Abstract
Neisseria meningitidis is a leading cause of meningitis and sepsis. The pathogenesis of meningococcal disease is determined by both bacterial virulence factors and the host inflammatory response. Toll-like receptors (TLRs) are prominent activators of the inflammatory response, and TLR2, -4, and -9 have been reported to be involved in the host response to N. meningitidis. While TLR4 has been suggested to play an important role in early containment of infection, the roles of TLR2 and TLR9 in meningococcal disease are not well described. Using a model for meningococcal sepsis, we report that TLR9−/− mice displayed reduced survival and elevated levels of bacteremia compared to wild-type mice. In contrast, TLR2−/− mice controlled the infection in a manner comparable to that of wild-type mice. TLR9 deficiency was also associated with reduced bactericidal activity in vitro, which was accompanied by reduced production of nitric oxide by TLR9-deficient macrophages. Interestingly, TLR9−/− mice recruited more macrophages to the bloodstream than wild-type mice and produced elevated levels of cytokines at late time points during infection. At the cellular level, activation of signal transduction and induction of cytokine gene expression were independent of TLR2 or TLR9 in macrophages and conventional dendritic cells. In contrast, plasmacytoid dendritic cells relied entirely on TLR9 to induce these activities. Thus, our data demonstrate an important role for TLR9 in host defense against N. meningitidis.
Neisseria meningitidis is a leading cause of bacterial meningitis and sepsis (29). This gram-negative diplococ, which is commonly carried asymptomatically by humans through colonization of the nasopharynx, may occasionally switch to invasive infection and rapid disease progression through a process where bacterial expression of virulence factors plays a pivotal role (29). Infection with N. meningitidis triggers an innate immune response aimed at eliminating the pathogen. Host factors known to contribute to restriction of N. meningitidis include the complement system (23), antimicrobial peptides (7), reactive nitrogen and oxygen radicals (24, 33), and a number of components of the inflammatory response (9). However, the potent inflammatory response evoked by invasive N. meningitidis infection is also associated with development of disease and is responsible for some of the severe symptoms characteristic of meningococcal disease (29).
Host defense against meningococcal infections is initiated by recognition of the bacteria by host pattern recognition receptors (PRRs), which recognize evolutionarily conserved pathogen-associated molecular patterns. Toll-like receptors (TLRs) represent the best-described class of PRRs involved in recognition of N. meningitidis. Upon ligand recognition, TLRs signal to the intracellular milieu and stimulate expression of inflammatory and antimicrobial genes (1, 2). Two important signaling pathways in this respect are the nuclear factor κB (NF-κB) and mitogen-activated protein kinase pathways, which are activated by TLRs and contribute to expression of several genes involved in the pathogenesis of meningococcal disease (9).
N. meningitidis is recognized by Toll-like receptor 2 (TLR2), TLR4, and TLR9, which recognize bacterial porins, lipooligosaccharide, and genomic DNA, respectively (18, 20, 35). It has been well described that TLR4 plays an important role in the host response against N. meningitidis. Polymorphisms in the TLR4 gene have been reported to be associated with meningococcal sepsis, as well as invasive disease in children (10, 31). We have previously demonstrated that nonlipooligosaccharide meningococcal pathogen-associated molecular patternss also contribute to disease progression in a MyD88-dependent manner (25), indicating a role for other TLRs. However, the roles of TLR2 and TLR9 in meningococcal disease have not been described.
Here we have investigated the roles of TLR2 and TLR9 in a murine model for meningococcal sepsis. We report that TLR9 is essential for bacterial clearance whereas TLR2 plays only a minor role in the model used in this study. Our data further suggest a dual role for TLR9 in the host response to meningococcal infection, both as a potent stimulator of proinflammatory signaling pathways and bactericidal activity and as a player in the eventual resolution of the inflammatory response.
MATERIALS AND METHODS
Bacteria and TLR ligands.
We used the N. meningitidis strain FAM20, which belongs to serogroup C, multilocus sequence type 11. The bacteria were grown overnight in brain heart infusion broth with 10% Levinthal broth, reaching a concentration of (18.0 ± 2.2) × 108 bacteria per ml as determined in a Thoma counting chamber. For stimulations with pure TLR ligands, we used Pam3CSK4, lipopolysaccharide (LPS) (Ultrapure LPS; E. coli 0111:B4), and ODN1826 (all from InvivoGen).
Mouse model of infection.
The mice used were 8- to 10-week-old female C57BL/6J, TLR2−/−, TLR9−/−, or TLR2/9−/− mice. The TLR single-knockout mice were obtained from Oriental Yeast Co Ltd. TLR2/9−/− animals were generated by mating TLR2−/− and TLR9−/− animals. The F1 animals were then intercrossed to get homozygous TLR2/9−/− mice, which were identified by PCR of tail snips. All mice were bred at Taconic M&B (Laven, Denmark). The animals had been backcrossed onto the background of the wild-type C57BL/6J strain for eight generations prior to use in experiments. The animal experiments were approved by the Swedish Ethical Committee. Mice were challenged intraperitoneally (i.p.) with 2 × 108 bacteria in 100 μl phosphate-buffered saline (PBS). Control mice were injected with 100 μl of PBS. In survival studies, mice were monitored for 7 days. Bacteremia was defined by recovery of bacteria from blood samples taken from the tail vein at different time points post-i.p. challenge. Briefly, 5 μl of blood was diluted in GC liquid and serial dilutions were plated on GCB plates; the CFU were enumerated the next day. Blood smears were made for analysis of neutrophil/macrophage recruitment by light microscopy after Wright's staining (Sigma, St. Louis, MO). Mice (n = 6 per group) were anesthetized by isoflurane (Forene, Abbott) before blood was collected by retro-orbital bleed. Blood was allowed to clot at 4°C, and sera were collected after centrifugation.
