Abstract
Exotoxins which belong to the family containing the RTX toxins (repeats in toxin) contribute to a variety of important human and animal diseases. One example of such a toxin is the potent leukotoxin (LKT) produced by the bovine respiratory pathogen Mannheimia haemolytica. LKT binds to CD18, resulting in the death of bovine leukocytes. In this study, we showed that internalized LKT binds to the outer mitochondrial membrane, which results in the release of cytochrome c and collapse of the mitochondrial membrane potential (ψm). Incubation of bovine lymphoblastoid cells (BL-3 cells) with the mitochondrial membrane-stabilizing agent cyclosporine (CSA) reduced LKT-mediated cytotoxicity, cytochrome c release, and collapse of the ψm. Coimmunoprecipitation and intracellular binding studies suggested that LKT binds to the mitochondrial matrix protein cyclophilin D. We also demonstrated that LKT mobilizes the vesicle scission protein dynamin-2 from mitochondria to the cell membrane. Incubation with CSA depleted mitochondrial dynamin-2 in BL-3 cells, making it unavailable for vesicle scission and LKT internalization. The results of this study show that LKT trafficking and LKT-mediated cell death involve dynamin-2 and cyclophilin D, in a process that can be prevented by the mitochondrial membrane-protecting function of CSA.
Several recent reports have shown that some bacterial toxins enter cells and are transported to mitochondria. For example, Clostridium difficile toxin A binds to the mitochondrial protein GRP 75 within 5 min after exposure, resulting in direct damage to mitochondria in Chinese hamster ovary cells (18). Similarly, vacuolating cytotoxin A of Helicobacter pylori and Panton-Valentine leukocidin of Staphylococcus aureus bind to mitochondria, which results in cytochrome c release and reduced mitochondrial membrane potential (ψm) in intoxicated cells (13, 56). Likewise, we have reported that Mannheimia haemolytica leukotoxin (LKT) causes gross morphological changes in mitochondria of bovine lymphoblastoid cells (BL-3 cells), consistent with mitochondrial dysfunction (4).
M. haemolytica is the principal bacterial pathogen in the bovine respiratory disease complex (19, 58). The most important virulence factor of M. haemolytica is the LKT, which has potent cytotoxic effects on ruminant leukocytes but not on leukocytes from other species (38). M. haemolytica LKT is a member of the RTX toxin (repeats in toxin) family, which includes LKTs and hemolysins produced by a number of gram-negative bacteria. It was originally thought that M. haemolytica LKT damages cells by inserting into the cell membrane, resulting in pore formation and necrosis (53). At lower LKT concentrations, however, susceptible cells die via caspase-dependent apoptotic pathways (8, 10, 45, 46). LKT was first reported to bind to the β2 integrin CD18/CD11a, also known as leukocyte functional antigen 1 (LFA-1) (1, 24, 28). Recent studies, however, have shown that Mac-1 (CD18/CD11b) also binds LKT and that CD18 is the functional receptor for LKT (9, 30). After binding to LFA-1, LKT induces apoptosis of bovine leukocytes as a result of activation of caspase 1, caspase 3, and caspase 9 (4, 10, 31, 46). The LFA-1-dependent cytotoxicity of LKT is a conundrum, because signaling through LFA-1 generally results in cell adhesion and promotes cell survival (34, 44, 51).
In this study we hypothesized that mitochondrial damage and caspase 9 activation could be due to direct binding of LKT to the mitochondrial outer membrane (MOM). In this paper we show that LKT directly targets mitochondria in BL-3 cells, creating lesions in the MOM that can lead to release of proapoptotic proteins, culminating in cell death. We also show that LKT targeting to mitochondria is dynamin-2 dependent and that mitochondria appear to be the principal source of dynamin-2 in BL-3 cells. Treating BL-3 cells with cyclosporine (CSA) depleted mitochondrial dynamin-2, thereby inhibiting LKT transport to mitochondria and cell death.
MATERIALS AND METHODS
LKT production and purification.
Crude LKT was prepared and purified as described previously and was stored at −80°C until it was used in experiments (4). Inactive toxin from an lktC mutant of M. haemolytica strain A1 (SH 1562), which produces an LKT protein with no biological activity (LKTinact) (generously provided by S. K. Highlander, Baylor College of Medicine, Houston, TX), was prepared in a similar manner.
Cell lines, cell cultures, and antibodies.
Nonadherent bovine lymphoblastoid cells (BL-3 cells) and adherent murine macrophages (RAW 264.7 cells) (kindly provided by Ronald Schultz, University of Wisconsin-Madison) were grown at 37°C in the presence of 5% CO2 in RPMI medium supplemented with 10% fetal bovine serum (Gibco BRL, Burlington, VT). Neutralizing anti-LKT monoclonal antibodies (MAbs) MM601 and MM605 were generous gifts from S. Srikumaran (Washington State University, Pullman). Anti-dynamin-2 MAb, anti-cytochrome c antibody (Ab), anti-pyruvate dehydrogenase Ab, anti-cyclophilin D Ab, and anti-porin Ab were purchased from U.S. Biologicals (Swampscott, MA), Calbiochem (San Diego, CA), and Molecular Probes (Carlsbad, CA).
Isolation of mitochondria.
BL-3 cells were incubated with 0.5 U of LKT or LKTinact for 30 to 60 min at 37°C, and their mitochondria were isolated as described previously (4, 26). A crude mitochondrial preparation was immunopurified using anti-porin-2 Ab-coated agarose protein A/G beads (10 μg of MAb on 50 μl of beads). The immunopurified mitochondrial pellet was boiled in loading buffer for 2 min and loaded on a 4 to 15% sodium dodecyl sulfate (SDS)-polyacrylamide gradient gel for electrophoresis. The resulting bands were blotted onto a polyvinylidene difluoride membrane and analyzed by immunoblotting for LKT, cytochrome c, dynamin-2, or mitochondrion-specific porin-2 proteins.
LKT treatment of BL-3 cells.
BL-3 cells (106 cells per ml) were incubated with LKT (0.5 U) for 60 min, washed, and resuspended in antibiotic-free RPMI medium. Cytotoxicity was quantified using the Cell Titre 96 AQ one-assay system (Promega Corporation, Madison, WI). In some experiments mitochondria were isolated from BL-3 cells as described above. Mitochondria were then incubated with CSA (5 μM for 30 min at 37°C) or propranolol (200 μM for 30 min at 37°C) in mitochondrial wash buffer before they were incubated with LKT (0.2 U) for 30 min at 37°C. The mitochondrial pellet was washed three times with mitochondrial wash buffer and then lysed before SDS-polyacrylamide gel electrophoresis (PAGE) and Western immunoblotting were performed. To investigate the effects of actin polymerization in response to LKT, BL-3 cells were incubated in RPMI medium with cytochalasin D (2 μg/ml; Sigma) for 1 h at 37°C. Following this, the cells were incubated with LKT (0.5 U) for 1 h, and toxin internalization and cytotoxicity were determined as described above.
siRNA inhibition of dynamin-2.
Dynamin-2 was knocked down with small interfering RNA (siRNA) designed for the sequence 5′-GGGATGTCCTGGAGAACAA-3′, using the T7 RiboMAX Express RNAi system (Promega Co., Madison, WI) as described previously (3).
