Abstract
The Lyme disease spirochete Borrelia burgdorferi alters the expression of outer surface protein (osp) genes as the bacterium cycles between ticks and mammals. OspA is produced as borreliae enter the tick vector and remains a major surface antigen during midgut colonization. To elucidate the role of OspA in the vector, we created an insertional deletion of ospA in strain B31-A3. The ospA mutant infects mice when it is injected intradermally and is acquired by larval ticks fed on these mice, where it persists through the molt to the nymph stage. Bacterial survival rates in artificially infected tick larvae fed on naïve mice were compared with those in the vector fed on immune mice. The ospA mutant proliferates in larvae if it is exposed to blood from naïve mice, but it declines in density after larval feeding if the blood is from immune mice. When uninfected larvae are fed on B-cell-deficient mice infected with the ospA mutant, larvae show borrelial densities and persistence that are significantly greater than those fed on infected, immunocompetent mice. We conclude that OspA serves a critical antibody-shielding role during vector blood meal uptake from immune hosts and is not required for persistence in the tick vector.
The Lyme disease agent Borrelia burgdorferi is maintained in natural enzootic cycles involving small mammals and blood-feeding ticks (1, 21, 38, 40). When borrelia-infected Ixodes scapularis ticks feed on humans, infection may occur with protean clinical manifestations. Outer surface protein A (OspA) was the first surface protein identified in this bacterium (5, 32). The vector-restricted pattern of OspA production was exploited in its development as a transmission-blocking vaccine (24), and in a purified recombinant form, it was the specific immunogenic component of the first Lyme disease vaccine for humans (61, 63, 66). OspB is the product of a gene cotranscribed with the OspA-encoding gene (31); it is homologous to OspA by inferred amino acid sequence (9) and by structural analysis (7). OspA and -B are differentially produced as borreliae alternate between vector and host, synthesized in ticks but only rarely in mammals (6, 60). During the uptake of an infected blood meal, antibodies to OspA elicited by vaccination appear to block the acquisition of borreliae by the tick (20). A significant portion of borreliae in the tick midgut downregulates transcription of the ospAB operon (49) and synthesis of the encoded proteins (16, 19, 59) during the 3- to 7-day feeding. Concurrent with the loss of OspA and -B, borreliae disseminate from the tick's midgut to the salivary glands (52).
Characterization of OspA as a predominantly tick-restricted surface protein prompted investigation of its function in the vector. OspA may function as a plasminogen binding protein (28, 36), a function necessary for efficient dissemination in the tick (16). OspA may also function to shield other conserved borrelial surface antigens, such as P66 (12) and P13 integral membrane proteins (43, 56), from antibody recognition; P66 is immunogenic in the mouse reservoir of B. burgdorferi in North America (11). Borreliae producing OspA in the tick may be targeted for immune recognition by natural antibodies, based on the relative levels of the B. burgdorferi OspA-positive N40 strain in the salivary glands of ticks fed on control and B-cell-deficient mice (8). OspA may act as a midgut adhesin, based on binding of recombinant OspA and of intact borreliae to tick midgut extracts (45, 47). Mutation of ospA in B. burgdorferi strain 297 is reported to give rise to borreliae that lose tick colonization function (69). Screening of a tick cDNA expression library indicates a specific tick midgut receptor for OspA (46); this paper also reports that the absence of OspA reduces but does not abolish borrelial binding to tick midgut extract. Mutagenesis of the ospB gene in the strain B31 borreliae that retain production of OspA is also reported to block tick acquisition of borreliae from infected mice (42). Of the strains used in these studies, strain 297 is a human cerebrospinal fluid isolate, and N40 and B31 are tick isolates: all are infectious in mice (30) but have not been directly compared in the mouse-tick infectious cycle. The strain B31 chromosome (27) and its 21 extrachromosomal elements (14) comprise the only complete Lyme disease borrelia genome published to date. In summary, both vector colonization (attachment and/or dissemination) and host immune avoidance are suggested as reasons for the temporal pattern of OspA production.
To further elucidate the function of OspA in borrelial colonization and survival in the tick vector, we created an ospA mutant in an infectious wild-type clone of strain B31 and an ospA-reconstituted OspA-producing derivative of the mutant to confirm the single-locus nature of the mutant phenotype. We introduced these clones and the parental clone into the mouse-tick infectious cycle and examined borrelial survival in ticks through the larval and nymphal stages of transmission.
MATERIALS AND METHODS
Construction of allelic replacement vectors for ospA and ospB mutagenesis.
DNA from the B. burgdorferi strain B31 MI (14, 15, 27) was PCR amplified (Expand high-fidelity PCR system; Roche Applied Science, Indianapolis, IN) with the primers LP54-F2+AscI and LP54-B3+AvrII (Table 1), corresponding to sequences within open reading frames (ORFs) BBA1 1 and BBA19 of the lp54 plasmid (14), to yield a 5,226-bp product containing the ospA (BBA15) and ospB (BBA16) genes. Primers POK12-F1+AscI and POK12-B1+AvrII (Table 1) were likewise used to amplify a 1,737-bp portion of pOK12 (67) containing the kanamycin resistance gene (kan) and the p15A low-copy origin of replication. PCR products, isolated by QIAquick purification (Qiagen, Valencia, CA) were separately digested with AscI and AvrII and ligated (T4 ligase; New England Biolabs, Ispwich, MA). The resultant DNA was used to transform Escherichia coli (One Shot TOP10F′; Invitrogen Corp., Carlsbad, CA). Plasmid DNA from colonies selected on kanamycin LB plates (50 μg/ml) was screened for predicted restriction fragment sizes by agarose gel electrophoresis, using PacI digest to identify desired products. One clone, pOKLP54, was chosen to produce internally deleted versions of the ospA and ospB genes bearing selectable markers. The pTAkanA vector, containing the kan resistance gene controlled by the flaB promoter of B. burgdorferi (flaBp-kan) (10), was digested with EcoRI and NheI to produce a flaBp-kan cassette fragment. This fragment and the 6,620-bp product of an EcoRI-SpeI restriction digest of pOKLP54 were isolated by agarose gel electrophoresis and QIAquick purification. The latter enzymes remove a 324-bp fragment from the ospA gene. The flaBP-kan cassette was inserted into the deleted ospA gene of pOKLP54, and transformants of E. coli TOP10 plated on 50 μg/ml kanamycin LB were screened by PCR and restriction digestion, resulting in pOKOSPAKO.
TABLE 1.
Strains, plasmids, and oligonucleotides used in this study
| Bacterium, plasmid, or oligonucleotide | Description or sequence | Reference or source |
|---|---|---|
| B. burgdorferi strains | ||
| B31 MI | Medimmune B31; nonclonal, low-passage infectious strain | 14 |
| A3 | Infectious, transformation-competent clone of B31 MI | 23 |
| 6 | Clonal derivative of A3 (WT) | This study |
| 2E6 | ospA mutant generated with pOKOSPAKO (ospA1) | This study |
| 7A | ospA restored, ospB mutant generated with pOKOSPA-C′ (ospA+B1) | This study |
| E. coli strain | ||
| TOP10F′ | Used for propagating plasmids | Invitrogen |
| Plasmids | ||
| pOK12 | P15A replicon, multiple cloning site lacZ′ vector | 67 |
| pTAkanA | B. burgdorferi flaBp-kan cassette in pCR2.1-TOPO | 10 |
| pTAGmA | B. burgdorferi flaBp-aacC1 cassette in pCR2.1-TOPO | 22 |
| pOKLP54 | pOK12 amplicon fused to LP54 amplicon | This study |
| pOKOSPAKO | ospA inactivation plasmid; pOKLP54 SpeI-EcoRI fragment ligated with NheI-EcoRI fragment of pTAkanA | This study |
| pOKOSPA-C′ | ospA restoration plasmid; pOKLP54 BclI-PvuII fragment ligated with BamHI-PvuII fragment of pTAGmA | This study |
| Oligonucleotides | ||
| POK12-F1+AscI | 5′-TGGCGCGCCTCGCCCTTCCCAACAGTTG | This study |
| POK12-B1+AvrII | 5′-ACCTAGGGCGTATTGGAGCTTTCGCG | This study |
| LP54-F2+AscI | 5′-AGGCGCGCCTTGGTGGCAAGGAAAACCATAC | This study |
| LP54-B3+AvrII | 5′-TCCTAGGCCTTTGGCTTGTTGTCTGCG | This study |
| OSP1A | 5′-TAGGATCCAAGCTTAATTAGAACCAAAC | 54 |
| OSP2 | 5′-CTTCCTACACTAGCTGATGC | 54 |
| KANA.5 | 5′-AGCCATATTCAACGGGAAACG | This study |
| KANA.3 | 5′-CAAGTCAGCGTAATGCTCTGCC | This study |
| flaB470F | 5′-TGGTTTGCTCCAACATGAACTC | This study |
| flaB547R | 5′-TGCAAAAATTAACACACCAGCAT | This study |
| flaB523TR | 5′-FAM-ACTTTCAGGGTCTCAAGCGTCTTGGACTTT-TAMRAa | This study |
FAM, 6-carboxyfluorescein; TAMRA, 6-carboxytetramethylrhodamine.
