Abstract
Infection with the trematode helminth Schistosoma mansoni results in a parasite egg-induced, CD4 T-cell-mediated, hepatointestinal granulomatous and fibrosing inflammation that varies greatly in severity, with a higher frequency of milder forms typically occurring in regions where the disease is endemic. One possible explanation for this is that in these regions the degree of inflammation is lessened by widespread concurrent infection with gastrointestinal nematodes. We tested this hypothesis by establishing a murine coinfection model in which mice were infected with the intestinal nematode parasite Heligmosomoides polygyrus prior to infection with S. mansoni. In CBA mice that naturally display a severe form of schistosomiasis, preinfection with H. polygyrus resulted in a marked reduction in schistosome egg-induced hepatic immunopathology, which was associated with significant decreases in the levels of interleukin-17 (IL-17), gamma interferon, tumor necrosis factor alpha, IL-23, IL-6, and IL-1β and with increases in the levels of IL-4, IL-5, IL-10, and transforming growth factor β in mesenteric lymph node cells, purified CD4 T cells, and isolated liver granuloma cells. There also were increases in liver Ym1 and forkhead box P3 transcription factor expression. In another model of high-pathology schistosomiasis induced in C57BL/6 mice by immunization with schistosome egg antigens in complete Freund's adjuvant, coinfection with the nematodes also resulted in a marked inhibition of hepatic immunopathology accompanied by similar shifts in cytokine production. These findings demonstrate that intestinal nematodes prevent Th1- and Th17-cell-mediated inflammation by promoting a strong Th2-polarized environment associated with increases in the levels of alternatively activated macrophages and T regulatory cells, which result in significant amelioration of schistosome-induced immunopathology.
Nearly one-half of the world's human population is infected with one or more of a variety of parasitic helminths. A majority of the infections are with gastrointestinal helminths, and they occur mostly in tropical developing regions. It also has been observed that the highest density of helminth infections coincides with the lowest incidence of allergic and autoimmune diseases. This observation has prompted the formulation of the “hygiene hypothesis,” which states that living in an exceedingly clean environment predisposes humans to such conditions and that helminth infections can prevent and protect against the development of aberrant adaptive immune responses to normally nonimmunogenic foreign or self antigens (12, 19, 41, 74, 75). This idea has been greatly strengthened by supporting evidence obtained using experimental models of asthma (34), type 1 diabetes (17, 58, 76), experimental allergic encephalomyelitis (37, 59), Graves' thyroiditis (51), and inflammatory bowel disease (22). Predictably, coinfections with helminths also lessen proinflammatory responses against other pathogens, usually resulting in reduced overall immunopathology, albeit sometimes at the risk of diminished protection (21, 50, 52, 62, 66, 68, 73). The ameliorating effect of helminths on disease susceptibility or magnitude has been attributed to the ability of these organisms to down-modulate the level of inflammation through induction of anti-inflammatory Th2-type cells and T-regulatory cells (Treg), as well as alternatively activated macrophages (AAM) (4).
Schistosomes are blood-dwelling trematode helminths that cause disease by eliciting a host granulomatous and fibrosing inflammatory reaction against tissue-trapped parasite eggs, which in the case of Schistosoma mansoni takes place in the liver and intestines. The immunopathology in schistosomiasis is mediated and orchestrated by CD4 T cells specific for schistosome egg antigens (SEA), and its severity varies greatly from person to person, as well as among inbred mouse strains. In human “hepatosplenic” schistosomiasis, severe liver pathology causes splenomegaly, portal hypertension, and death, whereas in the more prevalent “intestinal” schistosomiasis, there is significantly milder liver pathology and clinical disease (10). In mouse models of schistosomiasis, the CBA strain develops pronounced granulomatous inflammation compared with the smaller lesions in the C57BL/6 (BL/6) strain (13, 56). However, disease severity in the low-pathology BL/6 mice can be markedly exacerbated by concomitant immunization with soluble SEA in complete Freund's adjuvant (CFA) (SEA/CFA) (55). Both the natural and induced forms of severe schistosomiasis correlate with high levels of the proinflammatory cytokines gamma interferon (IFN-γ) and interleukin-17 (IL-17) (55-57) indicative of the Th1 and Th17 subpopulations of CD4 T lymphocytes, respectively, although the IL-23-driven Th17 subset has recently been shown to be a more potent mediator and faithful indicator of severe disease (54, 57). On the other hand, an unopposed Th2 response signaled by the production of IL-4, IL-5, IL-10, and IL-13 results in a milder pathology (55), although there is a risk of increased hepatic fibrosis at a late stage of the disease, mainly through the action of IL-13 (15, 23).
The immunopathology in schistosomiasis is the product of a CD4 T-cell hypersensitivity reaction and as such shares mechanistic features with many T-cell-mediated autoimmune diseases. Moreover, the severity of schistosomiasis in individuals from areas where the disease is endemic is generally less than that in accidentally infected nonresidents (10, 18). We surmised that this could at least in part be due to the widespread coinfection with gastrointestinal helminths. To test this hypothesis, we established a murine coinfection model with S. mansoni and the intestinal hookworm nematode parasite Heligmosomoides polygyrus to examine the effect of concurrent nematode infection on the severity of schistosomiasis. H. polygyrus induces a strong host Th2-polarized response, which, in turn, is essential for subsequent worm expulsion (25, 65). We report here that administration of H. polygyrus prior to infection with schistosomes resulted in a marked reduction in hepatic egg-induced granulomatous inflammation in both the natural (CBA) and SEA/CFA-induced (BL/6) forms of high pathology. Disease amelioration correlated with significant decreases in the levels of proinflammatory cytokines in granuloma and mesenteric lymph node cells (MLNC).
MATERIALS AND METHODS
Mice, parasites, infections, and immunizations.
Five- to six-week-old female CBA/J and BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and maintained at the Animal Facility at Tufts University School of Medicine in accordance with the American Association for the Assessment and Accreditation of Laboratory Animal Care guidelines. CBA and BL/6 mice were infected by intraperitoneal injection of 85 cercariae of S. mansoni (Puerto Rico strain) obtained from infected Biomphalaria glabrata snails provided by Fred Lewis of the Biomedical Research Institute (Rockville, MD). Some mice were infected by gastric gavage with 40 third-stage larvae of H. polygyrus (U.S. National Helminthological Collection no. 81930) (69). Some BL/6 mice were also immunized by subcutaneous injection of 50 μg of SEA/CFA, as described previously (55). Treatment of BL/6 mice with SEA/CFA results in marked exacerbation of hepatic egg-induced immunopathology; either SEA or CFA by itself is ineffective at enhancing disease (55). SEA from S. mansoni was obtained from the Biomedical Research Institute and was prepared as described previously (11).
Experimental protocol.
CBA and BL/6 mice were infected with S. mansoni as described above. Some mice were also infected with H. polygyrus 4 weeks and again 2 days prior to infection with schistosomes. Some BL/6 mice were also immunized with SEA/CFA 1 day prior to and again 4 weeks after schistosome infection. All mice were sacrificed 7 weeks after schistosome infection and 11 weeks after the initial H. polygyrus infection.