Scanning electron microscopy.
Mitochondria isolated from BL-3 cells treated with LKT (0.5 U for 0.5 or 1 h at 37°C) were blocked with 3% bovine serum albumin for 30 min at 25°C and incubated overnight with 18-nm colloidal gold-labeled anti-LKT MAb MM601 at 4°C. The mitochondria were then processed for scanning electron microscopy as described previously (4). Sample images were obtained by using an accelerative voltage of 5 kV. Both secondary electron microscopy imaging and back-scattered electron microscopy imaging were performed using a Hitachi S-900 field emission scanning electron microscope (Hitachi High Technologies, Pleasanton, CA).
Transmission electron microscopy.
LKT-treated or control BL-3 cells were fixed in modified Karnovsky's fixative (2% glutaraldehyde, 2% paraformaldehyde) with 0.1 M cacodylate buffer for 1 h at 25°C, washed twice with 0.1 M cacodylate buffer for 10 min, and stored overnight at 4°C in fresh buffer in a refrigerator. Cells were then fixed with 2% OsO4 buffer for 30 min, rinsed twice with water for 10 min, and embedded in 3% agar. Each sample was placed in 50% ethanol and dehydrated using a graded ethanol series (30 to 95% ethanol). The sample was treated with propylene oxide-100% ethanol (1:1) for 10 min, incubated twice in propylene oxide for 5 min, and infiltrated using a graded epoxy series (25 parts to 75 parts of propylene oxide) for 1 h. Samples were then embedded in a fresh epoxy mixture and polymerized in a 60°C oven for 26 h.
Laser confocal microscopy.
BL-3 cells were incubated with 0.5 U of LKT for 120 min at 37°C, fixed with 4% paraformaldehyde, washed three times with phosphate-buffered saline (PBS), and permeabilized with cold acetone (−20°C) for 10 min. Cells were washed three times with PBS and incubated with anti-LKT MAb MM601, Mitotracker Red (Molecular Probes, Carlsbad, CA), or anti-dynamin-2 MAb (U.S. Biologicals, Swampscott, MA). Cells were stained with fluorescein isothiocyanate (FITC)- or Texas Red-tagged secondary antibodies and visualized by confocal microscopy at emission wavelengths of 474 and 595 nm (Nikon C1 laser confocal microscope with fluorescent resonance energy transfer).
Flow cytometry of purified mitochondria from LKT-treated BL-3 cells.
Mitochondria were purified from LKT-treated BL-3 cells as described above. The mitochondria were fixed in 2% paraformaldehyde at 25°C for 15 min and washed three times in PBS by using centrifugation at 10,000 × g. Fixed mitochondria were then stained with anti-LKT FITC and Mitotracker Red for 1 h and 10 min, respectively. After three washes in PBS, they were analyzed by flow cytometry (BD Biosciences, Rockville, MD). Mitochondria isolated from BL-3 cells that were not treated with LKT and mitochondria purified from LKT-treated BL-3 cells stained with an unrelated primary antibody served as controls.
ψm of LKT-treated BL-3 cells and purified mitochondria.
Mitochondrial membrane potential ψm was determined using JC-1 (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide/chloride) as described in the ψm detection kit protocol (Cell Technology Inc., Mountain View, CA). Briefly, 106 BL-3 cells were washed and incubated with the JC-1 reagent at 37°C for 15 min in a 5% CO2 incubator. The cells were washed three times in PBS, and flow cytometry was performed at an emission wavelength of 595 nm. BL-3 cells were displayed and gated based on forward and side light scatter characteristics. Purified mitochondria isolated from 106 LKT-treated BL-3 cells were analyzed by flow cytometry in a similar manner. Mitochondria were gently fixed in 2% paraformaldehyde for 15 min on ice, washed three times in PBS, and stained simultaneously with FITC-tagged anti-LKT MAb and Texas Red-tagged anti-porin-2 MAb overnight at 4°C. The mitochondrial pellet was washed three times in PBS, and binding of LKT to mitochondria was analyzed using the Texas Red-positive (i.e., porin-2-positive) mitochondrion population.
Protein (antibody and LKT) transfection into BL-3 and RAW 264.7 cells.
Anti-LKT, anti-β-actin, and anti-cyclophilin D Ab transfection into BL-3 cells and LKT transfection into RAW 264.7 cells were performed using the Pro-ject protein transfection reagent according to the manufacturer's protocol (catalog no. 89850; Pierce Biotechnology Inc., Rockford, IL). Briefly, approximately 106 cells were serum starved for 1 h in Dulbecco modified Eagle medium at 37°C and then incubated with Pro-ject-Ab or Pro-ject-LKT complexes (10 μl of Pro-ject with 4 to 5 μg of Ab or 0.5 U of LKT) for 3 h at 37°C. BL-3 cells were washed three times in Dulbecco modified Eagle medium, and isolation of mitochondria or an LKT-mediated cytotoxicity assay was performed as described above. RAW 264.7 cells were fixed, permeabilized, and stained for LKT and mitochondria (with Mitotracker Red) as described previously.
Immunoprecipitation of LKT-protein complexes.
Agarose protein A/G beads (50 μl) were coated with anti-LKT MAb by incubating them with 20 μg of MAb in PBS for 1 h at 25°C. The beads were washed three times in PBS and then incubated with LKT (10 μl) and purified mitochondrial lysate (preabsorbed with agarose protein A/G beads) overnight at 4°C. Following incubation, the beads were washed five times with 0.05% Triton-X-PBS wash buffer, and protein was eluted by boiling the preparation with 5% SDS. The elute was subjected to SDS-PAGE, transferred to a polyvinylidene difluoride membrane, and probed for LKT and cyclophilin D. A separate SDS-PAGE gel stained with Coomassie blue contained two bands at molecular weights corresponding to the molecular weights of LKT and cyclophilin D (data not shown).
Statistical analysis.
Group means were compared by analysis of variance. Relevant comparisons were made using the Tukey-Kramer test as performed by the Instat statistical package (GraphPad, San Diego, CA). The level of statistical significance used was a P value of <0.05.
RESULTS
LKT detection in mitochondrial lysates and on mitochondria from LKT-treated BL-3 cells.