To generate a plasmid to restore the functional ospA allele, we inserted a gentamicin resistance marker in the ospB gene of pOKLP54, leaving ospA intact. pOKLP54 was digested with PvuII and BclI to remove 567 bp of ospB. The resistance cassette vector pTAGmA, containing the aminoglycoside acetyltransferase (aacC1) gene of Tn1696 linked to the flaB promoter of B. burgdorferi (22), was digested with PvuII and BamHI. The pOKLP54 backbone fragment and the flaBp-aacC1 cassette were isolated by agarose gel electrophoresis with BlueView stain (Sigma-Aldrich, St. Louis, MO), purified with QIAquick, and ligated. Transformants of E. coli TOP10 selected on 50 μg/ml kanamycin-5 μg/ml gentamicin LB plates were screened for the expected construct by PCR and restriction digest to yield pOKOSPA-C′.
Transformation of B. burgdorferi.
A single colony isolate of B. burgdorferi B31-A3 (23), designated the wild type (WT), was prepared for electroporation as described previously (53, 57), transformed with 25 μg of pOKOSPAKO and plated on Barbour-Stoenner-Kelly (BSK) agarose containing 100 μg/ml kanamycin. Twelve kanamycin-resistant borrelia colonies were recovered after 7 to 10 days of growth for expansion in liquid BSK II medium (2, 33, 62) containing the same concentration of kanamycin. PCR amplification of DNA from the resistant clones with primers flanking the ospAB operon (Table 1) gave 2.8-kb products, as expected from the excision of 324 bp from ospA and the insertion of the flaBp-kan cassette, while the untransformed WT gave a 1.9-kb product. All of the clones showed the expected product size of 857 bp when amplified with the kan gene primers KANA.5 and KANA.3 (Table 1). Five of twelve clones contained the WT complement of plasmids, detected with 29 plasmid-specific primer pairs as described previously (23); one, designated the ospA1 clone, was chosen for use in further work.
To restore OspA production, ospA1 was transformed with 30 μg of pOKOSPA-C′ (as described above) and plated on BSK medium containing 10 μg/ml gentamicin. Twelve colonies were expanded in liquid medium with gentamicin and screened by PCR with OSP1A and OSP2 to identify the ospAB locus products with the expected size of 2.48 kb and with KANA.5 and KANA.3 to test for the loss of the flaBp-kan cassette from ospAB; five of these products were then screened with strain B31 plasmid-specific PCR primer pairs, and two of them retained the full plasmid complement; one of these products was designated ospA+B1. Passages of the WT and mutant clones used for experiments were monitored for plasmid content by PCR with plasmid-specific primer pairs (23).
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblot analysis.
Lysates of low-passage, uncloned MI B31 and B31-A3 WT and the ospA1 and ospA+B1 clones, grown at 35°C to stationary phase were prepared as described previously (58), except that 50 mM dithiothreitol (Boehringer Mannheim, Indianapolis, IN) was substituted for 2-mercaptoethanol as the reducing agent in lysates prepared for analysis of the OspA protein (5). Lysates were subjected to SDS-PAGE (12.5% separating gel; acrylamide/bisacrylamide, 30:0.8) (37). Proteins in replicate gel panels were stained with Coomassie brilliant blue R-250 (Bio-Rad, Hercules, CA) or transferred to nitrocellulose membranes (64) as described previously. Membranes were blocked and reacted with monoclonal antibodies H5332 (anti-OspA) (5), H6831 (anti-OspB) (4), H4610 (anti-OspB) (54), mouse sera, and rabbit polyclonal J5750 (anti-B. hermsii FlaB; C. Guyard M. Schrumpf, and T. Schwan, unpublished data). Bound antibodies were reacted with horseradish peroxidase-conjugated protein A (Zymed, San Francisco, CA) and detected by enhanced chemiluminescence, as recommended by the manufacturer (Amersham ECL Western blotting detection reagents; GE Healthcare, Buckinghamshire, United Kingdom), using Hyperfilm ECL (Amersham).
Borrelial survival in ticks feeding on infected mice.
Rocky Mountain Laboratories (RML) mice, an outbred strain of Swiss-Webster mice, were injected intradermally with the WT, ospA1, and ospA+B1 clones at inoculating doses as described for individual experiments. C57BL/6J (B6) and B6.129S2-Igh-6tm1Cgn/J (B6.Igh−/−) (34) mice obtained from JAX (Bar Harbor, ME) were injected intradermally with 104 to 105 borreliae of the WT, ospA1, and ospA+B1 clones. Mice were tested at 3 weeks postinjection by immunofluorescence assay (IFA), as described previously (50), to test for seroconversion. Healthy Ixodes scapularis larvae (Tick Rearing Facility, Oklahoma State University, Stillwater, OK) were fed on these mice. At the times indicated for each experiment, tick midguts were macerated with forceps against the wells of Teflon-coated slides in phosphate-buffered saline, homogenized by pumping with a micropipette prior to drying and fixation, and reacted for IFA with rabbit anti-B. burgdorferi and fluorescein-labeled goat anti-rabbit immunoglobulin G (IgG) (KPL, Gaithersburg, MD) as described previously (50). To reduce fluorescence interference from blood, 0.1 volume of homogenate was aliquoted to a separate well before IFA. Molted, unfed nymphs were also analyzed by IFA. Statistical analyses of enumerated borreliae were performed with GraphPad Prism software, version 4.0c for Macintosh (GraphPad Software, San Diego, CA). The RML animal facility is accredited by the International Association for Assessment and Accreditation of Laboratory Animal Care. Experiments using animals were carried out using protocols approved by the RML animal care and use committee, prepared according to National Institutes of Health guidelines.
In vitro serum killing assay.
Based on a 24-h killing assay (8), but utilizing BSK II medium rather than glucose-supplemented phosphate-buffered saline as the assay medium, the WT, ospA1, and ospA+B1 clones grown to late exponential stage were adjusted to 2 × 106 to 5 × 106 borreliae per ml of BSK II medium. Duplicate 100-μl aliquots of each culture were mixed with 100 μl of BSK II, BSK II containing naïve mouse serum, or BSK II containing immune mouse serum and all containing an antibiotic mixture for borrelia (Sigma-Aldrich). Mouse sera pooled from three naïve and three WT-infected RML mice (8 weeks postinfection) were added to BSK II at a dilution of 1 part per 7 parts of medium (a final serum titer of 1:16) and filtered through a 0.22-μm syringe (Millex-GS; Millipore Corp., New Bedford, MA) before the addition to borreliae. Sealed tubes were incubated for 24 h, and aliquots of each culture were plated in BSK II agarose and counted after 10 days of incubation at 35°C in 2.5% CO2 to determine CFU.
Artificial infection of ticks and feeding on mice.
I. scapularis larvae were infected with the WT, ospA1, and ospA+B1 clones by 1.5-h immersion in culture suspensions of 1 × 107 to 3 × 107 borreliae per ml (50), with the following modification. Larvae were preexposed to a relative humidity of 80.5% maintained by a saturated solution of (NH4)2SO4 (68) immediately before immersion to stimulate rehydration behavior (35, 55). Exposure to 80.5% relative humidity was adjusted to 4 days for 1- to 2-month-old larvae, 3 days for 3- to 4-month-old larvae, and 1 day for 5- to 6-month-old larvae (larvae of >6 months postemergence were not used for immersion). Calibrated dehydration reproducibly increased the percentage of infected larvae (data not shown). At the times pre- and postfeeding as indicated in each experiment, infected tick midguts were examined by IFA as described above.
Borrelial survival in infected larvae fed on immune versus naïve mice.
“Immune” RML mice were first infected by tick bite using I. scapularis larvae artificially infected with the WT clone; “naïve” control mice were infested with noninfected larvae. At 4 weeks postfeeding, mice were tested by IFA for reactivity to B31. Both the immune and the naïve groups were then treated with ceftriaxone (50 mg/kg of body weight/dose; Sigma-Aldrich) intramuscularly for 5 days (41, 48) and tested for transmissible borrelia at 1 week posttreatment by xenodiagnosis (feeding of healthy ticks and examining midguts by IFA) to verify clearance of borrelial infection. Groups of tick larvae infected by immersion with the WT, ospA1, and ospA+B1 clones were then applied to immune and naïve mice (approximately 90 days after the initial infection). Dissected midguts of larvae were examined at 7 days postrepletion and after molt by IFA for the enumeration of borreliae.