Cell preparations, cell cultures, and cytokine determinations.
Livers and mesenteric lymph nodes (MLN) were removed aseptically, and single-cell suspensions were prepared from MLN by teasing the tissues in complete RPMI 1640 medium supplemented with 10% fetal calf serum (Atlanta Biologicals), 4 mM l-glutamine, 80 U/ml penicillin, 80 μg/ml streptomycin, 1 mM sodium pyruvate, 10 mM HEPES, and 1× nonessential amino acids (all obtained from BioWhittaker), as well as 0.1% 2-mercaptoethanol. Erythrocytes were lysed by exposure to Tris ammonium chloride buffer (pH 7.2) (Sigma) for 15 min on ice. Cells were washed, and live cells that excluded trypan blue were counted and resuspended at the desired concentrations in complete RPMI 1640 medium. For purification of CD4 T cells, MLNC were negatively selected on CD4 MACS columns (Miltenyi Biotec) by following the manufacturer's instructions. The resulting cell preparations contained >94% CD4+ cells as determined by flow cytometry. Granuloma cells (GC) were obtained by homogenization of the livers in a Waring blender, isolation of granulomas by sedimentation at 1 × g, extensive washing, and enzymatic digestion with 1 mg/ml of collagenase type H from Clostridium histolyticum (Sigma Chemical Co.).
Bulk MLNC and GC suspensions (5 × 106 cells/ml) or purified CD4 T cells from MLN (1 × 106 cells/ml) plus normal irradiated syngeneic splenic antigen-presenting cells (APC) (4 × 106 cells/ml) were incubated in the presence or absence of 15 μg/ml of SEA. After 48 h, the culture supernatants were removed, filtered, and stored at −36°C until they were analyzed by an enzyme-linked immunosorbent assay (ELISA). For IL-4, IL-5, IL-10, and transforming growth factor β (TGF-β), antibody, standard cytokines, and protocols were obtained from BD-PharMingen, and for IL-17, IFN-γ, and tumor necrosis factor alpha (TNF-α), antibody, standard cytokines, and protocols were obtained from R&D Systems, Inc.
Hepatic immunopathology.
Sections of liver samples fixed in 10% buffered formalin and processed by routine histopathologic technique were stained with hematoxylin and eosin and examined by optic microscopy. The sizes of the granulomatous lesions were determined by computer-assisted morphometric analysis, as described previously (54). Ten to 20 granulomas were evaluated for each liver.
Determination of worm and egg burdens.
The schistosome worm burden was assessed by perfusing the vasculature of infected mice with phosphate-buffered saline plus 25 mM sodium citrate. A small incision was made in the hepatic portal vein, and 20 ml of solution was injected into the aorta to flush out the worms. The worms were placed in medium and counted. The schistosome egg load was assessed by counting the number of eggs present in 1-mm2 fields of liver tissue in sections stained with hematoxylin and eosin.
Real-time quantitative RT-PCR.
Total RNA was isolated from the livers of infected CBA mice using Trizol according to the manufacturer's instructions (Invitrogen). RNA (1 μg) was subjected to DNase I treatment (Roche Molecular Biochemicals) and reverse transcribed using a high-capacity cDNA reverse transcription (RT) kit from Applied Biosystems. Real-time quantitative RT-PCR was performed with 10 ng of cDNA from each sample using either SYBR green analysis with a custom PCR array or Taqman analysis. All reactions were performed using an ABI 7300 instrument. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) levels were measured in a separate reaction and used to normalize the data. Reagents and protocols used for SYBR green and Taqman real-time quantitative RT-PCR were obtained from SuperArray Bioscience and Applied Biosystems, respectively. Using the average mean cycle threshold (CT) value for GAPDH and the gene of interest for each sample, the following equation was used to obtain normalized values (14): 1.8e(CT of GAPDH − CT of the gene of interest) × 104.
Statistical analysis.
Analysis of variance and Student's t tests were used to determine the statistical significance of the differences among groups. A P value of <0.05 was considered significant. Each individual experiment was conducted with groups of three to six mice.
RESULTS
Coinfection with H. polygyrus and S. mansoni markedly reduces schistosome egg-induced immunopathology but does not affect the schistosome worm or egg burden in CBA mice.
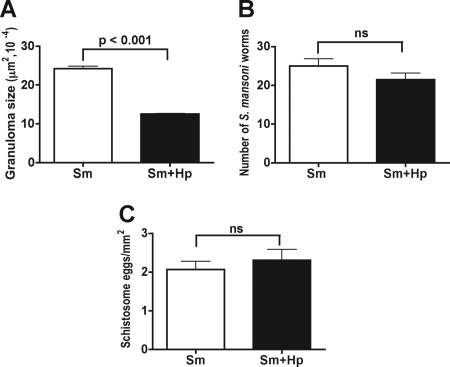
Naturally high-pathology CBA mice were infected with 40 H. polygyrus third-stage larvae 4 weeks and again 2 days prior to infection with schistosomes. This protocol was chosen in order to establish and maintain a Th2-dominant environment from the start of and throughout a subsequent 7-week schistosome infection (4, 25). Infection with H. polygyrus resulted in a marked reduction in the sizes of the hepatic granulomatous lesions induced by schistosome eggs (Fig. 1A). This inhibitory effect was strictly dependent on the sequence of the infections as it was completely abrogated when mice were infected simultaneously with H. polygyrus and schistosomes or when H. polygyrus was inoculated after the schistosome infection (data not shown). H. polygyrus had no significant effect on the schistosome worm burden (Fig. 1B) or the number of eggs present per unit of area in the hepatic tissue (Fig. 1C), implying that the coinfection affected the host egg-induced immunopathology but not the schistosomes themselves.
FIG. 1.
Hepatic immunopathology, worm burdens, and egg counts in CBA mice coinfected with H. polygyrus and S. mansoni. (A) Hepatic granulomatous inflammation, measured as described in Materials and Methods, was significantly decreased in CBA mice coinfected with H. polygyrus and S. mansoni compared with control CBA mice infected with S. mansoni alone. (B and C) There were no significant differences in the numbers of (B) S. mansoni worms or (C) S. mansoni eggs between CBA mice infected with schistosomes alone and CBA mice coinfected with H. polygyrus. Thirteen to 23 1-mm2 fields were counted for each liver section, and 10 livers were counted for each mouse group. The immunopathology, worm, and egg data are representative of the results of two or three independent experiments. Sm, S. mansoni; Hp, H. polygyrus; ns, not significant.
Coinfection with H. polygyrus causes a profound Th1/Th17-to-Th2 cytokine shift in schistosome-infected CBA mice.
