Abstract
Polyreactive immunoglobulins (Ig) and complement components are present in tissues and blood of healthy individuals. They facilitate pathogen uptake and inactivation in lysosomes of phagocytes and thereby provide rapid protection against infection. Dendritic cells (DCs) are phagocytes that can acquire peptides from phagocytosed antigen to elicit cytotoxic immune responses by CD8+ T lymphocytes. The mechanisms that select peptides for cross-presentation are not fully resolved. Here we investigated the role of polyreactive Ig and complement in directing phagosomal antigen processing for cross-presentation. Phagocytosis facilitated by serum opsonization required the presence of Ig for effective antigen cross-presentation of microbe-derived antigen. The presence of complement C3 in serum promoted phagocytosis, yet phagosomes were defective in antigen degradation. The small GTPase Rab27a was recently implicated in antigen cross-presentation and was rapidly recruited to phagosomes only when Ig was present. Our data suggest that prebinding of antigen by polyreactive Ig potentiates the efficiency of antigen cross-presentation to CD8+ T cells through recruitment of Rab27a.
Immunoglobulins (Ig) and complement factors are abundant serum constituents that are critically involved in the immediate protection against infection. One mechanism of protection concerns the ability of C3 degradation products and Ig to rapidly opsonize and neutralize pathogens, thereby enhancing receptor-mediated uptake and eventual hydrolysis of pathogens and their products in lysosomes of phagocytes (25). Dendritic cells (DCs) function as phagocytes and also play a pivotal role in the maintenance of immunological tolerance and in promotion of adaptive immune responses to foreign antigens. Adaptive immunity is initiated when DCs display antigenic peptides in complex with molecules of the major histocompatibility complex (MHC) to T cells. Antigens derived from the extracellular milieu serve as substrates for presentation as peptide/class II MHC complexes to CD4 T cells, whereas antigens that are synthesized intracellularly are mostly presented as peptide/class I MHC to CD8 T cells. A third pathway of presentation is termed cross-presentation, which is the process whereby exogenous proteins are presented on class I MHC. DCs selectively process some exogenous antigens for cross-presentation as peptide/MHC class I complexes, while degrading others to single amino acids. The mechanisms involved with selection of antigen for cross-presentation are not fully resolved.
Both complement components and Ig are present in the serum of healthy individuals without previous immunization. Polyreactive Ig of the IgM, IgG, or IgA isotype recognize a broad range of self and foreign antigens with low affinity (16). The binding of complement to antigens of invading microbes can be achieved by triggering of the classical, alternative, or lectin pathway of complement activation; all three converge at the covalent attachment of the large C3 component C3b to the target surface. C3b or its subsequent degradation products, iC3b and C3d, provide the opsonin piece that is recognized by the complement receptors CR1, CR2, and CR3 expressed by DCs and other phagocytes. The role of complement in promoting adaptive immunity has mostly been studied in B cells, showing that cross-linking of the B-cell receptor with the CD21 complement receptor enhances B-cell function (14). Immune complexes composed of antigen-specific Ig and antigen can efficiently be cross-presented to CD8 T cells by DCs (4, 15). Natural polyreactive Ig usually have moderate affinities for microbial antigens, and their abundance makes it important to understand their role in modulating phagocytosis and cross-presentation by DCs.
In macrophages, phagocytosis is primarily mediated by CR3 (a β2 integrin composed of CD11b and CD18) and Fc receptors (FcR). Whereas CD11b can directly recognize components of the microbial wall (38), phagocytosis via CR3 is most effective when particles are opsonized with complement C3-derived fragments. FcRγ mediate phagocytosis via recognition of the constant region of IgG bound to antigens (20). It is now understood that circulating and tissue-resident DCs also phagocytose particles by using receptors for active complement and IgG (18, 37). Moreover, DCs control whether antigens are fully degraded as part of innate immunity or shuttled toward presentation by MHC molecules for induction of adaptive immunity.
Research on antigen cross-presentation so far has mostly focused on the transfer of endocytosed proteins to the MHC class I loading pathway, an essential step toward cross-presentation. There is evidence to support antigen transfer from early endosomes or the endoplasmic reticulum into the cytosol (1, 2, 10), and cellular exchange of peptides via gap junctions may supply DCs with antigenic peptide for class I MHC-mediated presentation (26, 28). Antigens probably require proteasomal processing and further trimming by cytosolic peptidases to allow class I MHC binding. The formation of peptide/class I MHC complexes is thought to occur in the endoplasmic reticulum (possibly reached via retrograde transport through the secretory pathway) or in phagosomes themselves (12, 35). Regardless of where capture by class I MHC occurs, the stability of the antigen in endocytic compartments is likely to affect the subsequent induction of CD8+ T-cell-mediated adaptive immunity. Therefore, it is important to study the importance of antigen sorting and processing in the endocytic pathway. Several members of the Rab family of small GTPases are involved with regulation of endosomal transport and function (23). Rab27a was recently added to this list; it was thought to restrain phagosomal proteolysis, thereby stimulating antigen cross-presentation (22). Elucidation of the mechanisms involved with antigen proteolysis and cross-presentation should benefit the development of vaccines that stimulate strong CD8+ T-cell responses. We here show that DCs use distinct routes of phagocytosis depending on the opsonins involved in antigen uptake. Opsonin binding controls antigen phagocytosis, processing, and eventual cross-presentation as peptide/class I MHC.
MATERIALS AND METHODS
Mice and sera.
C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME). Class II enhanced green fluorescent protein (EGFP) knock-in mice (7), RAG1−/− mice (27), and C3−/− mice (39) were bred in-house. All mice were on a C57BL/6 background. Experiments were performed in accordance with institutional guidelines for animal use and care.
DC culture.