Isolation and culture of mouse peritoneal macrophages.
Peritoneal cells were isolated as described previously by lavage of the peritoneal cavity (21). The cells were seeded onto tissue culture plates and allowed to settle for 4 h. Nonmacrophage cells were removed by washing of the wells three times with ice-cold PBS. For determination of ex vivo cytokine production, peritoneal cells from infected or vehicle-treated mice were cultured for 20 h after isolation, at which point supernatants were harvested and cytokine levels were determined.
Generation of mouse bone marrow-derived dendritic cells (BM-DCs).
BM-DCs were prepared as described previously (4). Briefly, mice were sacrificed by cervical dislocation and femur and tibia were removed. Bone marrow cells were flushed from the bone shafts and cultured at 2 × 105 cells/ml in medium containing 40 ng/ml recombinant murine granulocyte-macrophage colony-stimulating factor (R&D systems). Fresh medium containing 40 ng/ml granulocyte-macrophage colony-stimulating factor was added after days 3 and 6. On days 7, nonadherent cells were harvested, washed, resuspended in fresh medium, and used for experiments. The cells were 70% CD11c positive as determined by flow cytometry.
Isolation of pDCs from mouse spleens.
Spleens were surgically removed and transferred to RPMI with 5% fetal calf serum (FCS). The spleens were then transferred to a 1-mg/ml suspension of collagenase D (Roche). The enzyme was injected into the organ, which was subsequently cut into small pieces, followed by incubation in the collagenase D suspension for 30 min at 37°C. The suspension was filtrated over a 70-μm-pore-size cell strainer (BD Falcon), spun down, and suspended in RPMI-5% FCS. Erythrocytes were removed using Lympholyte-M (CL5035; Cederlane Laboratories), following the instructions of the manufacturer. After centrifugation, the cells were resuspended in PBS with 2 mM EDTA and 0.5% bovine serum albumin (MACS running buffer) in concentrations according to the manufacturer's instruction (MACS; Miltenyi Biotec). Anti-mPDCA-1 microbeads were added, and after incubation for 15 min at 4°C, the suspension was spun down and suspended in running buffer. Plasmacytoid DCs (pDCs) were then isolated in an Auto-MACS separator by positive selection. Isolated fractions were spun down, suspended in medium, and counted. The purity of the pDC preparation was determined to be 75% by flow cytometry.
Stimulation of macrophages, BM-DCs, and pDCs in vitro.
For stimulation of cells in vitro, the cells were seeded in 96-well and 6-well tissue culture plates at densities of 3.5 × 105 and 5.0 × 106 cells per well, respectively, in RPMI supplemented with antibiotics and 10% FCS. The cells were stimulated as appropriate, and culture supernatants were harvested after the appropriate amount of time for measurement of cytokines. For measurement of intracellular signaling proteins, the cells were lysed using a cell lysis kit from Bio-Rad.
Determination of cytokines and chemokines.
Cytokines were measured by enzyme-linked immunosorbent assay and Luminex technology assay as described earlier (19, 21, 25, 26). Tumor necrosis factor alpha (TNF-α) was measured using a kit (Ultrasensitive; sensitively, 0.01 pg/ml) from Biosource. Type I interferon (IFN) bioactivity was measured using an L929-cell-based bioassay (28).
Measurement of signal transduction pathways.
To measure activation of signal transduction pathways, cells receiving the appropriate treatment were lysed using a Bio-Rad cell lysis kit. The levels of phosphorylated IκBα was measured using a Bio-Rad Luminex kit, following the instructions of the manufacturer, similar to the Luminex protocol described above.
Nitrite determination.
Nitrite is generated by the rapid oxidation of nitric oxide (NO). It is stable, and its accumulation in the culture medium reflects the amount of NO produced. To assay nitrite, we used the Griess assay. Aliquots of 100 μl culture supernatants were mixed with equal volumes of the Griess reagent (equal volumes of 0.1% N-(1-naphthyl)-ethylenediamine dihydrochloride and 1% p-aminobenzene sulfanilamide diluted in 2.5% phosphoric acid) in a 96-well microtiter plate (Maxisorb Immunoplate; Nunc). After a 10-min incubation at room temperature, the absorbance at a wavelength of 540 nm was measured in a microplate reader. A range of twofold dilutions of sodium nitrite (0.05 to 100 μM) in RPMI medium was run in each assay to generate a standard curve.
Phagocytosis of N. meningitidis by macrophages.
For fluorescein labeling, organisms (0.5 ml in RPMI 1640 medium without phenol red) at an A540 value of 1 were mixed with an equal volume of fluorescein isothiocyanate (FITC) solution (1 mg/ml in RPMI medium without phenol red) and incubated at 37°C for 30 min. The organisms were spun at 9,500 × g for 1 min and washed three times with 0.5 ml of RPMI medium. Organisms were fixed by the addition of 0.5% (wt/vol) (final concentration) paraformaldehyde and resuspended in RPMI medium. FITC-labeled organisms (50 μl) were incubated with peritoneal macrophages (five bacteria per macrophage) in a total volume of 1 ml, and the suspension was incubated at 37°C for 60 min. The cells were washed three times in 37°C RPMI and fixed, and phagocytosis was determined by flow cytometry.
Growth of meningococci in mouse whole blood.
Whole blood was collected from B6, TLR2−/−, TLR9−/−, and TLR2/9−/− mice (n = 3), pooled, and mixed 1:1 with bacteria suspended in Dulbecco's modified Eagle cell culture medium (1 × 105 CFU of FAM20/ml). The mixtures were incubated for 1 h at 37°C, and the number of surviving bacteria was determined by spreading 10-fold serially diluted supernatants on GCB plates incubated overnight in air plus 5% CO2 at 37°C. The number of CFU was determined the next day. Assays were performed twice in triplicate, and data show the percentage recovery of bacteria compared to the initial inoculum.
