Abstract
Recombinant Bacillus subtilis endospores have been used to vaccinate against tetanus and anthrax. In this work, we have developed spores that could be used to vaccinate against Clostridium perfringens alpha toxin and that could be used to protect against gas gangrene in humans and necrotic enteritis in poultry. The primary active agent in both cases is alpha toxin. A carboxy-terminal segment of the alpha toxin gene (cpa) fused to the glutathione-S-transferase (GST) gene was cloned in B. subtilis such that the encoded GST-Cpa247-370 polypeptide had been expressed in the following three different ways: expression in the vegetative cell, expression on the surface of the spore coat (fused to the CotB spore coat protein), and a combined approach of spore coat expression coupled with expression in the vegetative cell. Mice immunized orally or nasally with three doses of recombinant spores that carried GST-Cpa247-370 on the spore surface showed the most striking responses. This included seroconversion with anti-Cpa247-370-specific immunoglobulin G (IgG) responses in their sera, a Th2 bias, and secretory IgA responses in saliva, feces, and lung samples. Neutralizing IgG antibodies to alpha toxin were detected using in vitro and in vivo assays, and a toxin challenge established protection. Mice immunized nasally or orally with recombinant spores were protected against a challenge with 12 median lethal doses of alpha toxin. Existing use of spores as competitive exclusion agents in animal feeds supports their use as a potentially economical and heat-stable vaccine for the poultry industry.
The alpha toxin (phospholipase C) of Clostridium perfringens is a toxin produced by all strains of this bacterium but produced in large quantities by some type A strains (22). The alpha toxin has historically been associated with the ability of the bacterium to cause gas gangrene, and mutants unable to produce alpha toxin are almost entirely attenuated in murine models of gas gangrene (1). In previous studies, a fragment of alpha toxin comprising the carboxy-terminal domain of the toxin, which was produced in Escherichia coli using recombinant DNA technology, was shown to be nontoxic (33). Immunization of mice with the carboxy-terminal domain provided protection against experimental gas gangrene (37).
More recently, alpha toxin has been proposed to play a role in the pathogenesis of enterotoxaemia in various domesticated livestock (2, 20, 30). Over the past decade, there has been extensive debate over the possibility that alpha toxin plays a role in the pathogenesis of necrotic enteritis in chickens (36). This disease has recently become of significant economic importance worldwide, partly because the incidence of disease has increased as a consequence of the withdrawal of antibiotic growth promoters from dietary feedstuffs (26). While it is clear that C. perfringens type A is the main etiological agent of necrotic enteritis, an alpha toxin-negative mutant of C. perfringens did not show an impaired ability to cause disease in poultry (15).
Paradoxically, although the evidence with an alpha toxin-negative mutant of C. perfringens strongly indicates that the toxin does not play a role in disease, other recent studies have shown that immunization of poultry with an alpha toxoid significantly reduced the incidence of necrotic enteritis (16). However, the induction of good protective immunity was dependent on “boosting” of toxoid-immunized poultry with toxin, suggesting that some protective epitopes were destroyed by the toxoiding process. Recently, protective immunity against C. perfringens necrotic enteritis in chickens has been demonstrated using an attenuated live Salmonella strain that expresses the carboxy-terminal domain of alpha toxin (39). There are other circumstantial pointers to the role of antibody against alpha toxin in protection from necrotic enteritis. Although C. perfringens is commonly found in poultry gut, flocks which have high levels of antibody to the alpha toxin show reduced mortality from necrotic enteritis (11). Therefore, overall, the findings are that alpha toxin does not appear to play a significant role in the pathogenesis of necrotic enteritis, but antibody against the toxin can protect poultry from disease.
We have set out to develop a vaccine that could be given noninvasively to poultry and which would consistently induce good protective immunity against alpha toxin. We have selected a nonpathogenic Bacillus species as the carrier for the carboxy-terminal domain of alpha toxin. Bacillus subtilis has been used successfully as an antigen delivery system (14, 40) and, in murine models, has been shown to vaccinate against tetanus as well as anthrax (5, 6, 8, 25).
Bacillus species are in current use as probiotics for the livestock industry, with a number of European Union-approved products available, most notably BioPlus2B from Christian Hansen (12). Moreover, competitive exclusion studies have shown that spores of B. subtilis are able to inhibit C. perfringens infection in a poultry model (18). Bacillus species form heat-resistant bacterial endospores that can be stored without loss of viability at ambient temperatures. Most species are safe for consumption, and probiotic effects on poultry pathogens are well documented (17, 18). They are therefore suitable for use in animal feeds.
In this study, we have evaluated genetically engineered strains of B. subtilis that express the C-terminal domain of alpha toxin and show that these can protect orally immunized mice against a challenge with alpha toxin.
MATERIALS AND METHODS
Construction of recombinant strains.
B. subtilis strains used in this work for immunizations were PY79, a standard prototrophic laboratory strain, and a strain isogenic to the 168 type strain (38). Three recombinant derivatives of PY79 were made (HT230, HT251, and HT266) that expressed a carboxy-terminal domain of C. perfringens, Cpa247-370, as a fusion to glutathione-S-transferase (GST). This GST-Cpa247-370 fusion has been described previously (37); in this work, it was expressed in the vegetative cell (HT230; rrnO-gst-cpa247-370), on the spore surface as a fusion to the spore coat protein CotB (HT251; cotB-gst-cpa247-370), and finally, on the spore surface fused to CotB together with expression in the vegetative cell (HT266; rrnO-gst-cpa247-370 cotB-gst-cpa247-370).
HT230 was constructed by transforming competent cells of strain PY79 with linearized DNA of pHT223, followed by selection for chloramphenicol resistance (5 μg/ml). pHT223 was derived from a plasmid, pDL243, that carries an expression cassette, PrrnO-RBS-MCS, enabling vegetative gene expression at high levels. The cassette comprises the B. subtilis rrnO promoter, the sspA ribosome binding site and a multiple cloning site into which candidate open reading frames can be inserted. The plasmid also carries left and right flanking sequences of the amyE (amylase) gene that allows insertion of constructions into the chromosome of B. subtilis using the amyE gene and selection provided by a chloramphenicol resistance gene (cat). pDL243 is essentially identical to the plasmid pDL242 that has been described elsewhere (6), with the exception that the PrrnO-RBS-MCS cassette was subcloned into the plasmid pDG364 that allows ectopic insertion of gene sequences by a double-crossover recombination at the amyE locus (10). To construct pHT223, a 1,083-bp sequence encoding GST fused to the C terminus of alpha toxin (Cpa247-370) was amplified from the pGEX-3X-13 expression plasmid that carries gst-cpa247-370 (23). Forward and reverse primers, GST-αF1 and GST-αR1 (Table 1), provided NheI (GST-αF1) and NotI (GST-αR1) sites for cloning directly into the multiple cloning site of pDL243. Construction of pHT223 was confirmed by nucleotide sequencing.