Experiments were performed using mouse DCs obtained from spleen or cultured from mouse bone marrow (BM-DCs), which gave similar results. Spleen DCs were enriched using a magnetic bead-assisted separation (>90% purity, based on CD11c expression; Miltenyi Biotec Inc., Auburn, CA). BM-DCs were prepared by flushing the bone marrow cavity of C57BL/6 mouse femurs and tibias until a single cell suspension was obtained. Cells were cultured for 4 days in Dulbecco's modified Eagle's medium (Gibco, Invitrogen Corporation) without phenol red; supplemented with 10% fetal bovine serum (FBS), l-glutamine (2 mM), and penicillin-streptomycin (200 U/ml and 200 μg/ml, respectively) in the presence of interleukin-4 (1 ng/ml; PeproTech, Rocky Hill, NJ) and granulocyte-macrophage colony-stimulating factor (10 ng/ml; Roche Molecular Biochemicals, Somerville, NJ). Cells were plated at 2.5 × 105 cells/well in flat-bottomed 96-well plates and 2.5 × 106 cells/well on the coverslip in a six-well plate for imaging. Immediately prior to Escherichia coli phagocytosis experiments, DCs were rinsed and received warm medium lacking FBS (37°C).
Bacterial strains and growth conditions.
Escherichia coli strain DH5α and isogenic derivatives expressing OVA138-385 peptide, EGFP, or DsRed were generated in the laboratory of Michael Starnbach (Department of Microbiology and Molecular Genetics, Harvard Medical School). Bacteria were grown under aerobic conditions at 37°C in 3 ml LB broth in the presence of chloramphenicol (OVA138-385-E. coli and DsRed-E. coli) and ampicillin (EGFP-E. coli).
Opsonization of E. coli and phagocytosis by DCs.
Blood was obtained by cardiac puncture from sacrificed mice, from which serum was obtained by centrifugation (10 min at 5,000 rpm). Serum was immediately stored in aliquots sufficient for one experiment each at −20°C to prevent multiple freeze-thaw cycles. Serum was thawed on ice and was immediately used for opsonization of E. coli. Functionality of complement and Ig was comparable between once-thawed serum and fresh serum (data not shown). Where indicated, mouse serum was heat inactivated by incubation at 56°C for 30 min. E. coli bacteria were opsonized with 50 μl mouse serum by incubation at 37°C for 30 min and were subsequently added to DCs in 150 μl FBS-free medium at a multiplicity of infection (MOI) of 50:1. E. coli bacteria were added to DCs for 1 hour, after which unbound E. coli bacteria were removed by washing.
DC killing assay.
Killing of E. coli by DCs was determined using recombinant E. coli expressing a chloramphenicol resistance plasmid and RAG1−/− and C3−/− serum. E. coli bacteria were opsonized with serum and added to DCs for 1 or 6 hours. Then gentamicin was added to specifically kill all remaining extracellular E. coli bacteria. DCs were collected, and serial dilutions were plated in triplicate on LB agar plates containing chloramphenicol. After overnight incubation at 37°C, the numbers of viable colony-forming E. coli bacteria retrieved from DCs were calculated.
Flow cytometry.
Uptake of E. coli was determined by culture of DCs with serum-opsonized GFP-E. coli (1 hour). Immunostaining was performed for 15 min on ice with fluorophore-conjugated antibodies against CD11c (clone HL3; BD Pharmingen) and anti-I-Ab monoclonal antibody (MAb) (clone AF6-120.1; BD Pharmingen). Surface display of SIINFEKL/Kb complexes was determined using ovalbumin (OVA)-E. coli bacteria that were added to DCs for 2, 4, or 24 h, followed by immunostaining with culture supernatant from 25D1.16 hybridoma (30) and Alexa-647 goat anti-mouse IgG (Molecular Probes) and anti-CD11c MAb and anti-I-Ab MAb as described above. Flow cytometry analysis was performed on a fluorescence-activated cell sorting Canto flow cytometer (BD). Activation of DCs by culture with serum-opsonized E. coli was measured using MAb to CD40 (clone 1C10; eBiosciences), CD86 (clone GL1; BD Pharmingen), and I-Ab (clone AF6-120.1; BD Pharmingen).
Confocal microscopy.
Class II-EGFP DCs were cultured from bone marrow on coverslips in six-well plates as described above. To visualize phagocytosed E. coli, serum-opsonized DsRed-E. coli bacteria were added to class II-EGFP DCs at an MOI of 50:1 for 1 and 4 hours. Live cell imaging was performed using a Nikon TE2000U inverted microscope equipped with a 100× oil objective (1.4 numerical aperture) and a Perkin-Elmer UltraView RS system equipped with a spinning-disk confocal head. Perkin-Elmer acquisition software was used for image acquisition and data processing (Universal Imaging Corporation). Thirty micrograph fields that each contain several GFP-expressing DCs and DsRed-expressing E. coli were quantitated for binding of E. coli to the plasma membrane and for uptake into the endocytic route of DCs near the center of the cells. These quantitation experiments were done by Z-stack analysis of 10 to 15 separate sections (1-μm separation in depth) for each one of 30 micrograph fields.
Visualization of formation of SIINFEKL/Kb complexes and phagosome recruitment of Rab27a were performed using class II-EGFP DCs that were incubated with opsonized OVA-E. coli or opsonized DsRed-E. coli, respectively (MOI of 50:1, 2 h, 37°C). DCs were washed and fixed with 4% paraformaldehyde-10% sucrose-phosphate-buffered saline (10 min at 37°C) followed by permeabilization (0.05% saponin in Fc block, 24G2 hybridoma culture supernatant). Formation of SIINFEKL/Kb complexes was performed by immunostaining using culture supernatant from 25D1.16 hybridoma (30) and Alexa-568 goat anti-mouse IgG secondary antibody (Molecular Probes). Rab27a was visualized using anti-Rab27a polyclonal antibody (IBL America) and Alexa-647 goat anti-mouse IgG secondary antibody (Molecular Probes). Imaging of immunostained DCs was performed using an Olympus Fluoview confocal microscope.
Statistical analyses.