Detection of mouse infection.
Mice that were fed upon by larval and nymphal stage ticks were held for 3 to 4 weeks before IFA to determine their exposure to B31 as described previously (50). Mice were sacrificed, and bladder and joint tissues were cultured at 35°C in BSK II medium containing an antibiotic mixture for borrelia (Sigma-Aldrich) to control potential contaminating bacteria. Cultures containing borreliae detected by dark-field microscopy at 2 weeks postinoculation were extracted for DNA (DNeasy; Qiagen). DNA was amplified by PCR (Roche Corp., Indianapolis, IN) with primers OSP1A and OSP2 (Table 1), and products were analyzed by agarose gel electrophoresis to identify the size of the ospAB locus. To detect levels of borrelial DNA in infected tissue, DNA from mouse ears was extracted (DNeasy) and further purified by Qiaquick PCR purification (Qiagen). Aliquots of 100 ng were analyzed in triplicate for B. burgdorferi flaB DNA, using the primers flaB470F and flaB547R and the probe flaB523TR (Table 1), designed with ABI Primer Express software, version 2.0 (Applied Biosystems, Foster City, CA), by real-time quantitative PCR with an ABI Prism 7900HT sequence detection system (Applied Biosystems), using Brilliant II QPCR Master Mix (Stratagene, La Jolla, CA), as recommended by the manufacturer. DNA from strain B31 MI served as the standard for quantification of the flaB copies.
RESULTS
Inactivation and reconstitution of ospA.
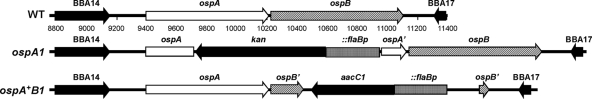
A segment of lp54 containing the ospAB locus from B. burgdorferi strain B31 cloned in a shuttle vector (pOKLP54) was modified by the placement of a flaBP-kan antibiotic resistance marker within an internally deleted ospA coding region. The resultant construct, pOKOSPAKO, was used to transform a clonal derivative of the B31-A3 WT. Five kanamycin-resistant transformants retained all plasmids found in the WT clone. The sequencing (data not shown) of one clone, 2E6, at the ospAB locus was compatible with a double crossover insertion of the flaBP-kan element from the transforming vector DNA into the ospA ORF (Fig. 1) located on the 54-kb linear plasmid (3). This is the ospA1 clone used in all experiments.
FIG. 1.
Physical maps of the lp54 ospAB locus in the B31-A3 WT, the ospA mutant, and the ospA restored strains. WT, segment of lp54 from bp 8750 to 11400, as indicated by numbers below the map (27), from locus tag BBA14 to BBA17 in clonal isolate A3-6. ospA1, mutation of ospA by insertion of a flaBP-kan marker (10) inserted in an internally deleted ospA gene via transformation of A3-6 with pOKOSPAKO (described in Materials and Methods), resulting in clone 2E6. ospA+B1, restoration of the ospA mutation by insertion of a flaBP-aacC1 marker (22) in an internally deleted ospB gene on the 3′ end of an intact ospA gene via transformation of 2E6 with pOKOSPA-C′ (described in Materials and Methods), resulting in clone 7A.
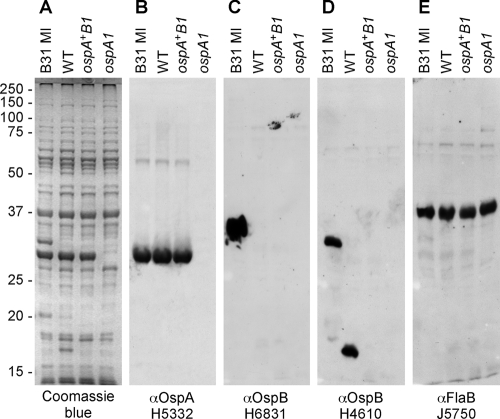
B31-A3 is a clone derived from strain B31 MI with a uniform extrachromosomal DNA profile that is competent for transformation, infectious when injected in mice, acquired by I. scapularis larvae from mice, and transmitted to mice by nymphal ticks (23, 29). Although it is designated WT for comparison to the ospAB mutants described here, it produces a full-length OspA protein and a truncated OspB protein (23). To identify ospAB products in the WT and the ospA1 mutant, we examined borrelial lysates by SDS-PAGE and immunoblotting (Fig. 2). B31 MI produced full-length OspA and -B proteins, the WT clone contained full-length OspA and truncated OspB, and the ospA1 mutant lacked both OspA and OspB. Both the full-length and truncated forms of OspB (Fig. 2D) are recognized by monoclonal antibody H4610 (54). The flaBP-kan insertion in the ospA ORF eliminates both OspA production and truncated OspB production, the latter by apparent polar effect on the ospB gene. As a control, FlaB presence was examined and found at equivalent levels in all three extracts (Fig. 2E).
FIG. 2.
Protein profiles of strain B31 MI and the WT and isogenic ospAB clones; detection of OspA, OspB, and FlaB. (A) Coomassie blue gel shows whole-cell lysates from 35°C cultures of B31 MI, WT, ospA+B1, and ospA1 strains separated by SDS-PAGE and stained with Coomassie blue. (B to E) Immunoblots of replicate SDS-PAGE separations reacted as described in Materials and Methods with OspA-specific monoclonal antibody H5332 (B), OspB-specific monoclonal antibody H6831 (C), OspB-specific monoclonal antibody H4610 (D), and FlaB-specific rabbit polyclonal antiserum J5750 (E). Migration of molecular mass standards in kDa is indicated at left. αOspA, anti-OspA.
An isogenic ospA restoration strain was constructed in ospA1 by a similar allelic exchange strategy. A flaBp-aacC1 resistance marker was inserted within ospB in pOKLP54 to create pOKOSPA-C′, which was electroporated into ospA1. The resultant gentamicin-resistant clones were screened by PCR analysis of the ospAB locus to identify those transformants that contained a single product of the expected size. Sequence analysis (data not shown) of clone 7A verified that an intact ospA gene, in tandem with an internally deleted ospB gene bearing the flaBp-aacC1 marker, had inserted in lp54 at the ospAB locus (Fig. 1). Immunoblot analysis of this clone (Fig. 2B) showed OspA production, and immunofluorescent staining of live borreliae (data not shown) verified that OspA was exposed on the borrelia surface; no OspB was detected by immunoblotting analysis (Fig. 2C and D). Clone 7A is the ospA+B1 clone in all experiments.
The tick vector acquires OspA-deficient borreliae from infected mice.
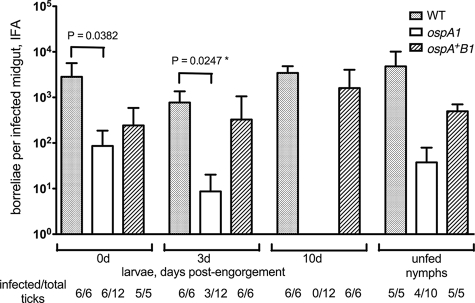
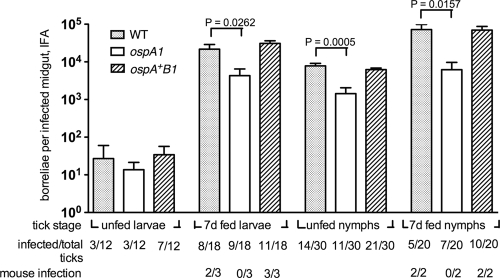
A previous study indicated that borreliae lacking OspA are not stably acquired by I. scapularis nymphs when they feed upon mice infected with an ospAB mutant of B. burgdorferi strain 297 or when ticks are inoculated via the rectal aperture with this mutant (69). We tested the ability of the B31-derived ospA1 strain to establish infection in mice and in I. scapularis larvae. Two mice per group were injected intradermally with 5 × 103 borreliae of the WT, ospA1, and ospA+B1 clones. At 3 weeks postinjection, we observed seroconversion in one WT-injected mouse, in both ospA1-injected mice, and in one ospA+B1-injected mouse (data not shown). Larvae fed on seropositive mice were dissected for IFA at 0, 3, and 10 days postrepletion. IFA is routinely used as an appropriate and accurate method for the evaluation of individual tick midguts for borrelial density (17, 20, 42, 44, 46, 47, 59, 69). As a test of viability of acquired borreliae, larvae fed on mice infected with the three ospA genotypes were homogenized and plated in BSK-agarose at day 0 postrepletion; all cultures gave rise to borrelia colonies. Larvae fed on mice infected with the ospA1 and ospA+B1 borreliae showed significantly lower borrelial densities than the larvae fed on mice infected with the WT at day 0, but at day 3 only the ospA1 group was significantly different from the WT (Fig. 3). At day 10 postrepletion, we found no borreliae in 12 midguts examined from the two ospA mutant-infected mouse feedings; the ospA+B1 clone was not significantly different from the WT at day 10. The absence of detectable ospA1 borreliae at day 10 appears to reflect a limit of detection by the IFA, because molted, unfed nymphs from all three groups showed the presence of midgut borreliae, including 4 of 10 midguts examined for ospA1. The densities of ospA1 and ospA+B1 borreliae infections in unfed nymphs were not statistically different from that of the WT. Acquisition of the mutant was not abolished by the deficiency of OspA and OspB, in contrast to results with the strain 297 ospAB mutant (69). The WT clone, producing a truncated OspB protein, and the ospA+B1 clone, lacking OspB, were both capable of establishing tick midgut infections that showed no population decline during the 10-day postengorgement period and persisted transstadially, in contrast to results with another strain B31 ospB mutant (42).