TABLE 1.
PCR primers used in this work
| Primer | 5′-3′ sequencea |
|---|---|
| GST-αF1 | CTAGCTAGCTCCCCTATACTAGGTTATTGGAAA |
| GST-αR1 | ATAAGAATGCGGCCGCTTATTTTATATTATAAGTTGAATTTCC |
| CotBF2 | CGCGGATCCACGGATTAGGCCGTTTGTC |
| CotBR1 | CCCAAGCTTGGATGATTGATCATCTGAAG |
| GST-αF2 | CCCAAGCTTTCCCCTATACTAGGTTATTGGAAA |
| GST-αR2 | CCGGAATTCTTATTTTATATTATAAGTTGAATTTCC |
Underlined residues correspond to a stop codon.
HT251 was constructed by transforming competent cells of strain PY79 with linearized DNA of pHT245, followed by selecting for erythromycin resistance (Ermr) (1 μg/ml). pHT245 is derived from the plasmid pDG1664 that allows ectopic insertion of cloned genes at the thrC (threonine) locus enabled by selection for Ermr and has been described in detail elsewhere (10). To fuse the cotB gene sequence to gst-cpa247-370, PCR was first used to amplify the entire cotB gene from PY79 chromosomal DNA and gst-cpa247-370 from pGEX-3X-13. These PCR products were then coligated and inserted into pDG1664. cotB was amplified using forward and reverse primers CotBF2 and CotBR1 (Table 1). The 1,105-bp amplified product was then cut with BamHI (CotBF2) and HindIII (CotBR1). Two primers were used to amplify the 1,083-bp gst-cpa247-370 fragment from pGEX-3X-13. These were GST-αF2 and GST-αR2 (data not shown). The 1,083-bp PCR product was cleaved with HindIII and EcoRI, ligated to the 1,105-bp cleaved cotB PCR product and then ligated to pDG1664 (cleaved with BamHI and EcoRI). Recombinants that carried the cotB gene fused to gst-cpa247-370 were confirmed by nucleotide sequencing.
HT266 was created by transforming competent cells of HT251 with chromosomal DNA of strain HT230, followed by selecting for Cmr.
Preparation of spores and general methods.
Spores used in all experiments were prepared by growth and sporulation in Difco sporulation medium as previously described by Nicholson and Setlow (24). Each batch of spores was heat treated (68°C for 30 min) to ensure that there were no viable vegetative cells, suspended in sterile phosphate-buffered saline (PBS), and stored in aliquots (1 × 1011 spores/ml) at −70°C until use. Spore counts were determined by serial dilution and plate counting. Extraction and analysis of spore coat proteins using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) were performed as described previously (24).
Simulated intestinal conditions.
Approximately 1 × 109 spores were incubated in simulated gastric fluid (SGF) or simulated intestinal fluid (SIF) as described previously (4). Incubation was done at 37°C for 1 h in SGF and 3 h in SIF, after which time the coat proteins were extracted from the spore, size fractionated by SDS-PAGE, and analyzed by Western blotting using a polyclonal antibody to GST-Cpa247-370 for retention of the expressed GST-Cpa247-370.
GST-Cpa247-370 protein.
The GST-Cpa247-370 protein was purified from an E. coli strain carrying the plasmid pGEX-3X-13 that expresses a fusion of GST with the carboxy-terminal domain of C. perfringens alpha toxin (Cpa247-370) protein (23). To induce expression of GST-Cpa247-370, IPTG (isopropyl-β-d-thiogalactopyranoside; 0.5 mM) was added, and the expressed protein was purified using a GST-binding column mounted on a Pharmacia AKTA liquid chromatography system.
Alpha toxin.
Alpha toxin was purified from an E. coli strain containing the expression plasmid pT2.2 that carries the C. perfringens NCTC 8237 alpha toxin gene (37).
Antibodies.
To specifically recognize GST-Cpa247-370-expressing clones, two mouse antibodies were used. First, a monoclonal antibody was raised against alpha toxin (Bio321; Bio-X Diagnostics, Belgium). Second, a polyclonal antibody was raised against 2 μg of purified GST-Cpa247-370 that had been administered (three doses in a 0.2-ml volume) by the intraperitoneal route to six mice (sera were pooled). These pooled sera were used for the in vitro, in vivo, and enzyme-linked immunosorbent assay (ELISA) experiments. Both antibodies were used at a 1:5,000 dilution. A third antibody used was an anti-GST polyclonal antibody raised in goats (Sigma) and used at a 1:5,000 dilution. Cross-reactive antibodies were detected using the ECL Western blotting system (Amersham).
Animals.
Animals used in this work were pathogen-free BALB/c female mice (6 to 8 weeks old; obtained from Harlan United Kingdom). Animals were housed in the Royal Holloway, University of London animal house, and work described in this paper was performed under the Home Office project license PPL 70/6126.
Immunizations.
For evaluation of immune responses, groups of eight mice (under light anesthesia) were dosed by the intraperitoneal (i.p.) route (0.2 ml) or mucosally using the oral (intragastric gavage; 0.2 ml) or nasal routes (no anesthesia, using a Gilson pipette tip; 40 μl). The dose for the i.p. route was 1 × 109 spores (in PBS; equivalent to 0.075 μg of GST-Cpa247-370/dose) on days 0, 14, and 28. For oral administration, the dose was 5 × 1010 spores (in PBS and equivalent to 3.6 μg of GST-Cpa247-370/dose) on days 1, 21, and 42. For nasal delivery, a dose of 2 × 109 spores (in PBS; equivalent to 0.15 μg of GST-Cpa247-370/dose) was used on days 1, 21, and 42. For sampling, serum, saliva, and fecal pellets were taken on days 1, 20, 40, and 60.
ELISA for detection of total antigen-specific serum (immunoglobulin G [IgG]).
Nunc-Immuno MaxiSorp plates were coated with 50 μl/well of GST-Cpa247-370-purified proteins (5 μg/ml in PBS buffer) and incubated at 4°C overnight. After being blocked with 2% bovine serum albumin (BSA) in PBS for 1 h at 37°C, serum samples were applied using a twofold dilution series starting with a 1/40 dilution in ELISA diluent buffer (0.01 M PBS [pH 7.2], 0.5% [wt/vol] BSA, 5% [vol/vol] fetal bovine serum [Sigma], 0.1% [vol/vol] Triton X-100, 0.05% [vol/vol] Tween 20). Every plate carried replicate wells of a negative control (a 1/40 dilution of preimmune serum) and a positive control (1/200 starting dilution of serum from mice immunized parenterally with purified GST-Cpa247-370; see “Antibodies”). Plates were incubated for 2 h at 37°C before appropriate anti-mouse Ig horseradish peroxidase conjugates were added (all were obtained from Sigma, with the exception of Serotec for the subclass conjugates). Plates were incubated for a further 1 h at 37°C and then developed using the substrate TMB (0.1 mg/ml 3.3′,5.5′-tetramethylbenzidine [Sigma] in 0.1 M sodium acetate buffer [pH 5.5]). Reactions were stopped using 2 M H2SO4. The optical density at 450 nm was read using a Rosys Anthos HT3 ELISA plate reader. Dilution curves were drawn for each sample, and the end-point titers of the specific antibody were estimated as the maximum dilution of serum giving an absorbance reading of 0.2 units over the background.