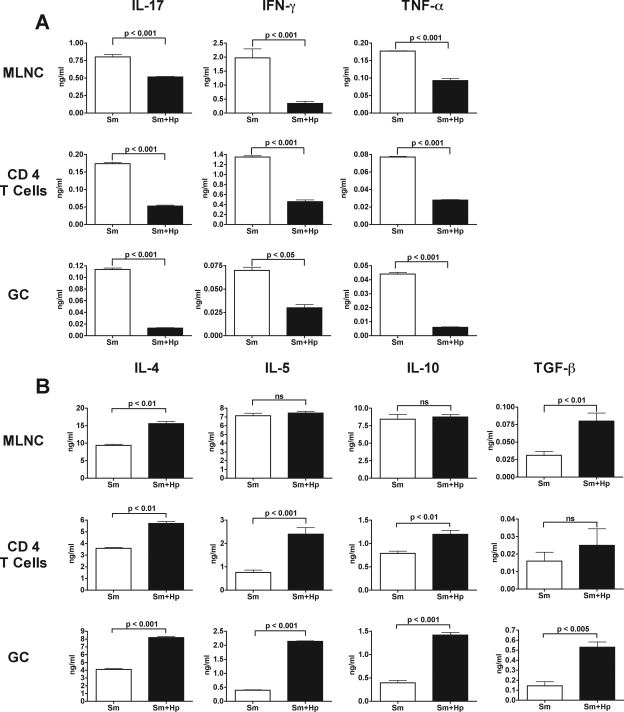
H. polygyrus coinfection significantly reduced the intensity of the normally severe immunopathology displayed by schistosome-infected CBA mice. To assess the underlying changes in the immune response of these mice, we measured cytokine production in supernatants from SEA-stimulated bulk MLNC, CD4 T cells isolated from the MLNC, and GC after a 7-week schistosome infection. Compared with the mice infected only with schistosomes, there were significant decreases in SEA-specific IL-17, IFN-γ, and TNF-α production in mice that were coinfected with H. polygyrus; these decreases were variable but evident in both bulk MLNC and MLN-derived CD4 T-cell populations, as well as in GC populations (Fig. 2A). On the other hand, the levels of IL-4, IL-5, and IL-10 were considerably higher in the coinfected mice, and more pronounced increases were observed in the SEA-stimulated CD4 T-cell and GC cultures than in the bulk MLNC cultures; in turn, the increases in the level of TGF-β were greater in MLNC and GC than in CD4 T cells (Fig. 2B).
FIG. 2.
Cytokine production by SEA-stimulated bulk MLNC, CD4 T cells, and GC in CBA mice coinfected with H. polygyrus and S. mansoni. Cytokine levels in 48-h supernatants from SEA-stimulated bulk MLNC, CD4 T cells plus APC, and GC were measured by ELISA. (A) The levels of IL-17, IFN-γ, and TNF-α produced by bulk MLNC, CD4 T cells, and GC were significantly lower in CBA mice coinfected with H. polygyrus and S. mansoni than in CBA mice infected with S. mansoni alone. (B) The levels of IL-4 and TGF-β, but not the level of IL-5 or IL-10, produced by bulk MLNC were significantly higher in CBA mice coinfected with H. polygyrus and S. mansoni than in CBA mice infected with S. mansoni alone. IL-4, IL-5, and IL-10 production, but not TGF-β production, was significantly increased in CD4 T cells, and production of all four cytokines was significantly increased in GC. The bars indicate the means of triplicate determinations, and the error bars indicate the standard deviations; background cytokine levels from unstimulated cells were subtracted. The results shown are from one experiment that was representative of three experiments in which similar results were obtained. Sm, S. mansoni; Hp, H. polygyrus; ns, not significant.
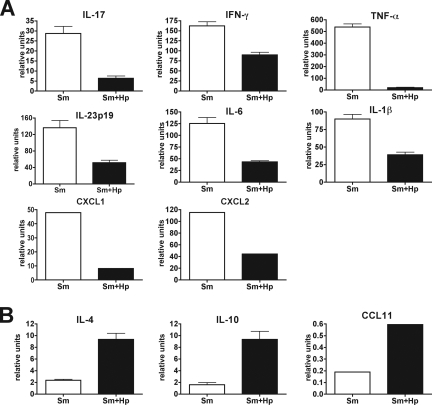
Significant decreases in the levels of IL-17, IFN-γ, and TNF-α induced by H. polygyrus in the schistosome-infected CBA mice were also observed at the mRNA level; importantly, the transcript levels of the innate immunity-associated proinflammatory cytokines IL-23, IL-6, and IL-1β, as well as the chemokines CXCL1 and CXCL2, were also markedly downregulated in the livers of the doubly infected mice (Fig. 3A). Conversely, in the coinfected mice there was marked upregulation of the IL-4 and IL-10 mRNA transcripts, as well as the mRNA transcripts of the chemokine CCL11 (Fig. 3B). These results indicate that the intestinal nematodes induced a strong Th2 shift in the Th1- and Th17-dominated response to schistosome infection in CBA mice.
FIG. 3.
mRNA transcripts from livers of CBA mice coinfected with H. polygyrus and S. mansoni. mRNA levels were measured by real-time quantitative RT-PCR as described in Materials and Methods. (A) The mRNA transcript levels of IL-17, IFN-γ, TNF-α, IL-23p19, IL-6, and IL-1β, as well as the chemokines CXCL1 and CXCL2, were decreased and (B) the levels of IL-4 and IL-10, as well as the chemokine CCL11, were increased in CBA mice coinfected with H. polygyrus and S. mansoni compared with CBA mice infected with S. mansoni alone. The bars indicate the means of two independent experiments using mRNA from three livers in each experiment, and the error bars indicate the standard deviations. All P values are at least <0.05. Sm, S. mansoni; Hp, H. polygyrus.
Coinfection with H. polygyrus causes upregulation of Ym1 and Foxp3 expression in schistosome-infected CBA mice.
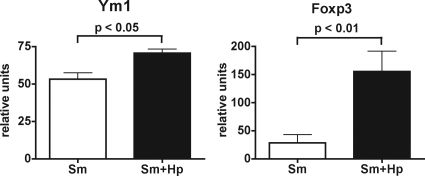
The significant increases in the IL-4, IL-10, and TGF-β levels in the coinfected CBA mice prompted us to examine two likely mechanisms that may underlie the amelioration of egg-induced immunopathology. IL-4 has been shown to be critical for the development of AAM (26, 40, 44), and IL-10 and TGF-β have been associated with Treg development and function (3, 43, 71). Both AAM and Treg have previously been implicated in the control of excessive inflammation against the schistosome eggs (8, 30, 31, 46, 67). We examined the differential expression of the lectin Ym1, which is induced by IL-4 and signal transducer and activator of transcription 6 and serves as a marker of AAM (27, 40, 72), as well as the forkhead box P3 (Foxp3) transcription factor, which is a marker of Treg (24). The levels of expression of Ym1 and Foxp3 (Fig. 4) were indeed significantly higher in the livers of the coinfected mice than in livers of the mice infected only with schistosomes. These findings suggest that early administration of H. polygyrus induces AAM and Treg, which are capable of inhibiting proinflammatory cytokines and thus preventing the development of severe egg-induced hepatic immunopathology.
