodaltons).
Immunoblotting with serum from negative control rats confirmed that rat IgG is also present in infected urine samples and accounts for some antigens detected in the RUIL sample (Fig. 4).
The serum from rat 18, which was infected and shedding leptospires in urine, as confirmed by dark-field microscopy and culture (Fig. 1), was reactive in immunoblots against IVCL when used at 1:100 (data not shown).
2-D gel electrophoresis of IVCL and RUIL.
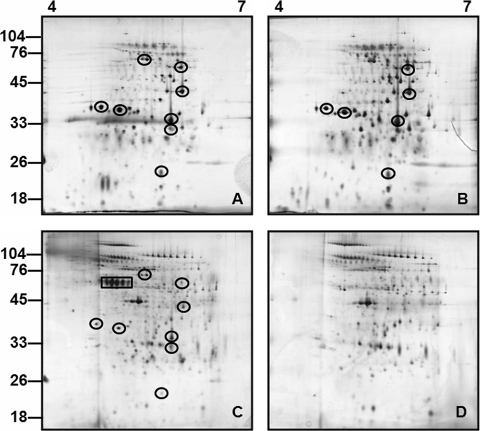
Total protein samples of IVCL, IVCL treated with methanol buffer, RUIL, and noninfected control urine were separated by 2-D gel electrophoresis over a pH range of 4 to 7 (Fig. 5). Immunoblotting with anti-OMV confirmed the presence of reactive antigens in IVCL and RUIL (Fig. 6). Results demonstrate clear differences in the antigenic composition of IVCL (Fig. 6A and B) from that of RUIL (Fig. 6C), which were not readily detected when samples were separated by 1-D SDS-PAGE (Fig. 3B). Differentially expressed antigens in RUIL (Fig. 6C) were compared relative to those in various amounts of IVCL (Fig. 6A and B) in order to confirm that differential antigen expression was not due to unequal loading amounts. Increased expression of antigens was observed relative to an apparent diminution of other antigens. No antigens were detected in noninfected control urine with anti-OMV (data not shown).
FIG. 5.
Total protein profiles by 2-D gel electrophoresis (pH 4 to 7) silver-staining analysis of IVCL (A), IVCL MeOH (B), RUIL (C), or negative urine (D). Each gel is loaded with 5 μg total protein. Molecular mass markers are indicated (in kilodaltons). Proteins common to RUIL and IVCL samples are circled, while proteins which are present in L. interrogans-infected rat urine (C) and absent from the urine of a negative rat (D) are boxed.
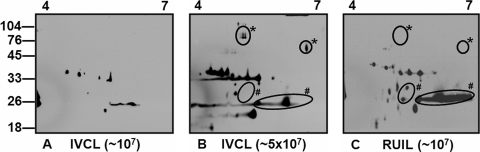
FIG. 6.
Antigens reactive with anti-OMV: 2-D gel electrophoresis (pH 4 to 7) immunoblot analysis of ∼1 × 107 IVCL organisms (A), 5 × 107 IVCL organisms (B), and ∼1 × 107 RUIL organisms (C) with anti-OMV. The number (#) or asterisk symbol indicates the antigens that are apparently upregulated or downregulated, respectively, during chronic infection. Molecular mass markers are indicated (in kilodaltons).
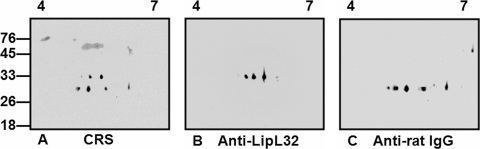
Finally, and in agreement with results shown in Fig. 4, relatively few antigens of the RUIL sample separated by 2-D gel electrophoresis were reactive with serum from chronically infected rats (Fig. 7A). These results suggest that leptospires derived from infected urine have limited reactivity with CRS compared to IVCL organisms, which in turn would enhance the ability of Leptospira species to persist in the presence of a specific host antibody response. The identities of those antigens of RUIL that were reactive with CRS were confirmed as LipL32 and rat IgG by immunoblotting with monospecific anti-LipL32 (Fig. 7B) and anti-rat IgG, respectively (Fig. 7C).