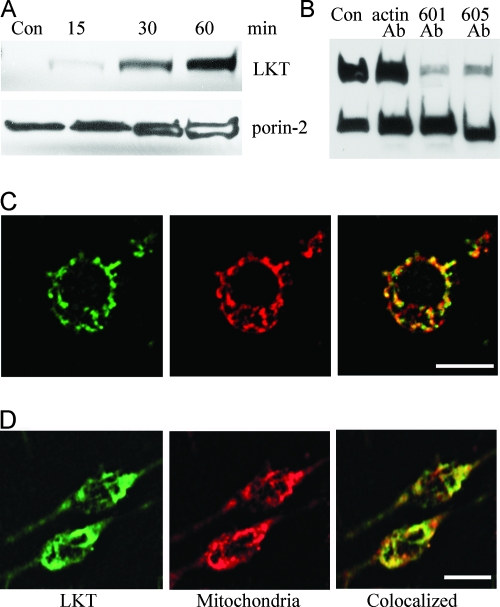
We previously reported that mitochondria from LKT-treated BL-3 cells exhibited gross morphological changes in the outer membrane that were larger than typical permeability transition pores (PTPs) (4). To investigate this process further, we immunopurified mitochondria from BL-3 cells incubated with LKT or LKTinact, prepared mitochondrial lysates, and analyzed for LKT binding by immunoblotting. The presence of porin-2 (a mitochondrion-specific protein) bands and the absence of β-actin bands on immunoblots confirmed that there was no cytosolic contamination of the mitochondrial preparations (data not shown). Substantial amounts of LKT but not LKTinact (data not shown) were detected in the mitochondrial lysates of BL-3 cells after 30 to 60 min of incubation. The estimated molecular mass of the LKT band on a Western blot was ∼104 kDa, which is consistent with the molecular mass of full-length LKT and suggests that LKT does not undergo cleavage or processing during binding to mitochondria (Fig. 1A; also see Fig. S1 in the supplemental material). Protein transfection of BL-3 cells with anti-LKT MAbs MM601 and MM605 inhibited binding of LKT to mitochondria within the cell (Fig. 1B; also see Fig. S1 in the supplemental material).
FIG. 1.
LKT binds to mitochondria from BL-3 cells and RAW 264.7 cells. (A) Immunopurified mitochondria from 106 BL-3 cells were incubated for 30 to 60 min with LKT (0.5 U). Lysates were prepared and immunoblotted for LKT and the mitochondrion-specific porin-2 protein. Con, control. (B) BL-3 cells were protein transfected with anti-LKT MAbs (5 μg of MM601 or MM605) or with anti-β-actin MAb as a negative control using the Pro-ject protein transfection reagent. The cells were then incubated with LKT (0.5 U) for 1 h. Mitochondria were immunopurified and lysed, and the lysates were immunoblotted for LKT and porin-2. (C) Single BL-3 cell stained for LKT (green), mitochondria (red), and colocalization of LKT with mitochondria (yellow). (D) RAW 264.7 cells were protein transfected with LKT (0.5 U) using the Pro-ject protein transfection reagent and stained for LKT (green) and mitochondria (red) as described above. Colocalization of LKT with mitochondria is indicated by yellow. Bars = 10 μm.
Next we performed a confocal microscopy analysis of LKT trafficking within BL-3 cells. BL-3 cells were incubated with LKT for 30 min and then permeabilized and stained with anti-LKT FITC to detect LKT and with Mitotracker Red to detect mitochondria. Fifty randomly selected BL-3 cells were spatially scanned to determine the positions of LKT (green) and mitochondria (red), and the degree of colocalization was calculated to be 58% ± 6.2% (mean ± standard error of the mean of three separate experiments with a probability of colocalization error of <5%) using Image J software (NIH; http://rsb.info.nih.gov/ij/) (Fig. 1C). Interestingly, there was LKT binding to the mitochondria when they were protein transfected with LKT in RAW 264.7 cells (a murine macrophage cell line), which are normally resistant to LKT (Fig. 1D).
LKT detected on the mitochondrial surface.
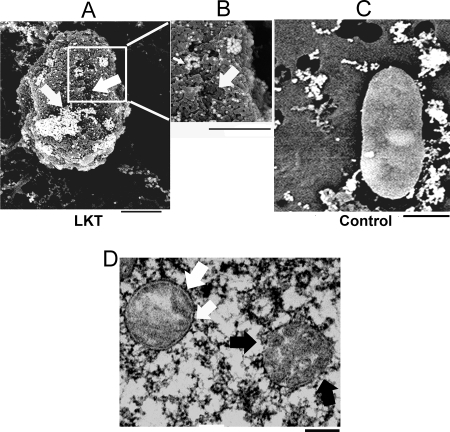
To visualize LKT binding to individual mitochondria, we performed a scanning electron microscopy analysis of mitochondria purified from LKT-treated BL-3 cells, using colloidal gold-labeled anti-LKT MAb. Clusters of 18-nm gold particles were found to be distributed over the mitochondrial surface. Punched-out lesions (diameter, 50 to 150 nm) were observed with gold particles on the periphery (Fig. 2A and B). The mitochondrial surface was very irregular, and there were regions where there was sloughing. Transmission electron microscopy of BL-3 cells incubated with LKT showed that there was partial to complete disruption of the mitochondrial outer and inner membrane architecture (Fig. 2D). In contrast, some mitochondria had a normal surface and internal architecture (Fig. 2D).
FIG. 2.
LKT on mitochondria isolated from BL-3 cells detected by scanning electron microscopy and transmission electron microscopy. Mitochondria isolated from BL-3 cells were incubated with 0.5 U LKT (A and B) or RPMI medium (control) (C), stained with anti-LKT colloidal gold, and visualized by scanning electron microscopy. The micrographs show areas of LKT binding (clusters of gold beads in panels A and B) and sloughing of the MOM, leaving punched-out areas (arrows in panels A and B). (D) Mitochondrion with a disrupted outer membrane and cristae (black arrows) and normal mitochondrion with an intact double membrane (white arrow). Bars = 200 nm.
Flow cytometry detection of LKT on mitochondria isolated from LKT-treated BL-3 cells.
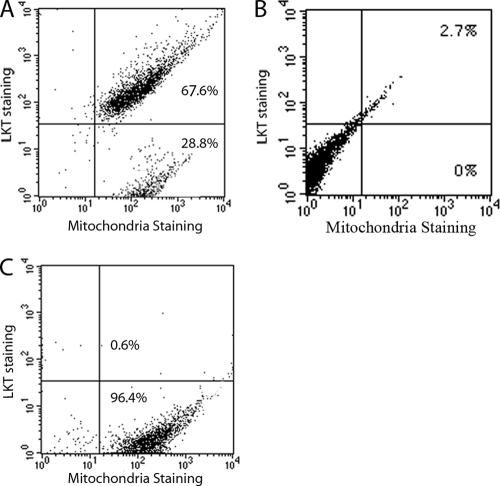
To quantify LKT binding to individual mitochondria, we performed a flow cytometry analysis of purified mitochondria from LKT-treated BL-3 cells that were double labeled with FITC-tagged anti-LKT MAb and mitochondrion-specific Mitotracker Red. The data obtained suggest that about 69% of the total mitochondrial population had LKT bound to the surface (Fig. 3).
FIG. 3.
LKT detected by flow cytometry of purified mitochondria from LKT-treated BL-3 cells. Mitochondria were stained with Mitotracker Red and FITC-conjugated anti-LKT MAb. (A) Mitochondria with positive staining for both LKT and Mitotracker Red. (B and C) Mitochondria from BL-3 cells not treated with LKT (B) and mitochondria from LKT-treated cells stained with Mitotracker Red and an isotype-matched primary Ab as controls (C). The data represent the results of three similar experiments that were performed.
CSA protects mitochondria against LKT-mediated damage.