FIG. 4.
Ym1 and Foxp3 expression in livers from CBA mice coinfected with H. polygyrus and S. mansoni. mRNA levels were measured by real-time quantitative RT-PCR as described in Materials and Methods. The mRNA transcript levels of Ym1 and Foxp3 were significantly greater in CBA mice coinfected with H. polygyrus and S. mansoni than in CBA mice infected with S. mansoni alone. The bars indicate the means of two independent experiments using mRNA from three livers in each experiment, and the error bars indicate the standard deviations. Sm, S. mansoni; Hp, H. polygyrus.
Coinfection with H. polygyrus and S. mansoni markedly reduces schistosome egg-induced immunopathology and promotes a Th2 shift in the cytokine response in SEA/CFA-immunized BL/6 mice.
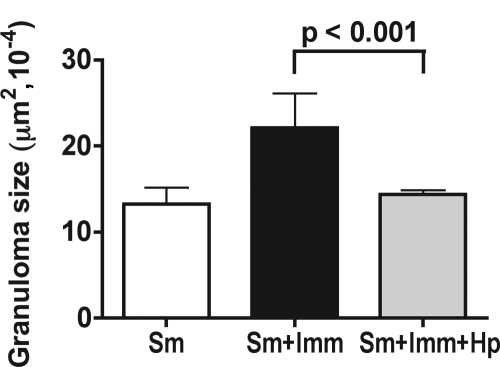
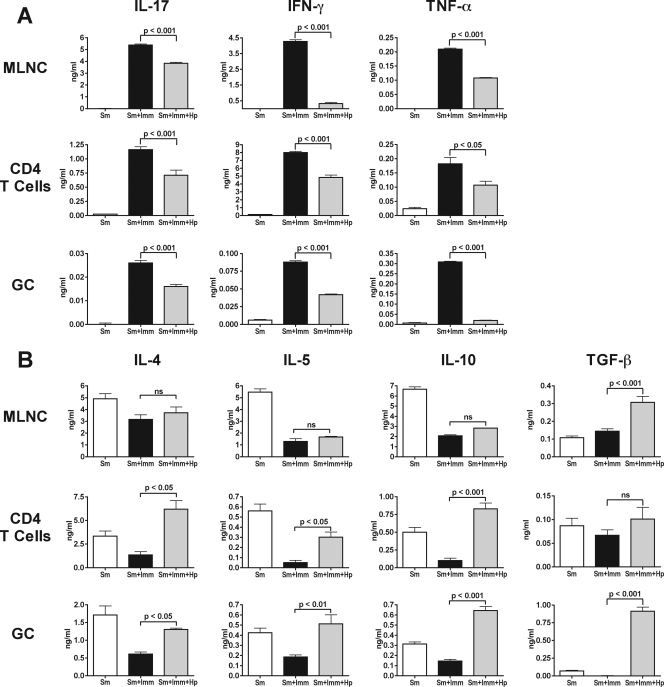
Schistosome-infected BL/6 mice typically exhibit small hepatic egg granulomas; however, SEA/CFA immunization results in marked exacerbation of the lesions and sharp increases in the levels of IFN-γ and IL-17. This phenotype is similar to that seen in CBA mice, but because of the existence of suitable “knockout” mice with the H-2b background, the immunized BL/6 mice have been very useful for discerning the role of different genes in the induction of severe immunopathology (54, 55, 57). Using the same coinfection protocol, we investigated whether the presence of H. polygyrus affected the disease exacerbation resulting from immunization with SEA/CFA. Histologic analysis of the livers revealed that coinfection with H. polygyrus virtually abrogated the development of the severe immunopathology caused by SEA/CFA immunization, and the observed lesions were more comparable to those seen in the unimmunized BL/6 mice (Fig. 5). In addition, the marked increases in SEA-specific IL-17, IFN-γ, and TNF-α production by bulk MLNC, CD4 T cells, and GC induced by SEA/CFA immunization were significantly abrogated in mice coinfected with H. polygyrus (Fig. 6A). In contrast, the levels of IL-4, IL-5, IL-10, and TGF-β production by bulk MLNC, CD4 T cells, and GC, which were generally reduced by SEA/CFA immunization, were partially or completely restored or even increased in the presence of H. polygyrus, although the differences were not always statistically significant (Fig. 6B).
FIG. 5.
Hepatic immunopathology in SEA/CFA-immunized BL/6 mice coinfected with H. polygyrus and S. mansoni. Hepatic granulomatous inflammation, measured as described in Materials and Methods, was significantly increased in SEA/CFA-immunized mice compared with unimmunized BL/6 mice (P < 0.001) but was significantly decreased in SEA/CFA-immunized BL/6 mice coinfected with H. polygyrus compared with SEA/CFA-immunized BL/6 mice infected with schistosomes alone. The results shown are from one experiment representative of three in which similar results were obtained. Sm, S. mansoni; Hp, H. polygyrus; Imm, SEA/CFA immunization.
FIG. 6.
Cytokine production by SEA-stimulated bulk MLNC, CD4 T cells, and GC in SEA/CFA-immunized BL/6 mice coinfected with H. polygyrus and S. mansoni. Cytokine levels in 48-h supernatants from SEA-stimulated bulk MLNC, CD4 T cells plus APC, and GC were measured by ELISA. (A) The levels of IL-17, IFN-γ, and TNF-α produced by bulk MLNC, CD4 T cells, and GC were significantly lower in SEA/CFA-immunized BL/6 mice coinfected with H. polygyrus than in SEA/CFA-immunized BL/6 mice infected with S. mansoni alone. (B) The levels of IL-4, IL-5, and IL-10 produced by bulk MLNC were not significantly different in SEA/CFA-immunized mice coinfected with H. polygyrus and SEA/CFA-immunized mice infected with S. mansoni alone, but the level of TGF-β was higher in SEA/CFA-immunized mice coinfected with H. polygyrus than in SEA/CFA-immunized mice infected with S. mansoni alone. IL-4, IL-5, and IL-10 production, but not TGF-β production, by CD4 T cells was significantly greater in SEA/CFA-immunized BL/6 mice coinfected with H. polygyrus than in SEA/CFA-immunized BL/6 mice infected with S. mansoni alone, and the levels of all four cytokines were significantly greater in GC from SEA/CFA-immunized BL/6 mice coinfected with H. polygyrus than in GC from SEA/CFA-immunized BL/6 mice infected with S. mansoni alone. The bars indicate the means of triplicate determinations, and the error bars indicate standard deviations; background cytokine levels from unstimulated cells were subtracted. The results shown are from one experiment that was representative of three experiments in which similar results were obtained. Sm, S. mansoni; Hp, H. polygyrus; Imm, SEA/CFA immunization; ns, not significant.