Determination of secretory IgA (sIgA) responses. (i) Saliva.
Whatman no. 1 filter strips (2 by 3 mm), inserted into the mouth, were used to collect saliva from mice. Strips were then incubated in 70 μl of PBS containing 0.1% BSA, 0.05% Tween 20, and 1 mM phenylmethylsulphonyl fluoride (PMSF; Sigma) at room temperature (RT) for 1 h and centrifuged (13,000 rpm for 10 min), and the supernatant was used for analysis.
(ii) Lung samples.
All of the excised lung (0.1 g) was washed with PBS containing 0.1% (wt/vol) BSA and 1 mM of PMSF at RT for 1 h. Samples were centrifuged (13,000 rpm for 10 min), and the supernatant was used for analysis.
(iii) Fecal samples.
Freshly voided fecal pellets were collected and frozen at −20°C until use. Approximately 0.1 g of each pellet was incubated in 400 μl PBS containing 0.05% Tween 20, 1% BSA, 1 mM PMSF, 0.1% Triton X-100, and 0.02% NaN3 at RT for about 1 h with occasional vortexing to disrupt solid material. Samples were centrifuged (13,000 rpm for 10 min), and supernatants were used for analysis.
Levels of total IgA in saliva, lungs, and feces were determined by ELISA using Nunc-Immuno MaxiSorp plates coated with 50 μl/well of GST-Cpa247-370-purified proteins (5 μg/ml in PBS) and incubated at 4°C overnight. After being blocked with 2% BSA in PBS for 1 h at 37°C, samples were applied using a twofold dilution series starting with a dilution of 1/10 for feces, 1/20 for saliva, and 1/40 for lungs in ELISA dilution buffer (0.01 M PBS, 0.1% BSA, 0.05% Tween 20, and 10 mM PMSF [Sigma]). Plates were incubated for 1 h at 37°C. After being washed and the addition of 1/1,000 anti-mouse IgA horseradish peroxidase conjugate, plates were further incubated for 1 h at 37°C. Color development was performed with the TMB substrate. The ELISA titer of specific antibody was estimated as the maximum dilution of serum, giving an absorbance reading of 0.1 units over the background.
Statistics.
Student's t test was used to compare data between groups. A P value of >0.05 was considered nonsignificant.
In vitro and in vivo neutralization assays.
The ability of antiserum to inhibit the activity of alpha toxin in vitro or in vivo was determined as described previously (19, 37). Positive control serum was obtained from mice injected (i.p.) with 2 μg of GST-Cpa247-370 (see “Antibodies”). For in vitro inhibition of hemolysis, pooled serum was serially diluted in microtiter plates, and dilutions were preadsorbed with 0.1 μg of alpha toxin. Preadsorbed mixtures were coincubated with mouse erythrocytes for 1 h, and hemolysis was measured. The end-point titer was defined as the dilution causing 50% hemolysis.
For in vivo assays, 2 median 50% lethal doses (LD50) of alpha toxin were adsorbed to pooled antiserum (diluted 1/5) from each mouse group. The mixture was incubated for 2 h at 37°C in a volume of 200 μl and then injected (i.p.) into individual mice. Animals were monitored for signs of toxicity. sIgA samples were not evaluated in either the in vitro or in vivo assays, primarily because of the low antibody titers and the small sample volumes.
Toxin challenge.
Mice (groups of six) were immunized with three oral or nasal doses of spores (HT251, HT266, and PY79) or GST-Cpa247-370 polypeptide as outlined in “Immunizations” above. Twenty-one days after the last immunization, animals were administered an i.p. injection (0.2 ml) of purified alpha toxin in PBS at either 6 or 12 LD50. The precise dose corresponding to 2 LD50 was first established by injecting mice with increasing amounts of alpha toxin (0.25 μg, 0.5 μg, 1 μg, 1.5 μg, and 2 μg) and, using the method of Reed and Muench, 1 LD50 was determined to be 0.35 μg (27). Animals were closely observed for 24 h. Animals showing clear signs of toxicity were considered susceptible, and the time was recorded.
RESULTS
Expression of C. perfringens alpha toxin in recombinant strains of B. subtilis.
Immunization of mice with the C-terminal domain (residues 247 to 370) of alpha toxin (Cpa) when fused to GST has been shown to confer protection against challenge with C. perfringens type A in a murine model of gas gangrene (37, 39). Accordingly, we chose to express GST-Cpa247-370 on the spore coat of B. subtilis since a similar strategy using the Clostridium tetani tetanus toxin fragment C (TTFC), fused to CotB, was shown to induce protective immunity in mice (8). It is possible that surface display might enhance degradation of particular antigens, especially following intragastric delivery. Accordingly, we also used an alternative approach of expressing the antigen within the live vegetative cell. In this method, spores are administered but the antigen is expressed only following spore germination (7), which has been shown to occur in the small intestine (32). A third approach was a combination of spore coat and vegetative cell expression.
A recombinant gene (gst-cpa247-370), encoding the 41-kDa GST-Cpa247-370 polypeptide, was expressed in vegetative cells of B. subtilis by being placed under the control of the PrrnO promoter that permits high levels of expression in the vegetative cell (7). HT230 (rrnO-gst-cpa247-370) cells expressed a 41-kDa protein species during vegetative growth that was recognized by anti-GST-Cpa247-370 antiserum (Fig. 1A). At stationary phase (4-h growth in LB medium at 37°C), expression was highest and approximately 0.017 pg of GST-Cpa247-370 was produced per cell, equivalent to 1.6% of total cellular protein.
FIG. 1.
Expression of Cpa247-370 in B. subtilis. (A) Western blots of SDS-PAGE-fractionated extracts of cells from recombinant strains that expressed the 41-kDa GST-Cpa247-370 polypeptide probed with a polyclonal antibody raised to GST-Cpa247-370 to confirm expression. Lanes 1 and 2 show extracts of vegetative cells that express the 41-kDa GST-Cpa247-370 polypeptide under the control of the PrrnO promoter (indicated with an arrow). Lane 1, HT230 (rrnO-gst-cpa247-370); lane 2, HT266 (rrnO-gst-cpa247-370 cotB-gst-cpa247-370). Lane 3 is an extract from nonrecombinant cells of strain PY79. Lane 4, 50 ng of GST-Cpa247-370 purified from an E. coli expression strain containing pGEX-3X-13. (B) Spore coat proteins were extracted from purified spores (1 × 109) of HT251 (cotB-gst-cpa247-370) size fractionated on SDS-PAGE gels and probed with a polyclonal antibody to GST-Cpa247-370 (lanes 1 and 4). Spore coat extractions after incubation of HT251 spores for 30 min (lane 2) and 60 min (lane 3) in SGF or after 60 min (lane 5) and 180 min (lane 6) of incubation in SIF. The principal 72-kDa polypeptide corresponding to CotB-GST-Cpa247-370 is indicated by an arrow. Identical results were obtained with HT266 spores (data not shown).