FIG. 3.
Acquisition of the WT and ospAB clones from infected mice. RML mice were injected intradermally with 5 × 103 bacteria of the WT, ospA1, and ospA+B1 clones, as designated, and normal I. scapularis larvae were allowed to feed on seropositive mice at 3 weeks postinjection. Borreliae in dissected midguts from each group were enumerated by IFA at 0, 3, and 10 days postengorgement and after they molted to nymphs (unfed nymphs); values represent means plus standard deviations (error bars) for infected midguts. Infection frequency (no. of infected/total ticks) of assayed tick groups is shown. The ospA1 and ospA+B1 groups were subjected to a t test comparison with the WT group at each time point; a significant t test P value is shown above bracket. *, the value for the WT and ospA1 clones at 3 days postengorgement was significant (P = 0.0247) compared by an unpaired t test with Welch's correction.
Density of mouse infection and immune response to the OspA-deficient clone.
Since the lack of OspA impaired but did not abolish acquisition of borreliae by tick larvae, we asked if aspects of mouse infection by the mutant, including relative levels of borreliae in mouse tissue and the quality of the humoral immune response to infection, might influence borrelial density upon vector acquisition. We repeated the injection of mice with the WT and ospA1 isolates, at an inoculating dose of 5 × 104 borreliae and observed seroconversion for all three animals in each group at 3 weeks postinjection. At 8 weeks postinfection, animals were sacrificed, and sera and tissue were recovered. All animals were culture positive. We assayed mouse ear DNA for B. burgdorferi flaB DNA copies by quantitative PCR (Table 2) to avoid variable efficiency in the recovery of viable borreliae from tissue samples. Values for flaB were significantly different between animals within the WT group and those within the ospA1 groups, but there were no statistically significant differences between the WT and ospA1 groups.
TABLE 2.
Copies of flaB DNA in mouse tissue
| Clone | Mouse | Mean no. ± SD of copies of flaB/100 ng DNAa |
|---|---|---|
| WT | 1 | 107.1 ± 15.5 |
| 2 | 235.7 ± 12.0 | |
| 3 | 4.9 ± 2.1 | |
| ospA1 | 1 | 9.9 ± 3.0 |
| 2 | 265.4 ± 39.6 | |
| 3 | 12.1 ± 4.8 |
Quantitative PCR was performed with triplicate 100-ng aliquots of mouse ear DNA for each sample to determine the mean number of copies of flaB DNA ± standard deviation (SD) relative to that of the B31 MI DNA standard, as described in Materials and Methods.
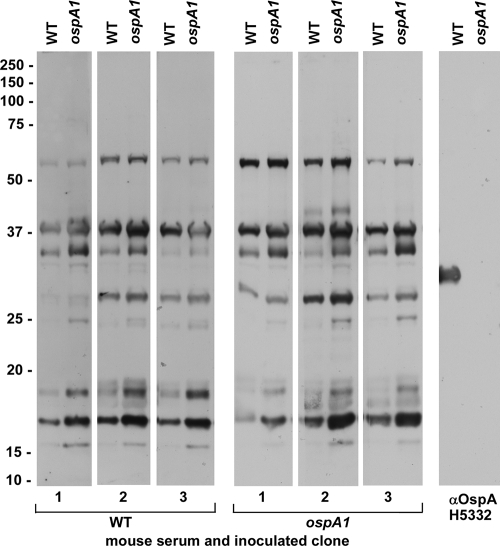
To compare the patterns of borrelial antigen recognition between mice infected with WT and those infected with the ospA1 clones, we reacted immunoblots of the WT and the ospA1 lysates with each serum sample (Fig. 4). With minor differences between the intensities of reactions, the two borrelial clones produced antibody responses in mice that resulted in recognition of similar antigens in the WT and ospA1 lysates. Recognition of OspA, as defined here and in Fig. 2 by reactivity to monoclonal antibody H5332, is absent in the mouse serum samples. Thus, similar antigens may be targeted for humoral immune recognition when ticks acquire borreliae from a blood meal, whether the mouse infection involves OspA-producing or OspA-deficient borreliae.
FIG. 4.
Recognition of B. burgdorferi antigens by sera from infected mice. RML mice were injected intradermally with 5 × 104 bacteria of the WT or ospA1 clone, as designated, and serum was recovered from blood collected 8 weeks postinjection. These infected mouse sera (1:200 dilution) and OspA-specific monoclonal antibody H5332 (1:500 dilution) were reacted with immunoblots of the WT and ospA1 clones as described in Materials and Methods. Migration of molecular mass standards in kDa is indicated at left. αOspA, anti-OspA.
OspA-deficient borreliae persist through larval and nymphal feeding on naïve mice.
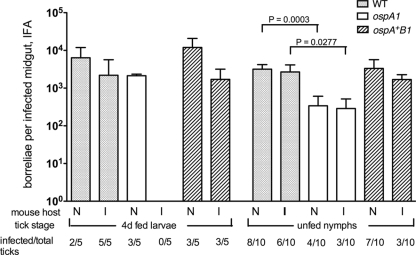
Since the ospA1 mutant was less effective than the controls at colonizing larvae when acquired from infected mice, we compared colonization and replication of the ospA isogenic clones in ticks by infecting larvae before blood meal exposure. We used calibrated dehydration of larvae (see Materials and Methods) to enhance the frequency of artificial infection by immersion. The ospA1 mutant was present in larval midgut samples before and after feeding on naïve mice, as were the WT and ospA+B1 clones (Fig. 5). There were no significant differences in relative densities between clones in unfed larvae, suggesting an ability of the ospA1 mutant to colonize the midgut in the absence of the blood meal. After the uptake of blood, the ospA1 colony density was reduced relative to that of the WT in 7-day fed larvae, while the ospA+B1 restored clone density showed no significant difference from that of the WT. Importantly, the blood meal from these naïve mice resulted in an average density of 4.3 × 103 ospA1 borreliae per infected midgut at 7 days postengorgement, whereas the same clone was not detectable in 10-day-engorged larvae acquired from infected mice (Fig. 3). The densities of midgut infections at 7 to 10 days postengorgement for the WT and ospA+B1 clones were similar, whether infection arose from the blood meal (Fig. 3) or from preliminary inoculation by immersion (Fig. 5). The results of these two experiments suggest that the density of midgut infection by the ospA1 mutant is influenced by the immune status of the mouse providing the blood meal. The ospA1 mutant replicates in larval midguts when exposed to blood from a naïve mouse but may not achieve the same midgut density as controls because of a natural antibody toward other borrelial antigens (8), a lack of plasminogen binding (16), or reduced midgut adhesion (46). Transmission of these clones to the naïve mice was examined by IFA and culture of mouse tissue. The WT clone infected two of three mice, the ospA1 clone infected none of three mice, and the ospA+B1 clone infected all three mice. These values were not subjected to statistical analysis but suggest that the ospA1 clone may not be transmitted from infected larvae.
FIG. 5.
Replication of the WT and ospAB borreliae in artificially infected larvae fed on naïve mice. I. scapularis larvae infected by immersion in the WT, ospA1, and ospA+B1 clones as indicated were fed on RML mice at larval and nymphal stages. Borrelial density in infected midguts from larvae and nymphs before feeding or at 7 days postengorgement was enumerated by IFA; values represent means plus standard deviations (error bars) for infected midguts. The infection frequency of assayed tick groups is shown as in Fig. 3. The transmission frequency of borreliae to mice (mouse infection) was determined as described in Materials and Methods. Ticks infected with the ospA1 and ospA+B1 clones were subjected to t test comparison with the WT at each time point; significant t test P values are shown above the brackets.