DISCUSSION
Although widespread in mostly tropical regions, infection with a variety of parasitic helminths generally results in relatively low morbidity and even less mortality. In the case of infection with schistosomes, there is predictable severe disease in about 5 to 10% of the population, and residents of areas where the disease is endemic generally suffer from less symptomatic and milder clinical forms of the disease. The immunopathology in schistosomiasis is mediated by CD4 effector T cells, and the milder pathology settings have been widely attributed to the generation of host-protective immunoregulatory mechanisms linked to genetic predisposition (45, 78) or induced by transplacental passage of parasite antigen or antiparasite antibody (7, 16, 48). In this study we tested the hypothesis that low pathology is at least in part determined by coinfection with intestinal nematodes. This hypothesis is based on the observations that nematode coinfection is prevalent in areas where schistosomiasis is endemic and that nematode infection creates a host immune environment associated with attenuated incidence of CD4 T-cell-dependent autoimmune diseases (19).
Using the murine model of schistosomiasis, we show here that coinfection with the trichostrongyle parasitic nematode H. polygyrus resulted in significant inhibition of the natural (CBA) or SEA/CFA immunization-induced (BL/6) form of severe hepatic granulomatous inflammation caused by schistosome eggs. Coinfection with intestinal nematodes had no measurable effect on the viability or fecundity of the schistosomes. The reduction in disease intensity was accompanied by marked decreases in IL-17, IFN-γ, and TNF-α levels in bulk MLNC, GC, and purified CD4 T cells. These cytokines correlate with and variously drive the immunopathology in schistosomiasis (1, 49, 54, 55, 57). In particular, the proinflammatory function of IL-17, which induces chemokine-mediated leukocyte recruitment, has also been demonstrated in the context of other infectious and autoimmune diseases (32, 35, 61). IL-17 production is associated with a distinct subset of CD4 T cells, Th17 cells (28, 53), which are variously promoted by an array of innate immunocyte-derived cytokines, including IL-6, TGF-β, IL-23, IL-21, and IL-1β (2, 9, 36, 38, 64, 70). In the CBA mice coinfected with H. polygyrus, we indeed detected significant decreases in expression of IL-23p19, IL-6, and IL-1β, which explains the decreases in expression of IL-17 and of the neutrophil chemoattractants CXCL1 (Gro-α) and CXCL2 (Gro-β) (54). On the other hand, there were marked increases in expression of IL-4, IL-5, IL-10, and TGF-β, as well as in expression of CCL11 (eotaxin), which collectively indicate that there was a Th1/Th17-to-Th2 shift in the cytokine environment.
The observed increases in IL-4, IL-10, and TGF-β production induced by coinfection with H. polygyrus suggested a role for AAM and Treg, which characterize immunoregulatory mechanisms that have been linked to the down-modulation of schistosome egg-induced immunopathology (30, 31, 42, 46, 67). IL-4 is critical for the development of AAM (26, 40, 44), and IL-10 and TGF-β are anti-inflammatory cytokines widely associated with Treg activity (3, 43, 71). Indeed, the increase in expression of TGF-β together with the downregulation of IL-6 and IL-1β caused by H. polygyrus is a setting conducive for Treg differentiation and development (9, 71). The levels of both the lectin Ym1 and the transcription factor Foxp3, which are markers of AAM and Treg, respectively, were significantly higher in coinfected mice than in mice infected with only schistosomes. These findings support the hypothesis that AAM and Treg have a role as effector mechanisms involved in the reduction of schistosome egg-induced immunopathology induced by H. polygyrus coinfection; in fact, both mechanisms have also been implicated in helminth-induced amelioration of inflammation in a number of infectious diseases, as well as autoimmune diseases (4, 12, 22, 37, 77).
The ameliorating effect of nematode coinfection on the severity of schistosomiasis is similar to that exerted on a variety of autoimmune diseases (17, 22, 34, 37, 51, 58, 59, 76), thus offering a collective explanation for the lower incidence of these T-cell-mediated conditions in areas where helminths are endemic. Such an effect of nematodes with relatively little intrinsic pathogenicity appears to be beneficial for the host and is currently being explored as a therapeutic means to control inflammatory bowel disease in humans (63) and possibly other autoimmune diseases (37). On the other hand, the helminths may be detrimental under conditions in which a strong proinflammatory response is necessary to control other infectious agents (20, 29, 33, 39, 66).
In summary, preexposure to intestinal nematodes effectively protected mice from severe schistosomiasis using a regimen that provided optimal Th2 conditioning at the time of the schistosome infection and subsequent downregulation of pathogenic Th1- and Th17-cell-mediated responses. This successful time sequence closely mimics the sequence observed in areas where the disease is endemic, where individuals typically acquire intestinal nematode infections before they are exposed to bodies of freshwater contaminated with schistosomes. It should be noted, however, that while nematode coinfection averted severe Th1/Th17-mediated schistosome egg-induced hepatic immunopathology, schistosome infection by itself induces in the vast majority of individuals a Th2 response (6, 47, 60) that downregulates inflammation against other pathogens (21, 50, 52, 62, 66, 68, 73). In some instances, however, pathogens can induce a proinflammatory milieu that is conducive to exacerbated schistosome pathology (5). Regardless, a concept supported by our findings is that, as a whole, natural or therapeutic helminth infections can be important elements in the prevention and amelioration of aberrant or excessive CD4 T-cell-mediated disease.
Acknowledgments
S. mansoni-infected B. glabrata snails were provided by Fred Lewis of the Biomedical Research Institute (Rockville, MD) through National Institutes of Health National Institute of Allergy and Infectious Diseases contract NO1-AI-55270. This work was supported by Public Health Service grants RO1-18919, DK38327, DK58755, and DK34928.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 18 August 2008.