For expression on the spore coat, GST-Cpa247-370 was fused to the C terminus of the CotB spore coat protein. HT251 cells carrying cotB-gst-cpa247-370 expressed a 72-kDa hybrid protein that was presented on the spore surface (Fig. 1B). This band corresponded in size to the predicted molecular mass of CotB-GST-Cpa247-370. Additional polypeptides of lower molecular masses of 58, 41, and 15 kDa were also present. The 58-kDa species was shown to be recognized by polyclonal antisera to CotB and to GST (data not shown) and corresponded in size to CotB-GST. The 41-kDa species was recognized by a polyclonal GST antiserum (data not shown) but not by the CotB antiserum and corresponds, in size, to GST-Cpa247-370. The 15-kDa species was not recognized by either the CotB or GST antisera and may be the Cpa247-370 species. Approximately 6.5 pg of CotB-GST-Cpa247-370 was produced per spore, equivalent to 0.33% of total spore coat protein.
A third strain, HT266 (cotB-gst-cpa247-370 rrnO-gst-cpa247-370), that expressed GST-Cpa247-370 on the spore coat (fused to CotB) and in the vegetative cell (under the PrrnO control) was also constructed, and expression was confirmed in vegetative cells (Fig. 1A) and on the spore coat (data not shown). In all of the cases described above, the recombinant genes had been integrated into the B. subtilis chromosome by a double-crossover recombination and were therefore maintained stably and in single copy. Expression of GST-Cpa247-370 was indistinguishable from that observed in HT230 during growth in liquid medium, with maximum levels produced at stationary phase. Similarly, expression of GST-Cpa247-370 on the HT266 spore coat, when fused to CotB, was identical to that observed for HT251.
The stability of the GST-Cpa247-370 moiety when fused to CotB was evaluated under conditions that simulated the gastrointestinal (GI) tract (Fig. 1B). Incubation of HT251 spores in SGF demonstrated that GST-Cpa247-370 was substantially degraded, with less than 15% of the protein remaining after a 30-min incubation in SGF. Incubation of HT251 spores in SIF showed that GST-Cpa247-370 was more stable, with approximately 50% of the protein surviving a 3-h incubation in SIF. Stability of the GST-Cpa247-370 was also evaluated over a period of 8 weeks in lyophilized preparations of HT251 spores stored at different temperatures (30°C, 37°C, and 42°C). No significant degradation of the 72-kDa species corresponding to CotB-GST-Cpa247-370 was observed, but there was a gradual reduction in the levels of the 58-kDa species when stored at 42°C (data not shown).
Serum anti-GST-Cpa247-370 responses following parenteral immunization.
Serum antibody responses to GST-Cpa247-370 were determined for mice that had been immunized with three doses of spores of HT230, HT251, and HT266 by the i.p. route (Table 2). The strongest antibody responses were measured on day 40 as the animals became hyperimmune. The amount of GST-Cpa247-370 expressed on the spore surface of HT251 and HT266 corresponded to 0.075 μg/dose. Animals receiving three injections of the same amount of purified GST-Cpa247-370 protein generated antibody responses not significantly different (P > 0.05) than those of the control groups. These results show that GST-Cpa247-370 delivered on the spore surface or in the germinating spore could generate potent antibody responses to GST-Cpa247-370 and, by enhancing the immune response, must provide a role as an adjuvant since injection of the same amount of polypeptide alone generated poor antibody responses. In previous studies, i.p. administration of GST-Cpa247-370 was shown to generate significant serum IgG responses, but in this case the protein was administered with Freund's adjuvant (37).
TABLE 2.
Anti-GST-Cpa247-370 titers following parenteral immunizationa
| Immunogen | Anti-GST- Cpa247-370 titer |
|---|---|
| HT230 | 849 |
| HT251 | 3,837 |
| HT266 | 1,869 |
| PY79 (nonrecombinant) | 86 |
| GST-Cpa247-370 (0.075 μg) | 46 |
| Naïve | 40 |
Groups of mice were given three i.p. injections. Serum anti-GST-Cpa247-370 titers were determined by ELISA at day 40.
Serum anti-GST-Cpa247-370 responses following oral and nasal immunization.
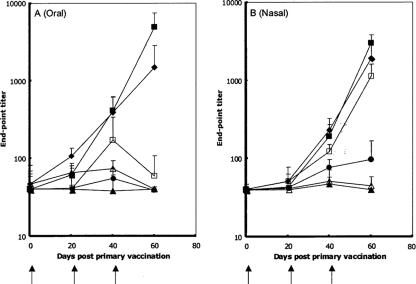
Groups of inbred mice were immunized either orally or intranasally with spores of HT230, HT251, and HT266. In addition to a naïve group, one group was dosed with nonrecombinant PY79 spores and another with pure GST-Cpa247-370 protein (at 3.6 μg or 0.15 μg for oral and nasal routes, respectively). In each case, the amount of protein equaled that expected to be delivered by surface display on the spore coat of HT251 and HT266 spores. Using the oral route, anti-GST-Cpa247-370 IgG titers were obtained at days 40 (second dose) and 60 (third dose) in mice immunized with HT251 and HT266 spores (Fig. 2A). These responses were significantly higher than those of the control groups (P < 0.05). Mice dosed with HT230 spores did show an increase in IgG titers at day 40 but at levels not significantly (P > 0.05) higher than those in mice immunized with nonrecombinant spores or those of the naïve group. The nasal immunizations showed somewhat different responses (Fig. 2B). Here, in addition to HT251 and HT266 spores, HT230 spores were also found to produce significant (P < 0.05) anti-GST-Cpa247-370 responses following the second and third boosts. No other significant responses were observed in the other groups. In addition to anti-GST-Cpa247-370 responses, antispore IgG levels following oral and intranasal immunization were determined as described previously for a recombinant spore vaccine against tetanus (8). Using either oral or intranasal administration of PY79, systemic spore-coat-specific IgG levels of HT230, HT251, or HT266 spores were significantly higher than those of the naïve group (P < 0.05) (data not shown).
FIG. 2.