An unpaired two-tailed t test was used for statistical analyses. P values below 0.05 are considered statistically significant and are indicated in the figures by an asterisk.
RESULTS
Phagocytosis routes in mouse DCs involving complement and Ig.
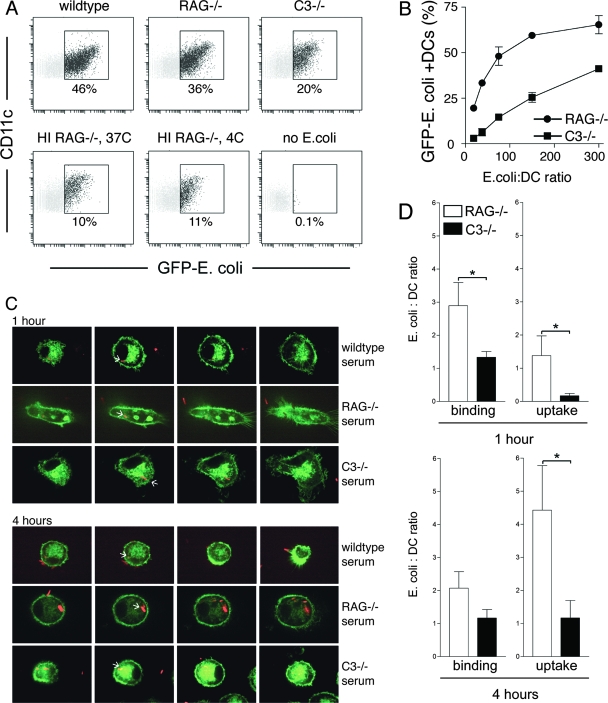
Pathogen recognition and phagocytosis involve multiple ligand-receptor interactions, including those mediated by complement components and Ig, most notably IgG (3). To test the involvement of complement C3 and Ig in phagocytosis, we used whole serum extracted from unstimulated knockout mice. Serum from C3−/− mice lacks all complement factor C3 and opsonins derived from C3 but contains normal levels of Ig (19). Serum from RAG1−/− mice lacks all Ig but is sufficient in complement components (27). Control serum that lacks both complement C3 and Ig was generated by enzymatic inactivation of RAG1−/− serum by heat treatment (33). Recombinant bacteria expressing GFP were used to visualize surface binding and internalization of E. coli by mouse DCs (Fig. 1A). E. coli bacteria were added to DCs for 1 hour, after which unbound E. coli bacteria were removed by washing.
FIG. 1.
C3-derived opsonins and Ig facilitate phagocytosis by mouse DCs. (A) Fresh sera from wild-type, RAG1−/−, and C3−/− mice, as well as heat-inactivated (HI) serum from RAG1−/− mice, were used to opsonize GFP-expressing E. coli. Wild-type serum contains complement and Ig; RAG1−/− serum is complement sufficient but Ig deficient; C3−/− serum lacks complement C3 but is sufficient in Ig; heat-inactivated RAG1−/− serum is deficient in both complement and Ig. GFP fluorescence was measured in DCs after 60 min of internalization using an MOI of 50:1. (B) GFP-expressing E. coli bacteria were opsonized using fresh sera from RAG1−/− and C3−/− mice, and titers were determined on DCs in twofold dilutions (MOIs of 300:1, 150:1, 75:1, 38:1, and 19:1). RAG−/− serum promoted superior E. coli phagocytosis at all MOIs. Data are representative of more than five independent experiments. (C) Opsonized DsRed-E. coli bacteria were added to DCs derived from class II-EGFP mice and analyzed for deposition into class II-EGFP-positive endosomal compartments, using class II-EGFP as a marker for late endosomes/lysosomes (7). Four consecutive Z-stack sections are shown through the center portion of DCs downward (farthest-right images show dendrites attached to the coverslip). White arrows point toward endosome-localized DsRed-E. coli. (D) Thirty fields were analyzed by Z-stack analysis, each visualizing several DCs (×100 magnification; at 1 and 4 hours of DC culture in the presence of DsRed-E. coli), and E. coli binding and uptake by DCs were enumerated. *, statistically significant at P < 0.05.
When wild-type serum was used for opsonization, 46% of DCs phagocytosed E. coli. Prebinding of E. coli with C3−/− serum allowed 20% of DCs to internalize bacteria. Opsonization with Ig-deficient RAG−/− serum resulted in 36% GFP-positive DCs. In contrast, only 10% of the DCs were able to bind or internalize the bacteria when heat-inactivated Ig-deficient serum from RAG−/− mice was used for opsonization. These 10% are likely accounted for by other serum factors, such as mannan-binding lectin (21). In summary, these results suggest that binding of both complement and Ig contributes to the phagocytosis of bacteria by DCs.
Next, we determined whether complement and polyclonal Ig promote phagocytosis of E. coli in a dose-dependent manner (Fig. 1B). Twofold serial dilutions of opsonized E. coli were added to DCs starting at an E. coli/DC ratio of 300:1. Opsonization using polyclonal Ig promoted E. coli phagocytosis in a dose-dependent manner. However, at all concentrations analyzed, complement opsonization using RAG−/− serum promoted most phagocytosis, reaching saturation at a ratio of 300:1. Complement and Ig confer immune protection through immediate binding upon microbial infection (8, 31). Complement and Ig opsonization both facilitate phagocytosis, with complement being critical especially at low concentrations.
Efficient delivery of antigen to endosomal/lysosomal compartments requires complement opsonization.
To determine whether complement and polyclonal Ig control the ability of DCs to process endocytosed antigen, we visualized the endosomal transport of phagocytosed E. coli, using DCs from MHC class II-EGFP mice, in which class II-EGFP serves as a marker for late endosomes and lysosomes (7). Red fluorescent protein-expressing E. coli (DsRed-E. coli) bacteria were added to DCs cultured on coverslips and were analyzed by live cell confocal imaging at 1 and 4 hours. Confocal Z-stack analysis allowed for enumeration of their binding and uptake of DsRed-E. coli. Four consecutive Z-stacks through the center portion of each DC are shown in Fig. 1C. Both complement and polyclonal Ig mediate deposition of E. coli into late endosomal and lysosomal compartments as early as within 1 hour. White arrows in Fig. 1C indicate the presence of DsRed-E. coli in MHC class II-EGFP-positive endosomal compartments. Quantitation of bacterial attachment and uptake by DCs was done by Z-stack confocal microscopy analysis of at least 50 DCs for each treatment (Fig. 1D, bar graph).