FIG. 7.
Identification of antigens reactive with chronic rat serum: 2-D gel electrophoresis (pH 4 to 7) immunoblot analysis of ∼1 × 107 RUIL organisms probed with chronic rat serum (A), anti-LipL32 (B), or anti-rat IgG (C). Molecular mass markers are indicated (in kilodaltons).
DISCUSSION
Rattus norvegicus is a reservoir host for L. interrogans serovar Copenhageni, a causative agent of SPFL, which is a severe, rapid disease process with mortality rates greater than 50% (3). Since human patients become infected by direct contact with urine from chronically infected animals or other environmental sources contaminated with such urine, it can be assumed that leptospires derived from such sources represent an inherently virulent form responsible for causing acute disease processes. The proteome of such excreted organisms has not yet been examined.
To date, the majority of proteomic studies seek to elucidate pathogenic mechanisms of leptospirosis by identifying the proteome of leptospires that have been cultured in vitro (16, 18). Results confirm the dynamic nature of the proteome, particularly in response to culture conditions designed to mimic host conditions (11, 15). In the present study, the development of an animal model of leptospirosis reflecting chronic disease was exploited to look at the proteome of Leptospira organisms as excreted during transmission in infected urine. Interestingly, early work on rodent carrier models of Leptospira infection suggested fundamental antigenic differences between IVCL organisms and leptospires derived from infected renal tissue, since antibody from infected urine, kidney suspensions, or serum of infected rodents was active against IVCL but inactive against leptospires derived from renal tissues (6, 7). Therefore, the present study sought to establish the nature of these antigenic differences using, for the first time, Leptospira organisms as excreted from chronically infected hosts.
Experimental infection of Rattus norvegicus results in an asymptomatic carrier state accompanied by a persistent chronic interstitial nephritis, aggregations of organisms within tubules, and chronic excretion of organisms in urine. Numbers of leptospires excreted in infected urine were sufficient for proteomic analysis, as confirmed by immunoblotting of samples with antiserum specific for OMV of Leptospira species (Fig. 3B). In addition, both IVCL and RUIL samples expressed the previously characterized outer membrane proteins Qlp42, LipL41, LipL32, LipL21, and Loa22. However, differential antigenic expression was observed in RUIL compared to IVCL when samples were separated by 2-D gel electrophoresis for immunoblotting with anti-OMV (Fig. 6).
Chronic infection of rats with Leptospira species exemplifies the equilibrium between the host immune response and persistent infection. Despite the fact that experimentally infected rats produce antibody specific for IVCL by 7 days postinfection (data not shown) and continue to produce specific antibody during chronic disease, this immune response does not stop the shedding of leptospires in urine. The detection of a lymphocyte-rich inflammatory infiltrate in association with leptospires in infected kidney sections was interesting; however, it remains unclear if such an immune response would ultimately be successful enough to remove organisms and eliminate renal excretion. Of equal interest was the observation of leptospiral organisms within tubules devoid of any immune response, suggesting a possibility that such organisms may be shielded from the host immune response (Fig. 2). Immunoblotting of IVCL with chronic rat serum detected many antigens. However, immunoblotting of RUIL with chronic rat serum reacted with relatively fewer antigens. This observation would explain previously described differences in activity of chronic rat serum against in vitro-derived Leptospira compared to kidney-derived Leptospira (7). It further confirms the dynamic nature of the leptospiral proteome in adapting to specific niche conditions and in this case regulating protein expression to minimize interaction with the host antibody response. While the results of immunoblotting using chronic rat serum shown in Fig. 4 might suggest a diminution of antigen expression by leptospires isolated from urine compared to that for IVCL, immunoblotting with serum specific for OMV of Leptospira species confirmed the presence of several antigens common to both samples. This in turn suggests that the expression of those antigens specifically reactive with chronic rat serum is downregulated during chronic disease (Fig. 4). However, downregulation of specific antigens may correlate with the release of leptospires from tissues into urine. This is a major possibility if some of these antigens are adhesins that mediate adherence of leptospires to host tissues. Finally, different environmental signals associated with kidney and urine, e.g., pH, could influence protein expression.