Statistical analysis.
Survival rates were assessed using Fisher's exact test. Bacterial counts in blood were analyzed using a nonparametric Mann-Whitney test. Cytokine concentrations, phagocytosis, and the serum bactericidal assay were evaluated with a two-tailed Student t test. P values of <0.05 were considered significant.
RESULTS
TLR9 deficiency is associated with impaired survival and increased bacteremia during infection with N. meningitidis.
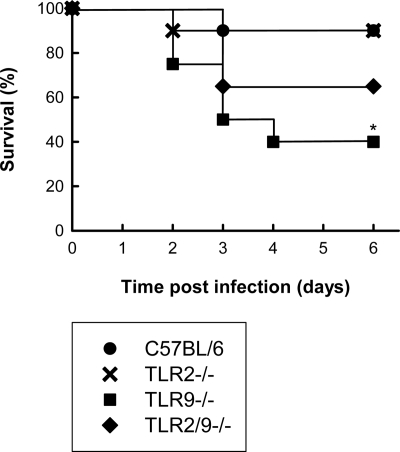
To examine the role of TLR2 and TLR9 in host defense against N. meningitidis infection, we used a murine model for meningococcal sepsis (25, 30). In a first series of experiments, we infected C57BL/6, TLR2−/−, TLR9−/−, and TLR2/9−/− mice i.p. with the FAM20 strain and monitored survival of the animals. As expected, the majority of the wild-type mice (10 out of 12) survived the infection with the dose of bacteria used (Fig. 1). TLR2 deficiency did not affect the survival rate of the mice, but mice lacking TLR9 displayed a significantly lower survival rate than wild-type mice. The TLR2/TLR9 double-deficient mice responded to the infection with a phenotype intermediate between those of the TLR2−/− and TLR9−/− mice.
FIG. 1.
Survival rates of mice after infection with N. meningitidis. C57BL/6, TLR2−/−, TLR9−/−, and TLR2/9−/− mice (n = 12 per group) were infected i.p. with FAM20 (2 × 108 CFU/mouse) and monitored for 6 days. *, P < 0.05 (Fisher's exact test).
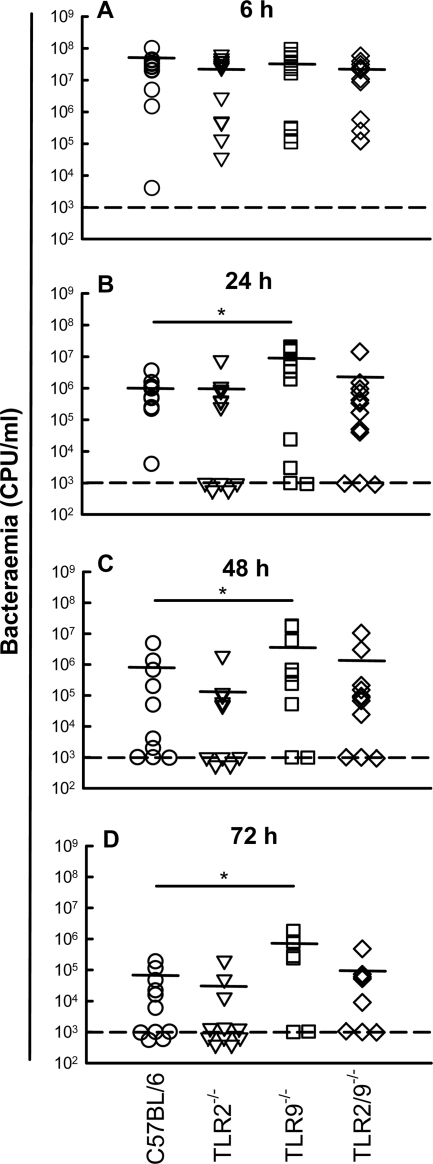
To examine if the differential survival rate of TLR9−/− mice versus wild-type and TLR2−/− mice was reflected in differences in bacterial loads in the bloodstream, we harvested blood samples from the mice at the indicated time points postinfection (p.i.) and measured bacteria in the samples. At 6 h p.i., a high bacterial load was observed for all mouse strains with no significant differences between the strains (Fig. 2A). However, at later time points (24, 48, and 72 h p.i.), the level of bacteremia for TLR9−/− mice was significantly higher than that for C57BL/6 mice (Fig. 2B and D). TLR2−/− and TLR2/9−/− mice were statistically indistinguishable from wild-type mice at all time points. Thus, TLR9 deficiency is associated with impaired survival and increased levels of bacteremia during infection with N. meningitidis.
FIG. 2.
Bacteremia levels in mice after infection with N. meningitidis. C57BL/6, TLR2−/−, TLR9−/−, and TLR2/9 −/− mice (n = 12 per group) were infected i.p. with FAM20 (2 × 108 CFU/mouse). The bacterial load in blood (CFU/ml) was quantified at 6 h (A), 24 h (B), 48 h (C), and 72 h (D) postinfection. The detection limit of this assay is 1,000 CFU per ml blood, as illustrated by the dashed line. Below this limit, mice were considered nonbacteremic. *, P < 0.05 (nonparametric Mann-Whitney test).
TLR9 is required for optimal activation of bactericidal activity.