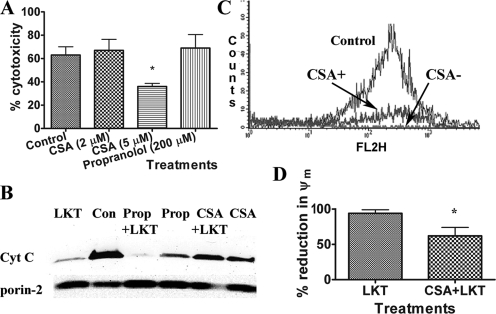
The data described above suggest that LKT binds to and damages the MOM. We used two pharmacological stabilizers of MOM (CSA and propranolol) to investigate damage to MOM caused by LKT. Pretreating BL-3 cells with CSA inhibited LKT-mediated killing of BL-3 cells by about 50%, whereas propranolol had no effect (Fig. 4A). Because CSA has other effects on cells (27, 57), we purified mitochondria from BL-3 cells, preincubated them with CSA or propranolol, and then incubated them briefly with LKT. Cell-free mitochondria preincubated with CSA, but not cell-free mitochondria preincubated with propranolol, were protected from LKT-mediated damage to the MOM and cytochrome c release (Fig. 4B; also see Fig. S2 in the supplemental material). Next, we investigated the effect of CSA on LKT-induced changes in ψm by staining cells with JC-1, a red fluorescent dye that is a sensitive indicator for ψm. Preincubation of BL-3 cells with CSA significantly inhibited the LKT-mediated reduction in ψm, presumably as a result of its ability to prevent mitochondrial membrane damage (Fig. 4C and D).
FIG. 4.
CSA protects BL-3 cells against LKT-mediated cytotoxicity, mitochondrial membrane damage, and collapse of ψm. (A) BL-3 cells (106 cells) were preincubated with CSA (2 or 5 μM) for 30 min before incubation with LKT (0.5 U) for 60 min. Cytotoxicity was measured using the Cell Titre 96 AQ one-assay system. (B) Mitochondria were immunopurified from untreated BL-3 cells, preincubated with CSA (5 μM) or with propranolol (Prop) (200 μM), and then incubated with LKT (0.2 U) for 30 min at 37°C. Immunoblot analysis of the resulting mitochondrial lysates demonstrated that CSA, but not propranolol, protected against LKT-mediated mitochondrial cytochrome c (Cyt c) release. Con, control. (C and D) BL-3 cells were preincubated with CSA or medium (control) and then incubated with LKT (0.5 U) for 1 h. Cells were then incubated with JC-1, a ψm-sensitive dye, at 37°C for 15 min and washed three times in PBS. Aggregated intramitochondrial JC-1 is red, and the monomeric cytoplasmic form is green. BL-3 cells were then analyzed by flow cytometry in the FL2 channel (absorption and emission maxima at 580 and 595 nm, respectively). The results show that CSA protected against collapse of ψm. The bars in panel D indicate the means of three separate experiments, and the error bars indicate the standard errors of the means. *, P < 0.05.
Mobilization of dynamin-2 in LKT-treated BL-3 cells.
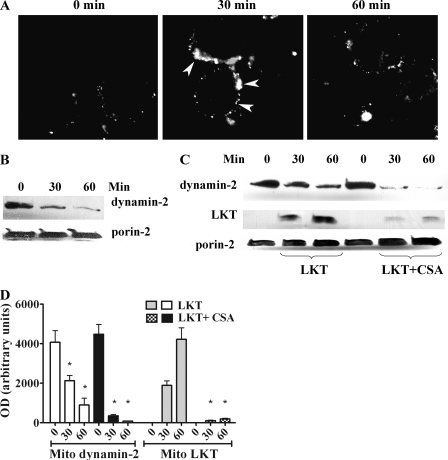
We previously reported that the vesicle scission protein dynamin-2 is important for LKT internalization and cytotoxicity in BL-3 cells. Here we observed mobilization of dynamin-2 to the periphery of the cell within 30 min after LKT treatment and dispersion of membrane-bound dynamin-2 into the cytoplasm by 60 min (Fig. 5A). Interestingly, we also found that dynamin-2 was significantly depleted in mitochondrial lysates from LKT-treated BL-3 cells (Fig. 5B).
FIG. 5.
Movement of dynamin-2 to the cell periphery in LKT-treated BL-3 cells. (A) BL-3 cells (106 cells) were incubated with LKT (0.5 U) for 30 or 60 min and then fixed, permeabilized, stained for cytoplasmic dynamin-2 (arrows), and visualized by confocal microscopy. (B) BL-3 cells were incubated with CSA (5 μM for 30 or 60 min at 37°C). Mitochondria were isolated and lysed, and the lysates were immunoblotted for dynamin-2 and porin-2. (C) BL-3 cells preincubated with CSA were treated with LKT for 30 or 60 min. Their mitochondria were then isolated and lysed, and the lysates were immunoblotted for LKT, dynamin-2, and porin-2. (D) Mitochondrial (Mito) dynamin-2 and LKT levels in immunoblots as determined by densitometry. The bars indicate the means of three separate experiments, and the error bars indicate the standard errors of the means (*, P < 0.05). OD, optical density.
We next investigated whether CSA inhibits binding of LKT to mitochondria. Incubation of BL-3 cells with CSA (5 μM) depleted mitochondrial dynamin-2 in a time-dependent manner (Fig. 5C). Furthermore, the translocation of dynamin-2 from mitochondria in response to LKT was augmented by pretreating BL-3 cells with CSA. Thus, pretreatment of BL-3 cells with CSA both depleted mitochondrial dynamin-2 and significantly inhibited LKT binding to mitochondria (Fig. 5C and D). Dynamin-2 was detected in mitochondrial fractions but not in membrane or cytoplasmic fractions from untreated BL-3 cells (data not shown).
Cytoskeletal actin and dynamin-2 are responsible for LKT targeting to mitochondria.
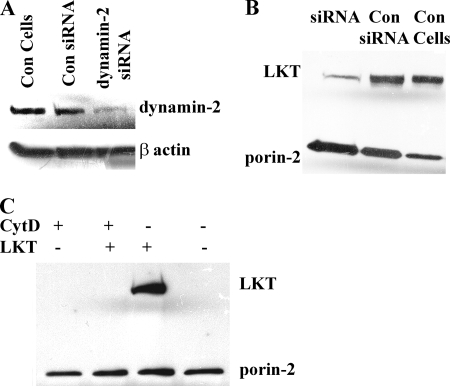
We previously observed that actin and dynamin-2 are responsible for surface trafficking and internalization of LKT in BL-3 cells (3). To investigate whether β-actin polymerization plays a role in LKT trafficking to mitochondria, we depolymerized β-actin in BL-3 cells by treating the cells with cytochalasin D or when dynamin-2 was knocked down with siRNA before incubating the cells with LKT. Mitochondria isolated from BL-3 cells in which β-actin was depleted or in which dynamin-2 was knocked down showed significantly less LKT binding than mitochondria from control BL-3 cells (Fig. 6B and C; also see Fig. S3 in the supplemental material).
FIG. 6.