REFERENCES
- 1.Adewusi, O. I., N. A. Nix, X. Lu, D. G. Colley, and W. E. Secor. 1996. Schistosoma mansoni: relationship of tumor necrosis factor-alpha to morbidity and collagen deposition in chronic experimental infection. Exp. Parasitol. 84115-123. [DOI] [PubMed] [Google Scholar]
- 2.Aggarwal, S., N. Ghilardi, M. H. Xie, F. J. de Sauvage, and A. L. Gurney. 2003. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J. Biol. Chem. 2781910-1914. [DOI] [PubMed] [Google Scholar]
- 3.Annacker, O., R. Pimenta-Araujo, O. Burlen-Defranoux, T. C. Barbosa, A. Cumano, and A. Bandeira. 2001. CD25+ CD4+ T cells regulate the expansion of peripheral CD4 T cells through the production of IL-10. J. Immunol. 1663008-3018. [DOI] [PubMed] [Google Scholar]
- 4.Anthony, R. M., L. I. Rutitzky, J. F. Urban, Jr., M. J. Stadecker, and W. C. Gause. 2007. Protective immune mechanisms in helminth infection. Nat. Rev. Immunol. 7975-987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Araujo, M. I., S. K. Bliss, Y. Suzuki, A. Alcaraz, E. Y. Denkers, and E. J. Pearce. 2001. Interleukin-12 promotes pathologic liver changes and death in mice coinfected with Schistosoma mansoni and Toxoplasma gondii. Infect. Immun. 691454-1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Araujo, M. I., A. R. de Jesus, O. Bacellar, E. Sabin, E. Pearce, and E. M. Carvalho. 1996. Evidence of a T helper type 2 activation in human schistosomiasis. Eur. J. Immunol. 261399-1403. [DOI] [PubMed] [Google Scholar]
- 7.Attallah, A. M., A. T. Abbas, M. I. Dessouky, H. M. El-Emshaty, and H. M. Elsheikha. 2006. Susceptibility of neonate mice born to Schistosoma mansoni-infected and noninfected mothers to subsequent S. mansoni infection. Parasitol. Res. 99137-145. [DOI] [PubMed] [Google Scholar]
- 8.Baumgart, M., F. Tompkins, J. Leng, and M. Hesse. 2006. Naturally occurring CD4+ Foxp3+ regulatory T cells are an essential, IL-10-independent part of the immunoregulatory network in Schistosoma mansoni egg-induced inflammation. J. Immunol. 1765374-5387. [DOI] [PubMed] [Google Scholar]
- 9.Bettelli, E., Y. Carrier, W. Gao, T. Korn, T. B. Strom, M. Oukka, H. L. Weiner, and V. K. Kuchroo. 2006. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441235-238. [DOI] [PubMed] [Google Scholar]
- 10.Bica, I., D. H. Hamer, and M. J. Stadecker. 2000. Hepatic schistosomiasis. Infect. Dis. Clin. N. Am. 14583-604, viii. [DOI] [PubMed] [Google Scholar]
- 11.Boros, D. L., and K. S. Warren. 1970. Delayed hypersensitivity-type granuloma formation and dermal reaction induced and elicited by a soluble factor isolated from Schistosoma mansoni eggs. J. Exp. Med. 132488-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Capron, A., D. Dombrowicz, and M. Capron. 2004. Helminth infections and allergic diseases: from the Th2 paradigm to regulatory networks. Clin. Rev. Allergy Immunol. 2625-34. [DOI] [PubMed] [Google Scholar]
- 13.Cheever, A. W., R. H. Duvall, T. A. Hallack, Jr., R. G. Minker, J. D. Malley, and K. G. Malley. 1987. Variation of hepatic fibrosis and granuloma size among mouse strains infected with Schistosoma mansoni. Am. J. Trop. Med. Hyg. 3785-97. [DOI] [PubMed] [Google Scholar]
- 14.Chen, Y., C. L. Langrish, B. McKenzie, B. Joyce-Shaikh, J. S. Stumhofer, T. McClanahan, W. Blumenschein, T. Churakovsa, J. Low, L. Presta, C. A. Hunter, R. A. Kastelein, and D. J. Cua. 2006. Anti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. J. Clin. Investig. 1161317-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiaramonte, M. G., D. D. Donaldson, A. W. Cheever, and T. A. Wynn. 1999. An IL-13 inhibitor blocks the development of hepatic fibrosis during a T-helper type 2-dominated inflammatory response. J. Clin. Investig. 104777-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colley, D. G., M. A. Montesano, G. L. Freeman, and W. E. Secor. 1999. Infection-stimulated or perinatally initiated idiotypic interactions can direct differential morbidity and mortality in schistosomiasis. Microbes Infect. 1517-524. [DOI] [PubMed] [Google Scholar]
- 17.Cooke, A., P. Tonks, F. M. Jones, H. O'Shea, P. Hutchings, A. J. Fulford, and D. W. Dunne. 1999. Infection with Schistosoma mansoni prevents insulin dependent diabetes mellitus in non-obese diabetic mice. Parasite Immunol. 21169-176. [DOI] [PubMed] [Google Scholar]
- 18.de Jesus, A. R., A. Silva, L. B. Santana, A. Magalhaes, A. A. de Jesus, R. P. de Almeida, M. A. Rego, M. N. Burattini, E. J. Pearce, and E. M. Carvalho. 2002. Clinical and immunologic evaluation of 31 patients with acute schistosomiasis mansoni. J. Infect. Dis. 18598-105. [DOI] [PubMed] [Google Scholar]
- 19.Dunne, D. W., and A. Cooke. 2005. A worm's eye view of the immune system: consequences for evolution of human autoimmune disease. Nat. Rev. Immunol. 5420-426. [DOI] [PubMed] [Google Scholar]
- 20.Dunne, D. W., and E. M. Riley. 2004. Immunity, morbidity and immunoepidemiology in parasite infections. Parasite Immunol. 26425-428. [DOI] [PubMed] [Google Scholar]
- 21.Elias, D., S. Britton, A. Kassu, and H. Akuffo. 2007. Chronic helminth infections may negatively influence immunity against tuberculosis and other diseases of public health importance. Expert Rev. Anti-Infect. Ther. 5475-484. [DOI] [PubMed] [Google Scholar]
- 22.Elliott, D. E., T. Setiawan, A. Metwali, A. Blum, J. F. Urban, Jr., and J. V. Weinstock. 2004. Heligmosomoides polygyrus inhibits established colitis in IL-10-deficient mice. Eur. J. Immunol. 342690-2698. [DOI] [PubMed] [Google Scholar]
- 23.Fallon, P. G., E. J. Richardson, G. J. McKenzie, and A. N. McKenzie. 2000. Schistosome infection of transgenic mice defines distinct and contrasting pathogenic roles for IL-4 and IL-13: IL-13 is a profibrotic agent. J. Immunol. 1642585-2591. [DOI] [PubMed] [Google Scholar]
- 24.Fontenot, J. D., M. A. Gavin, and A. Y. Rudensky. 2003. Foxp3 programs the development and function of CD4+ CD25+ regulatory T cells. Nat. Immunol. 4330-336. [DOI] [PubMed] [Google Scholar]
- 25.Gause, W. C., J. F. Urban, Jr., and M. J. Stadecker. 2003. The immune response to parasitic helminths: insights from murine models. Trends Immunol. 24269-277. [DOI] [PubMed] [Google Scholar]
- 26.Goerdt, S., and C. E. Orfanos. 1999. Other functions, other genes: alternative activation of antigen-presenting cells. Immunity 10137-142. [DOI] [PubMed] [Google Scholar]