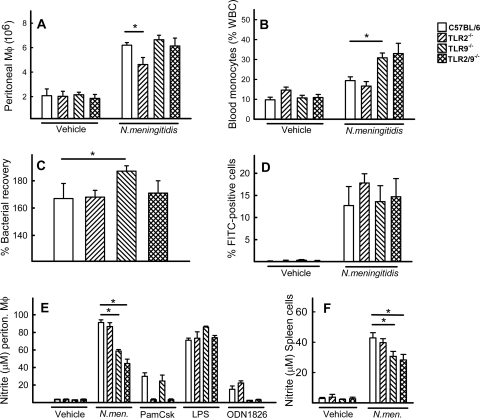
Given the elevated mortality and bacterial load of TLR9−/− mice, we were interested in knowing how TLR9−/− cells controlled meningococcal infection. We first examined recruitment of macrophages to the peritoneal cavity after infection. By 2 h postchallenge, significantly elevated levels of macrophages were found in the peritoneal cavity of all mouse strains compared to results for vehicle-treated mice (Fig. 3A). However, the peritoneal washes from infected TLR2−/− mice contained significantly fewer macrophages than the washes from the C57BL/6 mice. When looking in the bloodstream, we found that cell types of this lineage did migrate to the bloodstream during sepsis, particularly in TLR9−/− mice, from which a significantly higher number of blood monocytes were recovered than from C57BL/6 mice (Fig. 3B). In contrast, in TLR2−/− mice, the infection did not lead to significantly higher levels of blood monocytes. Next, we examined the ability of meningococci to grow in mouse whole blood. Interestingly, significantly more bacteria were recovered from the blood of TLR9−/− mice than from that of C57BL/6 mice (Fig. 3C). The bacterial growth in blood from TLR2−/− and TLR2/9−/− mice was comparable to what was observed in blood from C57BL/6 mice. The observed augmented bacterial growth in a TLR9-deficient environment was not due to an impaired ability of TLR9−/− macrophages to perform phagocytosis, since all four genotypes displayed similar abilities to take up N. meningitidis (Fig. 3D).
FIG. 3.
Killing of N. meningitidis is impaired in cells from TLR9−/− mice. (A and B) C57BL/6, TLR2−/−, TLR9−/−, and TLR2/9 −/− mice (n = 6 per group) were infected i.p. with FAM20 (2 × 108 CFU/mouse). Peritoneal cells (A) or blood smear samples (B) were collected at 2 h or 24 h postchallenge, respectively. Samples from vehicle-treated mice were collected as the control. Macrophage recruitment was analyzed by light microscopy after Wright's staining. Data are represented as means ± standard errors. P values below 0.05 (Student's t test) are shown (*). WBC, white blood cell. (C) Whole blood was collected from C57BL/6, TLR2−/−, TLR9−/−, and TLR2/9 −/− mice (n = 3), pooled within groups, and mixed 1:1 with FAM20 (105 CFU/ml). After incubation for 1 h at 37°C, the number of surviving bacteria was determined. Data shown represent the percentage recovery of bacteria relative to the initial inoculated dose. (D) Phagocytosis of N. meningitidis. Macrophages from C57BL/6, TLR2−/−, TLR9−/−, and TLR2/9−/− mice were incubated with mock- or FITC-labeled bacteria for 60 min and analyzed by flow cytometry. The data are presented as mean percent cells positive for FITC in triplicate cultures ± standard errors. (E and F) Production of NO. Peritoneal macrophages (E) or spleen cells (F) were treated with 2 × 106 bacteria (N.men.)/ml, pure TLR ligands (Pam3Csk2 [TLR2], 200 ng/ml; LPS [TLR4], 100 ng/ml; ODN1826 [TLR9], 1 μM) or vehicle control as indicated for 24 h. Supernatants were harvested, and levels of nitrite were quantified using the Griess assay. The data are shown as means for triplicate cultures ± standard errors.
One way through which macrophages kill meningococci is through production of reactive nitrogen and oxygen radicals (24, 33). To test if the production of NO in response to meningococcal infection was dependent on either TLR2 or TLR9, we harvested and cultured peritoneal macrophages and spleen cells from C57BL/6, TLR2−/−, TLR9−/−, and TLR2/9−/− mice and treated them as indicated in Fig. 3E and F. Nitrite, which is a stable oxidation product of NO, was measured 24 h postinfection. TLR2−/− peritoneal macrophages and spleen cells produced levels of nitrite comparable to those of wild-type cells. However, cells lacking TLR9 produced significantly less nitrite than wild-type cells. Thus, N. meningitidis displays augmented growth in blood from TLR9−/− mice, and leukocytes from these mice produce reduced levels of NO.
Prolonged cytokine production in TLR9-deficient mice.
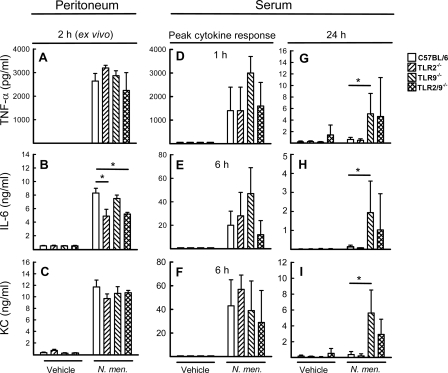
TLR9 agonists are well described as potent inducers of a number of cytokines and chemokines (11, 17), but important roles in negative control of the inflammatory response in vivo also have been ascribed to them (16). Therefore, we were interested in examining the production of cytokines in mice during meningococcal infection. First we infected mice with N. meningitidis. Two hours later, peritoneal cells were isolated and cultured for 20 h to allow ongoing cytokine production to continue. Supernatants were harvested, and cytokines were measured. The cells from infected mice produced large amounts of TNF-α, interleukin 6 (IL-6), and KC (Fig. 4A to C), and for IL-6 the response was partly dependent on TLR2, whereas the two other cytokines were produced independently of TLR2 and TLR9. Next, we harvested serum from uninfected mice and mice challenged for 1, 6, and 24 h with FAM20. For TNF-α, peak levels were observed 1 h postchallenge (Fig. 4D), while the other cytokines measured (IL-6, KC, IL-10, IL-12, and gamma IFN [IFN-γ]) reached the highest levels about 6 h postinfection (Fig. 4E and F; also data not shown). The level of the complement cleavage product C5a was also elevated, with peak levels at 6 h post-bacterial challenge (data not shown). For all parameters, no significant differences between the mouse strains were observed. However, after 24 h, the cytokine levels were close to background levels for C57BL/6 and TLR2−/− mice but remained elevated for TLR9−/− and TLR2/9−/− mice (Fig. 4G to I). For the former mouse strain, the difference was significant for all cytokines and C5a (relative to results for C57BL/6 mice), while for the latter strain, only the levels of IL-10 and IFN-γ were significantly higher. Thus, infection with N. meningitidis induced a potent cytokine response, which was prolonged in TLR9-deficienct mice.