Dynamin-2 and β-actin are required for targeting of LKT to mitochondria. (A) Representative immunoblot of cell lysates from BL-3 cells transfected with dynamin-2 siRNA, untreated cells (control [Con cells]), or cells transfected with scrambled siRNA (Con siRNA). (B) BL-3 cells were transfected with dynamin-2 siRNA or scrambled siRNA or were not treated (Con Cells) before incubation with LKT (0.5 U) at 37°C for 1 h. Mitochondria were purified and lysed, and the lysates were immunoblotted for LKT and porin-2 (mitochondrial marker). (C) BL-3 cells were pretreated with cytochalasin D (CytD) (2 μg/ml for 1 h at 37°C) before they were treated with LKT. Mitochondria were then isolated and immunoblotted for LKT and porin-2. The controls included cells treated with cytochalasin D alone and untreated BL-3 cells.
LKT binds to the mitochondrial the matrix protein cyclophilin D.
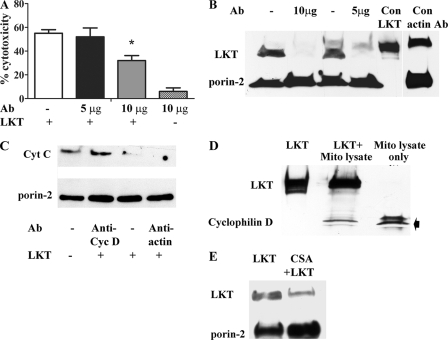
CSA binds to the mitochondrial matrix protein cyclophilin D and inhibits permeability transition and mitochondrion-dependent apoptosis (6, 33). Because CSA inhibits targeting of LKT to mitochondria from BL-3 cells, we investigated the possibility that LKT binds to the mitochondrial matrix protein cyclophilin D. Protein transfection of anti-cyclophilin D Abs (10 μg) into BL-3 cells inhibited LKT-mediated cytotoxicity and release of cytochrome c from mitochondria, whereas transfection with control β-actin Abs had no effect (Fig. 7A, B, and C; also see Fig. S4 in the supplemental material). In an immunoblot investigation, we coimmunoprecipitated cyclophilin D with LKT from BL-3 cell mitochondrial lysates (Fig. 7D). Furthermore, we were able to inhibit binding of LKT to purified mitochondria by preincubating them with CSA, which is known to bind mitochondrial cyclophilin D (Fig. 7E; also see Fig. S4 in the supplemental material).
FIG. 7.
LKT binds to the mitochondrial matrix protein cyclophilin D. (A) BL-3 cells were protein transfected with anti-cyclophilin D Ab (5 and 10 μg) and then incubated with LKT (0.5 U for 1 h at 37°C), and cytotoxicity was measured as described previously. BL-3 cells transfected with anti-cyclophilin D Ab (10 μg) alone or LKT-treated untransfected cells served as controls. (B) BL-3 cells were protein transfected with anti-cyclophilin D Ab (5 or 10 μg) or anti-β-actin MAb (unrelated Ab control) and then incubated with LKT (0.5 U for 1 h at 37°C). BL-3 cells incubated with LKT alone (Con LKT) and untreated BL-3 cells served as controls. Mitochondria were then purified, lysed, and immunoblotted for LKT and porin-2. (C) BL-3 cells were protein transfected with anti-cyclophilin D Ab or anti-β-actin MAb (unrelated control Ab) and incubated with LKT (0.5 U for 1 h at 37°C). Mitochondria were isolated and lysed, and the lysates were immunoblotted for cytochrome c (Cyt C) and porin-2. (D) LKT was bound to agarose protein A/G beads coated with anti-LKT Ab and incubated overnight at 4°C with a preabsorbed mitochondrial lysate (Mito lysate) from untreated BL-3 cells. The beads were then washed five times in 0.05% Triton X-100-PBS wash buffer, and proteins were eluted with Laemmli buffer. The eluates were immunoblotted for cyclophilin D and LKT. LKT bound to agarose protein A/G beads alone and mitochondrial lysate from untreated BL-3 cells served as controls. (E) Immunoblot demonstrating that preincubation with CSA (5 μM for 30 min at 37°C) reduced binding of LKT (0.5 U for 30 min at 37°C) to purified mitochondria from untreated BL-3 cells.
DISCUSSION
Numerous mechanisms have been suggested for how bacterial toxins kill susceptible mammalian cells. Several recent studies demonstrated the importance of mitochondrial targeting of toxins produced by H. pylori, C. difficile, and S. aureus to mitochondria (56). In these cases toxin-mediated cell death was caspase independent and did not result in typical PTPs in the MOM (14, 18).
Previously, we reported that M. haemolytica LKT induces apoptosis of BL-3 cells in a caspase-9-dependent manner and that in mitochondria isolated from LKT-intoxicated BL-3 cells there was gross damage to the MOM (4). Based on these observations, we hypothesized that LKT is transported into the cell and binds directly to mitochondria. In the present study, we first demonstrated that full-length LKT protein could be identified in purified mitochondrial lysates from LKT-treated BL-3 cells (Fig. 1A). Transfection of anti-LKT antibodies into BL-3 cells prevented binding of LKT to mitochondria. Confocal microscopy and flow cytometry confirmed that 58 to 70% of the mitochondria had LKT bound to their surfaces (Fig. 1C). As anticipated, the mitochondria to which LKT was bound were localized predominantly in the region of the cell where cytoplasm is abundant. Scanning electron microscopy confirmed that clusters of LKT were distributed along the edge of punched-out lesions in the MOM (Fig. 2A and B), suggesting that there is a relationship between clustering of LKT and the nature of lesions in the MOM. Interestingly, we observed that once transfected into cells, LKT binds to mitochondria of RAW 264.7 cells (a murine macrophage cell line). This suggests that mitochondria from different species have some structure that facilitates binding of LKT (Fig. 1D).
Oligomerization of proapoptotic proteins belonging to the Bcl-2 family, such as Bax and Bak, is responsible for formation of PTPs in mitochondria (7, 20, 22, 25, 37, 39, 40, 50). These PTPs release cytochrome c, AIF, Smac and EndoG from the intermembrane space of mitochondria, leading to apoptosis of the cells (2, 11, 23, 43, 48). In the present study, binding of LKT to the MOM resulted in lesions much larger (∼100 nm) than PTPs (∼10 nm), which might have accelerated apoptosis.
Numerous pharmacological substances stabilize the MOM by altering its physiochemical properties (36). Two such agents are CSA and propranolol, both of which inhibit apoptosis by preventing opening of PTPs in mitochondria (5, 15, 17, 32). We found that addition of CSA reduced LKT-induced cytochrome c release by about 65% but had a smaller effect on the collapse of Δψm, which suggests that events other than direct binding of LKT to mitochondria might contribute to the reduction in Δψm. This inference is consistent with the hypothesis that Δψm may be a more sensitive marker than cytochrome c for mitochondrial function. The relationship between Δψm and cytochrome c in mitochondrial dysfunction is complex and variable. For example, in cerebellar neurons, cytochrome c is released before any detectable changes in Δψm (29, 41, 47, 55).