Results illustrated prolonged survival of leptospires in renal tubules despite antibody production by the rat. It is likely that leptospires are exposed to antibody during their replication in renal tubules, since rat IgG is found in normal as well as infected rat urine (33); however, the specificity of urinary antibodies for Leptospira species in infected rats remains to be determined. The outer membrane protein LipL32 (also known as Hap-1) was expressed by RUIL and was reactive with chronic rat serum. This suggests that the antibody in chronic rat serum that is specific for LipL32 may not be able to bind leptospires in situ in renal tissues or if so is not functionally active.
This study has confirmed the utility of a chronic animal model of disease for studying the proteome of Leptospira species directly during the transmission process. Results highlight the dynamic nature of the leptospiral proteome in response to host conditions during infection and confirm that colonization of renal tubules is facilitated by differential protein expression. Identification of proteins expressed during persistent infection will provide insights into pathogenic mechanisms of disease and potential mechanisms that distinguish acute versus chronic disease processes.
Acknowledgments
This work was supported by grant number 05/YI2/B696, a President of Ireland Young Researcher Award from Science Foundation Ireland, to J.E.N.
We thank Sheila Worrall for assistance with light microscopy studies, Brian Cloak for photography, and Alfie Redmond for animal care. We thank D. Haake for kindly providing antiserum specific to Qlp42.
Editor: J. B. Bliska
Footnotes
Published ahead of print on 2 September 2008.
REFERENCES
- 1.Asakura, T., K. Adachi, and E. Schwartz. 1978. Stabilizing effect of various organic solvents on protein. J. Biol. Chem. 2536423-6425. [PubMed] [Google Scholar]
- 2.Babudieri, B. 1958. Animal reservoirs of leptospires. Ann N. Y. Acad. Sci. 70393-413. [DOI] [PubMed] [Google Scholar]
- 3.Bharti, A. R., J. E. Nally, J. N. Ricaldi, M. A. Matthias, M. M. Diaz, M. A. Lovett, P. N. Levett, R. H. Gilman, M. R. Willig, E. Gotuzzo, and J. M. Vinetz. 2003. Leptospirosis: a zoonotic disease of global importance. Lancet Infect. Dis. 3757-771. [DOI] [PubMed] [Google Scholar]
- 4.Chernukcha, Y. G., Y. V. Ananyina, and N. S. Zenkovitch. 1974. Pathogenicity of leptospires of various serological types for some species of wild rodents. Zentbl. Bakteriol. 228388-395. [PubMed] [Google Scholar]
- 5.Cullen, P. A., S. J. Cordwell, D. M. Bulach, D. A. Haake, and B. Adler. 2002. Global analysis of outer membrane proteins from Leptospira interrogans serovar Lai. Infect. Immun. 702311-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faine, S. 1962. Factors affecting the development of the carrier state in leptospirosis. J. Hyg. 60427-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faine, S. 1962. The growth of Leptospira australis B in the kidneys of mice in the incipient experimental carrier state. J. Hyg. 60435-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ido, Y., R. Hoki, H. Ito, and H. Wani. 1917. The rat as a carrier of Spirocheta icterohaemorrhagiae, the causative agent of Weil's disease (spirochaetosis icterohaemorrhagica). J. Exp. Med. 26341-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levett, P. N. 2001. Leptospirosis. Clin. Microbiol. Rev. 14296-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller, J. N. (ed.) 1971. Spirochetes in body fluids and tissues: manual of investigative methods. Charles C. Thomas, Springfield, IL.