FIG. 4.
Meningococcal infection induces prolonged release of proinflammatory cytokines in serum of TLR9-deficient mice. C57BL/6, TLR2−/−, TLR9−/−, and TLR2/9−/− mice (n = 6 per group) were infected i.p. with FAM20 (2 × 108 CFU/mouse). Peritoneal cells were harvested 2 h postchallenge and cultured for 20 h before isolation of supernatants (A to C), and sera were collected at the indicated time points postchallenge (D to I). Samples from mice challenged with PBS were collected as a control. Induction of TNF-α (A, D, and G), IL-6 (B, E, and H), or KC (C, F, and I) was quantified by enzyme-linked immunosorbent assay and is shown as means ± standard errors. *, P < 0.05 (Student's t test).
N. meningitidis is recognized by pDCs through TLR9.
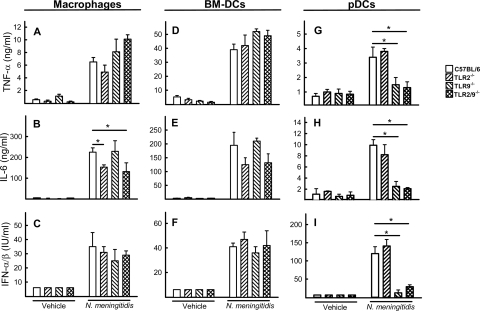
The data shown above suggest a role for TLR9 in protection against meningococcal sepsis. Therefore, we wanted to investigate the specific nature of the cells recognizing N. meningitidis in a TLR9-dependent manner. We focused on macrophages, BM-DCs, and pDCs, of which pDCs are known to be the major TLR9-expressing cell type (2). All three cell types responded to stimulation with the TLR9 agonist ODN1826 with expression of IFN-α/β (data not shown), suggesting that the receptor was expressed on these cell types. When the macrophages and BM-DCs were stimulated with N. meningitidis, expression of TNF-α and IL-6 was strongly induced (Fig. 5A and B and D and E). In addition, type I IFN (Fig. 5C and F), which has been suggested to be a negative modulator of the inflammatory response in certain settings (16), was detected in the culture supernatants. The IL-6 response was partly dependent on TLR2. The pDCs also responded to meningococcal treatment with expression of TNF-α and IL-6, although to a more modest extent than the two other cell types (Fig. 5G and H). Interestingly, the pDCs produced large amounts of IFN-α/β after bacterial stimulation, and this was dependent on TLR9 (Fig. 5I).
FIG. 5.
Cytokine production by macrophages, BM-DCs, and pDCs after infection in vitro. Macrophages (A to C), BM-DCs (D to F), and pDCs (G to I) from wild-type and KO mice were treated with 2 × 107 CFU of FAM20 per ml or vehicle control, and supernatants were harvested 16 h later for measurement of TNF-α, IL-6, and IFN-α/β. The data are shown as means for triplicate cultures ± standard errors. *, P < 0.05 (Student's t test).
To look further into the differential requirement for TLR9 in recognition of meningococci by macrophages, BM-DCs, and pDCs, we examined the ability of N. meningitidis to activate intracellular signaling in these cell types. Cell lysates were prepared from cells incubated with the bacteria for 2 h, and phosphorylation of IκBα was determined as a measure of activation of the NF-κB signaling pathway. In macrophages and BM-DCs, N. meningitidis strongly activated the NF-κB pathway in a TLR9-independent manner (Fig. 6A and B). In macrophages, the NF-κB response displayed a partial dependence on TLR2. The pDCs also mediated activation of the NF-κB pathway in response to bacteria, and in this cell type the response was dependent on TLR9 (Fig. 6C). Thus, N. meningitidis is recognized by pDCs in vitro in a TLR9-dependent manner, leading to activation of the NF-κB pathway and expression of a number of cytokines, including IL-6, TNF-α, and type I IFN.
FIG. 6.
Activation of intracellular signaling in macrophages, BM-DCs, and pDCs after infection in vitro. Macrophages (A), BM-DCs (B), and pDCs (C) from wild-type and KO mice were treated with 2 × 107 CFU of FAM20 per ml or vehicle control, and whole-cell lysates were prepared 2 h later. The levels of phospho-IκBα (P-IκBα) were determined by using Luminex. The data are shown as means for triplicate cultures ± standard errors.
DISCUSSION
TLRs are responsible for innate recognition of microbes, and TLR2, -4, and -9 have previously been reported to be involved in recognition of N. meningitidis (18, 20, 35). While the importance of TLR4 in the host response to meningococcal infection is well described (25, 31), the functions of TLR2 and TLR9 remain unknown. Here we have investigated the roles of TLR2 and TLR9 in a murine model for meningococcal sepsis. It has been reported that preinjection of iron compounds enhances meningococcal infection in mouse models (13, 34). However, the main disadvantage of this experimental model is the unknown effects of iron on the host immune response, especially on the innate immune defense system. Therefore, in this study we challenged mice i.p. without iron pretreatment. We report that TLR9−/− mice displayed elevated levels of N. meningitidis bacteremia and reduced survival compared to wild-type mice but also responded to the infection with enhanced recruitment of macrophages and elevated expression of cytokines at later stages of infection. In contrast, TLR2−/− mice controlled the infection in a manner comparable to that of wild-type mice.