Ig opsonization targets antigens to compartments with higher antigen degradation activity than does complement opsonization.
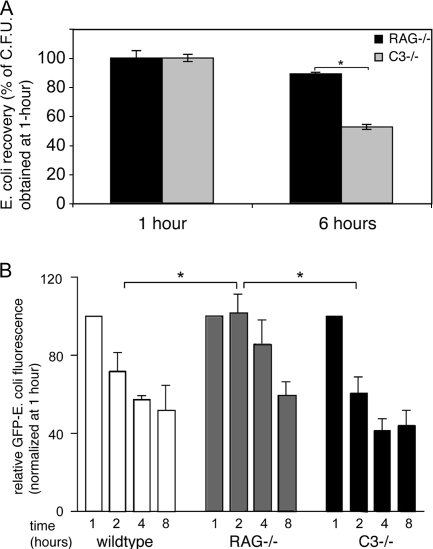
Phagosomal killing of intracellular bacteria occurs via the production of toxic reactive oxygen and nitrogen intermediates by activation of the NADPH oxidase complex (24, 34). We tested whether bacterial binding to complement or polyclonal Ig affects phagosomal killing by DCs. E. coli bacteria were opsonized using RAG1−/− and C3−/− serum, and DCs were allowed to phagocytose and kill E. coli for 1 and 6 hours. Remaining extracellular E. coli bacteria were depleted by addition of gentamicin, and phagosomal E. coli bacteria were recovered by culture on LB-agar plates. In accordance with a report showing that IgG-opsonized Salmonella enterica bacteria do not survive inside mouse DCs (37), opsonization of E. coli with C3−/− serum promoted intracellular killing. However, DC killing of complement-opsonized E. coli was relatively ineffective, as a larger fraction of RAG1−/− serum-opsonized E. coli bacteria formed CFU at 6 hours after phagocytosis (Fig. 2A). These results show that opsonization with polyclonal Ig but not complement promotes phagosomal killing by DCs.
FIG. 2.
Ig facilitate phagosomal degradation of E. coli antigen. (A) Opsonized E. coli bacteria were added to DCs for 1 or 6 hours, after which gentamicin was added to kill remaining extracellular E. coli bacteria. DCs were resuspended and plated in serial dilutions and in triplicate onto LB-agar plates. CFU from endocytosed E. coli were expressed as a fraction of the number recovered after 1 hour of incubation. (B) Live opsonized GFP-E. coli bacteria were added to DCs for 1, 2, 4, and 8 hours, after which DC uptake and processing were stopped and GFP fluorescence in DCs was determined by flow cytometry by counterstaining for the DC marker CD11c. GFP fluorescence at 2, 4, and 8 hours was plotted as a percentage of GFP fluorescence determined at 1 hour of culture using wild-type, RAG−/−, and C3−/− serum opsonization. *, statistically significant at P < 0.05.
We next employed E. coli expressing GFP, to monitor the loss of GFP fluorescence as a readout for processing of E. coli-derived antigen by DCs. E. coli bacteria were opsonized using serum sufficient in polyclonal Ig and complement or with serum that lacks polyclonal Ig or complement C3. As shown in Fig. 2B, when phagocytosis was facilitated by opsonization using Ig-sufficient serum from wild-type or C3−/− mice, GFP fluorescence from E. coli was rapidly lost. In contrast, phagocytosis facilitated by opsonization using complement-sufficient serum that lacks polyclonal Ig, using RAG1−/− serum, caused a restrained loss of GFP fluorescence (2 hours, Fig. 2B). Thus, complement-mediated phagocytosis results in delayed phagosomal/lysosomal killing and antigen processing.
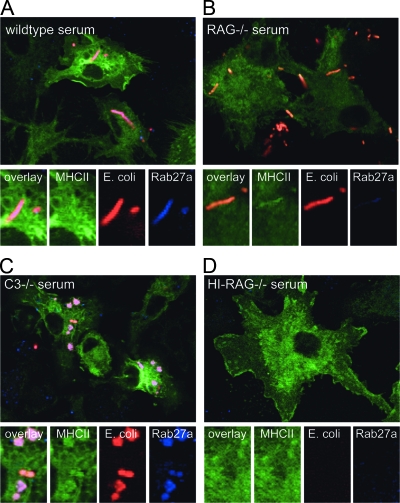
Ig-opsonized antigens, but not complement-opsonized antigens, are delivered into phagosomes that efficiently recruit Rab27a.
Several members of the Rab family of small GTPases localize to distinct endosomal compartments and are key regulators of endosomal transport and function (23). Phagocytosis of latex beads, for example, stimulates the rapid recruitment of the Rab27a to phagosomes, which limits the degradation of bead-coupled OVA and results in improved OVA cross-presentation (22). We here asked whether phagosomes facilitated by complement and polyclonal Ig binding can rapidly recruit Rab27a and whether Rab27a recruitment promotes cross-presentation of phagocytosed microbial antigen. DCs from class II MHC-EGFP mice were allowed to phagocytose DsRed-E. coli for 2 hours and were stained using antibody to Rab27a. Opsonization using C3−/− and wild-type serum, but not RAG1−/− serum, promoted Rab27a recruitment to phagosomes (Fig. 3). We had already established that polyclonal Ig opsonization using C3−/− or wild-type serum, but not complement opsonization, targets antigens to compartments with higher antigen degradation activity (Fig. 2). The rapid recruitment of Rab27a to phagosomes is therefore not necessary for restraining the antigen degradation, as complement-facilitated phagosomes did not recruit Rab27a but degraded GFP more slowly than did Ig-facilitated phagosomes that did recruit Rab27a. In phagosomes where Rab27a is recruited, however, Rab27a recruitment should affect cross-presentation. We next determined the functional consequence of Rab27a recruitment to cross-presentation of phagosomal antigen.