- 11.Nally, J. E., S. Artiushin, and J. F. Timoney. 2001. Molecular characterization of thermoinduced immunogenic proteins Qlp42 and Hsp15 of Leptospira interrogans. Infect. Immun. 697616-7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nally, J. E., C. Chantranuwat, X. Y. Wu, M. C. Fishbein, M. M. Pereira, J. J. Da Silva, D. R. Blanco, and M. A. Lovett. 2004. Alveolar septal deposition of immunoglobulin and complement parallels pulmonary hemorrhage in a guinea pig model of severe pulmonary leptospirosis. Am. J. Pathol. 1641115-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nally, J. E., E. Chow, M. C. Fishbein, D. R. Blanco, and M. A. Lovett. 2005. Changes in lipopolysaccharide O antigen distinguish acute versus chronic Leptospira interrogans infections. Infect. Immun. 733251-3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nally, J. E., M. C. Fishbein, D. R. Blanco, and M. A. Lovett. 2005. Lethal infection of C3H/HeJ and C3H/SCID mice with an isolate of Leptospira interrogans serovar Copenhageni. Infect. Immun. 737014-7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nally, J. E., J. F. Timoney, and B. Stevenson. 2001. Temperature-regulated protein synthesis by Leptospira interrogans. Infect. Immun. 69400-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nally, J. E., J. P. Whitelegge, R. Aguilera, M. M. Pereira, D. R. Blanco, and M. A. Lovett. 2005. Purification and proteomic analysis of outer membrane vesicles from a clinical isolate of Leptospira interrogans serovar Copenhageni. Proteomics 5144-152. [DOI] [PubMed] [Google Scholar]
- 17.Nally, J. E., J. P. Whitelegge, S. Bassilian, D. R. Blanco, and M. A. Lovett. 2007. Characterization of the outer membrane proteome of Leptospira interrogans expressed during acute lethal infection. Infect. Immun. 75766-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nally, J. E., J. P. Whitelegge, and J. A. Carroll. 2007. Proteomic strategies to elucidate pathogenic mechanisms of spirochetes. Proteomics Clin. Appl. 11185-1197. [DOI] [PubMed] [Google Scholar]
- 19.Pereira, M. M., J. J. Da Silva, M. A. Pinto, M. F. Da Silva, M. P. Machado, H. L. Lenzi, and R. S. Marchevsky. 2005. Experimental leptospirosis in marmoset monkeys (Callithrix jacchus): a new model for studies of severe pulmonary leptospirosis. Am. J. Trop. Med. Hyg. 7213-20. [PubMed] [Google Scholar]
- 20.Pereira, M. M., M. G. Matsuo, A. R. Bauab, S. A. Vasconcelos, Z. M. Moraes, G. Baranton, and I. Saint Girons. 2000. A clonal subpopulation of Leptospira interrogans sensu stricto is the major cause of leptospirosis outbreaks in Brazil. J. Clin. Microbiol. 38450-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roy, S., D. Biswas, P. Vijayachari, A. P. Sugunan, and S. C. Sehgal. 2005. A clone of Leptospira interrogans sensu stricto is the major cause of leptospirosis in the archipelago of Andaman and Nicobar Islands, India. Lett. Appl. Microbiol. 41179-185. [DOI] [PubMed] [Google Scholar]
- 22.Sarkar, U., S. F. Nascimento, R. Barbosa, R. Martins, H. Nuevo, I. Kalafanos, I. Grunstein, B. Flannery, J. Dias, L. W. Riley, M. G. Reis, and A. I. Ko. 2002. Population-based case-control investigation of risk factors for leptospirosis during an urban epidemic. Am. J. Trop. Med. Hyg. 66605-610. [DOI] [PubMed] [Google Scholar]