After they molted to the nymphal stage, WT, ospA1, and ospA+B1 clone-infected nymphs were tested by IFA to determine midgut borrelial densities before feeding (Fig. 5). Unfed nymphs infected with the ospA1 clone had significantly lower midgut borrelial densities than nymphs infected with the WT. After nymphs fed on naïve mice, the density of borreliae increased, but the ospA1 clone density was, again, lower than that of the WT. Each pair of mice in the WT and ospA+B1 clone-infected groups gave serological and cultural evidence of infection, but neither of the mice fed upon by nymphs infected with the ospA1 clone became infected. Therefore, the ospA1 mutant survived transstadially from larvae to nymphs and survived the nymphal blood meal but may not be competent to infect mice during nymphal feeding. Truncation of OspB in the WT and its absence in the ospA+B1 reconstituted clone did not prevent tick colonization or transmission to mice.
Serum killing of the OspA-deficient clone in vitro.
Since the ospA1 clone was found in larval midguts at a lower density than the WT after engorgement on infected mice (Fig. 3) but replicated in larvae after a naïve blood meal (Fig. 5), we compared the WT, ospA1, and ospA+B1 clones in vitro for their abilities to survive exposure to serum from naïve mice and mice infected with the WT borreliae, independent of the larval midgut environment. The WT-immune sera were identical to those collected and tested by immunoblotting in Fig. 4, as described above. We assayed viability in serum that was not heat treated to allow antibody interaction with complement activity in killing of borreliae. Incubation of the WT in BSK II supplemented with naïve or immune serum resulted in statistically significant increases in density relative to that of incubation in BSK II alone (Fig. 6). The OspA-deficient ospA1 clone also showed a significant increase in density in naïve serum but a significant decrease in immune serum; it declined 10-fold in viable count relative to that of naïve serum. The ospA+B1 clone showed enhanced survival in untreated naïve serum compared to that in BSK II alone, but immune serum did not significantly affect its growth. This test indicates that OspA deficiency can alter the viability of in vitro-grown borreliae upon exposure to mouse serum, consistent with previous results of serum sensitivity of borreliae lacking Osps (12, 56). The BSK II medium used in this assay allow continued growth of borreliae, which may obscure natural antibody killing, which has been detected in medium that does not support borrelial growth (8).
FIG. 6.
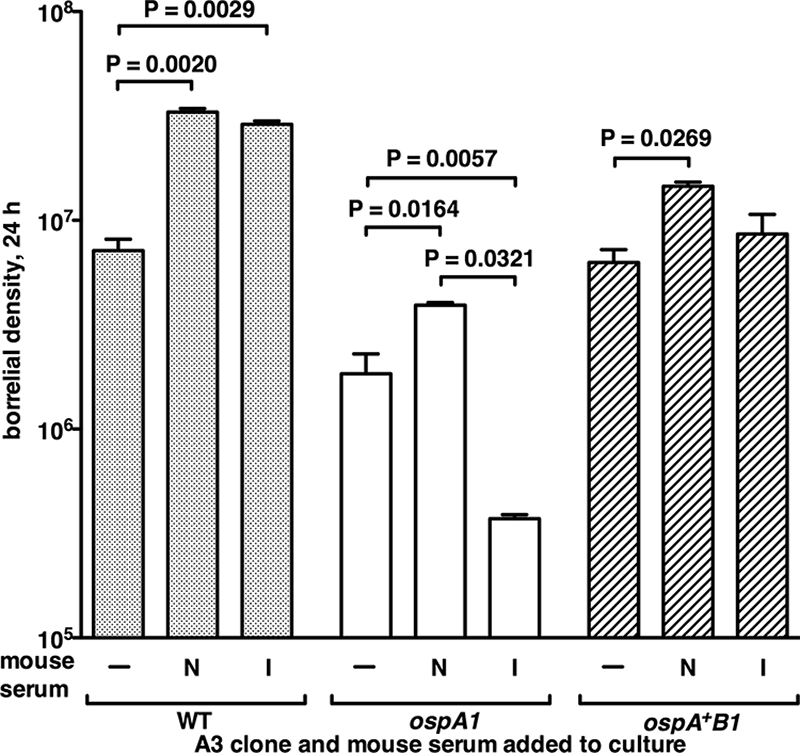
Survival of the OspA-deficient and control clones with mouse serum in vitro. The WT, ospA1, and ospA+B1 clones grown in BSK II (−) or BSK II containing naïve (N) or borrelia-infected (I) mouse sera, as described in Materials and Methods. Values are mean borrelial densities plus standard deviations (error bars) per ml, from colony counts of dilutions after 24 h of incubation (duplicate incubations for each condition). One-way analysis of variance posttest values for significant differences between conditions are shown above the brackets.
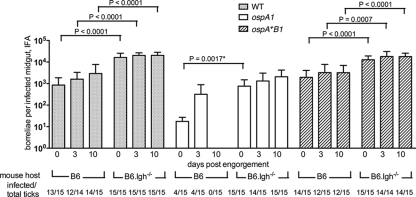
OspA-deficient borreliae are depleted in ticks after feeding on an immune mouse.
We wanted to determine whether the ospA1 mutant, in preinfected ticks, was selectively cleared from the midgut by a blood meal from an immune mouse. Such clearance could be inferred from the results shown in Fig. 3 and 5, but a more direct comparison of the effects of immune and naïve blood on resident borreliae was needed. Mice in an “immune” group were infected with WT borreliae and cured by antibiotic treatment, as verified by xenodiagnosis (see Materials and Methods). “Naïve” host mice were fed upon by uninfected larvae but also received antibiotic and xenodiagnosis. Test larvae were artificially infected with the WT, ospA1, and ospA+B1 clones, as in the previous experiment, and divided into groups fed upon the immune and naïve mice. At day 4 postengorgement, the densities of borreliae in ticks infected with the WT and ospA+B1 clones were not significantly different between the larvae fed on immune mice and those fed on naïve mice (Fig. 7). The density of borreliae in ticks infected with the ospA1 clone 4 days after naïve blood intake was not significantly different from the WT density. After immune blood meal uptake, however, there were no ospA1 borreliae detected in larval midguts at day 4. After molting to nymphs, ticks from these groups were examined by IFA, at which time the ospA1 mutant borreliae densities detected in ticks that had fed on naïve mice were similar to those that fed on immune mice at the larval stage, indicating they were not eliminated by the immune blood and were capable of replicating to comparable levels. These levels were still significantly reduced, relative to those of the WT borreliae, indicating an effect of OspA deficiency independent of the blood meal source. This experiment showed a significant if transient effect of an immune blood meal on the ospA1 clone and corroborates earlier findings that borreliae producing OspA show significant resistance to acquired immunity to other surface antigens, both in vitro (56) and in vivo (18).
FIG. 7.
Effect of acquired immunity to B. burgdorferi on the survival of OspA-deficient borreliae. Naïve, mock-infected RML mice (N) and immune mice (I) infected via larval tick bite with the A3-6 wild type, identified as seropositive and cured by ceftriaxone treatment, were used as hosts for feeding by larvae artificially infected with the WT, ospA1, and ospA+B1 clones. Borrelial densities in infected midguts from larvae 4 days postengorgement and in molted, unfed nymphs were enumerated by IFA; values represent means plus standard deviations (error bars) for infected midguts. The infection frequencies of assayed tick groups are shown as in Fig. 3. Student's t test values for significant differences between naïve- and immune-fed ticks at each tick stage are shown above the brackets.
The ospA1 mutant proliferates in larvae after acquisition from infected B-cell-deficient mice.
Since survival of the ospA1 mutant in larvae was diminished by specific immunity in the mouse host, we asked whether intake of borreliae from infected mice lacking humoral immunity would show enhanced ospA1 survival relative to that of immunocompetent mice. C57BL/6J (B6) control mice and B6.129S2-Igh-6tm1Cgn/J (B6.Igh−/−) B-cell-deficient congenic mice were injected intradermally with the WT, ospA1, and ospA+B1 borrelial clones. When mice were tested at 3 weeks postinjection, all B6 mice had seroconverted. B6.Igh−/− mice were not tested for serological response, but all mice gave evidence of infection upon culture for borreliae after tick feed; a single B6.Igh−/− mouse injected with the ospA1 clone died before culture but was positive when tested for infection by xenodiagnosis. Uninfected larvae were fed on these infected mice, and midguts were examined by IFA at 0, 3, and 10 days postengorgement for density of borrelial infection. The ospA1 clone was initially detected (at 0 and 3 days) in larval midguts after ticks fed on infected B6 mice (Fig. 8), but they were not observed at 10 days postengorgement, as previously observed with acquisition from RML mice (Fig. 3). After feeding on B6.Igh−/− mice, the ospA1 clone was detected at day 0 and remained at an elevated density up to 10 days postengorgement. It survived larval acquisition from B6.Igh−/− mice at a level significantly greater than the same clone acquired from B6 mice, assessed at day 0 postengorgement. Densities were not significantly different at day 3 postengorgement. The lack of any sign of infection in larvae 10 days after feeding on B6 mice did not allow statistical comparison but clearly demonstrated a difference in outcome relative to the ospA1 density in larvae feeding on B6.Igh−/− mice. We observed significant increases at 0, 3, and 10 days postengorgement in the WT and ospA+B1 borreliae densities in ticks fed on B6.Igh−/− mice compared to that of ticks fed on B6 mice, likely an effect of antibody on survival of borreliae that are competent for OspA production.