- 27.Gordon, S. 2003. Alternative activation of macrophages. Nat. Rev. Immunol. 323-35. [DOI] [PubMed] [Google Scholar]
- 28.Harrington, L. E., R. D. Hatton, P. R. Mangan, H. Turner, T. L. Murphy, K. M. Murphy, and C. T. Weaver. 2005. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 61123-1132. [DOI] [PubMed] [Google Scholar]
- 29.Helmby, H., M. Kullberg, and M. Troye-Blomberg. 1998. Altered immune responses in mice with concomitant Schistosoma mansoni and Plasmodium chabaudi infections. Infect. Immun. 665167-5174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herbert, D. R., C. Holscher, M. Mohrs, B. Arendse, A. Schwegmann, M. Radwanska, M. Leeto, R. Kirsch, P. Hall, H. Mossmann, B. Claussen, I. Forster, and F. Brombacher. 2004. Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity 20623-635. [DOI] [PubMed] [Google Scholar]
- 31.Hesse, M., C. A. Piccirillo, Y. Belkaid, J. Prufer, M. Mentink-Kane, M. Leusink, A. W. Cheever, E. M. Shevach, and T. A. Wynn. 2004. The pathogenesis of schistosomiasis is controlled by cooperating IL-10-producing innate effector and regulatory T cells. J. Immunol. 1723157-3166. [DOI] [PubMed] [Google Scholar]
- 32.Kastelein, R. A., C. A. Hunter, and D. J. Cua. 2007. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu. Rev. Immunol. 25221-242. [DOI] [PubMed] [Google Scholar]
- 33.Khan, I. A., R. Hakak, K. Eberle, P. Sayles, L. M. Weiss, and J. F. Urban, Jr. 2008. Coinfection with Heligmosomoides polygyrus fails to establish CD8+ T-cell immunity against Toxoplasma gondii. Infect. Immun. 761305-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kitagaki, K., T. R. Businga, D. Racila, D. E. Elliott, J. V. Weinstock, and J. N. Kline. 2006. Intestinal helminths protect in a murine model of asthma. J. Immunol. 1771628-1635. [DOI] [PubMed] [Google Scholar]
- 35.Kolls, J. K., and A. Linden. 2004. Interleukin-17 family members and inflammation. Immunity 21467-476. [DOI] [PubMed] [Google Scholar]
- 36.Korn, T., E. Bettelli, W. Gao, A. Awasthi, A. Jager, T. B. Strom, M. Oukka, and V. K. Kuchroo. 2007. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature 448484-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.La Flamme, A. C., K. Canagasabey, M. Harvie, and B. T. Backstrom. 2004. Schistosomiasis protects against multiple sclerosis. Mem. Inst. Oswaldo Cruz 9933-36. [DOI] [PubMed] [Google Scholar]
- 38.Langrish, C. L., Y. Chen, W. M. Blumenschein, J. Mattson, B. Basham, J. D. Sedgwick, T. McClanahan, R. A. Kastelein, and D. J. Cua. 2005. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 201233-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Legesse, M., B. Erko, and F. Balcha. 2004. Increased parasitaemia and delayed parasite clearance in Schistosoma mansoni and Plasmodium berghei co-infected mice. Acta Trop. 91161-166. [DOI] [PubMed] [Google Scholar]
- 40.Loke, P., M. G. Nair, J. Parkinson, D. Guiliano, M. Blaxter, and J. E. Allen. 2002. IL-4 dependent alternatively-activated macrophages have a distinctive in vivo gene expression phenotype. BMC Immunol. 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maizels, R. M. 2005. Infections and allergy—helminths, hygiene and host immune regulation. Curr. Opin. Immunol. 17656-661. [DOI] [PubMed] [Google Scholar]
- 42.Maizels, R. M., A. Balic, N. Gomez-Escobar, M. Nair, M. D. Taylor, and J. E. Allen. 2004. Helminth parasites—masters of regulation. Immunol. Rev. 20189-116. [DOI] [PubMed] [Google Scholar]
- 43.Maloy, K. J., L. Salaun, R. Cahill, G. Dougan, N. J. Saunders, and F. Powrie. 2003. CD4+ CD25+ T(R) cells suppress innate immune pathology through cytokine-dependent mechanisms. J. Exp. Med. 197111-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mantovani, A., A. Sica, and M. Locati. 2005. Macrophage polarization comes of age. Immunity 23344-346. [DOI] [PubMed] [Google Scholar]
- 45.Marquet, S., L. Abel, D. Hillaire, H. Dessein, J. Kalil, J. Feingold, J. Weissenbach, and A. J. Dessein. 1996. Genetic localization of a locus controlling the intensity of infection by Schistosoma mansoni on chromosome 5q31-q33. Nat. Genet. 14181-184. [DOI] [PubMed] [Google Scholar]
- 46.McKee, A. S., and E. J. Pearce. 2004. CD25+ CD4+ cells contribute to Th2 polarization during helminth infection by suppressing Th1 response development. J. Immunol. 1731224-1231. [DOI] [PubMed] [Google Scholar]
- 47.Montenegro, S. M., P. Miranda, S. Mahanty, F. G. Abath, K. M. Teixeira, E. M. Coutinho, J. Brinkman, I. Goncalves, L. A. Domingues, A. L. Domingues, A. Sher, and T. A. Wynn. 1999. Cytokine production in acute versus chronic human Schistosomiasis mansoni: the cross-regulatory role of interferon-gamma and interleukin-10 in the responses of peripheral blood mononuclear cells and splenocytes to parasite antigens. J. Infect. Dis. 1791502-1514. [DOI] [PubMed] [Google Scholar]
- 48.Montesano, M. A., D. G. Colley, M. T. Willard, G. L. Freeman, Jr., and W. E. Secor. 2002. Idiotypes expressed early in experimental Schistosoma mansoni infections predict clinical outcomes of chronic disease. J. Exp. Med. 1951223-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mwatha, J. K., G. Kimani, T. Kamau, G. G. Mbugua, J. H. Ouma, J. Mumo, A. J. Fulford, F. M. Jones, A. E. Butterworth, M. B. Roberts, and D. W. Dunne. 1998. High levels of TNF, soluble TNF receptors, soluble ICAM-1, and IFN-gamma, but low levels of IL-5, are associated with hepatosplenic disease in human schistosomiasis mansoni. J. Immunol. 1601992-1999. [PubMed] [Google Scholar]
- 50.Nacher, M., P. Singhasivanon, S. Yimsamran, W. Manibunyong, N. Thanyavanich, R. Wuthisen, and S. Looareesuwan. 2002. Intestinal helminth infections are associated with increased incidence of Plasmodium falciparum malaria in Thailand. J. Parasitol. 8855-58. [DOI] [PubMed] [Google Scholar]
- 51.Nagayama, Y., K. Watanabe, M. Niwa, S. M. McLachlan, and B. Rapoport. 2004. Schistosoma mansoni and alpha-galactosylceramide: prophylactic effect of Th1 immune suppression in a mouse model of Graves' hyperthyroidism. J. Immunol. 1732167-2173. [DOI] [PubMed] [Google Scholar]