FIG. 3.
Inability to recruit Rab27a to complement-facilitated phagosomes. DCs from MHC class II-EGFP mice were incubated with DsRed-E. coli opsonized with indicated sera to allow uptake. At 2 hours postinfection, DCs were collected and stained for Rab27a. Phagosomes induced by uptake of E. coli bound to wild-type or C3−/− serum showed strong recruitment of Rab27a (A and C), whereas phagosomes formed by E. coli opsonized with RAG−/− serum did not show recruitment of Rab27a (B). Heat-inactivated (HI) RAG−/− serum did not promote E. coli phagocytosis and served as a negative control for Rab27a staining (D).
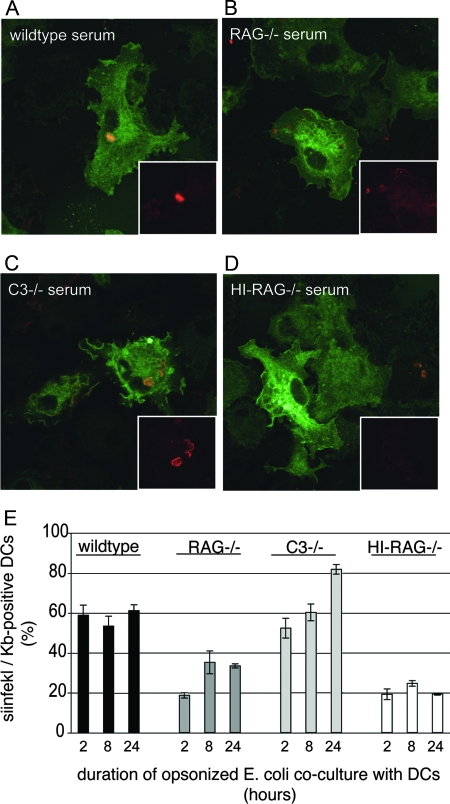
Ig opsonization but not complement opsonization mediates cross-presentation of phagosomal antigen.
Recent evidence suggests that the NADPH oxidase membrane subunit NOX2 is important for antigen cross-presentation by DCs (32). To determine whether the rapid recruitment of Rab27a to phagosomes, as mediated by polyclonal Ig but not by complement, yields cross-presentation of microbial antigen, we made use of E. coli expressing OVA. Expression of OVA by E. coli was confirmed by Western blot analysis using a polyclonal anti-OVA antibody for detection (data not shown). BM-DCs from MHC class II-EGFP mice were allowed to phagocytose opsonized OVA-E. coli for 2 hours and were fixed and analyzed for formation of SIINFEKL/Kb complexes by confocal microscopy (25D1.16 and Alexa-568 goat anti-mouse IgG). DCs that had phagocytosed E. coli opsonized with wild-type or C3−/− serum, but not RAG1−/− serum, show effective formation of SIINFEKL/Kb complexes (Fig. 4A to D). Heat-inactivated RAG1−/− serum served as a negative control, as such serum supports relatively little E. coli intake.
FIG. 4.
Formation of peptide/class I MHC complexes through cross-presentation requires Ig. (A to D) OVA-E. coli bacteria were opsonized with serum from wild-type (A), RAG1−/− (B), or C3−/− (C) mice or with RAG1−/− serum depleted of complement by heat inactivation (HI) (D). Opsonized nonfluorescent E. coli bacteria were then added to MHC class II-EGFP-expressing DCs, and at 2 hours, DCs were fixed and stained for SIINFEKL/Kb complexes using 25D1.16 antibody. Only wild-type and C3−/− serum-opsonized E. coli bacteria were processed sufficiently at this time to detect intracellular SIINFEKL/Kb staining. (E) Surface display of SIINFEKL/Kb complexes was analyzed by flow cytometry after coculture of OVA-E. coli with DCs for 2, 8, and 24 h. E. coli bacteria were opsonized as described for panel A and added to DCs. Opsonization using wild-type and C3−/− sera yields surface display of SIINFEKL/Kb complexes in greater than 50% of DCs starting at 2 hours postuptake. These data show that while RAG1−/− serum promotes phagocytosis, opsonization using RAG1−/− serum does not yield significant SIINFEKL/Kb presentation (20% of DCs express SIINFEKL/Kb complexes at 2 hours postuptake, which is similar to what is found using heat-inactivated RAG−/− serum).
To confirm that SIINFEKL/Kb complexes were displayed on the plasma membrane, DCs were allowed to phagocytose opsonized OVA-E. coli for 2, 8, or 24 h (MOI, 50:1) and were stained for CD11c and surface display of SIINFEKL/Kb. As shown in Fig. 4E, more than half of DCs that had phagocytosed wild-type and C3−/− serum-opsonized OVA-E. coli displayed SIINFEKL/Kb complexes starting at 2 hours of phagocytosis, suggesting that polyclonal Ig opsonization targets antigen to phagosomes dedicated to cross-presentation. At 24 h, DCs that had internalized C3−/− serum-opsonized OVA-E. coli displayed significantly more SIINFEKL/Kb than did DCs that had internalized wild-type serum-opsonized OVA-E. coli, supporting our notion that the presence of complement in wild-type serum counteracts antigen cross-presentation. In DCs that had phagocytosed complement-opsonized E. coli using RAG1−/− serum, the display of SIINFEKL/Kb complexes was delayed and significantly reduced at all time points compared to that for DCs that had phagocytosed wild-type or C3−/− serum-opsonized E. coli. Heat-inactivated RAG1−/− serum served as a negative control. Thus, polyclonal Ig promotes cross-presentation of bacterial antigen, whereas complement opsonization stimulates little if any cross-presentation.