TLR2 recognizes meningococcal porins and is a potent inducer of inflammatory cytokines (18). Accordingly, we found reduced macrophage recruitment to the peritoneal cavity in TLR2−/− mice after infection. In addition, bacterium-induced expression of IL-6 in vitro and ex vivo was reduced in the absence of TLR2. However, in the model used in this study, we did not observe any effect of TLR2 deficiency on bacteremia levels or survival. Thus, our data suggest that TLR2 plays only a minor role in triggering the inflammatory response during N. meningitidis sepsis. These data are in line with our previous observation that TLR2−/− mice displayed unaltered sensitivity to meningococcal infection and that production of inflammatory cytokines and chemokines was not impaired in TLR2−/− mice (26).
A much more pronounced phenotype was observed in the TLR9−/− mice, displaying both reduced bactericidal activity and prolonged production of cytokines. This prolonged expression of cytokines in TLR9−/− mice could indicate that this PRR plays a role in negative regulation of the host response or might alternatively be due to the higher bacterial load in the TLR9−/− mice. However, the elevated cytokine expression 24 h postchallenge was also observed in the TLR2/9−/− mice, which did not exhibit an elevated bacterial load, thus suggesting that TLR9 may play a role in the eventual resolution of the inflammatory response during meningococcal infection.
TLR9 is expressed on a number of cell types, with the highest abundance on pDCs, which are potent inducers of type I IFN (2). By in vitro stimulation, we found that pDCs produced type I IFN and other cytokines in response to live meningococci, and this was dependent on TLR9. The signaling pathways activated by TLR9 include the NF-κB and mitogen-activated protein kinase pathways, which stimulate potent proinflammatory activities (2), but also the IFN regulatory factor pathway, which together with the DNA-dependent protein kinase induces type I IFN and anti-inflammatory activities (2). For instance, stimulation through TLR9 protects mice from experimental colitis through induction of type I IFN (16). Also, stimulation of allergen-loaded macrophages with TLR9 agonists suppressed allergic airway inflammation in mice by a mechanism dependent on IL-10 (32). In contrast, type I IFN is essential for mounting the inflammatory response during Francisella infection through activation of the inflammasome, which triggers production of bioactive IL-1β and IL-18 (12). It remains unknown whether type I IFN is responsible for the prolonged cytokine production observed in our experimental model and more globally how IFN may either promote or inhibit the inflammatory response dependent on the conditions.
One of the key findings in this work is that TLR9 is essential for optimal host defense against N. meningitidis. This is the first report to demonstrate an essential role for this PRR in meningococcal disease. TLR9 recognizes DNA from meningococci and other bacteria (11, 20) and has previously been reported to play a role in defense against infections with Streptococcus pneumoniae and Klebsiella pneumoniae in vivo (3, 8). In the case of pneumococcal infection, TLR9 was essential for uptake and intracellular killing of the bacteria (3), whereas during infection with K. pneumoniae, the role of TLR9 was to recruit and activate leukocytes, including dendritic cells and macrophages, and to promote development of a Th1 response (8). Similar results have been obtained in other studies, where TLR9 has been ascribed important functions in establishment of an IL-12/IFN-γ response (15, 22). In our studies, TLR9 was essential for restriction of bacterial growth but not for phagocytosis. We observed that TLR9−/− and TLR2/9−/− macrophages, as well as total spleen cells, produced reduced levels of NO after meningococcal infection, suggesting that this may contribute to the impaired bactericidal activity of the TLR9−/− mice. In agreement with these data, TLR9-deficient macrophages have been reported to produce reduced levels of NO synthase in a model for mycobacterial antigen-elicited pulmonary granuloma (14). Collectively, TLR9 seems to play an important role in host defense against a range of bacteria and to utilize a whole array of mechanisms to shape the immune response and control bacterial growth.
It has recently been demonstrated that TLR9 contributes to the pathogenesis of polymicrobial sepsis following experimental peritonitis induced by cecal ligation and puncture (27). It was reported that TLR9−/− mice exhibited lower levels of both bacteremia and serum inflammatory cytokines than wild-type mice and had improved survival. These findings are somewhat in contrast to our observation that TLR9−/− mice displayed higher levels of bacteremia and prolonged expression of serum cytokines. However, similar to findings of Plitas et al. (27), we also observed that TLR9 deficiency led to enhanced migration of certain types of leukocytes. The microbial flora giving rise to polymicrobial sepsis in the model used by Plitas and colleagues is very different from the well-defined meningococcal strain used in our study, and the observed differences are most likely explained by this fact. Therefore, these studies suggest that induction as well as control of bacterial sepsis may rely on different immunological mechanisms, including the possible involvement of several different TLRs, depending on the invading pathogen.
TLR2 and TLR9 were previously reported to synergistically mount protective host responses against pathogens. TLR2/9−/− mice were severely impaired in defense against Mycobacterium tuberculosis and Trypanosoma cruzi (5, 6), and this involved reduced production of IL-12 and IFN-γ. However, in our model, no synergy between TLR2 and TLR9 was observed. In fact, the TLR2/9−/− mice displayed a phenotype which in many respects was intermediate between those of the two single knockout mouse strains. The apparent beneficial effect of TLR2 deficiency on the TLR9-deficient background may at least in part be explained by the proinflammatory activity of TLR2 in the model, as discussed above. TLR2 deletion may thus lead to reduced inflammation and hence less immunopathology.