- 52.Noland, G. S., J. F. Urban, Jr., B. Fried, and N. Kumar. 2008. Counter-regulatory anti-parasite cytokine responses during concurrent Plasmodium yoelli and intestinal helminth infections in mice. Exp. Parasitol. 119272-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park, H., Z. Li, X. O. Yang, S. H. Chang, R. Nurieva, Y. H. Wang, Y. Wang, L. Hood, Z. Zhu, Q. Tian, and C. Dong. 2005. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 61133-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rutitzky, L. I., L. Bazzone, M. G. Shainheit, B. Joyce-Shaikh, D. J. Cua, and M. J. Stadecker. 2008. IL-23 is required for the development of severe egg-induced immunopathology in schistosomiasis and for lesional expression of IL-17. J. Immunol. 1802486-2495. [DOI] [PubMed] [Google Scholar]
- 55.Rutitzky, L. I., H. J. Hernandez, and M. J. Stadecker. 2001. Th1-polarizing immunization with egg antigens correlates with severe exacerbation of immunopathology and death in schistosome infection. Proc. Natl. Acad. Sci. USA 9813243-13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rutitzky, L. I., H. J. Hernandez, Y. S. Yim, D. E. Ricklan, E. Finger, C. Mohan, I. Peter, E. K. Wakeland, and M. J. Stadecker. 2005. Enhanced egg-induced immunopathology correlates with high IFN-gamma in murine schistosomiasis: identification of two epistatic genetic intervals. J. Immunol. 174435-440. [DOI] [PubMed] [Google Scholar]
- 57.Rutitzky, L. I., J. R. Lopes da Rosa, and M. J. Stadecker. 2005. Severe CD4 T cell-mediated immunopathology in murine schistosomiasis is dependent on IL-12p40 and correlates with high levels of IL-17. J. Immunol. 1753920-3926. [DOI] [PubMed] [Google Scholar]
- 58.Saunders, K. A., T. Raine, A. Cooke, and C. E. Lawrence. 2007. Inhibition of autoimmune type 1 diabetes by gastrointestinal helminth infection. Infect. Immun. 75397-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sewell, D., Z. Qing, E. Reinke, D. Elliot, J. Weinstock, M. Sandor, and Z. Fabry. 2003. Immunomodulation of experimental autoimmune encephalomyelitis by helminth ova immunization. Int. Immunol. 1559-69. [DOI] [PubMed] [Google Scholar]
- 60.Silveira, A. M., G. Gazzinelli, L. F. Alves-Oliveira, J. Bethony, A. Gazzinelli, C. Carvalho-Queiroz, M. C. Alvarez, F. C. Lima-Silva, A. Prata, P. T. LoVerde, and R. Correa-Oliveira. 2004. Human schistosomiasis mansoni: intensity of infection differentially affects the production of interleukin-10, interferon-gamma and interleukin-13 by soluble egg antigen or adult worm antigen stimulated cultures. Trans. R. Soc. Trop. Med. Hyg. 98514-519. [DOI] [PubMed] [Google Scholar]
- 61.Stockinger, B., M. Veldhoen, and B. Martin. 2007. Th17 T cells: linking innate and adaptive immunity. Semin. Immunol. 19353-361. [DOI] [PubMed] [Google Scholar]
- 62.Su, Z., M. Segura, K. Morgan, J. C. Loredo-Osti, and M. M. Stevenson. 2005. Impairment of protective immunity to blood-stage malaria by concurrent nematode infection. Infect. Immun. 733531-3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Summers, R. W., D. E. Elliott, J. F. Urban, Jr., R. Thompson, and J. V. Weinstock. 2005. Trichuris suis therapy in Crohn's disease. Gut 5487-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sutton, C., C. Brereton, B. Keogh, K. H. Mills, and E. C. Lavelle. 2006. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J. Exp. Med. 2031685-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Svetic, A., K. B. Madden, X. D. Zhou, P. Lu, I. M. Katona, F. D. Finkelman, J. F. Urban, Jr., and W. C. Gause. 1993. A primary intestinal helminthic infection rapidly induces a gut-associated elevation of Th2-associated cytokines and IL-3. J. Immunol. 1503434-3441. [PubMed] [Google Scholar]
- 66.Talaat, K. R., R. E. Bonawitz, P. Domenech, and T. B. Nutman. 2006. Preexposure to live Brugia malayi microfilariae alters the innate response of human dendritic cells to Mycobacterium tuberculosis. J. Infect. Dis. 193196-204. [DOI] [PubMed] [Google Scholar]
- 67.Taylor, J. J., M. Mohrs, and E. J. Pearce. 2006. Regulatory T cell responses develop in parallel to Th responses and control the magnitude and phenotype of the Th effector population. J. Immunol. 1765839-5847. [DOI] [PubMed] [Google Scholar]
- 68.Tristao-Sa, R., R. Ribeiro-Rodrigues, L. T. Johnson, F. E. Pereira, and R. Dietze. 2002. Intestinal nematodes and pulmonary tuberculosis. Rev. Soc. Bras. Med. Trop. 35533-535. [DOI] [PubMed] [Google Scholar]
- 69.Urban, J. F., Jr., I. M. Katona, W. E. Paul, and F. D. Finkelman. 1991. Interleukin 4 is important in protective immunity to a gastrointestinal nematode infection in mice. Proc. Natl. Acad. Sci. USA 885513-5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Veldhoen, M., R. J. Hocking, C. J. Atkins, R. M. Locksley, and B. Stockinger. 2006. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24179-189. [DOI] [PubMed] [Google Scholar]
- 71.Vignali, D. A., L. W. Collison, and C. J. Workman. 2008. How regulatory T cells work. Nat. Rev. Immunol. 8523-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Welch, J. S., L. Escoubet-Lozach, D. B. Sykes, K. Liddiard, D. R. Greaves, and C. K. Glass. 2002. TH2 cytokines and allergic challenge induce Ym1 expression in macrophages by a STAT6-dependent mechanism. J. Biol. Chem. 27742821-42829. [DOI] [PubMed] [Google Scholar]
- 73.Weng, M., D. Huntley, I. F. Huang, O. Foye-Jackson, L. Wang, A. Sarkissian, Q. Zhou, W. A. Walker, B. J. Cherayil, and H. N. Shi. 2007. Alternatively activated macrophages in intestinal helminth infection: effects on concurrent bacterial colitis. J. Immunol. 1794721-4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yazdanbakhsh, M., P. G. Kremsner, and R. van Ree. 2002. Allergy, parasites, and the hygiene hypothesis. Science 296490-494. [DOI] [PubMed] [Google Scholar]
- 75.Yazdanbakhsh, M., and P. M. Matricardi. 2004. Parasites and the hygiene hypothesis: regulating the immune system? Clin. Rev. Allergy Immunol. 2615-24. [DOI] [PubMed] [Google Scholar]
- 76.Zaccone, P., Z. Fehervari, F. M. Jones, S. Sidobre, M. Kronenberg, D. W. Dunne, and A. Cooke. 2003. Schistosoma mansoni antigens modulate the activity of the innate immune response and prevent onset of type 1 diabetes. Eur. J. Immunol. 331439-1449. [DOI] [PubMed] [Google Scholar]
- 77.Zaccone, P., Z. Fehervari, J. M. Phillips, D. W. Dunne, and A. Cooke. 2006. Parasitic worms and inflammatory diseases. Parasite Immunol. 28515-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zinn-Justin, A., S. Marquet, D. Hillaire, A. Dessein, and L. Abel. 2001. Genome search for additional human loci controlling infection levels by Schistosoma mansoni. Am. J. Trop. Med. Hyg. 65754-758. [DOI] [PubMed] [Google Scholar]