DISCUSSION
The cross-presentation of peptide from an exogenous source, such as that derived from microbial antigens, depends on several factors, such as the concentration and the structure of antigen, its route of internalization, intracellular proteolytic potential, and the stability of peptide/MHC complexes. We considered it important that the mechanisms involved with microbe-directed immunity be elucidated, because microbial infections are still a major health risk. It is because of this risk that infants receive a large number of vaccinations against Corynebacterium diphtheriae (diphtheria), Clostridium tetani (tetanus), and Streptococcus pneumoniae, to name a few. Current vaccines provide only a narrow type of immunity that is likely to be different from broad natural immunity that exists in healthy individuals, as the vaccines induce large amounts of IgG specific to one or a few epitopes, as is the case for human papillomavirus and Streptococcus pneumoniae vaccines. We here studied the antigen phagocytosis, processing, and presentation mechanisms involved in natural protection against microbes.
Two distinct mechanisms of receptor-mediated phagocytosis were identified earlier in macrophages, which employ FcRγ and complement receptor CR3 for uptake of IgG-coated particles and C3bi on complement-opsonized particles, respectively (13). In mouse DCs the relevance of these receptors to phagocytosis had not been clear. We confirmed these two phagocytic routes for DCs by use of E. coli opsonized with sera deficient in Ig and C3. Additionally, we used DCs derived from FcR common γ-chain-knockout mice and blocking antibodies to the CR3 subunit CD11b (data not shown). RAG-deficient serum functioned as a source of serum that lacks Ig but additionally lacks T-cell-derived cytokines.
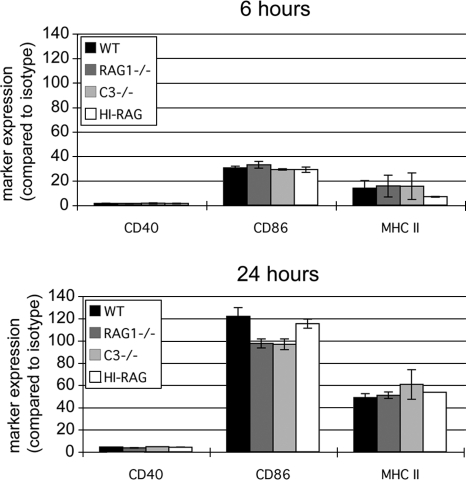
To determine whether phagosomal maturation coincides with general DC maturation, we exposed DCs to E. coli opsonized with serum that lacks or is sufficient in complement or polyclonal Ig. After 6 and 24 h of exposure, DCs were analyzed by staining using CD11c MAb and counterstained with MAb specific for CD40, CD86, and class II MHC or with isotype control MAb. Addition of opsonized E. coli caused the upregulation of CD40, CD86, and class II MHC (as analyzed at 6 and 24 h after addition, compared to isotype control antibodies). DC maturation was comparable between samples, regardless of the presence of complement or Ig (Fig. 5). Phagosome maturation is therefore compartmentalized and not generalized upon DC maturation. We conclude that the binding and uptake facilitated by polyclonal Ig in serum stimulate the maturation in DCs of involved phagosomes for optimal cross-presentation of microbe-derived antigen to CD8+ T cells.
FIG. 5.
Comparable upregulation of activation markers by stimulated DCs. E. coli bacteria were opsonized with serum from wild-type (WT), RAG1−/−, or C3−/− mice or with RAG1−/− serum depleted of complement by heat inactivation (HI). Opsonized E. coli bacteria were then added to BM-DCs. After 6 and 24 h, DCs were stained and analyzed by flow cytometry for surface display of CD40, CD86, and class II MHC or using isotype control antibodies. Opsonized E. coli bacteria induce potent DC activation regardless of the serum used.
The ability of DCs to cross-present phagocytosed antigen had earlier been correlated with phagosomal recruitment of a member of the Rab family, Rab27a. Rab27a is a member of the small GTP-binding protein family, which controls various types of intracellular membrane trafficking in eukaryotic cells (40). DCs from mice that lack Rab27a exhibit impaired cross-presentation of bead-coupled OVA (22). Those studies did not focus on the route through which beads are phagocytosed. The impairment in cross-presentation of bead-associated antigen had been attributed to excessive phagosomal acidification in Rab27a-deficient DCs, causing excessive OVA antigen degradation (22). The absence of Rab27a recruitment to phagosomes should therefore cause excessive degradation. Our findings using phagosomes facilitated by complement-mediated uptake, however, show moderate degradation and cross-presentation of E. coli-expressed antigen and limited phagosomal recruitment of Rab27a. Thus, while Rab27a is clearly important to generate a phagosome dedicated to cross-presentation and is recruited to phagosomes facilitated by Ig binding, Rab27a does not necessarily play this role by inhibition of degradation. It remains possible that the presence of Toll-like receptor ligands in our phagosomes (i.e., TLR4 ligand lipopolysaccharide expressed by E. coli) recruits additional adaptor proteins, possibly even other Rab family GTPases, to phagosomes that inhibit phagosomal degradation. A comparative proteomic analysis of purified DC phagosomes, which includes microbe-induced phagosomes, could elucidate such a protein.
Epithelial cell layers with tight junctions provide mechanical and chemical protection against pathogen infection. When this protection proves insufficient, pathogens enter the normally sterile tissue, where the various arms of the immune system convey protection. Ig and C3 are among the most potent opsonins that readily bind and neutralize pathogens to prevent bacterial outgrowth and uncontrolled inflammation. They are abundantly present in interstitial fluid in barrier tissues such as skin and mucosa, as well as in plasma (concentration of IgG isotypes combined, 6 to 14 mg/ml; concentration of complement C3, 1.2 to 1.3 mg/ml in humans). Their binding facilitates uptake by phagocytes including B cells, macrophages, and DCs. Studies of the role of opsonins in antigen processing had mostly been performed in B cells. Transfection of FcRγ in B cells, for example, stimulates the internalization of IgG-antigen immune complexes (5, 6) and induces cross-presentation via class I MHC (4). In these studies, the IgG antibodies used were antigen specific and had high affinity for the complexed antigen, as they were produced by B plasma cells that had undergone germinal center reactions. Antigen uptake via the C-type lectin DEC205 or mannose receptor mediates cross-presentation by DCs (9, 11). Importantly, natural Ig and complement recognize an epitope expressed by measles virus and mediate cross-presentation by DCs (17). Although DCs were not the focus of this study, the data corroborated our notion that Ig and complement may somehow mediate cross-presentation of phagocytosed material by DCs.