Systemic responses after mucosal immunizations. Serum anti-GST-Cpa247-370-specific IgG responses after oral (A) or intranasal (B) immunization with recombinant B. subtilis spores. Groups of eight mice were immunized (↑) with spores orally (5 × 1010/dose) or nasally (2 × 109/dose) of strain HT230 (rrnO-gst-cpa247-370) (□), HT251 (cotB-gst-cpa247-370) (▪), and HT266 (rrnO-gst-cpa247-370 cotB-gst-cpa247-370) (♦). Three other control groups were PY79 (nonrecombinant) (▵), naïve (▴), and pure GST-Cpa247-370 protein (•). Individual serum samples from groups were tested by ELISA for GST-Cpa247-370-specific IgG. Data are presented as arithmetic means, and error bars are the standard deviation.
Ratio of specific anti-GST-Cpa247-370 IgG1 and IgG2a titers.
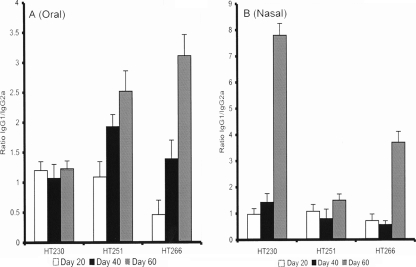
In order to determine whether there was a Th1 or Th2 bias, we examined the ratio of the IgG1 and IgG2a subclasses in serum samples (Fig. 3). For the oral immunizations, with the exception of HT230 spores (which failed to induce significant IgG responses when administered orally), there was a clear increase in the IgG1:IgG2a titers, which was indicative of a Th2 response (3, 13, 29, 35). IgG1:IgG2a ratios from mice nasally immunized with HT230 and HT266 spores showed clear increases after the second dose of spores. However, with HT251 spores there was no significant change in ratios.
FIG. 3.
Anti-GST-Cpa247-370-specific IgG1 and IgG2a ratios. Serum samples taken from selected oral (A) and nasal (B) immunization experiments (Fig. 2) were analyzed for the IgG1 and IgG2a subclasses. The relative ratios of IgG1 to IgG2a at days 20, 40, and 60 postdosing are shown. Samples were from HT230 (rrnO-gst-cpa247-370), HT251 (cotB-gst-cpa247-370), and HT266 (rrnO-gst-cpa247-370 cotB-gst-cpa247-370).
Mucosal anti-GST-Cpa247-370 IgA responses.
sIgA responses were determined from lung washes, saliva, and fresh fecal pellets in mice immunized orally and nasally. Figure 4 shows anti-GST-Cpa247-370 IgA responses at day 60 and demonstrates that strong sIgA responses could be achieved using recombinant spores. Lung washes gave the highest responses but are conceivably contaminated with serum IgA (as opposed to sIgA). Saliva and fecal responses showed a clear difference, with much higher levels being found in the saliva. In the case of HT230 spores, responses were considerably lower than those of the other strains (HT251 and HT266) when delivered orally but were significantly higher (P < 0.05) than those of the control groups. Further differences were found between immunogens, which were the same for both oral and nasal immunizations. For lung, saliva, and fecal samples, HT230 responses were always significantly lower (P > 0.05) than those of HT251. HT251 responses, though, were always significantly higher (P < 0.05) than those of HT266 for the saliva and lung samples but not in the case of the fecal samples.
FIG. 4.
GST-Cpa247-370-specific lung, saliva, and fecal IgA responses. Groups of mice immunized orally (A) or intranasally (B) with recombinant B. subtilis spores from the experiment shown in Fig. 2 were examined for their sIgA responses in lung, saliva, and fecal samples taken on day 60. Groups shown are mice immunized with spores of HT230 (rrnO-gst-cpa247-370), HT251 (cotB-gst-cpa247-370), and HT266 (rrnO-gst-cpa247-370 cotB-gst-cpa247-370). Two other control groups shown were naïve mice and mouse-administered nonrecombinant PY79 spores. Individual serum samples from groups were tested by ELISA for GST-Cpa247-370-specific IgG. Data are presented as arithmetic means, and error bars are the standard deviation.
In vitro neutralization.
Serum was taken from the nasally and orally immunized animals at day 60, pooled, and analyzed for its ability to neutralize, in vitro, the biological activity of alpha toxin (Table 3). As a positive control, serum was taken from mice injected (i.p.) with GST-Cpa247-370 protein (2 μg), and this was shown to neutralize alpha toxin-mediated hemolysis of erythrocytes, with a geometric mean titer (GMT) of neutralizing antibodies of 158.6. Our analysis showed that compared to control groups, which had GMTs of 1.1 (naïve) and 1.15 (PY79 spores), serum obtained from animals immunized with recombinant spores had significantly (P < 0.05) higher GMT levels. In the case of HT251 and HT266 spores, the levels were 10-fold higher. Although sIgA samples were not evaluated in this study due to the small sample volumes, it was clear that neutralizing activity was present in the serum from animals immunized with spores expressing GST-Cpa247-370 on the spore surface.
TABLE 3.
In vitro neutralization of alpha toxin activity
| Antiserum and/or route of administrationa | GMT | 95% CIb | P valuec |
|---|---|---|---|
| GST-Cpa247-370d | 158.6 | 154-164 | 0.001 |
| Naïve | 1.1 | 0.8-1.4 | >0.2 |
| Oral | |||
| PY79 | 1.15 | 0.75-1.8 | >0.2 |
| HT230 | 3.8 | 3.0-4.6 | 0.001 |
| HT251 | 19.9 | 13.8-36.1 | 0.02 |
| HT266 | 9.5 | 8.4-10.6 | 0.001 |
| Nasal | |||
| PY79 | 1.3 | 0.8-1.6 | >0.2 |
| HT230 | 3.7 | 3.0-17.7 | 0.001 |
| HT251 | 10.3 | 6.9-13.7 | 0.001 |
| HT266 | 10.5 | 9.4-11.6 | 0.001 |
Serum (day 60) obtained from mice immunized as outlined in Fig. 2 was pooled and serially diluted, and aliquots were preadsorbed with 0.1 μg of alpha toxin. Preadsorbed mixtures were coincubated with mouse erythrocytes for 1 h, and hemolysis was measured. The end-point titer was defined as the dilution causing 50% hemolysis.
CI, confidence interval.
P value compared to PY79 and naïve control groups.
Serum from mice immunized (i.p.) with 2 μg of pure GST-Cpa247-370 protein.
In vivo neutralization.
An in vivo neutralization assay was used to assess the inhibitory activity of anti-GST-Cpa247-370 antibodies. HT230 antiserum was not examined due to its relatively low titers determined using the in vitro assay. Mouse serum from immunized animals was incubated with 2 LD50 of purified alpha toxin (in a volume of 0.2 ml). Mice were injected (i.p.) with the antiserum-toxin mixture, and time to onset of toxicity was determined (Table 4). In control groups consisting of naïve mice and mice immunized with nonrecombinant PY79 spores, serum failed to inhibit the action of alpha toxin. In contrast, serum from mice immunized with recombinant spores, whether by the oral or nasal routes, showed inhibitory activity for at least 10 h after the injection. In the case of serum from mice immunized with HT251 spores, the highest levels of inhibition were observed, with two out of four animals showing no signs of toxicity after 24 h.