FIG. 8.
Relative survival of OspA-deficient borreliae acquired from intact and B-cell-deficient infected mice. C57BL/6J (B6) and C57BL/6J Igh−/− (B6.Igh−/−) congenic mice were injected with the WT, ospA1, and ospA+B1 clones, and B6 mice were tested at 3 weeks for serological response; normal I. scapularis larvae were fed at 4 to 5 weeks postinfection. Borrelial density in infected midguts from larvae at 0, 3, and 10 days postengorgement was enumerated by IFA; values represent means plus standard deviations (error bars) for infected midguts. The infection frequencies of assayed tick groups are shown as in Fig. 3. Statistically significant t test values of borrelial midgut density for ticks fed on B6 mice relative to ticks fed on the B6.Igh−/− mouse host genotype at each time point are shown above the brackets; *, the value for the ospA1 clone at day 0 postengorgement, with B6 versus B6.Igh−/− mice, was significant (P = 0.0017) compared by an unpaired t test with Welch's correction.
DISCUSSION
We created an ospA mutant from strain B31 to examine the relevance of OspA lipoprotein to borrelial colonization of the tick vector. The mutant declined to undetectable levels in the larval midgut when acquired from an immunocompetent mouse host (Fig. 3). However, when it was introduced by artificial infection by immersion, followed by larval engorgement on naïve mice, the ospA1 mutant proliferated in larval midguts (Fig. 5). The relative survival of the ospA1 mutant upon ingestion of naïve versus that on immune mouse blood is similar to the effects of naïve and immune serum on the ospA1 mutant in vitro (Fig. 6) and suggests that mouse humoral immunity, in the absence of OspA, recognized borrelial antigens that are conserved between in vitro and in vivo (tick midgut) conditions. The data for the ospA1 mutant midgut survival in naïve versus immune blood led us to propose that a role for OspA, which is not typically produced in the mammalian host and thus not recognized by immune serum, is to shield borreliae as they enter the midgut from specific antibodies in the mouse blood meal that recognize conserved borrelial proteins. We tested this hypothesis with immune mice that were cured of borrelial infection before feeding infected larvae (Fig. 7) and with normal larvae fed on infected mice that were deficient in humoral immune response (Fig. 8); the results of these experiments are consistent with the hypothesis that OspA provides an immunity-shielding function. The findings extend from previous observations with a strain B31 variant that lacks expression of OspA, -B, -C, and -D (12, 56). Those authors demonstrated in vitro growth inhibition of the mutant compared to that of the parental B31 by monoclonal antibodies against surface proteins P13 and P66, respectively, and proposed that OspA may act in vivo to shield borreliae from immunoglobulin recognition of these integral outer membrane proteins. Since OspA-deficient borreliae survived the host-vector transition at a significantly higher rate in the absence of the B-cell response, we conclude that borrelial antigens recognized by an intact immune system are shielded from antibody recognition in the tick vector by the presence of OspA. Antigens conserved between the vector and host stages of the borrelial infectious cycle were shielded from recognition in the WT and ospA+B1 strains because of the abundance of OspA on the surface of these clones. Additionally, OspA interaction with midgut receptors (46) or plasminogen (16, 28) may indirectly shield borreliae from exposure to antibodies in the blood meal. The midgut density of the ospA1 clone in the absence of mouse host B-cell function (Fig. 8) is particularly relevant to the separate function of OspA as an adhesin or a plasminogen binding factor; the mutant reached a density that was only a fraction of that of the control clones and therefore is still subject to growth restriction in the midgut.
The data are in agreement with a protective role for OspA against the acquired immunity of hosts repeatedly fed upon by infected ticks (8, 13). When I. scapularis larvae feed on a host infected with B. burgdorferi, borreliae from the epithelium mix with blood components as they enter the tick. In areas where Lyme disease is endemic, the majority of the reservoir host population has developed humoral immunity to borreliae infection (13, 40). The influx of antibody in the blood meal represents a deterrent to multiplication and transmission for any borreliae that do not shield constitutively produced surface antigens. Antibody shielding occurs in other bacterial pathogens in the context of serum resistance (25) but rarely seems to involve protein as the block to antigen recognition. I. scapularis anticomplement factor (65) may act to inhibit complement function in the tick midgut, but in vitro killing (Fig. 6) and in vivo midgut reduction in the density of the OspA-deficient clone (Fig. 3 and 8) may depend in part on complement activity.
Our findings for OspA-deficient borrelial survival in the vector are not consistent with previous research indicating that an OspA- and B-deficient strain 297 derivative does not colonize tick midgut (69). In that report, nymphs fed on infected mice and nymphs infected by rectal microinjection and fed on naïve mice did not show midgut survival of the ospAB mutant. The difference between the fate of OspA-deficient borreliae in that study and the present work may result from the mode of tick infection. The rectal microinjection method (51) was reported to result in significant attenuation of B. burgdorferi strain JD-1 tick infection compared to mouse-acquired infection. Delivery of borreliae to the rectal sac may not provide adequate signals for borrelial adaptation to the midgut environment. Borreliae appear to enter the midgut via the oral cavity during infection by immersion, as they are observed in the midgut directly after this exposure (29, 50). A more recent report indicates that the strain B31 ospB insertion mutant, which retains OspA production, is also defective in colonization of the tick midgut when borreliae are introduced via rectal injection (42). As in previous experiments (23, 29), we found that the truncated form of OspB in the B31-A3 WT clone did not prevent host transmission or vector acquisition. The absence of OspB in the ospA+B1 clone likewise did not prevent the transmission-acquisition cycle. We propose that this inconsistency between the ospB mutant phenotypes may also stem from differential survival between the rectal and oral modes of tick infection. Importantly, our measurement of midgut colonization differs from those of the studies by Yang et al. (69) and Neelakanta et al. (42); these authors evaluated borrelial density after washing dissected midguts, whereas we evaluated the entire midgut borrelial population after dispersion, as have previous studies (17, 20, 44, 47, 59).
The phenotype of the ospA1 mutant in relation to tick midgut epithelial cells and to components of the blood meal may result in a significant decrease in transmission to the mouse host at the nymphal feeding stage. Our data for feeding by the ospA1 mutant-infected nymphs (Fig. 5) provide insufficient sample numbers for a statistically valid test of this potential defect. However, the data presented here for the effects of humoral immunity on vector acquisition of borreliae provide a basis for future studies, including localization of the ospA1 mutant in the tick and the effects of OspA deficiency on the induction of borrelial RpoN-RpoS-dependent gene transcription, which is essential to transmission (26). The ability of the OspA-deficient strain to survive tick uptake of a naïve mouse blood meal provided a demonstration of the critical role for OspA as a shield for constitutively expressed surface components during the uptake of immune blood. It may also be possible to demonstrate by mutagenesis of the putative binding site at the C-terminal portion of OspA (39) that the adhesin function of OspA can be dissociated from the antibody-shielding function.
Acknowledgments
We thank Cyril Guyard and Kathy Wehrly for antibodies; Anita Mora and Gary Hettrick for assistance with illustrations; and Kena Swanson, Chris Bosio, and Phil Stewart for valuable comments on the manuscript.
We acknowledge the construction of an initial series of ospAB mutants in high-passage, noninfectious strain B31 by Aimee Geissler.
The Intramural Research Program of the NIAID, NIH, supported this research.
Editor: R. P. Morrison
Footnotes
Published ahead of print on 8 September 2008.