Our work described here shows a previously unrecognized role for Ig and complement in directing phagosomal processing of microbial antigen by DCs. An important functional consequence of phagosome maturation in DCs is to optimize the availability and selection of antigenic peptides for cross-presentation to CD8+ T cells. We propose that targeting antigen for cross-presentation may not require monoclonal antibodies. Pretreatment of vaccination antigens with polyclonal serum Ig in the absence of complement, such as in intravenous Ig preparations, may potentiate antigen-specific CD8 T-cell responses.
Acknowledgments
We acknowledge Edda Fiebiger for critically reading the manuscript and Michael Carroll, Michael Starnbach, and Ronald Germain for providing reagents.
Grant support is acknowledged from The Netherlands Organization for Scientific Research (NWO, Veni grant, to M.B.) and the Harvard Skin Disease Research Center (to M.B.).
Editor: A. J. Bäumler
Footnotes
Published ahead of print on 8 September 2008.
REFERENCES
- 1.Ackerman, A. L., A. Giodini, and P. Cresswell. 2006. A role for the endoplasmic reticulum protein retrotranslocation machinery during crosspresentation by dendritic cells. Immunity 25607-617. [DOI] [PubMed] [Google Scholar]
- 2.Ackerman, A. L., C. Kyritsis, R. Tampe, and P. Cresswell. 2003. Early phagosomes in dendritic cells form a cellular compartment sufficient for cross presentation of exogenous antigens. Proc. Natl. Acad. Sci. USA 10012889-12894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aderem, A., and D. M. Underhill. 1999. Mechanisms of phagocytosis in macrophages. Annu. Rev. Immunol. 17593-623. [DOI] [PubMed] [Google Scholar]
- 4.Amigorena, S. 2002. Fc gamma receptors and cross-presentation in dendritic cells. J. Exp. Med. 195F1-F3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amigorena, S., C. Bonnerot, J. R. Drake, D. Choquet, W. Hunziker, J. G. Guillet, P. Webster, C. Sautes, I. Mellman, and W. H. Fridman. 1992. Cytoplasmic domain heterogeneity and functions of IgG Fc receptors in B lymphocytes. Science 2561808-1812. [DOI] [PubMed] [Google Scholar]
- 6.Amigorena, S., J. Salamero, J. Davoust, W. H. Fridman, and C. Bonnerot. 1992. Tyrosine-containing motif that transduces cell activation signals also determines internalization and antigen presentation via type III receptors for IgG. Nature 358337-341. [DOI] [PubMed] [Google Scholar]
- 7.Boes, M., J. Cerny, R. Massol, M. Op den Brouw, T. Kirchhausen, J. Chen, and H. L. Ploegh. 2002. T-cell engagement of dendritic cells rapidly rearranges MHC class II transport. Nature 418983-988. [DOI] [PubMed] [Google Scholar]
- 8.Boes, M., A. P. Prodeus, T. Schmidt, M. C. Carroll, and J. Chen. 1998. A critical role of natural immunoglobulin M in immediate defense against systemic bacterial infection. J. Exp. Med. 1882381-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonifaz, L., D. Bonnyay, K. Mahnke, M. Rivera, M. C. Nussenzweig, and R. M. Steinman. 2002. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J. Exp. Med. 1961627-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burgdorf, S., A. Kautz, V. Bohnert, P. A. Knolle, and C. Kurts. 2007. Distinct pathways of antigen uptake and intracellular routing in CD4 and CD8 T cell activation. Science 316612-616. [DOI] [PubMed] [Google Scholar]
- 11.Burgdorf, S., V. Lukacs-Kornek, and C. Kurts. 2006. The mannose receptor mediates uptake of soluble but not of cell-associated antigen for cross-presentation. J. Immunol. 1766770-6776. [DOI] [PubMed] [Google Scholar]
- 12.Burgdorf, S., C. Scholz, A. Kautz, R. Tampe, and C. Kurts. 2008. Spatial and mechanistic separation of cross-presentation and endogenous antigen presentation. Nat. Immunol. 9558-566. [DOI] [PubMed] [Google Scholar]
- 13.Caron, E., and A. Hall. 1998. Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science 2821717-1721. [DOI] [PubMed] [Google Scholar]
- 14.Carter, R. H., M. O. Spycher, Y. C. Ng, R. Hoffman, and D. T. Fearon. 1988. Synergistic interaction between complement receptor type 2 and membrane IgM on B lymphocytes. J. Immunol. 141457-463. [PubMed] [Google Scholar]
- 15.den Haan, J. M., and M. J. Bevan. 2002. Constitutive versus activation-dependent cross-presentation of immune complexes by CD8(+) and CD8(-) dendritic cells in vivo. J. Exp. Med. 196817-827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dighiero, G. 1997. Natural autoantibodies, tolerance, and autoimmunity. Ann. N. Y. Acad. Sci. 815182-192. [DOI] [PubMed] [Google Scholar]
- 17.Durrbach, A., E. Baple, A. F. Preece, B. Charpentier, and K. Gustafsson. 2007. Virus recognition by specific natural antibodies and complement results in MHC I cross-presentation. Eur. J. Immunol. 371254-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fanger, N. A., K. Wardwell, L. Shen, T. F. Tedder, and P. M. Guyre. 1996. Type I (CD64) and type II (CD32) Fc gamma receptor-mediated phagocytosis by human blood dendritic cells. J. Immunol. 157541-548. [PubMed] [Google Scholar]