In conclusion, we report that N. meningitidis is recognized by TLR9 in vivo and contributes to production of cytokines and NO by pDCs and macrophages, respectively. Our work further demonstrates that TLR9 is essential for bacterial clearance in vivo, and hence mice deficient in this PRR are more susceptible to meningococcal sepsis.
Acknowledgments
This work was supported by grants from Beckett Fonden, Helga og Peter Kornings Fond, Gangstedfonden, The Danish Medical Research Council (grant no. 271-06-0438), The Swedish Research Council (Dnr 2007-3369, 2004-4831, 2002-6240, 2006-4112, 2006-5073, and 2006-2911, 2006-6304), Swedish Cancer Society, Magnus Bergvalls Foundation, Åke Wibergs Foundation, Swedish Society for Medicine, Torsten och Ragnar Söderbergs Foundation, Knut och Alice Wallenbergs Foundation, Petrus och Augusta Hedlunds Foundation, Tore Nilsons Foundation, Foundation of Goljes Minne, Foundation of Lars Hiertas Minne, Sigurd och Elsa Goljes mine, Laerdal Foundation for Acute Medicine, and Uppsala University.
We thank technicians Tove Findahl and Kirsten Stadel Pedersen for excellent assistance.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 15 September 2008.
REFERENCES
- 1.Akira, S., and K. Takeda. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4499-511. [DOI] [PubMed] [Google Scholar]
- 2.Akira, S., S. Uematsu, and O. Takeuchi. 2006. Pathogen recognition and innate immunity. Cell 124783-801. [DOI] [PubMed] [Google Scholar]
- 3.Albiger, B., S. Dahlberg, A. Sandgren, F. Wartha, K. Beiter, H. Katsuragi, S. Akira, S. Normark, and B. Henriques-Normark. 2007. Toll-like receptor 9 acts at an early stage in host defence against pneumococcal infection. Cell Microbiol. 9633-644. [DOI] [PubMed] [Google Scholar]
- 4.Ank, N., M. B. Iversen, C. Bartholdy, P. Staeheli, R. Hartmann, U. B. Jensen, F. Dagnaes-Hansen, A. R. Thomsen, Z. Chen, H. Haugen, K. Klucher, and S. R. Paludan. 2008. An important role for type III interferon (IFN-λ/IL-28) in TLR-induced antiviral activity. J. Immunol. 1802474-2485. [DOI] [PubMed] [Google Scholar]
- 5.Bafica, A., H. C. Santiago, R. Goldszmid, C. Ropert, R. T. Gazzinelli, and A. Sher. 2006. Cutting edge: TLR9 and TLR2 signaling together account for MyD88-dependent control of parasitemia in Trypanosoma cruzi infection. J. Immunol. 1773515-3519. [DOI] [PubMed] [Google Scholar]
- 6.Bafica, A., C. A. Scanga, C. G. Feng, C. Leifer, A. Cheever, and A. Sher. 2005. TLR9 regulates Th1 responses and cooperates with TLR2 in mediating optimal resistance to Mycobacterium tuberculosis. J. Exp. Med. 2021715-1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergman, P., L. Johansson, H. Wan, A. Jones, R. L. Gallo, G. H. Gudmundsson, T. Hokfelt, A. B. Jonsson, and B. Agerberth. 2006. Induction of the antimicrobial peptide CRAMP in the blood-brain barrier and meninges after meningococcal infection. Infect. Immun. 746982-6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhan, U., N. W. Lukacs, J. J. Osterholzer, M. W. Newstead, X. Zeng, T. A. Moore, T. R. McMillan, A. M. Krieg, S. Akira, and T. J. Standiford. 2007. TLR9 is required for protective innate immunity in Gram-negative bacterial pneumonia: role of dendritic cells. J. Immunol. 1793937-3946. [DOI] [PubMed] [Google Scholar]
- 9.Emonts, M., J. A. Hazelzet, R. de Groot, and P. W. Hermans. 2003. Host genetic determinants of Neisseria meningitidis infections. Lancet Infect. Dis. 3565-577. [DOI] [PubMed] [Google Scholar]
- 10.Faber, J., C. U. Meyer, C. Gemmer, A. Russo, A. Finn, C. Murdoch, W. Zenz, C. Mannhalter, B. U. Zabel, H. J. Schmitt, P. Habermehl, F. Zepp, and M. Knuf. 2006. Human toll-like receptor 4 mutations are associated with susceptibility to invasive meningococcal disease in infancy. Pediatr. Infect. Dis. J. 2580-81. [DOI] [PubMed] [Google Scholar]
- 11.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408740-745. [DOI] [PubMed] [Google Scholar]
- 12.Henry, T., A. Brotcke, D. S. Weiss, L. J. Thompson, and D. M. Monack. 2007. Type I interferon signaling is required for activation of the inflammasome during Francisella infection. J. Exp. Med. 204987-994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holbein, B. E., K. W. Jericho, and G. C. Likes. 1979. Neisseria meningitidis infection in mice: influence of iron, variations in virulence among strains, and pathology. Infect. Immun. 24545-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ito, T., M. Schaller, C. M. Hogaboam, T. J. Standiford, S. W. Chensue, and S. L. Kunkel. 2007. TLR9 activation is a key event for the maintenance of a mycobacterial antigen-elicited pulmonary granulomatous response. Eur. J. Immunol. 372847-2855. [DOI] [PubMed] [Google Scholar]
- 15.Kalis, C., M. Gumenscheimer, N. Freudenberg, S. Tchaptchet, G. Fejer, A. Heit, S. Akira, C. Galanos, and M. A. Freudenberg. 2005. Requirement for TLR9 in the immunomodulatory activity of Propionibacterium acnes. J. Immunol. 1744295-4300. [DOI] [PubMed] [Google Scholar]