REFERENCES
- 1.Anderson, J. F., R. C. Johnson, L. A. Magnarelli, and F. W. Hyde. 1985. Identification of endemic foci of Lyme disease: isolation of Borrelia burgdorferi from feral rodents and ticks (Dermacentor variabilis). J. Clin. Microbiol. 2236-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbour, A. G. 1984. Isolation and cultivation of Lyme disease spirochetes. Yale J. Biol. Med. 57521-525. [PMC free article] [PubMed] [Google Scholar]
- 3.Barbour, A. G., and C. F. Garon. 1987. Linear plasmids of the bacterium Borrelia burgdorferi have covalently closed ends. Science 237409-411. [DOI] [PubMed] [Google Scholar]
- 4.Barbour, A. G., S. L. Tessier, and S. F. Hayes. 1984. Variation in a major surface protein of Lyme disease spirochetes. Infect. Immun. 4594-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbour, A. G., S. L. Tessier, and W. J. Todd. 1983. Lyme disease spirochetes and ixodid tick spirochetes share a common surface antigenic determinant defined by a monoclonal antibody. Infect. Immun. 41795-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barthold, S. W., E. Fikrig, L. K. Bockenstedt, and D. H. Persing. 1995. Circumvention of outer surface protein A immunity by host-adapted Borrelia burgdorferi. Infect. Immun. 632255-2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Becker, M., J. Bunikis, B. D. Lade, J. J. Dunn, A. G. Barbour, and C. L. Lawson. 2005. Structural investigation of Borrelia burgdorferi OspB, a bactericidal Fab target. J. Biol. Chem. 28017363-17370. [DOI] [PubMed] [Google Scholar]
- 8.Belperron, A. A., and L. K. Bockenstedt. 2001. Natural antibody affects survival of the spirochete Borrelia burgdorferi within feeding ticks. Infect. Immun. 696456-6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergström, S., V. G. Bundoc, and A. G. Barbour. 1989. Molecular analysis of linear plasmid-encoded major surface proteins, OspA and OspB, of the Lyme disease spirochete Borrelia burgdorferi. Mol. Microbiol. 3479-486. [DOI] [PubMed] [Google Scholar]
- 10.Bono, J. L., A. F. Elias, J. J. Kupko, B. Stevenson, K. Tilly, and P. Rosa. 2000. Efficient targeted mutagenesis in Borrelia burgdorferi. J. Bacteriol. 1822445-2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brunet, L. R., C. Sellitto, A. Spielman, and S. R. Telford III. 1995. Antibody response of the mouse reservoir of Borrelia burgdorferi in nature. Infect. Immun. 63:3030-3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bunikis, J., and A. G. Barbour. 1999. Access of antibody or trypsin to an integral outer membrane protein (P66) of Borrelia burgdorferi is hindered by Osp lipoproteins. Infect. Immun. 672874-2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bunikis, J., J. Tsao, C. J. Luke, M. G. Luna, D. Fish, and A. G. Barbour. 2004. Borrelia burgdorferi infection in a natural population of Peromyscus leucopus mice: a longitudinal study in an area where Lyme borreliosis is highly endemic. J. Infect. Dis. 1891515-1523. [DOI] [PubMed] [Google Scholar]
- 14.Casjens, S., N. Palmer, R. Van Vugt, W. Mun Huang, B. Stevenson, P. Rosa, R. Lathigra, G. Sutton, J. Peterson, R. J. Dodson, D. Haft, E. Hickey, M. Gwinn, O. White, and C. M. Fraser. 2000. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol. Microbiol. 35:490-516. [DOI] [PubMed] [Google Scholar]
- 15.Casjens, S., R. van Vugt, K. Tilly, P. A. Rosa, and B. Stevenson. 1997. Homology throughout the multiple 32-kilobase circular plasmids present in Lyme disease spirochetes. J. Bacteriol. 179217-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coleman, J. L., J. A. Gebbia, J. Piesman, J. L. Degen, T. H. Bugge, and J. L. Benach. 1997. Plasminogen is required for efficient dissemination of B. burgdorferi in ticks and for enhancement of spirochetemia in mice. Cell 891111-1119. [DOI] [PubMed] [Google Scholar]
- 17.de Silva, A. M., and E. Fikrig. 1995. Growth and migration of Borrelia burgdorferi in Ixodes ticks during blood feeding. Am. J. Trop. Med. Hyg. 53397-404. [DOI] [PubMed] [Google Scholar]
- 18.de Silva, A. M., E. Fikrig, E. Hodzic, F. S. Kantor, S. R. Telford, and S. W. Barthold. 1998. Immune evasion by tickborne and host-adapted Borrelia burgdorferi. J. Infect. Dis. 177395-400. [DOI] [PubMed] [Google Scholar]
- 19.de Silva, A. M., S. R. Telford, L. R. Brunet, S. W. Barthold, and E. Fikrig. 1996. Borrelia burgdorferi OspA is an arthropod-specific transmission-blocking Lyme disease vaccine. J. Exp. Med. 183271-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Silva, A. M., N. S. Zeidner, Y. Zhang, M. C. Dolan, J. Piesman, and E. Fikrig. 1999. Influence of outer surface protein A antibody on Borrelia burgdorferi within feeding ticks. Infect. Immun. 6730-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donahue, J. G., J. Piesman, and A. Spielman. 1987. Reservoir competence of white-footed mice for Lyme disease spirochetes. Am. J. Trop. Med. Hyg. 3692-96. [DOI] [PubMed] [Google Scholar]
- 22.Elias, A. F., J. L. Bono, J. J. Kupko, P. E. Stewart, J. G. Krum, and P. A. Rosa. 2003. New antibiotic resistance cassettes suitable for genetic studies in Borrelia burgdorferi. J. Mol. Microbiol. Biotechnol. 629-40. [DOI] [PubMed] [Google Scholar]
- 23.Elias, A. F., P. E. Stewart, D. Grimm, M. J. Caimano, C. H. Eggers, K. Tilly, J. L. Bono, D. R. Akins, J. D. Radolf, T. G. Schwan, and P. Rosa. 2002. Clonal polymorphism of Borrelia burgdorferi strain B31 MI: implications for mutagenesis in an infectious strain background. Infect. Immun. 702139-2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fikrig, E., S. R. Telford, S. W. Barthold, F. S. Kantor, A. Spielman, and R. A. Flavell. 1992. Elimination of Borrelia burgdorferi from vector ticks feeding on OspA immunized mice. Proc. Natl. Acad. Sci. USA 895418-5421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finlay, B. B., and S. Falkow. 1997. Common themes in microbial pathogenicity revisited. Microbiol. Mol. Biol. Rev. 61136-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fisher, M. A., D. Grimm, A. K. Henion, A. F. Elias, P. E. Stewart, P. A. Rosa, and F. C. Gherardini. 2005. Borrelia burgdorferi sigma(54) is required for mammalian infection and vector transmission but not for tick colonization. Proc. Natl. Acad. Sci. USA 1025162-5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fraser, C. M., S. Casjens, W. M. Huang, G. G. Sutton, R. Clayton, R. Lathigra, O. White, K. A. Ketchum, R. Dodson, E. K. Hickey, M. Gwinn, B. Dougherty, J.-F. Tomb, R. F. Fleischmann, D. Richardson, J. Peterson, A. R. Kerlavage, J. Quackenbush, S. Salzberg, M. Hanson, R. van Vugt, N. Palmer, M. D. Adams, J. Gocayne, J. Weidman, T. Utterback, L. Watthey, L. McDonald, P. Artiach, C. Bowman, S. Gerland, C. Fujii, M. D. Cotton, K. Horst, K. Roberts, B. Hatch, H. O. Smith, and J. C. Venter. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390580-586. [DOI] [PubMed] [Google Scholar]
- 28.Fuchs, H., R. Wallich, M. M. Simon, and M. D. Kramer. 1994. The outer surface protein A of the spirochete Borrelia burgdorferi is a plasmin(ogen) receptor. Proc. Natl. Acad. Sci. USA 9112594-12598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grimm, D., K. Tilly, R. Byram, P. E. Stewart, J. G. Krum, D. M. Bueschel, T. G. Schwan, P. F. Policastro, A. F. Elias, and P. A. Rosa. 2004. Outer-surface protein C of the Lyme disease spirochete: a protein induced in ticks for infection of mammals. Proc. Natl. Acad. Sci. USA 1013142-3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hanson, M. S., D. R. Cassatt, B. P. Guo, N. K. Patel, M. P. McCarthy, D. W. Dorward, and M. Hook. 1998. Active and passive immunity against Borrelia burgdorferi decorin binding protein A (DbpA) protects against infection. Infect. Immun. 662143-2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Howe, T. R., F. W. LaQuier, and A. G. Barbour. 1986. Organization of genes encoding two outer membrane proteins of the Lyme disease agent Borrelia burgdorferi within a single transcriptional unit. Infect. Immun. 54:207-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Howe, T. R., L. W. Mayer, A. G. Barbour, S. L. Tessier, and W. J. Todd. 1985. A single recombinant plasmid expressing two major outer surface proteins of the Lyme disease spirochete. Science 227645-646. [DOI] [PubMed] [Google Scholar]