- 19.Fischer, M. B., M. Ma, S. Goerg, X. Zhou, J. Xia, O. Finco, S. Han, G. Kelsoe, R. G. Howard, T. L. Rothstein, E. Kremmer, F. S. Rosen, and M. C. Carroll. 1996. Regulation of the B cell response to T-dependent antigens by classical pathway complement. J. Immunol. 157549-556. [PubMed] [Google Scholar]
- 20.Gessner, J. E., H. Heiken, A. Tamm, and R. E. Schmidt. 1998. The IgG Fc receptor family. Ann. Hematol. 76231-248. [DOI] [PubMed] [Google Scholar]
- 21.Hansen, S., and U. Holmskov. 1998. Structural aspects of collectins and receptors for collectins. Immunobiology 199165-189. [DOI] [PubMed] [Google Scholar]
- 22.Jancic, C., A. Savina, C. Wasmeier, T. Tolmachova, J. El-Benna, P. M. Dang, S. Pascolo, M. A. Gougerot-Pocidalo, G. Raposo, M. C. Seabra, and S. Amigorena. 2007. Rab27a regulates phagosomal pH and NADPH oxidase recruitment to dendritic cell phagosomes. Nat. Cell Biol. 9367-378. [DOI] [PubMed] [Google Scholar]
- 23.Jordens, I., M. Marsman, C. Kuijl, and J. Neefjes. 2005. Rab proteins, connecting transport and vesicle fusion. Traffic 61070-1077. [DOI] [PubMed] [Google Scholar]
- 24.Karupiah, G., N. H. Hunt, N. J. King, and G. Chaudhri. 2000. NADPH oxidase, Nramp1 and nitric oxide synthase 2 in the host antimicrobial response. Rev. Immunogenet. 2387-415. [PubMed] [Google Scholar]
- 25.Lamm, M. E. 1997. Interaction of antigens and antibodies at mucosal surfaces. Annu. Rev. Microbiol. 51311-340. [DOI] [PubMed] [Google Scholar]
- 26.Mendoza-Naranjo, A., P. J. Saez, C. C. Johansson, M. Ramirez, D. Mandakovic, C. Pereda, M. N. Lopez, R. Kiessling, J. C. Saez, and F. Salazar-Onfray. 2007. Functional gap junctions facilitate melanoma antigen transfer and cross-presentation between human dendritic cells. J. Immunol. 1786949-6957. [DOI] [PubMed] [Google Scholar]
- 27.Mombaerts, P., J. Iacomini, R. S. Johnson, K. Herrup, S. Tonegawa, and V. E. Papaioannou. 1992. RAG-1-deficient mice have no mature B and T lymphocytes. Cell 68869-877. [DOI] [PubMed] [Google Scholar]
- 28.Neijssen, J., C. Herberts, J. W. Drijfhout, E. Reits, L. Janssen, and J. Neefjes. 2005. Cross-presentation by intercellular peptide transfer through gap junctions. Nature 43483-88. [DOI] [PubMed] [Google Scholar]
- 29.Reference deleted.
- 30.Porgador, A., J. W. Yewdell, Y. Deng, J. R. Bennink, and R. N. Germain. 1997. Localization, quantitation, and in situ detection of specific peptide-MHC class I complexes using a monoclonal antibody. Immunity 6715-726. [DOI] [PubMed] [Google Scholar]
- 31.Quezado, Z. M., W. D. Hoffman, J. A. Winkelstein, I. Yatsiv, C. A. Koev, L. C. Cork, R. J. Elin, P. Q. Eichacker, and C. Natanson. 1994. The third component of complement protects against Escherichia coli endotoxin-induced shock and multiple organ failure. J. Exp. Med. 179569-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Savina, A., C. Jancic, S. Hugues, P. Guermonprez, P. Vargas, I. C. Moura, A. M. Lennon-Dumenil, M. C. Seabra, G. Raposo, and S. Amigorena. 2006. NOX2 controls phagosomal pH to regulate antigen processing during crosspresentation by dendritic cells. Cell 126205-218. [DOI] [PubMed] [Google Scholar]
- 33.Schetters, T., J. Van Run-Van Breda, A. Van Zon, and W. Eling. 1988. Selective depletion of immunoglobulin isotypes after decomplementation of mouse sera by heat treatment. J. Immunol. Methods 109193-197. [DOI] [PubMed] [Google Scholar]
- 34.Segal, A. W. 2005. How neutrophils kill microbes. Annu. Rev. Immunol. 23197-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen, L., and K. L. Rock. 2006. Priming of T cells by exogenous antigen cross-presented on MHC class I molecules. Curr. Opin. Immunol. 1885-91. [DOI] [PubMed] [Google Scholar]
- 36.Reference deleted.
- 37.Tobar, J. A., P. A. Gonzalez, and A. M. Kalergis. 2004. Salmonella escape from antigen presentation can be overcome by targeting bacteria to Fc gamma receptors on dendritic cells. J. Immunol. 1734058-4065. [DOI] [PubMed] [Google Scholar]
- 38.Vetvicka, V., B. P. Thornton, and G. D. Ross. 1996. Soluble beta-glucan polysaccharide binding to the lectin site of neutrophil or natural killer cell complement receptor type 3 (CD11b/CD18) generates a primed state of the receptor capable of mediating cytotoxicity of iC3b-opsonized target cells. J. Clin. Investig. 9850-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wessels, M. R., P. Butko, M. Ma, H. B. Warren, A. L. Lage, and M. C. Carroll. 1995. Studies of group B streptococcal infection in mice deficient in complement component C3 or C4 demonstrate an essential role for complement in both innate and acquired immunity. Proc. Natl. Acad. Sci. USA 9211490-11494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zerial, M., and H. McBride. 2001. Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2107-117. [DOI] [PubMed] [Google Scholar]