TABLE 4.
In vivo neutralization of alpha toxin activity
| Antiserum and/or route of administrationa | No. of mice/group showing no signs of toxicity at time postinjection
|
|||
|---|---|---|---|---|
| <5 h | 10 h | 18 h | 24 h | |
| Naïve | 0 | |||
| Oral | ||||
| PY79 | 0 | |||
| HT251 | 4 | 4 | 3 | 2 |
| HT266 | 4 | 4 | 0 | |
| Nasal | ||||
| PY79 | 0 | |||
| HT251 | 4 | 4 | 2 | 2 |
| HT266 | 4 | 4 | 0 | |
Serum (day 60) obtained from mice immunized as outlined in Fig. 2 was pooled, diluted 1/5, and mixed with alpha toxin (2 h at 37°C) corresponding to 2 LD50. The preadsorbed toxin mixture was injected (i.p.) into individual mice, and symptoms were monitored.
Protection against toxin challenge.
Based on the in vitro and in vivo assessments of neutralizing antibodies mice immunized with HT251 and HT266 spores were most likely to gain protection. Accordingly, mice were dosed with HT251 or HT266 spores by the oral or nasal routes and then challenged with a subcutaneous dose of alpha toxin. We found that for orally immunized mice, they showed protection to 12 LD50 (Table 5). Mice dosed with nonrecombinant PY79 spores or with 3.6 μg GST-Cpa247-370 (corresponding to the dose of immunogen displayed on one oral dose of recombinant spores) exhibited symptoms in less than 3 h. Protection levels were higher for nasally immunized mice than for orally immunized mice, with all HT251-immunized mice surviving a challenge of 12 LD50.
TABLE 5.
Protection of orally immunized animals against a challenge with alpha toxina
| Immunogen and/or route of administration | Dose of alpha toxin (LD50) | No. of survivors/total no. of mice | Mean time (h) to symptoms ± standard error of the mean |
|---|---|---|---|
| Naïve | 2 | 0/4 | 4.5 ± 0.5 |
| Oral | |||
| GST-Cpa247-370 (3.6 μg) | 6 | 0/6 | 2.5 ± 0.5 |
| PY79 | 6 | 0/6 | 2.0 ± 0.5 |
| HT251 | 6 | 6/6 | |
| HT251 | 12 | 3/6 | |
| HT266 | 6 | 6/6 | |
| HT266 | 12 | 2/6 | |
| Nasal | |||
| GST-Cpa247-370 (0.015 μg) | 6 | 0/6 | 2.5 ± 0.3 |
| PY79 | 6 | 0/6 | 2.0 ± 0.5 |
| HT251 | 6 | 6/6 | |
| HT251 | 12 | 6/6 | |
| HT266 | 6 | 6/6 | |
| HT266 | 12 | 5/6 |
Animals immunized as described for the experiments shown in Fig. 2 but from a separate experiment.
DISCUSSION
Immunization and protection of mice with a genetically engineered vaccine expressing the C-terminal domain of C. perfringens alpha toxin are relevant to a potential vaccine against gas gangrene in humans. Our long-term aim is to develop an oral vaccine that could be used to protect poultry from necrotic enteritis, for which no vaccine is currently available and there is a clear economical need.
The use of heat-stable spores as vaccine delivery agents is attractive since they could be incorporated into feed and would require no refrigeration. The C-terminal domain of C. perfringens alpha toxin has been identified as a strong immunogen and an effective vaccine against alpha toxin in mice (31, 37) but only when administered by a parenteral route. In this work, we have shown that a C-terminal fragment of alpha toxin, Cpa247-370, when fused to GST, can be successfully displayed on the surfaces of B. subtilis spores as well as in the live bacterium and was immunogenic when administered by a parenteral route. Interestingly, we have shown that the spore itself appears to provide an adjuvant effect, boosting the immune response to this antigen. The antigen was degraded under conditions mimicking those of the stomach and small intestine when displayed on the spore surface. This has been noticed previously when using B. subtilis spores for expression of the TTFC-protective antigen (32). In these studies, despite partial degradation of TTFC, mucosal responses were still achieved in immunized animals as well as protection from challenge with tetanus toxin. It has been demonstrated that B. subtilis spores not only germinate in the murine GI tract but also grow and then resporulate as they pass through the gut (32). It is probable then that the GST-Cpa247-370 immunogen is expressed once again as the live bacteria resporulate in the GI tract, providing a second dose of immunogen. When dosed orally or nasally, the resulting immune responses did not plateau, indicating that hyperimmunity had not yet been reached. In this work, we used three doses based on prior work, showing that three doses of spores expressing the TTFC antigen could elicit protective levels to tetanus antibodies (34). It would appear then that additional doses might boost immune responses further. Necrotic enteritis results from overgrowth and proliferation of C. perfringens within the GI tract, and for this a strong sIgA response might be beneficial. It is encouraging then that with our vaccines we have shown very strong sIgA responses in both the saliva and feces following oral or nasal delivery of recombinant spores. Supporting this, the resulting immune response exhibited a clear Th2 bias. In other work where we have used spores for delivery of the C. tetani TTFC antigen, display of the antigen on the spore produced a Th1 bias indicative of the involvement of a cellular response, whereas expression in germinating spores produced a Th2 bias (8, 21). It is possible that the GST-Cpa247-370 moiety is partially degraded in the GI tract, leading to a source of exogenous antigen that, following uptake into antigen-presenting cells (APCs) by phagocytosis or endocytosis, leads to presentation in a major histocompatibility complex class II-restricted manner. Alternatively, following phagocytosis the antigen is rapidly stripped from the spore, leading to class II-restricted presentation.
Systemic IgG and local sIgA responses specific to GST-Cpa247-370 when HT230 spores were administered nasally could result only if the spores had germinated. Currently, we have no evidence that any proliferation of spores within the nasopharynx occurs and we cannot rule out the possibility of low levels of cell multiplication. Expression of GST-Cpa247-370 in the germinating spore was shown to generate humoral responses, but these quickly declined following the second dose when spores were administered orally. This had been observed in a similar study when using the C. tetani TTFC antigen (34). The fate of ingested spores is now understood in principle, and spores have been shown to germinate in the lumen of the small intestine (jejunum and ileum). Not all spores germinate, and some pass through the GI tract and are shed in feces. A percentage of spores, though, are taken up by M cells (28) where they enter the Peyer's patches (8). Phagocytosis of spores results in ingested spores that are able to germinate within the phagosome where they can persist for a number of hours (9). This interaction with APCs helps promote a potent antispore as well as an antivegetative cell response (7). It is likely that antispore sIgA may promote rapid uptake and destruction of opsonized spores following the second dose of immunogen, preventing germination of spores or enhancing their uptake by APCs. Interestingly, sIgA responses, as well as neutralization and protection, were lower in animals dosed with HT266 spores than in animals given an identical amount of HT251 spores. The amount of GST-Cpa247-370 displayed on the spore coat was equivalent in HT251 and HT266 spores (Fig. 1B), yet the immune response was somewhat lower in animals dosed with the latter. HT266 spores also expressed the GST-Cpa247-370 antigen following spore germination. It is not yet clear why responses are lower or whether this is significant, but it appears that the strategy for antigen presentation does affect humoral and protective immunity.