- 33.Kelly, R. 1971. Cultivation of Borrelia hermsii. Science 173443-444. [DOI] [PubMed] [Google Scholar]
- 34.Kitamura, D., J. Roes, R. Kuhn, and K. Rajewsky. 1991. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature 350423-426. [DOI] [PubMed] [Google Scholar]
- 35.Knülle, W. 1966. Equilibrium humidities and survival of some tick larvae. J. Med. Entomol. 2335-338. [DOI] [PubMed] [Google Scholar]
- 36.Kramer, M. D., R. Wallich, and M. M. Simon. 1996. The outer surface protein A (OspA) of Borrelia burgdorferi: a vaccine candidate and bioactive mediator. Infection 24190-194. [DOI] [PubMed] [Google Scholar]
- 37.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227680-685. [DOI] [PubMed] [Google Scholar]
- 38.Lane, R. S., J. Piesman, and W. Burgdorfer. 1991. Lyme borreliosis: relation of its causative agent to its vectors and hosts in North America and Europe. Annu. Rev. Entomol. 36587-609. [DOI] [PubMed] [Google Scholar]
- 39.Li, H., J. J. Dunn, B. J. Luft, and C. L. Lawson. 1997. Direct demonstration of antigenic substitution of Borrelia burgdorferi ex vivo: exploration of the paradox of the early immune response to outer surface proteins A and C in Lyme disease. Proc. Natl. Acad. Sci. USA 943584-3589.9108020 [Google Scholar]
- 40.Magnarelli, L. A., J. F. Anderson, K. E. Hyland, D. Fish, and J. B. McAninch. 1988. Serologic analyses of Peromyscus leucopus, a rodent reservoir for Borrelia burgdorferi, in northeastern United States. J. Clin. Microbiol. 261138-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moody, K. D., R. L. Adams, and S. W. Barthold. 1994. Effectiveness of antimicrobial treatment against Borrelia burgdorferi infection in mice. Antimicrob. Agents Chemother. 381567-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neelakanta, G., X. Li, U. Pal, X. Liu, D. S. Beck, K. Deponte, D. Fish, F. S. Kantor, and E. Fikrig. 2007. Outer surface protein B is critical for Borrelia burgdorferi adherence and survival within Ixodes ticks. PLoS Pathog. 3e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Noppa, L., Y. Östberg, M. Lavrinovicha, and S. Bergström. 2001. P13, an integral membrane protein of Borrelia burgdorferi, is C-terminally processed and contains surface-exposed domains. Infect. Immun. 693323-3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ohnishi, J., J. Piesman, and A. M. de Silva. 2001. Antigenic and genetic heterogeneity of Borrelia burgdorferi populations transmitted by ticks. Proc. Natl. Acad. Sci. USA 98670-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pal, U., A. M. de Silva, R. R. Montgomery, D. Fish, J. Anguita, J. F. Anderson, Y. Lobet, and E. Fikrig. 2000. Attachment of Borrelia burgdorferi within Ixodes scapularis mediated by outer surface protein A. J. Clin. Investig. 106561-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pal, U., X. Li, T. Wang, R. R. Montgomery, N. Ramamoorthi, A. M. De Silva, F. Bao, X. Yang, M. Pypaert, D. Pradhan, F. S. Kantor, S. Telford, J. F. Anderson, and E. Fikrig. 2004. TROSPA, an Ixodes scapularis receptor for Borrelia burgdorferi. Cell 119457-468. [DOI] [PubMed] [Google Scholar]
- 47.Pal, U., R. R. Montgomery, D. Lusitani, P. Voet, V. Weynants, S. E. Malawista, Y. Lobet, and E. Fikrig. 2001. Inhibition of Borrelia burgdorferi-tick interactions in vivo by outer surface protein A antibody. J. Immunol. 1667398-7403. [DOI] [PubMed] [Google Scholar]
- 48.Pavia, C., M. A. Inchiosa, and G. P. Wormser. 2002. Efficacy of short-course ceftriaxone therapy for Borrelia burgdorferi infection in C3H mice. Antimicrob. Agents Chemother. 46132-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Piesman, J., N. S. Zeidner, and B. S. Schneider. 2003. Dynamic changes in Borrelia burgdorferi populations in Ixodes scapularis (Acari:Ixodidae) during transmission: studies at the mRNA level. Vector Borne Zoonotic Dis. 3125-132. [DOI] [PubMed] [Google Scholar]
- 50.Policastro, P. F., and T. G. Schwan. 2003. Experimental infection of Ixodes scapularis larvae (Acari:Ixodidae) by immersion in low passage cultures of Borrelia burgdorferi. J. Med. Entomol. 40364-370. [DOI] [PubMed] [Google Scholar]
- 51.Pollack, R. J., S. R. Telford, and A. Spielman. 1991. Rectal infusion and aspiration of material through the guts of ixodid ticks (Acari:Ixodidae). J. Med. Entomol. 28809-815. [DOI] [PubMed] [Google Scholar]
- 52.Ribeiro, J. M., T. N. Mather, J. Piesman, and A. Spielman. 1987. Dissemination and salivary delivery of Lyme disease spirochetes in vector ticks (Acari:Ixodidae). J. Med. Entomol. 24201-205. [DOI] [PubMed] [Google Scholar]
- 53.Rosa, P., D. S. Samuels, D. Hogan, B. Stevenson, S. Casjens, and K. Tilly. 1996. Directed insertion of a selectable marker into a circular plasmid of Borrelia burgdorferi. J. Bacteriol. 1785946-5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rosa, P. A., T. Schwan, and D. Hogan. 1992. Recombination between genes encoding major outer surface proteins A and B of Borrelia burgdorferi. Mol. Microbiol. 63031-3040. [DOI] [PubMed] [Google Scholar]
- 55.Rudolph, D., and W. Knulle. 1974. Site and mechanism of water vapour uptake from the atmosphere in ixodid ticks. Nature 24984-85. [DOI] [PubMed] [Google Scholar]
- 56.Sadziene, A., D. D. Thomas, and A. G. Barbour. 1995. Borrelia burgdorferi mutant lacking Osp: biological and immunological characterization. Infect. Immun. 631573-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Samuels, D. S., K. E. Mach, and C. F. Garon. 1994. Genetic transformation of the Lyme disease agent Borrelia burgdorferi with coumarin-resistant gyrB. J. Bacteriol. 1766045-6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schwan, T. G., K. K. Kime, M. E. Schrumpf, J. E. Coe, and W. J. Simpson. 1989. Antibody response in white-footed mice (Peromyscus leucopus) experimentally infected with the Lyme disease spirochete (Borrelia burgdorferi). Infect. Immun. 573445-3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schwan, T. G., and J. Piesman. 2000. Temporal changes in outer surface proteins A and C of the Lyme disease-associated spirochete, Borrelia burgdorferi, during the chain of infection in ticks and mice. J. Clin. Microbiol. 38382-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schwan, T. G., J. Piesman, W. T. Golde, M. C. Dolan, and P. A. Rosa. 1995. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc. Natl. Acad. Sci. USA 282909-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Steere, A. C., V. K. Sikand, F. Meurice, D. L. Parenti, E. Fikrig, R. T. Schoen, J. Nowakowski, C. H. Schmid, S. Laukamp, C. Buscarino, and D. S. Krause for the Lyme Disease Vaccine Study Group. 1998. Vaccination against Lyme disease with recombinant Borrelia burgdorferi outer surface lipoprotein A with adjuvant. N. Engl. J. Med. 339209-215. [DOI] [PubMed] [Google Scholar]
- 62.Stoenner, H. G., T. Dodd, and C. Larsen. 1982. Antigenic variation of Borrelia hermsii. J. Exp. Med. 1561297-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thanassi, W. T., and R. T. Schoen. 2000. The Lyme disease vaccine: conception, development, and implementation. Ann. Intern. Med. 132661-668. [DOI] [PubMed] [Google Scholar]
- 64.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 764350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Valenzuela, J. G., R. Charlab, T. N. Mather, and J. M. Ribeiro. 2000. Purification, cloning, and expression of a novel salivary anticomplement protein from the tick, Ixodes scapularis. J. Biol. Chem. 27518717-18723. [DOI] [PubMed] [Google Scholar]
- 66.Van Hoecke, C., D. Fu, D. De Grave, P. Voet, and E. Lebacq. 1998. Clinical and immunological assessment of a candidate Lyme disease vaccine in healthy adults: antibody persistence and effect of a booster dose at month 12. Vaccine 161688-1692. [DOI] [PubMed] [Google Scholar]
- 67.Vieira, J., and J. Messing. 1991. New pUC-derived cloning vectors with different selectable markers and DNA replication origins. Gene 100189-194. [DOI] [PubMed] [Google Scholar]
- 68.Winston, P. W., and D. H. Bates. 1960. Saturated solutions for the control of humidity in biological research. Ecology 41232-237. [Google Scholar]
- 69.Yang, X. F. F., U. Pal, S. M. Alani, E. Fikrig, and M. V. Norgard. 2004. Essential role for OspA/B in the life cycle of the Lyme disease spirochete. J. Exp. Med. 199641-648. [DOI] [PMC free article] [PubMed] [Google Scholar]