Our data contrast with the finding in a previous report that CSA did not protect mitochondria against H. pylori VacA toxin, which causes mitochondrial damage without inducing a permeability transition (56). In vitro studies with synthetic lipid membranes revealed that CSA changes the membrane curvature from a rectangular form to an inverted-cone form that resists insertion of proapoptotic molecules into the MOM (21). CSA is a known ligand for cyclophilin D, a mitochondrial matrix protein belonging to the peptidyl-prolyl cis,trans-isomerase family that is associated with mitochondrial voltage-dependent ion channels. CSA prevents opening of the PTPs and release of cytochrome c into the cytoplasm in response to binding of the proapoptotic proteins Bax and Bak (12, 16, 42, 52). Perhaps the ability of CSA to protect mitochondria is not limited to inhibition of the opening of PTPs but instead represents a general strengthening of the physical properties of the MOM. The ability of CSA to inhibit binding of LKT to purified mitochondria raises the possibility that mitochondrial binding of LKT involves cyclophilin D. Because cyclophilin D is a mitochondrial matrix protein, it is not clear whether LKT is transported through PTPs into mitochondria or LKT binding to cyclophilin D is secondary to MOM damage. Our observation that LKT binding to mitochondria is inhibited by CSA and anti-cyclophilin D suggests that LKT might act as a microbial ligand for cyclophilin D.
Our results also suggest that the role of dynamin-2 in LKT-mediated cytotoxicity is complex. Dynamin-2 is a vesicle scission protein that is required for both clathrin- and caveola-mediated internalization of LKT (3, 35). Most mammalian mitochondrial membranes (both inner and outer membranes) and the trans-Golgi network are rich in dynamin-2 (49, 54). In the present study, we observed that mitochondria are an important source of dynamin-2 in BL-3 cells. In contrast, virtually undetectable amounts of dynamin-2 were present in the extramitochondrial cytoplasmic fractions (data not shown). We observed that dynamin-2 was mobilized from mitochondria to the cell periphery in LKT-treated BL-3 cells in a time-dependent manner. Perhaps trafficking of dynamin-2 molecules to the cell membrane amplifies internalization of LKT into cells.
Our observation that CSA depletes dynamin-2 from mitochondria and protects BL-3 cells against LKT-mediated cytotoxicity provides evidence for the role of dynamin-2 in LKT targeting to mitochondria. We propose that while LKT mobilizes mitochondrial dynamin-2 to the cell membrane, pretreatment of BL-3 cells with CSA depletes the mitochondria of dynamin-2 that is otherwise available for LKT trafficking into the cell. Thus, CSA seems to have two mechanisms for protection against LKT-induced mitochondrial damage in BL-3 cells. First, it inhibits trafficking of LKT by depleting dynamin-2; and second, it competes with LKT for binding to cyclophilin D.
In summary, the mechanisms of LKT-induced target cell death are complex. As shown previously, LKT binds LFA-1 and moves into lipid rafts that facilitate its internalization (3). LKT then traffics to the MOM, which in turn results in release of cytochrome c and a reduction in Δψm. The ability of CSA to prevent LKT-induced mitochondrial damage and the ability of LKT to localize with cyclophilin D suggest that LKT targets mitochondrial cell death as part of the mechanism of action. To our knowledge, this is the first report that any of the RTX toxins, which are produced by bacterial pathogens of both humans and animals, cause cell death by targeting mitochondria.
Supplementary Material
Acknowledgments
We thank Yoshi Kawaoka, Ian Duncan, Kelly Moore, Philip Oshel, and Yongjing Lee (University of Wisconsin-Madison) for helping with microscopy and Dagmara I. Kisiela for reviewing the manuscript.
This work was funded by grants 2004-14841 and 2006-17522 from the USDA National Research Initiative and by grant WIS04884 from the Wisconsin Agricultural Experiment Station.
Editor: V. J. DiRita
Footnotes
Published ahead of print on 2 September 2008.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Ambagala, T. C., A. P. Ambagala, and S. Srikumaran. 1999. The leukotoxin of Pasteurella haemolytica binds to beta(2) integrins on bovine leukocytes. FEMS Microbiol. Lett. 179161-167. [DOI] [PubMed] [Google Scholar]
- 2.Arnoult, D., B. Gaume, M. Karbowski, J. C. Sharpe, F. Cecconi, and R. J. Youle. 2003. Mitochondrial release of AIF and EndoG requires caspase activation downstream of Bax/Bak-mediated permeabilization. EMBO J. 224385-4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atapattu, D. N., and C. J. Czuprynski. 2007. Mannheimia haemolytica leukotoxin binds to lipid rafts in bovine lymphoblastoid cells and is internalized in a dynamin-2- and clathrin-dependent manner. Infect. Immun. 754719-4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atapattu, D. N., and C. J. Czuprynski. 2005. Mannheimia haemolytica leukotoxin induces apoptosis of bovine lymphoblastoid cells (BL-3) via a caspase-9-dependent mitochondrial pathway. Infect. Immun. 735504-5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Battino, M., S. Bompadre, L. Leone, A. Pugnaloni, C. Rubini, M. S. Ferreiro, I. Gallardo, and P. Bullon. 2003. The effect of cyclosporine A chronic administration on the antioxidant pattern of rat liver mitochondria: structural and functional consequences. Biofactors 18271-275. [DOI] [PubMed] [Google Scholar]
- 6.Capano, M., S. Virji, and M. Crompton. 2002. Cyclophilin-A is involved in excitotoxin-induced caspase activation in rat neuronal B50 cells. Biochem. J. 36329-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng, E. H., M. C. Wei, S. Weiler, R. A. Flavell, T. W. Mak, T. Lindsten, and S. J. Korsmeyer. 2001. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol. Cell 8705-711. [DOI] [PubMed] [Google Scholar]
- 8.Cudd, L. A., C. L. Ownby, C. R. Clarke, Y. Sun, and K. D. Clinkenbeard. 2001. Effects of Mannheimia haemolytica leukotoxin on apoptosis and oncosis of bovine neutrophils. Am. J. Vet. Res. 62136-141. [DOI] [PubMed] [Google Scholar]
- 9.Dassanayake, R. P., S. Shanthalingam, W. C. Davis, and S. Srikumaran. 2007. Mannheimia haemolytica leukotoxin-induced cytolysis of ovine (Ovis aries) leukocytes is mediated by CD18, the beta subunit of beta2-integrins. Microb. Pathog. 42167-173. [DOI] [PubMed] [Google Scholar]