Our studies show that genetically engineered spores can be used to mucosally vaccinate mice to at least 12 LD50. Necrotic enteritis results from the proliferation of C. perfringens in the GI tract of poultry. If used orally, or indeed nasally, we might expect immunization of poultry, with recombinant spores conferring even higher levels of protection to the live bacterium. This might occur because of the action of sIgA, which we have shown in this work to be stimulated to high levels and which would be expected to act as the first line of defense against C. perfringens alpha toxin produced in the GI tract. It is also important to consider that nonrecombinant B. subtilis is being used in a number of poultry feeds as a competitive exclusion agent (12) and has been shown to suppress C. perfringens infection in poultry (18). Using strain PY79, which was isogenic to the recombinant strains used in this work, a single dose of 1 × 109 spores produced a significant reduction in colonization of C. perfringens in the GI tract but only in animals where C. perfringens colonization was already established. This work showed that B. subtilis spores were able to interfere with colonization and persistence of C. perfringens, possibly by immune stimulation, which has been proposed as one way in which protection could occur (18). Coupled with the heat stability of the spore, it is worthwhile at least to consider further development of this antigen delivery system for vaccination against necrotic enteritis. As a first step, it is now necessary to evaluate protection in a poultry model of C. perfringens infection, addressing primarily issues of identification of the optimal dosing regimen, and these studies are in progress.
In conclusion, our data show that recombinant spores offer a new prototype vaccine that could have real value in the poultry industry as a heat-stable vaccine against C. perfringens infection.
Acknowledgments
This work was supported by two grants (LSH-2005-036871 and NMP4-CT-2004-013523) from the European Union's Sixth Framework to S.M.C.
Editor: S. R. Blanke
Footnotes
Published ahead of print on 8 September 2008.
REFERENCES
- 1.Awad, M. M., A. E. Bryant, D. L. Stevens, and J. I. Rood. 1995. Virulence studies on chromosomal alpha-toxin and theta-toxin mutants constructed by allelic exchange provide genetic evidence for the essential role of alpha-toxin in Clostridium perfringens-mediated gas gangrene. Mol. Microbiol. 15191-202. [DOI] [PubMed] [Google Scholar]
- 2.Baba, E., A. L. Fuller, J. M. Gilbert, S. G. Thayer, and L. R. McDougald. 1992. Effects of Eimeria brunetti infection and dietary zinc on experimental induction of necrotic enteritis in broiler chickens. Avian Dis. 3659-62. [PubMed] [Google Scholar]
- 3.Balloul, J.-M., J.-M. Grzych, R. J. Pierce, and A. Capron. 1987. A purified 28,000 dalton protein from Schistosoma mansoni adult worms protects rats and mice against experimental schistosomiasis. J. Immunol. 1383448-3453. [PubMed] [Google Scholar]
- 4.Barbosa, T. M., C. R. Serra, R. M. La Ragione, M. J. Woodward, and A. O. Henriques. 2005. Screening for bacillus isolates in the broiler gastrointestinal tract. Appl. Environ. Microbiol. 71968-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ciabattini, A., R. Parigi, R. Isticato, M. R. Oggioni, and G. Pozzi. 2004. Oral priming of mice by recombinant spores of Bacillus subtilis. Vaccine 224139-4143. [DOI] [PubMed] [Google Scholar]
- 6.Duc, L. H., H. A. Hong, H. S. Atkins, H. C. Flick-Smith, Z. Durrani, S. Rijpkema, R. W. Titball, and S. M. Cutting. 2007. Immunization against anthrax using Bacillus subtilis spores expressing the anthrax protective antigen. Vaccine 25346-355. [DOI] [PubMed] [Google Scholar]
- 7.Duc, L. H., H. A. Hong, and S. M. Cutting. 2003. Germination of the spore in the gastrointestinal tract provides a novel route for heterologous antigen presentation. Vaccine 214215-4224. [DOI] [PubMed] [Google Scholar]
- 8.Duc, L. H., H. A. Hong, N. Fairweather, E. Ricca, and S. M. Cutting. 2003. Bacterial spores as vaccine vehicles. Infect. Immun. 712810-2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duc, L. H., H. A. Hong, N. Q. Uyen, and S. M. Cutting. 2004. Intracellular fate and immunogenicity of B. subtilis spores. Vaccine 221873-1885. [DOI] [PubMed] [Google Scholar]
- 10.Guérout-Fleury, A. M., N. Frandsen, and P. Stragier. 1996. Plasmids for ectopic integration in Bacillus subtilis. Gene 18057-61. [DOI] [PubMed] [Google Scholar]
- 11.Heier, B. T., A. Lovland, K. B. Soleim, M. Kaldhusdal, and J. Jarp. 2001. A field study of naturally occurring specific antibodies against Clostridium perfringens alpha toxin in Norwegian broiler flocks. Avian Dis. 45724-732. [PubMed] [Google Scholar]
- 12.Hong, H. A., L. H. Duc, and S. M. Cutting. 2005. The use of bacterial spore formers as probiotics. FEMS Microbiol. Rev. 29813-835. [DOI] [PubMed] [Google Scholar]
- 13.Isaka, M., Y. Yasuda, M. Mizokami, S. Kozuka, T. Taniguchi, K. Matano, J. Maeyama, K. Mizuno, K. Morokuma, K. Ohkuma, N. Goto, and K. Tochikubo. 2001. Mucosal immunization against hepatitis B virus by intranasal co-administration of recombinant hepatitis B surface antigen and recombinant cholera toxin B subunit as an adjuvant. Vaccine 191460-1466. [DOI] [PubMed] [Google Scholar]
- 14.Isticato, R., G. Cangiano, H. T. Tran, A. Ciabattini, D. Medaglini, M. R. Oggioni, M. De Felice, G. Pozzi, and E. Ricca. 2001. Surface display of recombinant proteins on Bacillus subtilis spores. J. Bacteriol. 1836294-6301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keyburn, A. L., S. A. Sheedy, M. E. Ford, M. M. Williamson, M. M. Awad, J. I. Rood, and R. J. Moore. 2006. Alpha-toxin of Clostridium perfringens is not an essential virulence factor in necrotic enteritis in chickens. Infect. Immun. 746496-6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kulkarni, R. R., V. R. Parreira, S. Sharif, and J. F. Prescott. 2007. Immunization of broiler chickens against Clostridium perfringens-induced necrotic enteritis. Clin. Vaccine Immunol. 141070-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.La Ragione, R. M., G. Casula, S. M. Cutting, and M. Woodward. 2001. Bacillus subtilis spores competitively exclude Escherichia coli 070:K80 in poultry. Vet. Microbiol. 79133-142. [DOI] [PubMed] [Google Scholar]
- 18.La Ragione, R. M., and M. J. Woodward. 2003. Competitive exclusion by Bacillus subtilis spores of Salmonella enterica serotype Enteritidis and Clostridium perfringens in young chickens. Vet. Microbiol. 94245-256. [DOI] [PubMed] [Google Scholar]
- 19.Logan, A. J., E. D. Williamson, R. W. Titball, D. A. Percival, A. D. Shuttleworth, J. W. Conlan, and D. C. Kelly. 1991. Epitope mapping of the alpha-toxin of Clostridium perfringens. Infect. Immun. 594338-4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Long, J. R., and R. B. Truscott. 1976. Necrotic enteritis in broiler chickens. III. Reproduction of the disease. Can. J. Comp. Med. 4053-59. [PMC free article] [PubMed] [Google Scholar]
- 21.Mauriello, E. M. F., L. H. Duc, R. Isticato, G. Cangiano, H. A. Hong, M. De Felice, E. Ricca, and S. M. Cutting. 2004. Display of heterologous antigens on the Bacillus subtilis spore coat using CotC as a fusion partner. Vaccine 221177-1187. [DOI] [PubMed] [Google Scholar]
- 22.McDonel, J. L. 1986. Toxins of Clostridium perfringens types A, B, C, D and E, p. 477-517. In F. Dorner and J. Drews (ed.), Pharmacology of bacterial toxins. Permagon Press, Oxford, United Kingdom.