- 16.Katakura, K., J. Lee, D. Rachmilewitz, G. Li, L. Eckmann, and E. Raz. 2005. Toll-like receptor 9-induced type I IFN protects mice from experimental colitis. J. Clin. Investig. 115695-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malmgaard, L., J. Melchjorsen, A. G. Bowie, S. C. Mogensen, and S. R. Paludan. 2004. Viral activation of macrophages through TLR-dependent and -independent pathways. J. Immunol. 1736890-6898. [DOI] [PubMed] [Google Scholar]
- 18.Massari, P., P. Henneke, Y. Ho, E. Latz, D. T. Golenbock, and L. M. Wetzler. 2002. Cutting edge: immune stimulation by neisserial porins is toll-like receptor 2 and MyD88 dependent. J. Immunol. 1681533-1537. [DOI] [PubMed] [Google Scholar]
- 19.Mogensen, T. H., R. S. Berg, S. R. Paludan, and L. Ostergaard. 2008. Mechanisms of dexamethasone-mediated inhibition of Toll-like receptor signaling induced by Neisseria meningitidis and Streptococcus pneumoniae. Infect. Immun. 76189-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mogensen, T. H., S. R. Paludan, M. Kilian, and L. Ostergaard. 2006. Live Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis activate the inflammatory response through Toll-like receptors 2, 4, and 9 in species-specific patterns. J. Leukoc. Biol. 80267-277. [DOI] [PubMed] [Google Scholar]
- 21.Mogensen, T. H., S. R. Paludan, M. Kilian, and L. Ostergaard. 2006. Two Neisseria meningitidis strains with different ability to stimulate toll-like receptor 4 through the MyD88-independent pathway. Scand. J. Immunol. 64646-654. [DOI] [PubMed] [Google Scholar]
- 22.Newton, C. A., I. Perkins, R. H. Widen, H. Friedman, and T. W. Klein. 2007. Role of Toll-like receptor 9 in Legionella pneumophila-induced interleukin-12 p40 production in bone marrow-derived dendritic cells and macrophages from permissive and nonpermissive mice. Infect. Immun. 75146-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nicholson, A., and I. H. Lepow. 1979. Host defense against Neisseria meningitidis requires a complement-dependent bactericidal activity. Science 205298-299. [DOI] [PubMed] [Google Scholar]
- 24.Padron, J., Y. Bebelagua, M. Lastre, J. Lapinet, C. Zayas, Y. Quintero, M. Diaz, and O. Perez. 1999. Nitric oxide participates in the immune response against Neisseria meningitidis serogroup B. FEMS Immunol. Med. Microbiol. 25385-389. [DOI] [PubMed] [Google Scholar]
- 25.Plant, L., H. Wan, and A. B. Jonsson. 2006. MyD88-dependent signaling affects the development of meningococcal sepsis by nonlipooligosaccharide ligands. Infect. Immun. 743538-3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plant, L., H. Wan, and A. B. Jonsson. 2007. Non-lipooligosaccharide-mediated signalling via Toll-like receptor 4 causes fatal meningococcal sepsis in a mouse model. Cell Microbiol. 9657-669. [DOI] [PubMed] [Google Scholar]
- 27.Plitas, G., B. M. Burt, H. M. Nguyen, Z. M. Bamboat, and R. P. Dematteo. 2008. Toll-like receptor 9 inhibition reduces mortality in polymicrobial sepsis. J. Exp. Med. 2051277-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rasmussen, S. B., L. N. Sorensen, L. Malmgaard, N. Ank, J. D. Baines, Z. J. Chen, and S. R. Paludan. 2007. Type I interferon production during herpes simplex virus infection is controlled by cell-type-specific viral recognition through Toll-like receptor 9, the mitochondrial antiviral signaling protein pathway, and novel recognition systems. J. Virol. 8113315-13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenstein, N. E., B. A. Perkins, D. S. Stephens, T. Popovic, and J. M. Hughes. 2001. Meningococcal disease. N. Engl. J. Med. 3441378-1388. [DOI] [PubMed] [Google Scholar]
- 30.Sjolinder, H., and A. B. Jonsson. 2007. Imaging of disease dynamics during meningococcal sepsis. PLoS ONE 2e241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smirnova, I., N. Mann, A. Dols, H. H. Derkx, M. L. Hibberd, M. Levin, and B. Beutler. 2003. Assay of locus-specific genetic load implicates rare Toll-like receptor 4 mutations in meningococcal susceptibility. Proc. Natl. Acad. Sci. USA 1006075-6080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vissers, J. L., B. C. van Esch, P. V. Jeurink, G. A. Hofman, and A. J. van Oosterhout. 2004. Stimulation of allergen-loaded macrophages by TLR9-ligand potentiates IL-10-mediated suppression of allergic airway inflammation in mice. Respir. Res. 521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilks, K. E., K. L. Dunn, J. L. Farrant, K. M. Reddin, A. R. Gorringe, P. R. Langford, and J. S. Kroll. 1998. Periplasmic superoxide dismutase in meningococcal pathogenicity. Infect. Immun. 66213-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu, D., Y. Zhang, V. Barniak, L. Bernfield, A. Howell, and G. Zlotnick. 2005. Evaluation of recombinant lipidated P2086 protein as a vaccine candidate for group B Neisseria meningitidis in a murine nasal challenge model. Infect. Immun. 736838-6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zughaier, S. M., Y. L. Tzeng, S. M. Zimmer, A. Datta, R. W. Carlson, and D. S. Stephens. 2004. Neisseria meningitidis lipooligosaccharide structure-dependent activation of the macrophage CD14/Toll-like receptor 4 pathway. Infect. Immun. 72371-380. [DOI] [PMC free article] [PubMed] [Google Scholar]