- 10.Dileepan, T., M. S. Kannan, B. Walcheck, P. Thumbikat, and S. K. Maheswaran. 2005. Mapping of the binding site for Mannheimia haemolytica leukotoxin within bovine CD18. Infect. Immun. 735233-5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Er, E., L. Oliver, P. F. Cartron, P. Juin, S. Manon, and F. M. Vallette. 2006. Mitochondria as the target of the pro-apoptotic protein Bax. Biochim. Biophys. Acta 17571301-1311. [DOI] [PubMed] [Google Scholar]
- 12.Fournier, N., G. Ducet, and A. Crevat. 1987. Action of cyclosporine on mitochondrial calcium fluxes. J. Bioenerg. Biomembr. 19297-303. [DOI] [PubMed] [Google Scholar]
- 13.Galmiche, A., J. Rassow, A. Doye, S. Cagnol, J. C. Chambard, S. Contamin, V. de Thillot, I. Just, V. Ricci, E. Solcia, E. Van Obberghen, and P. Boquet. 2000. The N-terminal 34 kDa fragment of Helicobacter pylori vacuolating cytotoxin targets mitochondria and induces cytochrome c release. EMBO J. 196361-6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Genestier, A. L., M. C. Michallet, G. Prevost, G. Bellot, L. Chalabreysse, S. Peyrol, F. Thivolet, J. Etienne, G. Lina, F. M. Vallette, F. Vandenesch, and L. Genestier. 2005. Staphylococcus aureus Panton-Valentine leukocidin directly targets mitochondria and induces Bax-independent apoptosis of human neutrophils. J. Clin. Investig. 1153117-3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gogvadze, V., and C. Richter. 1993. Cyclosporine A protects mitochondria in an in vitro model of hypoxia/reperfusion injury. FEBS Lett. 333334-338. [DOI] [PubMed] [Google Scholar]
- 16.Hanada, H., K. Katsu, T. Kanno, E. F. Sato, A. Kashiwagi, J. Sasaki, M. Inoue, and K. Utsumi. 2003. Cyclosporin A inhibits thyroid hormone-induced shortening of the tadpole tail through membrane permeability transition. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 135473-483. [DOI] [PubMed] [Google Scholar]
- 17.Hay, R., K. Tammi, B. Ryffel, and M. J. Mihatsch. 1986. Alterations in molecular structure of renal mitochondria associated with cyclosporine A treatment. Clin. Nephrol. 25(Suppl 1)S23-S26. [PubMed] [Google Scholar]
- 18.He, D., S. J. Hagen, C. Pothoulakis, M. Chen, N. D. Medina, M. Warny, and J. T. LaMont. 2000. Clostridium difficile toxin A causes early damage to mitochondria in cultured cells. Gastroenterology 119139-150. [DOI] [PubMed] [Google Scholar]
- 19.Heddleston, K. L., R. C. Reisinger, and L. P. Watko. 1962. Studies on the transmission and etiology of bovine shipping fever. Am. J. Vet. Res. 23548-553. [PubMed] [Google Scholar]
- 20.Hemmati, P. G., D. Guner, B. Gillissen, J. Wendt, C. von Haefen, G. Chinnadurai, B. Dorken, and P. T. Daniel. 2006. Bak functionally complements for loss of Bax during p14(ARF)-induced mitochondrial apoptosis in human cancer cells. Oncogene 256582-6594. [DOI] [PubMed] [Google Scholar]
- 21.Henry-Mowatt, J., C. Dive, J. C. Martinou, and D. James. 2004. Role of mitochondrial membrane permeabilization in apoptosis and cancer. Oncogene 232850-2860. [DOI] [PubMed] [Google Scholar]
- 22.Hou, Q., and Y. T. Hsu. 2005. Bax translocates from cytosol to mitochondria in cardiac cells during apoptosis: development of a GFP-Bax-stable H9c2 cell line for apoptosis analysis. Am. J. Physiol. Heart Circ. Physiol. 289H477-H487. [DOI] [PubMed] [Google Scholar]
- 23.Ishitsuka, K., T. Hideshima, M. Hamasaki, N. Raje, S. Kumar, K. Podar, S. Le Gouill, N. Shiraishi, H. Yasui, A. M. Roccaro, Y. Z. Tai, D. Chauhan, R. Fram, K. Tamura, J. Jain, and K. C. Anderson. 2005. Novel inosine monophosphate dehydrogenase inhibitor VX-944 induces apoptosis in multiple myeloma cells primarily via caspase-independent AIF/Endo G pathway. Oncogene 245888-5896. [DOI] [PubMed] [Google Scholar]
- 24.Jeyaseelan, S., S. L. Hsuan, M. S. Kannan, B. Walcheck, J. F. Wang, M. E. Kehrli, E. T. Lally, G. C. Sieck, and S. K. Maheswaran. 2000. Lymphocyte function-associated antigen 1 is a receptor for Pasteurella haemolytica leukotoxin in bovine leukocytes. Infect. Immun. 6872-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kandasamy, K., S. M. Srinivasula, E. S. Alnemri, C. B. Thompson, S. J. Korsmeyer, J. L. Bryant, and R. K. Srivastava. 2003. Involvement of proapoptotic molecules Bax and Bak in tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced mitochondrial disruption and apoptosis: differential regulation of cytochrome c and Smac/DIABLO release. Cancer Res. 631712-1721. [PubMed] [Google Scholar]
- 26.Kluck, R. M., E. Bossy-Wetzel, D. R. Green, and D. D. Newmeyer. 1997. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science 2751132-1136. [DOI] [PubMed] [Google Scholar]
- 27.Knight, R. J., R. Kurrle, S. Stepkowski, F. Serino, T. C. Chou, and B. D. Kahan. 1994. Synergistic immunosuppressive actions of cyclosporine with a mouse anti-rat alpha/beta-T cell receptor monoclonal antibody. Transplantation 571544-1548. [PubMed] [Google Scholar]
- 28.Lally, E. T., I. R. Kieba, A. Sato, C. L. Green, J. Rosenbloom, J. Korostoff, J. F. Wang, B. J. Shenker, S. Ortlepp, M. K. Robinson, and P. C. Billings. 1997. RTX toxins recognize a beta2 integrin on the surface of human target cells. J. Biol. Chem. 27230463-30469. [DOI] [PubMed] [Google Scholar]
- 29.La Piana, G., E. Fransvea, D. Marzulli, and N. E. Lofrumento. 1998. Mitochondrial membrane potential supported by exogenous cytochrome c oxidation mimics the early stages of apoptosis. Biochem. Biophys. Res. Commun. 246556-561. [DOI] [PubMed] [Google Scholar]
- 30.Lawrence, P. K., W. R. Nelson, W. Liu, D. P. Knowles, W. J. Foreyt, and S. Srikumaran. 2008. Beta(2) integrin Mac-1 is a receptor for Mannheimia haemolytica leukotoxin on bovine and ovine leukocytes. Vet. Immunol. Immunopathol. 122285-294. [DOI] [PubMed] [Google Scholar]
- 31.Leite, F., S. O'Brien, M. J. Sylte, T. Page, D. Atapattu, and C. J. Czuprynski. 2002. Inflammatory cytokines enhance the interaction of Mannheimia haemolytica leukotoxin with bovine peripheral blood neutrophils in vitro. Infect. Immun. 704336-4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lesnefsky, E. J., D. He, S. Moghaddas, and C. L. Hoppel. 2006. Reversal of mitochondrial defects before ischemia protects the aged heart. FASEB J. 201543-1545. [DOI] [PubMed] [Google Scholar]