- 23.Neeson, B. N., G. C. Clark, H. S. Atkins, B. Lingard, and R. W. Titball. 2007. Analysis of protection afforded by a Clostridium perfringens alpha-toxoid against heterologous clostridial phospholipases C. Microb. Pathog. 43161-165. [DOI] [PubMed] [Google Scholar]
- 24.Nicholson, W. L., and P. Setlow. 1990. Sporulation, germination and outgrowth, p. 391-450. In C. R. Harwood and S. M. Cutting (ed.), Molecular biological methods for Bacillus. John Wiley & Sons Ltd., Chichester, United Kingdom.
- 25.Oggioni, M. R., A. Ciabattini, A. M. Cuppone, and G. Pozzi. 2003. Bacillus spores for vaccine delivery. Vaccine 2196-101. [DOI] [PubMed] [Google Scholar]
- 26.Phillips, I. 2007. Withdrawal of growth-promoting antibiotics in Europe and its effects in relation to human health. Int. J. Antimicrob. Agents 30466-468. [DOI] [PubMed] [Google Scholar]
- 27.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 27493-497. [Google Scholar]
- 28.Rhee, K. J., P. Sethupathi, A. Driks, D. K. Lanning, and K. L. Knight. 2004. Role of commensal bacteria in development of gut-associated lymphoid tissues and preimmune antibody repertoire. J. Immunol. 1721118-1124. [DOI] [PubMed] [Google Scholar]
- 29.Robinson, K., L. M. Chamberlain, K. M. Schofield, J. M. Wells, and R. W. F. Le Page. 1997. Oral vaccination of mice against tetanus with recombinant Lactococcus lactis. Nat. Biotechnol. 15653-657. [DOI] [PubMed] [Google Scholar]
- 30.Songer, J. G. 1996. Clostridial enteric diseases of domestic animals. Clin. Microbiol. Rev. 9216-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stevens, D. L., R. W. Titball, M. Jepson, C. R. Bayer, S. M. Hayes-Schroer, and A. E. Bryant. 2004. Immunization with the C-domain of alpha-toxin prevents lethal infection, localizes tissue injury, and promotes host response to challenge with Clostridium perfringens. J. Infect. Dis. 190767-773. [DOI] [PubMed] [Google Scholar]
- 32.Tam, N. M. K., N. Q. Uyen, H. A. Hong, L. H. Duc, T. T. Hoa, C. H. Serra, A. O. Henriques, and S. M. Cutting. 2006. The intestinal life cycle of Bacillus subtilis and close relatives. J. Bacteriol. 1882692-2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Titball, R. W., A. M. Fearn, and E. D. Williamson. 1993. Biochemical and immunological properties of the C-terminal domain of the alpha-toxin of Clostridium perfringens. FEMS Microbiol. Lett. 11045-50. [DOI] [PubMed] [Google Scholar]
- 34.Uyen, N. Q., H. A. Hong, and S. M. Cutting. 2007. Enhanced immunisation and expression strategies using bacterial spores as heat-stable vaccine delivery vehicles. Vaccine 25356-365. [DOI] [PubMed] [Google Scholar]
- 35.VanCott, J. L., H. F. Staats, D. W. Pascual, M. Roberts, S. N. Chatfield, A. Yamamoto, M. Coste, P. B. Carter, H. Kiyono, and J. R. McGhee. 1996. Regulation of mucosal and systemic antibody responses by T helper cell subsets, macrophages, and derived cytokines following oral immunisation with live recombinant Salmonella. J. Immunol. 1561504-1514. [PubMed] [Google Scholar]
- 36.Van Immerseel, F., J. De Buck, F. Pasmans, G. Huyghebaert, F. Haesebrouck, and R. Ducatelle. 2004. Clostridium perfringens in poultry: an emerging threat for animal and public health. Avian Pathol. 33537-549. [DOI] [PubMed] [Google Scholar]
- 37.Williamson, E. D., and R. W. Titball. 1993. A genetically engineered vaccine against the alpha-toxin of Clostridium perfringens protects mice against experimental gas gangrene. Vaccine 111253-1258. [DOI] [PubMed] [Google Scholar]
- 38.Youngman, P., J. Perkins, and R. Losick. 1984. Construction of a cloning site near one end of Tn917 into which foreign DNA may be inserted without affecting transposition in Bacillus subtilis or expression of the transposon-borne erm gene. Plasmid 121-9. [DOI] [PubMed] [Google Scholar]
- 39.Zekarias, B., H. Mo, and R. R. Curtiss III. 2008. Recombinant attenuated Salmonella enterica serovar Typhimurium expressing the carboxy-terminal domain of alpha toxin from Clostridium perfringens induces protective responses against necrotic enteritis in chickens. Clin. Vaccine Immunol. 15805-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou, Z., H. Xia, X. Hu, Y. Huang, C. Ma, X. Chen, F. Hu, J. Xu, F. Lu, Z. Wu, and X. Yu. 2008. Immunogenicity of recombinant Bacillus subtilis spores expressing Clonorchis sinensis tegumental protein. Parasitol. Res. 102293-297. [DOI] [PubMed] [Google Scholar]