- 23.Silva, J. J., M. O. Dalston, J. E. Carvalho, S. Setubal, J. M. Oliveira, and M. M. Pereira. 2002. Clinicopathological and immunohistochemical features of the severe pulmonary form of leptospirosis. Rev. Soc. Bras. Med. Trop. 35395-399. [DOI] [PubMed] [Google Scholar]
- 24.Simpson, F. G., K. A. Green, G. J. Haug, and D. L. Brookes. 1998. Leptospirosis associated with severe pulmonary haemorrhage in Far North Queensland. Med. J. Aust. 169151-153. [DOI] [PubMed] [Google Scholar]
- 25.Spichler, A., D. Athanazio, M. Buzzar, B. Castro, E. Chapolla, A. Seguro, and J. M. Vinetz. 2007. Using death certificate reports to find severe leptospirosis cases, Brazil. Emerg. Infect. Dis. 131559-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spichler, A., M. Moock, E. G. Chapola, and J. Vinetz. 2005. Weil's disease: an unusually fulminant presentation characterized by pulmonary hemorrhage and shock. Braz. J. Infect. Dis. 9336-340. [DOI] [PubMed] [Google Scholar]
- 27.Thaipadungpanit, J., V. Wuthiekanun, W. Chierakul, L. D. Smythe, W. Petkanchanapong, R. Limpaiboon, A. Apiwatanaporn, A. T. Slack, Y. Suputtamongkol, N. J. White, E. J. Feil, N. P. Day, and S. J. Peacock. 2007. A dominant clone of Leptospira interrogans associated with an outbreak of human leptospirosis in Thailand. PLoS Negl. Trop. Dis. 1e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thiermann, A. B. 1981. The Norway rat as a selective chronic carrier of Leptospira icterohaemorrhagiae. J. Wildl. Dis. 1739-43. [DOI] [PubMed] [Google Scholar]
- 29.Thiermann, A. B., and R. R. Frank. 1980. Human leptospirosis in Detroit and the role of rats as chronic carriers. Int. J. Zoonoses 762-72. [PubMed] [Google Scholar]
- 30.Trevejo, R. T., J. G. Rigau-Perez, D. A. Ashford, E. M. McClure, C. Jarquin-Gonzalez, J. J. Amador, J. O. de los Reyes, A. Gonzalez, S. R. Zaki, W. J. Shieh, R. G. McLean, R. S. Nasci, R. S. Weyant, C. A. Bolin, S. L. Bragg, B. A. Perkins, and R. A. Spiegel. 1998. Epidemic leptospirosis associated with pulmonary hemorrhage-Nicaragua, 1995. J. Infect. Dis. 1781457-1463. [DOI] [PubMed] [Google Scholar]
- 31.Tucunduva de Faria, M., D. A. Athanazio, E. A. Goncalves Ramos, E. F. Silva, M. G. Reis, and A. I. Ko. 2007. Morphological alterations in the kidney of rats with natural and experimental Leptospira infection. J. Comp. Pathol. 137231-238. [DOI] [PubMed] [Google Scholar]
- 32.Vinetz, J. M., G. E. Glass, C. E. Flexner, P. Mueller, and D. C. Kaslow. 1996. Sporadic urban leptospirosis. Ann. Intern. Med. 125794-798. [DOI] [PubMed] [Google Scholar]
- 33.Wait, R., E. Gianazza, I. Eberini, L. Sironi, M. J. Dunn, M. Gemeiner, and I. Miller. 2001. Proteins of rat serum, urine, and cerebrospinal fluid. VI. Further protein identifications and interstrain comparison. Electrophoresis 223043-3052. [DOI] [PubMed] [Google Scholar]
- 34.Yersin, C., P. Bovet, F. Merien, J. Clement, M. Laille, M. Van Ranst, and P. Perolat. 2000. Pulmonary haemorrhage as a predominant cause of death in leptospirosis in Seychelles. Trans. R. Soc. Trop. Med. Hyg. 9471-76. [DOI] [PubMed] [Google Scholar]