- 33.Li, Y., N. Johnson, M. Capano, M. Edwards, and M. Crompton. 2004. Cyclophilin-D promotes the mitochondrial permeability transition but has opposite effects on apoptosis and necrosis. Biochem. J. 383101-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lum, A. F., C. E. Green, G. R. Lee, D. E. Staunton, and S. I. Simon. 2002. Dynamic regulation of LFA-1 activation and neutrophil arrest on intercellular adhesion molecule 1 (ICAM-1) in shear flow. J. Biol. Chem. 27720660-20670. [DOI] [PubMed] [Google Scholar]
- 35.Marks, B., and H. T. McMahon. 1998. Calcium triggers calcineurin-dependent synaptic vesicle recycling in mammalian nerve terminals. Curr. Biol. 8740-749. [DOI] [PubMed] [Google Scholar]
- 36.Martinez-Caballero, S., L. M. Dejean, and K. W. Kinnally. 2004. Some amphiphilic cations block the mitochondrial apoptosis-induced channel, MAC. FEBS Lett. 56835-38. [DOI] [PubMed] [Google Scholar]
- 37.Mathai, J. P., M. Germain, and G. C. Shore. 2005. BH3-only BIK regulates BAX, BAK-dependent release of Ca2+ from endoplasmic reticulum stores and mitochondrial apoptosis during stress-induced cell death. J. Biol. Chem. 28023829-23836. [DOI] [PubMed] [Google Scholar]
- 38.Narayanan, S. K., T. G. Nagaraja, M. M. Chengappa, and G. C. Stewart. 2002. Leukotoxins of gram-negative bacteria. Vet. Microbiol. 84337-356. [DOI] [PubMed] [Google Scholar]
- 39.Nutt, L. K., V. Gogvadze, W. Uthaisang, B. Mirnikjoo, D. J. McConkey, and S. Orrenius. 2005. Indirect effects of Bax and Bak initiate the mitochondrial alterations that lead to cytochrome c release during arsenic trioxide-induced apoptosis. Cancer Biol. Ther. 4459-467. [DOI] [PubMed] [Google Scholar]
- 40.Nutt, L. K., A. Pataer, J. Pahler, B. Fang, J. Roth, D. J. McConkey, and S. G. Swisher. 2002. Bax and Bak promote apoptosis by modulating endoplasmic reticular and mitochondrial Ca2+ stores. J. Biol. Chem. 2779219-9225. [DOI] [PubMed] [Google Scholar]
- 41.Rego, A. C., S. Vesce, and D. G. Nicholls. 2001. The mechanism of mitochondrial membrane potential retention following release of cytochrome c in apoptotic GT1-7 neural cells. Cell Death Differ. 8995-1003. [DOI] [PubMed] [Google Scholar]
- 42.Ryffel, B., B. M. Foxwell, A. Gee, B. Greiner, G. Woerly, and M. J. Mihatsch. 1988. Cyclosporine-relationship of side effects to mode of action. Transplantation 4690S-96S. [DOI] [PubMed] [Google Scholar]
- 43.Saelens, X., N. Festjens, L. Vande Walle, M. van Gurp, G. van Loo, and P. Vandenabeele. 2004. Toxic proteins released from mitochondria in cell death. Oncogene 232861-2874. [DOI] [PubMed] [Google Scholar]
- 44.Stewart, M. P., C. Cabanas, and N. Hogg. 1996. T cell adhesion to intercellular adhesion molecule-1 (ICAM-1) is controlled by cell spreading and the activation of integrin LFA-1. J. Immunol. 1561810-1817. [PubMed] [Google Scholar]
- 45.Thumbikat, P., T. Dileepan, M. S. Kannan, and S. K. Maheswaran. 2005. Characterization of Mannheimia (Pasteurella) haemolytica leukotoxin interaction with bovine alveolar macrophage beta2 integrins. Vet. Res. 36771-786. [DOI] [PubMed] [Google Scholar]
- 46.Thumbikat, P., T. Dileepan, M. S. Kannan, and S. K. Maheswaran. 2005. Mechanisms underlying Mannheimia haemolytica leukotoxin-induced oncosis and apoptosis of bovine alveolar macrophages. Microb. Pathog. 38161-172. [DOI] [PubMed] [Google Scholar]
- 47.Tsofina, L. M., E. A. Liberman, T. V. Vygodina, and A. A. Konstantinov. 1986. Hexaammineruthenium as an electron donor to mitochondrial cytochrome oxidase: membrane potential generation in the absence of cytochrome c. Biochem. Int. 12103-110. [PubMed] [Google Scholar]
- 48.Uren, R. T., G. Dewson, C. Bonzon, T. Lithgow, D. D. Newmeyer, and R. M. Kluck. 2005. Mitochondrial release of pro-apoptotic proteins: electrostatic interactions can hold cytochrome c but not Smac/DIABLO to mitochondrial membranes. J. Biol. Chem. 2802266-2274. [DOI] [PubMed] [Google Scholar]
- 49.Urrutia, R., J. R. Henley, T. Cook, and M. A. McNiven. 1997. The dynamins: redundant or distinct functions for an expanding family of related GTPases? Proc. Natl. Acad. Sci. USA 94377-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.von Haefen, C., B. Gillissen, P. G. Hemmati, J. Wendt, D. Guner, A. Mrozek, C. Belka, B. Dorken, and P. T. Daniel. 2004. Multidomain Bcl-2 homolog Bax but not Bak mediates synergistic induction of apoptosis by TRAIL and 5-FU through the mitochondrial apoptosis pathway. Oncogene 238320-8332. [DOI] [PubMed] [Google Scholar]
- 51.Wagrowska-Danilewicz, M., and M. Danilewicz. 1998. Intercellular adhesion molecule-1 (ICAM-1), leucocyte function-associated antigen-1 (LFA-1) and leucocyte infiltration in proliferative human glomerulonephritis. Acta Histochem. 100201-215. [DOI] [PubMed] [Google Scholar]
- 52.Waldmeier, P. C., K. Zimmermann, T. Qian, M. Tintelnot-Blomley, and J. J. Lemasters. 2003. Cyclophilin D as a drug target. Curr. Med. Chem. 101485-1506. [DOI] [PubMed] [Google Scholar]
- 53.Welch, R. A. 1991. Pore-forming cytolysins of gram-negative bacteria. Mol. Microbiol. 5521-528. [DOI] [PubMed] [Google Scholar]
- 54.Wiejak, J., and E. Wyroba. 2004. Structure and function of dynamin and dynamin-like proteins. Postepy Biochem. 50240-247. (In Polish.) [PubMed] [Google Scholar]
- 55.Wigdal, S. S., R. A. Kirkland, J. L. Franklin, and M. Haak-Frendscho. 2002. Cytochrome c release precedes mitochondrial membrane potential loss in cerebellar granule neuron apoptosis: lack of mitochondrial swelling. J. Neurochem. 821029-1038. [DOI] [PubMed] [Google Scholar]
- 56.Willhite, D. C., and S. R. Blanke. 2004. Helicobacter pylori vacuolating cytotoxin enters cells, localizes to the mitochondria, and induces mitochondrial membrane permeability changes correlated to toxin channel activity. Cell. Microbiol. 6143-154. [DOI] [PubMed] [Google Scholar]
- 57.Williams, R. O., C. Mauri, L. J. Mason, L. Marinova-Mutafchieva, S. E. Ross, M. Feldmann, and R. N. Maini. 1998. Therapeutic actions of cyclosporine and anti-tumor necrosis factor alpha in collagen-induced arthritis and the effect of combination therapy. Arthritis Rheum. 411806-1812. [DOI] [PubMed] [Google Scholar]
- 58.Yates, W. D. 1982. A review of infectious bovine rhinotracheitis, shipping fever pneumonia and viral-bacterial synergism in respiratory disease of cattle. Can. J. Comp. Med. 46225-263. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.